Abstract
Loss of sirtuin 1 (SIRT1) activity may be associated with metabolic diseases, including diabetes. The aim of the present study was to investigate the potential effects of overexpressed endothelial nitric oxide synthase (eNOS) on cell proliferation and apoptosis with SIRT1 activation in the Min6 mouse pancreatic β cell line. A pcDNA3.0-eNOS plasmid was constructed and transfected into Min6 cells for 24 h prior to harvesting. eNOS expression was validated and SIRT1 expression was detected following plasmid transfection using reverse transcription-quantitative polymerase chain reaction and western blot analysis, which demonstrated that the expression levels of eNOS and SIRT1 were significantly upregulated. Furthermore, the cell proliferation and cell apoptosis of the Min6 cells were evaluated, using a cell counting kit-8 assay and flow cytometry, respectively. The results suggested that overexpressed eNOS promoted cell proliferation and inhibited cell apoptosis in Min6 cells. The interaction between eNOS and SIRT1 was explored through co-immunoprecipitation, and it found that there was a strong interaction between eNOS and SIRT1. In conclusion, overexpressed eNOS may induce SIRT1 activation, which is implied to play a protective role in Min6 cells, and eNOS may be a new therapeutic target for diseases such as type 2 diabetes.
Keywords: diabetes, sirtuin 1, endothelial nitric oxide synthase, protein-protein interaction, protective effect
Introduction
Diabetes leads to vascular changes and dysfunction with the most critical factor of insulin resistance, and morbidity and mortality in diabetic patients are mainly caused by diabetic complications (1). According to the International Diabetes Federation Atlas in 2014, the estimated diabetes prevalence for 2015 has risen to 387 million, representing 8.3% of the world's adult population, and it has been predicted that by 2035 the number of people with diabetes will have risen to 592 million (2–4).
Sirtuin 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD+)-dependent histone deacetylase, is involved in the regulation of metabolism, cell survival, differentiation and longevity (5), and exerts beneficial effects on glucose-lipid homeostasis and insulin sensitivity in diabetes in both animal studies and clinical research (6,7). This suggests that SIRT1 may be a promising novel therapeutic target for diabetic complications and recognized as a key regulator of vascular endothelial homeostasis, controlling angiogenesis, endothelial senescence and dysfunction (8). It is reported that the activation of SIRT1 prevents hyperglycemia-induced vascular cell senescence and protects against vascular dysfunction in mice with diabetes, which suggests a protective role of SIRT1 in the pathogenesis of diabetic vasculopathy (9). According to another study, mitochondrial biogenesis can be enhanced by resveratrol through the 5′-adenosine monophosphate-activated kinase/SIRT1 pathway in muscle and liver, resulting in extension of life span or amelioration of high-fat diet-induced metabolic impairment, including obesity and insulin resistance (10).
Recently, it has been indicated that SIRT1 regulates endothelial nitric oxide synthase (eNOS), which generates endothelial nitric oxide (NO) (11). Furthermore, another study demonstrated that the production of NO, stimulated by caloric restriction, increases SIRT1 expression, which implies that eNOS may be involved in regulation of the expression of SIRT1 in murine white adipocytes (12). Therefore, it may be hypothesized that there is an interaction between eNOS and SIRT1. The aim of the present study was to investigate the potential effects of overexpressed eNOS on cell proliferation and apoptosis with SIRT1 activation in the mouse pancreatic β cell line, Min6. The results of the present study indicated that SIRT1 expression was significantly upregulated following eNOS recombinant plasmid transfection, which induced cell proliferation and decreased cell apoptosis. Furthermore, we explored the underlying mechanisms between eNOS and SIRT1, which demonstrated that there was a strong interaction between these two proteins.
