Abstract
Introduction
Traumatic brain injury (TBI) and posttraumatic stress disorder (PTSD) have previously been reported to be associated with increased risk of Alzheimer's disease (AD). We are using biomarkers to study Vietnam Veterans with/without mild cognitive impairment with a history of at least one TBI and/or ongoing PTSD to determine whether these contribute to the development of AD.
Methods
Potential subjects identified by Veterans Administration records underwent an initial telephone screen. Consented subjects underwent clinical evaluation, lumbar puncture, structural magnetic resonance imaging, and amyloid positron emission tomography (PET) scans.
Results
We observed worse cognitive functioning in PTSD and TBI + PTSD groups, worse global cognitive functioning in the PTSD group, lower superior parietal volume in the TBI + PTSD group, and lower amyloid positivity in the PTSD group, but not the TBI group compared to controls without TBI/PTSD. Medial temporal lobe atrophy was not increased in the PTSD and/or TBI groups.
Discussion
Preliminary results do not indicate that TBI or PTSD increase the risk for AD measured by amyloid PET. Additional recruitment, longitudinal follow-up, and tau-PET scans will provide more information in the future.
Keywords: Traumatic brain injury, Posttraumatic stress disorder, Alzheimer's disease, Vietnam veterans, Mild cognitive impairment
1. Introduction
Alzheimer's disease (AD) is characterized by the presence of amyloid plaques and tau fibrils, which are associated with synaptic loss and neurodegeneration, leading to cognitive impairments and dementia. After age, family history, and apolipoprotein E (APOE) ε4 risk allele [1], traumatic brain injury (TBI) has been reported as a risk factor in many [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33] but not all [13] epidemiological studies (reviewed in [34], [35], [36]). Some studies have suggested that a history of TBI is associated with earlier onset of AD [3], [6], [13], [18], [20], [34], [35], [37]. Others, but not all, have shown an interaction of TBI with the APOE ε4 allele [5], [6], [15], [17], [38], [39], [40], [41], [42], [43], [44], [45], [46]. After TBI, young subjects appear to have had Aβ pathology in postmortem brain tissue [47], [48]. A comprehensive consensus analysis of the literature concluded that there was some evidence for a relationship between TBI in males and future development of AD (odds ratio 2.29 [1.47–3.58]) [20]. One study [33], which showed a significantly increased risk of developing AD after TBI, used information provided by the US Department of Defense to identify and prospectively enroll subjects with nonpenetrating head injury suffered during WWII and the Korean War, and non–head-injured controls. Recently, one study from the Mayo Clinic Study of Aging [49] suggested that MCI, but not cognitively normal subjects, who reported a history of concussion had higher brain Aβ levels, measured with Aβ positron emission tomography (PET) scans, than noninjured MCI participants. With the exception of one publication [50], studies of the effects of TBI on AD have used “self-report” information to determine a history of concussion; few studies have used imaging and cerebrospinal fluid biomarkers to investigate the relationship of TBI to AD.
Posttraumatic stress disorder (PTSD) is an anxiety disorder following exposure to traumatic stress [51]. The prevalence of combat-related PTSD in US military Veterans since the Vietnam War ranges from 10% to 15% [52], [53]. PTSD is associated with worse cognitive functioning [50], [54], [55], [56], [57], [58], [59], [60], [61], [62]. Furthermore, PTSD is associated with alterations in the hippocampus [63], [64], [65], [66], [67], anterior cingulate [65], and prefrontal structures [63], [64], [65], [66], [67], [68], [69], whereas improvement of symptoms is associated with less-progressive atrophy [67]. The relationship of PTSD to impaired cognitive function and hippocampal abnormalities suggests that PTSD could be a risk factor for the development of AD. A study of the national Veterans Administration (VA) clinical database showed that Veterans with PTSD are twice as likely to develop a dementia diagnosis compared to Veterans without PTSD, even when controlling for known risk factors for dementia [70]. Aside from magnetic resonance imaging (MRI) studies, to our knowledge, there are no reports of the use of Aβ PET scans or other biomarkers to examine whether the mechanism accounting for the higher risk for dementia in PTSD is related to AD pathology.
The Alzheimer's Disease Neuroimaging Initiative (ADNI) is a large, multisite study, aimed at validating biomarkers for AD clinical trials. ADNI has enrolled over 1500 subjects at 57 sites in the United States and Canada and followed them longitudinally with clinical/cognitive testing, genetic analysis, MRI, lumbar puncture for cerebrospinal fluid (CSF) analysis, 18F-fluorodeoxyglucose PET, Aβ PET, and most recently tau-PET (reviewed in [71]). To investigate the relationship of a past history of TBI and PTSD on the development of AD, the Department of Defense funded three proposals: (1) Effects of Traumatic Brain Injury (TBI) and Posttraumatic Stress Disorder on Alzheimer's Disease in Veterans Using Imaging and Biomarkers in the AD Neuroimaging Initiative; (2) Effects of Traumatic Brain Injury and Posttraumatic Stress Disorder on Alzheimer's Disease in Veterans with Mild Cognitive Impairment Using the Alzheimer's Disease Neuroimaging Initiative; and (3) Effects of Traumatic Brain Injury and Posttraumatic Stress Disorder and Alzheimer's Disease on Brain Tau in Vietnam Veterans Using ADNI. These studies are collectively termed “DOD ADNI,” and aim to enroll approximately 400 elderly Veterans who served in Vietnam.
The initial DOD ADNI grant enrolled Vietnam Veterans with PTSD and/or TBI, as well as controls with service-connected disorders not related to PTSD or TBI who met criteria for normal cognition. The second grant expanded the original study to include subjects who also met criteria for MCI. The study now includes cohorts of Vietnam Veterans, both with and without MCI, who have histories of TBI, PTSD, and both TBI and PTSD, as well as controls without PTSD/TBI. Potential subjects are initially contacted by mail, prescreened for eligibility by telephone and those meeting criteria for one of the cohorts are referred for clinical examination, cognitive tests, Aβ PET using 18F-labeled florbetapir, MRI (structural, diffusion tensor, and resting state functional), lumbar puncture for CSF markers of tau, phosphorylated tau (p-tau181), β-amyloid (Aβ42), and blood collection for genetic analysis. The clinical/cognitive battery and MRI is repeated after 1 year, and tau-PET scans are obtained at baseline and after 1 year. Results from the CSF assays and tau-PET imaging are pending and will not be reported in this article.
This interim report uses baseline data to determine the extent to which a prior history of TBI and/or documented past history and present diagnosis of PTSD increase the risk of developing AD. Our primary a priori hypothesis is that Vietnam Veterans with a history of TBI or presence of ongoing PTSD will have lower cognitive functioning and increased prevalence of brain AD pathology (measured by Aβ florbetapir PET and medial temporal lobe atrophy) after accounting for effects of age and presence of the APOE ε4 allele.
2. Methods
2.1. Identification of potential study subjects
For this study, we used an operational definition of TBI as head injury with loss of consciousness for more than 5 minutes, and/or having amnesia, and/or being dazed and confused for more than 24 hours. Using military and VA Compensation and Pension records, as well as VA Health records, and/or response to advertisements, Vietnam Veterans with a service connection related to a possible traumatic head injury and/or a history of at least one moderate/severe TBI and/or evidence of ongoing PTSD, and demographically comparable Veteran controls without PTSD/TBI were identified. Because TBI was not a common diagnostic code during the Vietnam War, the study requested a sample of subjects from the Veteran Benefits Administration who were service-connected for several diagnostic codes that might be related to a traumatic brain injury (traumatic brain disease, posttrauma headache, acute posttrauma headache, chronic posttrauma headache, post-concussion syndrome, brain hemorrhage, limited motion of the jaw, and other head-related injuries). We also obtained separate samples of subjects who are service-connected for PTSD as well as a sample of controls who were service-connected for something other than PTSD or TBI. Study invitation letters, brochures, and response postcards were mailed to Veterans who met these initial broad criteria and who lived within 150 miles of the closest ADNI clinic.
