Abstract
The aim of the present study was to investigate the protective effect of salvianolic acid A (SAA) on myocardial ischemia/reperfusion injury in rats. SAA (10 mg/kg) or Tirofiban (60 µg/kg) was administered to rats by jugular vein injection 10 min before the initiation of reperfusion. After 3 h of reperfusion, platelet aggregation was measured using an aggregometer and levels of nitric oxide (NO) were detected using an ultraviolet spectrophotometer. Serum levels of cardiac troponin T (cTnT), creatine kinase isoenzyme MB (CK-MB), p-selectin, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were also measured 3 and 24 h after reperfusion. Furthermore, morphology of the ischemic myocardium was histopathologically analyzed by hematoxylin and eosin staining, and the infarct area was evaluated by Evans blue and triphenyltetrazolium chloride staining. In rats subjected to reperfusion, it was observed that pretreatment with SAA significantly increased the survival rate (P<0.05), and that increased survival rate was due to a significant decrease in infarct size, as evidenced by significantly reduced serum levels of cTnT and CK-MB (P<0.05). In addition, decreases in infarct size occurred through the inhibition of platelet aggregation and inflammation associated with reperfusion-induced myocardial cell damage, as indicated by reduced serum levels of p-selectin, TNF-α, IL-1β and NO. In conclusion, SAA was protective against myocardial ischemia/reperfusion injury in rats by serving antiplatelet and anti-inflammation roles.
Keywords: salvianolic acid A, myocardial ischemia reperfusion, infarct area, platelet aggregation rate, inflammation
Introduction
Acute myocardial infarction (AMI) is a major public health problem. In 2010, age-standardized AMI incidence in all ages was 195.3 per 100,000 in males and 115.0 in females (1). In Europe, ~1.8 million individuals succumb to coronary heart disease each year (2). Reperfusion of the ischemic myocardium is necessary to salvage tissue and limit cardiomyocyte death. While reperfusion is beneficial, it also induces new pathophysiological changes through the release of oxygen free radicals, cytokines and other pro-inflammatory factors that initiate an inflammatory cascade. This inflammation typically enhances damage to myocytes and induces myocardial dysfunction (3–5). Platelets serve key roles in myocardial reperfusion injury, including platelet adhesion, aggregation, activation and inhibiting the platelet activation, which reduces myocardial infarct size (6). Furthermore, they induce new thrombus formation and microembolization at sites of atherosclerotic plaques in coronary arteries, and facilitate interactions between the endothelium and leukocytes, consequently prompting microcirculation disturbance and inflammation (7–9). Suppression of platelet activation using Tirofiban to inhibit glycoprotein IIb/IIIa may alleviate ischemia-reperfusion (I/R) injury (10,11).
The Traditional Chinese Medicine Salvia miltiorrhiza is widely used in the treatment of coronary artery disease and cerebrovascular diseases and as a remedy to improve microcirculation (12,13). Salvianolic acid A (SAA), as a water-soluble polyphenolic compound, is a primary constituent of Salvia miltiorrhiza and is extracted from Salvia miltiorrhiza Bunge (14). A previous study by our group revealed that SAA dose-dependently inhibited platelet aggregation induced by adenosine diphosphate (ADP), thrombin, collagen and U46619, and inhibited arterial thrombosis via the inhibition of phosphoinositide 3-kinase in vitro (15). In the present study, the cardioprotective effects of SAA against platelet activation and inflammation during myocardial I/R injury were investigated by ligating the left anterior descending branch of coronary artery. Results indicated that the cardioprotective effects of SAA were comparable to those of the positive control agent, Tirofiban.
Materials and methods
Animals
A total of 150, male Sprague-Dawley rats (aged 6–8 weeks and weighing 250–300 g) were purchased from the Zhejiang Laboratory Animal Centre, China [certificate no. SCXL (Zhe) 2008–0033]. Animals were acclimated for at least 1 week at room temperature (18–25°C), 55±5% humidity and a 12-h light/dark cycle. The animals were given free access to a standard diet and tap water. All experimental procedures were approved by the Ethics Review of Animal Use Application of Fifth Affiliated Hospital of Wenzhou Medical University (Zhejiang, China).
