Abstract
Autism spectrum disorder (ASD) is often accompanied by gastrointestinal disturbances, which also may impact behavior. Alterations in autonomic nervous system functioning are also frequently observed in ASD. The relationship between these findings in ASD is not known. We examined the relationship between gastrointestinal symptomatology, examining upper and lower gastrointestinal tract symptomatology separately, and autonomic nervous system functioning, as assessed by heart rate variability and skin conductance level, in a sample of 120 individuals with ASD. Relationships with co-occurring medical and psychiatric symptoms were also examined. While the number of participants with significant upper gastrointestinal tract problems was small in this sample, 42.5% of participants met criteria for functional constipation, a disorder of the lower gastrointestinal tract. Heart rate variability, a measure of parasympathetic modulation of cardiac activity, was found to be positively associated with lower gastrointestinal tract symptomatology at baseline. This relationship was particularly strong for participants with co-occurring diagnoses of anxiety disorder and for those with a history of regressive ASD or loss of previously acquired skills. These findings suggest that autonomic function and gastrointestinal problems are intertwined in children with ASD; although it is not possible to assess causality in this data set. Future work should examine the impact of treatment of gastrointestinal problems on autonomic function and anxiety, as well as the impact of anxiety treatment on gastrointestinal problems. Clinicians should be aware that gastrointestinal problems, anxiety, and autonomic dysfunction may cluster in children with ASD and should be addressed in a multidisciplinary treatment plan.
Keywords: autism spectrum disorder, gastrointestinal, autonomic nervous system, sympathetic, parasympathetic, anxiety, constipation
Introduction
Autism spectrum disorder (ASD) is characterized by persistent deficits in social communication and interaction and restricted, repetitive patterns of behavior that occur early in development [American Psychiatric Association, 2013]. Co-occurring medical conditions, including gastrointestinal (GI) disorders, are common in ASD, though reports vary on the prevalence of GI disorders relative to typically developing children [McElhanon, McCracken, Karpen, & Sharp, 2014; Doshi-Velez, Ge, & Kohane, 2014; Chaidez, Hansen, & Hertz-Picciotto, 2014; Chandler et al., 2013; Gorrindo et al., 2012; Bauman, 2010; Buie et al., 2010; Mouridsen, Rich, & Isager, 2010; Ibrahim, Voigt, Katusic, Weaver, & Barbaresi, 2009]. Recent data suggests that GI symptomatology arises early in the course of ASD [Bresnahan et al., 2015]. Many individuals with ASD are non-verbal and are unable to report painful GI symptoms. As such, non-GI problem behavior may serve as a marker of abdominal pain and discomfort in ASD [Buie et al., 2010]. For instance, irritability, sleep disturbance, and aggression have been shown to be significantly increased in those with ASD relative to their typically developing siblings [Hovarth & Perman, 2002a,b]. Furthermore, children with ASD and regression/loss of previously acquired skills have been shown to have a higher frequency of GI symptoms when compared to those with ASD without regression [Valicenti-McDermott, McVicar, Cohen, Wershil, & Shinnar, 2008]. Despite these significant behavioral patterns, the pathophysiology associated with GI problems in ASD is poorly understood.
Recent data suggests that the brain and gut communicate with each other in a bidirectional manner through the central, autonomic, and enteric nervous systems [Collins, Surette, & Bercik, 2012; Mayer, 2011; Scott, Clarke, & Dinan, 2013]. The vagus nerve, a component of the parasympathetic branch of the autonomic nervous system (ANS), couples the gut to the nucleus of the solitary tract in the brain stem, and is the primary afferent pathway from the abdomen to the brain [Gillis, Quest, Pagini, & Norman, 1989]. Postganglionic sympathetic efferents project to the gut from the spinal cord, and synapse on the myenteric plexus to inhibit GI function [Aziz & Thompson, 1998]. Thus, investigating the relationship between ANS function and gastrointestinal symptomatology in ASD appears to be an important priority. Many psychophysiological studies suggest that individuals with ASD have altered ANS functioning relative to typically developing controls. Electrodermal activity (EDA), defined as the electrical conductivity between two electrodes on the skin over time, provides an index of sympathetic nervous system activity, due to the fact that eccrine sweat glands are innervated by the sympathetic but not parasympathetic branch of the ANS [Boucsein, 2012]. Studies have shown increased EDA in those with ASD relative to controls at baseline [van Engeland, 1984; Hirstein, Iversen, & Ramachandran, 2001], in response to visual and auditory stimuli [Barry & James, 1988], in response to facial stimuli [Joseph, Ehrman, & McNally, 2008; Kylliäinen & Hietanen, 2006], and in response to repetitive stimuli over time [Toichi & Kamio, 2003]. These findings suggest an enhanced stress response in ASD relative to typically developing controls. Examination of heart rate variability in the time-domain, or the variation between heart beats over time, yields information on the modulation of sympathetic and parasympathetic inputs to the sinus node of the heart. Studies have shown low cardiac vagal tone at rest in individuals with ASD relative to controls [Ming, JuLu, Brimacombe, Conner, & Daniels, 2005; Toichi & Kamio, 2003], suggesting altered parasympathetic tone. Taken together, these psychophysiological studies suggest a hyporesponsive parasympathetic system in ASD, with some associated changes in the sympathetic system as well [Kushki et al., 2013; Neuhaus, Bernier, & Beauchaine, 2014].
