Abstract
Cystathionine β-synthase (CBS) deficiency (Online Mendelian Inheritance in Man [OMIM] 236200) is an autosomal recessive disorder that is caused by mutations in the CBS gene. It is the most common inborn error of sulfur metabolism and is the cause of classical homocystinuria, a condition characterized by very high levels of plasma total homocysteine and methionine. Although recognized as an inborn error of metabolism over 60 years ago, these is still much we do not understand related to how this specific metabolic defect gives rise to its distinct phenotypes. To try and answer these questions, several groups have developed mouse models on CBS deficiency. In this article, we will review various mouse models of CBS deficiency and discuss how these mouse models compare to human CBS deficient patients.
Keywords: Homocystinuria, aminoaciduria, mouse model, methionine, recessive, genetic disorder
1. Introduction to human CBS deficiency
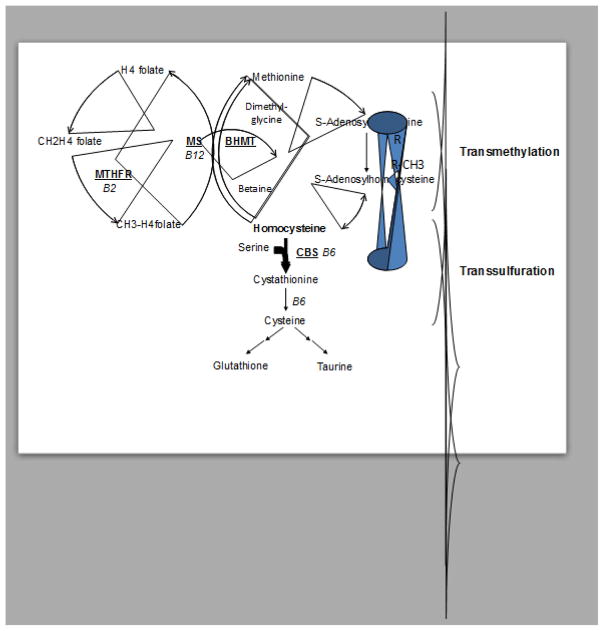
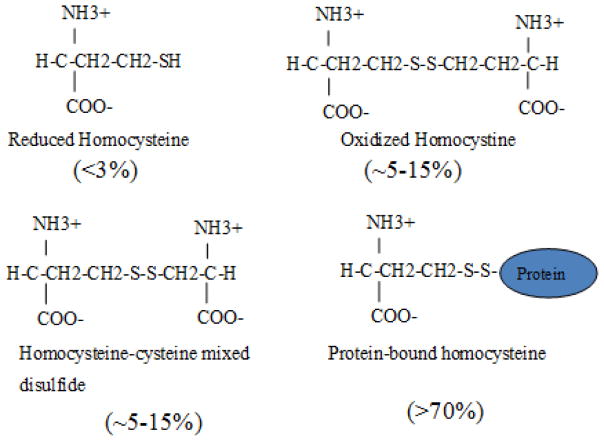
Cystathionine beta synthase deficiency (Online Mendelian Inheritance in Man [OMIM] 236200) is an autosomal recessive disorder that is caused by mutations in the CBS gene [1]. It is the most common inborn error of sulfur metabolism and is the cause of classical homocystinuria, a condition initially characterized by very high levels of homocystine in the urine [2]. Homocysteine is an intermediary amino acid metabolite that lies a critical junction point in methionine, folate, and cysteine biosynthesis (Fig. 1). Because homocysteine is a thiol with a reactive sulfur group, it can form disulfide bonds with a variety of thiol compounds including homocysteine, cysteine (both free and protein bound), and glutathione (Fig. 2). Today, most clinical labs measure total homocysteine (tHcy) in which samples are first treated with a reducing agent to break all disulfide bonds, thus allowing all homocysteine species to be liberated [3].
Fig. 1.
Homocysteine metabolism. Metabolites are in black, while key enzymes are shown underlined and in red.
Fig. 2.
Major forms of homocysteine found in blood. Approximate percentages for each form are shown as indicated.
CBS catalyzes the condensation of homocysteine with serine to form cystathionine, which is cleaved by the enzyme cystathionine δ-lyase to produce cysteine [4]. In the absence of CBS, homocysteine accumulates in tissue and is exported into the blood. In untreated patients, tHcy levels increase more than 10 to 20-fold, going from 10 μM to at least 300 μM [5]. CBS deficient patients can also have greatly elevated plasma methionine (Met), in some cases as much as 50-fold normal. This increase in Met is the basis for most neonatal screening for CBS deficiency [6]. CBS deficient patients also have a tendency for decreased plasma cystathionine, decreased total cysteine (tCys), increased S-adenosylmethionine (SAM), and increased S-adenosylhomocysteine (SAH) [5]. The incidence of CBS deficiency varies greatly by country and the methodology used to estimate severity. For western countries, the overall estimate is around 1/100,000 [7]. The highest reported frequency (1/1,800) is in the country of Qatar, due to a founder mutation and high consanguinity rates [8]. Untreated CBS-deficient patients suffer from various pathologies including thrombosis, osteoporosis, mental retardation, and dislocated lenses [9]. They often exhibit marfanoid habitus and are sometimes mistaken for individuals with mutations in fibrillin 1 [10]. Dislocated lenses (Ectopia lentis) are the most common finding in CBS deficiency, with greater than 90% of patients exhibiting this phenotype by age 25. Because this is an unusual finding in young people, it is often the way that patients are first identified [11]. Osteoporosis, as detected by radiography of the lumbar spine, is also quite common, appearing in over half the untreated patients by age 20 [9]. The major cause of morbidity in patients is thrombosis, with about 50% of untreated patients having a thrombotic event by age 30. Histopathologic and ultrasound studies show that homocystinuric patients have substantial narrowing of both major and minor vessels due to increased intima-media thickness [12]. Finally, CBS deficient patients have been reported to have a variety of central nervous system phenotypes including mental retardation, personality disorders, and behavioral abnormalities [13].
Treatment strategies for CBS deficiency generally focus on ways to lower the patients’ tHcy, as this seems to be the best predictor of clinical severity. Some patients can achieve lowering by giving pharmacologic doses of pyridoxine (vitamin B6), the precursor to pyridoxal phosphate, which is a co-factor for the CBS enzyme [14]. These so-called “B6-responders” generally produce mutant CBS proteins that have some residual enzyme activity [15], but the exact mechanism behind pyridoxine response is not well understood. For patients that do not respond to pyridoxine, a low-methionine diet in combination with B-vitamins is used to control tHcy levels [16]. While this approach can be effective, compliance with a low-methionine diet can be difficult. Betaine supplementation has also been used to lower tHcy levels [17]. This therapy works by stimulating the alternative remethylation pathway that utilizes the enzyme betaine-dependent homocysteine methyltransferase (BHMT) to convert homocysteine to methionine (Fig. 1). In addition, some patients are also given supplementation of cysteine, as cysteine and its downstream product glutathione are important in protecting cells from oxidative stress. In clinical practice, many of these strategies will be tried together along with regular monitoring of tHcy. Regardless of the exact treatment, it is clear that treated CBS deficient patients have significantly fewer clinical problems than untreated patients [11, 18].