Animal groups and drug pretreatment
All animals were randomly assigned to one of four groups. Each group was divided into n1 and n2 subgroups and exposed to reperfusion for 3 and 24 h, respectively. The model control group (I/R; n1=10, n2=30) underwent I/R and received an intravenous administration of glucose (5%), while the SAA group (I/R + SAA, n1=10, n2=30) underwent I/R and received an intravenous administration of SAA (10 mg/kg; Zhengda Qingchunbao Pharmaceutical Co., Ltd., Zhejiang, China) dissolved in 5% glucose. The Tirofiban group (I/R + Tirofiban, n1=10, n2=30) underwent I/R and received an intravenous administration of Tirofiban (60 µg/kg; Wuhan Grand Pharmaceutical Co., Ltd., Wuhan, China) dissolved in 5% glucose. The sham group (sham; n1=10, n2=20) received an intravenous administration of glucose (5%) alone. All drugs were administered intravenously 10 min before reperfusion was initiated.
Myocardial I/R model protocol
Rats were anesthetized with an intraperitoneal injection of 4% chloral hydrate (400 mg/kg). Following intubation, a left thoracotomy was performed. The left anterior descending coronary artery (LAD) was surgically occluded for 45 min through ligation of a silk suture, and coronary occlusion was confirmed by elevation of the ST segment (>0.1 mV) on an electrocardiogram (MedLab-U/4C50; Nanjing Medease Science and Technology Co., Ltd., Nanjing, China). Reperfusion of the coronary artery was initiated by release of the ligation tie. Following the procedure, the chest was closed and rats in each group; n1 and n2 were monitored in the animal facility for 3 and 24 h, respectively, anesthetized with an intraperitoneal injection of 4% chloral hydrate (400 mg/kg), prior to sacrifice with 10% potassium chloride solution (400 mg/kg; 1–2 ml) administered to the inferior vena cava. All groups underwent the same surgical procedure, though the LAD suture was not tied for rats in the sham group.
Measurement of myocardial infarct size
Myocardial infarct size was evaluated by Evans blue and 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) staining (both from Sigma-Aldrich, Inc.; Merck KGaA, Darmstadt, Germany), as described previously (16). Briefly, after 24 h of reperfusion, rats were sacrificed and the LAD was occluded with a silk suture in the same location as for the I/R procedure. The abdomen was opened and 5 ml of 1% Evans blue dye was injected into the aorta. The heart was immediately excised and the right ventricle and left and right atria were removed. The left ventricle was transversally cut into 1 mm thick slices and incubated in 1% TTC at 37°C for 10 min. Evans blue was used to stain the area outside of the risk area (RA), and the area unstained by TTC represented the ischemic area. The infarct area (ischemic area), RA and left ventricle wall size were also measured digitally using Image J software (version 1.38; National Institutes of Health, Bethesda, MA, USA).
Determination of platelet maximum aggregation rate
Blood was collected from the abdominal aorta in an anticoagulant solution containing 3.8% sodium citrate (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), 1:9 citrate: whole blood) after 3 h of reperfusion. The platelet-rich plasma (PRP) fraction was obtained by centrifugation at 174 × g at 25°C for 10 min, and the remaining blood was further centrifuged at 1,570 × g at 25°C for 10 min to prepare the platelet-poor plasma (PPP) fraction. The platelet concentration, according to a previously described method, using 1×20 l Coulter Isoton® II Dilutent (cat. no. 8546719; Beckman Coulter, Brea, CA, USA) (15), was adjusted to 250×106 platelets/ml and incubated at room temperature for 30 min to allow the platelets to clot. The platelet agonist ADP disodium (5 µM final concentration; Helena Laboratories, Beaumont, TX, USA) was used to stimulate platelet aggregation as a positive control. The level of platelet aggregation was measured using an aggregometer (AggRAM; cat. no. 8JF52001; Helena Laboratories, Beaumont, Texas, USA).
Enzyme-linked immunosorbent assay (ELISA)
After 3 and 24 h of reperfusion, blood samples were collected from the abdominal aorta using 3.8% sodium citrate as the anticoagulant, and samples were centrifuged at 25°C and 3,500 × g for 15 min to isolate the plasma. Quantikine ELISA kits (96Test; Abcam, Cambridge, UK) were used to measure the plasma levels of creatine kinase isoenzyme MB (CK-MB; cat. no. XF020852B), cardiac troponin T (cTnT; cat. no. XF03363B), p-selectin (cat. no. XF03259B), interleukin-1β (IL-1β; cat. no. XF01588B) and tumor necrosis factor-α (TNF-α; cat. no. XF01721B), according to the manufacturer's protocol.
