Abstract
Myosin is the principal component of the thick filaments that, through interactions with the actin thin filaments, mediates force production during muscle contraction. Myosin is a hexamer, consisting of two heavy chains, each associated with an essential (ELC) and a regulatory (RLC) light chain, which bind the lever-arm of the heavy chain and play important modulatory roles in striated muscle contraction. Nevertheless, a comprehensive assessment of the sequences of the ELC and RLC isoforms, as well as their post-translational modifications, in the heart remains lacking. Herein, utilizing top-down high-resolution mass spectrometry (MS), we have comprehensively characterized the sequences and N-terminal modifications of the atrial and ventricular isoforms of the myosin light chains from human and swine hearts, as well as the sites of phosphorylation in the swine proteins. In addition to the correction of disparities in the database sequences of the swine proteins, we show for the first time that, whereas the ventricular isoforms of the ELC and RLC are methylated at their N-termini, which is consistent with previous studies, the atrial isoforms of the ELC and RLC from both human and swine are Nα-methylated and Nα-acetylated, respectively. Furthermore, top-down MS with electron capture dissociation enabled localization of the sites of phosphorylation in swine RLC isoforms from the ventricles and atria to Ser14 and Ser22, respectively. Collectively, these results provide new insights into the sequences and modifications of myosin light chain isoforms in the human and swine hearts, which will pave the way for a better understanding of their functional roles in cardiac physiology and pathophysiology.
Keywords: Myosin light chain, Top-down mass spectrometry, Post-translational modification, Phosphorylation, Acetylation, Methylation
1. Introduction
The thick filaments of muscles, including cardiac, skeletal, and smooth muscles, are predominantly composed of myosin that, through interactions with actin (the principal component of thin filaments), mediates force production during muscle contraction [1, 2]. Myosin is a hexamer consisting of two heavy chains (MHCs), each of which is associated with an essential (or alkali) light chain (ELC) and a regulatory (or phosphorylatable) light chain (RLC) [3]. In striated muscles both the ELC and RLC, which bind and stabilize the lever-arm of the MHC [4, 5], play critical roles in the modulation of contractile function [6-11]. In support of this, mutations in both the ELC and RLC are associated with the development of cardiac and skeletal muscle myopathies [12]. Additionally, phosphorylation of the RLC by the Ca2+/calmodulin-dependent myosin light chain kinase (MLCK) represents a critical mechanism regulating contractility, especially in the heart [6, 11, 13-16]. In particular, recent studies have convincingly demonstrated that loss of RLC phosphorylation leads to pathological cardiac hypertrophy and heart failure [16-19]. These findings have generated substantial interest in the roles played by myosin light chains and their modifications in cardiac physiology and pathophysiology; thus, a comprehensive assessment of myosin light chain isoforms and their post-translational modifications (PTMs) in the heart is apropos.
A number of different myosin light chain isoforms have been identified to date, all of which belong to the EF-hand protein super-family of Ca2+-binding proteins [20]. Four of these genes are expressed in the heart, with expression patterns that vary by chamber, developmentally, and in response to pathological stimuli [7, 10, 15]. The MYL3 and MYL4 genes encode the ventricular (ELCv) and atrial (ELCa) isoforms of the ELC, while the ventricular (RLCv) and atrial (RLCa) isoforms of the RLC are encoded by the MYL2 and MYL7 genes, respectively. Although expression of ELCv is primarily restricted to the ventricles of the heart, ELCa is expressed in both the atria and ventricles during normal embryonic development [7, 10]. In adulthood, however, expression of the ELCa is restricted to the atria, although re-expression in the ventricles occurs in response to pressure overload and heart failure [7, 10, 21]. On the other hand, RLCv expression is restricted to the ventricles both in the developing and adult heart [15]. Conversely, RLCa, like ELCa, is expressed throughout the heart early in development, and becomes restricted to the atria later in development [15].
Top-down mass spectrometry (MS) has gained considerable popularity as the premier approach for comprehensively characterizing proteins [22-24]. Unlike in conventional bottom-up MS, in which proteins are digested and the resulting peptides are analyzed by MS, intact proteins are analyzed in top-down MS, providing a global or “bird's eye” view of all protein species, including those containing sequence variations (due to mutations/polymorphisms or alternative splicing) and/or PTMs [22-30]. Following intact protein analysis, specific protein species of interest can be isolated and fragmented using a variety of tandem MS (MS/MS) techniques, including, but not limited to, electron capture dissociation (ECD) and collision induced dissociation (CID), to obtain sequence information and localize PTMs [22-30]. In particular, top-down MS with ECD represents a powerful method for the comprehensive characterization of proteins; especially those containing labile PTMs such as phosphorylation, which are frequently lost when proteins are fragmented using energetic dissociation methods such as CID [25]. Additionally, the use of high-resolution mass spectrometers in top-down MS studies offers unparalleled mass accuracy, which not only increases confidence in protein identification, but also in the identification of protein PTMs [31]. Herein, utilizing top-down high-resolution MS, we have characterized the sequences and N-terminal modifications of cardiac myosin light chain isoforms from human and swine, as well as the sites of phosphorylation in the swine proteins, towards a better understanding of the functional roles of these proteins in cardiac physiology and pathophysiology. Interestingly, we found that, whereas the ventricular ELC and RLC are Nα-tri-methylated, the atrial ELC is methylated at its N-terminus while the atrial RLC in both swine and human is Nα-acetylated; making the atrial RLC unique among cardiac myosin light chain isoforms. Importantly, we have also precisely localized the sites of phosphorylation in swine RLC isoforms from the ventricles and atria to Ser14 and Ser22, respectively. Although prior studies have reported atrial RLC phosphorylation, this represents the first study to definitively localize a site of phosphorylation in this isoform.
2. Methods
Detailed methods are found in the Supporting Information. To comprehensively characterize the sequences and PTMs of swine myosin light chain isoforms, myofilament-enriched extracts were prepared from the atrial and ventricular myocardium of 1-3 healthy adult Yorkshire domestic swine (Sus scrofa) (approximately 3 months of age) using the two-step extraction procedure described by Van Eyk et al. [32, 33] with modifications. Subsequently, protein extracts prepared from swine atrial or ventricular myocardium were separated by 1D reverse phase liquid chromatography (LC) coupled directly online with a linear ion trap (LTQ) mass spectrometer (Thermo Scientific, Bremen, Germany) (Fig. S1), and eluting myosin light chain isoforms were fraction collected. MS/MS analysis of the fraction collected swine myosin light chain isoforms was conducted on a 7T LTQ/FT Ultra high-resolution mass spectrometer (Thermo Scientific) (Fig. 1). Moreover, to characterize the sequences and N-terminal modifications of human cardiac myosin light chain isoforms, myofilament-enriched extracts were prepared from donor atrial and ventricular myocardium as above, and online liquid chromatography (LC)-MS/MS analysis was carried out on a maXis II mass spectrometer (Bruker, Billerica, MA, USA). Accession numbers for all myosin light chain sequences utilized in this study can be found in Table S1.
Fig. 1.
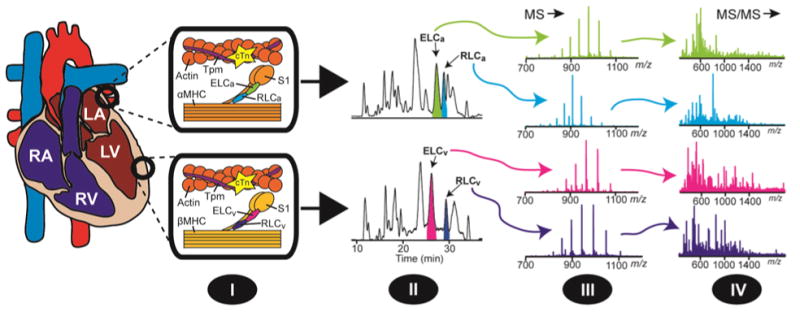
Overview of top-down MS strategy for the identification and characterization of myosin light chain isoforms in the atria and ventricles of the heart. (I) Extraction of the myofilament sub-proteome from atrial and ventricular tissue. (II) 1DLC separation of myofilament sub-proteomes and fraction collection of myosin light chain isoforms. (III) MS profiling of myosin light chain isoforms and the quantification of RLC phosphorylation. (IV) Comprehensive MS/MS characterization of isolated myosin light chain isoforms. RA, right atrium; RV, right ventricle; LA, left atrium; LV, left ventricle; Tpm, tropomyosin; cTn, cardiac troponin; αMHC, α myosin heavy chain; βMHC, β myosin heavy chain; S1, myosin heavy chain subfragment-1.
