Abstract
Background
Proteomic approaches identifying biomarkers have been applied to asthma to only a very limited extent.
Methods
With an antibody array (RayBiotech, Norcross, GA) the relative intensity and rank differences of 444 proteins were compared in 24 plasma samples obtained at age 3, 11 from children with and 12 without asthma diagnoses at ages 5 and 9. Protein candidates identified by antibody array were quantitated by ELISA in an enlarged sample. Proteins found to differentiate children with and without asthma were also examined for association with known Year 1 asthma risk factors, eczema and wheeze.
Results
In the antibody array, four proteins had rank differences between asthma and non-asthma groups (FDR <0.1). By ELISA, mean log (±SEM) erythropoietin (EPO) level (IU/L) was lower (0.750±0.048 vs. 0.898±0.035; p=0.006) and mean (±SEM) soluble GP130 (sGP130) level (ng/mL) was higher in the asthma vs. the non-asthma group (302±13 vs. 270±8; p=0.041). The other 2 array proteins (galactin-3, eotaxin-3) did not differ by ELISA by asthma. EPO related to the asthma risk factor, first year eczema, whereas sGP130 related to first year wheeze.
Conclusions
Through two independent assessments, age 3 plasma levels of EPO and sGP130 were found related to childhood asthma.
Keywords: asthma, plasma, biomarker, erythropoietin, soluble GP130
INTRODUCTION
Asthma is a lung disease which features chronic inflammation, episodic bronchospasm, airway hyper responsiveness, and airway remodeling [reviewed in Elias et al. (1)], and is a serious public health problem worldwide. The pathobiology remains relatively obscure. Studies have identified associations of asthma with variants in various genes [reviewed in Vercelli (2)], but these account for only a small portion of the familial inheritance patterns. The emerging area of plasma proteomics holds promise for biomarker discovery in complex diseases. Thus far plasma proteomic methodologies have been applied to the study the biology of asthma to only a very limited extent (3, 4)
Antibody array is a technique of profiling multiple protein analytes simultaneously in samples or tissues (5). Different from quantitative methods, antibody arrays generate signal intensities that allow assessment of relative concentrations of analytes among samples. Findings of significant relative differences can be assessed for quantitative validation by other methods.
We hypothesize that asthma development in childhood is accompanied by alterations in plasma proteins. Potential biomarkers for childhood asthma were sought through application of antibody array for 444 proteins in plasma obtained at age 3 from a subset of children enrolled in a longitudinal study and followed through age 9 for development of asthma. Top candidates were tested for quantitative differences with ELISA assays. The results point to potential biomarkers for childhood asthma that open the possibility of distinct pathways, and that if found to be generalizable, will allow for new directions of possible asthma treatment and/or prevention.
METHODS
Birth Cohort Subgroups and Samples
Cryopreserved plasma samples were from subjects in the Tucson Infant Immune Study (IIS), a birth cohort enrolled without selection and followed longitudinally through age 9 (6). Samples obtained at age 3 were from either children whose parents had reported the child had a physician diagnosis of asthma at ages 5 and 9 and active wheezing in the past year at both ages (asthma group) or children whose parents reported the child did not wheeze or have a diagnosis of asthma at age 3, 5, or 9 (non-asthma group).
Sample sizes, Assays, and Analyses
Samples (n=12 each from asthma and non-asthma groups) were sent to RayBiotech (Norcross, GA) where they were assayed by biotin-label-based antibody array (L-series 507) for 507 proteins. Group size was determined by availability of asthma group subjects meeting all criteria at point samples were submitted to Raybiotech and (for nonasthma group) availability of funds. The intensity values of signals from plate for each subject were evaluated by Axon GenePix laser scanner. To adjust for plate-to-plate variation, normalization by RayBiotech was done by multiplying the intensity values on each plate by the ratio of the positive controls on that plate to the mean of the positive controls on one randomly selected plate. Background was removed by subtracting each plate’s mean negative control from each value as described (5). One sample from the asthma group had background intensity values much greater than the other 23 and was excluded from further analysis. Proteins with mean intensities (mean of all samples) near background levels (less than 100, n=63) were excluded from further analysis. This value of 100 was selected as 3 times the mean background noise. Intensity values for remaining proteins (ranging from 100 to 19250) were ranked within each subject. Group mean ranks for each protein were compared using Student’s t-test and were judged noteworthy if the false discovery rate (FDR) adjusted p value was less than 0.1 (7). Noteworthy proteins were further considered for ELISA evaluation.
