Summary
The notochord, a conserved axial structure required for embryonic axis elongation and spine development, consists of giant vacuolated cells surrounded by an epithelial sheath [1–3]. During morphogenesis, vacuolated cells maintain their structural integrity despite being under constant mechanical stress [4]. We hypothesized that the high density of caveolae present in vacuolated cells [5, 6] could buffer mechanical tension. Caveolae are 50–80 nm membrane invaginations lined by cage-like polygonal structures [7, 8] formed by caveolin 1 (Cav1) or Cav3, and one of the cavin proteins [6, 9–11]. Recent in vitro work has shown that plasma membrane caveolae constitute a membrane reservoir that can buffer mechanical stresses such as stretching or osmotic swelling [12]. Moreover, mechanical integrity of vascular and muscle cells is partly dependent on caveolae [13–15]. However, the in vivo mechano-protective roles of caveolae have only begun to be explored. Using zebrafish mutants for cav1, cav3 and cavin1b, we show that caveolae are essential for notochord integrity. Upon loss of caveolae function, vacuolated cells collapse at discrete positions under the mechanical strain of locomotion. Then, sheath cells invade the inner notochord and differentiate into vacuolated cells, thereby restoring notochord function and allowing normal spine development. Our data further indicate that nucleotides released by dying vacuolated cells promote sheath cell vacuolization and trans-differentiation. This work reveals a novel structural role for caveolae in vertebrates and provides unique insights into the mechanisms that safeguard notochord and spine development.
Keywords: Notochord, zebrafish, caveolae, mechano-protective, vacuolated cells, nucleus pulposus, sheath cells, vacuolization, trans-differentiation
eTOC
Garcia et al. show that plasma membrane caveolae play a mechanoprotective role in vacuolated cells of the zebrafish notochord. Loss of caveolae causes vacuolated cell collapse during locomotion. Then, invasion of neighboring sheath cells and their trans-differentiation into vacuolated cells repair the damage, allowing normal spine development.
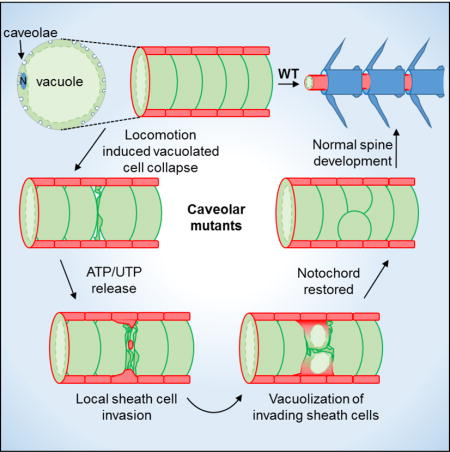
Results and discussion
Caveolin 1 is a conserved vacuolated cell plasma membrane protein in the vertebrate notochord
We previously generated a BAC transgenic line expressing Cav1-GFP under its own regulatory sequences [16]. Live confocal microscopy of 48 hours post fertilization (hpf) embryos revealed robust expression in both sheath (marked by col9a2:mcherry) and vacuolated cells of the notochord (Fig.S1A–C). To determine where Cav1-GFP localizes we isolated vacuolated cells from 48 hpf Cav1-GFP embryos expressing a cytoplasmic mcherry fusion protein or labeled the cell surface with fluorescent wheat germ agglutinin (WGA). Using live confocal microscopy we observed that Cav1-GFP was confined to the plasma membrane, marked with WGA, often in small punctae, and could not be detected in the vacuole membrane or in intracellular compartments (Fig.S1D–I). This is consistent with the high concentration of caveolae present at the plasma membrane of notochord vacuolated cells [5] (see also Fig. S4). Next, we wanted to determine whether caveolin 1 expression and localization is conserved in mammalian notochord cells. In vertebrates, notochord vacuolated cells can be found in the nucleus pulposus (NP) at the center of intervertebral discs (IVDs) [17]. We obtained pig spines, dissected NPs, and dissociated the tissue to generate isolated notochord cells [18]. DIC microscopy revealed a striking resemblance in structure and even in size between pig NP cells and vacuolated cells isolated from embryonic zebrafish notochords (Fig.S1J, K). Confocal microscopy of pig NP cells stained for CAV1 showed a dense concentration of punctae on the plasma membrane, labeled with WGA, which may correspond to caveolae (Fig.S1L, M). In agreement, using electron microscopy (EM), we detected abundant caveolae at the plasma membrane of NP cells (Fig.S1J). These data indicate that caveolae are conserved plasma membrane structures in notochord vacuolated cells.
Loss of caveolae renders notochord vacuolated cells prone to mechanical disruption during locomotion
To investigate the function of caveolae in the zebrafish notochord, we generated mutants for the caveolar genes cav3 and cavin1b by genome editing, and used the previously generated cav1pd1104 allele, which has a mutation that disrupts both cav1 transcripts [16]. These three genes constitute the only genes essential for caveolae formation expressed in the notochord ([5, 6] and our unpublished data). The cav3pd1149 mutant allele was generated using two CRISPRs that remove a 765 bp region between exon 1 and intron 1, resulting in the deletion of 90 base pairs of coding sequence, and a predicted early stop codon after amino acid (aa) 13 (Fig.S2A, D). Using RT-PCR, we found that cav1pd1104 is subject to nonsense mediated decay (Fig.S2F). The cavin1bbns110 allele contains a 7-nucleotide deletion that creates an early stop codon at aa 155, i.e., before the end of the second coiled coil domain (Fig.S2G). This mutation truncates the predicted protein from both cavin1b transcripts and causes decay of the long transcript, but does not eliminate the short transcript (Fig.S2H).