3. Results
3.1 Inhibition of phosphatase activity towards the RLC
To quantify RLC phosphorylation we employed quantitative online top-down LC-MS. This method provided robust and highly-reproducible measurement of RLC phosphorylation in cardiac tissue extracts prepared using the two-step extraction procedure described by Van Eyk et al. [32, 33] with modifications (Fig. S2, Supplemental Results). By varying the concentration of phosphatase inhibitors used in the HEPES-based extraction buffer [32, 33], we determined that supplementation with 600 mM NaF (in combination with 5 mM sodium pyrophosphate and 100 mM β-glycerophosphate) provided maximal inhibition of phosphatase activity towards the RLC in myocardial extracts (Figs. S3-S4, Supplemental Results). Therefore, HEPES-based buffer containing 600 mM NaF, 5 mM sodium pyrophosphate, and 100 mM β-glycerophosphate was used for all subsequent experiments to prevent de-phosphorylation of RLC during the preparation of myofilament-enriched protein extracts from atrial and ventricular myocardium.
3.2 Sequencing and N-terminal modification characterization of swine and human RLCa
Top-down MS profiling of RLCa protein species in extracts prepared from the atrial myocardium of swine revealed the presence of major and minor protein species presumably corresponding to un-phosphorylated RLCa (Mr 19,372.59 Da), mono-phosphorylated RLCa (pRLCa; Mr 19,452.55 Da), and bis-phosphorylated RLCa (ppRLCa; 19,532.51 Da) (Fig. 2A). Top-down LC-MS-based quantification yielded a mean value of 0.16 ± 0.03 mol of Pi/mol of RLC for swine RLCa (n=5) (Fig. 2B).
Fig. 2.
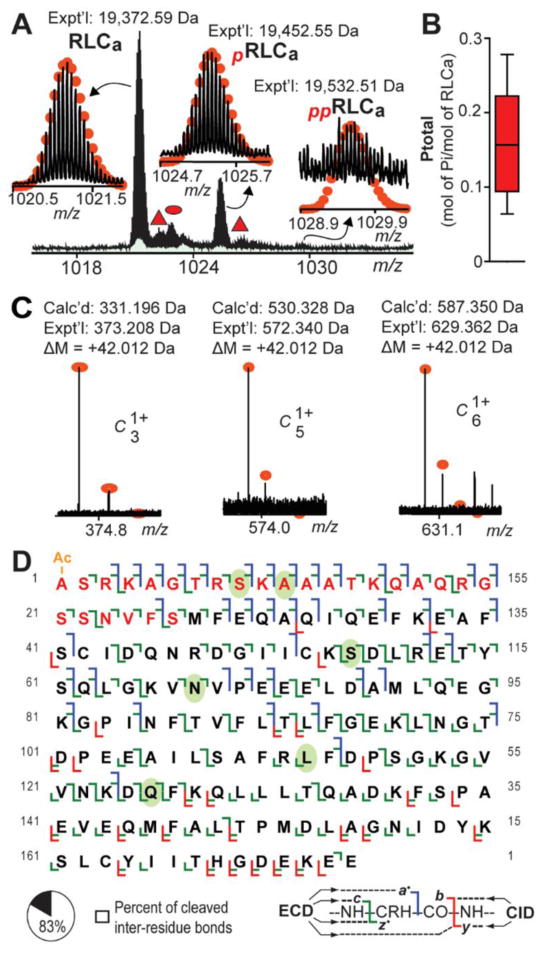
Top-down MS analysis of swine RLCa. A. Zoomed-in mass spectrum showing detected RLCa species in extracts prepared from swine atrial myocardium. Triangle and oval represent RLCa associated non-covalently with sodium and potassium, respectively. B. Graph showing MS-based quantification of swine RLCa phosphorylation, which had a mean value of 0.16 ± 0.03 mol of Pi/mol of RLC (n=5). C. N-terminal fragment ions showing approximate 42.010 Da mass increase compared to the calculated masses for the corresponding un-modified fragment ions, indicating N-terminal acetylation of swine RLCa. D. Sequence table showing bond cleavages for RLCa (∼83% of all inter-residue bonds). Residues in red are amino acids not present in the previous incorrect swine RLCa sequence in the UniProtKB/Swiss-Prot database. Ovals indicate amino acids differing between swine and human RLCa. “Ac-” denotes acetylation. “p” and “pp” indicate mono- and bis-phosphorylated protein forms, respectively.
However, the expected mass of swine RLCa, predicted based on the current unconfirmed sequence in the UniProtKB/Swiss-Prot database (Mr 16,694.24 Da), was substantially different than the experimentally determined mass for this protein (Fig. 2A), indicating potential sequence discrepancies in the unconfirmed sequence. Therefore, to correct potential sequence discrepancies in the database sequence, we first verified the sequence of the human protein using online top-down LC-MS/MS (Fig. S5), and, subsequently, utilized the confirmed human RLCa sequence as a template to characterize the swine protein. Interestingly, although previous studies have shown that ventricular myosin light chain isoforms are Nα-tri-methylated [34], high accuracy mass measurement revealed that human RLCa is N-terminally acetylated (+42.010 Da) rather than tri-methylated (+42.046 Da) (Fig. S5A). Similarly, swine RLCa was also Nα-acetylated rather than N-terminally methylated (Fig. 2C).
Comprehensive top-down MS analysis permitted the confirmation of 6 amino acid differences between the swine and human proteins (Fig. 2D; Fig. S6-S7; Supplementary Results). Moreover, swine RLCa contained a 26 amino acid N-terminal stretch not present in the unconfirmed swine sequence in the UniProtKB/Swiss-Prot database (Fig. 2D; Fig. S8). After sequencing, a total of 41 a·, 89 c, 28 y, and 87 z· ions could be matched to the sequence of swine RLCa (from a combined 2 ECD tandem mass spectra), representing cleavage of 144 out of 173 inter-residue bonds (∼83% sequence coverage) (Fig. 2D). Note that a·, b, and c ions contain the N-terminus, while x, y, and z· ions contain the C-terminus. Comparison of the confirmed sequences for human and swine RLCa with sequences in the UniProtKB/Swiss-Prot database showed that human RLCa is more similar in sequence to swine RLCa (approximately 97% sequence similarity) than mouse RLCa (Table S2).
3.2 Localization of the phosphorylation site in swine pRLCa
Previous studies have reported the existence of mono- and bis-phosphorylated forms of RLCa in human atrial myocardium [35]; however, the specific sites of phosphorylation in RLCa have not been determined. To localize the site(s) of mono-phosphorylation in swine RLCa, peaks corresponding to pRLCa were isolated and fragmented using ECD. Analysis of the MS/MS data revealed that all c ions prior to and including c21 were observed only in their un-phosphorylated states (Fig. 3A, B), which indicates that the site(s) of phosphorylation in pRLCa is located after Ser21. On the other hand, all c ions following and including c22 were detected only in their mono-phosphorylated state (Fig. 3C, D). This data indicates that Ser22 is a site of phosphorylation in swine RLCa. Additionally, all z· ions prior to and including z·152 were detected only in their un-phosphorylated state (Fig. 3E), whereas all z· ions after and including z·154 were observed only in their mono-phosphorylated state (Fig. 3F). Collectively, these results indicate that Ser22 is the sole site of phosphorylation in pRLCa from the swine heart (Fig. 3G). Unfortunately, the very low abundance of ppRLCa precluded MS/MS analysis of this species and, thus, localization of the second phosphorylation site in swine RLCa. To our knowledge this represents the first study to definitively localize a site of phosphorylation in RLCa.
Fig. 3.
