Abstract
Taxanes (docetaxel and paclitaxel) are among the most commonly prescribed anticancer drugs approved for the treatment of metastatic or locally advanced breast, non-small cell lung, prostate, gastric, head and neck, and ovarian cancers, as well as in the adjuvant setting for operable node-positive breast cancers. Although the true incidence of dermatological adverse events (AEs) in patients receiving taxanes is not known, and has never been prospectively analysed, they clearly represent one of the major AEs associated with these agents. With an increase in the occurrence of cutaneous AEs during treatment with novel targeted and immunological therapies when used in combination with taxanes, a thorough understanding of reactions attributable to this class is imperative. Moreover, identification and management of dermatological AEs is critical for maintaining the quality of life in cancer patients and for minimizing dose modifications of their antineoplastic regimen. This analysis represents a systematic review of the dermatological conditions reported with the use of these drugs, complemented by experience at comprehensive cancer centres. The conditions reported herein include skin, hair, and nail toxicities. Lastly, we describe the dermatological data available for the new, recently FDA-and EMA- approved, solvent-free nab-paclitaxel.
Keywords: hair, nab-paclitaxel, nail, skin, taxanes, toxicity, docetaxel, paclitaxel
Paclitaxel and docetaxel belong to the taxane class of cytotoxic agents, and are among the most commonly prescribed chemotherapeutic agents in oncology, especially in gynaecological cancers. Paclitaxel is a natural extract derived from the bark of the pacific yew tree (Taxus brevifolia) that became commercially available in 1992. On the other hand, docetaxel is a semisynthetic analogue of paclitaxel synthesised from the needles of the European yewtree (Taxus baccata). Both drugs act as antimicrotubule agents by promoting polymerisation of tubulin into highly stable intracellular microtubules, thus disrupting mitosis and normal cell division, and eventually leading to cell death [1–4]. Because of their highly hydrophobic properties, they require the use of solvents (non-ionic polyoxyethylated castor oil -Cremophor EL®- for paclitaxel; non-ionic surfactant polysorbate 80 for docetaxel) to facilitate parenteral administration. The drugs are approved for a number of indications in the US and Europe. Paclitaxel is generally administered by weekly infusion (80 mg/m2), while docetaxel is infused (100 mg/m2) every three weeks.
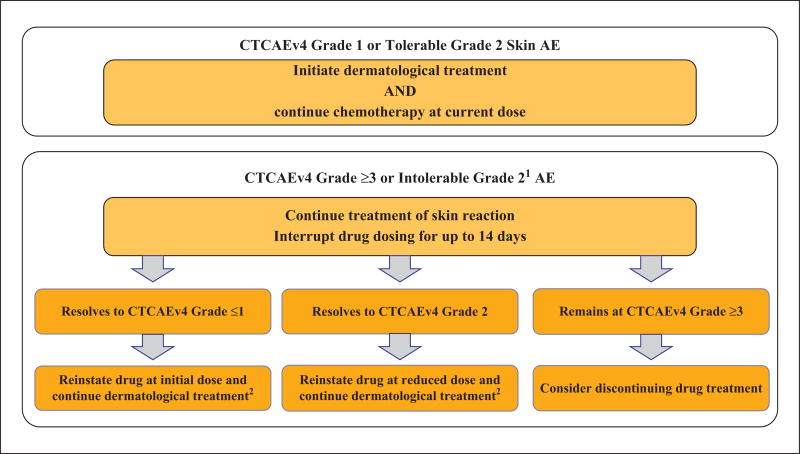
The main adverse events (AEs) with the use of taxanes include fatigue (27 to 74%), myalgia (23 to 33%), arthralgia (20 to 30%), nausea (20 to 45%) and symmetrical peripheral sensory neuropathy (20 to 64%) [1–3; 5–10], with neutropenia (45 to 95%) being the major dose-limiting AE [1, 3, 5, 9]. Dermatological AEs (dAEs) are frequent and most treated patients are affected [2, 11], but the true incidence has not been estimated and tends to vary significantly in the literature (6 to 81%) [2, 7, 12–14]. Most data are derived either from studies on oncology, or from case reports and/or very short case series [11, 13]; few studies have prospectively evaluated these toxicities from a dermatological standpoint. Indeed, dAEs are commonly under-reported and not systematically ascertained. They may affect the skin (and its appendages) or mucosae and are often characteristic, but generally mild to moderate (CTCAE Grade 1/2) in severity and self-limiting [2, 4, 9, 13, 14]. The more severe dAEs (Grade 3 and higher) are mainly induced by a toxic and non-immunoallergic mechanism [13]. Consequently, they are usually dose-dependent, and therefore sometimes necessitate transient dose interruptions, reductions, or even termination of the taxane chemotherapy (figure 1). While the dAEs appear to be a “class effect”, head-to-head comparisons with individual drugs are scarce [6, 7], but paclitaxel perhaps portrays a better dAE profile than docetaxel [13]. The incidence and clinical presentation, however, may vary by the dose frequency, dose intensity, cumulative dose, premedication regimen, chemotherapeutic regimen (e.g. combinations), and sometimes the underlying cancer [13, 15].
Figure 1.
Recommended dose modifications for dermatological adverse events.
1intolerable Grade 2 is defined as a Grade 2 adverse event (AE) that does not decrease to Grade ≤1 after two weeks of therapy R that is considered intolerable as per patient.
2If a patient was previously started on oral corticosteroids, they should be continued for at least one week after resumption of chemotherapy.
Herein, we systematically review the dAEs encountered with the use of docetaxel and paclitaxel (table 1), and supplement the available literature after narrative review, with our own multicentre, specialist, clinical experience acquired at comprehensive cancer centres in the US and Europe. In the last section, we also describe the dermatological data available for the new, recently FDA-and EMA-approved, solvent-free nab-paclitaxel.
Table 1.
Global incidence of dermatological toxicities induced by paclitaxel and docetaxel.
| “Class-effect” dermatological manifestations | Global incidence |
|---|---|
| Skin changes | |
| dorsal hand foot syndrome and PATEO syndrome1 | ++ |
| palmoplantar hand foot syndrome | + |
| Flexural and intertriginous rashes1 | ++ |
| Maculopapular rashes1 | + |
| Pustular eruptions | +/− |
| Pigmentary changes | + |
| Drug-induced lupus erythematosus | +/− |
| Scleroderma-like changes | +/− |
| Radiation and UV recall | +/− |
| Inflammation of actinic keratoses | +/− |
| Hot flashes | + |
| Oedema | ++ |
|
| |
| Appendage changes | |
| Acute reversible alopecia | +++ |
| Persistent alopecia | +/− |
| Onycholysis1 | ++ |
| Other nail changes (onychomadesis, Beau lines, melanonychia/leukonychia, paronychia, onychorrhexis) | + |
|
| |
| Mucosal changes | |
| Mucositis | ++ |
| Dysgueusia | ++ |
higher incidence with docetaxel
Acute reactions during parenteral infusion
Immediate hypersensitivity reactions
Besides the platinum-based antineoplastic drugs, the taxane chemotherapeutic agents are most often associated with hypersensitivity reactions, encountered either during or shortly after infusion [16]. With both paclitaxel and docetaxel, the onset is usually very rapid, and seen within a few minutes of starting the infusion [16–18]. The incidence is around 30% without premedication for both agents [1, 17–19], and seems to be on the rise based on recent years [18]. The reaction is generally observed during the first or second cycle of treatment [1, 16–18, 20, 21], and is most often moderate in severity, but severe anaphylactoid manifestations are possible [18, 19, 22]. Severe reactions leading to death are more common with docetaxel [22].
The underlying mechanism is poorly understood and does not appear to be IgE-mediated, however, the symptomatology is similar to those of a type I hypersensitivity reaction [17–19, 23]. While the solvent for paclitaxel (Cremophor EL®, castor oil vehicle) may play a crucial role in these hypersensitivity reactions [1, 3, 5], the solvent for docetaxel (Tween 80, polyoxyethylene-20-sorbitan monooleate) is less frequently incriminated [3]. However, it has been recently suggested that the relative risk of acute infusion reaction significantly increases with the use of some generic docetaxel formulations (containing variable amounts of tween 80 and ethanol).
The clinical manifestations include varying degrees of urticaria, morbilliform rash, flushing, angio-oedema, and pruritus, presenting in conjunction with systemic signs (e.g. hypotension, bronchospasm, dyspnoea, chills, and back pain) [17, 18].
The incidence of the immediate hypersensitivity reaction can be reduced to less than 10% with appropriate premedication [5, 16–18, 23–25]. This involves a systematic, protocol-based administration of corticosteroids (oral/intravenous), with or without H1/H2 blockers [5, 16, 17, 24]. Sometimes, it may be necessary to reduce the infusion rate [23], but generally, taxane treatment can be resumed the same day despite an initial mild-to-moderate immediate reaction [1, 5, 17]. In the event of severe and/or recurrent reactions despite these measures, the pros and cons of continuing treatment should be discussed jointly with the oncology team. In clinical practice, a couple of approaches can be adopted.
Change of treatment, if an alternative is available
While some studies show cross-reactivity between taxanes in 41–90% of the cases [16, 17, 20, 26], switching from paclitaxel to docetaxel and vice versa, may be viable. Alternatively, the nanoparticle albumin-bound (nab) paclitaxel (nab-paclitaxel) may be considered, in particular, because it does not contain solvents [21, 26]. While reactions may also occur with this agent, they tend to be less frequent [16, 18, 22] (see below).
Desensitization
Several rapid drug desensitization protocols have recently been proposed and used successfully [17, 18, 20, 27]. This involves a 12-step protocol with gradual increments of doses over several hours. Unlike platinum-based drugs, skin testing (skin prick test/ intradermal testing) prior to re-introduction is not recommended with taxanes [27], but could be a promising diagnostic, as well as a potential risk stratification tool. However, this needs to be better defined prior to wide-spread implementation [16].
Extravasation reactions
The extravasation of chemotherapeutic drugs into soft tissues in the vicinity of an infusion site can occur in 0.1%–6% of patients. Incidence of all injection-site reactions including extravasation is 1.6% with paclitaxel and <1% with docetaxel [28]. These reactions are usually mild but severe extravasations may occur with either paclitaxel [28–30], docetaxel [31–33], or nab-paclitaxel [28]. Taxanes are considered “irritants” [34], but they may produce a “vesicant” reaction [29, 35–37] when a higher volume or concentration of drug leaks into soft tissues [28]. Severe complications, including necrosis or chronic ulceration, may occur [29, 32]. Local dilution with perilesional subcutaneous injections of hyaluronidase (250 units diluted in 6 ml saline) has been reported to improve resolution in some cases [36]; no specific antidote, however, is universally accepted. Furthermore, the beneficial effects of both warm compresses (which encourage dispersion and dilution of the product) [28, 32, 36] and cold compresses (which limit the diffusion of the product to the affected area) [29, 37, 38] have been reported. However, reports of worsening at extravasation sites with warm compresses suggest that application of ice packs may be more prudent [29]. Additional management includes limb elevation, aspiration of the drug to prevent further extravasation of any drug remaining in the needle/catheter, close clinical monitoring, wound care, strict surveillance, and a prompt surgical opinion where necessary. Central infusion via a central venous access device, which reduces the risk of peripheral extravasation, should be recommended [29].
Cutaneous toxicities
Macular and papular eruptions
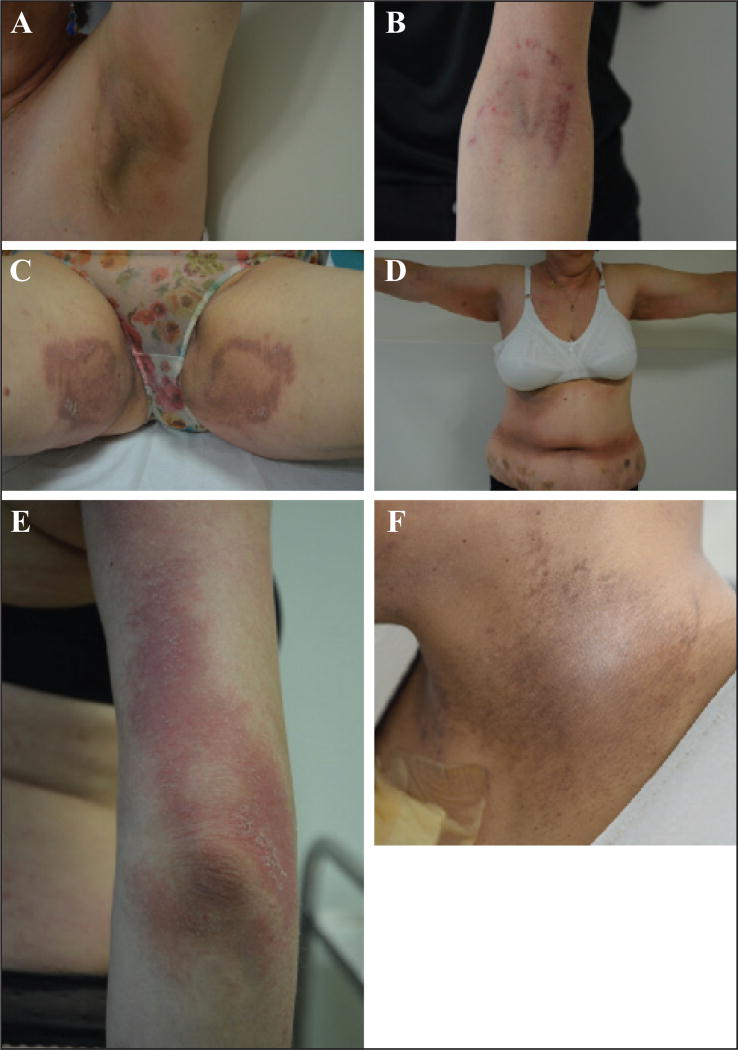
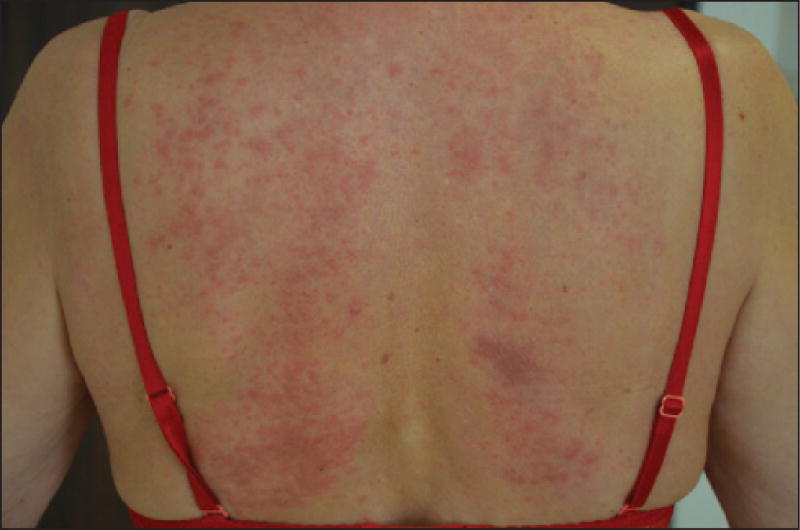
Although “rashes” are relatively common, especially with docetaxel [1, 2, 9, 14, 25, 39], the clinical characteristics have received little attention in the literature. Diffuse macules and papules may manifest as a mild morbilliform eruption [2, 9, 14], and may be associated with moderate-to-severe pruritus and burning. Taxane-induced rash is predominantly found on warm sites prone to trauma, such as the folds, contact areas, or under dressings and pads (figures 2 A–C) [2, 11, 40]. The most characteristic presentation is the development of painful, bilateral, inflammatory intertriginous patches in the axillary and inguinal regions or neck folds, especially with docetaxel as adjuvant therapy for breast cancer (figure 2 D). A concomitant PATEO (periarticular thenar erythema and onycholysis) syndrome is frequently noted [41] (see below); the face can be also involved. It may develop several days after the first treatment cycle and it is felt to be a dose-dependent toxic effect. Involvement of the extensor surfaces of arms and lateral aspects of the thighs and knees, in an “inverse” fashion, has also been described with paclitaxel (figure 2 E) [42].