The single cav1 and cav3 zygotic or maternal zygotic (mz) mutants show no gross morphological defects and are adult viable and fertile. Close examination of the notochord revealed no apparent defects in either zygotic or mz mutants (Fig.S3A). We then examined cav1; cav3 double mutants (cav1, 3−/− henceforth), and single cavin1b mutants and found they present no gross anatomical defects (Fig.1A–F). However, close examination of zygotic cav1, 3 and cavin1b mutants revealed disruptions of their notochord structure, starting around the time of embryo hatching (between 48 and 72 hpf). By DIC microscopy, vacuolated cells in 72 hpf larvae appeared disrupted in both cav1, 3 and cavin1 mutants (Fig.1A–F). The penetrance and severity of the notochord lesions are essentially the same for both zygotic mutants (p>0.1, t-test) (Fig.S3C, D). We then used a BODIPY TR methylester dye (MED) to visualize vacuole membranes in live larvae [2] and observed a dramatic collapse of vacuolated cells and the presence of cellular debris in some areas (Fig.1A–F), which became more extensive and pronounced by 96 hpf (Fig.1G, H). We then examined mz mutants and found that while the penetrance and severity of the notochord phenotype is higher at 72 hpf in mz compared to zygotic cav1, 3 or cavin1b mutants (Fig.S3B–F), the onset still occurs after 48 hpf. Because notochord vacuoles are required for axis elongation [2], we measured body length and found that mz but not zygotic mutants present a small but significant reduction in body length compared to heterozygous larvae at 72 and 120 hpf (Fig.S3G–I). This difference is likely due to the later onset of notochord phenotype in zygotic compared to mz mutants. In spite of presenting severe notochord defects, neither cav1, 3 nor cavin1b mutants present spine defects (Fig.S3J–M). At the ultra-structural level, the plasma membrane of mz cav1, 3 mutants showed a sharp reduction in caveolae formation compared to WT as well as the presence of finger-like invaginations that may correspond to misshapen caveolae (Fig.S4A–C). The unexpected finding of a few caveolae still present prompted us to explore whether alternative cav3 transcripts are generated. RT-PCR revealed that in mz cav1, 3 mutants, but not in heterozygous fish, the cav3pd1149 transcript is spliced, generating a predicted alternative start site in the first ATG of the second exon (Fig.S2B, C). Translation of the mutant transcript would generate a smaller protein missing the N-terminus and part of the oligomerization domain, but retaining the rest of the protein (Fig.S2E). This striking compensatory splicing event may allow mz cav1, 3 mutants to form the few normal and the dysmorphic caveolae we detected. In mz cavin1b mutants, we also observed a sharp reduction in caveolae formation compared to WT and the presence of dysmorphic caveoale (Fig.S4D–H). The small number of caveolae still present suggests that the mutated protein retains some residual activity. Altogether, these data indicate that in our cav1, 3 and cavin1b mutant alleles, caveolae formation and function is severely impaired to a similar extent and that the remaining caveolae are insufficient in number and/or are not functional. Because the notochord phenotype of cav1, 3 and cavin1b mutants is essentially identical, subsequent studies were done using cavin1b mutants only.
Figure 1. Loss of caveolae renders notochord vacuolated cells prone to mechanical disruption during locomotion.
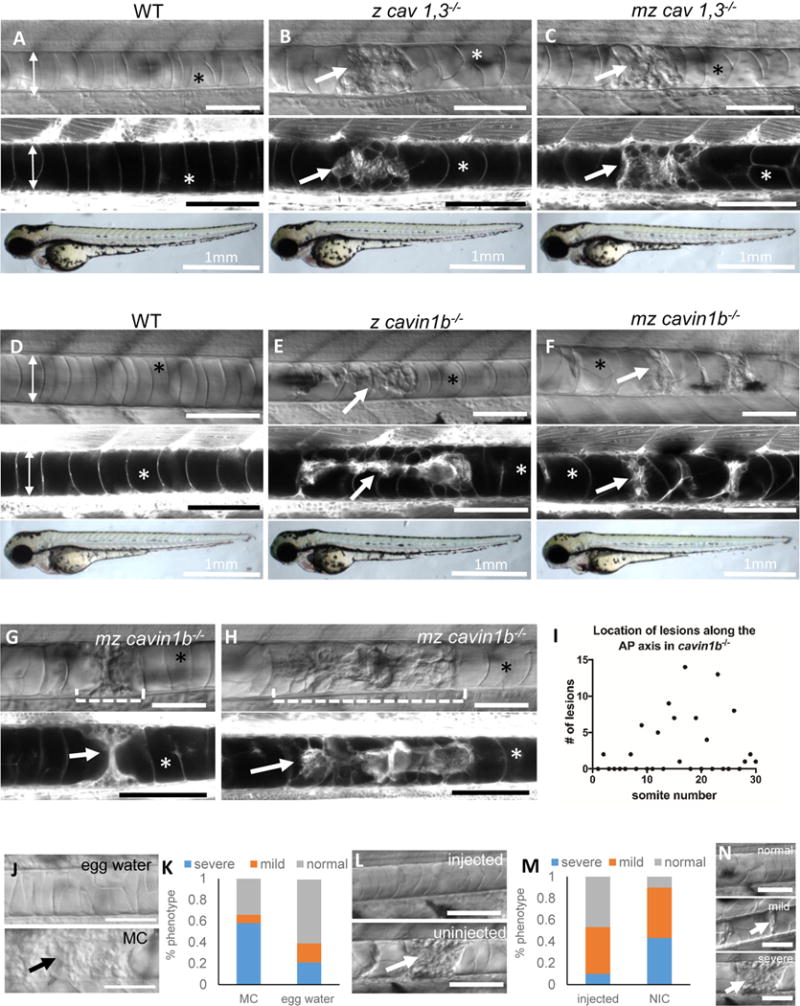
A–F: DIC (top panels), confocal (middle panels) and bright field images (bottom panels) of 72 hpf live MED labeled WT, zygotic (z) cav1, 3−/−, maternal zygotic (mz) cav1, 3−/−, z cavin1b−/−, and mz cavin1b−/− larvae. G–H: DIC and confocal images of a single live MED labeled cavin1b−/− mutant at 72 and 96 hpf. I: Distribution of notochord lesions along the AP axis of 72 hpf z cavin1b−/− larvae (n=30 fish). J–K: 24 hpf embryos from a cavin1b+/− cross were de-chorionated and placed in 3% methyl cellulose (MC) or egg water and scored for notochord lesion severity at 72 hpf. Fisher’s exact test p<0.001, n=83 (MC), n=89 (egg water). L–M: Embryos were injected with 200 pg α-Bungarotoxin and scored at 72 hpf. Fisher’s exact test p<0.001, n=94 (injected), n=74 (non-injected control=NIC). N: Classification of lesion severity. Scale bar: 100μm. Double arrows mark the width of the notochord, arrows point to notochord lesions, asterisks mark intact vacuoles. See also Figure S1, S2, S3 and S4.