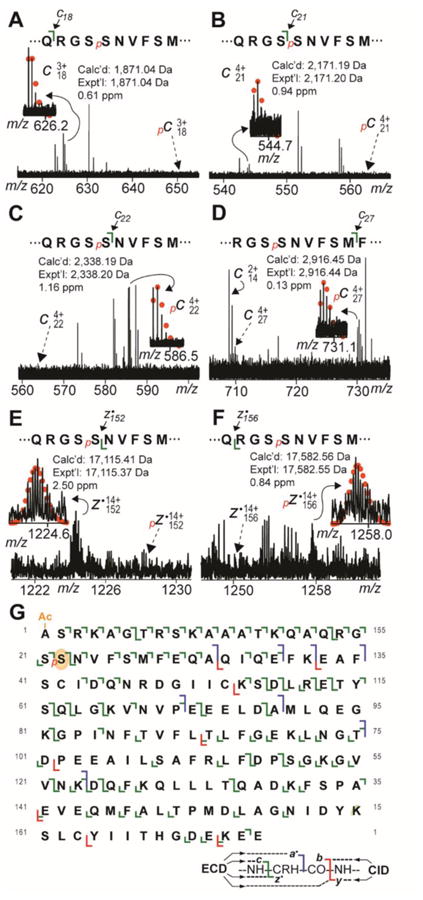
Localization of the phosphorylation site in swine pRLCa to Ser22 by top-down ECD MS/MS. A. Zoomed-in tandem mass spectrum showing observation of un-phosphorylated c18 ion without corresponding pc18 ion (expected position denoted by broken arrow). B. Zoomed-in tandem mass spectrum showing observation of un-phosphorylated c21 ion without corresponding pc21 ion (expected position denoted by broken arrow). C. Zoomed-in tandem mass spectrum showing observation of pc22 ion without corresponding un-phosphorylated c22 ion (expected position denoted by broken arrow). D. Zoomed-in tandem mass spectrum showing observation of pc27 ion without corresponding un-phosphorylated c27 ion (expected position denoted by broken arrow). E. Zoomed-in tandem mass spectrum showing observation of z·152 ion without corresponding pz·152 ion (expected position denoted by broken arrow). F. Zoomed-in tandem mass spectrum showing observation of pz·156 without corresponding un-phosphorylated z·156 ion (expected position denoted by broken arrow). G. Sequence table showing bond cleavages and site of phosphorylation in pRLCa. Oval indicates phosphorylated residue (Ser22) in swine pRLCa. “Ac-” denotes acetylation. “p” indicates mono-phosphorylation.
3.3 Sequencing and N-terminal modification characterization of ELCa from swine and human
Top-down MS profiling of swine ELCa protein species revealed the presence of a peak likely corresponding to un-modified ELCa (Mr 21,489.84 Da) (Fig. 4A). Peaks corresponding to mono-phosphorylated ELCa (pELCa) were not detected in any of the spectra and, thus, would represent less than 0.1% of the ELCa population based on the signal intensity at the expected position of pELCa (Fig. 4A).
Fig. 4.
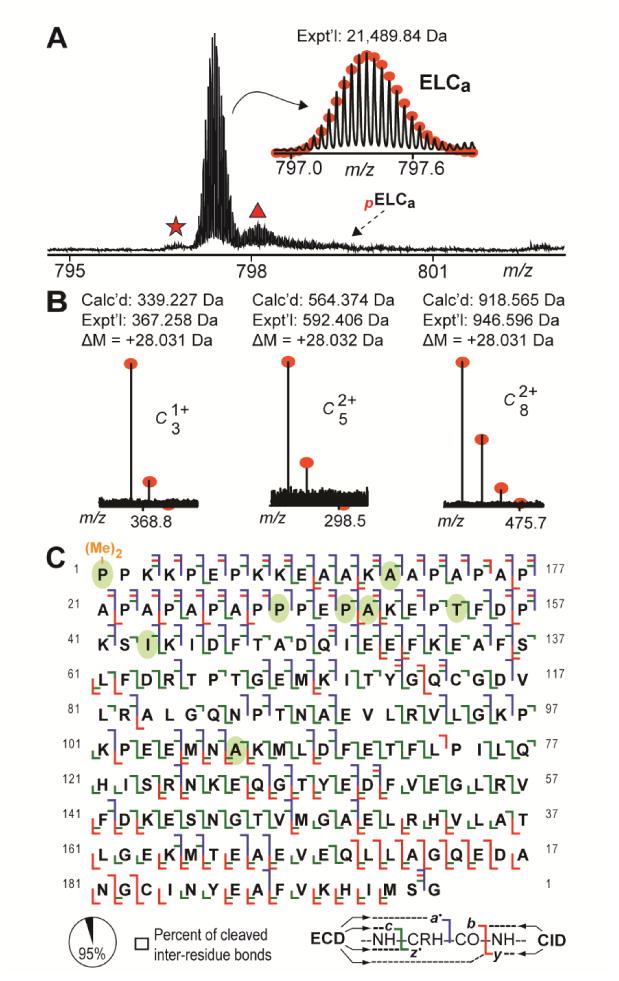
Top-down MS and MS/MS analyses of swine ELCa. A. Zoomed-in mass spectrum showing detected ELCa protein species in extracts prepared from the atrial myocardium of swine. Broken arrow indicates expected position of un-observed pELCa species. Star and triangle mark peaks corresponding to ELCa with NH3 loss and ELCa associated non-covalently with sodium. B. N-terminal fragment ions showing approximate 28.031 Da mass increase compared to the calculated masses for the corresponding un-modified fragment ions, indicating N-terminal di-methylation of swine ELCa. C. Sequence table for ELCa showing bond cleavages (∼95% of all inter-residue bonds). Ovals indicate amino acids differing between swine and human ELCa. “(Me)2-” denotes di-methylation. “p” indicates mono-phosphorylation.
As with swine RLCa, the experimentally determined mass of swine ELCa did not match with that calculated based on the unconfirmed sequence for this protein in the UniProtKB/Swiss-Prot database. Therefore, as above, we first verified the sequence of the human protein (Fig. S9), and utilized the confirmed human ELCa sequence as a template to characterize the swine protein.
Comprehensive MS/MS analysis of swine ELCa, using a combination of ECD and CID, allowed for the identification of di-methylation as the N-terminal modification in swine ELCa (Fig. 4B; Supplementary Results), as well as the localization of 8 amino acid differences between the swine and human proteins (Fig. 4C; Fig. S10-S11; Supplemental Results). Notably, determination of the swine ELCa sequence revealed that the initial five N-terminal amino acids present in the unconfirmed swine sequence from the UniProtKB/Swiss-Prot database were not present in the protein (Fig. S12). Following in-depth sequencing of swine ELCa, a total of 85 a·, 41 b, 125 c, 66 y, and 126 z· ions could be matched to the determined ELCa sequence (from a combined 7 ECD and 1 CID tandem mass spectra), representing cleavage of 185 out of 195 inter-residue bonds (∼95% sequence coverage) (Fig. 4C). Based on the determined sequences, swine ELCa shared the highest sequence similarity with the human protein (approximately 96% sequence similarity with human ELCa) (Table S2).
3.4 Sequencing and N-terminal modification characterization of swine and human RLCv
Analysis of myofilament-enriched protein extracts prepared from swine ventricular myocardium revealed the presence of un-modified and mono-phosphorylated forms of RLCv in agreement with our previous findings (Fig. S13, Supplemental Results) [29]. Quantitative LC-MS analysis yielded a mean of 0.12 ± 0.01 mol of Pi/mol of RLCv in the swine ventricular myocardium (n=6) (Fig. S13C). Sequence characterization of swine RLCv was in good agreement with our previous analysis of this protein (Fig. S13, Supplemental Results) [29].
In addition to verifying the sequence of swine RLCv, we have also confirmed the sequence of human RLCv, which matched well with the UniProtKB/Swiss-Prot database sequence for this protein (accession number P10916; Table S2) after considering removal of the N-terminal Met and tri-methylation of Ala1 (Fig. S14). Surprisingly, despite high sequence similarity with human RLCv, the sequence of swine RLCv was most similar to the rabbit protein (Fig. S15; Table S2). Similarly, human RLCv was most similar in sequence to rabbit RLCv (approximately 98% sequence similarity), with swine and mouse showing identical sequence similarity to the human protein (∼96% sequence similarity) (Fig. S15; Table S2).