2.2. Initial telephone prescreening recruitment calls
To include all subjects who may have a history of a TBI and to confirm whether or not the TBI-related diagnostic codes were related to a head injury, all subjects were contacted by telephone, and verbal consent was obtained for the administration of screening questions which determined eligibility criteria for current PTSD symptoms and history of a TBI. The Ohio State University TBI Identification Method–Interview Form [72] was used to ask about head injuries at three separate times in a subject's life: before, during, and after Vietnam. This ensured we included TBIs that may have occurred before or after a subject's military service. In addition, a safety assessment ruled out subjects who were not safe to participate in the study procedures. The purpose of the initial telephone prescreening recruitment call was both to (1) rule out exclusions such as dementia, history of psychosis or bipolar affective disorder, history of schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria), history of alcohol or substance abuse/dependence within the past 5 years, MRI-related exclusions (metal in the body, pacemakers), contraindications for lumbar puncture, PET scan, or other procedures in this study and (2) assess placement in study cohorts. During this initial screen, MCI was assessed using an adapted version of the AD8 (70, 71) and the Telephone Interview for Cognitive Status, as the latter contains a 10-word list recall which combined with the AD8 can help to identify cognitive impairment [73], [74]. Subjects meeting inclusion criteria were then mailed a written consent form for the telephone psychiatric assessment (clinical interview) portion of the study and audio recording, with a stamped addressed return envelope and the following self-report questionnaires: (1) MRI safety, (2) Medical history, (3) Current medications, (4) Symptom Check List 90 (revised) for general psychopathology [75], (5) Pittsburgh Sleep Quality [76], (6) Smoking/Life Time Smoking, (6) SF-12 Health Survey [77], and (7) Combat Exposure Scale [78].
After documented consent was received, all self-report questionnaires described previously were reviewed as well as the subjects' VA medical records to ensure that there were no medical problems or exclusionary medications that the subject failed to report and to ensure that proper cohort assignment had been determined. VA medical records were reviewed for any documentary evidence of a TBI at anytime in a subject's life regardless of whether or not a subject reported a TBI during the administration of the Ohio State University TBI Identification Method–Interview Form. If the medical records specifically mentioned a TBI incident or a service connection related to a TBI (as indicated by the diagnostic codes discussed previously), or if a subject self-reported a TBI (that met the operational criteria of TBI discussed previously) during anytime in the subject's life, this subject was not placed in the control group.
2.3. SCID CAPS evaluation by telephone
All eligible subjects were next referred to the telephone clinical evaluation which used the Structured Clinical Interview 1 of the Diagnostic and Statistical Manual of Mental Disorders, Version IV, (Axis 1)–Text Revision (SCID) and the Clinician Administered PTSD Scale for DSM-IV (CAPS) [79] to assess current and lifetime PTSD and to rule out psychosis and/or drug and alcohol issues. SCID CAPS, as well as the Life Stressor Checklist [80] and the Addiction Severity Index Lite [81], was conducted by telephone by the PTSD Core at the San Francisco Veterans Administration Medical Center.
2.4. Assignment of subjects to cohorts
After completing the SCID CAPS, eligible subjects were placed into one of eight cohorts, four with no MCI and four who met initial criteria for MCI, as described in the following:
-
•
TBI only: Subjects with no service connection for PTSD, no history of lifetime, and/or current PTSD on SCID CAPS (scores of <30 for each) but with a service connection for a TBI-related diagnostic code, medical documentation of a TBI-related incident, and/or a self-report of a TBI which met the operational definition of TBI described previously.
-
•
PTSD only: Subjects who were service-connected for PTSD and who scored ≥40 for both lifetime and current PTSD, with no self-report history of TBI, no diagnostic service connection related to TBI, and no medical documentation of TBI.
-
•
Both TBI + PTSD: Subjects who scored ≥30 for either lifetime and/or current PTSD and had a service connection related to TBI, medical documented evidence of a TBI-related incident, and/or a self-report history of TBI meeting the operational criteria of TBI described previously.
-
•
Controls: Subjects with no service connection for PTSD, no history of lifetime, and/or current PTSD on SCID CAPS (scores of <30 for each), and no service connection for TBI-related diagnostic codes, no medical documentation of a TBI-related incident, and no self-report of a TBI that met the operational definition of TBI.
Eligible subjects were next referred to one of the 19 ADNI Clinic sites to sign a clinic consent and complete study procedures.
2.5. Clinical evaluation, neuropsychological testing, MRI, and Aβ florbetapir PET scans
After the clinical telephone interview, subjects were referred to the closest ADNI site for in-person studies using the identical methods used by ADNI. These include clinical evaluation, neuropsychological testing, lumbar puncture (results not available at this time), genetic sampling for the APOE ε4 allele, structural MRI at 3T, and Aβ florbetapir PET scans. These methods have been widely reported [82], [83], [84], [85], [86]. Longitudinal data and tau-PET scan results will be reported at a later time.
2.6. Power analysis
Our original study was designed to have 80% power to detect a minimum difference in means of 0.55 standard deviations, assuming 65 participants in each group, an alpha of 0.025, and a two-sided test. In this interim report, we have not yet achieved our desired sample size in each group, particularly in the TBI and TBI + PTSD groups. For comparison of the TBI group (n = 22) with the no TBI/PTSD group (n = 63), we have 80% power to detect a difference as small as 0.78 standard deviations (0.68 standard deviations for the TBI + PTSD vs. no TBI/PTSD group). Minimum detectable differences are larger for the imaging outcomes, where sample sizes are even smaller.
2.7. Statistical analysis
Means and standard deviations were computed for all continuous variables of interest (age, education, neuropsychological testing, MRI volumetrics, and florbetapir standardized uptake value ratio [SUVR]), whereas percentages were calculated for categorical variables (gender, race/ethnicity, diagnosis, APOE ε4 status). Analysis of variance or Fisher's exact test was used to compare groups (PTSD, TBI, both, neither) on demographic and clinical characteristics. Linear regression was used to compare groups on most neuropsychological testing scores and MRI volumetrics. Volume of white-matter hyperintensities was transformed with natural log to meet the assumptions of the models. Poisson regression [87] was used to compare groups on the clinical dementia rating (CDR) Sum of Boxes because this outcome did not meet the assumptions of linear regression. Mini–Mental State Examination (MMSE) was rescaled as the number of errors (30 MMSE) and analyzed with a negative binomial regression due to overdispersion. Finally, logistic regression was used to compare amyloid positivity based on Aβ florbetapir cortical SUVR across the groups. All models were adjusted for age, education, and APOE ε4 status by including these variables in the models. Primary comparisons of interest for all analyses were between the patient groups (PTSD, TBI, both) and the control group (no PTSD or TBI). All analyses were conducted using SAS software version 9.4 [88] with a P-value <.05 considered statistically significant.
3. Results
3.1. Description of the recruitment assessment process
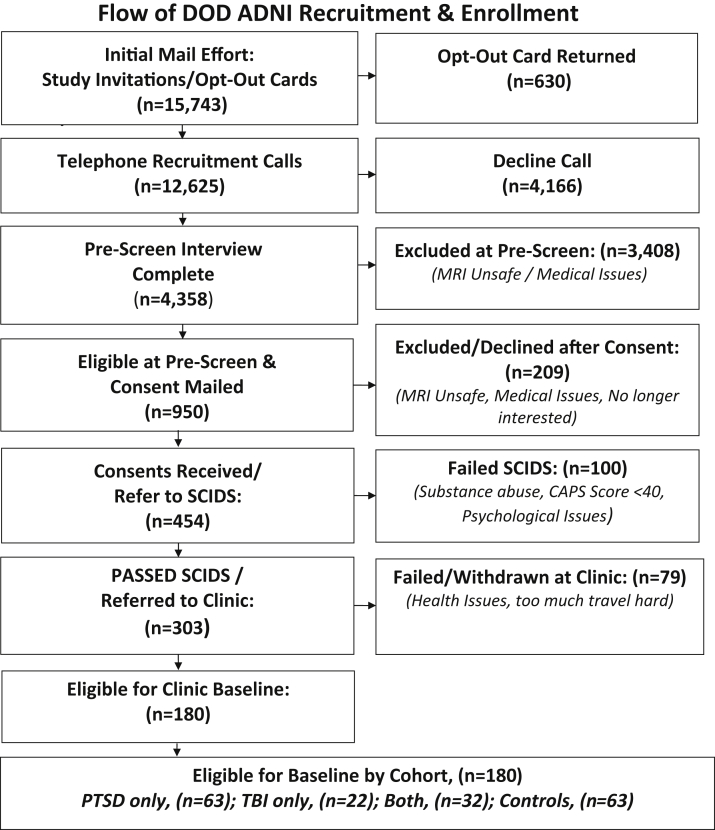
The number of subjects contacted by mailing brochures, the initial telephone screening effort, including number of subjects called and screened, the numbers of consents mailed and SCID evaluations by telephone, and finally the number of completed baseline clinic visits and 12-month follow-up visits are outlined in Fig. 1.
