Dear Editor
Sweat glands secrete up to liters of sweat each day to maintain stable body temperature. Dynamic ion traffic across secretory cells is a key driving force for sweat secretion in the standard Na+–K+–Cl− cotransporter model [1]. In this model, acetylcholine released from sympathetic nerve endings activates K+ and Cl− channels, most likely via Ca2+ action, resulting in KCl efflux from the secretory cells. The chemical gradient created by KCl efflux activates the Nkcc1 cotransporter, leading to massive transport of Na+, K+ and Cl− ions and associated water movement for sweat formation [1,2]. Consistent with their critical early involvement, inhibition of K+ channels by Ba2+ abruptly blocked sweat secretion [3,4]. However, individual K+ or Cl− channels and their functions are unknown in sweat glands.
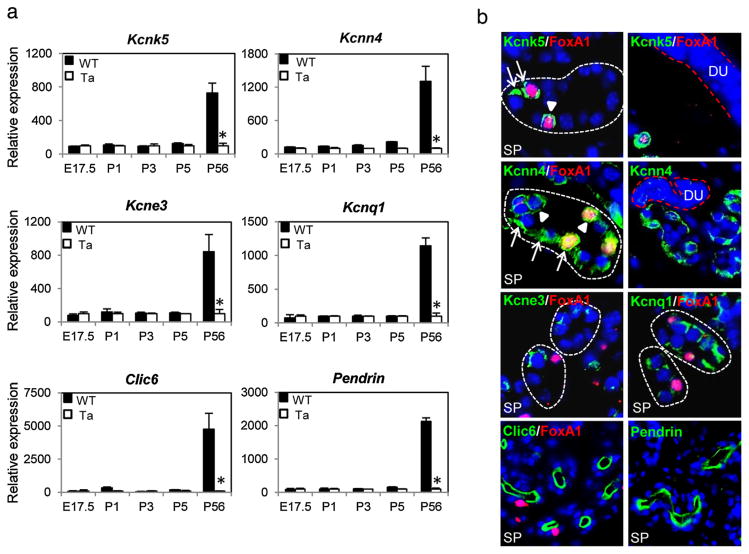
To identify K+ and Cl− channels in thermoregulatory eccrine sweat glands, we carried out expression profiling of mouse footpad skin, the sole location of sweat glands in mice [5]. At several developmental time points, including E17.5, P1, P3, P5 and 8 weeks, footpads were excised from wild-type mice and from Eda mutant Tabby mice that lack sweat glands. Isolated RNAs were profiled with NIA Mouse 44K cDNA Microarrays [5]. Dozens of genes were significantly affected in Tabby mice at different stages (profiling data deposited to Gene Expression Omnibus, #GSE73715). Our focus was of the secretory actions of mature sweat glands, and thus we looked for affected ion channels, especially K and Cl channels that are required for initiation of sweat secretion. Even in a tissue carrying out the one process of sweat secretion, four potassium channels – Kcnk5 (Task2), Kcnn4, Kcne3 and Kcnq1, and two Cl− channels – Clic6 and Pendrin (Slc26a4), all newly detected in sweat glands, were at least 7-fold more highly expressed in adult wild-type footpads compared to the Tabby footpads lacking sweat glands (Fig. 1a) [and additional K+ channels have been suggested from expression profiles of isolated sweat gland cells [6]]. Consistent with the known secretion of sweat only in mature mice, expression levels of the channels in wild-type and Tabby mice were at the background level in developing sweat glands and very high in adults (Fig. 1a, P56).
Fig. 1.
Expression patterns of newly identified K+ and Cl− channels in mouse sweat glands. (a) Expression profiling of wild-type and Tabby footpad skin revealed high expression levels of 4 K+ channels, Kcnk5, Kcnn4, Kcne3, Kcnq1, and 2Cl− channels, Clic6 and Pendrin in adult stage sweat glands. (b) Immunostaining confirmed expression of the K+ and Cl− channels in sweat gland secretory cells (SP, secretory portion enclosed by broken white lines), but not sweat ducts (DU, red broken lines). Note that, Kcnk5 is expressed only in Foxa1-positive cells. Arrows and arrowheads indicate basolateral and apical membranes of secretory cells, respectively. SP, secretory portion; DU, sweat duct. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
By immunostaining (Supplementary Materials and Methods), Kcnk5 (Fig. 1b, SP, green fluorescence) was specifically expressed in adult mice in the apical (arrowheads) and basolateral membranes (arrows) of secretory cells that are positive for a master regulator of sweat secretion, Foxa1 (Fig. 1b, red fluorescence) [7]. Kcnn4 was expressed in both Foxa1-positive and -negative secretory cells, though more intensely in Foxa1-positive cells (Fig. 1b, yellow superposition on red and green fluorescence). Kcne3 and Kcnq1 were more weakly expressed, mainly in the basolateral membranes of secretory cells. By contrast, the two Cl− channels, Clic6 and Pendrin, were abundantly expressed on the luminal membrane of secretory portions (Fig. 1b, green). Notably, none of these channels expressed more than the background level in sweat ducts (Fig. 1b, DU, Kcnk5 and Kcnn4 as examples), suggesting their primary involvement in sweat secretion rather than reabsorption.