Quantitation of the candidate proteins identified by antibody array screening was performed using Quantikine ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. Sample size of asthma group (our study being longitudinal) could be expanded to18 samples and included all of the subjects in the IIS population who both met the criteria for persistent childhood asthma (n=33) and had sufficient samples at age 3 for analysis. The non-asthma group was increased to 54 (3 times larger than the asthma group) randomly selected from among 146 in the IIS population who met the criteria and had sufficient samples at age 3. Samples were run as 2–4 replicates and values below the lowest standard were assigned one-half the lowest standard. For proteins with less than 15% undetectable values, distributions of values were assessed for normality and if skewed to the right were log transformed. Groups were compared by Student’s two-sample t test. For proteins with greater than 15% undetectable values, groups were compared using Mann–Whitney comparison of medians. Odds ratios with mutual adjustment were calculated to assess independence of the associations between two protein biomarkers and asthma. Proteins differing by asthma status were further assessed by dividing the asthma group into those diagnosed by or after age 3. These groups were compared by ANOVA and post-hoc Fisher’s protected LSD test. A p-value <0.05 was considered significant.
Blood samples were assayed for hemoglobin by Sonora Quest Laboratories (Tucson, AZ). Total IgE values at 3y were available from assays previously run by Immulite 2000 as previously described (8).
RESULTS
Identification of Plasma Protein Candidate Biomarkers for Childhood Asthma through Antibody Arrays
In comparing the asthma (n=11) and non-asthma (n=12) groups based on the ranks of intensity values from the antibody array assays, 4 proteins were identified as differentiating the two groups (FDR adjusted p-value <0.1; Figure 1). The mean ranks of erythropoietin (EPO) and galectin-3 were lower and the mean ranks of eotaxin-3 and soluble GP130 (sGP130) were higher in the asthma compared to the non-asthma group. The 444 proteins assessed are listed in E Table 1 or http://www.raybiotech.com/l-series-507-label-based-human-array-1-membrane-2.html.
Figure 1.
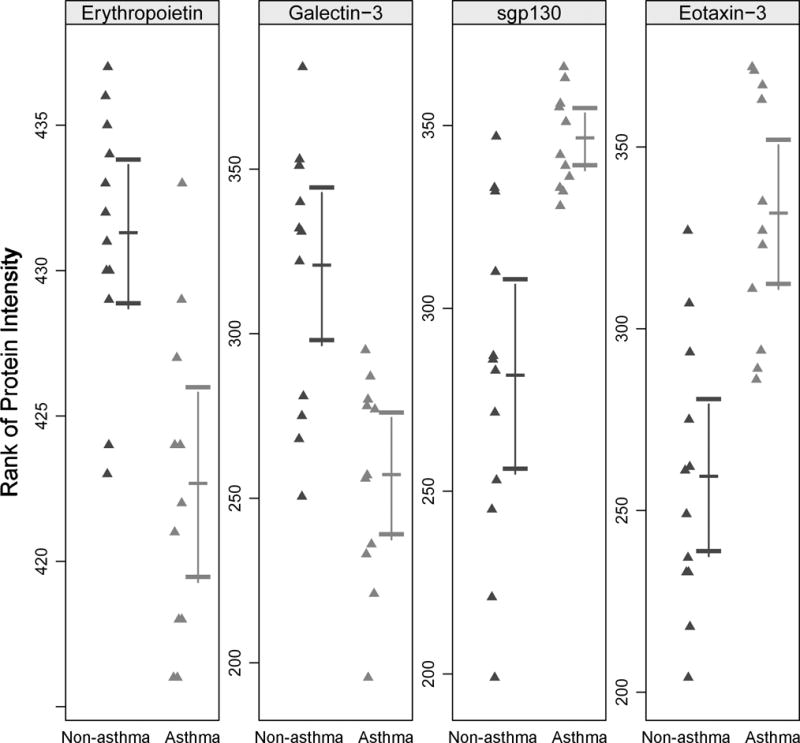
Rank of intensity signals for four proteins with significant rank differences between non-asthma and asthma groups from antibody array analysis (FDR adjusted p-value < 0.1).