Fig. 1.
Overall summary of study recruitment and enrollment. Abbreviations: ADNI, Alzheimer's Disease Neuroimaging Initiative; PTSD, posttraumatic stress disorder; TBI, traumatic brain injury.
3.1.1. Demographic and clinical characteristics
The demographic and clinical characteristics of the cohort are given in Table 1, Table 2. The groups differed in age, with the control group without PTSD/TBI significantly older than the PTSD (P < .001), TBI (P = .005), and TBI + PTSD (P < .001) groups. Education also differed between the groups, with the PTSD group significantly less educated than the control group (P = .004). There were no differences in gender, race/ethnicity, or APOE ε4 allele status. The percentage of subjects with an MCI diagnosis was significantly different across the groups (P = .03), with a significantly higher percentage in the group with both TBI and PTSD and the group with PTSD than in the controls.
Table 1.
Participant characteristics
| No TBI/PTSD (n = 63) | PTSD (n = 63) | TBI (n = 22) | TBI + PTSD (n = 32) | P value∗ | |
|---|---|---|---|---|---|
| Age | 71.1 (5.9) | 67.8 (3.6) | 67.9 (4.5) | 68.7 (3.1) | <.001 |
| Education | 16.0 (2.2) | 14.7 (2.5) | 15.8 (2.2) | 15.2 (2.4) | .01 |
| Male (%) | 100 | 100 | 100 | 96.9 | .30 |
| Ethnicity (%) | .81 | ||||
| African American | 6.3 | 4.8 | 9.1 | 12.5 | |
| Asian | 4.8 | 0 | 0 | 0 | |
| Caucasian | 80.9 | 82.5 | 81.8 | 78.1 | |
| Hispanic | 6.3 | 11.1 | 9.1 | 9.4 | |
| Other | 1.6 | 1.6 | 0 | 0 | |
| E4+ (%)† | 25.9 | 30.4 | 36.8 | 29.6 | .81 |
| MCI (%)‡ | 3.3 | 15.5 | 10.0 | 20.7 | .03 |
Abbreviations: TBI, traumatic brain injury; PTSD, posttraumatic stress disorder; E4+, carriers of the ε4 allele of apolipoprotein E; MCI, mild cognitive impairment.
NOTE. Means and standard deviations are presented unless otherwise stated.
P value presented is for the overall group difference.
E4 data available for all but 5 No TBI/PTSD (controls), 7 PTSD, 3 TBI, and 5 TBI + PTSD.
Diagnosis available for 61 No TBI/PTSD, 58 PTSD, 20 TBI, and 29 TBI + PTSD.
Table 2.
Neuropsychological performance by group
| No TBI/PTSD (n = 63) | PTSD (n = 63) | TBI (n = 22) | TBI + PTSD (n = 32) | P value∗ | |
|---|---|---|---|---|---|
| ADAS-Cog† | 10.9 (4.6) | 12.8 (3.9) | 9.4 (3.6) | 11.0 (5.2) | .10 |
| MMSE | 28.7 (1.3) | 28.0 (1.7) | 28.8 (1.1) | 28.4 (1.5) | .14 |
| CDR Sum of Boxes‡ | 0.19 (0.40) | 0.62 (0.82) | 0.33 (0.56) | 0.47 (0.81) | .02 |
| RAVLT§ | 41.3 (9.2) | 39.3 (7.4) | 41.3 (9.4) | 41.2 (9.1) | .46 |
| Delayed Recall|| | 11.1 (3.8) | 9.7 (3.8) | 10.9 (3.3) | 11.2 (4.1) | .63 |
Abbreviations: TBI, traumatic brain injury; PTSD, posttraumatic stress disorder; ADAS-cog, Alzheimer's Disease Assessment Scale–cognitive; MMSE, Mini–Mental State Examination; CDR, clinical dementia rating; RAVLT, Rey's auditory verbal learning test.
NOTE. Means and standard deviations are presented.
P value presented is for the overall group difference in models that adjust for age, education, and APOE ε4 allele status.
Total score based on 13 items; missing for 1 No TBI/PTSD, 3 PTSD, 1 TBI, and 1 TBI + PTSD.
Missing for 2 No TBI/PTSD, 3 PTSD, 1 TBI, and 1 TBI + PTSD.
Rey Auditory Verbal Learning Test: sum of five trials; missing for 1 No TBI/PTSD, 3 PTSD, 1 TBI, and 1 TBI + PTSD.
Logical Memory II.
In models adjusted for age, education, and APOE ε4 allele status, there was no significant difference in verbal memory (RAVLT, Delayed Recall), but there was a trend for a difference in global cognition (MMSE, ADAS-Cog) with the PTSD group having worse global cognition than the controls. There was a significant difference in CDR Sum of Boxes, with the PTSD group (P = .009) and the TBI + PTSD group (P = .007) having higher CDR Sum of Boxes scores (indicating worse cognitive functioning) than the controls. This finding and the trend for worse global cognitive function remained even after further accounting for amyloid burden in the brain as measured by Aβ florbetapir PET (MMSE: PTSD vs. control: P = .052; CDR Sum of Boxes: PTSD vs. control: P = .03, TBI + PTSD vs. control: P = .008).
3.2. Brain structure measured by MRI
Table 3 describes the brain regional volumes and volume of white-matter hyperintensities across the groups. There were no significant differences between the groups in models adjusted for age, education, and APOE ε4 allele status except in the superior parietal region (P = .046), which had a slightly lower volume in the TBI + PTSD group than in the control group (P = .04). A further analysis that considered a measure of PTSD (CAPS) rather than the specific exposure groups found no association between level of PTSD and hippocampal volume (P > .7) or superior parietal volume (P > .3) for either the current CAPS or lifetime CAPS, in a model that included age, education, and APOE ε4 allele status.
Table 3.
MRI volumetrics by group
| No TBI/PTSD (n = 52) | PTSD (n = 37) | TBI (n = 16) | TBI + PTSD (n = 15) | P value∗ | |
|---|---|---|---|---|---|
| Hippocampus† | 0.51 (0.06) | 0.53 (0.06) | 0.52 (0.05) | 0.51 (0.08) | .81 |
| Entorhinal cortex† | 0.26 (0.04) | 0.28 (0.04) | 0.28 (0.02) | 0.27 (0.03) | .56 |
| Inferior parietal† | 1.70 (0.17) | 1.74 (0.18) | 1.82 (0.15) | 1.77 (0.13) | .21 |
| Superior parietal† | 1.64 (0.16) | 1.69 (0.20) | 1.71 (0.15) | 1.56 (0.13) | .046 |
| Inferior temporal† | 1.38 (0.15) | 1.44 (0.14) | 1.41 (0.15) | 1.36 (0.12) | .35 |
| Superior temporal† | 1.42 (0.15) | 1.44 (0.15) | 1.41 (0.18) | 1.47 (0.15) | .71 |
| White-matter hyperintensities‡ | 6.1 (6.4) | 4.4 (3.8) | 5.0 (5.5) | 4.0 (3.9) | .47 |
Abbreviations: MRI, magnetic resonance imaging; TBI, traumatic brain injury; PTSD, posttraumatic stress disorder.
NOTE. Means and standard deviations are presented.