Materials and methods
Plasmid construction
The mouse eNOS cDNA (BC052636.1) was cloned into the eukaryotic expression vector pcDNA3.0 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) using NotI and HindIII restriction sites. The forward primer was 5′-ATAAGAATGCGGCCGCATGGGCAACTTGAAGAGTGTGG-3′ (NotI site underlined) and the reverse primer was 5′-CCCAAGCTTTCAGGAACCAGGTGTTTCTTGGG-3′ (HindIII site underlined). Subsequently, the recombinant vector was amplified in DH5α Escherichia coli (Sangon Biotech Co., Ltd., Shanghai, China) and plasmid DNA was purified with an endotoxin-free plasmid purification kit (Qiagen, Inc., Valencia, CA, USA) according to the manufacturer's instructions. Each segment was amplified by polymerase chain reaction (PCR) with Takara LA Taq or Primestar (Takara Bio Inc., Otsu, Japan) and cloned into the pcDNA3.0 vector. Furthermore, all joints in the constructs were confirmed by sequencing (Sangon Biotech Co., Ltd.). The present study was approved by the Ethics Committee of Guilin Medical University (Guilin, China).
Cell line culture and treatment
The mouse pancreatic β cell line Min6 (American Type Culture Collection, Manassas, VA, USA) was maintained in Dulbecco's modified Eagle's medium with high glucose, 10% heat inactivated newborn calf serum, and 1% antibiotic-antimycotic solution (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Min6 cells were treated with pcDNA3.0-eNOS plasmid (1 µg) using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for 24, 48, 72 and 96 h. Empty pcDNA3.0 vector (1 µg) was used as a negative control.
Cell proliferation activity
Min6 cells were seeded at a density of 1.0×105 cells/ml in 6-well plates in order to achieve ~50% confluence the next day, and were then transfected with pcDNA3.0-eNOS (50 µM). Thereafter, 100 µl cell counting kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) solution was added to each well and were incubated with the cells for 1 h. The absorbance was then measured at 450 nm using a microplate reader.
Apoptosis assay
Following plasmid transfection for 24 h, the apoptotic cells were quantified using an Annexin V/propidium iodide (PI) apoptosis kit (Multi Sciences Biotech Co., Ltd., Hangzhou, China). Min6 cells were collected, washed with PBS and resuspended in 200 µl binding buffer containing 5 µl Annexin V (10 µg/ml) for 10 min in the dark. The cells were then incubated with 10 µl PI (20 µg/ml), and the samples were immediately analyzed using flow cytometry (Beckman Coulter, Inc., Brea, CA, USA). Data acquisition and analysis was performed using CellQuest software (CellQuest Pro, version 5.1; BD Biosciences, Franklin Lakes, NJ, USA).
RNA isolation and reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated using a UNIQ-10 column and TRIzol total RNA isolation kit (Sangon Biotech Co., Ltd.). In total, 3 µg total RNA was used for reverse transcription in a reaction volume of 20 µl using Cloned AMV Reverse Transcriptase (Invitrogen; Invitrogen; Thermo Fisher Scientific, Inc.). In addition, 3 µl cDNA was used for qPCR using a Takara Ex Taq RT-PCR version 2.1 kit (Takara Bio, Inc.). Gene-specific PCR primers for eNOS, SIRT1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) are listed in Table I, and PCR signals were detected with a DNA Engine Opticon 2 Continuous Fluorescence Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). PCR was monitored for 45 cycles using an annealing temperature of 60°C. At the end of the PCR cycles, melt curve analysis and 2% agar electrophoresis was performed in order to assess the purity of the PCR products. Negative control reactions (no template) were routinely included to monitor potential contamination of reagents. Relative quantities of eNOS mRNA and SIRT1 mRNA were normalized to the quantity of GAPDH mRNA using the 2−∆∆Cq method (13).
Table I.
Sequences of the primers used for eNOS and SIRT1 detection by quantitative polymerase chain reaction.
| Gene | Primer sequence (5′-3′) |
|---|---|
| eNOS | Forward: TTCCTGGACATCACTTCCCC |
| Reverse: CTTCCATTCTTCGTAGCGCC | |
| SIRT1 | Forward: TGCCATCATGAAGCCAGAGA |
| Reverse: AACATCGCAGTCTCCAAGGA | |
| GAPDH | Forward: CGGAGTCAACGGATTTGGTCGTAT |
| Reverse: AGCCTTCTCCATGGTGGTGAAGAC |
eNOS, endothelial nitric oxide synthase; SIRT1, sirtuin 1.