In the general population, there is a strong relationship between psychological and physical stress and gastrointestinal disorders, and this may interact directly with gut bacteria to increase bacterial growth and infectivity [Lyte, Vulchanova, & Brown, 2011]. Stress activates the hypothalamic-pituitary-adrenal axis, resulting in the neuronal release of catecholamines, activating the sympathetic nervous system [Elenkov & Chrousos, 2006], which has been shown to affect the gut mucosa [Lyte, et al., 2003]. Sympathetic efferents can inhibit gut motility [Lomax, harkey, & Furness, 2010; Hirst & McKirdy, 1974], suggesting a mechanism for constipation. This may involve bidirectional communication between the enteric nervous system, the intrinsic, reflexive nervous system of the GI tract, and the central nervous system. Increased sympathetic functioning and decreased parasympathetic functioning have both been noted in individuals with constipation predominant irritable bowel syndrome (IBS) [Mazur Fugala, Jablonski, Mach, & Thor, 2012], in association with a range of autonomic disturbances in IBS [Martinez-Martinez, Mora, Vargas, Fuentes-Iniestra, & Martinez-Lavin, 2014; Pellissier, Dantzer, Canini, Mathieu, & Bonaz, 2010], though this literature is still evolving [Mazurak, Seredyuk, Sauer, Teufel, & Enck, 2012]. Diarrhea can also be frequently observed, however, as part of the stress reaction, which includes sympathetic activation, in patients with irritable bowel syndrome [Bouchoucha, Hejnar, Devroede, Babba, & Benamouzig, 2013]. Despite the literature describing alterations in both ANS and GI function in ASD, little is known about the relationship between these two systems in ASD.
Altered autonomic functioning in ASD may play a role in the etiology of GI disorders in ASD. GI disturbance, however, may also impact ANS function. In the present study, our aim was to investigate the relationship between GI symptoms and psychophysiological measures of autonomic functioning at rest and during challenge by mild stressors in children and adolescents with a confirmed diagnosis of ASD. To our knowledge, the relationship between GI symptoms and markers of autonomic function has not been studied previously in this population. Given the presence of autonomic disturbances in ASD, and the prevalence of GI disorders in ASD, understanding this relationship in the ASD population is an important exploratory first step in identifying potentially salient biomarkers that may impact treatment approaches. In order to explore this relationship, we examined sympathetic and parasympathetic correlates of GI symptoms by measuring heart rate variability and skin conductance in a large sample of children and adolescents with ASD with varying degrees of GI dysfunction. To gain a better understanding of the impact of this association, we also explored the relationships among GI symptoms, adaptive functioning, and other co-occurring symptoms. Given the high frequency of constipation in ASD [McElhanon et al., 2014, Buie et al., 2010], parasympathetic alterations in ASD [Kushki et al., 2013; Neuhaus et al., 2014], and the finding of decreased parasympathetic functioning in those with IBS without ASD, we predicted that decreased parasympathetic activity, both at baseline and in response to mild stress, will be associated with greater lower GI tract symptoms, in particular, constipation. Furthermore, due to the relationship between stress and upper GI tract problems in the general population such as gastroesophageal reflux disease [Perlman et al., 2011] and Crohn’s disease [Stasi & Orlandelli, 2008], we hypothesized that positive relationships would exist, both at baseline and in response to mild stress, between sympathetic markers of stress and upper GI tract symptoms.