2. Mouse model of CBS deficiency: Overcoming neonatal lethality
The first mouse model for CBS deficiency was created in 1995 [19]. In this model, exons 3 and 4 of the mouse Cbs gene were replaced with a neomycin gene as a selectable marker in a C57BL6 strain background. Homozygous animals were born about half as often as expected, but the vast majority of these animals (>90%) died between two and four weeks after birth. Using this same model, Maclean et al., observed an even more severe shortage of homozygous animals, only appearing at 33% of the expected frequency [20]. In our lab, where we genotype animals at 10–14 days of age, we too have noted a shortage of homozygous animals, with 23% fewer than the expected number and a mortality rate at five weeks greater than 90% (W.K., unpublished). The slight differences in the rate of homozygous animal production may have to do with differences in how the animals are housed, at what age they are genotyped, and other environmental differences that may occur at different facilities. Treatment of parent mice with betaine-laced drinking water (2%) had extremely modest effects on increasing the number of Cbs−/− animals born and did not appear to increase their median survival [20].
The cause of the neonatal death is liver failure. Moribund Cbs−/− mice exhibit severe fibrosis and neutrophil invasion [19, 20]. Surviving Cbs−/− mice exhibit hapatomegaly with profound steatosis and evidence of early fibrosis [20]. They also show significantly elevated levels of ALT. Livers of surviving Cbs−/− mice express elevated levels of profibrogenic and proinflammatory factors such as TNF-α and IL-6 [21]. Although neonatal Cbs−/− mice have extremely elevated plasma tHcy (>200 μM), it is not clear if the tHcy itself is directly responsible for the liver failure (see below). It should also be noted that liver damage associated in human CBS deficiency seems to be quite rare [22].
To overcome the issue of neonatal lethality, our lab created a transgenic mouse in which the human CBS encoding cDNA was placed downstream of the mouse metallothionein promoter (Tg-hCBS) [23]. These mice were then crossed to the Maeda knockout strain to generate heterozygous transgene positive animals (Tg-hCBS Cbs+/−), which were then intercrossed on zinc water to generate Tg-hCBS Cbs−/− mice. These animals are born in the expected Mendelian ratio and have near 100% survival through adulthood. While on Zinc, female Tg-hCBS Cbs−/− mice have serum tHcy that is only slightly elevated, but when zinc is removed, these levels increase to a mean of 190 μM. Male Tg-hCBS Cbs−/− animals are more variable with regards to transgene induction by zinc for reasons that are not known. Long-term studies show that Tg-hCBS Cbs−/− mice maintained on normal water from weaning have a median survival of 927 days, no different than sibling heterozygous animals [24].
Our lab also found that expression of a CBS transgene containing a patient derived I278T mutation can also rescue neonatal lethality [25]. Tg-I278T Cbs−/− mice have mean tHcy levels around 290 μM even when placed on zinc water [24]. Since the levels of tHcy in Tg-I278T Cbs−/− mice are comparable to those observed in the few non-transgenic Cbs−/− mice that make it into adulthood [25], this suggests that high tHcy levels may not be the direct cause of the acute liver failure and high neonatal lethality rates observed in non-transgenic Cbs−/− mice. Unlike the Maeda survivors, adult Tg-I278T Cbs−/− mice have normal looking livers, with very limited amounts of steatosis, and only a small elevation in ALT ([25] and WK, unpublished). However, Tg-I278T Cbs−/− mice have a shorter median lifespan than Tg-hCBS Cbs−/− mice (both on regular water) and have several other phenotypes that are either not found or greatly reduced in Tg-hCBS Cbs−/− mice [24]. These phenotypes will be discussed in more detail later.
A different transgenic strategy to rescue neonatal lethality was used by the Patterson/Kraus labs. Initially, a transgenic mouse was created by injecting a bacterial artificial chromosome (BAC) with the entire human CBS gene into the mouse germline [26]. This mouse was then crossed with the Maeda knockout mouse to generate mice lacking mouse Cbs and containing the BAC transgene [27]. Intercrossing these animals to homozygosity for Cbs− resulted in the production of the HO (“human only”) mouse. These mice have low but detectable levels of CBS activity in liver, kidney and brain. Significantly, these mice show no signs of neonatal lethality and at least half are alive at one year of age. Like Tg-I278T Cbs−/− mice, they also have only a modest increase in ALT and show very limited liver pathology [27]. One issue with these mice is that they seem to have high variability with regards to tHcy levels ranging from 56–300 μM [28]. The reason for this variability is not known.
A final strategy to overcome neonatal lethality has been to cross the Maeda knockout allele into different mouse strain backgrounds. Akahoshi et al. compared survival rates of Cbs homozygotes in four different strain backgrounds; C57/BL6J, BABL/cA, C3H/HeJ, and DBA/2J. Survival rates at 8 weeks were; 7.7%, 20%, 30.8%, and 0%, respectively [29]. The strain with the highest survival rate, C3H/HeJ, did have the lowest tHcy levels at two weeks, but the second most viable strain, BABL/cA, appeared to have increased tHcy relative to C57/BL6J, suggesting that tHcy levels are not the only reason for the differential survival.
The effects of tHcy lowering by treatment with betaine-laced (2%) drinking water has been performed on three different models. The original Maeda Cbs−/− mice fail to respond to betaine, while both the HO mice and Tg-I278T Cbs−/− mice showed significant lowering of plasma tHcy [30, 31]. Since the HO and Tg-I278T Cbs−/− mice show much less liver damage compared to the Maeda Cbs−/− animals, it is possible that betaine lowering is dependent on good liver function. Also, it has been observed that the effectiveness of betaine treatment seems to decline over time [30].
3. Metabolic sequelae
Human CBS deficiency is characterized by extreme elevations in both plasma tHcy and Met. In all the mouse models, tHcy is severely elevated, in most cases even higher than the human patients (Table 1). In adult mice, Met are fairly normal except in the Tg-I278T Cbs−/− model where they are slightly elevated [24]. The lack of hypermethioninemia may be partly due to the fact that normal mouse serum Met levels are already elevated compared to humans (22.4 vs. 60 μM) [32, 33]. However, it has been observed that two-week old CBS-deficient mice have much higher plasma Met levels, suggesting that the level of Met elevation may be related to developmental stage [29, WK unpublished]. It has not been reported if the degree of hypermethioninemia in human CBS deficient patients varies with age. CBS-deficient mice, like humans, have elevated plasma/serum SAM and SAH and reduced SAM/SAH ratio [5, 20, 34–36]. These changes are also observed in mouse tissues including liver, brain, heart, and kidney [37].