Figure 2.
A–C) Characteristic presentation of toxic erythema, predominantly located to contact areas (flexural areas [A, C] or under dressing [B]). D) Development of painful, bilateral, inflammatory patches on contact areas (axillary, inguinal, and belt regions). E) Severe lesions involving the extensor surface of the elbow (paclitaxel). F) Post-inflammatory hyperpigmentation of the neck fold.
In general, lesions can be easily managed with topical steroids. However, dose reduction or temporary interruption of taxanes is sometimes required (table 2 A). Post-inflammatory hyperpigmentation following these eruptions is commonplace (figure 2 F).
Table 2.
| A. Maculopapular and intertriginous rashes. | |
|---|---|
|
| |
| Severity (CTCAE v.4) |
Intervention |
| Grade 0 | Gentle skin care instructions given |
|
| |
| Grade 1 | Continue drug at current dose and monitor for change in severity |
|
| |
| Topical low/moderate-strength steroid to affected areas bid1 AND | |
| If infection is suspected, apply topical antibiotic or anti-fungal agent | |
|
| |
| Reassess after 2 weeks (either by healthcare professional or patient self-report); if reactions worsen proceed to next step | |
|
| |
| Grade 2 | Continue drug at current dose and monitor for change in severity; obtain bacterial/viral/fungal cultures if infection is suspected; continue treatment of skin reaction with the following: |
|
| |
| Topical moderate-strength steroid to affected areas bid1 AND | |
| If infection is suspected, apply topical antibiotic or anti-fungal agent | |
|
| |
| Reassess after 2 weeks (either by healthcare professional or patient self-report); if reactions worsen or do not improve proceed to next step | |
|
| |
| Grade 3 | Interrupt treatment until severity decreases to Grade 0–1; obtain bacterial/viral/fungal cultures if infection is suspected; and continue treatment of skin reaction with the following: |
|
| |
| Topical moderate-strength steroid to affected areas bid1 AND | |
| If infection is suspected, apply topical antibiotic or anti-fungal agent | |
|
| |
| Reassess after 2 weeks; if reactions worsen or do not improve, consider dose interruption or discontinuation per protocol and switch to another antineoplastic agent2 | |
| B. Palmar plantar or dorsal Hand Foot Syndrome/ PATEO syndrome. | |
|---|---|
|
| |
| Severity (CTCAE v.4) |
Intervention |
| Grade 0 | Gentle skin care instructions given; avoid irritation to the hands and feet |
|
| |
| Grade 1 | Continue drug at current dose and monitor for change in severity |
|
| |
| Topical high-potency steroid bid | |
|
| |
| Reassess after 2 weeks (either by healthcare professional or patient self-report); if reactions worsen proceed to next step | |
|
| |
| Grade 2 | Continue drug at current dose and monitor for change in severity |
|
| |
| Topical high-potency steroid bid AND | |
| Pain control with NSAIDs/GABA agonists/narcotics AND | |
| +/− oral steroids (dexamethasone/prednisone) | |
|
| |
| Grade ≥3 or intolerable Grade 2 | Reassess after 2 weeks (either by healthcare professional or patient self-report); if reactions worsen or do not improve proceed to next step |
|
| |
| Interrupt treatment until severity decreases to Grade 0–1, and continue treatment of skin reaction with the following: | |
|
| |
| Topical high-potency steroid bid AND | |
| pain control with NSAIDs/GABA agonists/narcotics | |
| +/− oral steroids (dexamethasone/prednisone) | |
|
| |
| Reassess after 2 weeks; if reactions worsen or do not improve, consider dose interruption or discontinuation per protocol and switch to another antineoplastic agent1 | |
Oral antihistamines can be also prescribed
If applicable, switch docetaxel to paclitaxel (or vice versa)
If applicable, switch docetaxel to paclitaxel (or vice versa)
Life-threatening skin reactions
There have been only few inconsistent reports of severe life-threatening dermatological AEs with docetaxel, and even fewer with paclitaxel [43]. These include rare instances of erythema multiforme (EM) [44], toxic epidermal necrolysis (TEN) [45], and Stevens-Johnson syndrome (SJS) [46, 47].
Drug-induced lupus erythematosus (DILE)
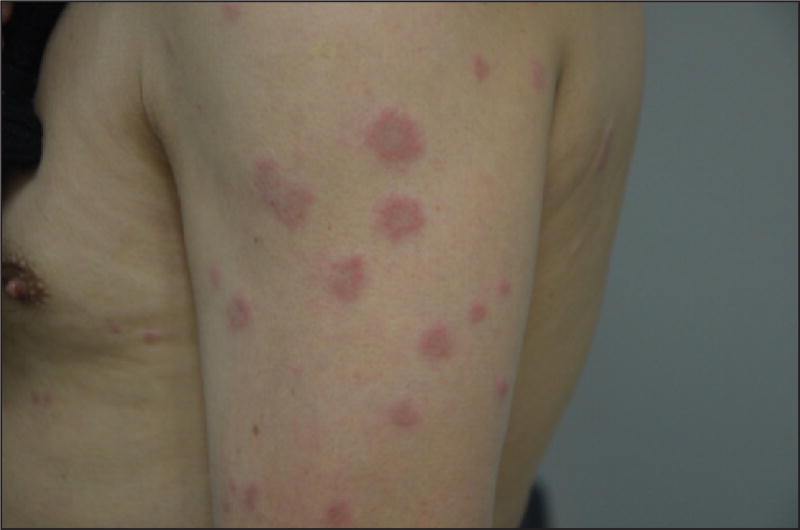
Both paclitaxel [48, 49] and docetaxel [50–52] can predispose to and/or cause DILE, which most commonly manifests as subacute cutaneous lupus erythematosus (SCLE). The latter is clinically indistinguishable from idiopathic SCLE [49, 51], and is marked by erythematous, papulosquamous and/or more characteristically, annular-appearing lesions in photo-exposed areas (figure 3) [48, 50–53]. Although patients may report pruritus [48], there are no associated systemic symptoms. This reaction may occur de novo, after a few weeks/months of treatment, or develop in the presence of a known autoimmune predisposing condition (such as Sjögren’s syndrome or pre-existing systemic lupus erythematosus [48, 51]), where it could be extensive and severe [52]. HLA typing has not been specifically characterised in this setting but, as with the idiopathic forms, patients who develop drug-induced SCLE are generally HLA DR2- or DR3-positive [49].
Figure 3.
Annular-appearing lesions related to subacute cutaneous lupus erythematosus.
The laboratory findings are remarkable for anti-SSA/Ro antibodies, which are almost always positive. The SSB or antinuclear antibodies can also be positive, [48, 50–53], but the anti-DNA antibodies are negative. On histological examination, an interface dermatitis with basal cell vacuolisation, epidermal atrophy, and some keratinocyte necrosis is apparent, often with a superficial perivascular lymphocytic infiltrate and mucin deposition [48, 50]. Direct immunofluorescent stainings generally do not reveal a lupus band, but instead show granular IgG, IgM, and/or C3 deposits in the intraepidermal keratinocytes [48, 50]. Taxanes are hypothesized to lead the appearance of serum anti-SSA antibodies [51, 52]. In addition, the apoptotic phenomena in keratinocytes, initiated by taxanes, may promote the release of nucleosomes, leading to a secondary local autoimmune reaction [50]. Furthermore, taxanes may also allow the exposure of Ro52 (also known as TRIM21, an antigen recognised by the anti-SSA antibody) to the immune system, through microtubule-stabilizing action at the time of mitotic inhibition [51].
The lesions of SCLE regress upon discontinuation of chemotherapy [50, 51, 53], without scarring [48], but anti-SSA antibodies tend to remain positive for a prolonged period of time [48, 49]. Synthetic antimalarials, photo-protection, and topical steroids can be instituted [49], particularly if treatment needs to be continued in the absence of a therapeutic alternative.
The possibility of developing lupus as a result of other concomitant chemotherapeutic drugs, including capecitabine, doxorubicin, and fluorouracil, also needs to be borne in mind [49]. SCLE has also been reported with nab-paclitaxel, portraying the same clinical, histological or laboratory abnormalities [54].
Photosensitivity
In addition to specific photo-induced dermatoses, such as SCLE or UV recall reaction (see below), both paclitaxel and docetaxel can lead to the development of an inflammatory rash predominantly on photo-exposed areas (phototoxicity) [11, 55]. The photosensitivity is thought to be related to induced aberrations in porphyrin biosynthesis and triggered by UVB [56]. The incidence, however, appears to be inferior to 1% in breast cancer patients treated with paclitaxel or docetaxel [6]. Photo-distributed erythema multiforme has also been reported [56, 57].
Pustular eruptions
Acute generalized exanthematous pustulosis (AGEP)
The development of typical AGEP, which is characterized by an acute cutaneous eruption with subcorneal non-follicular pustules on an oedematous erythema and accompanied by fever, has been reported with docetaxel [58]. An entirely similar form of generalized pustulosis has also been reported with paclitaxel [59]. On the other hand, a localized variant of AGEP confined strictly to the face, the acute localized exanthematous pustulosis (ALEP), is a rare event [60].
Folliculitis
An inflammatory folliculitis may be observed with taxanes [61], but it is usually non-dose-limiting. In our experience, it occurs predominantly on the upper part of the body, particularly the scalp and shoulders, and microbial cultures are usually negative (figure 4).
Figure 4.
Sterile scalp folliculitis induced by paclitaxel.
Recall phenomena
Radiation recall dermatitis and radiation enhancement
This well-recognised phenomenon refers to the development of an acute inflammatory reaction, strictly localized to an area of skin that was previously irradiated. The initial radiation treatment may have occurred several years prior to the chemotherapy. The recall reaction may be noted during chemotherapy (first/subsequent cycles), immediately after, or in the succeeding days [62]. The clinical manifestations may include erythema, oedema, induration, desquamation, and sometimes bullous detachment or necrosis of skin, typically confined to a previously irradiated area [37, 63]. These are of variable intensity and may be accompanied by pruritus, burning or pain, and sometimes constitutional symptoms.
Various chemotherapeutic agents, including docetaxel [64–66] and paclitaxel [62, 67], can precipitate the reaction, which is rare and affects less than 2% of the previously irradiated patients treated with docetaxel [66]. Involvement of the mucosae and organs prone to the risk of secondary failure has not been reported with taxanes, however, it can occur with other chemotherapies, especially gemcitabine [62].
The pathophysiological mechanisms remain obscure, however, some of the proposed theories include a cytotoxic chemotherapy-induced activation of memory-type inflammatory cells (that survived radiotherapy) [65, 68], an idiosyncratic hypersensitivity reaction akin to fixed drug eruption (FDE), and a deficiency in the individual’s nutritional status leading to an impairment in endogenous scavenging activity [62].
In general, the reaction does not mandate discontinuation of chemotherapy [37, 62, 66], and with subsequent treatment cycles, its development may be attenuated or absent altogether [62, 64]. Premedication with corticosteroid therapy or dose reductions may also be necessary, the pathophysiological rationale for which remains to be established [62, 64–66, 68].
In contrast, radiation enhancement, which refers to an increased reaction to radiotherapy after concomitant administration of certain types of chemotherapeutic agents (by definition <7 days after the end of radiotherapy), tends to be rare with these drugs [62, 69].
UV recall reaction
This phenomenon is well-described in oncology, and was first reported with methotrexate, and subsequently with gemcitabine and etoposide [37, 70–72]. Both paclitaxel and docetaxel can induce a photo or UV recall (or reactivation of solar erythema) [68, 72;73]. Typically, an episode of solar erythema will have occurred (and regressed), a few days or weeks prior to chemotherapy. After infusion of chemotherapy, the skin lesions re-appear (UV recall) on the previously affected photo-distributed areas [37], with the same clinical presentation even without exposure to sunlight (figure 5). The post-infusion secondary reaction bears the potential to be much more severe than the initial episode of solar erythema [71–73].
Figure 5.
Inflammatory skin lesions reminiscent of a previous sunburn (UV recall).
Symptomatic management with topical anti-inflammatory agents may be necessary, and photo-protective measures should be recommended prior to subsequent cycles. The reaction gradually attenuates over the course of these cycles [37] and does not necessitate interruptions of chemotherapy [72, 73].