3.5 Localization of the phosphorylation site in swine pRLCv
The phosphorylation site in swine RLCv has been localized previously [13, 29]. To confirm the phosphorylation site in swine RLCv, peaks corresponding to pRLCv were selected for fragmentation by ECD. Comprehensive ECD MS/MS analysis revealed that swine pRLCv is phosphorylated at a single site (Ser14), which is in good agreement with our previous findings and those of others (Figs. S16, Supplemental Results) [13, 29].
3.6 Sequencing and N-terminal modification characterization of ELCv from swine and human
Top-down MS profiling of ELCv protein species in the swine ventricle revealed the presence of a protein species with an experimental mass (Mr 21,753.86 Da) that corresponded well with the predicted mass of swine ELCv calculated based on the sequence in the UniProtKB/Swiss-Prot database (accession number F1SNW4; Table S1) after considering removal of the N-terminal Met and Nα-tri-methylation of Ala1 (Fig. 5A, B) [34]. Peaks corresponding to mono-phosphorylated ELCv (pELCv) were not detected in any of the mass spectra analyzed and, thus, would represent less than approximately 0.5% of the entire ELCv population based on the signal intensity at the expected position of pELCv (Fig. 5A).
Fig. 5.
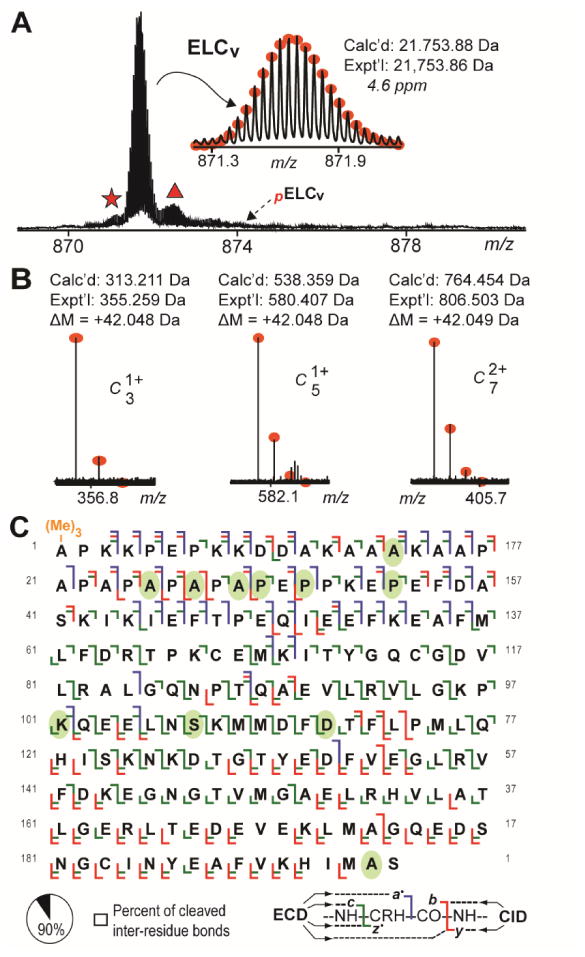
Top-down MS and MS/MS analyses of swine ELCv. A. Zoomed-in mass spectrum showing swine ELCv protein species in extracts prepared from the ventricular myocardium of swine. Broken arrow indicates expected position of un-observed pELCv species. Star and triangle mark peaks corresponding to ELCv with NH3 loss and ELCv associated non-covalently with sodium, respectively. B. N-terminal fragment ions showing approximate 42.046 Da mass increase compared to the calculated masses for the corresponding un-modified fragment ions, indicating N-terminal tri-methylation of swine ELCv. C. Sequence table for ELCv showing bond cleavages (∼90% of all inter-residue bonds). Ovals indicate amino acids differing between swine and human ELCv. “(Me)3-” denotes tri-methylation. “p” indicates mono-phosphorylation.
To confirm the sequence of swine ELCv, peaks corresponding to the un-modified protein were isolated and fragmented using CID and ECD. Comprehensive sequencing allowed for confirmation of 11 amino acid differences between the swine and human proteins (Fig. 5C; Fig. S17-S18; Supplemental Results). Following MS/MS analysis, a total of 41 a·, 34 b, 102 c, 62 y, and 104 z· ions could be matched to the swine ELCv sequence with high mass accuracy (from a combined 7 ECD and 3 CID tandem mass spectra) (Fig. 5C). This represents cleavage of 176 out of 195 inter-residue bonds (approximately 90% sequence coverage), providing high confidence in the sequence of swine ELCv (Fig. 5C). In addition to verification of the swine ELCv sequence, we also confirmed the sequence of human ELCv, which matched well with the sequence in the UniProtKB/Swiss-Prot database (accession number P08590; Table S1) after considering N-terminal Met removal and tri-methylation of Ala1 (Fig. S19). Comparison of the confirmed sequences for human and swine ELCv with sequences in the UniProtKB/Swiss-Prot database showed that, while human ELCv is closest in sequence to the swine protein (approximately 94% sequence similarity), swine ELCv shared the highest sequence similarity with rat ELCv (approximately 95% sequence similarity) (Table S2).
4. Discussion
4.1 Characterization of cardiac myosin light chain isoform sequences
In this study, we utilized top-down high-resolution MS/MS to characterize the sequences of cardiac myosin light chain isoforms in the human and swine hearts (Figs. 2, 4, 5; Figs. S5, S9, S13, S14, S19) towards a better understanding of the functional roles of these proteins in cardiac physiology and pathophysiology.
In addition to verifying the sequence of human RLCa, we found that swine RLCa contained a 26 amino acid stretch not present in the UniProtKB/Swiss-Prot database sequence for this protein (Fig. 2D; Fig. S8). Comprehensive top-down MS/MS sequencing also enabled the confirmation of six amino acid differences between human and swine RLCa (Fig. 2D; Fig. S6). These differences were distributed throughout the protein sequence, with two occurring near the N-terminus of the protein, one within the first EF-hand domain, one immediately adjacent to the first EF-hand domain, and two within the second EF-hand domain (Fig. S7). Sequence comparison using the sequences for human and swine RLCa confirmed herein, as well as protein sequences from other species in the UniProtKB/Swiss-Prot database, revealed that swine RLCa was most similar in sequence to the human protein (∼97% sequence similarity) (Table S2). Likewise, the sequence of human RLCa was more similar in sequence to the swine protein than it was to the mouse protein (Table S2). Six amino acid differences were also confirmed between swine and human RLCv isoforms (Figs. S13, S15). Two of these amino acid differences are located at/near the N-terminus, two are located within the second EF-hand domain, and the final two can be found in the third EF-hand domain (Fig. S15). Interestingly, although the sequences of the swine and human RLCv isoforms were highly similar (∼96% sequence similarity), the isoforms from both species shared a higher degree of sequence similarity with RLCv from rabbit (approximately 97% and 98% sequence similarity with swine and human RLCv, respectively) (Table S2). Nevertheless, the high degree of sequence similarity between swine and human protein isoforms in the heart highlights the usefulness of swine as a model system for modeling human cardiovascular diseases [36, 37].
In comparison, a greater number of differences were identified in the sequences of swine and human ELC isoforms. In addition to determining that the first five amino acids in the UniProtKB/Swiss-Prot database sequence for swine ELCa are not present in the protein (Fig. S12), a total of eight and eleven amino acid differences between human and swine ELCa and ELCv, respectively, were localized in this study (Figs. 4, 5; Figs. S11, S17). Both of the ELC isoforms expressed in the heart are long ELC isoforms (so-called A1-type ELCs), which differ from the short (A2-type) isoforms (expressed in fast skeletal and smooth muscles) in that they contain a unique N-terminal extension that is approximately 40-46 amino acids in length and is comprised of a cluster of Lys residues at the extreme N-terminus followed by a stretch of predominantly Pro and Ala residues (Figs. S12, S18) [3]. Notably, the preponderance of the amino acid differences between ELCa and ELCv isoforms in swine and human, as well as other species, were located within the N-terminal extensions of these isoforms (Figs. S12, S18). Given that previous studies have shown that the N-terminal extensions of A1-type ELCs interact with actin [10, 38-40], differences in the N-terminal sequences of these isoforms may be, in part, responsible for differences in their actin affinities [39].