P value presented is for the overall group difference in models that adjust for age, education, and APOE ε4 allele status.
Expressed as percentage of intracranial volume.
White-matter hyperintensities data based on 59 No TBI/PTSD, 53 PTSD, 18 TBI, and 21 TBI + PTSD participants.
3.3. β amyloid florbetapir positron emission tomography
Table 4 and Fig. 2 illustrate the distribution of Aβ florbetapir cortical SUVR across the groups. When categorized according to amyloid positivity using a previously validated cutoff of 1.11 [89], there was a significant difference by group (P = .04) in a model adjusted for age, education, and APOE ε4 allele status, with the PTSD group having significantly lower odds of being amyloid positive (odds ratio: 0.21; 95% CI: 0.05–0.93; P = .04) than the controls. The proportion of controls in this study classified as amyloid positive was comparable to previous ADNI studies (data not shown).
Table 4.
Florbetapir cortical SUVR by group
| No TBI/PTSD (n = 54) | PTSD (n = 52) | TBI (n = 19) | TBI + PTSD (n = 21) | P value∗ | |
|---|---|---|---|---|---|
| Cortical SUVR | 1.07 (0.14) | 1.01 (0.07) | 1.08 (0.20) | 1.06 (0.08) | .21 |
| % amyloid positive (SUVR > 1.11) | 24.1 | 5.8 | 21.0 | 33.3 | .04 |
Abbreviations: SUVR, standardized uptake value ratio; TBI, traumatic brain injury; PTSD, posttraumatic stress disorder.
NOTE. Means and standard deviations are presented.
P value presented is for the overall group difference in a model that adjusts for age, education, and APOE ε4 allele status.
Fig. 2.
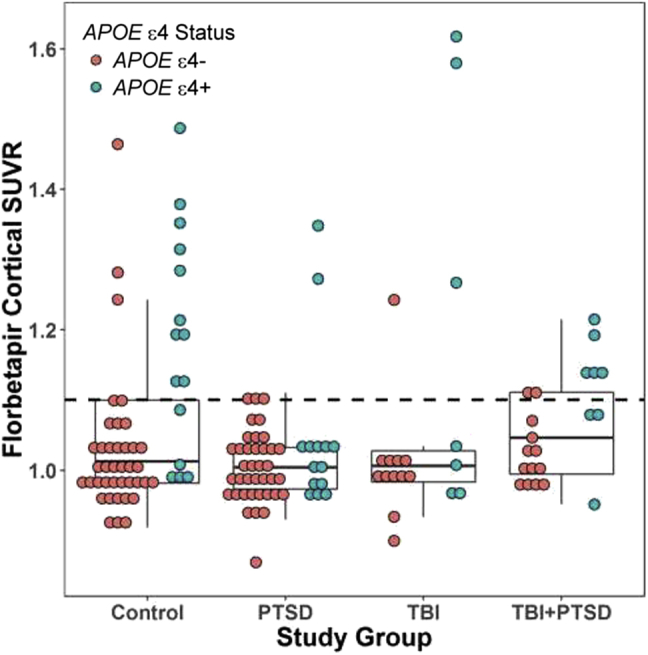
Florbetapir cortical SUVR by group and APOE ε4 allele status. The dotted line represents a previously validated amyloid positivity cutoff of 1.11 [89]. Abbreviations: APOE ε4, apolipoprotein E ε4 allele; PTSD, posttraumatic stress disorder; SUVR, standardized uptake value ratio; TBI, traumatic brain injury.
4. Discussion
The major findings of this project are as follows: (1) worse cognitive functioning as measured by the CDR Sum of Boxes in the PTSD and both TBI + PTSD groups relative to the controls; (2) a trend for worse global cognitive functioning (ADAS-Cog, MMSE) in the PTSD group relative to the controls; (3) slightly lower superior parietal volume in the group with both TBI + PTSD relative to controls; (4) lower odds of amyloid positivity based on cortical amyloid SUVR in the PTSD group relative to the controls; and (5) no evidence for increased brain amyloid associated with TBI and no evidence for medial temporal lobe atrophy in those with PTSD and/or TBI relative to the controls. Taken together, these results do not support the hypothesis that prior TBI or PTSD is associated with increased brain amyloid. Furthermore, the worse cognitive functioning in the PTSD and TBI + PTSD groups is not associated with increased brain amyloid. Overall, this interim analysis of an ongoing study does not support the view that TBI or PTSD is associated with increased development of AD.
Our first finding of worse cognitive functioning in the PTSD group confirms previous reports [90], [91], [92], [93], [94]. Furthermore, at least one previous study indicates that PTSD is associated with an increased incidence of dementia [95]. However, there are no autopsy reports demonstrating increased AD pathology in Veterans with PTSD. Moreover, levels of white-matter hyperintensities did not differ among groups suggesting that the worsening cognitive function in the PTSD group is not explained by cerebrovascular disease. Therefore, the cause of cognitive decline or dementia in Veterans with PTSD is not known. The interim results of our ongoing study do not show increased brain amyloid in the PTSD group compared to controls. In fact, there is a trend for reduced brain amyloid in the PTSD group. Although these results are preliminary, they suggest that the cognitive impairment and dementia associated with PTSD may not be due to AD pathology.
Hippocampal atrophy has been associated with PTSD in some studies [66], [96], [97], [98], but not others [99], [100], [101], [102]. Hippocampal atrophy is typically found in AD dementia but is not specific for this condition [103]. Therefore, our failure to detect hippocampal atrophy in the PTSD or TBI + PTSD group in this study further suggests that the cognitive impairments in the PTSD subjects are not due to AD pathology.
Thus far, our study shows no evidence for increased brain amyloid load in subjects with prior history of TBI or TBI + PTSD in contrast with previous reports of TBI as a risk factor for AD. In contrast to most of these reports which used “self-reported” TBI, we identified subjects from the VA records who receive VA compensation for diagnostic codes that might be related to a traumatic brain injury as well as by self-report of TBI before, during, and after Vietnam. In addition, there are no previous studies using Aβ florbetapir PET to determine brain amyloid load, or MRI to detect hippocampal atrophy in elders with a past history of TBI. One study reported greater amyloid deposition in MCI subjects measured by amyloid PET using Pittsburgh compound B but head trauma was again self-reported [49]. Although AD is considered an amyloid-mediated tauopathy, and we have yet to analyze CSF tau levels or tau-PET imaging results, our findings thus far lead us to conclude that we have not found evidence of a connection between past history of TBI and development of amyloid pathology characteristic of AD. The present study suggests that TBI may be associated with cognitive decline unrelated to AD and is, in this regard, consistent with a report [104] instead showing an association of TBI with an increased risk of Parkinson's disease.
5. Limitations of the study
We wish to emphasize the limitations of this interim report. The first limitation is the small sample size of the TBI group. Many Veterans who were contacted did not return our calls, declined to participate, or were unable to participate for a variety of reasons. Compared to subjects who were excluded or declined, the baseline subjects were slightly younger, and there were more Caucasians, fewer African Americans, and more Latinos. Furthermore, for unknown reasons, African American Veterans had a higher “passive refusal rate” (less likely to answer the phone, return phone calls, keep an appointment, return consent forms, etc.). These results reflect a combination of factors including willingness to participate and comorbidities which led to exclusions. Therefore, the population which was studied in the clinic differs considerably from the population of subjects who were initially contacted for this project. Despite our efforts to contact and enroll all Vietnam Veterans receiving compensation from the VA diagnostic codes that may be related to TBI, we found that a very high proportion of Veterans with history of TBI had comorbidities which prevented them from enrolling in the study. In particular, we found that large numbers of these Veterans reported metal in the body, which prevented MRI examinations. The prevalence of this exclusion was much higher than we have experienced in civilian populations studied in ADNI. However, to increase the number of subjects, the decision was recently made to waive the MRI for subjects in the TBI/both cohorts, if the only reason for exclusion was that MRI was unsafe. In addition, Veterans with both TBI and PTSD had exclusions for many other reasons and overall the “screen fail” rate in this study is much higher than we have experienced with ADNI. These findings serve to emphasize the well-known observation that clinical research studies which impose strict inclusion/exclusion criteria to eliminate comorbidities run the risk of studying a sample that does not resemble the “general population.” Furthermore, research studies which include biomarker measurements such as MRI, PET scans, and lumbar punctures also run the risk of reducing generalizability.