Extravasation recall phenomenon
Increased permeability of microvasculature and micro-trauma at the site of prior extravasation-damaged tissue allows leakage and accumulation of the chemotherapeutic agent upon re-infusion, thus resulting in an inflammatory reaction. This has been described following reinfusion of paclitaxel or docetaxel, albeit rarely, and is characterized by erythema recurring at sites of prior inflammation [12, 29, 31, 69, 74]. Biopsy reveals epidermal dysmaturation with dyskeratotic keratinocytes, vacuolar degeneration in the basal layer, and eccrine squamous syringometaplasia [31].
Palmar-plantar erythrodysaesthesia
Dorsal hand-foot syndrome (HFS)
The development of a HFS is relatively more common with docetaxel [11, 75–80], as opposed to paclitaxel [6, 13, 81, 82]. While less frequent than with other chemotherapeutic agents, such as fluorouracil, capecitabine, and doxorubicin [83], it occurs in 5–10% of patients treated with taxanes [6, 41, 78]. However, given the widespread use of taxanes, HFS is not uncommonly encountered in clinical practice, especially with the weekly regimen [41, 84].
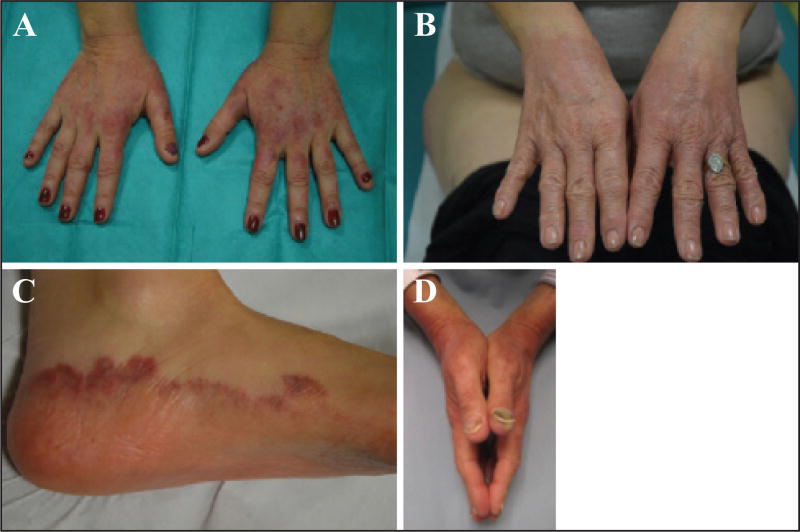
The most characteristic form is a distinct dorsal hand presentation which is specific to the taxanes and fairly common. Scaly erythematous lesions are seen predominantly on the dorsal aspects of hands (overlying the joints) and thenar eminences (figures 6 A–B) [11, 13, 75–77, 81, 85], and more rarely on the dorsum of the feet (figure 6C) or the peri-malleolar and Achilles regions [75, 82]. The dorsal hand eruption typically does not cross the Wallace’s line, a transitional zone that runs along the medial and lateral borders of the hands. Occasionally, palmoplantar involvement may be noted [75, 77, 82]. The constellation of PeriArticular Thenar Erythema and Onycholysis characterize the “PATEO syndrome” seen in patients on docetaxel (figure 6D) [41]; not all nails are universally affected, although their involvement is common [75]. The lesions may start from the first treatment cycle or develop progressively during subsequent cycles [75], and may sometimes be heralded by prodromal symptoms such as tingling, burning pain, or numbness. The dorsal HFS is bilateral, but not necessarily strictly symmetric, and pruritus, pain, or a burning sensation are generally the most prominent symptoms. Ectopic localisations have also been reported, for example in the sacral region [11].
Figure 6.
A–C) Specific taxane-induced dorsal hand foot syndrome, involving the dorsal aspects of the hands (A, B) and the dorsum of the feet (C). D) PATEO syndrome (visible dorsal erythematous lesions associated with onycholysis).
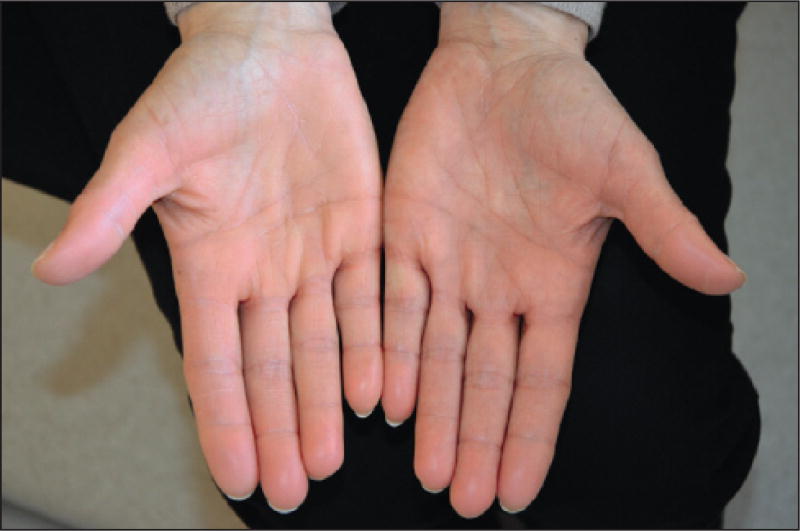
Rarely, patients may develop the HFS conventionally described with chemotherapy, which affects the bilateral palmar and/or plantar surfaces with varying degrees of erythema, pain, or tingling (figure 7) [13, 78, 79].
Figure 7.
Hand foot syndrome affecting the bilateral palmar surfaces.
The mechanisms governing HFS have not been established, although a direct cytotoxic effect of chemotherapy on basal keratinocytes has been proposed [76, 77, 82]. The predilection for palmoplantar regions may be explained by a high number of capillaries, rapid renewal of keratinocytes, preferential secretion of various chemotherapeutic agents by eccrine glands (present in a very high density), and repeated trauma [75, 76, 78, 84] in these areas. However, none of these hypotheses explain the dorsal localisation observed with taxanes, although a predilection for sun-exposed sites begins to address this.
Functional impairment is variable, but quality of life may be impacted and it may be dose-limiting [86]. Therefore, active preventive strategies are of paramount importance. Frozen gloves (or ice packs) used for the prevention of onycholysis also protect against the development of HFS [87]. Skin barrier-enhancing emollient use is advised. The benefit of oral pyridoxine is not proven [88] and the utility of COX-2 inhibitors needs to be further established [78, 89]. If erythema develops, systemic corticosteroids (i.e. prednisone equivalent) or high-potency topical steroids (i.e. clobetasol) are prescribed, sometimes under an occlusive dressing. Lidocaine patches (4% or 5%) are helpful in pain management. Global assessment of pain and its effect on instrumental and self-care activities of daily living must be considered; dose reduction or temporary discontinuation of treatment is sometimes necessary (table 2 B). In the setting of docetaxel-induced HFS, switching to paclitaxel, when possible, is an alternative [75].
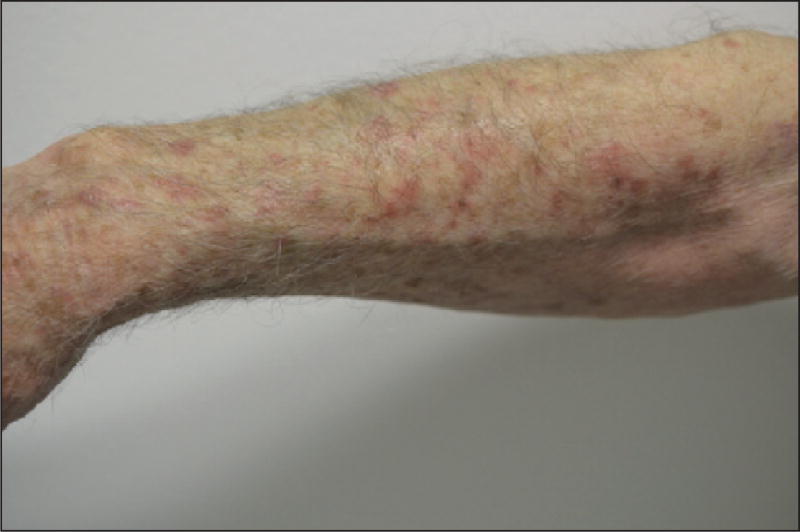
Fixed erythrodysaesthesia
Fixed erythrodysaesthesia appears to be specific to docetaxel [11, 12, 34, 85, 90, 91] and often manifests as a solitary, well-circumscribed, erythematous, tender plaque that develops a few days after a cycle of chemotherapy (weekly, or every three weeks) [85, 91]. It preferentially localises on the forearm, sometimes close to the infusion site, which then must be differentiated from extravasation [85]. Subsequently, it may develop desquamation with secondary hyperpigmentation, and is unlikely to recur with continued treatment [12, 92]. Some authors consider fixed erythrodysaesthesia as a variant of HFS [34].
Finally, a similar presentation of fixed drug eruption has been described in a woman, a day after the initiation of adjuvant therapy with paclitaxel following surgery for ovarian cancer [93].
Pigmentary changes
Serpentine supravenous hyperpigmentation (SSH)
Described initially with fluorouracil (and observed in particular with this drug), SSH has since been reported sporadically with several other drugs, including docetaxel [94–96]. This specific complication occurs almost exclusively after peripheral infusion [92]. The clinical presentation is highly characteristic; the superficial venous networks become pigmented and conspicuous, with infusion sites representing the starting point [70, 92]. These may be initially inflamed and erythematous, and accompanied by burning and/or pruritus [94, 96]. The condition does not require specific treatment or dose interruption/reduction of taxanes. To our knowledge, this has not been noted with paclitaxel.
Flagellate and reticulate hyperpigmentation
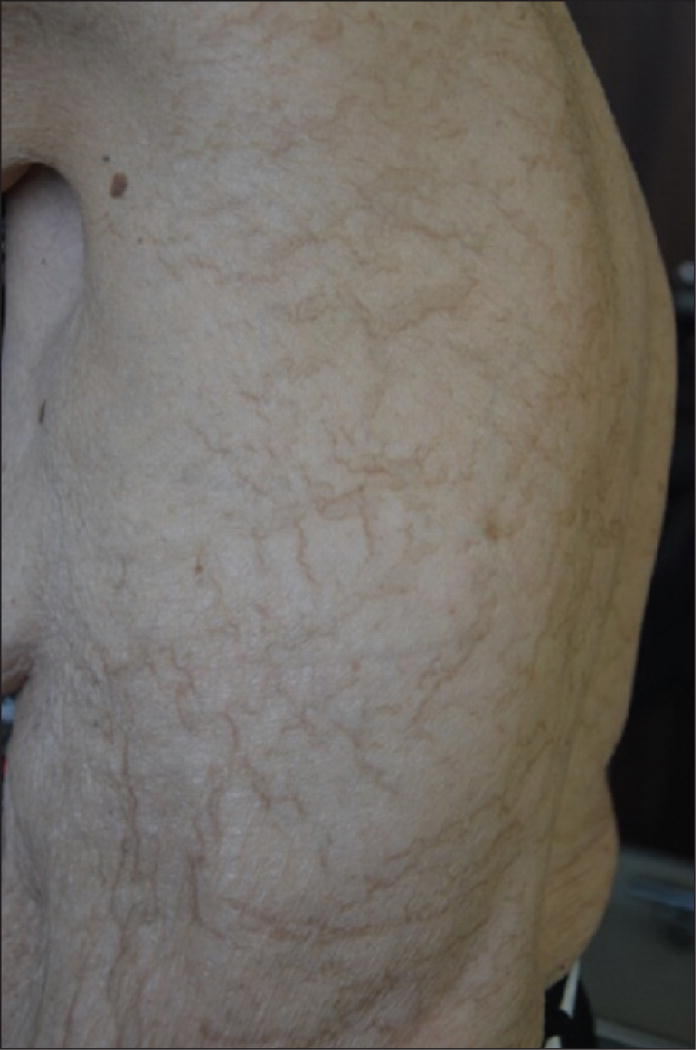
Flagellate erythema is traditionally attributed to bleomycin, but it can occur with docetaxel [13]. In addition, we have also personally observed exceptional cases of reticulate hyperpigmentation with paclitaxel (figure 8) [97].
Figure 8.
Reticulate hyperpigmentation of the trunk induced by paclitaxel.
Oedema/cutaneous sclerosis
Peripheral oedema
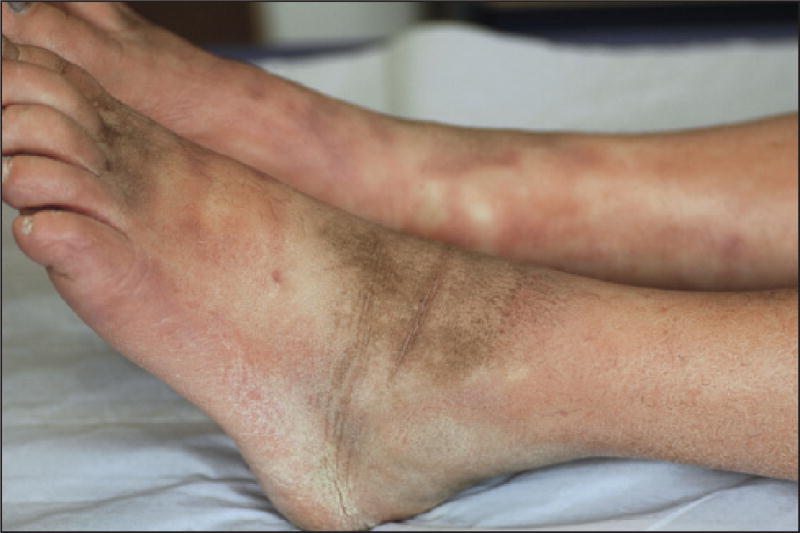
Taxanes, in general, can lead to reversible peripheral oedema, although it has been mostly reported with docetaxel. The underlying pathophysiological mechanism is thought to be related to a capillary protein leak syndrome, and at later stages, varying degrees of insufficiency in lymphatic drainage [1, 98]. Clinically, it manifests with peripheral soft pitting oedema of the lower limbs that may sometimes progress to lymphoedema [2, 7, 9, 25, 39, 99], and affects between 20% to over 60% of the patients treated for advanced cancer. In the more severe Grade 3 disease, ascites and/or pleural effusion may ensue [5, 7, 10, 39, 100]. Corticosteroid premedication, used for the prevention of hypersensitivity reactions, may restrict its development [2, 5, 25, 101]. There appears to be a relationship between the cumulative dose of docetaxel (>400 mg/m2, or at around the 4th–5th cycle) [2] and fluid retention, [97, 98, 102], but sometimes it may occur even with the first cycle of treatment. Persistence after discontinuation of treatment is infrequent [102], but secondary scleroderma-like changes can sometimes develop [103–108].