4.2 Identification of cardiac myosin light chain isoform N-terminal modifications
Here, we demonstrate that ELCv and RLCv from human and swine are Nα-tri-methylated (Fig. 5; Figs. S13, S14, S19), which is in good agreement with the findings of previous studies in human and cow [34]. Additionally, for the first time, we show that ELCa from human and swine is also methylated at its N-terminus, whereas RLCa is Nα-acetylated (Figs. 2, 4; Figs. S5, S9). In particular, the discovery that RLCa is N-terminally acetylated is interesting as this modification appears to make RLCa distinct from myosin light chain isoforms from ventricular myocardium and fast skeletal muscle as the latter isoforms contain the consensus X-Pro-Lys motif required for methylation by the N-terminal methyltransferase [41].
The Nα-acetylation of proteins is a common phenomenon, with predictions estimating that approximately 77% of all proteins in humans are N-terminally acetylated [42]. Nevertheless, the purpose(s) of N-terminal acetylation remains poorly understood. Traditionally, Nα-acetylation has been thought to protect proteins from degradation [43, 44]; however, recent studies have suggested diverse functions for this modification, including roles in protein-protein interactions [45], intracellular targeting to specific sub-cellular compartments [46, 47], and even in protein degradation via a ubiquitin-dependent pathway [46]. Given that RLCa Nα-acetylation has not been previously reported, the functional significance of this modification is unclear.
In comparison to N-terminal acetylation, methylation of the α-amino group of proteins is comparatively quite rare [48], although this modification seems to be relatively widespread in nature [34]. Furthermore, while the function(s) of protein Nα-acetylation remains poorly understood, even less is known about the consequences of protein N-terminal methylation. In contrast to N-terminal acetylation, tri-methylation (or di-methylation in the case of Pro) of the α-amino group of the protein dramatically alters the chemistry of the N-terminus. Specifically, quaternization of the α-amino group induces a permanent positive charge at the protein N-terminus and abrogates reactivity of the α-amino nitrogen [48]. Studies have previously suggested that charged residues at the extreme N-termini of the cardiac ELCs are important for interactions with actin [49]; therefore, it is conceivable that the permanent positive charge induced at the N-terminus by methylation of the α-amino groups of ELCa and ELCv may also be involved in electrostatic interactions with negatively charged amino acids on the surface of actin.
4.3 Regulatory light chain phosphorylation
Reported values for endogenous RLC phosphorylation vary widely in the literature. While many groups have reported relatively constant values of 0.4-0.6 mol Pi/mol of RLC across species [13, 50, 51], others have reported significantly lower values [52-55], even within the same species. Here, measurement of RLC phosphorylation using conditions under which maximal inhibition of phosphatase activity towards RLC during protein extraction was achieved (Fig. S4), we measured mean values of 0.16 ± 0.03 and 0.12 ± 0.01 mol of Pi/mol of RLC for swine RLCa and RLCv, respectively (Fig. 2; Fig. S13). These values are higher than those reported previously by our group for swine (due to complete inhibition of phosphatase activity towards the RLC during protein extraction in this study). Nevertheless, these values are lower than those reported by others for swine [13, 51]. Given that a number of studies, including those from our own lab, have demonstrated that RLC phosphorylation does not vary transmurally in large animals (swine) and humans [26, 56, 57], as it does in rodents [58], this discrepancy is unlikely to be from differences in sampling location within the ventricular myocardium. One possibility for the differences between the values measured in this study and in other studies [13, 51], is that different methods were used for measuring RLC phosphorylation (top-down MS versus ProQ Diamond stain or SDS-PAGE PhosTag™ analysis). Differences in the time taken for dissection of the heart following excision (prior to flash freezing in liquid nitrogen) [59], and swine strain differences in basal RLC phosphorylation are also possible. Regardless, we are confident that we have detected all phosphorylated forms of the atrial and ventricular RLCs as even groups reporting high levels of RLC phosphorylation (0.4-0.6 mol of Pi/mol of RLC) have observed only a singly phosphorylated form of RLCv and both singly and doubly phosphorylated forms of RLCa in swine and humans [13, 35, 51].
In contrast to RLC phosphorylation, which is well documented [6, 13-15], phosphorylation of the ELC has not been widely observed, with reports in only a handful of species, including rabbits, humans, and rats [33, 56, 60]. In this study, phosphorylated forms of ELCa and ELCv were not detected in either human or swine myocardium (Figs. 4, 5). Phosphorylation of the ELC was initially identified in vivo in a proteomic analysis of rabbit ventricular myocardium, and was found to increase in response to pharmacological preconditioning with adenosine [33]. It was subsequently shown that this modification was associated with increased MMP-2-mediated degradation of the ELC [60]. This modification has also been detected in the myocardium of patients with heart failure and was shown to be inversely correlated with myocardial power production, possibly as a consequence of ELC degradation by MMP-2 [56]. Consequently, given that the available evidence indicates that ELC phosphorylation is deleterious and occurs under conditions of cardiac stress, it is perhaps not surprising that phosphorylated forms of ELCa and ELCv were not detected by top-down high-resolution MS-based profiling of healthy swine atrial and ventricular tissue. Nevertheless, we cannot rule out the possibility that a small sub-population of phosphorylated ELC may exist in the atrial or ventricular myocardium of healthy swine but was below the detection limit (less than approximately 0.1 and 0.5% of the total ELCa and ELCv populations, respectively) (Figs. 4, 5).
4.4 Localization of regulatory light chain phosphorylation sites in the swine heart
Here, we show, for the first time, that Ser22 is the sole site of basal phosphorylation in pRLCa in the swine heart (Fig. 3). In accordance with observations from previous studies in humans [35], a bis-phosphorylated form of RLCa was also observed in swine atrial myocardium (Fig. 2); however, the low abundance of this species did not permit MS/MS analysis and the localization of the second site of phosphorylation in swine ppRLCa. In addition we also definitively show that the RLCv is basally mono-phosphorylated at a single site, Ser14, in the ventricles of the swine heart (Fig. S16), which is in good agreement with the findings of our previous study [29]. Although ppRLCv has been observed in mice [61], this species was not detected in the swine heart (Fig. S13), likely as a consequence of the replacement of Ser13 (mouse) with a non-phosphorylatable Asn residue in swine RLCv (Fig. S15) [29]. It is noteworthy that an Asn residue is also present at position 13 in human RLCv (Fig. S15), which suggests that RLCv from human is also basally mono-phosphorylated and further supports the use of large animals to model human cardiovascular diseases [36, 37].
Until recently, the identity of the kinase responsible for RLC phosphorylation in the heart was unclear. While both the smooth and skeletal muscle isoforms of MLCK are expressed in the heart, previous work suggested that these isoforms do not play a significant role in RLC phosphorylation in the heart [62, 63]. In 2008, Chan et al. discovered a previously unidentified cardiac-specific isoform of MLCK, encoded by the Mylk3 gene, and expressed in both the ventricles and atria of the mouse heart [64]. Subsequent work by Ding et al. showed that phosphorylation of RLC in the heart is predominantly mediated by cardiac MLCK [65], although the potential involvement of other kinases, such as ZIPK [66], cannot be ruled out.
4.5 High-resolution top-down MS/MS for myofilament protein isoform characterization
In this study, we utilized high-resolution top-down targeted proteomics to comprehensively characterize the sequences and N-terminal modifications of cardiac ELC and RLC isoforms in the human and swine heart, as well as the phosphorylation sites in the swine proteins. In general, high-resolution MS/MS has significant advantages for protein characterization, including increased confidence in protein and PTM identification as a consequence of enhanced mass accuracy [31]. Here, top-down high-resolution MS enabled the comprehensive sequence characterization of myosin light chain isoforms from both human and swine hearts with high sequence coverage and mass accuracy. Furthermore, high mass accuracy allowed for determination of N-terminal modifications in myosin light chain isoforms in the heart, including the assignment of Nα-acetylation versus Nα-tri-methylation—two modifications differing in mass by only ∼36 mDa (Fig. 6). We show that ELCv and RLCv from human and swine are Nα-tri-methylated (Fig. 5; Figs. S13, S14, S19), which is in good agreement with previous studies [34]. Additionally, for the first time, we show that human and swine ELCa is also methylated at its N-terminus, whereas RLCa is Nα-acetylated (Figs. 2, 4; Figs. S5, S9). Although the significance of Nα-acetylation versus Nα-tri-methylation in RLC remains unclear, these results showcase the great potential of top-down high-resolution MS/MS for PTM identification.