Methods
Participants
Children and adolescents were recruited through the Autism Speaks Autism Treatment Network (AS-ATN) registries at the University of Missouri Thompson Center for Autism and Neurodevelopmental Disorders in Columbia, Missouri and the Vanderbilt Kennedy Center and Monroe Carrell Jr. Children’s Hospital at Vanderbilt University in Nashville, Tennessee. To expand the sample, additional individuals were recruited outside of the AS-ATN registry at both sites. Individuals were included in the study if they were between the ages of 6 and 18 years and had a diagnosis of ASD. All participants were diagnosed with ASD based on the Diagnostic and Statistical Manual for Mental Disorders IV-TR criteria [American Psychiatric Association, 2000] and administration of the Autism Diagnostic Observation Schedule (ADOS) [Lord et al., 1989] to verify diagnosis. Individuals were excluded from the study if they had a known metabolic or genetic disorder, or a bleeding disorder.
Individuals that provided previous consent to be contacted about participating in research studies and that met inclusion and exclusion criteria were initially recruited by telephone or e-mail. Those interested in participating were administered the complete Questionnaire on Pediatric Gastrointestinal Disorders Rome III [QPGS Rome III; Walker, Caplan, & Rasquin, 2000]. The phone screen QPGS Rome III used parent-report to assess the frequency, severity, and duration of GI symptoms on a 5-point scale, in addition to several Yes/No questions regarding the presence or absence of specific symptoms. An effort was made to recruit an equal number of participants with and without a GI disorder at each study site. A total of 80 participants were recruited at the University of Missouri, and 40 at Vanderbilt University, for an overall total of 120 participants. A summary of the participant demographics is shown in Table 1.
Table 1.
Demographic Characteristics of the Sample
| % (n) Mean (std) |
Range | N | ||
|---|---|---|---|---|
| Male gender | 90.0% (108) | 120 | ||
| Caucasian, including multiracial | 92.5% (111) | 120 | ||
| Age at consent (years) | 11.8 (3.8) | 6–18 | 120 | |
| FSIQ | 84.0 (22.6) | 36–130 | 100 | |
| Vineland Standard Score | Composite | 72.3 (12.0) | 45–111 | 83 |
| Communication | 74.4 (14.6) | 44–129 | 83 | |
| Daily Living Skills | 77.4 (14.3) | 33–114 | 83 | |
| Socialization | 71.2 (13.3) | 40–103 | 83 | |
| ABC (calculated) | Irritability | 12.6 (10.4) | 0–42 | 117 |
| Lethargy | 10.2 (8.7) | 0–43 | 117 | |
| Stereotypy | 5.3 (4.9) | 0–21 | 117 | |
| Hyperactivity | 16.7 (11.9) | 0–46 | 117 | |
| Inappropriate speech | 3.8 (3.1) | 0–11 | 117 | |
| CSHQ total score | 45.5 (8.9) | 31–71 | 86 | |
| Upper GI tract score | 4.9 (5.4) | 0–24 | 120 | |
| Lower GI tract score | 17.9 (12.4) | 1–48 | 120 | |
| Rome III diagnoses | Functional constipation | 42.5% (51) | 120 | |
| Irritable bowel syndrome | 11.7% (14) | 120 | ||
| Lower abdominal pain associated with bowel symptoms | 9.2% (11) | 119 | ||
| Upper abdominal pain associated with bowel symptoms | 7.5% (9) | 120 | ||
| Aerophagia | 5.8% (7) | 120 | ||
| Abdominal migraine | 5.0% (6) | 119 | ||
| Functional abdominal pain | 3.3% (4) | 120 | ||
| Nonretentive fecal incontinence | 3.4% (4) | 119 |
(FSIQ: Full Scale Intelligence Quotient; ABC: Aberrant Behavior Checklist; CSHQ: The Children’s Sleep Habits Questionnaire.
Assessment of gastrointestinal symptomatology
To allow for the analysis of GI symptoms on a continuum, the QPGS Rome III was scored for each participant using a scoring rubric created by the research team. The multiple choice responses to the questions pertaining to the ten functional pediatric GI disorders assessed by the QPGS Rome III were assigned ratings, and a quantitative score was created by summing over the ratings (scored on scales of 1–3, 0–4, 1–5, or 0–5, in accordance with the QPGS Rome III scoring criteria for each designated item; Yes/No responses were assigned 1 point each). Separate scores were summed for upper and lower GI tract disorders to study their psychophysiological profiles independently (see Supporting Information Table 1 for a list of the Rome III upper and lower GI symptoms assessed in this study). Furthermore, items that were included multiple times throughout the scoring rubric for different GI disorders (e.g., item A1, “upper abdominal pain or discomfort ‘several times a week’ or more often,” is scored 4 times throughout the questionnaire, each contributing to different categories of functional pediatric GI disorders, such as Functional Abdominal Pain and Irritable Bowel Syndrome) were only scored once. For items where lower numbers indicated greater severity, the scoring was reversed such that greater scores indicated greater severity (i.e., items A6, B5, C1, C2). Items answered as “It depends” or “Don’t know” were scored as missing. These quantitative scores represented the duration, frequency, and severity of upper and lower GI tract symptomatology (See Supporting Information Table 1 for the complete scoring rubric). Given the age range of the participants and varying levels of verbal and cognitive functioning, the parent-report forms were administered to most families, and were completed by the participant’s caretaker. In four higher functioning individuals 17 years of age and older, where the parent indicated the participant would give the most reliable response, the child/adolescent self-report form was completed by the participant. The QPGS Rome III has been shown to be a reliable measure of functional GI disorders [Van Tilburg, Squires, Blois-Martin, Leiby, & Langseder, 2013], and Rome III criteria show adequate construct validity [Saps et al., 2014].