Scleroderma-like skin changes
Infiltrative cutaneous sclerosis has been reported with both paclitaxel [103–106] and docetaxel [106, 107], and may be marked by inflammation of the skin [103, 106, 107]. It can develop progressively over several months, and may not manifest until very late, sometimes appearing even after the end of chemotherapy [108, 109]. The extremities are most commonly affected [12, 104, 107], especially the lower limbs (figure 9) [105–107], which can lead to severe limitations in the mobility of underlying joints [103–106], besides the development of trophic ulcers [105]. Although the trunk [103] and neck [108, 109] may also be affected, there are no systemic findings or laboratory markers suggestive of progressive systemic sclerosis [103, 106, 107]. Rarely, an association with Raynaud’s syndrome may be noted [109]. Based on the features in published cases, the oedema tends to precede the scleroderma-like lesions [103–108], and HFS may also be noted in the same areas [107].
Figure 9.
Scleroderma-like changes with progressive development of cutaneous sclerosis (lower limbs).
Skin biopsy reveals fibrosis [105, 107] with proliferation of collagen bundles in the dermis [103, 106, 109], along with an occasional perivascular inflammatory cell infiltrate [104–106]. The mechanisms underlying these fibroblastic proliferative changes are not known, although taxanes are capable of stimulating the process, besides the expression of a number of cytokines (IL-2, IL-6, TNF-α) [104, 106]. Interestingly, such sclerotic changes are not unique to taxanes, and have been reported with capecitabine, bleomycin, hydroxyurea, topotecan, raltitrexed, cyclophosphamide, pemetrexed, or gemcitabine [34, 70, 104].
Discontinuation of chemotherapy may sometimes lead to clinical regression of the lesions [103, 106, 107], which is consistent with our experience and the cases published to date [105, 106, 108, 109]. In the management of oedema, physical therapy assumes importance [106], as it may offset the development of secondary sclerosis. While diuretics may not be helpful, the utility of corticosteroids in this setting needs to be established [104]. At our centres, we initiate treatment with prednisone, often in combination with oral methotrexate, with the aim of arresting sclerosis. Other modalities of questionable value include topical steroids under occlusion, intralesional steroids, and phototherapy (UV-A).
Hair and nail changes
Chemotherapy-induced alopecia (CIA)
Chemotherapy-induced acute reversible alopecia
Paclitaxel and docetaxel rank among the top CIA-inducing drugs [110, 111], chiefly by a mechanism that induces dystrophic anagen effluvium [112, 113]. The onset is typically after the first treatment cycle, whether or not in an adjuvant treatment setting. CIA represents one of the most distressing adverse events of taxanes, affecting the scalp in a diffuse pattern (Grade 2) in at least 60% of treated patients [6, 8, 9]. Other hair-bearing areas (eyelashes, eyebrows, beard, axillae, pubis, and body) may also be involved, especially when the treatment is prolonged, involves higher doses, or entails multiple exposures [1, 112].
Typically, the lost hair gradually regrows starting three to six months after the last cycle, and progressively returns to baseline. However, more than a third of patients observe a change in both the texture (usually more curly) and colour (usually more grey, or rarely darker) of the regrowing hair [113].
Chemotherapy-induced persistent alopecia (CIPAL)
In recent years, several reports of “permanent” and “irreversible” alopecia following treatment with taxanes have emerged. The hair loss manifests as suboptimal regrowth and/or absence of scalp and body hair regrowth, more than six months after the discontinuation of chemotherapy [111]. Here, it is important to note that there are no long-term studies addressing the (final) clinical outcome, and since these terms may imply a grave prognosis to patients, we suggest the usage of “persistent” instead of “permanent” in the acronym “CIPAL” (Chemotherapy-Induced Permanent Alopecia) proposed by Palamaras et al. to connote hair loss in this setting [114].
The clinical picture was initially well-described with busulfan, and to a lesser extent with thiotepa, during conditioning for allogeneic bone marrow transplantation [111, 114, 115]. In this scenario, the contribution of cutaneous graft-versus-host disease (GvHD) needs to be excluded. In the case of taxanes, Kluger et al. estimated the incidence to be 2% with docetaxel [116]. However, findings of the French Drugs and Emerging Therapeutics Observatory (OMIT), and our personal experience, suggest that the incidence is higher and is likely underestimated [117].
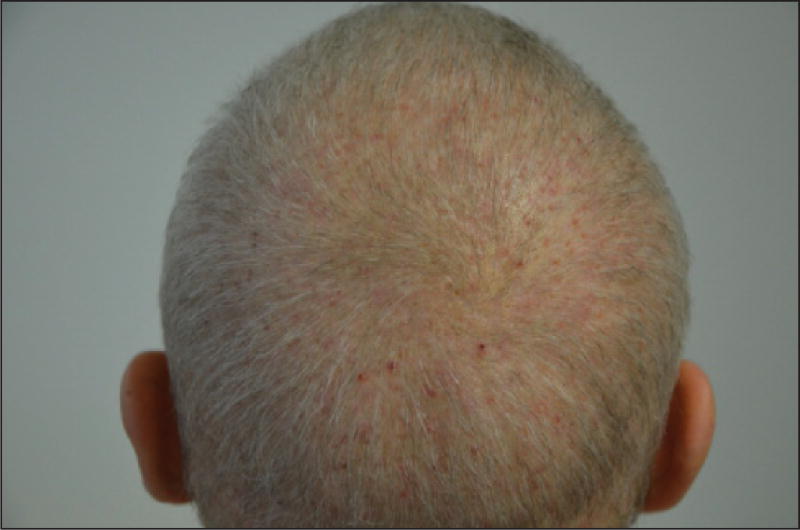
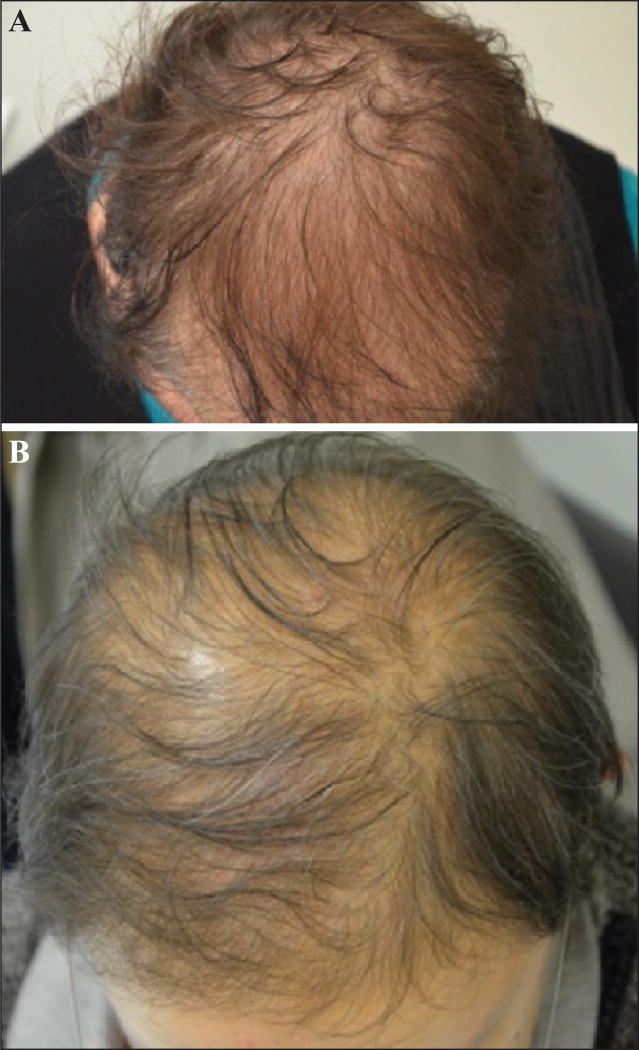
The hair loss in CIPAL is not total but rather relatively diffuse [111, 114], and tends to be accentuated in areas prone to androgenetic alopecia [115, 116, 118]. The hair is finer and shorter (generally <10 cm) [114–116], but the scalp appears healthy, without features of cicatricial alopecia or fibrosis (figures 10 A–B) [111, 116]. Hair at other sites (eyelashes, eyebrows, axillae, pubis, and body) can be affected as well [114, 116, 118]. It is perceived that CIPAL is usually irreversible, but there are no long-term prospective studies confirming this, although the psychological impact can be substantial [115, 116, 119]. On histological examination, the number of follicular units appears unchanged, but a reduction in capillary density correlates with a decrease in the number of hairs, to the detriment of those in the anagen phase (there is a parallel increase in miniaturized hairs and telogen hairs) [111, 115, 116, 118]. The terminal hair/miniaturized hair (vellus hair) ratio is 1:1, in other words, even lower than that in androgenetic alopecia [115]. Telogen germinal units (at the end stage of the telogen phase after hair loss) are also increased [119]. Conversely, no fibrosis or inflammation is noted [111, 115, 116]. A moderate peribulbar lymphocytic infiltrate has sometimes been noted [116, 118], which is interesting when considering therapeutic approaches.
Figure 10.
A, B) Taxane-induced persistent alopecia. Note that hair loss is relatively diffuse but not all over; the hair appears finer and shorter.
The mechanism of CIPAL has not been elucidated, although a separation of the matrix cells from the dermal papilla has been postulated, as well as a direct cytotoxic action of taxanes on hair matrix keratinocytes or hair bulge stem cells [111, 115, 116]. The risk factors for its development have not been identified. Neither a history of androgenetic alopecia, nor abnormal laboratory findings (zinc, iron, hormonal or thyroid abnormalities, etc.) appear to play a role [115, 116]. It must be noted that the long-term (2–10 years) adjuvant endocrine-based anticancer therapies (antioestrogens and aromatase inhibitors) may also cause and/or exacerbate hair loss [120].
Management of acute reversible alopecia
Prior to the initiation of chemotherapy, patients may be advised to cut their hair short before it falls out, or even shave their heads, to offset scalp irritation and any impending psychosocial stress (embarrassment from shedding). Similarly, a wig and/or scarf or other head coverings (e.g. turban) may also be advised. Topical minoxidil (2%) applications initiated at the start of chemotherapy, although not helpful in preventing CIA [110, 113], may accelerate the rate of hair regrowth which is significant for many patients. Scalp cooling is not universally instituted in the prevention of CIA. It tends to confer a protective effect, leading to a 50% reduction in (maximal) hair loss in around 50–75% of cases, depending on the series [113, 121, 122]. There are limited studies conducted specifically with taxanes [123, 124], but it has been suggested that the preventive efficacy of scalp cooling is best with low-dose paclitaxel and docetaxel monotherapies, as compared to the combination regimens [124, 125]. The mechanisms underlying scalp cooling benefits appear to be vasoconstriction and more importantly, a reduction in hair follicle metabolism during chemotherapy infusion (time of peak plasma concentration), thus rendering them less vulnerable to the toxic effects of chemotherapy [126].
The global patient safety profile is generally acceptable [121, 122, 125], however, the study parameters appear heterogenous in terms of the type of devices used, the degree or duration of cooling, the type or dose of chemotherapy, the study population, and the clinical assessment criteria [121, 126]. There is a dearth of well-conceived randomised clinical trials [127] and the implementation of large-scale prospective studies on homogeneous populations [113], accompanied by more objective measurements, appears mandatory.
Management of CIPAL
It is imperative that patients are forewarned of the potential for CIPAL with taxanes [114, 128], for ethical and medico-legal reasons. This is particularly true in the adjuvant treatment setting, especially in the female breast cancer population, where the overall prognosis is generally good and patients are younger.
In limited studies conducted so far, minoxidil, spironolactone, and phototherapy have not shown therapeutic benefit, when used prophylactically, or following the development of CIPAL [114, 116]. Further, it remains unclear whether mitigating or preventing CIA in the acute stages would reduce the risk of eventually developing CIPAL.
Nevertheless, for CIPAL, we recommend topical minoxidil (up to 5%), since it could potentially stimulate some hair regrowth [118]. This would also benefit any associated AGA, the presence of which can be confirmed by performing a trichogram. If inflammation is suspected or noted on biopsy, topical steroids can be instituted. In select cases, hair transplantation can be offered, but in most cases, hair wigs will suffice. Topical bimatoprost may be recommended for the management of eyelash loss (not available in Europe) [129]. In all cases, the impact of CIPAL on patients’ self-esteem or self-image and quality of life should be formally assessed and addressed.