Table 1.
Metabolic Sequela in of human and mouse models of CBS deficiency
| Species | Genotype/Model/Strain | Age | Met | tHcy | Cysteine | Cystathionine | Ref. |
|---|---|---|---|---|---|---|---|
| Human | Controls | Adults | 22.4 μM | 7.4 μM | 289 μM | 157 nM | [5]2 |
| Human | CBS−/− | Unknown | 160 μM | 124.8 μM | 136 μM | 40 nM | [5]2 |
| Mouse | Controls | 8–12 weeks | 66.9 μM | 5.4 μM | 268 μM | 956 nM1 | [24]3 |
| Mouse | Tg-hCBS Cbs−/− (not induced) | 8–12 weeks | 62.6 μM | 165 μM | 198 μM | 4036 nM1 | [24]3 |
| Mouse | Tg-I278T Cbs−/− | 8–12 weeks | 115 μM | 295 μM | 167 μM | 310 nM1 | [24]3 |
| Mouse | HO Cbs−/− | Unknown | 60 μM | 290 μM | 45 μM | 8100 nM | [27]3 |
| Mouse | Cbs−/− (C57BL6) | 2 weeks | 2445 μM | 353 μM | nd | nd | [29]3 |
| Mouse | CBS−/− (C57BL6) | 3 weeks | 130 | 212 | 117 | bd | [19]3 |
Cystathionine values were determined by Stabler and Kruger, unpublished. Average values given.
Median values reported in these papers.
Average values reported in these papers.
There are differences regarding plasma/serum cystathionine levels in human and certain mouse models of CBS deficiency. In control mice, plasma/serum cystathionine levels are about six-times higher than in human patients. In human CBS patients, median serum cystathionine is about 25% of that found in control individuals, with more than half the patients with levels below the lowest value in the control range [5]. However, in both HO and the Tg-hCBS Cbs−/− models, cystathionine levels are actually elevated compared to control mice [24, 27]. In the case of HO mice, this elevation is 400% that of wild-type. Since these mice have extremely low levels of CBS activity, it has been hypothesized that this increase may be due to inhibition in cystathionine gamma-lyase (CGL) activity by homocysteine. Consistent with this idea is the finding that lowering tHcy in HO mice with betaine resulted in a three-fold decrease in cystathionine levels [27]. Interestingly, HO mice are resistant to hepatic steatosis induced by either a methyl deficient diet, or the ER stress agent tunicamycin [36]. In addition, inhibition of CGL by the inhibitor proparaglycine (PPG) attenuates steatotic renal enlargement in wild-type mice treated with tunicamycin. These findings seem to imply that cystathionine has cellular function in addition to its role as an intermediary in methionine synthesis.
With regards to downstream metabolism, total plasma/serum cysteine (tCys) levels are also lower in both human and mouse models of CBS deficiency (Table 1), roughly about a 50% reduction. Tg-I278T Cbs−/− mice also have decreased levels of liver glutathione (GSH), the major intracellular anti-oxidant [38]. However, it is unlikely that the lower level of cysteine observed in blood is a direct consequence of lack of transsulfuration activity. It has been shown that treatments that lower tHcy in Cbs−/− animals, such as dietary methionine restriction or betaine supplementation, actually raise tCys even though these treatments have no ability to increase flux from methionine [27, 39]. Also, cysteine is abundant in mouse chow. The more likely explanation is that the loss of cysteine is due to excretion of homocysteine-cysteine mixed disulfides in urine. In the HO mouse model, plasma and hepatic taurine levels were also shown to be lower [40]. This was accompanied by a large decrease in the steady state level of cysteine dioxygenase and an increase in cyteinesulfanate decarboxylase protein. Interestingly, taurine supplementation could entirely reverse these effects. Whether these cysteine downstream effects are found in human patients is unknown.
Cbs deficient mice also show elevation in homocysteine thiolactone (HTL). Hcy can be converted to HTL as a result of the error-editing function of some amino-acyl-tRNA synthase enzymes [41]. HTL, which contains an internal thioester, is extremely chemically active and reacts with the free amino group of lysine residues in proteins, forming a stable isopeptide bond [42]. CBS-deficient patients have at least a 20-fold increase in plasma HTL and in N-homocysteinylated plasma proteins [43]. Like humans, both Tg-I278T Cbs−/− and Tg-hCBS Cbs−/− mice (regular water) have significantly increased levels of N-homocysteinylated protein in both plasma and liver compared to sibling heterozygous and wild-type control animals [44]. The greatest increase was observed in the livers of Tg-I278T Cbs−/− mice, with a 12-fold increase in the levels of N-homocysteinylated protein. However, it should be noted that even in extreme cases, like Tg-I278T Cbs−/− mice, the overall amount of N-homocysteinylated lysine in protein is small; perhaps, 0.5% of all protein incorporated lysine. Given the fact that most lysine is not N-homocysteinylated, it remains an open question as to whether this modification is responsible for the in vivo phenotypes observed in hyperhomocysteinemic humans and mice.
Cbs deficient mice also exhibit alterations in liver lipid profiles. Maclean and colleagues found that the original Maeda Cbs−/− mice have significantly reduced levels of phosphotidylcholine and phosphotidylethanolamine (PEMT), a key SAM-utilizing enzyme that produces phosphatidylcholine. However, HO mice do not show this reduction, suggesting that this is secondary to liver damage [45]. Consistent with this finding are earlier studies that showed that two-week-old Watanabe mice exhibiting steatosis had significantly altered hepatic lipid profiles, but these differences were much less severe in eight-week-old survivors that showed much less steatosis [46].
4. Skeletal, fat mass, and hair abnormalities
The appearance of untreated CBS deficient patients often resembles that of individuals with Marfan syndrome, characterized by arachnodactyly, scoliosis, low BMI, and reduced fat [47–49]. Adult patients with CBS deficiency show radiologic evidence of osteoporosis or decreased bone mineral density [50]. In surviving Maeda Cbs−/−, radiography was used to show that these animals had kyphoscoliosis and temporal shortening of long bones [51]. In the Tg-I278T Cbs−/− mouse model, micro-CT studies showed reduced bone density in the femur epiphysis and vertebrate of animals as young as six months old [24]. However, in Tg-hCBS Cbs−/− mice, which have significantly lower tHcy, no differences were observed. Duel X-ray absorptiometry on 240 day old Tg-I278T Cbs−/− mice showed a decrease in bone mineral density (BMD) of 12% in males and 9% in females [52]. These same studies reveal that fat mass, but not lean mass, is significantly reduced in these animals. Dietary intervention studies show that both the low BMD phenotype and loss of fat mass could be reversed by a low methionine diet, which lowered mean tHcy from 357 to 81 μM [39]. However, betaine supplementation, which only lowered tHcy to 159 μM, was not as effective in reversing the phenotype [31]. These findings suggest that, in mice, there is a tHcy threshold that must be crossed before either osteoporosis or loss of fat mass can occur. Interestingly, in humans, it appears that patients who are compliant with treatment also have minimal changes in bone mineral density overtime [53, 54].