Histological analysis by light microscopy
After 24 h of reperfusion, myocardial samples from the RA were rinsed with PBS (pH 7.4) and fixed in 4% paraformaldehyde and 25°C. After 24 h fixation, tissues were dehydrated in graded alcohol (75, 85 and 95% twice and then 100% twice) at a temperature of 25°C, embedded in paraffin and cut into 3–5 µm thin sections. The tissue sections were then stained with hematoxylin and eosin and histologically examined under a light microscope.
Measurement of myocardial nitric oxide (NO) content
The myocardial NO concentration was measured as described previously (17). Briefly, after 3 h of reperfusion, myocardial samples from the RA were rinsed and homogenized in deionized water (1:10 w/v) prior to centrifugation at 3,000 × g at 25°C for 5 min. The concentration of NO in the supernatant was determined using an NO detection kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the manufacturer's instructions.
Statistical analysis
All data are presented as the mean ± standard error of the mean and were analyzed by one-way analysis of variance, followed by a least-significant-difference test for multiple comparisons. Survival rate was analyzed by Kaplan-Meier analysis with SPSS, version 19.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was determined to represent statistically significant differences following a log-rank test.
Results
SAA treatment reduces myocardial injury and mortality following myocardial I/R
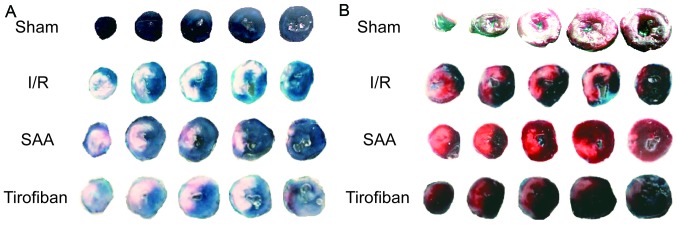
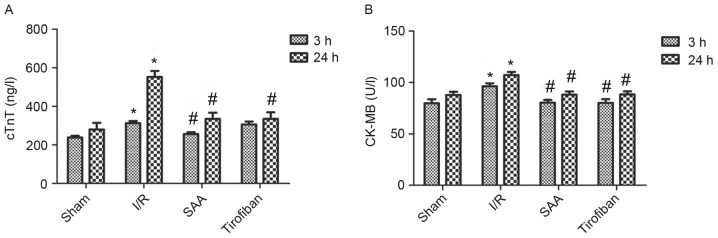
After 24 h of reperfusion, the RAs of the SAA, Tirofiban and control I/R groups were all of a similar size. The infarct areas were significantly reduced in both the SAA and Tirofiban groups compared to the I/R group (both P<0.05; Table I; Fig. 1). Furthermore, survival rates of rats in the sham, I/R, SAA and Tirofiban groups were 100, 61, 82 and 63%, respectively (log-rank test P=0.033 SAA group vs. I/R group; Fig. 2), indicating that rats in the SAA group had an improved survival rate relative to those in the I/R and Tirofiban groups. In addition, after 3 and 24 h of reperfusion, levels of cTnT and CK-MB in the serum were significantly increased in the I/R group, relative to the sham group (both P<0.05). This effect was significantly reversed by pretreatment with SAA or Tirofiban after 24 h reperfusion (all P<0.05; Fig. 3).
Table I.
Effect of SAA and Tirofiban on myocardial I/R injury in rats.
| Group | Dosage | Risk area, % | Infarct area, % |
|---|---|---|---|
| Sham | – | NA | NA |
| I/R | – | 30±2 | 29±5 |
| SAA | 10 mg/kg | 29±4 | 19±11a |
| Tirofiban | 60 µg/kg | 29±3 | 19±4a |
Values are presented as the mean ± standard error of the mean.
P<0.05 vs. I/R. SAA, salvianolic acid A; I/R, ischemia-reperfusion; NA, not applicable.
Figure 1.
Morphological assessment of left ventricles exposed to I/R. Representative Evans blue staining of the left ventricles in the (A) Sham, I/R, SAA and Tirofiban group was used to determine the RA. Normal myocardium was stained blue and the RA was stained white. Representative tetrazolium chloride staining of the left ventricles in the (B) Sham, I/R, SAA and Tirofiban group was used to determine infarct size. Normal myocardium was stained red and the infarct area was stained pale red and/or pink. I/R, ischemia-reperfusion; SAA, salvianolic acid A; RA, risk area.
Figure 2.