Recently a Foxa1-Best2 cascade was identified as a critical regulator of sweat secretion [7]. Especially because Kcnk5 was exclusively expressed in Foxa1-positive secretory cells, we further analyzed whether Kcnk5, like Best2, is transcriptionally regulated by Foxa1. We isolated total RNAs from adult stage wild-type and Foxa1 mutant footpad skin and carried out qRT-PCR assays. As expected, Best2 was dramatically downregulated in the mutant footpads (Fig. S1a). However, expression levels of all six ion channels in the mutants were comparable with wild-type controls (Fig. S1a). Immunostaining showed normal expression of Kcnk5, Kcnn4 and Clic6 proteins as well in Foxa1 mutant sweat glands (Fig. S1b). These data suggested that expression of these channels requires transcription factors other than Foxa1 that need to be elucidated [2].
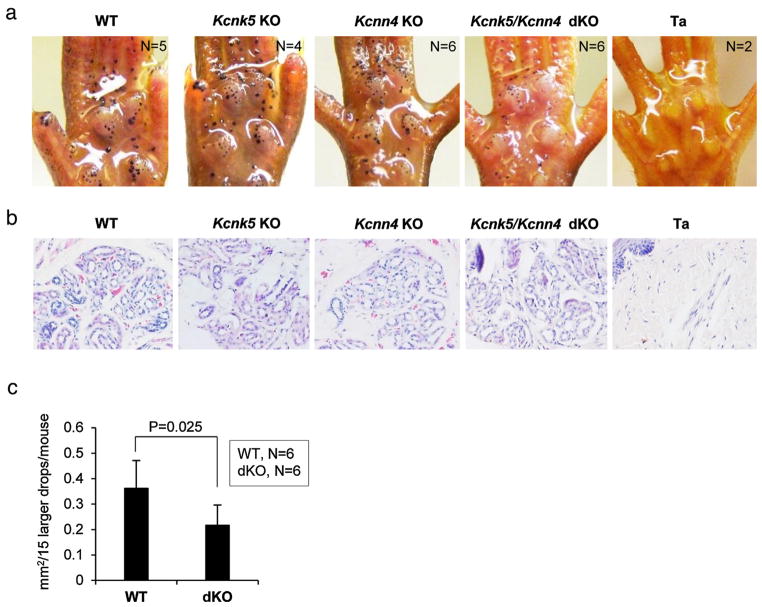
To further assess their possible involvement in sweat secretion, we analyzed knockout mouse models for Kcnk5 [8] and Kcnn4 [9]. Kcnk5 is a widely expressed pH-sensitive potassium channel [10]. Sweat glands were fully formed in the Kcnk5 knockout mice, and sweat tests showed sweating capacity comparable to wild-type controls (Fig. 2a,b). For Kcnn4, a Ca2+-activated potassium channel reported as expressed in salivary glands, stomach, colon, lung, and hematopoietic cells [9], knockout mice again had structurally normal sweat glands, and showed sweating comparable to wild-type mice (Fig. 2a,b).
Fig. 2.
Kcnk5 and Kcnn4 double knockout mice (dKO) show smaller sweat drops. (a) Iodine-starch sweat test was carried out with adult stage wild-type and knockout mice for Kcnk5, Kcnn4, dKO and Tabby. Mutant mice for Kcnk5 or Kcnn4 showed sweating capacity comparable to wild-type controls. dKO mice had reduced sweat drop size, easily discernible for larger drops. (b) Histological sections showed fully formed sweat glands in wild-type and each knockout mouse, and absence of sweat gland in Tabby. (c) The size of the larger sweat drops was reduced about 40% in dKO mice.
By contrast, a discernible phenotype was observed when Kcnk5 and Kcnn4 were simultaneously ablated (Fig. 2a and Fig. S2). The structure and numbers of sweat glands in the double-knockout (dKO) mice remained comparable to wild-type controls, and were positive in sweat tests (Fig. 2a,b). Corresponding to the variable size and capacity of individual sweat glands, the size of sweat drops was variable in all mice. However, although smaller drops were comparable in size in dKOs and wild-type controls (Fig. 2a), larger drops were visually bigger in wild-type sweat glands compared to dKOs (Fig. 2a). We measured the size of the larger drops that could be clearly defined, and found a reduction of about 40% on average in the dKO mice (Fig. 2c, Fig. S2). Thus, we infer that Kcnk5 and Kcnn4 account jointly for a fraction of sweat secretion.