Protein isolation and western blot analysis
The concentration of protein in extracts from mice pancreatic β cells was determined using a bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). Protein lysates were prepared using NP-40 buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) on ice for 20 min before centrifuging at 30, 000 × g for 30 min at 4°C. The supernatant was subsequently separated by 10% SDS-PAGE (20 µg protein/lane) followed by transfer onto nitrocellulose membranes. Western blot analysis was performed as previously described (14), and the signals were detected using an enhanced chemiluminescence (ECL) system (Millipore, Billerica, MA, USA). Antibodies used in the present study included anti-mouse eNOS (cat. no. SA-201-0100; 1:4,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-mouse SIRT1 (cat. no. sc-74465; 1:4,000; Santa Cruz Biotechnology, Inc.) and anti-mouse GAPDH (cat. no. sc-365062; 1:20,000; Santa Cruz Biotechnology, Inc.). The blots were subsequently incubated with a secondary antibody (horseradish peroxidase-conjugated AffiniPure Goat Anti-Rabbit IgG; cat. no. 111-035-003; 1:10,000; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) at room temperature for 2 h. and exposed to ECL reagent according to the manufacturer's protocol for the detection of protein expression.
Co-immunoprecipitation (Co-IP) assay
Protein-protein interactions were analyzed by co-IP experiments. The cells were collected, and the proteins were solubilized in IP buffer [50 mM Tris (pH 8.0), 150 mM NaCl, 1% protease inhibitor mixture]. Co-IP was performed according to the standard protocols previously described (15,16). Briefly, the Min6 cells were washed with ice-cold PBS twice and lysed in ice-cold radioimmunoprecipitation assay buffer (cat. no. P0013B; Beyotime Institute of Biotechnology, Haimen, China). The supernatant was incubated with 10 µl mouse anti-eNOS (cat. no. SA-201-0100; 1:100; Santa Cruz Biotechnology, Inc.) or mouse anti-SIRT1 (cat. no. sc-74465; 1:100; Santa Cruz Biotechnology, Inc.) monoclonal antibody and agarose ligand (Catch and Release v2.0; EMD Millipore, Billerica, MA, USA), followed by incubation at 4°C for 1 h. The immune complexes were washed, eluted by boiling in 2X SDS sample buffer, separated by 10% SDS-PAGE and transferred onto membranes. The blots were then incubated with a horseradish-peroxidase-conjugated secondary antibody (cat. no. BHR101-1; 1:10,000; Bersee, Biomart company, Beijing, China) at room temperature for 1 h and exposed to ECL reagent for the detection of protein expression according to the manufacturer's protocol.
Statistical analysis
Differences between each group were expressed as the mean ± standard deviation. Statistical significance was assessed by Student's t-test and one-way analysis of variance followed by a Tukey post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Validation assay of eNOS overexpression at the mRNA and protein levels
eNOS expression was upregulated following pcDNA3.0-eNOS transfection (Fig. 1). The mRNA (P<0.001; Fig. 1A) and protein (Fig. 1B) levels of eNOS in Min6 cells were clearly increased following pcDNA3.0-eNOS transfection for 24 h.
Figure 1.