A second limitation is that our selection of cognitively normal and MCI participants able to be followed over the timeframe of the study, rather than of Veterans already exhibiting dementia, may automatically eliminate Veterans susceptible to the development of AD. The remaining cohort may represent a portion of the population that does not develop AD as a result of TBI and/or PTSD.
It is important to emphasize that this is an interim analysis of an ongoing study. In addition to our continued enrollment and baseline assessments, we are obtaining 12-month follow-up data. We are also performing tau-PET imaging at baseline and 12-month follow-up. Our preliminary results do not support the hypothesis that either TBI or PTSD increases the risk for AD measured with biomarkers. Subjects with PTSD do appear to have worse cognition, but this is not associated with increased amounts of brain amyloid measured by Aβ florbetapir PET scans. In the present study, we controlled for APOE genotype, the strongest genetic risk factor for late onset AD. In the future, genome-wide genotyping will be available so that other major candidate genes for AD, PTSD, and TBI can be assessed. When we complete enrollment, we will test the major hypotheses again, and the large sample size will provide sufficient statistical power for additional hypothesis testing and data exploration. Longitudinal data and tau-PET data will also be reported.
6. Conclusions
Epidemiological studies have inconsistently linked history of TBI or PTSD as risk factors for AD although few have investigated the relationship using imaging or CSF biomarkers. The study aims to determine the relationship between a history of TBI and/or PTSD and AD pathology. This preliminary report includes results from baseline clinical examinations, cognitive tests, structural MRI, and Aβ florbetapir PET imaging. Despite finding lower cognitive functioning in the PTSD and TBI + PTSD groups, and lower superior parietal volume in the TBI + PTSD group relative to controls, there was no evidence to suggest that the observed cognitive decline or atrophy was related to amyloid deposition. The study was limited by the small sample size of the TBI group and does not include results from ongoing tau-PET imaging or CSF biomarker analysis. However, we conclude that these interim results do not support the hypothesis that either TBI or PTSD increases the risk for AD as measured by biomarkers.
Research in Context.
-
1.
Systematic review: The authors reviewed literature pertaining to the associations between traumatic brain injury, posttraumatic stress disorder, and cognitive decline, and the incidence and prevalence of these risk factors in military populations using traditional sources such as PubMed.
-
2.
Interpretation: Although we observed worse cognitive functioning in veterans with traumatic brain injury (TBI) and/or posttraumatic stress disorder, these risk factors did not increase AD risk measured by amyloid positron emission tomography (PET). Limitations include difficulty in recruitment due to exclusions resulting in a small sample size of TBI subjects and a study population that does not resemble the “general population.”
-
3.
Future directions: The study will continue to enroll participants and performed follow-up assessments of existing participants. Tau-PET imaging at baseline and 12 months will be performed and major hypotheses will be tested again.
Acknowledgments
Department of Defense Grants: Effects of Traumatic Brain Injury (TBI) and Post-Traumatic Stress Disorder (PTSD) on Alzheimer's Disease (AD) in Veterans Using Imaging and Biomarkers in the (AD) Neuroimaging Initiative (ADNI), W81XWH-12-2-0012. Effects of Traumatic Brain Injury and Post-Traumatic Stress Disorder on Alzheimer's Disease (AD) in Veterans with Mild Cognitive Impairment (MCI) Using the Alzheimer's Disease Neuroimaging Initiative (ADNI), W81XWH-13-1-0259. Effects of Traumatic Brain Injury and Post-Traumatic Stress Disorder and Alzheimer's Disease on Brain Tau in Vietnam Veterans Using ADNI, W81XWH-14-1-0462.
The authors very greatly appreciate the efforts of the clinical sites which recruited and assessed the subjects in this study. The principal investigators and study coordinators of the clinical sites are as follows: University of California, San Francisco, Howard Rosen, MD, Samuel Stark; Georgetown University, Brigid Reynolds, RN, MS, NP, Maysa Jawdat; University of Rochester Medical Center; Anton Porsteinsson, MD, Kim Martin, Nancy Kowalski; Banner Sun Health Research Institute, Edward Zamrini, MD, Sherye Sirrel; University of California, San Diego, James Brewer, MD, PhD, Helen Vanderswag; Rush University Medical Center, Debra Fleischman, PhD, Jamie Plenge, David Sedillo; Duke University Medical Center, P. Murali Doraiswamy, MD, Cammie Hellegers; University of Wisconsin, Madison, Sterling Johnson, PhD, Paul Cary; Brigham and Women's Hospital, Gad Marshall, MD, Lidya Poni; University of Southern California, Lon Schneider, MD, Mauricio Becerra, Karen Stevens-Dagerman; Liberty Teodoro, Sonia Pawluczyk, MD; Stanford University, Jerome Yesavage, MD, Michael Nolasco, Joy Taylor, PhD, Steven Chao, MD, Ann-Mary Salib; University of California, Irvine School of Medicine, Gaby Thai, MD, Beatriz Yanez; Mount Sinai Medical Center, Wien Center, Ranjan Duara, MD, Maria Greig, MD; Columbia University, Yaakov Stern, PhD, Siobhan Lawless; University of Washington Medical Center, Elaine Peskind, MD, Anita Ranta; Premiere Research Institute, Carl Sadowsky, MD, FAAN, Daisy Acevedo, Theresa Villena, MD; Howard University, Thomas Obisesan, MD, Saba Wolday; The Weill Cornell Memory Disorders Program, Michael Tai-ju-Lin, MD, David Kwak; Medical University of South Carolina, Jacobo Mintzer, MD, MBA, Courtney O'Neill.
The authors would like to thank San Francisco Veterans Administration Medical Center recruiters Laura Aronow, Morgan Blackburn, Viktoriya Bourakova, Lauren Chang, Diane Chao, Jason Falamino, Shannon Finley, Stacy Hatcher, Winnie Kwang, Cynthia Leo, Esther Rah, and Omar Rutledge.
Michael W. Weiner has served on the scientific advisory boards for Lilly, Araclon and Institut Catala de Neurociencies Aplicades, Gulf War Veterans Illnesses Advisory Committee, VACO, Biogen Idec, and Pfizer; has served as a consultant for AstraZeneca, Araclon, Medivation/Pfizer, Ipsen, TauRx Therapeutics Ltd., Bayer Healthcare, Biogen Idec, Exonhit Therapeutics, SA, Servier, Synarc, Pfizer, and Janssen; has received funding for travel from NeuroVigil, Inc., CHRU-Hopital Roger Salengro, Siemens, AstraZeneca, Geneva University Hospitals, Lilly, University of California, San Diego–ADNI, Paris University, Institut Catala de Neurociencies Aplicades, University of New Mexico School of Medicine, Ipsen, CTAD (Clinical Trials on Alzheimer's Disease), Pfizer, AD PD meeting, Paul Sabatier University, Novartis, Tohoku University; has served on the editorial advisory boards for Alzheimer's & Dementia and MRI; has received honoraria from NeuroVigil, Inc., Insitut Catala de Neurociencies Aplicades, PMDA/Japanese Ministry of Health, Labour, and Welfare, and Tohoku University; has received commercial research support from Merck and Avid; has received government research support from DOD and VA; has stock options in Synarc and Elan; and declares the following organizations as contributors to the Foundation for NIH and thus to the NIA funded Alzheimer's Disease Neuroimaging Initiative: Abbott, Alzheimer's Association, Alzheimer's Drug Discovery Foundation, Anonymous Foundation, AstraZeneca, Bayer Healthcare, BioClinica, Inc. (ADNI 2), Bristol-Myers Squibb, Cure Alzheimer's Fund, Eisai, Elan, Gene Network Sciences, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson & Johnson, Eli Lilly & Company, Medpace, Merck, Novartis, Pfizer Inc., Roche, Schering Plough, Synarc, and Wyeth.