Nail changes
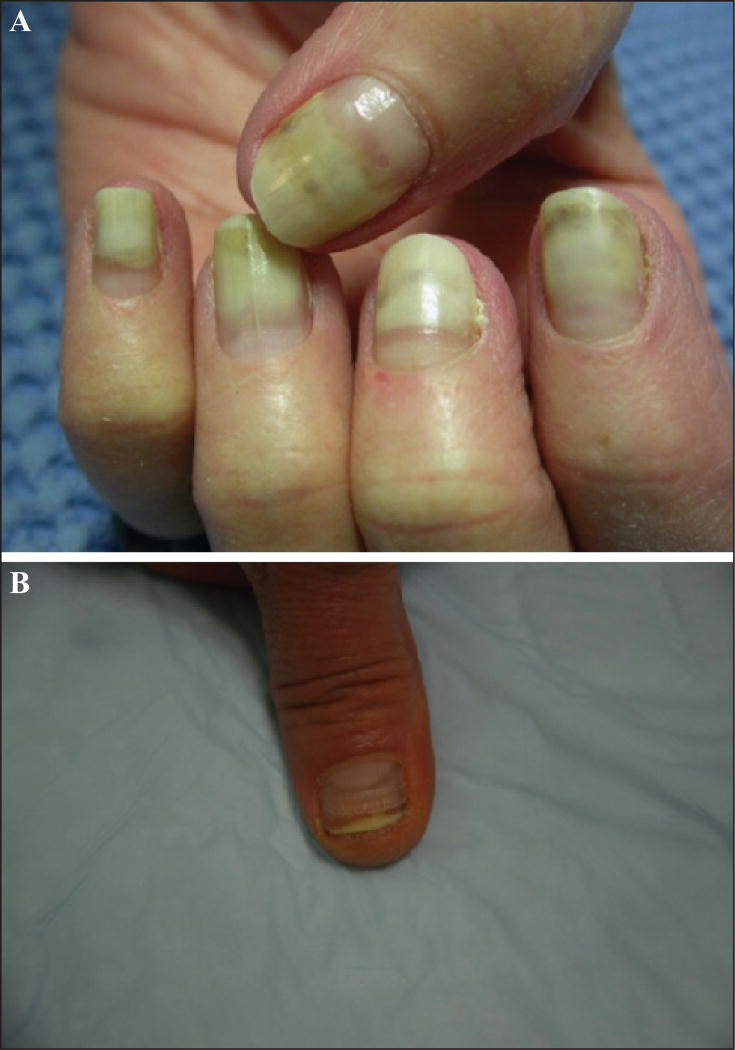
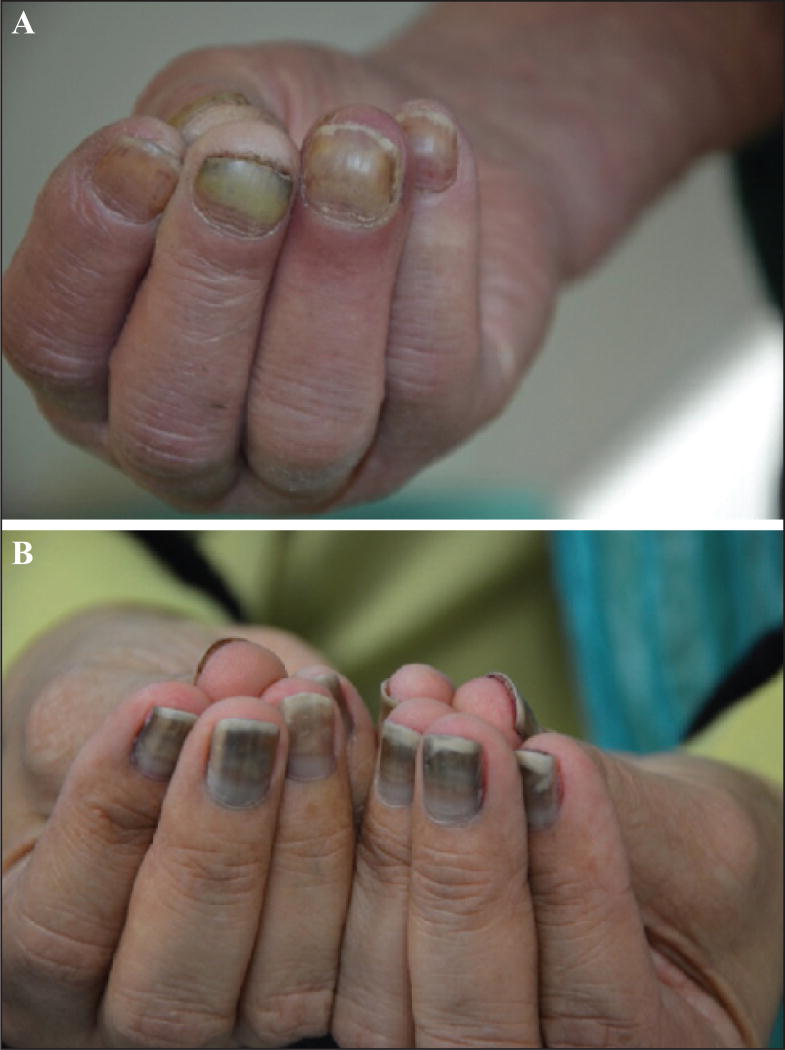
Nail changes with taxanes are very common [83, 87, 130–132], with some series reporting rates as high as 89% after three treatment cycles [133]. Recently, the overall incidence of taxane-induced nail changes has been systematically investigated [134]; all-grade incidence was 43.7% with paclitaxel (95% CI: 18.0–73.3%) and 34.9% (95% CI: 29.9–40.2%) with docetaxel. For the latter, the relative risk was 77.74 (95% CI: 41.88–144.32; p<0.001) as compared to controls. It is noteworthy that docetaxel and paclitaxel are the most frequent chemotherapeutic agents that induce nail changes [87, 130, 135, 136], resulting from a direct toxic effect. The changes may affect both the nail matrix (melanonychia, true leuconychia, Beau’s lines and onychomadesis, brittle nails with ridging and thinning, onychorrhexis, and koilonychias), the nail bed (onycholysis and apparent leuconychia) (figures 11 A–B), or the periungual tissue (paronychia) [34, 136, 137]. Nail lesions are evident after several weeks of treatment [133] because of the slow growth rate of the nail plate [135], and tend to increase with the number of treatment cycles [132, 133, 135, 136]. Although they are more common in patients receiving the once-weekly regimens [6, 135, 136, 138], they can also be observed with the every three-week regimen [90, 133, 139, 140]. In an overwhelming majority, these changes eventually regress after cessation of treatment [30, 132, 141], notwithstanding the fact that persistent sequelae affecting the nail plate and its growth may be encountered (figure 12).
Figure 11.
A) Partial detachment of the nail plate from the underlying nail bed (onycholysis). B) Beau’s lines associated with longitudinal melanonychia and mild onycholysis in the same fingernail.
Figure 12.
Persistent onycholysis secondary to subungual hyperkertosis.
Onycholysis represents the most characteristic lesion and occurs because of detachment of the nail plate from the underlying nail bed [137]; this may culminate in loss of the nail. The fingernails are more often affected than toenails [133, 142] and the number of digits involved varies, although involvement may be diffuse [30, 135, 138, 143]. The impact on the quality of life and activities of daily living can be significant [132, 133, 144] and result in treatment interruption; effects depend on the number of digits involved, the degree of detachment, and the extent of pain.
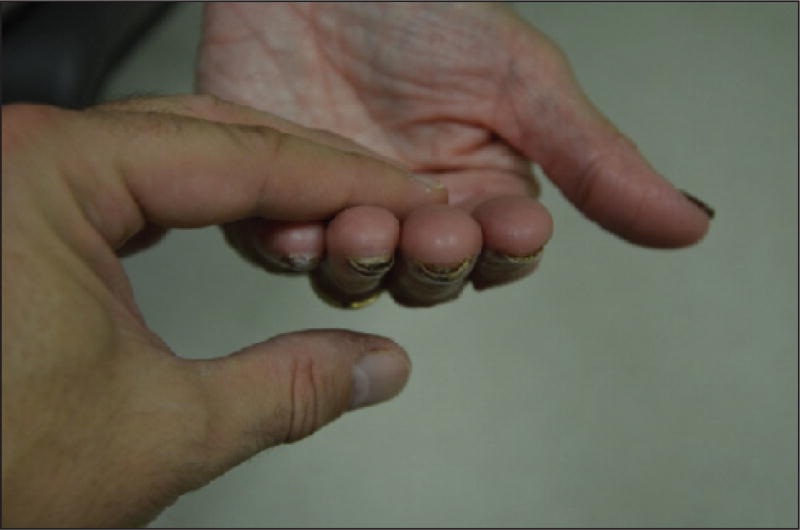
The development of a subungual haematoma, haemorrhage, or abscess with purulent discharge (figures 13 A–B) is not uncommon [90, 130, 140, 141, 143] and can be debilitating with involvement of multiple nails. The detached area can take on a black, white, or brown-red colour, depending on the type of lesion [90, 135, 139, 140]. Paronychia is also often present [55, 135, 139, 144–146]. In addition, chronic onycholysis can lead to nail bed keratinization and subungual hyperkeratosis [90, 141, 145]. In consequence, it is crucial to promote reattachment as soon as possible, otherwise onycholysis may become irreversible.
Figure 13.
A) Diffuse fingernail onycholysis associated with subungual abscesses and paronychia; a partial avulsion of the nail plate is required. B) Development of painful subungual haematomas resulting from toxic effects of taxanes.
The presence of regular transverse Beau’s lines (figure 11 B) in the nail plate is also very common and reflects the temporary cessation of matrix proliferation during each chemotherapy cycle [30, 90, 140, 141, 145] In its extreme form, proximal detachment of the plate with onychomadesis may be observed (figure 14) [130, 137].
Figure 14.
Onychomadesis affecting all fingernails (the nail plate is divided into two parts by a transverse thick groove).
The pathophysiological origin of these nail changes is not clearly established. A direct cytotoxic effect on the nail matrix and epithelial cells of the nail bed, as well as an intrinsic antiangiogenic activity of taxanes, have been postulated [130, 132, 138]. Similarly, a phototoxic mechanism for photo-onycholysis has been advanced by some authors [135], which is pending confirmation [137, 138]. Lastly, a taxane-induced neurotropic effect (neurogenic or prostaglandin-mediated inflammation) has also been entertained [146].
Management of nail changes depends on the type of nail affliction and the impact on activities of daily living. Prophylactic measures include the use of emollients (following hydration of nail folds), protective varnish on the nail plate, and cotton gloves (water-resistant if exposure is excessive). All trauma must be avoided, especially manipulation of the cuticles and nail biting, overzealous salon manicures or use of fingernails as “tools”, prolonged soaking in water, exposure to solvents, and application of artificial (“fake”) nails [137]. The nails should be cut regularly until the nail plate grows reattached. The importance of wearing frozen gloves/socks during chemotherapy cannot be overstressed. Scotte et al. have demonstrated a significant reduction in nail changes from 51% to 11% (p=0.0001) in fingernails, and from 21% to 0% in toenails, with a trend towards a prolongation (albeit non-significant) of the median time to development of these lesions [87, 142]. Importantly, the Grade 2 or greater nail AEs were reduced from 22% to 0% (p=0.0001). Alternatively, the use of ice packs (with interruptions) as a tolerable option may be a less expensive and effective strategy [131]. It is intriguing that despite the simplicity and effectiveness of this intervention, it is not universally employed. While discontinuation of chemotherapy is only rarely necessary, dose interruptions are commonplace.
Once the AE develops, management is directed primarily at symptom control. It may be necessary to remove the nail plate in cases of severe and/or painful onycholysis, or when associated with a haematoma or subungual abscess. The nail bed must be cleaned and cultured at the same time [137], and any infection should be promptly treated with an appropriate antibiotic. In the setting of paronychia without infection, potent topical steroids are recommended.
Oral mucosal involvement
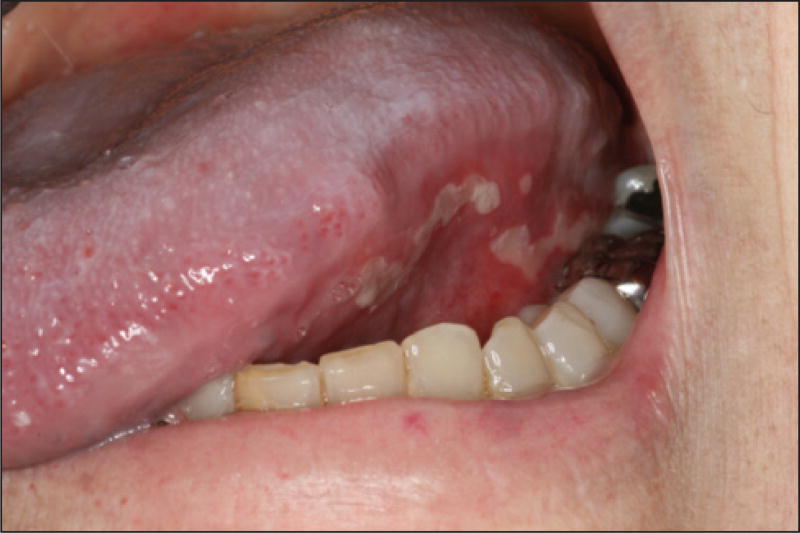
Oral mucositis is a dose-dependent AE known to occur with both paclitaxel and docetaxel [1, 5, 37, 68], although it is more common with the latter [6;7], and affects 29–63% of the treated patients [2, 7, 9, 10, 14]. The oral lesions are non-specific and affect the non-keratinized mucosa; they often begin with erythema and are followed by localized ulcerations, which become confluent and are sometimes covered with pseudomembranes. Sites involved include the soft palate, tongue (ventral and lateral aspects), floor of the mouth, and buccal mucosa (figure 15). The severity of mucositis usually does not exceed Grades 1 or 2 [2], with less than 10% of patients suffering from a higher grade with either drugs [6, 7, 10, 14, 100, 101]. However, functional impairment can be severe and dose-limiting [3]. Treatment is primarily supportive; topical steroids and low-level laser therapy may be helpful in reducing pain as well as the duration of lesions.
Figure 15.
Oral mucositis with apparent pseudomembranes.
Dysgeusia is frequently concomitant [10, 39] and predominantly occurs four to seven days following initiation of treatment with taxanes. The burden of dysgeusia can be in the forefront of complaints, and can result in poor eating behaviours [147].
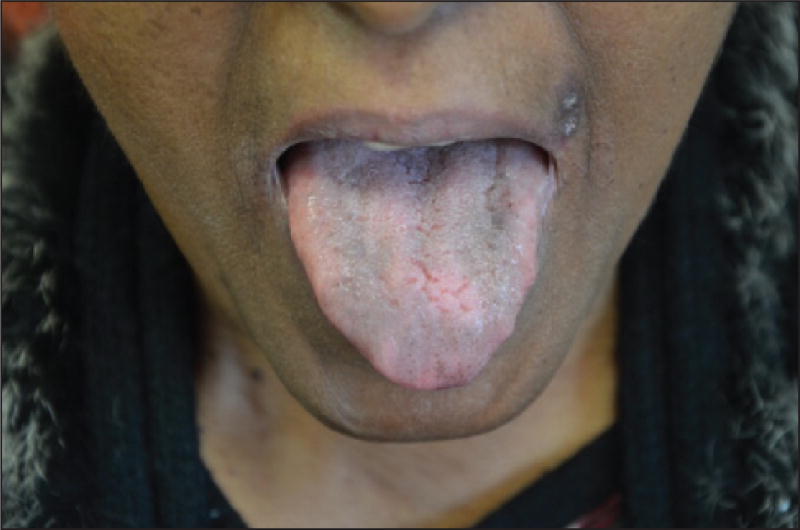
Residual mucosal pigmentation is also sometimes observed (figure 16) [92].