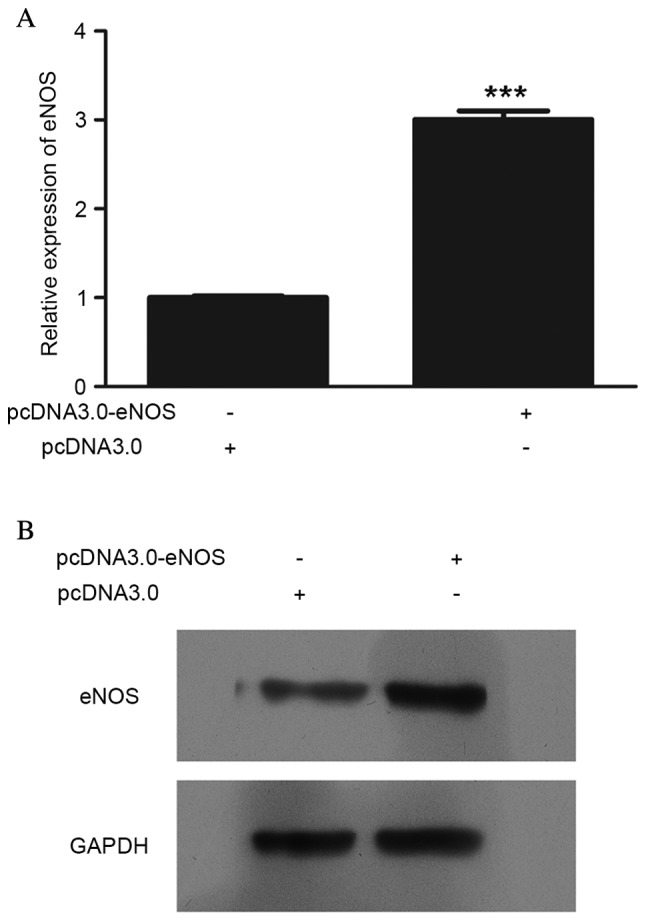
eNOS overexpression in vitro was validated by reverse transcription-quantitative polymerase chain reaction and western blot analysis. (A) mRNA level of eNOS after pcDNA3.0-eNOS transfection for 24 h, compared with the negative control group (pcDNA3.0); (B) protein level of eNOS following pcDNA3.0-eNOS transfection for 24 h, compared with the negative control group. All detections were repeated independently three times. ***P<0.001 vs. the negative control group. eNOS, endothelial nitric oxide synthase.
Effect of overexpressed eNOS on SIRT1 expression at the mRNA and protein levels
In order to determine the effect of eNOS on SIRT1, SIRT1 expression at the mRNA and protein levels was detected, as shown in Fig. 2. The mRNA (P<0.001; Fig. 2A) and protein (Fig. 2B) levels in Min6 cells were clearly upregulated following transfection with pcDNA3.0-eNOS for 24 h.
Figure 2.
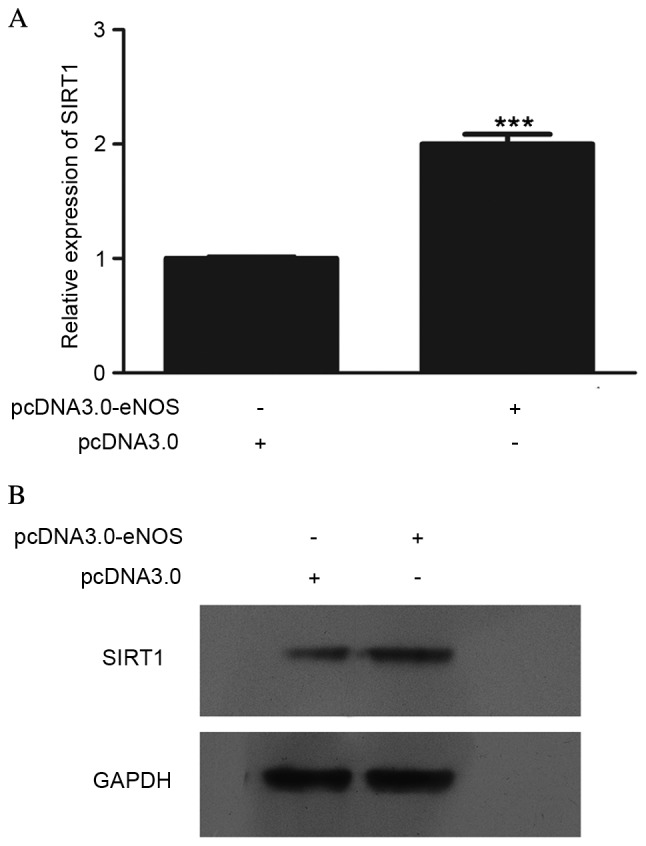
Effect of overexpressed eNOS on SIRT1 expression in vitro was detected by reverse transcription-quantitative polymerase chain reaction and western blot analysis. (A) mRNA level of SIRT1 after pcDNA3.0-eNOS transfection for 24 h, compared with the negative control group (pcDNA3.0); (B) protein level of SIRT1 following pcDNA3.0-eNOS transfection for 24 h compared with the negative control group. All detections were repeated independently three times. ***P<0.001 vs. the negative control group. eNOS, endothelial nitric oxide synthase; SIRT1, sirtuin 1.