Dr. Jack has provided consulting services for Eli Lily and Pfizer and owns stock in Johnson and Johnson. He receives research funding from the National Institutes of Health (R01-AG011378, RO1-AG041851, U01-AG06786, U01-AG024904, R01-AG37551, R01-AG043392, R01-NS092625), and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Clinic.
Dr. Landau has consulted for Genentech and Biogen.
Dr. Neylan serves on the Scientific Advisory Board for Resilience Therapeutics.Dr Petersen's consults for Roche, Inc., Merck, Inc., Genentech, Inc., Biogen, Inc. and Eli Lilly and Company: consultant/adviser.
Dr. Saykin received support from multiple NIA, NCI, NSF and DOD grants, Eli Lilly (collaborative research project), Avid Radiopharmaceuticals (AV1451 precursor), and Arkley BioTek (SBIR grant).
References
- 1.Daviglus M.L., Plassman B.L., Pirzada A., Bell C.C., Bowen P.E., Burke J.R. Risk factors and preventive interventions for Alzheimer disease: state of the science. Arch Neurol. 2011;68:1185–1190. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 2.Salib E., Hillier V. Head injury and the risk of Alzheimer's disease: a case control study. Int J Geriatr Psychiatry. 1997;12:363–368. doi: 10.1002/(sici)1099-1166(199703)12:3<363::aid-gps515>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 3.van Duijn C.M., Tanja T.A., Haaxma R., Schulte W., Saan R.J., Lameris A.J. Head trauma and the risk of Alzheimer's disease. Am J Epidemiol. 1992;135:775–782. doi: 10.1093/oxfordjournals.aje.a116364. [DOI] [PubMed] [Google Scholar]
- 4.Mortimer J.A., van Duijn C.M., Chandra V., Fratiglioni L., Graves A.B., Heyman A. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20 Suppl 2:S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 5.Mayeux R., Ottman R., Tang M.X., Noboa-Bauza L., Marder K., Gurland B. Genetic susceptibility and head injury as risk factors for Alzheimer's disease among community-dwelling elderly persons and their first-degree relatives. Ann Neurol. 1993;33:494–501. doi: 10.1002/ana.410330513. [DOI] [PubMed] [Google Scholar]
- 6.O'Meara E.S., Kukull W.A., Sheppard L., Bowen J.D., McCormick W.C., Teri L. Head injury and risk of Alzheimer's disease by apolipoprotein E genotype. Am J Epidemiol. 1997;146:373–384. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 7.Graves A.B., White E., Koepsell T.D., Reifler B.V., van Belle G., Larson E.B. The association between head trauma and Alzheimer's disease. Am J Epidemiol. 1990;131:491–501. doi: 10.1093/oxfordjournals.aje.a115523. [DOI] [PubMed] [Google Scholar]
- 8.Schofield P.W., Tang M., Marder K., Bell K., Dooneief G., Chun M. Alzheimer's disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry. 1997;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heyman A., Wilkinson W.E., Stafford J.A., Helms M.J., Sigmon A.H., Weinberg T. Alzheimer's disease: a study of epidemiological aspects. Ann Neurol. 1984;15:335–341. doi: 10.1002/ana.410150406. [DOI] [PubMed] [Google Scholar]
- 10.Williams D.B., Annegers J.F., Kokmen E., O'Brien P.C., Kurland L.T. Brain injury and neurologic sequelae: a cohort study of dementia, parkinsonism, and amyotrophic lateral sclerosis. Neurology. 1991;41:1554–1557. doi: 10.1212/wnl.41.10.1554. [DOI] [PubMed] [Google Scholar]
- 11.Katzman R., Aronson M., Fuld P., Kawas C., Brown T., Morgenstern H. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol. 1989;25:317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- 12.Launer L.J., Andersen K., Dewey M.E., Letenneur L., Ott A., Amaducci L.A. Rates and risk factors for dementia and Alzheimer's disease: results from EURODEM pooled analyses. EURODEM Incidence Research Group and Work Groups. European Studies of Dementia. Neurology. 1999;52:78–84. doi: 10.1212/wnl.52.1.78. [DOI] [PubMed] [Google Scholar]
- 13.Mehta K.M., Ott A., Kalmijn S., Slooter A.J., van Duijn C.M., Hofman A. Head trauma and risk of dementia and Alzheimer's disease: The Rotterdam Study. Neurology. 1999;53:1959–1962. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 14.Mayeux R. Apolipoprotein e4 and head trauma: synergistic or additive risks? Neurology. 1996;46:889–891. Letter. [PubMed] [Google Scholar]
- 15.Katzman R., Galasko D.R., Saitoh T., Chen X., Pay M.M., Booth A. Apolipoprotein-epsilon4 and head trauma: synergistic or additive risks? Neurology. 1996;46:889–891. [PubMed] [Google Scholar]
- 16.Frankowski R.F., Annegers J.F., Whitman S. Epidemiology and descriptive studies. Part 1. The descriptive epidemiology of head trauma in the United States. In: Becker D.P., Povlishock J., editors. Central Nervous System Trauma Status Report –1985. NIH, NINDS; Bethesda, Maryland: 1985. pp. 33–43. [Google Scholar]
- 17.Mayeux R., Ottman R., Maestre G., Ngai C., Tang M.X., Ginsberg H. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer's disease. Neurology. 1995;45:555–557. doi: 10.1212/wnl.45.3.555. [DOI] [PubMed] [Google Scholar]
- 18.Nemetz P.N., Leibson C., Naessens J.M., Beard M., Kokmen E., Annegers J.F. Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. Am J Epidemiol. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- 19.Gedye A., Beattie B.L., Tuokko H., Horton A., Korsarek E. Severe head injury hastens age of onset of Alzheimer's disease. J Am Geriatr Soc. 1989;37:970–973. doi: 10.1111/j.1532-5415.1989.tb07283.x. [DOI] [PubMed] [Google Scholar]
- 20.Fleminger S., Oliver D.L., Lovestone S., Rabe-Hesketh S., Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rasmusson D.X., Brandt J., Martin D.B., Folstein M.F. Head injury as a risk factor in Alzheimer's disease. Brain Inj. 1995;9:213–219. doi: 10.3109/02699059509008194. [DOI] [PubMed] [Google Scholar]
- 22.Broe G.A., Henderson A.S., Creasey H., McCusker E., Korten A.E., Jorm A.F. A case-control study of Alzheimer's disease in Australia. Neurology. 1990;40:1698–1707. doi: 10.1212/wnl.40.11.1698. [DOI] [PubMed] [Google Scholar]
- 23.Amaducci L.A., Fratiglioni L., Rocca W.A., Fieschi C., Livrea P., Pedone D. Risk factors for clinically diagnosed Alzheimer's disease: a case-control study of an Italian population. Neurology. 1986;36:922–931. doi: 10.1212/wnl.36.7.922. [DOI] [PubMed] [Google Scholar]
- 24.Chandra V., Kokmen E., Schoenberg B.S., Beard C.M. Head trauma with loss of consciousness as a risk factor for Alzheimer's disease. Neurology. 1989;39:1576–1578. doi: 10.1212/wnl.39.12.1576. [DOI] [PubMed] [Google Scholar]
- 25.Chandra V., Philipose V., Bell P.A., Lazaroff A., Schoenberg B.S. Case-control study of late onset “probable Alzheimer's disease”. Neurology. 1987;37:1295–1300. doi: 10.1212/wnl.37.8.1295. [DOI] [PubMed] [Google Scholar]
- 26.Forster D.P., Newens A.J., Kay D.W., Edwardson J.A. Risk factors in clinically diagnosed presenile dementia of the Alzheimer type: a case-control study in northern England. J Epidemiol Community Health. 1995;49:253–258. doi: 10.1136/jech.49.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferini-Strambi L., Smirne S., Garancini P., Pinto P., Franceschi M. Clinical and epidemiological aspects of Alzheimer's disease with presenile onset: a case control study. Neuroepidemiology. 1990;9:39–49. doi: 10.1159/000110750. [DOI] [PubMed] [Google Scholar]
- 28.Fratiglioni L., Ahlbom A., Viitanen M., Winblad B. Risk factors for late-onset Alzheimer's disease: a population-based, case-control study. Ann Neurol. 1993;33:258–266. doi: 10.1002/ana.410330306. [DOI] [PubMed] [Google Scholar]
- 29.Li G., Shen Y.C., Li Y.T., Chen C.H., Zhau Y.W., Silverman J.M. A case-control study of Alzheimer's disease in China. Neurology. 1992;42:1481–1488. doi: 10.1212/wnl.42.8.1481. [DOI] [PubMed] [Google Scholar]