Next, we plotted the location of notochord lesions in 72 hpf zygotic cavin1b mutants along the body axis and found that they peak around somite number 17 (Fig.1I). This point coincides with the region of maximum axial bending during the propulsive stroke of swimming larvae [19].
The spatial distribution of notochord lesions and their onset suggested that notochord lesions are triggered by locomotion. To test this hypothesis, we devised a simple experiment to either enhance or reduce the effect of locomotion. To increase mechanical strain, we de-chorionated 24 hpf mz cavin1b−/− embryos and placed them in egg water or in 3% methylcellulose (MC) to increase the viscosity of the medium as previously shown [14]. To abrogate the effect of locomotion, we injected one-cell stage mz cavin1b−/− embryos with α-Bungarotoxin cRNA to paralyze them [20] and incubated them (without removing the chorion) in egg water. Then, at 72 hpf we used DIC microscopy to score the notochord phenotype into three categories: normal (no lesions), mild (one or more areas with limited vacuole collapse), and severe (one or more areas with extended vacuole collapse and debris) (Fig.1N). Interestingly, incubation in MC significantly increased the number and severity of notochord lesions, whereas α-bungarotoxin injection significantly rescued the notochord phenotype (Fig.1J–M). Dechorionation reduced the severity of notochord lesions (compare both controls), likely due to the absence axial bending inside the chorion and hatching movements.
Together, these data indicate that caveolae function is necessary to resist the mechanical load exerted on the notochord by the bending of the axis during swimming strokes.
Collapse of vacuolated cells triggers sheath cell invasion
We next wanted to characterize in better detail the nature and progression of lesions by monitoring both notochord cell types. To this end, we isolated a promoter element from col9a2 to drive expression of transgenes in sheath cells, and a sequence from the col8a1a promoter for vacuolated cells, and established several new sheath and vacuolated cell-specific transgenic lines. In addition, we also used a previously published Gal4 line that drives expression of UAS transgenes in vacuolated cells [1].
We first examined cavin1b mutants and WT siblings expressing col9a2:mcherry and col8a1a:GFPCaaX labeling the cytoplasm of sheath cells and the plasma membrane of vacuolated cells respectively. Using light sheet microscopy (LSM) [21] we found that notochord lesions judged to be mild by DIC microscopy correspond to collapsed vacuolated cells (Fig.2C). Unexpectedly, we also found sheath cells inside the notochord that were tightly associated with the collapsed vacuolated cells (Fig.2D). In contrast, in WT siblings we never observed a sheath cell within the notochord core (Fig.2A, H). LSM imaging of cavin1b mutants with severe lesions revealed extended areas with vacuolated cell collapse with sheath cells surrounding them (Fig.2E, F). However, these clusters of collapsed cells did not seem to affect nearby areas, as we could find intact vacuolated cells without ectopic sheath cells between two close lesions (Fig.2G). Importantly, in all cases, the notochord sheath appeared intact, with a continuous and seemingly normal epithelium.
Figure 2. Collapse of vacuolated cells triggers sheath cell invasion.
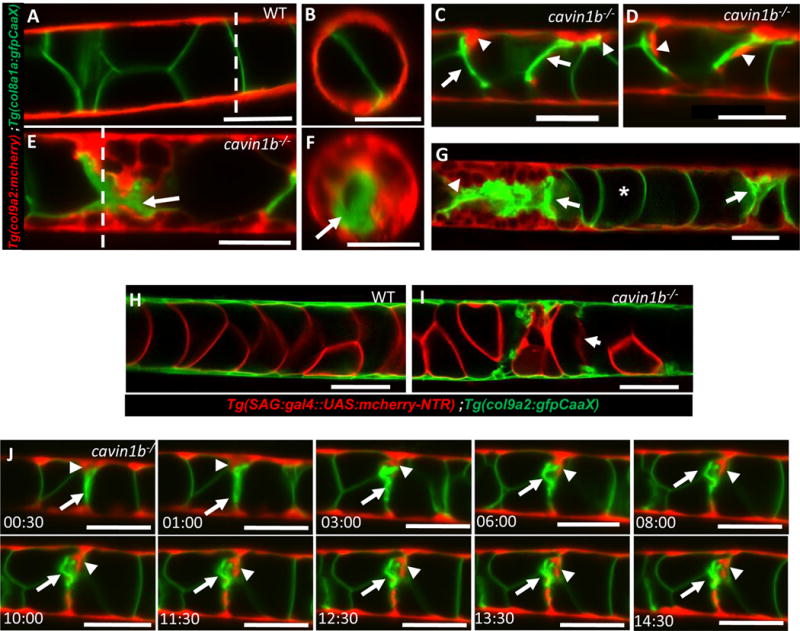
A–B: Lateral and orthogonal views of a live 72 hpf WT larva expressing col8a1a:GFPCaaX in vacuolated cells and col9a2:mcherry in sheath cells. C–D: In cavin1b−/− mutants with mild lesions individual sheath cells wedge and invade the inner notochord where vacuolated cells have collapsed. E–F: Lateral and orthogonal views of a live 72 hpf cavin1b−/− mutant with severe lesions showing multiple invading sheath cells. G: Confocal image of a live 72 hpf cavin1b−/− mutant with two notochord lesions in close proximity (arrows). H–I: Confocal images of live 72 hpf WT and cavin1b−/− mutant expressing sag:gal4::UAS:mcherry-NTR in vacuolated cells and col9a2:GFPCaaX in sheath cells. Collapsed vacuolated cells lose cytoplasmic contents. J: Still frames from a 15-hour LSM time-lapse movie showing sheath cells expressing col9a2:mcherry invading the inner notochord next to a collapsed vacuolated cell expressing col8a1a:GFPCaax. Arrows point to a collapsed vacuolated cell, arrowheads to an invading sheath cell. Scale bars: 50μm. See also Figure S2, S3 and S4, and Movie S1 and Movie S2.