Effect of overexpressed eNOS on Min6 cell proliferation
The effects of eNOS overexpression on Min6 cell proliferation were examined. As shown in the CCK-8 assay results in Fig. 3, the cellular population was increased time-dependently in the pcDNA3.0-eNOS transfection group compared with the negative control (pcDNA3.0) group, particularly at 48 h (P<0.01) and 72 h (P<0.001).
Figure 3.
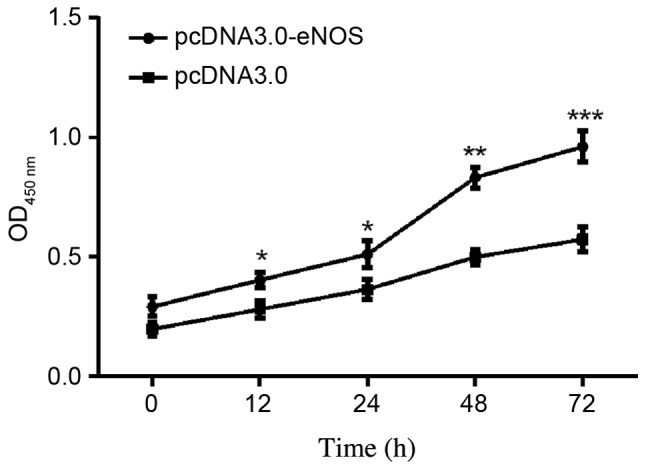
Upregulation of eNOS promoted the growth of Min6 cells in vitro. Cell numbers were counted at the following time points: 12, 24, 48 and 72 h. Cell viability was measured using a cell counting kit-8 assay. Data are shown as the mean ± standard deviation. All experiments were repeated independently three times. *P<0.05, **P<0.01 and ***P<0.001 vs. the data from pcDNA3.0 only group detected at the same time. eNOS, endothelial nitric oxide synthase.
Overexpression of eNOS reduced apoptosis of the Min6 cell line
Cell apoptosis was analyzed using flow cytometry following pcDNA3.0-eNOS transfection for 24 h based on the CCK-8 results. Exposure of Min6 cells to the recombinant eNOS plasmid inhibited the apoptosis of the cells compared with that in the negative control group. Furthermore, the occurrence of apoptosis was significantly lower (P<0.001) in the pcDNA3.0-eNOS group compared with the pcDNA3.0 group (negative control), as shown in Fig. 4.
Figure 4.
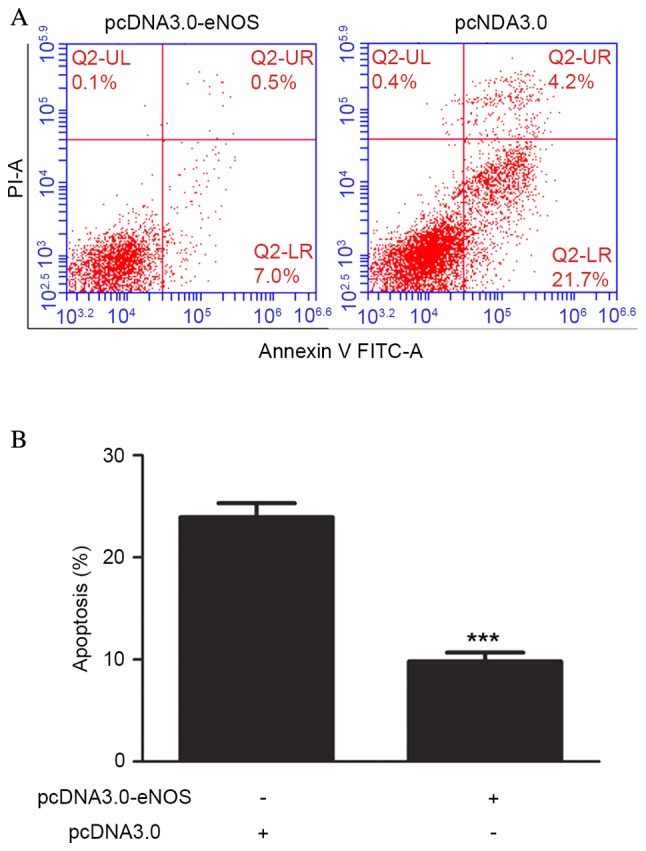
Upregulation of eNOS inhibited apoptosis of Min6 cells in vitro. (A) Min6 cells were transfected with pcDNA3.0-eNOS for 24 h prior to being harvested for apoptosis testing. (B) Percentage of apoptotic Min6 cells at the 72 h time point. Data are shown as the mean ± standard deviation. All experiments were repeated independently three times. ***P<0.001 vs. the Min6 group. eNOS, endothelial nitric oxide synthase.