- 30.Anonymous. The Canadian Study of Health and Aging: risk factors for Alzheimer's disease in Canada. Neurology. 1994;44:2073–2080. doi: 10.1212/wnl.44.11.2073. [DOI] [PubMed] [Google Scholar]
- 31.Mortimer J.A., French L.R., Hutton J.T., Schuman L.M. Head injury as a risk factor for Alzheimer's disease. Neurology. 1985;35:264–267. doi: 10.1212/wnl.35.2.264. [DOI] [PubMed] [Google Scholar]
- 32.Tsolaki M., Fountoulakis K., Chantzi E., Kazis A. Risk factors for clinically diagnosed Alzheimer's disease: a case-control study of a Greek population. Int Psychogeriatr. 1997;9:327–341. doi: 10.1017/s104161029700447x. [DOI] [PubMed] [Google Scholar]
- 33.Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 34.Johnson V.E., Stewart W., Smith D.H. Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams J.W., Plassman B.L., Burke J., Benjamin S. Preventing Alzheimer's disease and cognitive decline. Evid Rep Technol Assess (Full Rep) 2010;193:1–727. [PMC free article] [PubMed] [Google Scholar]
- 36.Plassman B.L., Grafman J. Traumatic brain injury and late-life dementia. Handb Clin Neurol. 2015;128:711–722. doi: 10.1016/B978-0-444-63521-1.00044-3. [DOI] [PubMed] [Google Scholar]
- 37.Jellinger K.A. Head injury and dementia. Curr Opin Neurol. 2004;17:719–723. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Jordan B.D., Relkin N.R., Ravdin L.D., Jacobs A.R., Bennett A., Gandy S. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA. 1997;278:136–140. [PubMed] [Google Scholar]
- 39.Friedman G., Froom P., Sazbon L., Grinblatt I., Shochina M., Tsenter J. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology. 1999;52:244–248. doi: 10.1212/wnl.52.2.244. [DOI] [PubMed] [Google Scholar]
- 40.Lichtman S.W., Seliger G., Tycko B., Marder K. Apolipoprotein E and functional recovery from brain injury following postacute rehabilitation. Neurology. 2000;55:1536–1539. doi: 10.1212/wnl.55.10.1536. [DOI] [PubMed] [Google Scholar]
- 41.Jellinger K.A., Paulus W., Wrocklage C., Litvan I. Effects of closed traumatic brain injury and genetic factors on the development of Alzheimer's disease. Eur J Neurol. 2001;8:707–710. doi: 10.1046/j.1468-1331.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- 42.Diaz-Arrastia R., Gong Y., Fair S., Scott K.D., Garcia M.C., Carlile M.C. Increased risk of late posttraumatic seizures associated with inheritance of APOE epsilon4 allele. Arch Neurol. 2003;60:818–822. doi: 10.1001/archneur.60.6.818. [DOI] [PubMed] [Google Scholar]
- 43.Nathoo N., Chetty R., van Dellen J.R., Barnett G.H. Genetic vulnerability following traumatic brain injury: the role of apolipoprotein E. Mol Pathol. 2003;56:132–136. doi: 10.1136/mp.56.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ariza M., Pueyo R., Matarin Mdel M., Junque C., Mataro M., Clemente I. Influence of APOE polymorphism on cognitive and behavioural outcome in moderate and severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77:1191–1193. doi: 10.1136/jnnp.2005.085167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houlden H., Greenwood R. Apolipoprotein E4 and traumatic brain injury. J Neurol Neurosurg Psychiatry. 2006;77:1106–1107. doi: 10.1136/jnnp.2006.095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nicoll J.A., Roberts G.W., Graham D.I. Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med. 1995;1:135–137. doi: 10.1038/nm0295-135. [DOI] [PubMed] [Google Scholar]
- 47.Roberts G.W., Gentleman S.M., Lynch A., Murray L., Landon M., Graham D.I. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1994;57:419–425. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uryu K., Chen X.H., Martinez D., Browne K.D., Johnson V.E., Graham D.I. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol. 2007;208:185–192. doi: 10.1016/j.expneurol.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mielke M.M., Savica R., Wiste H.J., Weigand S.D., Vemuri P., Knopman D.S. Head trauma and in vivo measures of amyloid and neurodegeneration in a population-based study. Neurology. 2014;82:70–76. doi: 10.1212/01.wnl.0000438229.56094.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkins M.A., Langlais P.J., Delis D.A., Cohen R.A. Attentional dysfunction associated with posttraumatic stress disorder among rape survivors. Clin Neuropsychol. 2000;14:7–12. doi: 10.1076/1385-4046(200002)14:1;1-8;FT007. [DOI] [PubMed] [Google Scholar]
- 51.Yehuda R., Ledoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Kulka R.A., Schlenger W.E., Fairbank J.A., Hough R.L., Jordan B.K., Marmar C.R. Brunner/Mazel; New York: 1990. Trauma and the Vietnam War Generation. [Google Scholar]
- 53.Dohrenwend B.P., Turner J.B., Turse N.A., Adams B.G., Koenen K.C., Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313:979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samuelson K.W., Neylan T.C., Metzler T.J., Lenoci M., Rothlind J., Henn-Haase C. Neuropsychological functioning in posttraumatic stress disorder and alcohol abuse. Neuropsychology. 2006;20:716–726. doi: 10.1037/0894-4105.20.6.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbertson M.W., Gurvits T.V., Lasko N.B., Orr S.P., Pitman R.K. Multivariate assessment of explicit memory function in combat veterans with posttraumatic stress disorder. J Trauma Stress. 2001;14:413–432. doi: 10.1023/A:1011181305501. [DOI] [PubMed] [Google Scholar]
- 56.Vasterling J.J., Duke L.M., Brailey K., Constans J.I., Allain A.N., Jr., Sutker P.B. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5–14. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 57.Bremner J.D., Scott T.M., Delaney R.C., Southwick S.M., Mason J.W., Johnson D.R. Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry. 1993;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- 58.Golier J., Yehuda R., Cornblatt B., Harvey P., Gerber D., Levengood R. Sustained attention in combat-related posttraumatic stress disorder. Integr Physiol Behav Sci. 1997;32:52–61. doi: 10.1007/BF02688613. [DOI] [PubMed] [Google Scholar]
- 59.Yehuda R., Keefe R.S., Harvey P.D., Levengood R.A., Gerber D.K., Geni J. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]
- 60.Uddo M., Vasterling J.J., Brailey K., Sutker P.B. Memory and attention in combat-related post-traumatic stress disorder (PTSD) J Psychopathol Behav Assess. 1993;15:43–52. [Google Scholar]
- 61.Vasterling J.J., Brailey K., Constans J.I., Sutker P.B. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology. 1998;12:125–133. doi: 10.1037//0894-4105.12.1.125. [DOI] [PubMed] [Google Scholar]
- 62.Johnsen G.E., Asbjornsen A.E. Verbal learning and memory impairments in posttraumatic stress disorder: the role of encoding strategies. Psychiatry Res. 2009;165:68–77. doi: 10.1016/j.psychres.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Schuff N., Marmar C.R., Weiss D.S., Neylan T.C., Schoenfeld F., Fein G. Reduced hippocampal volume and n-acetylaspartate in post traumatic stress disorder. Ann N Y Acad Sci. 1997;821:516–520. doi: 10.1111/j.1749-6632.1997.tb48319.x. [DOI] [PubMed] [Google Scholar]