Next, we examined 72 hpf cavin1b−/− larvae expressing col9a2:GFPCaaX to label the plasma membrane of sheath cells and SAG214:Gal4; UAS:mcherry-NTR to label the cytoplasm of vacuolated cells. Live imaging revealed sheath cells within the notochord core, and diffuse or absent mcherry signal in collapsed vacuolated cells, suggesting that the plasma membrane had ruptured in those cells (Fig.2I). Because movement triggers vacuolated cell collapse, we were unfortunately unable to film a collapsing cell. However, direct observation of fish before and after vigorous movement suggests the collapse process is rapid.
In notochord areas with vacuolated cell collapse, we readily found sheath cells wedging toward the remnants of vacuolated cells (Fig. 2C), suggesting that, cells delaminate from the sheath epithelium into the inner notochord in cavin1b mutants. To test this hypothesis we identified 72 hpf cavin1b mutants expressing col9a2:mcherry and col8a1a:GFPCaaX with mild notochord lesions and imaged them using LSM. Under these conditions the larvae are immobilized and no new lesions are generated. We observed single sheath cells within the sheath epithelium wedging toward a collapsed vacuolated cell, then delaminating and moving slowly over the collapsed cell in a span of 15 hours (Fig.2J and Moive S1 and S2).
Collectively, these data indicate that upon loss of caveolae vacuolated cells collapse, likely due to rupture of the plasma membrane, leading to the ingression of sheath cells through a slow delamination and migration process.
Release of vacuolated cell contents triggers sheath cell invasion
We next investigated whether sheath cell invasion is triggered specifically by the loss of caveolare, or by the death of vacuolated cells. To this end, we took 24 hpf WT embryos expressing nitroreductase (NTR) in vacuolated cells, and col9a2:GFPCaaX in sheath cells and treated them with metronidazole (mtz). Nitroreductase (NTR) converts mtz into a toxic compound that kills the expressing, but not neighboring cells [22]. The mtz treatment we used (1.5 mM for 24 hours) typically kills only some of the NTR-expressing notochord cells. At 48 hpf in mtz treated, but not in control animals, we observed clusters of sheath cells wedging towards the notochord core (Fig.3C). One day later, the same region was devoid of vacuolated cells and was filled with sheath cells (Fig.3D). These data indicate that disruption of vacuolated cells triggers sheath cell invasion.
Figure 3. Release of vacuolated cell contents triggers sheath cell invasion.

A–B: Confocal images of live WT embryos expressing sag:gal4::UAS:mcherry-NTR in vacuolated cells and col9a2:GFPCaaX in sheath cells. C–D: In embryos treated with 1.5mM metronidazole (MTZ), sheath cells invade areas of vacuolated cell death. Arrows point to invading sheath cells; asterisks mark intact vacuolated cells. E: LC-MS quantitation of ATP and UTP levels in nucleus pulposus (NP) and annulus fibrosis (AF) tissue isolated from 6 month old pig spines. n=12 for NP and 4 for AF, ***p<0.001, Student’s t-test. See also Figure S3 and S4.
We then hypothesized that the release of vacuolated cell contents promotes sheath cell invasion. To identify candidate molecules enriched in vacuolated cells, we performed a metabolomics analysis [23] of pig NP, which is largely composed of vacuolated cells [18], and the surrounding annulus fibrosus (AF) tissue. Using liquid chromatography-mass spectrometry (LC-MS) we analyzed a set of 200 small molecules and found among the most highly enriched compounds in NP versus AF tissue several nucleotides including UTP, UDP, and ATP (not shown). We then performed a quantitative LC-MS [23] for UTP and ATP and found that both were significantly enriched in NP compared to AF (Fig.3E). Interestingly, nucleotide release has been shown to promote cell migration in zebrafish [24], and mammals [25, 26]. Moreover, UTP has been found accumulating within lysosomes [27, 28], and we showed previously that notochord vacuoles are lysosome related organelles [2]. Together, these data show that vacuolated cell death releases NTPs, and likely also other signals including mechanical stimuli, that may promote sheath cell invasion. The short half-life of extracellular NTPs [29] may help explain why we always found invading sheath cells in close proximity to collapsed vacuolated cells.
Invading sheath cells differentiate into vacuolated cells
We noticed that the invading sheath cells enlarge in size and develop an internal compartment free of cytoplasmic mcherry (see Fig. 2E, G and Fig.S4G), suggesting that they may form a vacuole upon exposure to vacuolated cell contents. To test this possibility we imaged 72 hpf rcn3:GFPRab32a; col9a2:mcherry larvae and found in cavin1b−mutants with severe notochord lesions a dramatic upregulation of rcn3:GFPRab32a in sheath cells next to regions of vacuolated cell collapse (Fig.4A, B). We also observed in sheath cells GFP-Rab32a clearly lining the newly formed vacuoles (Fig.4C, D). Next, we tested whether vacuolization of invading sheath cells is triggered by extracellular NTPs. Because after 48 hpf the notochord sheath is highly impermeable to many compounds [2], we dissociated notochords with or without the addition of purinergic receptor inhibitors suramin (1 mM) and AR-C 118925XX (AR-C) (20 μM) [30] to block the action of NTPs released during the procedure. Then, we plated the dissociated tissues on laminin-coated plates and treated them with a cocktail of hydrolysis-resistant ATP and UTP analogues (ATPγS and UTPγS) +/− AR-C and suramin. Interestingly, upon treatment with ATPγS/UTPγS for 2 hours we found a sharp and significant increase in the fraction of cells forming vacuoles lined by GFP-Rab32a [2], that was abrogated by the inhibitors (Fig.4C). These data indicate that NTPs released from collapsed vacuolated cells act on sheath cells to promote vacuolization.
Figure 4. Invading sheath cells differentiate into vacuolated cells.