Some mouse models of CBS deficiency show a distinct facial alopecia phenotype. This has been observed in both non-transgenic Cbs−/− survivors and in the Tg-I278T Cbs−/− mice on the C57BL6 background [25, 55]. Histopathological studies indicate that Cbs−/− mice have wrinkled skin that is characterized by hyperkeritinosis of the epidermis and thinning of the dermal layer. Also, the mean diameter of the hair is significantly reduced. In Tg-I278T Cbs−/− mice, the facial alopecia first becomes apparent between 105 and 120 days of age and becomes more noticeable as the animals age [52]. The alopecia phenotype can be entirely eliminated by feeding the animals a low methionine diet after weaning [39]. The alopecia phenotype is much weaker in transgenic Cbs−/− mice in the C57BL6 background with tHcy levels lower than 250 μM [24, 34]. This phenotype has not been reported in either HO mice or Cbs−/− mice on other strain backgrounds. Interestingly, hair and skin phenotypes have also been noted in human CBS deficient patients, and in some cases, these phenotypes subsided upon treatment [56–58].
5. Eye phenotypes
One of the most frequent and earliest clinical signs of human CBS deficiency is the presence of a dislocated lens (ectopia lentis) caused by the fraying and disruption of the zonular fibers around the lens. Less frequent eye problems include glaucoma, optic atrophy, retinal degeneration/detachment, cataracts, and corneal abnormalities. It has been observed that about 20% of Cbs−/− mice show defects in lens opacity (Amany Twfik, personal communication). In addition, there seems to be defects in the retinal architecture. Three-week-old Cbs−/− mice have a seven-fold elevation of tHcy in the retina and have distinct alterations in retinal architecture and abnormal electroretinograms [59, 60]. Follow-up studies indicated that there was vascular leakage in the retina as determined by angiography [61]. More recently, it has been shown that Cbs−/− mice have altered retinal pigment epithelial structure [62].
6. Thrombosis and vascular function and CBS deficiency
In humans, the major cause of morbidity and mortality in CBS deficient patients is vascular thrombosis, and this has also been examined extensively in mice. These studies have involved characterization of thrombus formation, endothelial function, and atherosclerotic plaque formation. We will examine each of these areas below.
The most extensive studies examining thrombus formation have been performed in theTg-I278T Cbs−/− mouse model by Dayal and colleagues [63]. They found no difference in the rates of either arterial or venous thrombosis using a photochemical injury model in the carotid artery, a chemical injury model in both the carotid artery and mesenteric arterioles, or in a ligation model of the inferior vena cava. They also observed no differences in the mice’s hematologic profiles or in tail bleeding times. In contrast, Maclean et al. reported that the HO mice (but not the original Maeda strain) had decreased clotting times as assessed by tail vein bleeding assays [27], and that these effects could be partially reversed by treatment with betaine. Given the difficulties in standardizing and interpreting tail bleed data [64], the lack of reported changes in any of the other hematological parameters, and the fact that the shorter tail bleed times were only observed in HO mice, we think that this observation needs to be repeated in other models.
The vascular endothelium plays an important role in regulating proper haemostatic balance. Assays for endothelial function usually involve the isolation of a section of a blood vessel and examining contraction in response to agonists such as acetylcholine. Using this type of assay, Wang and colleagues reported that small mesenteric arteries from Tg-hCBS Cbs−/− mice (–Zn) exhibited reduced levels of contraction in response to acetylcholine, and presented evidence that this was caused by homocysteine-induced oxidative stress [65]. Similar endothelial dysfunction was also observed by Dayal in cerebral arterioles [63]. Taken together, these studies do seem to suggest CBS deficient mice do have some endothelial dysfunction.
Cbs−/− mice have also been examined specifically for atherosclerosis. Since mice normally do not develop atherosclerosis, it is first necessary to create an atherosclerosis prone animal. In this case, this was achieved by crossing transgenic Tg-hCBS Cbs−/− mice to the ApoE−/− mouse model of atherosclerosis [66]. Tg-hCBS Cbs−/− ApoE−/− (not on zinc) develop larger atherosclerotic lesions and show increased numbers of Ly6C+ monocytes in the lesions. In plasma, Tg-hCBS Cbs−/− ApoE−/− had increased levels of the proinflammatory cytokine TNFα, the chemotactic factor MCP-1, and elevated numbers of Ly6Chi/mid peripheral monocytes. These studies, in combination with diet-induced models of hyperhomocysteinemia [67], suggest that tHcy induced vascular problems in humans may be related to its effect on inflammatory processes.
Loss of Cbs in mice also affects certain proteins associated with HDL function. Cbs null mice have reduced plasma levels of paroxynase and arylestase activities, but HO null mice do not [28]. However, HO mice show reduced levels of hepatic apoA-1, a key component of HDL. These reduced apoA-1 levels can be partially rescued by treatment with betaine. Interestingly, these findings in mice have also been confirmed in the plasma human CBS-deficient patients, with treated patients having higher apoA-1 levels than untreated patients. These studies are particularly interesting as they show the usefulness of the mouse model for identifying potentially new phenotypes in human patients.
7. Brain phenotypes
Several neurologic phenotypes in human CBS deficient patients have been described including developmental delay and a variety of psychiatric problems including personality disorder, anxiety, depression, obsessive-compulsive behavior, and psychotic episodes [13, 68]. Two and ten-week old Cbs-deficient mice in both the C57BL6 backgrounds have been reported to have cerebellar malformation as characterized by small lobules and small intercrural fissures. However, Cbs-deficient mice in the C3H/HeJ background showed only small fissures, but not small lobules. Behavioral analysis of Cbs−/− mice on the C3H/HeJ background revealed no differences in open-field, hole-board, and elevated maze tests. However, Cbs−/− animals did perform significantly worse on a passive avoidance step-through test designed to examine learning and memory.
There have also been reports of altered levels of specific brain proteins in Cbs−/− mice. Overall protein arginine methylation was decreased in the brains of Tg-I278T Cbs−/− [37]. It has also been shown that upregulation of 5-liooxygenase protein and mRNA, which is observed in human Alzheimer’s disease, is observed in these same mice [69]. Perhaps most interestingly, it has been shown that the dentate gyrus region of the hippocampus of Tg-I278T Cbs−/− mice has elevated levels of the Alzheimer’s disease markers phospho-Tau and amyloid [70]. The same study also observed a 2.5-fold increase in degenerating neurons in Cbs−/− brains. It is not known if these findings are relevant to human patients.