Psychophysiology protocol
In order to examine ANS functioning and reactivity to stress, heart rate variability (pNN50, as described below, to assess parasympathetic modulation of cardiac activity) [Kleiger, Stein, & Bigger, 2005; Task Force, 1996] and skin conductance level, (as described below, to assess sympathetic nervous system activity) [Lidberg & Wallin, 1981] were collected. A BIOPAC MP 150 modular data acquisition and analysis system attached to a laptop computer was used to collect all psychophysiology data (BIOPAC Systems, Inc., Goleta, CA). Electrocardiogram (ECG) data were collected utilizing a BIOPAC ECG-100C amplifier outfitted with an MEC110C module extension cable and LEAD110 electrode leads (BIOPAC Systems, Inc., Goleta, CA) attached to a MP150 data acquisition system. The ECG-100C amplifier was set at a gain of 1000, and a low pass filter of 0.05 Hz. Participants were outfitted with a 2-lead ECG setup consisting of BIOPAC EL503 Ag/AgCl disposable electrodes with a moderate adhesive backing for contact with the skin. One lead was placed below the right clavicle, in the mid-clavicular line within the frame of the rib cage, and the other on the lower left abdomen within the rib cage frame. A ground was obtained through the VInconnection on the EDA100C amplifier, and so it follows that a grounding ECG lead was not placed on the chest. After placing electrodes on the participant, the ECG signal was verified by observing a QRS complex. Electrodes were replaced on the participant if the initial ECG signal was not suitable for analysis. Skin conductance data were collected using two reusable skin conductance transducers filled with isotonic gel connected to a BIOPAC GSR-100 amplifier attached to the MP150. Transducers were placed on the distal phalanges of the participant’s hand to measure skin conductance response. All psychophysiology data were acquired using AcqKnowledge Data Acquisition and Analysis Software Version 4.2 (BIOPAC Systems, Inc., Goleta, CA).
Stress reactivity protocol
Participants were seated at a table, directly opposite the investigator. Participants who were not able to remain seated at the table by themselves were permitted to sit in their caregiver’s lap for the duration of the study or until they felt comfortable being seated on their own. Seven participants were excluded from the study for not being able to comply with these instructions. At the beginning of the stress reactivity protocol, the researcher instructed the participant to sit still, remain quiet, and breathe normally during which 3 min of baseline ECG and skin conductance data were collected. Next, the participant engaged in either vibrotactile stimulation or cold pressor stimulation to the hands in a counter-balanced fashion, where the order of vibrotactile and cold pressor stimulation was reversed after every 10 participants to account for potential order effects. Although the cold pressor test has been established in the research literature as a method for eliciting a momentary increase in sympathetic nervous activity, [Zvan, Zaletel, Pretnar, Pogacnik, & Kiauta, 1998], we also wanted to also test the effects of vibrotactile stimulation to the hands given a recent report suggesting that vibrotactile stimulation can elicit changes in heart rate and blood pressure, indicating an increase in sympathetic nervous system activity [Foster et al., 2013]. This condition was also implemented as a secondary stressor given the expectation that some children would not tolerate the cold pressor test. For the vibrotactile condition, participants were instructed to grasp a vibrating stimulator (Conair WM200X, Stamford, CT) that was held by the researcher at an approximate height of the participant’s chest by placing the palmar surface of their hand on the middle of the stimulator and wrapping their fingers around the top edge of the device. The stimulator was then switched on “high,” and ECG data were collected for 30 sec. The stimulator produces 80 Hz oscillations at 1-mm amplitude on the “high” setting. Since the vibrotactile stimulator would result in artifact during skin conductance data collection, skin conductance data were not analyzed for the vibrotactile stimulation condition. However, the skin conductance transducers remained on the participant’s fingers as this connection provided the electrical ground for the ECG amplifier. Immediately following this procedure, the stimulator was removed from the participant’s hand, and a 3-min rest period was initiated. After the 3-min rest period, the skin conductance transducers were moved to the opposite hand, and the vibrotactile protocol explained above was repeated, with a 3-min rest period after vibrotactile stimulation. For the cold pressor test, a cooler was calibrated to a target temperature of 48C using ice and tap water. To encourage test compliance, a small yellow rubber duck was placed in the cooler, and participants were instructed to press the duck to the bottom of the cooler with their hand and hold it down for 30 sec. ECG and skin conductance data were collected from the opposite hand for 30 sec, immediately followed by a 3-min rest period. After the rest period, the skin conductance transducers were switched to the participant’s other hand, and the cold pressor test was repeated as above. A timeline of the order of tasks in the stress reactivity protocol is demonstrated in Figure 1 for the vibrotactile stimulation-first condition.