Fig. 6.
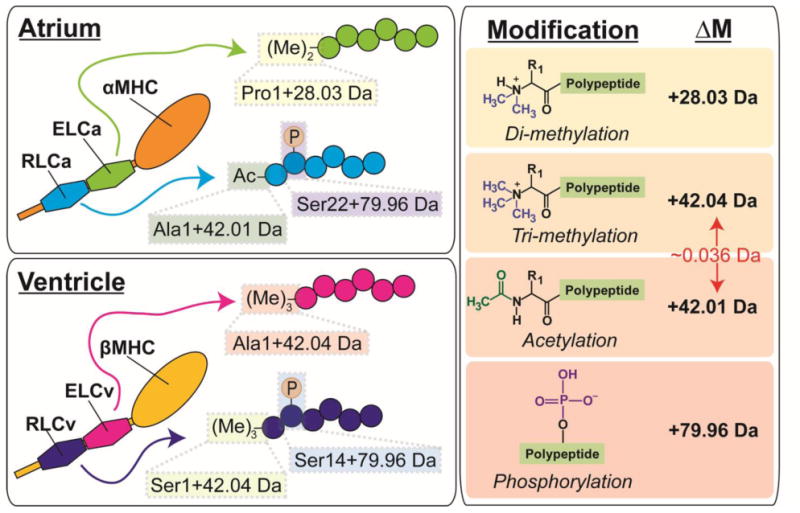
Study overview showing localized mass differences in the atrial and ventricular isoforms of the ELC and RLC (left panels) from swine, as well as the modifications they represent (right panel). Highlighted is the approximate 36 mDa (0.036 Da) mass difference between tri-methylation and acetylation, which were distinguished using high-resolution top-down MS/MS in the present study.
The use of ECD allowed unambiguous localization of the basal phosphorylation sites in swine pRLCv (Ser14) and pRLCa (Ser22) (Fig. 3; Fig. S16). It is well-known that dissociation with ECD preserves labile PTMs (e.g., phosphorylation), thereby allowing for their localization [22-24, 67]. This represents a significant advantage of electron-based dissociation methods over energetic dissociation methods, such as CID. For un-modified proteins or those with stable PTMs (e.g. acetylation), fragmentation using CID results in the cleavage of CO-NH bonds in the protein backbone, producing b and y fragment ions; however, for phosphorylated proteins, dissociation with CID leads to the preferential cleavage of the O-phosphoester bond, resulting in the neutral loss of phosphoric acid (H3PO4, 98 Da) or metaphosphoric acid (HPO3, 80 Da), and precluding localization of the phosphorylated site [25].
Also of note is the advantage conferred by top-down MS/MS utilizing a combination of ECD and CID for comprehensive protein characterization. Studies have demonstrated that peptide/protein fragmentation by ECD is more evenly distributed along the protein backbone and, thus, tends to produce higher sequence coverage than that achieved with CID alone [67, 68]. Nevertheless, one drawback of ECD is that fragmentation N-terminal to Pro does not occur due to the cyclic structure of this amino acid and the fact that only a single bond cleavage event occurs upon electron capture [67]. Therefore, CID is a good complement to ECD as cleavage N-terminal to Pro is highly favorable for CID [69]. Here, we utilized top-down MS/MS with a combination of ECD and CID to characterize the sequences of ELCv and ELCa, which both contain Pro-rich N-terminal extensions that bind to actin and are important for cardiac contractile function [7, 38]. Such Pro-rich sequence extensions would traditionally pose a challenge for sequencing using either ECD or CID alone; however, as demonstrated here, top-down MS/MS employing a combination of ECD and CID enabled sequencing of ELC isoforms from the swine heart with high sequence coverage (approximately 95% and 90% sequence coverage for ELCa and ELCv, respectively), including extensive cleavage in the Pro-rich N-termini of these proteins. These results highlight the benefit of a top-down MS/MS strategy employing a combination of ECD and CID for comprehensive protein characterization, especially for proteins with Pro-rich stretches.
Supplementary Material
Acknowledgments
The authors would like to thank Timothy A. Hacker for swine heart tissue samples and Rachel Heuer for critical reading of this manuscript. Financial support was kindly provided by NIH F31 HL128086 (to Z.G.), and NIH R01 HL109810 and R01 HL096971 (to Y.G.). YG would also like to acknowledge NIH R01 GM117058 and S10 OD018475.
Abbreviations
- MHCs
myosin heavy chains
- ELC
essential light chain
- RLC
regulatory light chain
- MLCK
myosin light chain kinase
- PTMs
post-translational modifications
- ELCv
ventricular isoform of ELC
- ELCa
atrial isoform of ELC
- RLCv
ventricular isoform of RLC
- RLCa
atrial isoform of RLC
- MS
mass spectrometry
- MS/MS
tandem MS
- ECD
electron capture dissociation
- CID
collision induced dissociation
- LC
liquid chromatography
- LTQ
linear ion trap
- FT-ICR
Fourier transform ion cyclotron resonance
- pRLCa
mono-phosphorylated RLCa
- ppRLCa
bis-phosphorylated RLCa
- pELCa
mono-phosphorylated ELCa
- pRLCv
mono-phosphorylated RLCv
- ppRLCv
bis-phosphorylated RLCv
- pELCv
mono-phosphorylated ELCv
Footnotes
Disclosures: None
References
- 1.Huxley HE. The mechanism of muscular contraction. Science. 1969;164(3886):1356–65. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- 2.Huxley AF, Simmons RM. Proposed mechanism of force generation in striated muscle. Nature. 1971;233(5321):533–8. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- 3.Weeds AG, Lowey S. Substructure of the myosin molecule. II. The light chains of myosin. J Mol Biol. 1971;61(3):701–25. doi: 10.1016/0022-2836(71)90074-x. [DOI] [PubMed] [Google Scholar]
- 4.Rayment I, Rypniewski WR, Schmidt-Bäse K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993;261(5117):50–8. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- 5.Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney HL, Bowman BF, Stull JT. Myosin light chain phosphorylation in vertebrate striated muscle: regulation and function. Am J Physiol. 1993;264(5 Pt 1):C1085–95. doi: 10.1152/ajpcell.1993.264.5.C1085. [DOI] [PubMed] [Google Scholar]
- 7.Morano I. Tuning the human heart molecular motors by myosin light chains. J Mol Med (Berl) 1999;77(7):544–55. doi: 10.1007/s001099900031. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann PA, Metzger JM, Greaser ML, Moss RL. Effects of partial extraction of light chain 2 on the Ca2+ sensitivities of isometric tension, stiffness, and velocity of shortening in skinned skeletal muscle fibers. J Gen Physiol. 1990;95(3):477–98. doi: 10.1085/jgp.95.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowey S, Trybus KM. Role of skeletal and smooth muscle myosin light chains. Biophys J. 1995;68(4 Suppl):120S–126S. discussion 126S-127S. [PMC free article] [PubMed] [Google Scholar]
- 10.Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol. 2007;292(4):H1643–54. doi: 10.1152/ajpheart.00931.2006. [DOI] [PubMed] [Google Scholar]
- 11.Szczesna D. Regulatory light chains of striated muscle myosin. Structure, function and malfunction. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3(2):187–97. doi: 10.2174/1568006033481474. [DOI] [PubMed] [Google Scholar]
- 12.Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, Rayment I, Sellers JR, Fananapazir L, Epstein ND. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet. 1996;13(1):63–9. doi: 10.1038/ng0596-63. [DOI] [PubMed] [Google Scholar]
- 13.Scruggs SB, Solaro RJ. The significance of regulatory light chain phosphorylation in cardiac physiology. Arch Biochem Biophys. 2011;510(2):129–34. doi: 10.1016/j.abb.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol. 2004;287(6):H2712–8. doi: 10.1152/ajpheart.01067.2003. [DOI] [PubMed] [Google Scholar]