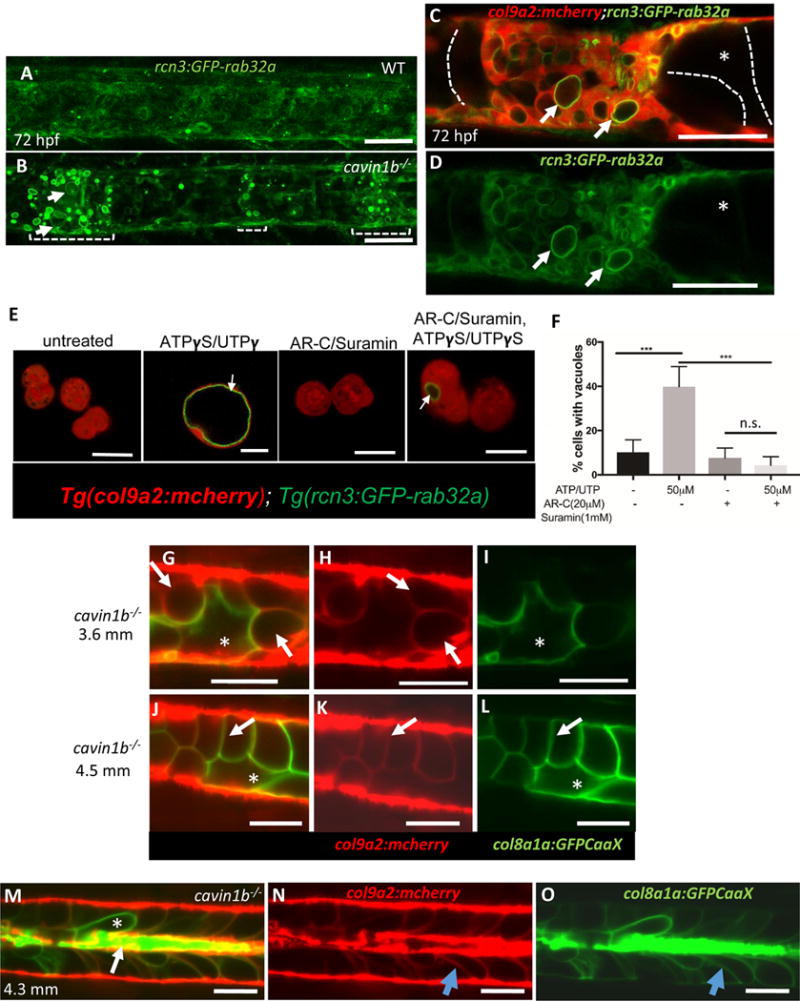
A–B: Projections of confocal stacks of 72 hpf WT and cavin1b−/− larvae expressing rcn3:GFP-rab32a and col9a2:mcherry. Brackets mark areas of vacuolated cell collapse and sheath invasion; arrows point to new vacuoles in sheath cells. C–D: Following invasion, sheath cell vacuoles (arrows) enlarge. Intact primary vacuolated cells are traced with dotted lines. E: Confocal images of live sheath cells obtained from 48 hpf embryos expressing col9a2:mcherry and rcn3:GFP-rab32a. Cells were treated with suramin and AR-C 118925XX (AR-C) or ATPγS/UTPγS or left untreated for 2 hours. Arrows point to newly formed vacuoles. F: Quantitation using one-way ANOVA followed by multiple comparisons using Tukey’s test, p<0.001, n.s.=not significant, n=3 experiments.
G–O: LSM imaging of cavin1b−/− mutants expressing col8a1a:GFPCaax and col9a2:mcherry. G–I: 3.6 mm fish. Arrows point to vacuolated sheath cells, asterisks mark a primary vacuolated cell. J–L: 4.5 mm cavin1b−/− fish showing new vacuolated cells that retained col9a2:mcherry expression (arrow) next to a primary vacuolated cell (asterisk). M–O: 4.3 mm cavin1b−/− fish with remnants of primary vacuolated cells (arrow), a primary vacuolated cell (asterisk), and newly differentiated vacuolated cells (blue arrow). Scale bars: 50μm. See also Figure S3 and S4.
Next, we imaged cavin1b mutants expressing col9a2:mcherry and col8a1a:GFPCaaX at the 3.6 mm stage [31], and found that the internalized sheath cells continue to enlarge, filling all the space created by the collapse of vacuolated cells (Fig.4G–I). Until the 3.6-mm stage, sheath cells retain the sheath marker (col9a2) and do not express vacuolated cell markers (i.e. col8a1a, cyb5r2). However, at around 4.25 mm we could detect expression of col8a1a:GFPCaaX in cells that retained col9a2:mcherry, indicating that they have switched to the mature vacuolated cell profile (Fig.4J–L). We also observed that remnants of collapsed vacuolated cells persisted at the center of the notochord for several days (Fig4M–O), possibly leading to the formation of a scar. Together, these data reveal that the internalized sheath cells differentiate into new vacuolated cells in response to signals from the dying vacuolated cells.
In this study, we demonstrate a mechano-protective role for caveolae in the zebrafish notochord. Loss of caveolar function results in a dramatic collapse of vacuolated cells due to the mechanical strain of locomotion. Strikingly, we found that vacuolated cell collapse leads to sheath cell invasion and trans-differentiation into new vacuolated cells, thereby replacing the collapsed cells and supporting normal spine development. Biochemical assays and experimental manipulations indicate that the release of NTPs from collapsed vacuolated cells triggers sheath cell vacuolization and possibly also invasion. Our data also suggest that chordomas, rare and aggressive notochord cell tumors [32], originate from the invasion of sheath cells into other tissues. Altogether, this work demonstrates a critical role for caveolae in maintaining the structural integrity of the notochord and reveals a novel protective response that safeguards spine development.
STAR methods
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact Michel Bagnat (m.bagnat@cellbio.duke.edu).
Experimental model and subject details
Animal experiments were approved by the Duke Institutional Animal Care and Use Committee (IACUC).
Fish stocks
Zebrafish (Danio rerio) stocks were maintained at 28 °C and bred as previously described [33]. Zebrafish were raised in a circulating aquarium in tanks housing 1–10 fish/L). Zebrafish stocks were healthy and of normal immune status, not involved in previous procedures, and were drug test naïve. Genotype of zebrafish are specified in each figure legend. Male and female breeders from 3–9 months of age were used to generate fish for all experiments. 3–5dpf zebrafish larvae from the Ekkwill (EK) background were used in this study. Strains generated for this study: Tg(col9a2:mcherry)pd1150, Tg(col8a1a:GFPCaaX)pd1152, Tg(rcn3:GFPrab32a)pd1153, Tg(col9a2:GFPCaaX)pd1151, cavin1bbns110, cav3pd1149. Previously published strains: cav1pd1104 [16],Tg(sag:gal4::UAS:mcherry-NTR) [2], Tg(cav1-spGFP)pd1096 [16].