Figure 16.
Residual pigmentation of the dorsum of the tongue.
Miscellaneous
Hot flashes
Outside the context of an immediate hypersensitivity reaction, hot flashes may occur in up to 10% of cases during treatment with paclitaxel or docetaxel [6]. The transient flushing typically affects the face, neck, and upper chest. They are not necessarily accompanied by hyperhidrosis (“wet” flush), but they can be socially disabling for the patient. Although the pathophysiological basis has not been elucidated in this setting, transient vasodilation is the underlying event, which is known to occur either as a result of disturbances in autonomic nervous system or by the direct action of circulating vasodilator mediators on vascular smooth muscle [37]. Clinically, such hot flashes may, however, be difficult to distinguish from hot flashes secondary to chemotherapy-induced premature menopause [80, 148].
Inflammation of actinic keratoses
Pre-existing actinic keratosis or foci of actinic damage on photo-exposed areas may become acutely inflamed, which is a well-appreciated phenomenon that occurs following chemotherapy with a variety of drugs [37] (figure 17). This is particularly frequent with fluorouracil or its prodrug, capecitabine, but may also be observed with docetaxel [11, 149]. Supportive counselling is recommended, as once the lesions crust and resolve, subsequent cycles do not cause a similar eruption. If the inflammation is symptomatic, topical steroids are helpful.
Figure 17.
Inflammation of actinic keratoses with docetaxel (forearms).
Xerosis
Xerosis and scaling are very common with both docetaxel [2, 25] and paclitaxel [6]. These are managed with liberal bland emollients. Taxane-related xerosis develops after skin barrier function damage, which induces increased transepidermal water loss and leads to a low content of skin water into the stratum corneum [150].
Psoriasis and taxanes
Psoriatic patients are generally believed to improve during treatment with taxanes as a result of their antiangiogenic and antiproliferative activity [151]. Recently, the use of paclitaxel (in micellar form) has even been proposed for the treatment of patients with a severe form of psoriasis [151, 152]. In our experience, both improvement and exacerbation of psoriasis can occur with these two agents [153].
Nab-paclitaxel
Nab-paclitaxel is a novel, solvent-free, albumin-bound particle formulation (with a size of 130 nm) of paclitaxel that has led to a significant improvement in progression-free survival, median overall survival, and overall response rates in patients with metastatic breast and pancreatic cancers [4]. It has recently received FDA and EMA approvals for the treatment of certain forms of both cancers [154]. It is also approved in the US for metastatic non-small cell lung cancer [155] (figure 1) and is still under evaluation for several other indications, such as metastatic urothelial tumours [156].
Nab-paclitaxel was developed to circumvent the highly hydrophobic properties of taxanes and to improve intratumoural paclitaxel penetration [154, 157]. Since it is devoid of Cremophor EL (the solvent for paclitaxel), several significant AEs, such as hypersensitivity reactions [154, 157], are less likely to be incurred. Severe immediate hypersensitivity reactions are significantly less common [16, 156], affecting 2–4% of treated patients. The use of premedication, however, is not required. Depending on the underlying oncological indication, it may be beneficial to substitute paclitaxel or docetaxel with nab-paclitaxel for those experiencing a severe immediate reaction (see above) [16, 21, 26].
The other reported AEs, such as neuropathy, neutropenia, thrombocytopenia, nausea, and fatigue, appear generally comparable to those with the solvent-based paclitaxel, but the overall safety profile is favourable [154, 157, 158]. For example, Grade 3/4 neutropenia and fatigue are less frequent, and neuropathies appear to resolve more rapidly [4, 8, 154, 155, 159].
Dermatological AEs have not yet been specifically studied. The incidence of alopecia (with monotherapy) ranges between 47% and 100% depending on the series [8, 156, 159, 160], but CIPAL has not yet been reported. The skin rashes have not been characterised clinically, although involvement of body folds and areas of friction has been reported, as have pigmentary changes [158]. Diffuse maculopapular rashes, including SJS and TEN, can occur. The incidence of rash is comparable to that observed with the solvent-based paclitaxel (overall incidence of 4%), except in Asian populations where it appeared to affect more than a third of patients in some series [158, 160]. Nab-paclitaxel-induced SCLE is similar in all aspects to that observed with paclitaxel (see above) [54]. Photosensitivity reaction can also occur [161]. The occurrence of peripheral oedema may be noted in 10% of cases [157]. While mucositis appears to affect 15% of patients, it tends to be less severe (<2% are Grade 3) [157]. Nail changes are also frequent with nab-paclitaxel [159], with an estimated overall all-grade incidence of 19.4% (95% CI: 11.8–30.3%) [134]. Finally, to the best of our knowledge, HFS has, to date, not been reported with nab-paclitaxel.
Acknowledgments
Disclosure. Financial support: none. VS has a speaking, consultant or advisory role with Roche, GlaxoSmithKline, Pierre Fabre, Merck, Bristol-Myers Squibb, Bayer, and Boehringer Ingelheim. MEL has a speaking, consultant or advisory role with Advancell, Amgen, AstraZeneca, Augmentium, Aveo, Bayer, Berg Pharma, Biopharm Communications, Boehringer Ingelheim, Brickell Biotech, Bristol-Myers Squibb, Clinical Assistance Programs, Clinical Care Options, EMD Serono, Envision Communications, Foamix, Galderma, Genentech, GlaxoSmithKline, Helsinn, Institute for Medical Education and Research, Integro-MC, Lindi Skin, Medscape, Medtrend International, Merck, Nerre Therapeutics, Novartis, Novocure, Oncology Specialty Group, OSI Pharmaceuticals, Permanyer, Physicians Education Resource, Pierre Fabre, Pfizer, Reata Pharmaceuticals, Roche, Sandoz, Sanofi Aventis, and Threshold.
Footnotes
Conflict of interest: NRL, HR, FD, VRB, AE, LG, MM, MD and EV have no conflicts of interest to declare.
References
- 1.Rowinsky EK, Donehower RC. Paclitaxel (taxol) N Engl J Med. 1995;332:1004–14. doi: 10.1056/NEJM199504133321507. [DOI] [PubMed] [Google Scholar]
- 2.Cortes JE, Pazdur R. Docetaxel. J Clin Oncol. 1995;13:2643–55. doi: 10.1200/JCO.1995.13.10.2643. [DOI] [PubMed] [Google Scholar]
- 3.Gelmon K. The taxoids: paclitaxel and docetaxel. Lancet. 1994;344:1267–72. doi: 10.1016/s0140-6736(94)90754-4. [DOI] [PubMed] [Google Scholar]
- 4.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markman M. Managing taxane toxicities. Support Care Cancer. 2003;11:144–7. doi: 10.1007/s00520-002-0405-9. [DOI] [PubMed] [Google Scholar]
- 6.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–71. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones SE, Erban J, Overmoyer B, et al. Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol. 2005;23:5542–51. doi: 10.1200/JCO.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Gradishar WJ, Krasnojon D, Cheporov S, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–9. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]
- 9.Trudeau ME. Docetaxel (Taxotere): an overview of first-line monotherapy. Semin Oncol. 1995;22:17–21. [PubMed] [Google Scholar]
- 10.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy REVEL: a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–73. doi: 10.1016/S0140-6736(14)60845-X. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerman GC, Keeling JH, Burris HA, et al. Acute cutaneous reactions to docetaxel, a new chemotherapeutic agent. Arch Dermatol. 1995;131:202–6. [PubMed] [Google Scholar]
- 12.Payne AS, James WD, Weiss RB. Dermatologic toxicity of chemotherapeutic agents. Semin Oncol. 2006;33:86–97. doi: 10.1053/j.seminoncol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Poi MJ, Berger M, Lustberg M, et al. Docetaxel-induced skin toxicities in breast cancer patients subsequent to paclitaxel shortage: a case series and literature review. Support Care Cancer. 2013;21:2679–86. doi: 10.1007/s00520-013-1842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poikonen P, Sjostrom J, Klaar S, et al. Skin toxicity as a risk factor for major infections in breast cancer patients treated with docetaxel. Acta Oncol. 2004;43:190–5. doi: 10.1080/02841860310022977. [DOI] [PubMed] [Google Scholar]
- 15.Susnjar S, Bosnjak SM, Radulovic S. Severe skin toxicity observed with the combination of capecitabine and weekly paclitaxel in metastatic breast cancer patients. Support Care Cancer. 2008;16:1415–8. doi: 10.1007/s00520-008-0495-0. [DOI] [PubMed] [Google Scholar]
- 16.Caiado J, Picard M. Diagnostic tools for hypersensitivity to platinum drugs and taxanes: skin testing, specific IgE, and mast cell/basophil mediators. Curr Allergy Asthma Rep. 2014;14:451. doi: 10.1007/s11882-014-0451-7. [DOI] [PubMed] [Google Scholar]
- 17.Lee C, Gianos M, Klaustermeyer WB. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann Allergy Asthma Immunol. 2009;102:179–87. doi: 10.1016/S1081-1206(10)60078-6. [DOI] [PubMed] [Google Scholar]
- 18.Castells M, Sancho-Serra MC, Simarro M. Hypersensitivity to antineoplastic agents: mechanisms and treatment with rapid desensitization. Cancer Immunol Immunother. 2012;61:1575–84. doi: 10.1007/s00262-012-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piovano E, Pivetta E, Modaffari P, et al. A search for predictive factors for hypersensitivity reactions to paclitaxel and platinum salts in chemotherapy for gynecologic pelvic neoplasms. Gynecol Obstet Invest. 2012;74:21–7. doi: 10.1159/000336772. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Munoz A, Jimenez B, Garcia-Tapiador A, et al. Cross-sensitivity between taxanes in patients with breast cancer. Clin Transl Oncol. 2011;13:904–6. doi: 10.1007/s12094-011-0753-3. [DOI] [PubMed] [Google Scholar]
- 21.Kimura K, Tanaka S, Iwamoto M, et al. Safety of nanoparticle albumin-bound paclitaxel administered to breast cancer patients with clinical contraindications to paclitaxel or docetaxel: four case reports. Oncol Lett. 2013;6:881–4. doi: 10.3892/ol.2013.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raisch DW, Campbell W, Garg V, et al. Description of anaphylactic reactions to paclitaxel and docetaxel reported to the FDA, with a focus on the role of premedication. Expert Opin Drug Saf. 2011;10:521–8. doi: 10.1517/14740338.2011.582865. [DOI] [PubMed] [Google Scholar]
- 23.Syrigou E, Dannos I, Kotteas E, et al. Hypersensitivity reactions to docetaxel: retrospective evaluation and development of a desensitization protocol. Int Arch Allergy Immunol. 2011;156:320–4. doi: 10.1159/000324454. [DOI] [PubMed] [Google Scholar]
- 24.O’Cathail SM, Shaboodien R, Mahmoud S, et al. Intravenous versus oral dexamethasone premedication in preventing Paclitaxel infusion hypersensitivity reactions in gynecological malignancies. Int J Gynecol Cancer. 2013;23:1318–25. doi: 10.1097/IGC.0b013e31829f1799. [DOI] [PubMed] [Google Scholar]
- 25.Schrijvers D, Wanders J, Dirix L, et al. Coping with toxicities of docetaxel Taxotere. Ann Oncol. 1993;4:610–1. doi: 10.1093/oxfordjournals.annonc.a058599. [DOI] [PubMed] [Google Scholar]
- 26.de Leon MC, Bolla S, Greene B, et al. Successful treatment with nab-paclitaxel after hypersensitivity reaction to paclitaxel and docetaxel. Gynecol Oncol Case Rep. 2013;5:70–1. doi: 10.1016/j.gynor.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madrigal-Burgaleta R, Berges-Gimeno MP, Angel-Pereira D, et al. Hypersensitivity and desensitization to antineoplastic agents: outcomes of 189 procedures with a new short protocol and novel diagnostic tools assessment. Allergy. 2013;68:853–61. doi: 10.1111/all.12105. [DOI] [PubMed] [Google Scholar]
- 28.Barbee MS, Owonikoko TK, Harvey RD. Taxanes: vesicants, irritants, or just irritating? Ther Adv Med Oncol. 2014;6:16–20. doi: 10.1177/1758834013510546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanford BL, Hardwicke F. A review of clinical experience with paclitaxel extravasations. Support Care Cancer. 2003;11:270–7. doi: 10.1007/s00520-003-0441-0. [DOI] [PubMed] [Google Scholar]
- 30.Link CJ, Jr, Sarosy GA, Kohn EC, et al. Cutaneous manifestations of Taxol therapy. Invest New Drugs. 1995;13:261–3. doi: 10.1007/BF00873811. [DOI] [PubMed] [Google Scholar]
- 31.Ley BD, Millan GG, Perez JS, et al. Docetaxel recall phenomenon at the site of previous drug extravasation. Arch Dermatol. 2010;146:1190–1. doi: 10.1001/archdermatol.2010.291. [DOI] [PubMed] [Google Scholar]
- 32.Ascherman JA, Knowles SL, Attkiss K. Docetaxel (taxotere) extravasation: a report of five cases with treatment recommendations. Ann Plast Surg. 2000;45:438–41. doi: 10.1097/00000637-200045040-00016. [DOI] [PubMed] [Google Scholar]
- 33.Gallo E, Llamas-Velasco M, Navarro R, et al. Eccrine squamous syringometaplasia secondary to cutaneous extravasation of docetaxel: report of three cases. J Cutan Pathol. 2013;40:326–9. doi: 10.1111/cup.12041. [DOI] [PubMed] [Google Scholar]