Quantitation of Candidate Biomarkers for Childhood Asthma
In the enlarged sample set, we quantified by ELISA each of the four plasma proteins that were identified by rank analysis in the antibody arrays. EPO had a lower mean log concentration in the asthma compared to the non-asthma group (Figure 2A). The direction of change agreed with that of the rank differences in the antibody array. Also in accord with rank difference direction, mean concentrations of sGP130 were increased in the asthma group (Figure 2B). Mean galectin-3 concentrations did not differ significantly between the two groups (Figure 2C). For eotaxin-3, for which 55% had at least one undetectable value, a nonparametric comparison indicated there was no difference in median values between the asthma and non-asthma groups (data not shown). Limiting the analysis to those samples also assayed in the antibody array revealed a similar significant difference in the two groups for EPO although the difference for sGP130 was not significant.
Figure 2.
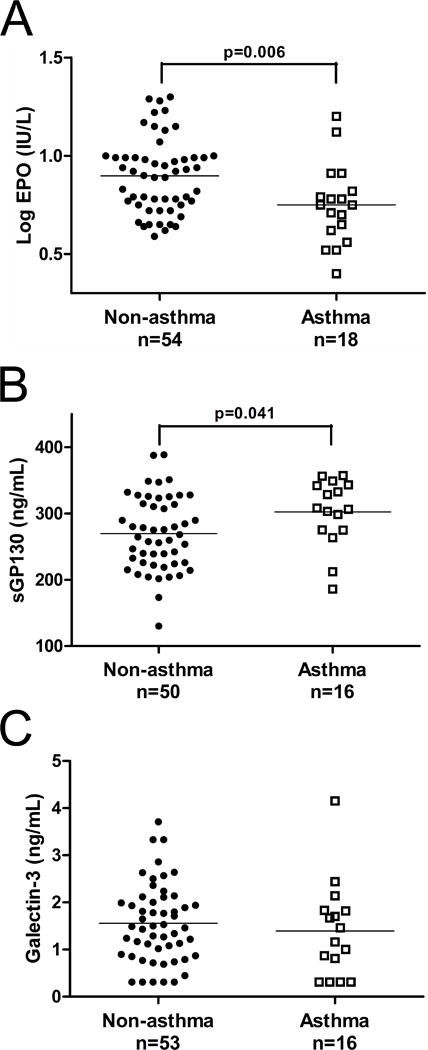
Comparisons of non-asthma and asthma groups for plasma levels of analytes assayed by ELISA for (A) erythropoietin (EPO; 0.898±0.035 vs. 0.750±0.048); (B) soluble GP130 (sGP130; 270±8 vs. 302±13); and (C) galectin-3 (1.56±0.11 vs. 1.39±0.25). Groups are compared by Student’s t-test.
When modeled together, logistic regression analysis revealed the independence of the associations of EPO and sGP130 plasma levels for asthma risk (Table 1). Levels for these two plasma biomarkers did not correlate with each other (r=0.12, p=0.33). Total IgE, well known to be increased in asthma, was increased in the plasma samples of the children with asthma compared to those without asthma (mean log±SEM: 1.775±0.141 vs. 1.104±0.089 IU/ml, respectively, p<0.001). Total IgE correlated inversely with EPO (r=-0.36; p=0.002, n=70) but not with sGP130 (r=-0.19; p=0.14, n=66). Hemoglobin levels did not differ by asthma group, although levels in the combined groups tend toward their known inverse relation with EPO (r=-.20, p=0.07).
Table 1.
Biomarker Odds Ratios for Asthma Before and After Mutual Adjustment.
| Asthma Biomarker | Marginal Odds Ratio | 95% CI | p | Mutually Adjusted Odds Ratio | 95% CI | p |
|---|---|---|---|---|---|---|
| EPO | 0.01 | 0.00–0.36 | 0.011 | 0.01 | 0.00–0.50 | 0.022 |
| SGP130 | 1.01 | 1.00–1.02 | 0.047 | 1.02 | 1.00–1.03 | 0.018 |
In a post-hoc analysis, the asthma group was subdivided into those who had a diagnosis of asthma already by the age of 3 and those who gained their diagnosis after age 3. A concentration response was evident with lower levels of EPO in those already diagnosed at age 3 (Table 2). Similar stepwise increases were observed for total IgE. In contrast, sGP130 levels did not differ by age of diagnosis in the asthmatic children.