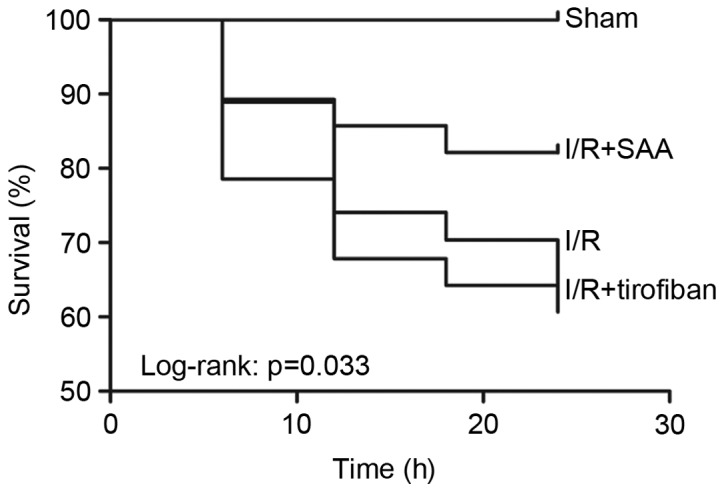
Survival rate 24 h after myocardial ischemia-reperfusion injury. I/R, ischemia-reperfusion; SAA, salvianolic acid A.
Figure 3.
SAA and Tirofiban reduced serum levels of cTnT and CK-MB following myocardial I/R. (A) cTnT and (B) CK-MB activity were quantified by ELISA. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. sham, #P<0.05 vs. I/R. cTnT, cardiac troponin T; CK-MB, creatine kinase isoenzyme MB; I/R, ischemia-reperfusion; SAA, salvianolic acid A.
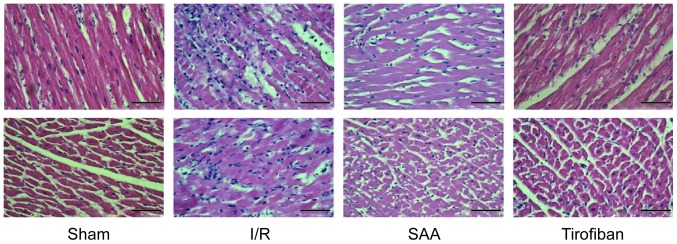
Under light microscopy, the myocardial samples of the I/R group exhibited a disordered myocardial cell arrangement and robust inflammatory cell infiltration, relative to the Sham group. In turn, myocardial injury and inflammatory exudation were attenuated in both the SAA and Tirofiban groups (Fig. 4).
Figure 4.
Cross-sectional and longitudinal sections of hematoxylin and eosin stained myocardial tissue at 3 h after reperfusion. Magnification, ×40. Scale bar, 100 µm. I/R, ischemia-reperfusion; SAA, salvianolic acid A.
SAA treatment inhibits platelet aggregation during myocardial I/R
After 3 h of reperfusion, the maximum rate of platelet aggregation in the I/R group was similar to that in the sham group (P=0.195). This rate was significantly reduced by pretreatment with SAA or Tirofiban (both P<0.05 vs. I/R group). The platelet aggregation rates of the SAA and Tirofiban groups did not differ significantly (P=0.59; Fig. 5).
Figure 5.
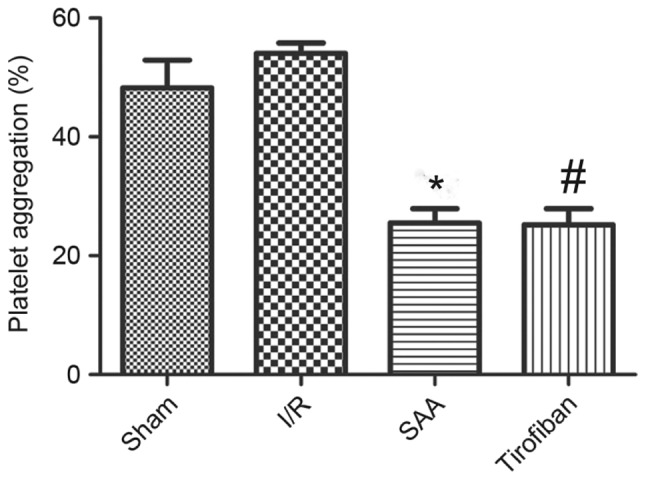
SAA and Tirofiban reduced platelet aggregation induced by ADP stimulation. Platelets were isolated from the blood of sham rats or rats following I/R. Data shown are presented as the mean ± standard error of the mean. *P<0.05 SAA vs. I/R group, #P<0.05 Tirofiban vs. I/R group. SAA, salvianolic acid A; ADP, adenosine diphosphate; I/R, ischemia-reperfusion.