8. Pyridoxine response and mice
Nearly half of human CBS deficient patients show significant lowering of plasma tHcy in response to pharmacologic doses of pyridoxine [71]. Specific mutations in the CBS gene seem to be associated with pyridoxine response, such as I278T or R266K [72, 73]. Despite a large amount of research, the exact mechanism by which pyridoxine lowers tHcy is not known. A large number of in vitro studies, using either cell extracts or purified mutant proteins, have failed to consistently show an increase in CBS activity in the presence of excess pyridoxine or pyridoxal phosphate [74, 75]. The most consistent finding is that the presence of some level of detectable residual activity is required for pyridoxine response [15].
The I278T alteration is the most common pyridoxine responsive mutation observed in CBS deficiency. In Tg-I278T Cbs−/− mice, the mutant protein is expressed in the liver and kidney and generally exhibits 1–5% of the specific activity of wild-type human CBS [25, 75]. This lack of activity is associated with decreased levels of tetramer formation as judged by native gel electrophoresis. When Tg-I278T Cbs−/− mice were given pyridoxine at 19.5 mg/kg/day via their drinking water, there was no significant change in serum tHcy despite an 8-fold increase in the level of serum PLP. Thus, the mouse model behaves consistently with respect to the studies done in all other systems.
It is interesting that while pyridoxine does not seem to enhance I278T CBS activity, other genetic and pharmacologic treatments can. In yeast, it was shown that cis-acting second site suppressor mutations, such as deletion of the C-terminal 142 amino acids or specific point mutations in the C-terminal regulatory domain, could restore function to I278T CBS protein [76, 77]. Later studies showed that addition of pharmacologic chaperones to the yeast media or genetic manipulation of certain heat shock proteins could also restore function [78–80]. In Tg-I278T Cbs−/− mice, treatment with the proteasome inhibitors bortezomib and ONX0912 could restore significant enzymatic function and lower tHcy levels [81]. These treatments are also associated with over expression of the Hsp70 chaperone protein. These findings indicate that it is possible to significantly stimulate enzyme activity I278T protein in a mouse liver. We speculate that subtle differences in the intracellular folding environment between human and mouse liver may help explain the lack of pyridoxine response, but clearly additional experiments will have to be performed to get at the bottom of this problem.
9. Concluding remarks
Overall, mouse models of CBS deficiency have proven to be a good, but not perfect models for human CBS deficiency. An unexpected problem is that homozygous null mice, unlike humans, have a large degree of neonatal lethality caused by liver failure. Fortunately, this problem can be solved by expressing either low levels of wild-type or mutant human CBS proteins. The resulting models are best at mimicking the metabolic sequela of the disease and seem to do a good job presenting similar skeletal defects and body composition differences. The mice also seem to be a good system to study the effects of dietary alterations on these phenotypes. For example, mice on a low methionine diet, like treated human patients, show a significant reduction in homocysteine-related phenotypes. With regards to vascular effects, the mouse models clearly show vascular differences, but, unlike the human condition, it is not a major source of morbidity. The reason for this probably has to do with innate differences between humans and mice with regards to the clotting cascade and atherosclerosis in general. Specifically, plaque rupture, the main source of thrombotic events in humans, is almost never observed in mice. The dislocated lens phenotype is another phenotype that is not observed in the mouse models. However, mouse models for Marfan syndrome also fail to show defects in lenses, suggesting that the lens structure in the mouse is somehow different than that in humans. Perhaps the biggest mystery is the inability to replicate the pyridoxine responsiveness of the human I278T allele when expressed in mice. Whether this is due to differences in pyridoxine metabolism or differences between the intracellular folding environment of mouse and human liver is unknown.
Highlights.
One sentence summary: In this review we compare and contrast several mouse models of human CBS deficiency to each other, and to the human condition.
Acknowledgments
Funding
We wish to thank Kathy Ireton for secretarial assistance. This work was supported by grants from the NIH (CA06927, DK101404, GM098772), the homocystinuria research foundation, and an appropriation from the Commonwealth of Pennsylvania.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mudd SH, Levy HL, Kraus JP. Disorders in transsulfuration. In: Scriver CR, Beaudet A, Sly W, Valle D, editors. The Metabolic Basis of Inherited Disease. McGraw-Hill; New York: 2001. pp. 2007–2056. [Google Scholar]
- 2.Carson NA, Cusworth DC, Dent CE, Field CM, Neill DW, Westall RG. Homocystinuria: A new Inborn error of metabolism associated with mental deficiency. Arch Dis Child. 1963;38:425–436. doi: 10.1136/adc.38.201.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobsen DW. Homocysteine and vitamins in cardiovascular disease. Clin Chem. 1998;44:1833–1843. [PubMed] [Google Scholar]
- 4.Mudd SH, Finkelstein JD, Irreverre F, Laster L. Transsulfuration in mammals: microassays and tissue distribution of three enzymes of the pathway. J Biol Chem. 1965;240:4382–4392. [PubMed] [Google Scholar]
- 5.Stabler SP, Korson M, Jethva R, Allen RH, Kraus JP, Spector EB, Wagner C, Mudd SH. Metabolic profiling of total homocysteine and related compounds in hyperhomocysteinemia: utility and limitations in diagnosing the cause of puzzling thrombophilia in a family. JIMD Rep. 2013;11:149–163. doi: 10.1007/8904_2013_235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mudd SH. Hypermethioninemias of genetic and non-genetic origin: A review. Am J Med Genet C Semin Med Genet. 2011;157:3–32. doi: 10.1002/ajmg.c.30293. [DOI] [PubMed] [Google Scholar]
- 7.Moorthie S, Cameron L, Sagoo GS, Bonham JR, Burton H. Systematic review and meta-analysis to estimate the birth prevalence of five inherited metabolic diseases. J Inherit Metab Dis. 2014;37:889–898. doi: 10.1007/s10545-014-9729-0. [DOI] [PubMed] [Google Scholar]