Table 2.
Mean levels of Plasma Biomarkers at Age 3 in Relation to Age of Asthma Diagnosis
| n | No asthma | n | Asthma Diagnosed After Age 3 | n | Asthma Diagnosed by Age 3 | ANOVA P | |
|---|---|---|---|---|---|---|---|
| Mean (SEM) log EPO IU/ml | 54 | 0.898 (0.026) | 7 | 0.830 (0.070) | 10 | 0.706 (0.068) | 0.018* |
| Mean (SEM)sGP130ng/ml | 50 | 260 (8) | 7 | 304 (23) | 8 | 305 (14) | 0.10 |
| Mean (SEM) log IgE IU/ml | 54 | 1.14 (0.09) | 7 | 1.43 (0.19) | 10 | 2.00 (0.19) | 0.001* |
Asthma diagnosed by age 3 and no asthma groups differ significantly by Fisher’s protected LSD post hoc test. P=0.006 for EPO and <0.001 for IgE.
Relation of plasma protein biomarkers to early life asthma risk factors
Eczema and wheezing in the first year of life are well-established, independent risk factors for childhood asthma (9) and in the subset of IIS children show odds of more than 5 and 9 respectively (Table 3). Interestingly, the mean log plasma EPO was lower in children with eczema in the first year of life but was unrelated to first year wheezing (Table 4). In contrast, mean sGP130 showed no relation to eczema but was higher in the children with first year wheezing (Table 4). Maternal asthma, also a significant risk factor for asthma in this subset, was unrelated to either plasma protein biomarker (data not shown).
Table 3.
Year 1 Risk Factor Odds Ratios for Asthma Before and After Mutual Adjustment
| Risk Factor | Marginal Odds Ratio | 95% CI | p | Mutually Adjusted Odds Ratio | 95%CI | p |
|---|---|---|---|---|---|---|
| Eczema Yr 1 | 5.0 | 1.6–15.9 | 0.007 | 5.9 | 1.5–22.7 | 0.009 |
| Wheeze Yr 1 | 8.3 | 2.5–27.7 | 0.001 | 9.4 | 2.5–36.2 | 0.001 |
Table 4.
Relation of Mean Levels of the Candidate Biomarkers to Eczema and Wheezing in the First Year of Life
| Risk Factor | n | mean (SEM) log EPO IU/ml | n | Mean (SEM) sGP130 ng/ml | |
|---|---|---|---|---|---|
| Eczema Year 1 | No | 49 | 0.900 (0.028) | 45 | 279 (9) |
| Yes | 21 | 0.770 (0.043) | 19 | 276 (11) | |
| ANOVA p | 0.014 | 0.85 | |||
| Wheeze Year 1 | No | 51 | 0.871 (0.028) | 49 | 266 (8) |
| Yes | 19 | 0.830 (0.053) | 15 | 320 (9) | |
| ANOVA p | 0.44 | 0.001 |
DISCUSSION
Our study identified four candidate protein biomarkers of asthma development using rank analysis of 444 proteins by antibody array in plasma samples obtained at age 3 from a subset children enrolled in the Tucson Infant Immune Study, a birth cohort. Of these four proteins, we identified quantitative differences by ELISA for two: EPO had lower mean levels in the asthma compared to the non-asthma group and sGP130 had higher levels in the asthma group. Neither eotaxin-3 nor galactin-3 yielded quantitative differences between the two groups.
Plasma proteomics offers the opportunity to follow hypothesis generating approaches to study of the biology of a complex disease like asthma. Differences in plasma proteins so identified suggest that the disease may have systemic involvement (in contrast to being completely organ constrained). Given that plasma contains several thousand proteins (10), antibody arrays do not assess the entire protein spectrum and carry some bias in regard to limitations based on availability of antibody pairs for protein detection. In contrast, though, they are much less limited by relative concentrations than are mass spectrometry-based methods.