- 64.Schuff N., Neylan T.C., Lenoci M.A., Du A.T., Weiss D.S., Marmar C.R. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schuff N., Neylan T.C., Fox-Bosetti S., Lenoci M., Samuelson K.W., Studholme C. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res. 2008;162:147–157. doi: 10.1016/j.pscychresns.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Z., Neylan T.C., Mueller S.G., Lenoci M., Truran D., Marmar C.R. Magnetic resonance imaging of hippocampal subfields in posttraumatic stress disorder. Arch Gen Psychiatry. 2010;67:296–303. doi: 10.1001/archgenpsychiatry.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Apfel B.A., Ross J., Hlavin J., Meyerhoff D.J., Metzler T.J., Marmar C.R. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry. 2011;69:541–548. doi: 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodward S.H., Schaer M., Kaloupek D.G., Cediel L., Eliez S. Smaller global and regional cortical volume in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1373–1382. doi: 10.1001/archgenpsychiatry.2009.160. [DOI] [PubMed] [Google Scholar]
- 69.Koenigs M., Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yaffe K., Vittinghoff E., Lindquist K., Barnes D., Covinsky K.E., Neylan T. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Weiner M.W., Veitch D.P., Aisen P.S., Beckett L.A., Cairns N.J., Cedarbaum J., Alzheimer's Disease Neuroimaging Initiative 2014 update of The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. 2015;11:e1–e120. doi: 10.1016/j.jalz.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corrigan J.D., Bogner J. Initial reliability and validity of the Ohio State University TBI identification method. J Head Trauma Rehabil. 2007;22:318–329. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- 73.Gallo J.J., Breitner J.C. Alzheimer's disease in the NAS-NRC Registry of aging twin veterans, IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer's dementia. Psychol Med. 1995;25:1211–1219. doi: 10.1017/s0033291700033183. [DOI] [PubMed] [Google Scholar]
- 74.Breitner J.C., Welsh K.A. Diagnosis and management of memory loss and cognitive disorders among elderly persons. Psychiatr Serv. 1995;46:29–35. doi: 10.1176/ps.46.1.29. [DOI] [PubMed] [Google Scholar]
- 75.Müller J.M., Postert C., Beyer T., Furniss T., Achtergarde S. Comparison of eleven short versions of the Symptom Checklist 90-Revised (SCL-90-R) for use in the assessment of general psychopathology. J Psychopathol Behav Assess. 2010;32:246–254. [Google Scholar]
- 76.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 77.Gandek B., Ware J.E., Aaronson N.K., Apolone G., Bjorner J.B., Brazier J.E. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. J Clin Epidemiol. 1998;51:1171–1178. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 78.Keane T.M., Fairbank J.A., Caddell J.M., Zimering R.T., Taylor K.L., Mora C.A. Clinical evaluation of a measure to assess combat exposure. Psychol Assess A J Consulting Clin Psychol. 1989;1:53. [Google Scholar]
- 79.Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 80.Wolfe J., Kimerling R., Brown P., Chrestman K. National Center for PTSD, Boston VA Medical Center; Boston: 1993. The Life Stressor Checklist. [Google Scholar]
- 81.Cacciola J.S., Alterman A.I., McLellan A.T., Lin Y.T., Lynch K.G. Initial evidence for the reliability and validity of a “Lite” version of the Addiction Severity Index. Drug Alcohol Depend. 2007;87:297–302. doi: 10.1016/j.drugalcdep.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 82.Kang J.H., Korecka M., Figurski M.J., Toledo J.B., Blennow K., Zetterberg H. The Alzheimer's Disease Neuroimaging Initiative 2 Biomarker Core: a review of progress and plans. Alzheimers Dement. 2015;11:772–791. doi: 10.1016/j.jalz.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saykin A.J., Shen L., Foroud T.M., Potkin S.G., Swaminathan S., Kim S. Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement. 2010;6:265–273. doi: 10.1016/j.jalz.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jack C.R., Jr., Bernstein M.A., Fox N.C., Thompson P., Alexander G., Harvey D. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jagust W.J., Landau S.M., Koeppe R.A., Reiman E.M., Chen K., Mathis C.A. The Alzheimer's Disease Neuroimaging Initiative 2 PET Core: 2015. Alzheimers Dement. 2015;11:757–771. doi: 10.1016/j.jalz.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aisen P.S., Petersen R.C., Donohue M., Weiner M.W. Alzheimer's Disease Neuroimaging Initiative 2 Clinical Core: progress and plans. Alzheimers Dement. 2015;11:734–739. doi: 10.1016/j.jalz.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCullagh P., Nelder J. 2nd ed. Chapman and Hall; London: 1989. Generalized Linear Models. [Google Scholar]
- 88.SAS SIIVotSSfWSaaoSIIposnartoto.
- 89.Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann Neurol. 2012;72:578–586. doi: 10.1002/ana.23650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scott Mackin R., Lesselyong J.A., Yaffe K. Pattern of cognitive impairment in older veterans with posttraumatic stress disorder evaluated at a memory disorders clinic. Int J Geriatr Psychiatry. 2012;27:637–642. doi: 10.1002/gps.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cohen B.E., Neylan T.C., Yaffe K., Samuelson K.W., Li Y., Barnes D.E. Posttraumatic stress disorder and cognitive function: findings from the mind your heart study. J Clin Psychiatry. 2013;74:1063–1070. doi: 10.4088/JCP.12m08291. [DOI] [PubMed] [Google Scholar]
- 92.Greenberg M.S., Tanev K., Marin M.F., Pitman R.K. Stress, PTSD, and dementia. Alzheimers Dement. 2014;10:S155–S165. doi: 10.1016/j.jalz.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 93.Meziab O., Kirby K.A., Williams B., Yaffe K., Byers A.L., Barnes D.E. Prisoner of war status, posttraumatic stress disorder, and dementia in older veterans. Alzheimers Dement. 2014;10:S236–S241. doi: 10.1016/j.jalz.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Qureshi S.U., Long M.E., Bradshaw M.R., Pyne J.M., Magruder K.M., Kimbrell T. Does PTSD impair cognition beyond the effect of trauma? J Neuropsychiatry Clin Neurosci. 2011;23:16–28. doi: 10.1176/jnp.23.1.jnp16. [DOI] [PubMed] [Google Scholar]
- 95.Qureshi S.U., Kimbrell T., Pyne J.M., Magruder K.M., Hudson T.J., Petersen N.J. Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J Am Geriatr Soc. 2010;58:1627–1633. doi: 10.1111/j.1532-5415.2010.02977.x. [DOI] [PubMed] [Google Scholar]
- 96.Bremner J.D., Randall P., Scott T.M., Bronen R.A., Seibyl J.P., Southwick S.M. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gurvits T.V., Shenton M.E., Hokama H., Ohta H., Lasko N.B., Gilbertson M.W. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hedges D.W., Allen S., Tate D.F., Thatcher G.W., Miller M.J., Rice S.A. Reduced hippocampal volume in alcohol and substance naive Vietnam combat veterans with posttraumatic stress disorder. Cogn Behav Neurol. 2003;16:219–224. doi: 10.1097/00146965-200312000-00003. [DOI] [PubMed] [Google Scholar]
- 99.Woodward S.H., Kaloupek D.G., Streeter C.C., Kimble M.O., Reiss A.L., Eliez S. Hippocampal volume, PTSD, and alcoholism in combat veterans. Am J Psychiatry. 2006;163:674–681. doi: 10.1176/ajp.2006.163.4.674. [DOI] [PubMed] [Google Scholar]
- 100.Bonne O., Brandes D., Gilboa A., Gomori J.M., Shenton M.E., Pitman R.K. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Bellis M.D., Keshavan M.S., Clark D.B., Casey B.J., Giedd J.N., Boring A.M. A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- 102.Fennema-Notestine C., Stein M.B., Kennedy C.M., Archibald S.L., Jernigan T.L. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- 103.Schuff N., Woerner N., Boreta L., Kornfield T., Shaw L.M., Trojanowski J.Q. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crane P.K., Gibbons L.E., Dams-O'Connor K., Trittschuh E., Leverenz J.B., Keene C.D. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73:1062–1069. doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]