Interaction between eNOS and SIRT1
Finally, the possibility that eNOS interacts with SIRT1 was investigated. To this end, Min6 cell lysates were harvested, and then were subjected to Co-IP and the results in Fig. 5 clearly indicated that there is an interaction between exogenous SIRT1 and eNOS proteins.
Figure 5.
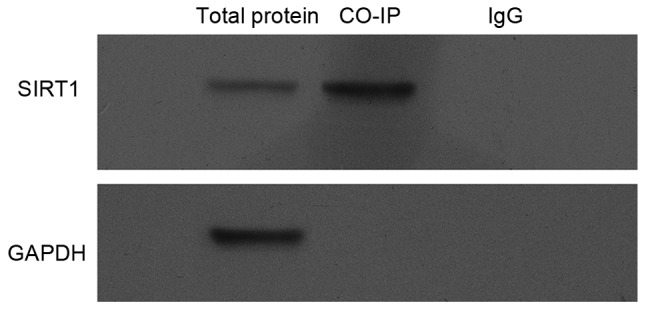
Interaction between eNOS and SIRT1. Min6 cells were treated with pcDNA3.0-eNOS for 24 h, and proteins were extracted for co-immunoprecipitation with anti-SIRT1 and probed with anti-eNOS or anti-SIRT1. The bands for SIRT1-immunoprecipitated eNOS were scanned and normalized with GAPDH. All experiments were repeated independently three times. eNOS, endothelial nitric oxide synthase; SIRT1, sirtuin 1.
Discussion
In the present study, eNOS has been indicated to be a regulator of SIRT1 in the mouse pancreatic β cell line Min6. This conclusion is based on several novel observations. Firstly, evidence that SIRT1 was activated by overexpressed eNOS was provided, which was achieved through recombinant plasmid transfection. Secondly, overexpressed eNOS promoted mouse pancreatic β cell proliferation and protected mouse pancreatic β cells from cell apoptosis. Thirdly, a strong protein-protein interaction between eNOS and SIRT1 was demonstrated. Furthermore, the present study implied that overexpressed eNOS may induce SIRT1 activation, which is indicated to have a protective role in Min6 cells.
NO is produced by three isoforms of NO synthase (NOS): Neuronal nNOS (NOS I), inducible iNOS (NOS II) and endothelial eNOS (NOS III) (17). Under physiological conditions, vascular NO is mostly produced by eNOS. It is known that NO reduces oxidative stress and the progression of atherosclerosis (18), and exerts cardioprotective and vasoprotective effects in endothelial cells though a regulatory effect for inhibition of platelet aggregation, blood flow and inflammatory cell adhesion (19,20), while SIRT1 has previously been identified as a critical regulator of vascular endothelial homeostasis, controlling angiogenesis, endothelial senescence and dysfunction (8,21). A recent study has shown that SIRT1 is an endogenous protective molecule and a promising novel therapeutic target against myocardial ischemia/reperfusion (MI/R)-induced injury, which reduces oxidative stress and diabetes-exacerbated injury via the activation of eNOS in diabetic rats (11). As Lemarie et al (22) reported, some effective antioxidants, including resveratrol, have been shown to act via the stimulation of endothelial SIRT1, which regulates endothelium-dependent vasodilation and bioavailable NO, stimulates eNOS activity and increases endothelial NO. However, eNOS-mediated NO also regulates SIRT1 expression during the aging of endothelial cells; the uncoupling of eNOS results in decreased expression of SIRT1, and ultimately to increased stress-induced senescence (22). It has also been reported that the interaction of SIRT1 with eNOS is important in the augmentation of the protective effect of statins against endothelial senescence, as it was shown that testosterone induced eNOS activity, and subsequently increased SIRT1 expression in endothelial cells (23). In type 2 diabetic rats, a reduction in the cellular redox status and an increase in oxidant stress may work together to reduce vascular SIRT1 expression (24–26). Furthermore, eNOS expression levels have also been shown to be low within cerebral arteries, which implies a connection between SIRT1 and eNOS (27).