Method details
Genome editing
Mutant lines were generated using CRISPR/Cas9. cav3 mutants were generated using CRISPRs targeting exon 1 and intron 1. cavin1b mutants were generated a CRISPR that targets exon 1. Guide RNAs: cavin1b-5′CGTGAACGTCAAGTCGGTGCGGG3′, cav3-1-5′CTACTTCTAGTTGTAGG 3′, cav3-2-5′GGACCAGTACAACACTAACG 3′. Zebrafish embryos were injected at the one-cell stage with 100pg total of CRISPR RNA. Genotyping for cav3 was performed using primers: forward, TCTCCTATCGGACACTTCTGC; reverse, TGTCTGTTTGCTGACCTTCAA. Genotyping for cavin1b was performed using primers: forward, CACAGCCAACACCGTCAATA; reverse, CAGCCTGTTTCTCCAGGTTC.
Locomotion dependency of notochord lesions
Methyl cellulose treatment: 24hpf mz cavin 1b−/− embryos were de-chorionated and placed in 3% methyl cellulose. Control animals were incubated in egg water. At 72hpf, larvae were evaluated for notochord lesions as indicated in the manuscript using a Nikon SMZ800 microscope. This experiment was performed 3 times with an n=30 animals for each condition per experiment.
α-bungarotoxin injection: One-cell stage mz cavin 1b−/− embryos were injected with α-bungarotoxin cRNA (1ng/nL). Control animals were not injected. Injected and non-injected controls were left in their chorions. At 72hpf, larvae were evaluated for notochord lesions as indicated in the manuscript. This experiment was performed 3 times with an n=30 animals for each condition per experiment.
Calcein staining and skeletal preparations
Larvae were stained with calcein (Sigma-Aldrich) for 15–30 minutes and then live imaged on a AX10 Zoom V116 Zeiss microscope equipped with a Plan neofluar Z 1× objective. Zebrafish between 21–30dpf were eviscerated and fixed in 4% PFA. They were then stained with alizarin red as previously described [2], and treated with 1% KOH to clear tissue from the bone. Once the skeleton was clear of tissue, the spine was imaged as indicated above.
Microscopy
Whole-mount live imaging, and fixed section imaging were performed on a confocal microscope (SP5; Leica) with 10×/0.40 HC PL APO air objective and 20×/0.70 HC PL APO oil objective objective and Application Suite software (Leica). Dissected notochord cells were imaged using a 710 inverted confocal microscope (Carl Zeiss) with 63×/1.40 Oil Plan-Apochromat objective (Carl Zeiss) and Zen software. Pig vacuolated cell were imaged on an Axio Imager.M1 microscope with 10×/0.3 EC Plan-NeoFluar objective and 20×/0.8 Plan-Apochromat objective, an AxioCamMRm camera, and AxioVision software (all from Carl Zeiss). Body length measurements were done on a Setero Discovery.V20 microscope with 1.0× Achromat S FWD 63 mm objective, an AxioCamHRc camera, and AxioVision software (all from Carl Zeiss). Light sheet microscopy fish were mounted in low melt agarose and imaged using using a Lightsheet Z.1 detection optics 20×/1.0 (water immersion) (Carl Zeiss). Where necessary, images were minimally post-processed in ImageJ software (National Institutes of Health) for overall brightness and contrast or to realign channels to correct for drift that occurs during live imaging.
Dissociated notochord assay
Notochord cells were obtained from various transgenic lines as indicated following previously published methods [2], with minor modifications. Whole larval zebrafish were placed in a solution containing Trypsin-EDTA and 1% collagenase. The fish were kept at 28 °C on a shaker to dissociate tissue surrounding the notochord. Individual vacuolated cells were then isolated from this preparation. As indicated, some preparations included 1mM suramin (Sigma-Aldrich), 20 μM AR-C 118925XX (Tocris), and/or 50μM ATP-γ-S, UTP-γ-S (Tocris). Then cells were plated in L15 medium (Gibco) in laminin-coated glass plates and incubated for 2 hours in presence of various chemicals as indicated. Cells were then imaged using a 710 inverted confocal microscope (Carl Zeiss). The experiment was repeated 3 times.
Metabolomics
Nucleus pulposus and annulus fibrosus cells were extracted from the intervertebral discs of 6 month old pigs. Once the material was collected with tweezers it was placed in 1.5mL tubes and immediately placed in liquid nitrogen. Once frozen, the tissue was pulverized into a powder that was then processed and analyzed for metabolomic analysis as previously described [23].
Mass Spectrometry
The QE-MS is equipped with a HESI probe, and the relevant parameters are as listed: heater temperature, 120 °C; sheath gas, 30; auxiliary gas, 10; sweep gas, 3; spray voltage, 3.6 kV for the positive mode and 2.5 kV for the negative mode. Capillary temperature was set at 320 °C, and S-lens was 55. A full scan range from 60 to 900 (m/z) was used. The resolution was set at 70000. The maximum injection time (max IT) was 200 ms with typical injection times around 50 ms. These settings resulted in a duty cycle of around 550 ms to carry out scans in both the positive and negative modes. Automated gain control (AGC) was targeted at 3 Å~ 106 ions. For MS/MS, the isolation width of the precursor was set at 2.5, HCD collision energy was 35%, and max IT is 100 ms. The resolution and AGC were 35000 and 200000, respectively. Full scan with resolution at 35000 and IT of 100 ms) was run together with MS/MS. Customized mass calibration was performed before any sample analysis.