- 34.Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: an update. J Am Acad Dermatol. 2008;58:545–70. doi: 10.1016/j.jaad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Schulmeister L. Extravasation management: clinical update. Semin Oncol Nurs. 2011;27:82–90. doi: 10.1016/j.soncn.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Perez Fidalgo JA, Garcia FL, Cervantes A. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(7):vii167–73. doi: 10.1093/annonc/mds294. [DOI] [PubMed] [Google Scholar]
- 37.Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999;40:367–98. doi: 10.1016/s0190-9622(99)70488-3. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez T. Chemotherapy extravasations: prevention, identification, management, and documentation. Clin J Oncol Nurs. 2013;17:61–6. doi: 10.1188/13.CJON.61-66. [DOI] [PubMed] [Google Scholar]
- 39.Araujo JC, Trudel GC, Saad F, et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer READY: a randomised, double-blind phase 3 trial. Lancet Oncol. 2013;14:1307–16. doi: 10.1016/S1470-2045(13)70479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524–9. doi: 10.1016/j.jaad.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Childress J, Lokich J. Cutaneous hand and foot toxicity associated with cancer chemotherapy. Am J Clin Oncol. 2003;26:435–6. doi: 10.1097/01.coc.0000026486.56886.18. [DOI] [PubMed] [Google Scholar]
- 42.Spicknall KE, Mutasim DF. Localized toxic erythema of chemotherapy during treatment with paclitaxel. Int J Dermatol. 2014;53:e3–5. doi: 10.1111/j.1365-4632.2012.05573.x. [DOI] [PubMed] [Google Scholar]
- 43.Rosen AC, Balagula Y, Raisch DW, et al. Life-threatening dermatologic adverse events in oncology. Anticancer Drugs. 2014;25:225–34. doi: 10.1097/CAD.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moisidis C, Mobus V. Erythema multiforme major following docetaxel. Arch Gynecol Obstet. 2005;271:267–9. doi: 10.1007/s00404-004-0643-9. [DOI] [PubMed] [Google Scholar]
- 45.Dourakis SP, Sevastianos VA, Alexopoulou A, et al. Treatment side effects. Case 2. Toxic, epidermal, necrolysis-like reaction associated with docetaxel chemotherapy. J Clin Oncol. 2002;20:3030–2. doi: 10.1200/JCO.2002.20.13.3030. [DOI] [PubMed] [Google Scholar]
- 46.Hiraki A, Aoe K, Murakami T, Maeda T, Eda R, Takeyama H. Stevens-Johnson syndrome induced by paclitaxel in a patient with squamous cell carcinoma of the lung: a case report. Anticancer Res. 2004;24:1135–7. [PubMed] [Google Scholar]
- 47.Sawada Y, Sugita K, Kabashima R, et al. Docetaxel-induced Stevens-Johnson syndrome with regenerating epidermis composed of atypical keratinocytes. J Eur Acad Dermatol Venereol. 2009;23:1333–5. doi: 10.1111/j.1468-3083.2009.03183.x. [DOI] [PubMed] [Google Scholar]
- 48.Adachi A, Horikawa T. Paclitaxel-induced cutaneous lupus erythematosus in patients with serum anti-SSA/Ro antibody. J Dermatol. 2007;34:473–6. doi: 10.1111/j.1346-8138.2007.00313.x. [DOI] [PubMed] [Google Scholar]
- 49.Lowe G, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164:465–72. doi: 10.1111/j.1365-2133.2010.10110.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen M, Crowson AN, Woofter M, et al. Docetaxel (taxotere) induced subacute cutaneous lupus erythematosus: report of 4 cases. J Rheumatol. 2004;31:818–20. [PubMed] [Google Scholar]
- 51.Marchetti MA, Noland MM, Dillon PM, et al. Taxane associated subacute cutaneous lupus erythematosus. Dermatol Online J. 2013;19:19259. [PubMed] [Google Scholar]
- 52.Wong NY, Parsons LM, Trotter MJ, et al. Drug-induced subacute cutaneous lupus erythematosus associated with docetaxel chemotherapy: a case report. BMC Res Notes. 2014;7:785. doi: 10.1186/1756-0500-7-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang C, Gershwin ME. Drug-induced lupus erythematosus: incidence, management and prevention. Drug Saf. 2011;34:357–74. doi: 10.2165/11588500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 54.Lamond NW, Younis T, Purdy K, et al. Drug-induced subacute cutaneous lupus erythematosus associated with nab-paclitaxel therapy. Curr Oncol. 2013;20:e484–7. doi: 10.3747/co.20.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferreira O, Baudrier T, Mota A, et al. Docetaxel-induced acral erythema and nail changes distributed to photoexposed areas. Cutan Ocul Toxicol. 2010;29:296–9. doi: 10.3109/15569527.2010.498397. [DOI] [PubMed] [Google Scholar]
- 56.Tokunaga M, Iga N, Endo Y, et al. Elevated protoporphyrin in patients with skin cancer receiving taxane chemotherapy. Eur J Dermatol. 2013;23:826–9. doi: 10.1684/ejd.2013.2235. [DOI] [PubMed] [Google Scholar]
- 57.Cohen PR. Photodistributed erythema multiforme: paclitaxel-related, photosensitive conditions in patients with cancer. J Drugs Dermatol. 2009;8:61–4. [PubMed] [Google Scholar]
- 58.Ji YZ, Geng L, Qu HM, et al. Acute generalized exanthematous pustulosis induced by docetaxel. Int J Dermatol. 2011;50:763–5. doi: 10.1111/j.1365-4632.2009.04434.x. [DOI] [PubMed] [Google Scholar]
- 59.Weinberg JM, Egan CL, Tangoren IA, et al. Generalized pustular dermatosis following paclitaxel therapy. Int J Dermatol. 1997;36:559–60. doi: 10.1111/j.1365-4362.1997.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 60.Kim SW, Lee UH, Jang SJ, et al. Acute localized exanthematous pustulosis induced by docetaxel. J Am Acad Dermatol. 2010;63:e44–6. doi: 10.1016/j.jaad.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Belda-Iniesta C, Casado E, Corral DLC, Castelo B, Baron MG. Re: Folliculitis associated with weekly paclitaxel treatment. J Natl Cancer Inst. 2003;95:410. doi: 10.1093/jnci/95.5.410. [DOI] [PubMed] [Google Scholar]
- 62.Burris HA, 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227–37. doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212–6. doi: 10.1097/00001622-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Borgia F, Guarneri C, Guarneri F, et al. Radiation recall dermatitis after docetaxel administration: absolute indication to replace the drug? Br J Dermatol. 2005;153:674–5. doi: 10.1111/j.1365-2133.2005.06801.x. [DOI] [PubMed] [Google Scholar]
- 65.Morkas M, Fleming D, Hahl M. Challenges in oncology. Case 2. Radiation recall associated with docetaxel. J Clin Oncol. 2002;20:867–9. doi: 10.1200/JCO.2002.20.3.867. [DOI] [PubMed] [Google Scholar]
- 66.Mizumoto M, Harada H, Asakura H, et al. Frequency and characteristics of docetaxel-induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys. 2006;66:1187–91. doi: 10.1016/j.ijrobp.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 67.Phillips KA, Urch M, Bishop JF. Radiation-recall dermatitis in a patient treated with paclitaxel. J Clin Oncol. 1995;13:305. doi: 10.1200/JCO.1995.13.1.305. [DOI] [PubMed] [Google Scholar]
- 68.Wyatt AJ, Leonard GD, Sachs DL. Cutaneous reactions to chemotherapy and their management. Am J Clin Dermatol. 2006;7:45–63. doi: 10.2165/00128071-200607010-00005. [DOI] [PubMed] [Google Scholar]
- 69.Sanborn RE, Sauer DA. Cutaneous reactions to chemotherapy: commonly seen, less described, little understood. Dermatol Clin. 2008;26:103–19. ix. doi: 10.1016/j.det.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Koppel RA, Boh EE. Cutaneous reactions to chemotherapeutic agents. Am J Med Sci. 2001;321:327–35. doi: 10.1097/00000441-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Hood AF. Cutaneous side effects of cancer chemotherapy. Med Clin North Am. 1986;70:187–209. doi: 10.1016/s0025-7125(16)30976-2. [DOI] [PubMed] [Google Scholar]
- 72.Droitcourt C, Le Ho H, Adamski H, et al. Docetaxel-induced photo-recall phenomenon. Photodermatol Photoimmunol Photomed. 2012;28:222–3. doi: 10.1111/j.1600-0781.2012.00669.x. [DOI] [PubMed] [Google Scholar]
- 73.Ee HL, Yosipovitch G. Photo recall phenomenon: an adverse reaction to taxanes. Dermatology. 2003;207:196–8. doi: 10.1159/000071795. [DOI] [PubMed] [Google Scholar]
- 74.Kazandjieva J, Gergovska M, Darlenski R, et al. Recall dermatitis after systemic treatment with paclitaxel. Int J Dermatol. 2010;49:956–9. doi: 10.1111/j.1365-4632.2010.04499.x. [DOI] [PubMed] [Google Scholar]
- 75.Corazza M, Minghetti S, Borghi A, et al. Hand-foot syndrome caused by docetaxel with no recurrence after switch to paclitaxel, a different taxane. Int J Dermatol. 2014;53:e180–2. doi: 10.1111/j.1365-4632.2012.05782.x. [DOI] [PubMed] [Google Scholar]
- 76.Eich D, Scharffetter-Kochanek K, Eich HT, et al. Acral erythrodysesthesia syndrome caused by intravenous infusion of docetaxel in breast cancer. Am J Clin Oncol. 2002;25:599–602. doi: 10.1097/00000421-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 77.Stravodimou A, Voutsadakis IA. Hand and foot syndrome associated with docetaxel treatment. Acta Oncol. 2012;51:554–6. doi: 10.3109/0284186X.2011.636755. [DOI] [PubMed] [Google Scholar]
- 78.Harris CS, Wang D, Carulli A. Docetaxel-associated palmarplantar erythrodysesthesia: a case report and review of the literature. J Oncol Pharm Pract. 2014;20:73–80. doi: 10.1177/1078155213475466. [DOI] [PubMed] [Google Scholar]
- 79.Bardia A, Loprinzi CL, Goetz MP. Hand-foot syndrome after dose-dense adjuvant chemotherapy for breast cancer: a case series. J Clin Oncol. 2006;24:e18–9. doi: 10.1200/JCO.2006.06.1143. [DOI] [PubMed] [Google Scholar]
- 80.Kawaguchi K, Ishiguro H, Morita S, et al. Correlation between docetaxel-induced skin toxicity and the use of steroids and H(2) blockers: a multi-institution survey. Breast Cancer Res Treat. 2011;130:627–34. doi: 10.1007/s10549-011-1641-9. [DOI] [PubMed] [Google Scholar]
- 81.Housholder AL, Adams BB. Chemotherapy-induced acral erythema sparing the palms. J Am Acad Dermatol. 2012;67:e116–7. doi: 10.1016/j.jaad.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 82.de Argila D, Dominguez JD, Iglesias L. Taxol-induced acral erythema. Dermatology. 1996;192:377–8. doi: 10.1159/000246419. [DOI] [PubMed] [Google Scholar]
- 83.Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced handfoot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71:787–94. doi: 10.1016/j.jaad.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 84.Nagore E, Insa A, Sanmartin O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (“hand-foot”) syndrome. Incidence, recognition and management. Am J Clin Dermatol. 2000;1:225–34. doi: 10.2165/00128071-200001040-00004. [DOI] [PubMed] [Google Scholar]
- 85.Chew L, Chuen VS. Cutaneous reaction associated with weekly docetaxel administration. J Oncol Pharm Pract. 2009;15:29–34. doi: 10.1177/1078155208096111. [DOI] [PubMed] [Google Scholar]
- 86.Sibaud V, Dalenc F, Chevreau C, et al. HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist. 2011;16:1469–78. doi: 10.1634/theoncologist.2011-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scotte F, Tourani JM, Banu E, et al. Multicenter study of a frozen glove to prevent docetaxel-induced onycholysis and cutaneous toxicity of the hand. J Clin Oncol. 2005;23:4424–9. doi: 10.1200/JCO.2005.15.651. [DOI] [PubMed] [Google Scholar]
- 88.Chen M, Zhang L, Wang Q, et al. Pyridoxine for prevention of hand-foot syndrome caused by chemotherapy: a systematic review. PLoS One. 2013;8:e72245. doi: 10.1371/journal.pone.0072245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Macedo LT, Lima JP, Dos Santos LV, et al. Prevention strategies for chemotherapy-induced hand-foot syndrome: a systematic review and meta-analysis of prospective randomised trials. Support Care Cancer. 2014;22:1585–93. doi: 10.1007/s00520-014-2129-z. [DOI] [PubMed] [Google Scholar]
- 90.Correia O, Azevedo C, Pinto FE, et al. Nail changes secondary to docetaxel Taxotere. Dermatology. 1999;198:288–90. doi: 10.1159/000018132. [DOI] [PubMed] [Google Scholar]
- 91.Chu CY, Yang CH, Yang CY, et al. Fixed erythrodysaesthesia plaque due to intravenous injection of docetaxel. Br J Dermatol. 2000;142:808–11. doi: 10.1046/j.1365-2133.2000.03432.x. [DOI] [PubMed] [Google Scholar]
- 92.Sibaud V, Fricain JC, Baran R, et al. Pigmentary disorders induced by anticancer agents. Part I: chemotherapy. Ann Dermatol Venereol. 2013;140:183–96. doi: 10.1016/j.annder.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 93.Baykal C, Erkek E, Tutar E, et al. Cutaneous fixed drug eruption to paclitaxel; a case report. Eur J Gynaecol Oncol. 2000;21:190–1. [PubMed] [Google Scholar]
- 94.Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16. [PubMed] [Google Scholar]
- 95.Schrijvers D, Van Den BJ, Vermorken JB. Supravenous discoloration of the skin due to docetaxel treatment. Br J Dermatol. 2000;142:1069–70. doi: 10.1046/j.1365-2133.2000.03518.x. [DOI] [PubMed] [Google Scholar]
- 96.Aydogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345–7. doi: 10.1111/j.1468-3083.2005.01088.x. [DOI] [PubMed] [Google Scholar]
- 97.Masson Regnault M, Gadaud N, Boulinguez S, et al. Chemotherapy-related reticulate hyperpigmentation: a case series and review of the literature. Dermatology. 2015;231:312–8. doi: 10.1159/000439047. [DOI] [PubMed] [Google Scholar]
- 98.Semb KA, Aamdal S, Oian P. Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J Clin Oncol. 1998;16:3426–32. doi: 10.1200/JCO.1998.16.10.3426. [DOI] [PubMed] [Google Scholar]