SAA treatment reduces serum levels of p-selectin
After 3 or 24 h of reperfusion, levels of p-selectin in the serum were significantly increased in the I/R group, compared with the sham group (P<0.05). This effect was significantly reversed by pretreatment with SAA or Tirofiban (P<0.05 vs. I/R). Serum levels of p-selectin did not differ significantly between the SAA and Tirofiban groups (P>0.05; Fig. 6).
Figure 6.
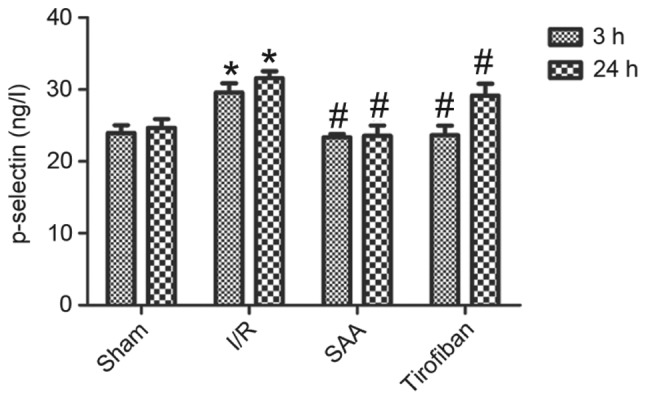
SAA reduced serum levels of p-selectin following myocardial I/R. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. sham; #P<0.05 vs. I/R. SAA, salvianolic acid A; I/R, ischemia-reperfusion.
SAA treatment reduces serum levels of TNF-α and IL-1β
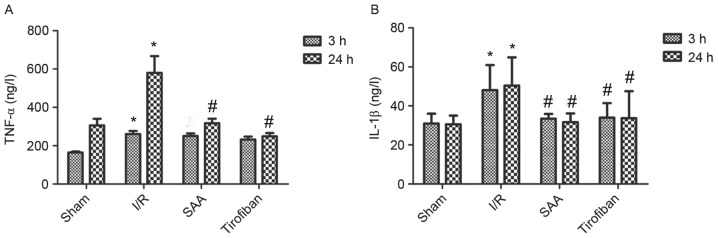
After 24 h of reperfusion, levels of TNF-α in the serum significantly increased in the I/R group compared to the sham group (both P<0.05). In turn, pretreatment with SAA or Tirofiban significantly reduced serum levels of TNF-α after 24 h of reperfusion (both P<0.05; Fig. 7A).
Figure 7.
SAA and Tirofiban reduced serum levels of IL-β and TNF-α following myocardial I/R. (A) TNF-α and (B) IL-1β were quantified by ELISA. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. sham; #P<0.05 vs. I/R. SAA, salvianolic acid A; I/R, ischemia-reperfusion; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α.
Similarly, after 3 or 24 h of reperfusion, levels of IL-1β in the serum were significantly increased in the I/R group, relative to the sham group (both P<0.05). In turn, pretreatment with SAA significantly reduced the serum levels of IL-1β levels (both P<0.05 compared to I/R) such that the serum levels of IL-1β did not differ significantly between the SAA and Tirofiban groups (P>0.05; SAA vs Tirofiban group for 3 or 24 h; Fig. 7B).
SAA treatment increases the NO concentration in myocardial tissue
After 3 h of reperfusion, concentrations of NO in the RA were similar between the I/R and sham groups (P>0.05). Relative to the I/R the SAA group, but not the Tirofiban group, exhibited significantly increased concentrations of NO in the RA (P<0.05; Fig. 8).
Figure 8.
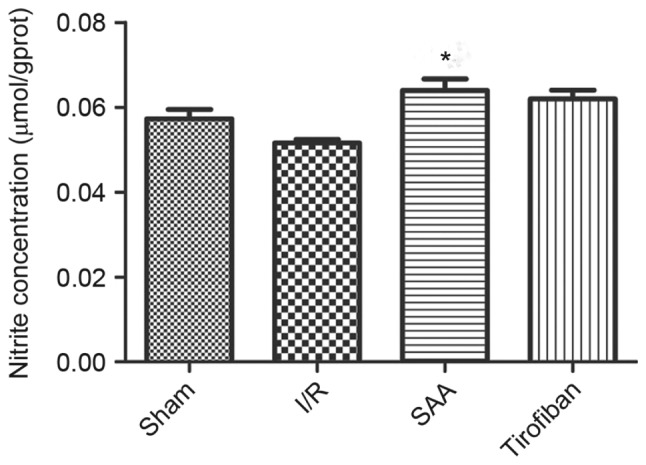
SAA treatment increased NO concentration in the left ventricle following myocardial I/R. Data are presented as the mean ± standard error of the mean. *P<0.05 vs. I/R. SAA, salvianolic acid A; I/R, ischemia-reperfusion.