In conclusion, the aim of the present study was to explore the interaction of SIRT1 and eNOS and elucidate the mechanism and potential therapeutic targets in diabetes. The results showed that eNOS was upregulated significantly through recombinant plasmid transfection, and subsequently increased SIRT1 expression through direct protein-protein interaction in the mouse pancreatic β cell line, Min6, which may assist future research. Further research may also focus on the identification of an effective drug playing a protective and therapeutic role for diabetes through the targeting of eNOS and regulation of SIRT1.
Acknowledgements
The present study was supported by the Science and Technology Research Projects of Guangxi Universities (grant no. YB2014266) and the National Natural Science Foundation of China (grant nos. 81460164 and 31060161).
References
- 1.Pernický M, Papinčák J, Reptová A, Kiňová S, Murín J. What may cause diabetes. Vnitr Lek. 2015;61:447–450. [PubMed] [Google Scholar]
- 2.Linnenkamp U, Guariguata L, Beagley J, Whiting DR, Cho NH. The IDF Diabetes Atlas methodology for estimating global prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014;103:186–196. doi: 10.1016/j.diabres.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Chan JC, Cho NH, Tajima N, Shaw J. Diabetes in the Western Pacific Region - past, present and future. Diabetes Res Clin Pract. 2014;103:244–255. doi: 10.1016/j.diabres.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 4.IDF Diabetes Atlas Group, corp-author. Update of mortality attributable to diabetes for the IDF Diabetes Atlas: Estimates for the year 2013. Diabetes Res Clin Pract. 2015;109:461–465. doi: 10.1016/j.diabres.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Codocedo JF, Allard C, Godoy JA, Varela-Nallar L, Inestrosa NC. SIRT1 regulates dendritic development in hippocampal neurons. PLoS One. 2012;7:e47073. doi: 10.1371/journal.pone.0047073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kitada M, Koya D. SIRT1 in type 2 diabetes: Mechanisms and therapeutic potential. Diabetes Metab J. 2013;37:315–325. doi: 10.4093/dmj.2013.37.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitada M, Kume S, Kanasaki K, Takeda-Watanabe A, Koya D. Sirtuins as possible drug targets in type 2 diabetes. Curr Drug Targets. 2013;14:622–636. doi: 10.2174/1389450111314060002. [DOI] [PubMed] [Google Scholar]
- 8.Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y. SIRT1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb. 2010;17:431–435. doi: 10.5551/jat.3525. [DOI] [PubMed] [Google Scholar]
- 9.Orimo M, Minamino T, Miyauchi H, Tateno K, Okada S, Moriya J, Komuro I. Protective role of SIRT1 in diabetic vascular dysfunction. Arterioscler Thromb Vasc Biol. 2009;29:889–894. doi: 10.1161/ATVBAHA.109.185694. [DOI] [PubMed] [Google Scholar]
- 10.Shiota A, Shimabukuro M, Fukuda D, Soeki T, Sato H, Uematsu E, Hirata Y, Kurobe H, Maeda N, Sakaue H, et al. Telmisartan ameliorates insulin sensitivity by activating the AMPK/SIRT1 pathway in skeletal muscle of obese db/db mice. Cardiovasc Diabetol. 2012;11:139. doi: 10.1186/1475-2840-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding M, Lei J, Han H, Li W, Qu Y, Fu E, Fu F, Wang X. SIRT1 protects against myocardial ischemia-reperfusion injury via activating eNOS in diabetic rats. Cardiovasc Diabetol. 