Only a few studies have yet pursued examining plasma or serum for asthma-related differences by either of these approaches. In one study utilizing two-dimensional gel electrophoresis and matrix assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry. Verrills et al. (3) identified 14 proteins that showed relative differences in serum of adult asthmatics vs. controls (without correcting for multiple comparisons). Validation of a few of the 14 proteins was attempted by ELISA: one yielded concentrations that validated the direction of change identified in the proteomic analysis (haptoglobin), one was not significant in the proteomic analysis but was increased in ELISA (ceruloplasmin), and one showed a difference opposite in direction (anti-thrombin III). None of the 14 proteins was among the proteins on the antibody arrays employed in our study. In a recent report, Reubsaet et al. (4) performed a limited multiplex antibody-based assay in plasma from a group of children all of whom exhibited wheezing in the first 3 years of life. Among 16 cytokines and chemokines assessed, they reported 3 analytes (CXCL10, CCL17, CCL22) that differed between children who subsequently gained a diagnosis of asthma by age 6 and those who did not. Although these analytes were among the 444 we assessed in the antibody array employed herein, they did not show a difference related to asthma in our study, possibly related to differences in study design. Reubsaet et al. studied only children with a history of early life wheezing, required evidence of allergic sensitization for a diagnosis of asthma, and included no consideration for multiple comparisons. Thus, it is possible that their findings may relate to an allergy susceptible group within asthma.
ELISA for plasma EPO levels provided quantitative validation of the rank differences and agreed in direction with data generated by antibody array. Thus, decreased concentration of EPO in plasma at age 3 appears to serve as a biomarker differentiating subjects with asthma from those without. The quantitative differences were greatest in children who had already acquired a diagnosis of asthma by age 3.
Although EPO has long been known for its actions in increasing production of red blood cells, other effects noted more recently involve protective effects on various cell types and include anti-inflammatory (11–13), anti-apoptotic (14, 15), and antioxidant effects (16, 17).
EPO functions through two classes of receptor made up of combinations of monomeric EPO receptor (EpoR) and CD131, the common beta chain (18, 19). While EpoR homodimerizes upon binding of its ligand, common beta chain has been shown to heterodimerize with monomeric EpoR (19, 20). It has been suggested that the protective effects on various tissues act through the heterodimerized receptor (21).
To our knowledge, altered EPO levels have not been implicated in human asthma. However, a recent study suggested that EPO is decreased in plasma of subjects experiencing exacerbations of COPD (22). A few studies in mice have suggested the potential therapeutic value of EPO. Following EPO administration, Tyagi et al. (23) found significant inhibition of bronchoconstriction induced by carbachol and In a model of chronic asthma, Karaman et al. (24) reported improvement in histological assessments of ovalbumin-induced airway hyper responsiveness and airway inflammation. Such findings support the possibility that the reduced levels of EPO associated with asthma shown in our study may increase risk for cell damage and also show a need for future studies to examine the local airway milieu for differences in EPO.
Soluble GP130 has also not been clearly associated with asthma or early life wheeze, but such roles are not without plausibility as sGP130 participates in a complex network with a membrane-bound form of GP130 together with IL-6 and both soluble and membrane-bound IL-6R (25). sGP130 is suggested to serve as a natural inhibitor of IL-6 (26) and its inflammatory activity. Soluble IL6R was shown (27) increased in airways of asthmatics.
We confirm that eczema and wheezing are associated independently with risk for asthma, suggesting that they may identify distinct paths to asthma. In this regard, our two biomarkers align differentially with these two risk factors: low plasma EPO levels associate with eczema in the first year of life whereas high sGP130 associate with wheeze. Further studies are needed to explore the possibility that the two biomarkers may mark separate paths involved in asthma development.
Previous reports, differ as to whether eotaxin-3 is increased in asthma (28, 29). Our results could not corroborate an increased plasma level associated with asthma. Galectin-3, has been reported to function as a soluble receptor for IgE (30), but has not been clearly associated with asthma and we were unable to identify differences in plasma levels between our asthma and non-asthma groups.
Our study has several advantages: the use of samples from a non-selected birth cohort that has longitudinal detailed information on respiratory symptoms and age of acquiring asthma diagnoses; a broad, hypothesis-generating, screening approach to identifying candidate plasma biomarkers; and a step validating the screening results through quantitation. A limitation of our study is a relatively small sample size and thus other markers in the antibody array may be present differentially in asthma but such differences were not detected because of insufficient power.