Discussion
The present study demonstrated that pretreatment with SAA significantly ameliorated cardiac injury and improved the survival rate of rats exposed to I/R. SAA reduced serum levels of cTnT and CK-MB and myocardial infarct size. Furthermore, SAA inhibited ADP-induced platelet aggregation, decreased IL-1β and TNF-α activity, reduced swelling of ischemic myocardial cells and inflammatory cell infiltration, and increased NO synthesis in the area at risk. These observations indicate that SAA may be a potential therapeutic agent in the treatment of myocardial I/R injury.
Platelets serve critical roles in I/R injury. Following reperfusion, platelet contact with subendothelial collagen stimulates platelet adhesion and activation (18). Activated platelets generate a variety of factors that induce a feed forward loop to reinforce the activation process. For instance, thromboxane A2 enhances platelet activation and adhesion, while ADP and thrombin recruit circulating platelets and promote platelet aggregation. Concomitant with these events, tissue factor XIII is activated to promote the formation of clots (both large thrombi and small platelet microthrombi). In the present study, SAA exhibited strong antiplatelet activity, as observed in an in vitro platelet aggregation assay. The in vitro experiments of the present study demonstrated that treatment with SAA significantly decreased ADP-induced platelet aggregation, eliciting an inhibitory effect similar to Tirofiban. Therefore, SAA may reduce coronary thrombus by inhibiting platelet activation and improving coronary blood flow.
The inflammatory response and platelet aggregation have been implicated in myocardial I/R injury (19). Within min of reperfusion, an inflammatory cascade is triggered and numerous pro-inflammatory cytokines are released into the region, including TNF-α, IL-1β, IL-6 and IL-8. These pro-inflammatory cytokines, particularly TNF-α, function as key mediators in cardiac dysfunction, serving to activate endothelial cells and neutrophils and aggravate myocardial I/R injury (20). In addition to regulating thrombosis, SAA modulates the inflammatory process (21). During adhesion, platelets are activated and release a variety of potent chemotactic factors, as previously described (22). Moreover, platelets may modulate the chemotactic properties of other cells through platelet-leukocyte (23) and platelet-endothelium interactions (24).
P-selectin is present on activated platelets and mediates loose contact between circulating platelets and the vascular endothelium. Once platelets are activated during ischemia and reperfusion, p-selectin may be cleaved from the membrane to generate a soluble form that is readily detected in the plasma (25). The present study observed that levels of p-selectin were significantly elevated in the myocardium after 3 and 24 h of reperfusion when compared to the sham group, indicating that platelet activation occurs as a result of I/R. Furthermore, it was observed that SAA decreased levels of serum p-selectin and plasma IL-1β at 3 and 24 h after reperfusion, and reduced plasma levels of TNF-α at 24 h after reperfusion. Reduced cytokine levels are both a cause and consequence of reduced inflammatory cell infiltration. These data suggest that SAA functions to reduce platelet activation and inflammation.
NO is a diatomic free-radical gas that serves key roles in a numerous of biological processes, including inhibited platelet aggregation via a cyclic GMP-dependent mechanism and relaxed vascular endothelium as an endothelium derived relaxation factor (16). Maintaining a basal level of NO is critical for various physiological processes, including vascular smooth muscle cell relaxation, prevention of neutrophil and platelet adhesion to the endothelium, and maintenance of an anti-apoptotic environment in the vessel wall. I/R alters the levels of NO to further aggravate I/R injury (26). Furthermore, it has been documented that Tirofiban increased NO production, and that SAA alleviated impaired expression of endothelial nitric oxide synthase and NO formation in response to I/R (10,27). Similarly, results of the present study indicated that SAA protected endothelial cells by improving NO production within the vessel wall.
SAA has been demonstrated to prevent I/R-induced myocardial damage by reducing cardiomyocyte necrosis and apoptosis (28,29). The present study revealed that SAA and Tirofiban share similarities in terms of inhibiting the ADP-induced platelet aggregation rate, reducing plasma levels of p-selectin, reducing inflammatory factors and increasing the generation of NO to reduce myocardial infarct size. These similarities suggest that SAA may protect myocardial cells principally through antiplatelet activity. Notably, Tirofiban does not increase the survival rate of rats following exposure to I/R, potentially due to severe hemorrhage complications associated with its use (30–32).