- 15.Sheikh F, Lyon RC, Chen J. Functions of myosin light chain-2 (MYL2) in cardiac muscle and disease. Gene. 2015;569(1):14–20. doi: 10.1016/j.gene.2015.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheikh F, Ouyang K, Campbell SG, Lyon RC, Chuang J, Fitzsimons D, Tangney J, Hidalgo CG, Chung CS, Cheng H, Dalton ND, Gu Y, Kasahara H, Ghassemian M, Omens JH, Peterson KL, Granzier HL, Moss RL, McCulloch AD, Chen J. Mouse and computational models link Mlc2v dephosphorylation to altered myosin kinetics in early cardiac disease. J Clin Invest. 2012;122(4):1209–21. doi: 10.1172/JCI61134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren SA, Briggs LE, Zeng H, Chuang J, Chang EI, Terada R, Li M, Swanson MS, Lecker SH, Willis MS, Spinale FG, Maupin-Furlowe J, McMullen JR, Moss RL, Kasahara H. Myosin light chain phosphorylation is critical for adaptation to cardiac stress. Circulation. 2012;126(22):2575–88. doi: 10.1161/CIRCULATIONAHA.112.116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan CC, Muthu P, Kazmierczak K, Liang J, Huang W, Irving TC, Kanashiro-Takeuchi RM, Hare JM, Szczesna-Cordary D. Constitutive phosphorylation of cardiac myosin regulatory light chain prevents development of hypertrophic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2015;112(30):E4138–46. doi: 10.1073/pnas.1505819112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczesna D, Ghosh D, Li Q, Gomes AV, Guzman G, Arana C, Zhi G, Stull JT, Potter JD. Familial hypertrophic cardiomyopathy mutations in the regulatory light chains of myosin affect their structure. Ca2+ binding, and phosphorylation, J Biol Chem. 2001;276(10):7086–92. doi: 10.1074/jbc.M009823200. [DOI] [PubMed] [Google Scholar]
- 20.Grabarek Z. Structural basis for diversity of the EF-hand calcium-binding proteins. J Mol Biol. 2006;359(3):509–25. doi: 10.1016/j.jmb.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 21.Morano I, Hädicke K, Haase H, Böhm M, Erdmann E, Schaub MC. Changes in essential myosin light chain isoform expression provide a molecular basis for isometric force regulation in the failing human heart. J Mol Cell Cardiol. 1997;29(4):1177–87. doi: 10.1006/jmcc.1996.0353. [DOI] [PubMed] [Google Scholar]
- 22.Gregorich ZR, Chang YH, Ge Y. Proteomics in heart failure: top-down or bottom-up? Pflugers Arch. 2014;466(6):1199–209. doi: 10.1007/s00424-014-1471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregorich ZR, Ge Y. Top-down proteomics in health and disease: challenges and opportunities. Proteomics. 2014;14(10):1195–210. doi: 10.1002/pmic.201300432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siuti N, Kelleher NL. Decoding protein modifications using top-down mass spectrometry. Nat Methods. 2007;4(10):817–21. doi: 10.1038/nmeth1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Zhang H, Ayaz-Guner S, Chen YC, Dong X, Xu Q, Ge Y. Phosphorylation, but not alternative splicing or proteolytic degradation, is conserved in human and mouse cardiac troponin T. Biochemistry. 2011;50(27):6081–92. doi: 10.1021/bi2006256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregorich ZR, Peng Y, Lane NM, Wolff JJ, Wang S, Guo W, Guner H, Doop J, Hacker TA, Ge Y. Comprehensive assessment of chamber-specific and transmural heterogeneity in myofilament protein phosphorylation by top-down mass spectrometry. J Mol Cell Cardiol. 2015;87:102–12. doi: 10.1016/j.yjmcc.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng Y, Yu D, Gregorich Z, Chen X, Beyer AM, Gutterman DD, Ge Y. In-depth proteomic analysis of human tropomyosin by top-down mass spectrometry. J Muscle Res Cell Motil. 2013;34(3-4):199–210. doi: 10.1007/s10974-013-9352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng Y, Chen X, Zhang H, Xu Q, Hacker TA, Ge Y. Top-down targeted proteomics for deep sequencing of tropomyosin isoforms. J Proteome Res. 2013;12(1):187–98. doi: 10.1021/pr301054n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Y, Gregorich ZR, Valeja SG, Zhang H, Cai W, Chen YC, Guner H, Chen AJ, Schwahn DJ, Hacker TA, Liu X, Ge Y. Top-down proteomics reveals concerted reductions in myofilament and Z-disc protein phosphorylation after acute myocardial infarction. Mol Cell Proteomics. 2014;13(10):2752–64. doi: 10.1074/mcp.M114.040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ge Y, Rybakova IN, Xu Q, Moss RL. Top-down high-resolution mass spectrometry of cardiac myosin binding protein C revealed that truncation alters protein phosphorylation state. Proc Natl Acad Sci U S A. 2009;106(31):12658–63. doi: 10.1073/pnas.0813369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mann M, Kelleher NL. Precision proteomics: the case for high resolution and high mass accuracy. Proc Natl Acad Sci U S A. 2008;105(47):18132–8. doi: 10.1073/pnas.0800788105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane LA, Neverova I, Van Eyk JE. Subfractionation of heart tissue: the “in sequence” myofilament protein extraction of myocardial tissue. Methods Mol Biol. 2007;357:87–90. doi: 10.1385/1-59745-214-9:87. [DOI] [PubMed] [Google Scholar]
- 33.Arrell DK, Neverova I, Fraser H, Marbán E, Van Eyk JE. Proteomic analysis of pharmacologically preconditioned cardiomyocytes reveals novel phosphorylation of myosin light chain 1. Circ Res. 2001;89(6):480–7. doi: 10.1161/hh1801.097240. [DOI] [PubMed] [Google Scholar]
- 34.Henry GD, Trayer IP, Brewer S, Levine BA. The widespread distribution of alpha-N-trimethylalanine as the N-terminal amino acid of light chains from vertebrate striated muscle myosins. Eur J Biochem. 1985;148(1):75–82. doi: 10.1111/j.1432-1033.1985.tb08809.x. [DOI] [PubMed] [Google Scholar]
- 35.Morano I, Wankerl M, Böhm M, Erdmann E, Rüegg JC. Myosin P-light chain isoenzymes in the human heart: evidence for diphosphorylation of the atrial P-LC form. Basic Res Cardiol. 1989;84(3):298–305. doi: 10.1007/BF01907977. [DOI] [PubMed] [Google Scholar]
- 36.Kooij V, Venkatraman V, Tra J, Kirk JA, Rowell J, Blice-Baum A, Cammarato A, Van Eyk JE. Sizing up models of heart failure: Proteomics from flies to humans. Proteomics Clin Appl. 2014;8(9-10):653–64. doi: 10.1002/prca.201300123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2(3):262–71. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morano I, Ritter O, Bonz A, Timek T, Vahl CF, Michel G. Myosin light chain-actin interaction regulates cardiac contractility. Circ Res. 1995;76(5):720–5. doi: 10.1161/01.res.76.5.720. [DOI] [PubMed] [Google Scholar]
- 39.Morano I, Haase H. Different actin affinities of human cardiac essential myosin light chain isoforms. FEBS Lett. 1997;408(1):71–4. doi: 10.1016/s0014-5793(97)00390-6. [DOI] [PubMed] [Google Scholar]
- 40.Muthu P, Wang L, Yuan CC, Kazmierczak K, Huang W, Hernandez OM, Kawai M, Irving TC, Szczesna-Cordary D. Structural and functional aspects of the myosin essential light chain in cardiac muscle contraction. FASEB J. 2011;25(12):4394–405. doi: 10.1096/fj.11-191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb KJ, Lipson RS, Al-Hadid Q, Whitelegge JP, Clarke SG. Identification of protein N-terminal methyltransferases in yeast and humans. Biochemistry. 2010;49(25):5225–35. doi: 10.1021/bi100428x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K. Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc Natl Acad Sci U S A. 2009;106(20):8157–62. doi: 10.1073/pnas.0901931106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persson B, Flinta C, von Heijne G, Jörnvall H. Structures of N-terminally acetylated proteins. Eur J Biochem. 1985;152(3):523–7. doi: 10.1111/j.1432-1033.1985.tb09227.x. [DOI] [PubMed] [Google Scholar]