- 99.Behar A, Pujade-Lauraine E, Maurel A, et al. The pathophysiological mechanism of fluid retention in advanced cancer patients treated with docetaxel, but not receiving corticosteroid comedication. Br J Clin Pharmacol. 1997;43:653–8. doi: 10.1046/j.1365-2125.1997.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ellis P, Barrett-Lee P, Johnson L, et al. Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an openlabel, phase III, randomised controlled trial. Lancet. 2009;373:1681–92. doi: 10.1016/S0140-6736(09)60740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Von Hoff DD. The taxoids: same roots, different drugs. Semin Oncol. 1997;24:S13. [PubMed] [Google Scholar]
- 102.Ohsumi S, Shimozuma K, Ohashi Y, et al. Subjective and objective assessment of edema during adjuvant chemotherapy for breast cancer using taxane-containing regimens in a randomized controlled trial: The National Surgical Adjuvant Study of Breast Cancer 02. Oncology. 2012;82:131–8. doi: 10.1159/000336480. [DOI] [PubMed] [Google Scholar]
- 103.Kupfer I, Balguerie X, Courville P, et al. Scleroderma-like cutaneous lesions induced by paclitaxel: a case study. J Am Acad Dermatol. 2003;48:279–81. doi: 10.1067/mjd.2003.30. [DOI] [PubMed] [Google Scholar]
- 104.Konishi Y, Sato H, Sato N, et al. Scleroderma-like cutaneous lesions induced by paclitaxel and carboplatin for ovarian carcinoma, not a single course of carboplatin, but re-induced and worsened by previously administrated paclitaxel. J Obstet Gynaecol Res. 2010;36:693–6. doi: 10.1111/j.1447-0756.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- 105.Lauchli S, Trueb RM, Fehr M, et al. Scleroderma-like drug reaction to paclitaxel Taxol. Br J Dermatol. 2002;147:619–21. doi: 10.1046/j.1365-2133.2002.488210.x. [DOI] [PubMed] [Google Scholar]
- 106.Itoh M, Yanaba K, Kobayashi T, et al. Taxane-induced scleroderma. Br J Dermatol. 2007;156:363–7. doi: 10.1111/j.1365-2133.2006.07597.x. [DOI] [PubMed] [Google Scholar]
- 107.Battafarano DF, Zimmerman GC, Older SA, et al. Docetaxel (Taxotere) associated scleroderma-like changes of the lower extremities. A report of three cases. Cancer. 1995;76:110–5. doi: 10.1002/1097-0142(19950701)76:1<110::aid-cncr2820760117>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 108.Farrant PB, Mortimer PS, Gore M. Scleroderma and the taxanes. Is there really a link? Clin Exp Dermatol. 2004;29:360–2. doi: 10.1111/j.1365-2230.2004.01519.x. [DOI] [PubMed] [Google Scholar]
- 109.Pedersen JV, Jensen S, Krarup-Hansen A, et al. Scleroderma induced by paclitaxel. Acta Oncol. 2010;49:866–8. doi: 10.3109/02841861003702510. [DOI] [PubMed] [Google Scholar]
- 110.Trueb RM. Chemotherapy-induced hair loss. Skin Therapy Lett. 2010;15:5–7. [PubMed] [Google Scholar]
- 111.Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333–6. doi: 10.1016/j.jaad.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 112.Trueb RM. Chemotherapy-induced anagen effluvium: diffuse or patterned? Dermatology. 2007;215:1–2. doi: 10.1159/000102025. [DOI] [PubMed] [Google Scholar]
- 113.Paus R, Haslam IS, Sharov AA, et al. Pathobiology of chemotherapy-induced hair loss. Lancet Oncol. 2013;14:e50–9. doi: 10.1016/S1470-2045(12)70553-3. [DOI] [PubMed] [Google Scholar]
- 114.Palamaras I, Misciali C, Vincenzi C, et al. Permanent chemotherapy-induced alopecia: a review. J Am Acad Dermatol. 2011;64:604–6. doi: 10.1016/j.jaad.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 115.Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345–50. doi: 10.1097/DAD.0b013e3181fcfc25. [DOI] [PubMed] [Google Scholar]
- 116.Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879–84. doi: 10.1093/annonc/mds095. [DOI] [PubMed] [Google Scholar]
- 117.Bourgeois H, Denis F, Kerbrat P, et al. Long-term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69:s703–4. [Google Scholar]
- 118.Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883–5. doi: 10.1111/j.1365-2133.2009.09043.x. [DOI] [PubMed] [Google Scholar]
- 119.Tosti A, Palamaras I, Miteva M, et al. Docetaxel and permanent alopecia. J Am Acad Dermatol. 2013;68:e151. doi: 10.1016/j.jaad.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 120.Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126–34. doi: 10.1634/theoncologist.2013-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mols F, van den Hurk CJ, Vingerhoets AJ, et al. Scalp cooling to prevent chemotherapy-induced hair loss: practical and clinical considerations. Support Care Cancer. 2009;17:181–9. doi: 10.1007/s00520-008-0475-4. [DOI] [PubMed] [Google Scholar]
- 122.van den Hurk CJ, Peerbooms M, van de Poll-Franse LV, et al. Scalp cooling for hair preservation and associated characteristics in 1411 chemotherapy patients - results of the Dutch Scalp Cooling Registry. Acta Oncol. 2012;51:497–504. doi: 10.3109/0284186X.2012.658966. [DOI] [PubMed] [Google Scholar]
- 123.van den Hurk CJ, Breed WP, Nortier JW. Short post-infusion scalp cooling time in the prevention of docetaxel-induced alopecia. Support Care Cancer. 2012;20:3255–60. doi: 10.1007/s00520-012-1465-0. [DOI] [PubMed] [Google Scholar]
- 124.Betticher DC, Delmore G, Breitenstein U, et al. Efficacy and tolerability of two scalp cooling systems for the prevention of alopecia associated with docetaxel treatment. Support Care Cancer. 2013;21:2565–73. doi: 10.1007/s00520-013-1804-9. [DOI] [PubMed] [Google Scholar]
- 125.Komen MM, Smorenburg CH, van den Hurk CJ, et al. Factors influencing the effectiveness of scalp cooling in the prevention of chemotherapy-induced alopecia. Oncologist. 2013;18:885–91. doi: 10.1634/theoncologist.2012-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432–42. doi: 10.1111/j.1529-8019.2011.01430.x. [DOI] [PubMed] [Google Scholar]
- 127.Lotfi-Jam K, Carey M, Jefford M, et al. Nonpharmacologic strategies for managing common chemotherapy adverse effects: a systematic review. J Clin Oncol. 2008;26:5618–29. doi: 10.1200/JCO.2007.15.9053. [DOI] [PubMed] [Google Scholar]
- 128.Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13–4. doi: 10.1188/09.CJON.13-14. [DOI] [PubMed] [Google Scholar]
- 129.Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73–6. doi: 10.1038/jidsymp.2013.30. [DOI] [PubMed] [Google Scholar]
- 130.Minisini AM, Tosti A, Sobrero AF, et al. Taxane-induced nail changes: incidence, clinical presentation and outcome. Ann Oncol. 2003;14:333–7. doi: 10.1093/annonc/mdg050. [DOI] [PubMed] [Google Scholar]
- 131.Matsumoto K, Hino C, Fukada K, et al. Prospective study of ice gel pack as less expensive alternative for prevention of skin and nail toxicity in patients with breast cancer receiving docetaxel. Cancer Res. 2009;69 Abstract 1114. [Google Scholar]
- 132.Can G, Aydiner A, Cavdar I. Taxane-induced nail changes: predictors and efficacy of the use of frozen gloves and socks in the prevention of nail toxicity. Eur J Oncol Nurs. 2012;16:270–5. doi: 10.1016/j.ejon.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 133.Winther D, Saunte DM, Knap M, et al. Nail changes due to docetaxel-a neglected side effect and nuisance for the patient. Support Care Cancer. 2007;15:1191–7. doi: 10.1007/s00520-007-0232-0. [DOI] [PubMed] [Google Scholar]
- 134.Capriotti K, Capriotti JA, Lessin S, et al. The risk of nail changes with taxane chemotherapy: a systematic review of the literature and meta-analysis. Br J Dermatol. 2015;173:842–5. doi: 10.1111/bjd.13743. [DOI] [PubMed] [Google Scholar]
- 135.Hussain S, Anderson DN, Salvatti ME, et al. Onycholysis as a complication of systemic chemotherapy: report of five cases associated with prolonged weekly paclitaxel therapy and review of the literature. Cancer. 2000;88:2367–71. doi: 10.1002/(sici)1097-0142(20000515)88:10<2367::aid-cncr22>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 136.Gilbar P, Hain A, Peereboom VM. Nail toxicity induced by cancer chemotherapy. J Oncol Pharm Pract. 2009;15:143–55. doi: 10.1177/1078155208100450. [DOI] [PubMed] [Google Scholar]
- 137.Sibaud V, Robert C, Mateus C, et al. Nail toxicities induced by systemic anticancer agents. Lancet Oncol. 2015;16:e181–9. doi: 10.1016/S1470-2045(14)71133-7. [DOI] [PubMed] [Google Scholar]
- 138.Mackay-Wiggan J, Nair KG, Halasz CL. Onycholysis associated with paclitaxel. Cutis. 2003;71:229–32. [PubMed] [Google Scholar]
- 139.Mourad YA, Matta-Muallem M, Shamseddine A. Nail toxicity related to taxanes. Dermatol Online J. 2003;9:15. [PubMed] [Google Scholar]
- 140.Obermair A, Binder M, Barrada M, et al. Onycholysis in patients treated with docetaxel. Ann Oncol. 1998;9:230–1. doi: 10.1023/a:1008218824342. [DOI] [PubMed] [Google Scholar]
- 141.Pavithran K, Doval DC. Nail changes due to docetaxel. Br J Dermatol. 2002;146:709–10. doi: 10.1046/j.1365-2133.2002.46824.x. [DOI] [PubMed] [Google Scholar]
- 142.Scotte F, Banu E, Medioni J, et al. Matched case-control phase 2 study to evaluate the use of a frozen sock to prevent docetaxel-induced onycholysis and cutaneous toxicity of the foot. Cancer. 2008;112:1625–31. doi: 10.1002/cncr.23333. [DOI] [PubMed] [Google Scholar]
- 143.Ghetti E, Piraccini BM, Tosti A. Onycholysis and subungual haemorrhages secondary to systemic chemotherapy (paclitaxel) J Eur Acad Dermatol Venereol. 2003;17:459–60. doi: 10.1046/j.1468-3083.2003.00774.x. [DOI] [PubMed] [Google Scholar]
- 144.Spazzapan S, Crivellari D, Lombardi D, et al. Nail toxicity related to weekly taxanes: an important issue requiring a change in common toxicity criteria grading? J Clin Oncol. 2002;20:4404–5. doi: 10.1200/JCO.2002.99.147. [DOI] [PubMed] [Google Scholar]
- 145.Almagro M, Pozo JD, Garcia J, et al. Nail alterations secondary to pactitaxel therapy. Eur J Dermatol. 2000;10:146–7. [PubMed] [Google Scholar]
- 146.Wasner G, Hilpert F, Baron R, et al. Clinical picture: nail changes secondary to docetaxel. Lancet. 2001;357:910. doi: 10.1016/s0140-6736(00)04210-0. [DOI] [PubMed] [Google Scholar]
- 147.Speck RM, DeMichele A, Farrar JT, et al. Taste alteration in breast cancer patients treated with taxane chemotherapy: experience, effect, and coping strategies. Support Care Cancer. 2013;21:549–55. doi: 10.1007/s00520-012-1551-3. [DOI] [PubMed] [Google Scholar]
- 148.Morrow PK, Mattair DN, Hortobagyi GN. Hot flashes: a review of pathophysiology and treatment modalities. Oncologist. 2011;16:1658–64. doi: 10.1634/theoncologist.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Makdsi F, Deversa R. Inflammation of actinic keratosis with combination of alkylating and taxane agents: a case report. Cases J. 2009;2:6946. doi: 10.4076/1757-1626-2-6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fabbrocini G, Cameli N, Romano MC, et al. Chemotherapy and skin reactions. J Exp Clin Cancer Res. 2012;31:50. doi: 10.1186/1756-9966-31-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ehrlich A, Booher S, Becerra Y, et al. Micellar paclitaxel improves severe psoriasis in a prospective phase II pilot study. J Am Acad Dermatol. 2004;50:533–40. doi: 10.1016/j.jaad.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 152.Halverstam CP, Lebwohl M. Nonstandard and off-label therapies for psoriasis. Clin Dermatol. 2008;26:546–53. doi: 10.1016/j.clindermatol.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 153.Kerob D, Le Maignan C, Vignon-Pennamen MD, et al. Docetaxel-induced psoriasis. Ann Dermatol Venereol. 2010;137:738–40. doi: 10.1016/j.annder.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 154.Cecco S, Aliberti M, Baldo P, et al. Safety and efficacy evaluation of albumin-bound paclitaxel. Expert Opin Drug Saf. 2014;13:511–20. doi: 10.1517/14740338.2014.893293. [DOI] [PubMed] [Google Scholar]
- 155.Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055–62. doi: 10.1200/JCO.2011.39.5848. [DOI] [PubMed] [Google Scholar]
- 156.Ko YJ, Canil CM, Mukherjee SD, et al. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol. 2013;14:769–76. doi: 10.1016/S1470-2045(13)70162-1. [DOI] [PubMed] [Google Scholar]
- 157.Yamamoto Y, Kawano I, Iwase H. Nab-paclitaxel for the treatment of breast cancer: efficacy, safety, and approval. Onco Targets Ther. 2011;4:123–36. doi: 10.2147/OTT.S13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang LC, Wang BY, Sun S, et al. Higher rate of skin rash in a phase II trial with weekly nanoparticle albumin-bound paclitaxel and cisplatin combination in Chinese breast cancer patients. BMC Cancer. 2013;13:232. doi: 10.1186/1471-2407-13-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23:7794–803. doi: 10.1200/JCO.2005.04.937. [DOI] [PubMed] [Google Scholar]
- 160.Takashima S, Kiyoto S, Takahashi M, et al. Clinical experience with nanoparticle albumin-bound paclitaxel, a novel taxane anticancer agent, and management of adverse events in females with breast cancer. Oncol Lett. 2015;9:1822–6. doi: 10.3892/ol.2015.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Beutler BD, Cohen PR. Nab-paclitxel-associated photosensitivity: report in a woman with non-small lung cancer and review of taxane-related photodermatoses. Dermatol Pract Concept. 2015;5:121–4. doi: 10.5826/dpc.0502a24. [DOI] [PMC free article] [PubMed] [Google Scholar]