2015;14:143. doi: 10.1186/s12933-015-0299-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lempiainen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol (Oxf) 2013;208:410–421. doi: 10.1111/apha.12120. [DOI] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Yan X, Hao Q, Mu Y, Timani KA, Ye L, Zhu Y, Wu J. Nucleocapsid protein of SARS-CoV activates the expression of cyclooxygenase-2 by binding directly to regulatory elements for nuclear factor-kappa B and CCAAT/enhancer binding protein. Int J Biochem Cell Biol. 2006;38:1417–1428. doi: 10.1016/j.biocel.2006.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 16.Mohr T, Van Soeren M, Graham TE, Kjaer M. Caffeine ingestion and metabolic responses of tetraplegic humans during electrical cycling. J Appl Physiol (1985) 1998;85:979–985. doi: 10.1152/jappl.1998.85.3.979. [DOI] [PubMed] [Google Scholar]
- 17.Eghbalzadeh K, Brixius K, Bloch W, Brinkmann C. Skeletal muscle nitric oxide (NO) synthases and NO-signaling in ‘diabesity’-what about the relevance of exercise training interventions? Nitric Oxide. 2014;37:28–40. doi: 10.1016/j.niox.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Strawn WB. Pathophysiological and clinical implications of AT(1) and AT(2) angiotensin II receptors in metabolic disorders: Hypercholesterolaemia and diabetes. Drugs 62. 2002;1:31–41. doi: 10.2165/00003495-200262991-00004. (In French) [DOI] [PubMed] [Google Scholar]
- 19.Schafer A, Bauersachs J. Endothelial dysfunction, impaired endogenous platelet inhibition and platelet activation in diabetes and atherosclerosis. Curr Vasc Pharmacol. 2008;6:52–60. doi: 10.2174/157016108783331295. [DOI] [PubMed] [Google Scholar]
- 20.Monti M, Solito R, Puccetti L, Pasotti L, Roggeri R, Monzani E, Casella L, Morbidelli L. Protective effects of novel metal-nonoates on the cellular components of the vascular system. J Pharmacol Exp Ther. 2014;351:500–509. doi: 10.1124/jpet.114.218404. [DOI] [PubMed] [Google Scholar]
- 21.Paschalaki KE, Starke RD, Hu Y, Mercado N, Margariti A, Gorgoulis VG, Randi AM, Barnes PJ. Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence. Stem Cells. 2013;31:2813–2826. doi: 10.1002/stem.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemarie CA, Shbat L, Marchesi C, Angulo OJ, Deschênes ME, Blostein MD, Paradis P, Schiffrin EL. Mthfr deficiency induces endothelial progenitor cell senescence via uncoupling of eNOS and downregulation of SIRT1. Am J Physiol Heart Circ Physiol. 2011;300:H745–H753. doi: 10.1152/ajpheart.00321.2010. [DOI] [PubMed] [Google Scholar]
- 23.Ota H, Akishita M, Akiyoshi T, Kahyo T, Setou M, Ogawa S, Iijima K, Eto M, Ouchi Y. Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: Protective role of eNOS and SIRT1. PLoS One. 2012;7:e29598. doi: 10.1371/journal.pone.0029598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajbakhsh S, Aliakbari K, Hussey DJ, Lower KM, Donato AJ, Sokoya EM. Differential telomere shortening in blood versus arteries in an animal model of type 2 diabetes. J Diabetes Res. 2015;2015:153829. doi: 10.1155/2015/153829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo R, Liu W, Liu B, Zhang B, Li W, Xu Y. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: An insight into endoplasmic reticulum stress response mechanism. Int J Cardiol. 2015;191:36–45. doi: 10.1016/j.ijcard.2015.04.245. [DOI] [PubMed] [Google Scholar]
- 26.Gencoglu H, Tuzcu M, Hayirli A, Sahin K. Protective effects of resveratrol against streptozotocin-induced diabetes in rats by modulation of visfatin/sirtuin-1 pathway and glucose transporters. Int J Food Sci Nutr. 2015;66:314–320. doi: 10.3109/09637486.2014.1003534. [DOI] [PubMed] [Google Scholar]
- 27.Tajbakhsh N, Sokoya EM. Sirtuin 1 is upregulated in young obese Zucker rat cerebral arteries. Eur J Pharmacol. 2013;721:43–48. doi: 10.1016/j.ejphar.2013.09.057. [DOI] [PubMed] [Google Scholar]