- 8.Zschocke J, Kebbewar M, Gan-Schreier H, Fischer C, Fang-Hoffmann J, Wilrich J, Abdoh G, Ben-Omran T, Shahbek N, Lindner M, Al Rifai H, Al Khal AL, Hoffmann GF. Molecular neonatal screening for homocystinuria in the Qatari population. Hum Mutat. 2009;30:1021–1022. doi: 10.1002/humu.20994. [DOI] [PubMed] [Google Scholar]
- 9.Mudd SH, Skovby F, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GH, Bromberg IL, Cerone R, et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 10.Summers KM, West JA, Peterson MM, Stark D, McGill JJ, West MJ. Challenges in the diagnosis of Marfan syndrome. Med J Aust. 2006;184:627–631. doi: 10.5694/j.1326-5377.2006.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 11.Mulvihill A, Yap S, O’Keefe M, Howard PM, Naughten ER. Ocular findings among patients with late-diagnosed or poorly controlled homocystinuria compared with a screened, well-controlled population. J AAPOS. 2001;5:311–315. doi: 10.1067/mpa.2001.118219. [DOI] [PubMed] [Google Scholar]
- 12.McCully KS. Vascular pathology of homocysteinemia: implications for the pathogenesis of arteriosclerosis. Am J Pathol. 1969;56:111–128. [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott MH, Folstein SE, Abbey H, Pyeritz RE. Psychiatric manifestations of homocystinuria due to cystathionine beta-synthase deficiency: prevalence, natural history, and relationship to neurologic impairment and vitamin B6-responsiveness. Am J Med Genet. 1987;26:959–969. doi: 10.1002/ajmg.1320260427. [DOI] [PubMed] [Google Scholar]
- 14.Barber GW, Spaeth GL. The successful treatment of homocystinuria with pyridoxine. J Pediatr. 1969;75:463–478. doi: 10.1016/s0022-3476(69)80274-x. [DOI] [PubMed] [Google Scholar]
- 15.Fowler B, Kraus J, Packman S, Rosenberg LE. Homocystinuria. Evidence for three distinct classes of cystathionine beta-synthase mutants in cultured fibroblasts. J Clin Invest. 1978;61:645–653. doi: 10.1172/JCI108976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komrower GM, Lambert AM, Cusworth DC, Westall RG. Dietary treatment of homocystinuria. Arch Dis Child. 1966;41:666–671. doi: 10.1136/adc.41.220.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcken DE, Wilcken B, Dudman NP, Tyrrell PA. Homocystinuria--the effects of betaine in the treatment of patients not responsive to pyridoxine. N Engl J Med. 1983;309:448–453. doi: 10.1056/NEJM198308253090802. [DOI] [PubMed] [Google Scholar]
- 18.Yap S, Naughten ER, Wilcken B, Wilcken DE, Boers GH. Vascular complications of severe hyperhomocysteinemia in patients with homocystinuria due to cystathionine beta-synthase deficiency: effects of homocysteine-lowering therapy [In Process Citation] Semin Thromb Hemost. 2000;26:335–340. doi: 10.1055/s-2000-8100. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maclean KN, Sikora J, Kozich V, Jiang H, Greiner LS, Kraus E, Krijt J, Crnic LS, Allen RH, Stabler SP, Elleder M, Kraus JP. Cystathionine beta-synthase null homocystinuric mice fail to exhibit altered hemostasis or lowering of plasma homocysteine in response to betaine treatment. Mol Genet Metab. 2010;101:163–171. doi: 10.1016/j.ymgme.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robert K, Nehme J, Bourdon E, Pivert G, Friguet B, Delcayre C, Delabar JM, Janel N. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology. 2005;128:1405–1415. doi: 10.1053/j.gastro.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 22.Snyderman SE. Liver failure and neurologic disease in a patient with homocystinuria. Mol Genet Metab. 2006;87:210–212. doi: 10.1016/j.ymgme.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Jhee KH, Hua X, DiBello PM, Jacobsen DW, Kruger WD. Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ Res. 2004;94:1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S, Kühnisch J, Mustafa A, Lhotak S, Schlachterman A, Slifker MJ, Klein-Szanto A, High KA, Austin RC, Kruger WD. Mouse models of cystathionine β-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 2009;23:883–893. doi: 10.1096/fj.08-120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Chen X, Tang B, Hua X, Klein-Szanto A, Kruger WD. Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum Mol Genet. 2005;14:2201–2208. doi: 10.1093/hmg/ddi224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butler C, Knox AJ, Bowersox J, Forbes S, Patterson D. The production of transgenic mice expressing human cystathionine beta-synthase to study Down syndrome. Behav Genet. 2006;36:429–438. doi: 10.1007/s10519-006-9046-y. [DOI] [PubMed] [Google Scholar]
- 27.Maclean KN, Sikora J, Kozich V, Jiang H, Greiner LS, Kraus E, Krijt J, Overdier KH, Collard R, Brodsky GL, Meltesen L, Crnic LS, Allen RH, Stabler SP, Elleder M, Rozen R, Patterson D, Kraus JP. A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment. Mol Genet Metab. 2010;101:153–162. doi: 10.1016/j.ymgme.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang H, Stabler SP, Allen RH, Maclean KN. Altered expression of apoA-I, apoA-IV and PON-1 activity in CBS deficient homocystinuria in the presence and absence of treatment: possible implications for cardiovascular outcomes. Mol Genet Metab. 2012;107:55–65. doi: 10.1016/j.ymgme.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 29.Akahoshi N, Kobayashi C, Ishizaki Y, Izumi T, Himi T, Suematsu M, Ishii I. Genetic background conversion ameliorates semi-lethality and permits behavioral analyses in cystathionine beta-synthase-deficient mice, an animal model for hyperhomocysteinemia. Hum Mol Genet. 2008;17:1994–2005. doi: 10.1093/hmg/ddn097. [DOI] [PubMed] [Google Scholar]
- 30.Maclean KN, Jiang H, Greiner LS, Allen RH, Stabler SP. Long-term betaine therapy in a murine model of cystathionine beta-synthase deficient homocystinuria: decreased efficacy over time reveals a significant threshold effect between elevated homocysteine and thrombotic risk. Mol Genet Metab. 2012;105:395–403. doi: 10.1016/j.ymgme.2011.11.190. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Wang L, Kruger WD. Betaine supplementation is less effective than methionine restriction in correcting phenotypes of CBS deficient mice. J Inherit Metab Dis. 2015;39:39–46. doi: 10.1007/s10545-015-9883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mustafa A, Gupta S, Hudes GR, Egleston BL, Uzzo RG, Kruger WD. Serum amino acid levels as a biomarker for renal cell carcinoma. J Urol. 2011;186:1206–1212. doi: 10.1016/j.juro.2011.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang B, Mustafa A, Gupta S, Melnyk S, James SJ, Kruger WD. Methionine-deficient diet induces post-transcriptional downregulation of cystathionine beta-synthase. Nutrition. 2010;26:1170–1175. doi: 10.1016/j.nut.