The model of Sato et al. [1] implies that Ca2+ ions induce KCl efflux, perhaps through Ca2+-activated K+ or Cl− channels. However, ablation of Ca2+-activated Kcnn4 was not clearly linked to sweat reduction. Knockout of Kcnk5 alone again did not reduce sweat secretion significantly. Clear sweating decrement seen in double knockout of Kcnk4 and Kcnk5, however, suggested that there may be some redundancy of K+ channels in sweat glands, and sufficient activity remains or compensates when only one is lost. As a less likely speculative alternative, different channels might function differentially in different cells, with one cell type(s) able to increase its activity to compensate for the loss of function in another type.
Supplementary Material
Acknowledgments
This work was supported in part by Intramural Research Program of National Institute on Aging. Kcnk5 knockout mice and Kcnn4 knockout mice were kindly provided by Dr. Bayliss and Dr. Melvin, respectively.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jdermsci.2015.11.001.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Chang-Yi Cui, Laboratory of Genetics, National Institute on Aging, National Institutes of Health, 251 Bayview Blvd., Suite 100, Baltimore, MD, USA.
Jian Sima, Laboratory of Genetics, National Institute on Aging, National Institutes of Health, 251 Bayview Blvd., Suite 100, Baltimore, MD, USA.
Mingzhu Yin, Laboratory of Genetics, National Institute on Aging, National Institutes of Health, 251 Bayview Blvd., Suite 100, Baltimore, MD, USA.
Marc Michel, Laboratory of Genetics, National Institute on Aging, National Institutes of Health, 251 Bayview Blvd., Suite 100, Baltimore, MD, USA.
Makoto Kunisada, Division of Dermatology, Department of Internal Related, Graduate School of Medicine, Kobe University, Kobe, Japan.
David Schlessinger, Laboratory of Genetics, National Institute on Aging, National Institutes of Health, 251 Bayview Blvd., Suite 100, Baltimore, MD, USA.
References
- 1.Sato K, Kang WH, Saga K, Sato KT. Biology of sweat glands and their disorders. I. Normal sweat gland function. J Am Acad Dermatol. 1989;20:537–563. doi: 10.1016/s0190-9622(89)70063-3. [DOI] [PubMed] [Google Scholar]
- 2.Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Exp Dermatol. 2015;24:644–650. doi: 10.1111/exd.12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato F, Sato K. Effect of periglandular ionic composition and transport inhibitors on rhesus monkey eccrine sweat gland function in vitro. J Physiol. 1987;393:195–212. doi: 10.1113/jphysiol.1987.sp016819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saga K. Structure and function of human sweat glands studied with histochemistry and cytochemistry. Prog Histochem Cytochem. 2002;37:323–386. doi: 10.1016/s0079-6336(02)80005-5. [DOI] [PubMed] [Google Scholar]
- 5.Kunisada M, Cui CY, Piao Y, Ko MS, Schlessinger D. Requirement for Shh and Fox family genes at different stages in sweat gland development. Hum Mol Genet. 2009;18:1769–1778. doi: 10.1093/hmg/ddp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu CP, Polak L, Rocha AS, et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell. 2012;150:136–150. doi: 10.1016/j.cell.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui CY, Childress V, Piao Y, et al. Forkhead transcription factor FoxA1 regulates sweat secretion through Bestrophin 2 anion channel and Na–K–Cl cotransporter 1. Proc Natl Acad Sci U S A. 2012;109:1199–1203. doi: 10.1073/pnas.1117213109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warth R, Barrière H, Meneton P, et al. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci U S A. 2004;101:8215–8220. doi: 10.1073/pnas.0400081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begenisich T, Nakamoto T, Ovitt CE, et al. Physiological roles of the intermediate conductance, Ca2+-activated potassium channel Kcnn4. J Biol Chem. 2004;279:47681–47687. doi: 10.1074/jbc.M409627200. [DOI] [PubMed] [Google Scholar]
- 10.Gabriel A, Abdallah M, Yost CS, Winegar BD, Kindler CH. Localization of the tandem pore domain K+ channel KCNK5 (TASK-2) in the rat central nervous system. Brain Res Mol Brain Res. 2002;98:153–163. doi: 10.1016/s0169-328x(01)00330-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.