In summary, our study demonstrates the utility of antibody array as a screening tool for proteomic identification of candidate biomarkers in human plasma for childhood asthma and the importance of quantitation for follow-up of candidates so identified. We suggest that children with asthma have decreased levels of circulating EPO and increased levels of sGP130. In addition, EPO relates to the early life risk factor, eczema, and sGP130 relates to early life wheezing. Further studies are warranted to assess generalizability and to establish the biological actions and possible therapeutic effects of treatment with EPO and/or approaches to decrease sGP130 in childhood asthma.
Supplementary Material
Electronic Repository: E Table 1. List of Proteins Assessed in the Antibody Array.
Acknowledgments
The authors wish to thank I. Carla Lohman and Amber Spangenberg for their technical assistance in ELISA assays.
Financial Support
Arizona Center for the Biology of Complex Diseases (D.V.), NIEHS grants ES018328 and ES006694 (both to S.S.L.), NIAID grant AI42268 (A.L.W.) and Human Genes and The Environment Research Training Program (T32 ES016652, H.X.).
References
- 1.Elias J, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003 Feb;111:291–7. doi: 10.1172/JCI17748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008 Mar;8:169–82. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 3.Verrills N, Irwin JA, He XY, Wood LG, Powell H, Simpson JL, McDonald VM, Sim A, Gibson PG. Identification of novel diagnostic biomarkers for asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011 Jun;183:15. 1633–43. doi: 10.1164/rccm.201010-1623OC. [DOI] [PubMed] [Google Scholar]
- 4.Reubsaet LL, Meerding J, de Jager W, de Kleer IM, Hoekstra MO, Prakken BJ, van Wijk F, Arets HG. Plasma chemokines in early wheezers predict the development of allergic asthma. Am J Respir Crit Care Med. 2013 Oct;188:15. 1039–40. doi: 10.1164/rccm.201212-2330LE. [DOI] [PubMed] [Google Scholar]
- 5.Huang R, Jiang W, Yang J, Mao YQ, Zhang Y, Yang W, Yang D, Burkholder B, Huang RF, Huang RP. A biotin label-based antibody array for high-content profiling of protein expression. Cancer Genomics Proteomics. 2010 May-Jun;7:129–41. [PubMed] [Google Scholar]
- 6.Oddy W, Halonen M, Martinez FD, Lohman IC, Stern DA, Kurzius-Spencer M, Guerra S, Wright AL. TGF-beta in human milk is associated with wheeze in infancy. J Allergy Clin Immunol. 2003;112:723–8. doi: 10.1016/s0091-6749(03)01941-9. [DOI] [PubMed] [Google Scholar]
- 7.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc (Series B) 1995;57:289–300. [Google Scholar]
- 8.Rothers J, Halonen M, Stern DA, Lohman IC, Mobley S, Spangenberg A, Anderson D, Wright AL. Adaptive cytokine production in early life differentially predicts total IgE levels and asthma through age 5 years. J Allergy Clin Immunol. 2011 Aug;128:397–402. doi: 10.1016/j.jaci.2011.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halonen M, Lohman IC, Stern DA, Ellis WL, Rothers J, Wright AL. Perinatal tumor necrosis factor-α production, influenced by maternal pregnancy weight gain, predicts childhood asthma. Am J Respir Crit Care Med. 2013 Jul;188:1. 35–41. doi: 10.1164/rccm.201207-1265OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.States DJ, Omenn GS, Blackwell TW, Fermin D, Eng J, Speicher DW, Hanash SM. Challenges in deriving high-confidence protein identifications from data gathered by a HUPO plasma proteome collaborative study. Nat Biotechnol. 2006 Mar;24:333–8. doi: 10.1038/nbt1183. [DOI] [PubMed] [Google Scholar]
- 11.Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, Ghezzi P. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–34. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Takemura G, Okada H, Miyata S, Maruyama R, Li L, Higuchi M, Minatoguchi S, Fujiwara T, Fujiwara H. Reduction of inflammatory cytokine expression and oxidative damage by erythropoietin in chronic heart failure. Cardiovasc Res. 2006;71:684–94. doi: 10.1016/j.cardiores.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, Tilg H, Moser PL, Weiss G. Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity. 2011;34:61–74. doi: 10.1016/j.immuni.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirén A, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–9. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uddin S, Kottegoda S, Stigger D, Platanias LC, Wickrema A. Activation of the Akt/FKHRL1 pathway mediates the antiapoptotic effects of erythropoietin in primary human erythroid progenitors. Biochem Biophys Res Commun. 2000;275:16–9. doi: 10.1006/bbrc.2000.3266. [DOI] [PubMed] [Google Scholar]