In conclusion, the present study demonstrated that SAA protected the myocardium against I/R injury and improved survival rate by reducing platelet activation and inflammation. The protective effects of SAA may be principally related to the antiplatelet effects of SAA. Thus, SAA may be viable as a novel antiplatelet drug for use in both ischemic heart disease and cardiac surgeries associated with I/R injury.
Acknowledgements
The present study was supported by the Technology Bureau of Lishui (grant no. 20110410).
References
- 1.Moran AE, Forouzanfar MH, Roth GA, Mensah GA, Ezzati M, Flaxman A, Murray CJ, Naghavi M. The global burden of ischemic heart disease in 1990 and 2010: The Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fröhlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: Looking beyond primary PCI. Eur Heart J. 2013;34:1714–1722. doi: 10.1093/eurheartj/eht090. [DOI] [PubMed] [Google Scholar]
- 3.Becker LC, Ambrosio G. Myocardial consequences of reperfusion. Prog Cardiovasc Dis. 1987;30:23–44. doi: 10.1016/0033-0620(87)90009-0. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell SR, Lip GY. Reperfusion injury: A review of the pathophysiology, clinical manifestations and therapeutic options. Int J Cardiol. 1997;58:95–117. doi: 10.1016/S0167-5273(96)02854-9. [DOI] [PubMed] [Google Scholar]
- 5.Piper HM, Meuter K, Schäfer C. Cellular mechanisms of ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S644–S648. doi: 10.1016/S0003-4975(02)04686-6. [DOI] [PubMed] [Google Scholar]
- 6.Pachel C, Mathes D, Arias-Loza AP, Heitzmann W, Nordbeck P, Deppermann C, Lorenz V, Hofmann U, Nieswandt B, Frantz S. Inhibition of platelet GPVI protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2016;36:629–635. doi: 10.1161/ATVBAHA.115.305873. [DOI] [PubMed] [Google Scholar]
- 7.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 9.Skyschally A, Erbel R, Heusch G. Coronary microembolization. Circ J. 2003;67:279–286. doi: 10.1253/circj.67.279. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Tao GZ. Effects of tirofiban on the reperfusion-related no-reflow in rats with acute myocardial infarction. J Geriatr Cardiol. 2013;10:52–58. doi: 10.3969/j.issn.1671-5411.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard JP, Jones DA, Gallagher S, Rathod K, Antoniou S, Wright P, Knight C, Mathur A, Weerackody R, Wragg A. Glycoprotein IIb/IIIa inhibitors use and outcome after percutaneous coronary intervention for non-ST elevation myocardial infarction. Biomed Res Int. 2014;2014:643981. doi: 10.1155/2014/643981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng TO. Cardiovascular effects of Danshen. Int J Cardiol. 2007;121:9–22. doi: 10.1016/j.ijcard.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Han JY, Fan JY, Horie Y, Miura S, Cui DH, Ishii H, Hibi T, Tsuneki H, Kimura I. Ameliorating effects of compounds derived from Salvia miltiorrhiza root extract on microcirculatory disturbance and target organ injury by ischemia and reperfusion. Pharmacol Ther. 2008;117:280–295. doi: 10.1016/j.pharmthera.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Lian-Niang L, Rui T, Wei-Ming C. Salvianolic acid A, a new depside from roots of Salvia miltiorrhiza. Planta Med. 1984;50:227–228. doi: 10.1055/s-2007-969684. [DOI] [PubMed] [Google Scholar]
- 15.Huang ZS, Zeng CL, Zhu LJ, Jiang L, Li N, Hu H. Salvianolic acid A inhibits platelet activation and arterial thrombosis via inhibition of phosphoinositide 3-kinase. J Thromb Haemost. 2010;8:1383–1393. doi: 10.1111/j.1538-7836.2010.03859.x. [DOI] [PubMed] [Google Scholar]
- 16.Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 17.Liu X, Tao GZ. Effects of tirofiban on the reperfusion-related no-reflow in rats with acute myocardial infarction. J Geriatr Cardiol. 2013;10:52–58. doi: 10.3969/j.issn.1671-5411.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 19.Hansen PR. Inflammatory alterations in the myocardial microcirculation. J Mol Cell Cardiol. 1998;30:2555–2559. doi: 10.1006/jmcc.1998.0827. [DOI] [PubMed] [Google Scholar]