- 44.Hershko A, Heller H, Eytan E, Kaklij G, Rose IA. Role of the alpha-amino group of protein in ubiquitin-mediated protein breakdown. Proc Natl Acad Sci U S A. 1984;81(22):7021–5. doi: 10.1073/pnas.81.22.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coulton AT, East DA, Galinska-Rakoczy A, Lehman W, Mulvihill DP. The recruitment of acetylated and unacetylated tropomyosin to distinct actin polymers permits the discrete regulation of specific myosins in fission yeast. J Cell Sci. 2010;123(Pt 19):3235–43. doi: 10.1242/jcs.069971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang CS, Shemorry A, Varshavsky A. N-terminal acetylation of cellular proteins creates specific degradation signals. Science. 2010;327(5968):973–7. doi: 10.1126/science.1183147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Behnia R, Panic B, Whyte JR, Munro S. Targeting of the Arf-like GTPase Arl3p to the Golgi requires N-terminal acetylation and the membrane protein Sys1p. Nat Cell Biol. 2004;6(5):405–13. doi: 10.1038/ncb1120. [DOI] [PubMed] [Google Scholar]
- 48.Stock A, Clarke S, Clarke C, Stock J. N-terminal methylation of proteins: structure, function and specificity. FEBS Lett. 1987;220(1):8–14. doi: 10.1016/0014-5793(87)80866-9. [DOI] [PubMed] [Google Scholar]
- 49.Sweeney HL. Function of the N terminus of the myosin essential light chain of vertebrate striated muscle. Biophys J. 1995;68(4 Suppl):112S–118S. discussion 118S-119S. [PMC free article] [PubMed] [Google Scholar]
- 50.Jeacocke SA, England PJ. Phosphorylation of myosin light chains in perfused rat heart. Effect of adrenaline and increased cytoplasmic calcium ions. Biochem J. 1980;188(3):763–8. doi: 10.1042/bj1880763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toepfer C, Caorsi V, Kampourakis T, Sikkel MB, West TG, Leung MC, Al-Saud SA, MacLeod KT, Lyon AR, Marston SB, Sellers JR, Ferenczi MA. Myosin regulatory light chain (RLC) phosphorylation change as a modulator of cardiac muscle contraction in disease. J Biol Chem. 2013;288(19):13446–54. doi: 10.1074/jbc.M113.455444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silver PJ, Buja LM, Stull JT. Frequency-dependent myosin light chain phosphorylation in isolated myocardium. J Mol Cell Cardiol. 1986;18(1):31–7. doi: 10.1016/s0022-2828(86)80980-4. [DOI] [PubMed] [Google Scholar]
- 53.Sanbe A, Fewell JG, Gulick J, Osinska H, Lorenz J, Hall DG, Murray LA, Kimball TR, Witt SA, Robbins J. Abnormal cardiac structure and function in mice expressing nonphosphorylatable cardiac regulatory myosin light chain 2. J Biol Chem. 1999;274(30):21085–94. doi: 10.1074/jbc.274.30.21085. [DOI] [PubMed] [Google Scholar]
- 54.Hidalgo C, Wu Y, Peng J, Siems WF, Campbell KB, Granzier H. Effect of diastolic pressure on MLC2v phosphorylation in the rat left ventricle. Arch Biochem Biophys. 2006;456(2):216–23. doi: 10.1016/j.abb.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 55.Kampourakis T, Irving M. Phosphorylation of myosin regulatory light chain controls myosin head conformation in cardiac muscle. J Mol Cell Cardiol. 2015;85:199–206. doi: 10.1016/j.yjmcc.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haynes P, Nava KE, Lawson BA, Chung CS, Mitov MI, Campbell SG, Stromberg AJ, Sadayappan S, Bonnell MR, Hoopes CW, Campbell KS. Transmural heterogeneity of cellular level power output is reduced in human heart failure. J Mol Cell Cardiol. 2014;72:1–8. doi: 10.1016/j.yjmcc.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Velden J, Merkus D, de Beer V, Hamdani N, Linke WA, Boontje NM, Stienen GJ, Duncker DJ. Transmural heterogeneity of myofilament function and sarcomeric protein phosphorylation in remodeled myocardium of pigs with a recent myocardial infarction. Front Physiol. 2011;2:83. doi: 10.3389/fphys.2011.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, Aletras AH, Wen H, Epstein ND. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107(5):631–41. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 59.Walker LA, Medway AM, Walker JS, Cleveland JC, Buttrick PM. Tissue procurement strategies affect the protein biochemistry of human heart samples. J Muscle Res Cell Motil. 2011;31(5-6):309–14. doi: 10.1007/s10974-010-9233-6. [DOI] [PubMed] [Google Scholar]
- 60.Cadete VJ, Sawicka J, Jaswal JS, Lopaschuk GD, Schulz R, Szczesna-Cordary D, Sawicki G. Ischemia/reperfusion-induced myosin light chain 1 phosphorylation increases its degradation by matrix metalloproteinase 2. FEBS J. 2012;279(13):2444–54. doi: 10.1111/j.1742-4658.2012.08622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scruggs SB, Reisdorph R, Armstrong ML, Warren CM, Reisdorph N, Solaro RJ, Buttrick PM. A novel, in-solution separation of endogenous cardiac sarcomeric proteins and identification of distinct charged variants of regulatory light chain. Mol Cell Proteomics. 2010;9(9):1804–18. doi: 10.1074/mcp.M110.000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ohlmann P, Tesse A, Loichot C, Ralay Ranaivo H, Roul G, Philippe C, Watterson DM, Haiech J, Andriantsitohaina R. Deletion of MLCK210 induces subtle changes in vascular reactivity but does not affect cardiac function. Am J Physiol Heart Circ Physiol. 2005;289(6):H2342–9. doi: 10.1152/ajpheart.00511.2004. [DOI] [PubMed] [Google Scholar]
- 63.Zhi G, Ryder JW, Huang J, Ding P, Chen Y, Zhao Y, Kamm KE, Stull JT. Myosin light chain kinase and myosin phosphorylation effect frequency-dependent potentiation of skeletal muscle contraction. Proc Natl Acad Sci U S A. 2005;102(48):17519–24. doi: 10.1073/pnas.0506846102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan JY, Takeda M, Briggs LE, Graham ML, Lu JT, Horikoshi N, Weinberg EO, Aoki H, Sato N, Chien KR, Kasahara H. Identification of cardiac-specific myosin light chain kinase. Circ Res. 2008;102(5):571–80. doi: 10.1161/CIRCRESAHA.107.161687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding P, Huang J, Battiprolu PK, Hill JA, Kamm KE, Stull JT. Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo. J Biol Chem. 2010;285(52):40819–29. doi: 10.1074/jbc.M110.160499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang AN, Chen G, Gerard RD, Kamm KE, Stull JT. Cardiac myosin is a substrate for zipper-interacting protein kinase (ZIPK) J Biol Chem. 2010;285(8):5122–6. doi: 10.1074/jbc.C109.076489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Electron capture dissociation for structural characterization of multiply charged protein cations. Anal Chem. 2000;72(3):563–73. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 68.Axelsson J, Palmblad M, Håkansson K, Håkansson P. Electron capture dissociation of substance P using a commercially available Fourier transform ion cyclotron resonance mass spectrometer. Rapid Commun Mass Spectrom. 1999;13(6):474–7. doi: 10.1002/(SICI)1097-0231(19990330)13:6<474::AID-RCM505>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 69.Schaaff TG, Cargile BJ, Stephenson JL, McLuckey SA. Ion trap collisional activation of the (M + 2H)2+ - (M + 17H)17+ ions of human hemoglobin beta-chain. Anal Chem. 2000;72(5):899–907. doi: 10.1021/ac991344e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