- 16.Bany-Mohammed F, Slivka S, Hallman M. Recombinant human erythropoietin: possible role as an antioxidant in premature rabbits. Pediatr Res. 1996;40:381–7. doi: 10.1203/00006450-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Noyan T, Onem O, Ramazan Sekeroğlu M, Köseoğlu B, Dülger H, Bayram I, Yalçinkaya AS, Bakan V. Effects of erythropoietin and pentoxifylline on the oxidant and antioxidant systems in the experimental short bowel syndrome. Cell Biochem Funct. 2003;21:49–54. doi: 10.1002/cbf.991. [DOI] [PubMed] [Google Scholar]
- 18.Budarf M, Huebner K, Emanuel B, Croce CM, Copeland NG, Jenkins NA, D’Andrea AD. Assignment of the erythropoietin receptor (EPOR) gene to mouse chromosome 9 and human chromosome 19. Genomics. 1990;8:575–8. doi: 10.1016/0888-7543(90)90047-x. [DOI] [PubMed] [Google Scholar]
- 19.Jubinsky P, Krijanovski OI, Nathan DG, Tavernier J, Sieff CA. The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood. 1997;90:1867–73. [PubMed] [Google Scholar]
- 20.Watowich S, Hilton DJ, Lodish HF. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994;14:3535–49. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–12. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sala E, Balaguer C, Villena C, Ríos A, Noguera A, Núñez B, Agustí A. Low erythropoietin plasma levels during exacerbations of COPD. Respiration. 2010;80:190–7. doi: 10.1159/000264604. [DOI] [PubMed] [Google Scholar]
- 23.Tyagi M, Goyal S, Sathyakumar K, Subbana PK. Influence of erythrocyte function-enhancing drugs on the bronchoprotective actions of chemokine receptor blockers in mice. Med Sci Monit. 2006;12:BR279–82. [PubMed] [Google Scholar]
- 24.Karaman M, Firinci F, Kiray M, Tuncel T, Bagriyanik A, Yilmaz O, Uzuner N, Karaman O. Beneficial effects of erythropoietin on airway histology in a murine model of chronic asthma. Allergol Immunopathol (Madr) 2012;40:75–80. doi: 10.1016/j.aller.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 25.Simon D, Denniston AK, Tomlins PJ, Wallace GR, Rauz S, Salmon M, Murray PI, Curnow SJ. Soluble gp130, an antagonist of IL-6 transsignaling, is elevated in uveitis aqueous humor. Invest Ophthalmol Vis Sci. 2008 Sep;49:3988–91. doi: 10.1167/iovs.08-1953. [DOI] [PubMed] [Google Scholar]
- 26.Jostock T, Müllberg J, Ozbek S, Atreya R, Blinn G, Voltz N, Fischer M, Neurath MF, Rose-John S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001 Jan;268:160–7. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 27.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad el-B, Lehr HA, Schmitt E, Bopp T, Kallen KJ, Herz U, Schmitt S, Luft C, Hecht O, Hohlfeld JM, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Renz H, Neurath MF, Galle PR, Finotto S. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005 Feb;115:313–25. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min J, Jang AS, Park SM, Lee SH, Lee JH, Park SW, Park CS. Comparison of plasma eotaxin family level in aspirin-induced and aspirin-tolerant asthma patients. Chest. 2005;128:3127–32. doi: 10.1378/chest.128.5.3127. [DOI] [PubMed] [Google Scholar]
- 29.Berkman N, Ohnona S, Chung FK, Breuer R. Eotaxin-3 but not Eotaxin gene expression is upregulated in asthmatics 24 hours after allergen challenge. Am J Respir Cell Mol Biol. 2001 Jun;24:682–7. doi: 10.1165/ajrcmb.24.6.4301. [DOI] [PubMed] [Google Scholar]
- 30.Robertson MW, Albrandt K, Keller D, Liu FT. Human IgE-binding protein: a soluble lectin exhibiting a highly conserved interspecies sequence and differential recognition of IgE glycoforms. Biochemistry. 1990 Sep;29:4. 8093–100. doi: 10.1021/bi00487a015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic Repository: E Table 1. List of Proteins Assessed in the Antibody Array.