- 20.Vinten-Johansen J, Jiang R, Reeves JG, Mykytenko J, Deneve J, Jobe LJ. Inflammation, proinflammatory mediators and myocardial ischemia-reperfusion injury. Hematol Oncol Clin North Am. 2007;21:123–145. doi: 10.1016/j.hoc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Gu T, Fu X, Zhao R. Effect of salvianolic acid A and C compatibility on inflammatory cytokines in rats with unilateral ureteral obstruction. J Tradit Chin Med. 2015;35:564–70. doi: 10.1016/S0254-6272(15)30140-0. [DOI] [PubMed] [Google Scholar]
- 22.Rendu F, Brohard-Bohn B. The platelet release reaction: Granules' constituents, secretion and functions. Platelets. 2001;12:261–273. doi: 10.1080/09537100120068170. [DOI] [PubMed] [Google Scholar]
- 23.Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, Sticherling C, Meinl C, May A, Schömig A. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation. 1997;95:2387–2394. doi: 10.1161/01.CIR.95.10.2387. [DOI] [PubMed] [Google Scholar]
- 24.Gawaz M, Neumann FJ, Dickfeld T, Koch W, Laugwitz KL, Adelsberger H, Langenbrink K, Page S, Neumeier D, Schömig A, Brand K. Activated platelets induce monocyte chemotactic protein-1 secretion and surface expression of intercellular adhesion molecule-1 on endothelial cells. Circulation. 1998;98:1164–1171. doi: 10.1161/01.CIR.98.12.1164. [DOI] [PubMed] [Google Scholar]
- 25.Massberg S, Enders G, Leiderer R, Eisenmenger S, Vestweber D, Krombach F, Messmer K. Platelet-endothelial cell interactions during ischemia/reperfusion: The role of P-selectin. Blood. 1998;92:507–515. [PubMed] [Google Scholar]
- 26.Rafikov R, Fonseca FV, Kumar S, Pardo D, Darragh C, Elms S, Fulton D, Black SM. eNOS activation and NO function: Structural motifs responsible for the posttranslational control of endothelial nitric oxide synthase activity. J Endocrinol. 2011;210:271–284. doi: 10.1530/JOE-11-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang D, Xie P, Liu Z. Ischemia/reperfusion-induced MKP-3 impairs endothelial NO formation via inactivation of ERK1/2 pathway. PLoS One. 2012;7:e42076. doi: 10.1371/journal.pone.0042076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan H, Li D, Fang F, Chen D, Qi L, Zhang R, Xu T, Sun H. Salvianolic acid A demonstrates cardioprotective effects in rat hearts and cardiomyocytes after ischemia/reperfusion injury. J Cardiovasc Pharmacol. 2011;58:535–542. doi: 10.1097/FJC.0b013e31822de355. [DOI] [PubMed] [Google Scholar]
- 29.Fan H, Yang L, Fu F, Xu H, Meng Q, Zhu H, Teng L, Yang M, Zhang L, Zhang Z, Liu K. Cardioprotective effects of salvianolic acid A on myocardial ischemia-reperfusion injury in vivo and in vitro. Evid Based Complement Alternat Med. 2012;2012:508938. doi: 10.1155/2012/508938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirre FV, Topol EJ, Ferguson JJ, Anderson K, Blankenship JC, Heuser RR, Sigmon K, Taylor M, Gottlieb R, Hanovich G, et al. Bleeding complications with the chimeric antibody to platelet glycoprotein IIb/IIIa integrin in patients undergoing percutaneous coronary intervention. EPIC Investigators. Circulation. 1995;91:2882–2890. doi: 10.1161/01.CIR.91.12.2882. [DOI] [PubMed] [Google Scholar]
- 31.Kellert L, Hametner C, Rohde S, Bendszus M, Hacke W, Ringleb P, Stampfl S. Endovascular stroke therapy: Tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. 2013;44:1453–1455. doi: 10.1161/STROKEAHA.111.000502. [DOI] [PubMed] [Google Scholar]
- 32.Ilhan E, Güvenc TS, Güzelburc O, Altay S, Özer N, Soylu O, Hasdemir H, Ergelen M. A fatal complication of tirofiban in an octogenarian: Diffuse alveolar hemorrhage. J Cardiol Cases. 2010;2:e48–e51. doi: 10.1016/j.jccase.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]