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta S, Wang L, Hua X, Krijt J, Kozich V, Kruger WD. Cystathionine β-synthase p.S466L mutation causes hyperhomocysteinemia in mice. Hum Mutat. 2008;29:1048–1054. doi: 10.1002/humu.20773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keating AK, Freehauf C, Jiang H, Brodsky GL, Stabler SP, Allen RH, Graham DK, Thomas JA, Van Hove JL, Maclean KN. Constitutive induction of pro-inflammatory and chemotactic cytokines in cystathionine beta-synthase deficient homocystinuria. Mol Genet Metab. 2011;103:330–337. doi: 10.1016/j.ymgme.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maclean KN, Greiner LS, Evans JR, Sood SK, Lhotak S, Markham NE, Stabler SP, Allen RH, Austin RC, Balasubramaniam V, Jiang H. Cystathionine protects against endoplasmic reticulum stress-induced lipid accumulation, tissue injury, and apoptotic cell death. J Biol Chem. 2012;287:31994–32005. doi: 10.1074/jbc.M112.355172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esse R, Imbard A, Florindo C, Gupta S, Quinlivan EP, Davids M, Teerlink T, Tavares de Almeida I, Kruger WD, Blom HJ, Castro R. Protein arginine hypomethylation in a mouse model of cystathionine beta-synthase deficiency. FASEB J. 2014;28:2686–2695. doi: 10.1096/fj.13-246579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta S, Kuhnisch J, Mustafa A, Lhotak S, Schlachterman A, Slifker MJ, Klein-Szanto A, High KA, Austin RC, Kruger WD. Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 2009;23:883–893. doi: 10.1096/fj.08-120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta S, Melnyk SB, Kruger WD. Cystathionine beta-synthase-deficient mice thrive on a low-methionine diet. FASEB J. 2014;28:781–790. doi: 10.1096/fj.13-240770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, Stabler SP, Allen RH, Abman SH, Maclean KN. Altered hepatic sulfur metabolism in cystathionine beta-synthase-deficient homocystinuria: regulatory role of taurine on competing cysteine oxidation pathways. FASEB J. 2014;28:4044–4054. doi: 10.1096/fj.14-253633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jakubowski H. Proofreading in vivo: editing of homocysteine by methionyl-tRNA synthetase in Escherichia coli. Proc Natl Acad Sci U S A. 1990;87:4504–4508. doi: 10.1073/pnas.87.12.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jakubowski H. Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J. 1999;13:2277–2283. [PubMed] [Google Scholar]
- 43.Jakubowski H, Boers GH, Strauss KA. Mutations in cystathionine beta-synthase or methylenetetrahydrofolate reductase gene increase N-homocysteinylated protein levels in humans. FASEB J. 2008;22:4071–4076. doi: 10.1096/fj.08-112086. [DOI] [PubMed] [Google Scholar]
- 44.Jakubowski H, Perla-Kajan J, Finnell RH, Cabrera RM, Wang H, Gupta S, Kruger WD, Kraus JP, Shih DM. Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. FASEB J. 2009;23:1721–1727. doi: 10.1096/fj.08-127548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobs RL, Jiang H, Kennelly JP, Orlicky DJ, Allen RH, Stabler SP, Maclean KN. Cystathionine beta-synthase deficiency alters hepatic phospholipid and choline metabolism: Post-translational repression of phosphatidylethanolamine N-methyltransferase is a consequence rather than a cause of liver injury in homocystinuria. Mol Genet Metab. 2017;120:325–336. doi: 10.1016/j.ymgme.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda K, Kubo A, Akahoshi N, Yamada H, Miura N, Hishiki T, Nagahata Y, Matsuura T, Suematsu M, Taguchi R, Ishii I. Triacylglycerol/phospholipid molecular species profiling of fatty livers and regenerated non-fatty livers in cystathionine beta-synthase-deficient mice, an animal model for homocysteinemia/homocystinuria. Anal Bioanal Chem. 2011;400:1853–1863. doi: 10.1007/s00216-011-4703-2. [DOI] [PubMed] [Google Scholar]
- 47.Brenton DP, Dow CJ, James JI, Hay RL, Wynne-Davies R. Homocystinuria and Marfan’s syndrome. A comparison. Journal of Bone and Joint Surgery British Volume. 1972;54:277–298. [PubMed] [Google Scholar]
- 48.Gibson JB, Carson NA, Neill DW. Pathological findings in homocystinuria. J Clin Pathol. 1964;17:427–437. doi: 10.1136/jcp.17.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poloni S, Leistner-Segal S, Bandeira IC, D’Almeida V, de Souza CF, Spritzer PM, Castro K, Tonon T, Nalin T, Imbard A, Blom HJ, Schwartz IV. Body composition in patients with classical homocystinuria: body mass relates to homocysteine and choline metabolism. Gene. 2014;546:443–447. doi: 10.1016/j.gene.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Parrot F, Redonnet-Vernhet I, Lacombe D, Gin H. Osteoporosis in late-diagnosed adult homocystinuric patients. J Inherit Metab Dis. 2000;23:338–340. doi: 10.1023/a:1005618927729. [DOI] [PubMed] [Google Scholar]
- 51.Robert K, Maurin N, Vayssettes C, Siauve N, Janel N. Cystathionine beta synthase deficiency affects mouse endochondral ossification. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:1–7. doi: 10.1002/ar.a.20145. [DOI] [PubMed] [Google Scholar]
- 52.Gupta S, Kruger WD. Cystathionine beta-synthase deficiency causes fat loss in mice. PLoS One. 2011;6:e27598. doi: 10.1371/journal.pone.0027598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yap S, Naughten E. Homocystinuria due to cystathionine beta-synthase deficiency in Ireland: 25 years’ experience of a newborn screened and treated population with reference to clinical outcome and biochemical control [In Process Citation] J Inherit Metab Dis. 1998;21:738–747. doi: 10.1023/a:1005445132327. [DOI] [PubMed] [Google Scholar]
- 54.Lim JS, Lee DH. Changes in bone mineral density and body composition of children with well-controlled homocystinuria caused by CBS deficiency. Osteoporos Int. 2013;24:2535–2538. doi: 10.1007/s00198-013-2351-4. [DOI] [PubMed] [Google Scholar]
- 55.Robert K, Maurin N, Ledru A, Delabar J, Janel N. Hyperkeratosis in cystathionine beta synthase-deficient mice: an animal model of hyperhomocysteinemia. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:1072–1076. doi: 10.1002/ar.a.20082. [DOI] [PubMed] [Google Scholar]
- 56.Reish O, Townsend D, Berry SA, Tsai MY, King RA. Tyrosinase inhibition due to interaction of homocyst(e)ine with copper: the mechanism for reversible hypopigmentation in homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1995;57:127–132. [PMC free article] [PubMed] [Google Scholar]
- 57.Rao TN, Radhakrishna K, Mohana Rao TS, Guruprasad P, Ahmed K. Homocystinuria due to cystathionine beta synthase deficiency. Indian J Dermatol Venereol Leprol. 2008;74:375–378. doi: 10.4103/0378-6323.42916. [DOI] [PubMed] [Google Scholar]