High-Performance Liquid Chromatography
The HPLC (Ultimate 3000 UHPLC) is coupled to QE-MS (Thermo Scientific) for metabolite separation and detection. An Xbridge amide column (100 Å~ 2.1 mm i.d., 3.5μm; Waters) is employed for compound separation at room temperature. The mobile phase A is 20 mM ammonium acetate and 15 mM ammonium hydroxide in water with 3% acetonitrile, pH 9.0, and mobile phase B is acetonitrile. The linear gradient used is as follows: 0 min, 85% B; 1.5 min, 85% B, 5.5 min, 35% B; 10 min, 35% B, 10.5 min, 35% B, 14.5 min, 35% B, 15 min, 85% B, and 20 min, 85% B. The flow rate was 0.15 mL/min from 0 to 10 min and 15 to 20 min and 0.3 mL/min from 10.5 to 14.5 min.
For quantitative LC-MS Uridine-13C9,15N2 5′-triphosphate sodium salt and Adenosine-13C10,15N5 5′-triphosphate sodium salt (Sigma-Aldrich) were used as standards.
Electron Microscopy
NP tissue was fixed overnight at 4°C in 0.1M sodium cacodylate, 2% para-formaldehide (PFA), and 2.5% glutaraldehyde (GA). Zebrafish larvae were fixed in 0.1M sodium cacodylate, and 2.5% GA. Specimen preparation and staining was done as previously described[34]. Briefly, after fixation, samples were stained with osmium tetroxide and uranyl acetate. Then samples were then dehydrated in ethanol solutions of increasing concentration and embedded in resin blocks overnight and then embedded and cured in a 60 degree oven for 48 hours. Then thin sections were cut and post-stained with lead citrate and uranyl acetate and placed on copper grids for imaging. Caveolae formation was quantified by counting the number of omega shaped structures present per 1μM of plasma membrane.
Quantification and statistical analysis
GraphPad Prism version 7.0c for Mac, GraphPad Software, La Jolla California USA, www.graphpad.com was used to plot and analyze data. Specific statistical data (n values) can be found within the figure legends. The n values listed in each figure legend represent the number of animals or the number of experimental replicates. In Figure 1, n represents the number of animals. A Fisher’s exact test was used here to compare the nominal variables. In Figure 3, n represents the number for samples. In Figure 4, n represents the number of experimental replicates. One-way ANOVA followed by Tukey’s multiple comparisons test was performed for this data set. For body length experiments in Supplemental Figure 3, n represents the number of animals. One-way ANOVA followed by Tukey’s multiple comparisons test was performed on this data set.
Supplementary Material
Movie S1. Sheath cells invade the inner notochord following vacuolated cells collapse. Related to figure 2. LSM time-lapse movie of a live 72 hpf cavin1b−/− larva expressing col9a2:mcherry in sheath cells and col8a1a:GFPCaaX in vacuolated cells. A stack of 271 slices was taken every 30 min for 15 hours. The movie was generated from slice 84.
Movie S2. Sheath cells invasion into the inner notochord occurs at sites of vacuolated cell collapse. Related to figure 2. Live LSM time-lapse movie of a 72 hpf cavin1b−/− larva expressing col9a2:mcherry in sheath cells and col8a1a:GFPCaaX in vacuolated cells. A stack of 508 slices was taken every 30 min for 15 hours. The movie was generated from slices 201–212.
Highlights.
Caveolae are conserved cell surface structures in notochord vacuolated cells
Loss of caveolae causes motion-dependent vacuolated cell collapse in zebrafish
Vacuolated cell collapse causes NTP release, sheath cell invasion and vacuolization
Differentiation of sheath cells restores the notochord and safeguards spine formation
Acknowledgments
We thank the Duke zebrafish facility for fish care, the Duke light microscopy facility for help with image acquisition, Janice Williams for EM, Jun Chen for help in obtaining IVD tissue, Stefano di Talia for the use of his confocal microscope, Cagla Eroglu and Ken Poss for critical reading of this manuscript.
This work was supported by National Institutes of Health grants AR065439 (M. Bagnat), AR065439-04S1 (J. Garcia), T32DK007568-26 (D. Levic), and CA193256 (J. Locasale), a Capes-Humboldt Fellowship (B. Njaine), and the Max Planck Society (D. Stainier). The research of Michel Bagnat was supported in part by a Faculty Scholar grant from the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, M.B.; Methodology, M.B, D.Y.R.S., J.B., J.G., D.S.L., X.L. and J.W.L.; Validation, J.G., J.B., D.S.L. and J.N.; Formal Analysis, M.B., J.G., X.L and D.S.L; Investigation, J.G., J.B., B.N., S.W., X.L., D.S.L. S.E.M., J.N., and M.B.; Writing- Original Draft, M.B.; Writing, Review and Editing, M.B., D.Y.R.S. and J.W.L.; Visualization, J.B and M.B.; Supervision, M.B., D.Y.R.S. and J.W.L.; Project Administration, M.B. and J.B.; Funding Acquisition, M.B. and D.Y.R.S.
The authors declare no competing financial interests.
Online supplemental material
Supplemental information contains four figures and two movies.