- 58.Reish O, Berry SA, King RA. Spontaneous hair hyperpigmentation in response to vitamin intake in pregnancy--a clue for homocystinuria. Am J Obstet Gynecol. 1995;173:1640–1641. doi: 10.1016/0002-9378(95)90677-0. [DOI] [PubMed] [Google Scholar]
- 59.Ganapathy PS, Moister B, Roon P, Mysona BA, Duplantier J, Dun Y, Moister TK, Farley MJ, Prasad PD, Liu K, Smith SB. Endogenous elevation of homocysteine induces retinal neuron death in the cystathionine-beta-synthase mutant mouse. Invest Ophthalmol Vis Sci. 2009;50:4460–4470. doi: 10.1167/iovs.09-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu M, Sturgill-Short G, Ganapathy P, Tawfik A, Peachey NS, Smith SB. Age-related changes in visual function in cystathionine-beta-synthase mutant mice, a model of hyperhomocysteinemia. Exp Eye Res. 2012;96:124–131. doi: 10.1016/j.exer.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tawfik A, Al-Shabrawey M, Roon P, Sonne S, Covar JA, Matragoon S, Ganapathy PS, Atherton SS, El-Remessy A, Ganapathy V, Smith SB. Alterations of retinal vasculature in cystathionine-Beta-synthase mutant mice, a model of hyperhomocysteinemia. Invest Ophthalmol Vis Sci. 2013;54:939–949. doi: 10.1167/iovs.12-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ibrahim AS, Mander S, Hussein KA, Elsherbiny NM, Smith SB, Al-Shabrawey M, Tawfik A. Hyperhomocysteinemia disrupts retinal pigment epithelial structure and function with features of age-related macular degeneration. Oncotarget. 2016;7:8532–8545. doi: 10.18632/oncotarget.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dayal S, Chauhan AK, Jensen M, Leo L, Lynch CM, Faraci FM, Kruger WD, Lentz SR. Paradoxical absence of a prothrombotic phenotype in a mouse model of severe hyperhomocysteinemia. Blood. 2012;119:3176–3183. doi: 10.1182/blood-2011-09-380568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greene TK, Schiviz A, Hoellriegl W, Poncz M, Muchitsch EM. Animal Models Subcommittee of the Scientific And Standardization Committee Of The Isth, Towards a standardization of the murine tail bleeding model. J Thromb Haemost. 2010;8:2820–2822. doi: 10.1111/j.1538-7836.2010.04084.x. [DOI] [PubMed] [Google Scholar]
- 65.Cheng ZJ, Jiang XH, Kruger WD, Pratico D, Gupta S, Mallilankaraman K, Madesh M, Schafer AI, Durante W, Yang XF, Wang H. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood. 2011;118:1998–2006. doi: 10.1182/blood-2011-01-333310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X, Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang D, Fang P, Jiang X, Nelson J, Moore JK, Kruger WD, Berretta RM, Houser SR, Yang X, Wang H. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in LDLr/CBS-deficient mice. Circ Res. 2012;111:37–49. doi: 10.1161/CIRCRESAHA.112.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yap S, Rushe H, Howard PM, Naughten ER. The intellectual abilities of early-treated individuals with pyridoxine-nonresponsive homocystinuria due to cystathionine beta-synthase deficiency. J Inherit Metab Dis. 2001;24:437–447. doi: 10.1023/a:1010525528842. [DOI] [PubMed] [Google Scholar]
- 69.Li JG, Barrero C, Gupta S, Kruger WD, Merali S, Pratico D. Homocysteine modulates 5-lipoxygenase expression level via DNA methylation. Aging Cell. 2017;16:273–280. doi: 10.1111/acel.12550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khayati K, Antikainen H, Bonder EM, Weber GF, Kruger WD, Jakubowski H, Dobrowolski R. The amino acid metabolite homocysteine activates mTORC1 to inhibit autophagy and form abnormal proteins in human neurons and mice. FASEB J. 2017;31:598–609. doi: 10.1096/fj.201600915R. [DOI] [PubMed] [Google Scholar]
- 71.Mudd SSHF, Levy HL, Pettigrew KD, Wilcken B, Pyeritz RE, Andria G, Boers GJH, Bromberg IL, Cerone R, Fowler B, Grobe H, Schmidt H, Schweitzer L. The natural history of homocystinuria due to cystathionine beta synthase deficiency. Am J Hum Genet. 1983;37:1–26. [PMC free article] [PubMed] [Google Scholar]
- 72.Shih VE, Fringer JM, Mandell R, Kraus JP, Berry GT, Heidenreich RA, Korson MS, Levy HL, Ramesh V. A missense mutation (I278T) in the cystathionine beta-synthase gene prevalent in pyridoxine-responsive homocystinuria and associated with mild clinical phenotype. Am J Hum Genet. 1995;57:34–39. [PMC free article] [PubMed] [Google Scholar]
- 73.Kim CE, Gallagher PM, Guttormsen AB, Refsum H, Ueland PM, Ose L, Folling I, Whitehead AS, Tsai MY, Kruger WD. Functional modeling of vitamin responsiveness in yeast: a common pyridoxine-responsive cystathionine beta-synthase mutation in homocystinuria. Hum Mol Genet. 1997;6:2213–2221. doi: 10.1093/hmg/6.13.2213. [DOI] [PubMed] [Google Scholar]
- 74.Uhlendorf BW, Conerly EB, Mudd SH. Homocystinuria: studies in tissue culture. Pediatr Res. 1973;7:645–658. doi: 10.1203/00006450-197307000-00008. [DOI] [PubMed] [Google Scholar]
- 75.Chen X, Wang L, Fazlieva R, Kruger WD. Contrasting behaviors of mutant cystathionine beta-synthase enzymes associated with pyridoxine response. Hum Mutat. 2006;27:474–482. doi: 10.1002/humu.20320. [DOI] [PubMed] [Google Scholar]
- 76.Shan X, Kruger WD. Correction of disease-causing CBS mutations in yeast. Nat Genet. 1998;19:91–93. doi: 10.1038/ng0598-91. [DOI] [PubMed] [Google Scholar]
- 77.Shan X, Dunbrack RL, Jr, Christopher SA, Kruger WD. Mutations in the regulatory domain of cystathionine beta-synthase can functionally suppress patient-derived mutations in cis. Hum Mol Genet. 2001;10:635–643. doi: 10.1093/hmg/10.6.635. [DOI] [PubMed] [Google Scholar]
- 78.Singh LR, Chen X, Kozich V, Kruger WD. Chemical chaperone rescue of mutant human cystathionine β-synthase. Mol Genet Metab. 2007;91:335–342. doi: 10.1016/j.ymgme.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh LR, Kruger WD. Functional rescue of mutant human cystathionine β-synthase by manipulation of Hsp26 and Hsp70 levels in Saccharomyces cerevisiae. J Biol Chem. 2009;284:4238–4245. doi: 10.1074/jbc.M806387200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh LR, Gupta S, Honig NH, Kraus JP, Kruger WD. Activation of mutant enzyme function in vivo by proteasome inhibitors and treatments that induce Hsp70. PLoS Genet. 2010;6:e1000807. doi: 10.1371/journal.pgen.1000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta S, Wang L, Anderl J, Slifker MJ, Kirk C, Kruger WD. Correction of cystathionine beta-synthase deficiency in mice by treatment with proteasome inhibitors. Hum Mutat. 2013;34:1085–1093. doi: 10.1002/humu.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]