References
- 1.Yamamoto M, Morita R, Mizoguchi T, Matsuo H, Isoda M, Ishitani T, Chitnis AB, Matsumoto K, Crump JG, Hozumi K, et al. Mib-Jag1-Notch signalling regulates patterning and structural roles of the notochord by controlling cell-fate decisions. Development. 2010;137:2527–2537. doi: 10.1242/dev.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellis K, Bagwell J, Bagnat M. Notochord vacuoles are lysosome-related organelles that function in axis and spine morphogenesis. J Cell Biol. 2013;200:667–679. doi: 10.1083/jcb.201212095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellis K, Hoffman BD, Bagnat M. The vacuole within: how cellular organization dictates notochord function. Bioarchitecture. 2013;3:64–68. doi: 10.4161/bioa.25503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 5.Nixon SJ, Carter A, Wegner J, Ferguson C, Floetenmeyer M, Riches J, Key B, Westerfield M, Parton RG. Caveolin-1 is required for lateral line neuromast and notochord development. J Cell Sci. 2007;120:2151–2161. doi: 10.1242/jcs.003830. [DOI] [PubMed] [Google Scholar]
- 6.Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, et al. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoeber M, Schellenberger P, Siebert CA, Leyrat C, Helenius A, Grunewald K. Model for the architecture of caveolae based on a flexible, net-like assembly of Cavin1 and Caveolin discs. Proc Natl Acad Sci U S A. 2016;113:E8069–E8078. doi: 10.1073/pnas.1616838113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14:98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 9.Ludwig A, Howard G, Mendoza-Topaz C, Deerinck T, Mackey M, Sandin S, Ellisman MH, Nichols BJ. Molecular composition and ultrastructure of the caveolar coat complex. PLoS Biol. 2013;11:e1001640. doi: 10.1371/journal.pbio.1001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ariotti N, Parton RG. SnapShot: caveolae, caveolins, and cavins. Cell. 2013;154:704–704 e701. doi: 10.1016/j.cell.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Shvets E, Ludwig A, Nichols BJ. News from the caves: update on the structure and function of caveolae. Curr Opin Cell Biol. 2014;29:99–106. doi: 10.1016/j.ceb.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–413. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng JP, Mendoza-Topaz C, Howard G, Chadwick J, Shvets E, Cowburn AS, Dunmore BJ, Crosby A, Morrell NW, Nichols BJ. Caveolae protect endothelial cells from membrane rupture during increased cardiac output. J Cell Biol. 2015;211:53–61. doi: 10.1083/jcb.201504042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo HP, Nixon SJ, Hall TE, Cowling BS, Ferguson C, Morgan GP, Schieber NL, Fernandez-Rojo MA, Bastiani M, Floetenmeyer M, et al. The caveolin-cavin system plays a conserved and critical role in mechanoprotection of skeletal muscle. J Cell Biol. 2015;210:833–849. doi: 10.1083/jcb.201501046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo HP, Hall TE, Parton RG. Mechanoprotection by skeletal muscle caveolae. Bioarchitecture. 2016;6:22–27. doi: 10.1080/19490992.2015.1131891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao J, Navis A, Cox BD, Dickson AL, Gemberling M, Karra R, Bagnat M, Poss KD. Single epicardial cell transcriptome sequencing identifies Caveolin 1 as an essential factor in zebrafish heart regeneration. Development. 2016;143:232–243. doi: 10.1242/dev.130534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawson L, Harfe BD. Notochord to Nucleus Pulposus Transition. Curr Osteoporos Rep. 2015;13:336–341. doi: 10.1007/s11914-015-0284-x. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 3):S303–311. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller UK, van den Boogaart JG, van Leeuwen JL. Flow patterns of larval fish: undulatory swimming in the intermediate flow regime. J Exp Biol. 2008;211:196–205. doi: 10.1242/jeb.005629. [DOI] [PubMed] [Google Scholar]
- 20.Swinburne IA, Mosaliganti KR, Green AA, Megason SG. Improved Long-Term Imaging of Embryos with Genetically Encoded alpha-Bungarotoxin. PLoS One. 2015;10:e0134005. doi: 10.1371/journal.pone.0134005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huisken J, Swoger J, Del Bene F, Wittbrodt J, Stelzer EH. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science. 2004;305:1007–1009. doi: 10.1126/science.1100035. [DOI] [PubMed] [Google Scholar]
- 22.Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Ser Z, Locasale JW. Development and quantitative evaluation of a high-resolution metabolomics technology. Anal Chem. 2014;86:2175–2184. doi: 10.1021/ac403845u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gault WJ, Enyedi B, Niethammer P. Osmotic surveillance mediates rapid wound closure through nucleotide release. J Cell Biol. 2014;207:767–782. doi: 10.1083/jcb.201408049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider G, Glaser T, Lameu C, Abdelbaset-Ismail A, Sellers ZP, Moniuszko M, Ulrich H, Ratajczak MZ. Extracellular nucleotides as novel, underappreciated pro-metastatic factors that stimulate purinergic signaling in human lung cancer cells. Mol Cancer. 2015;14:201. doi: 10.1186/s12943-015-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corriden R, Insel PA. New insights regarding the regulation of chemotaxis by nucleotides, adenosine, and their receptors. Purinergic Signal. 2012;8:587–598. doi: 10.1007/s11302-012-9311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarowski ER. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Q, Zhao K, Zhong XZ, Zou Y, Yu H, Huang P, Xu TL, Dong XP. SLC17A9 protein functions as a lysosomal ATP transporter and regulates cell viability. J Biol Chem. 2014;289:23189–23199. doi: 10.1074/jbc.M114.567107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8:437–502. doi: 10.1007/s11302-012-9309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Kugelgen I, Hoffmann K. Pharmacology and structure of P2Y receptors. Neuropharmacology. 2016;104:50–61. doi: 10.1016/j.neuropharm.2015.10.030. [DOI] [PubMed] [Google Scholar]
- 31.Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn. 2009;238:2975–3015. doi: 10.1002/dvdy.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X, Hornicek F, Schwab JH. Chordoma: an update on the pathophysiology and molecular mechanisms. Curr Rev Musculoskelet Med. 2015;8:344–352. doi: 10.1007/s12178-015-9311-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Westerfield M. The Zebrafish Book A Guide for the Laboratory Use of Zebrafish (Danio rerio) Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- 34.Bagnat M, Navis A, Herbstreith S, Brand-Arzamendi K, Curado S, Gabriel S, Mostov K, Huisken J, Stainier DY. Cse1l is a negative regulator of CFTR-dependent fluid secretion. Curr Biol. 2010;20:1840–1845. doi: 10.1016/j.cub.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Sheath cells invade the inner notochord following vacuolated cells collapse. Related to figure 2. LSM time-lapse movie of a live 72 hpf cavin1b−/− larva expressing col9a2:mcherry in sheath cells and col8a1a:GFPCaaX in vacuolated cells. A stack of 271 slices was taken every 30 min for 15 hours. The movie was generated from slice 84.
Movie S2. Sheath cells invasion into the inner notochord occurs at sites of vacuolated cell collapse. Related to figure 2. Live LSM time-lapse movie of a 72 hpf cavin1b−/− larva expressing col9a2:mcherry in sheath cells and col8a1a:GFPCaaX in vacuolated cells. A stack of 508 slices was taken every 30 min for 15 hours. The movie was generated from slices 201–212.