Figure 1.
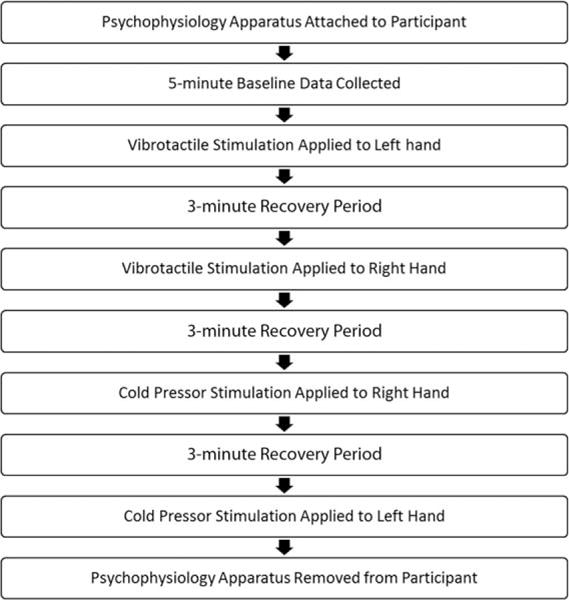
Illustration of the order of tasks in the stress reactivity protocol, for the vibrotactile stimulation-first condition.
Additional measures
The participant’s caretaker completed the Vineland Adaptive Behavior Scales, Second Edition (Vineland-II) [Sparrow, Cicchetti, & Balla, 2005], Aberrant Behavior Checklist (ABC) [Aman, Singh, Stewart, & Fields, 1985], the Sensory Over-Responsivity Scale (SensOR) [Schoen, Miller, & Green, 2008], the Children’s Sleep Habits Questionnaire (CSHQ) [Owens, Spirito, & McGuinn, 2000] and provided a self-reported/caregiver-reported list of co-occurring disorders from the Autism Treatment Network Parent Baseline Questionnaire (see Supporting Information Table 2). During the study visit, participants also provided blood and saliva samples after the initial psychophysiology baseline recording and provided a second saliva sample at the conclusion of the study, for use in a separate study. Participants were given approximately 10 min to rest and consume a small snack after the blood draw.
Data processing and statistical methods
ECG data were visually inspected, and records with excessive motion artifacts were excluded from further analysis. Data were then imported into Kubios HRV, Version 2.2 [Tarvainen, Niskanen, Lipponen, Ranta-aho, & Karjalainen, 2014]. R-R intervals, or the amount of time between heart beats, were determined by QRS detection, and were then visually inspected for errors. The R-R interval is influenced by vagal nerve (i.e., parasympathetic) activity [Katona, Poitras, Barnett, & Terry, 1970], and increases in the R-R interval are associated with increases in parasympathetic tone, whereas decreases in the R-R interval are associated with decreases in parasympathetic tone. Increases and decreases in parasympathetic activity to the heart create beat-to-beat variations in heart rate, termed heart rate variability (HRV). For the present study, HRV was assessed by determining the percentage of pairs of consecutive R-R intervals that differed by more than 50 ms, widely known as pNN50 [Bigger et al., 1988]. pNN50 values were calculated for the initial baseline reading and each vibrotactile and cold pressor stimulus condition.
Skin conductance (SCL) data were processed in AcqKnowledge, Version 4.2 (BIOPAC, Goleta, CA). The data were visually inspected by a study team member with extensive experience in ECG and skin conductance data collection and analysis, and those with excessive artifacts due to motion were removed from further analysis. Mean skin conductance level data in microsiemens (mS) were then determined for each baseline and the cold pressor condition.
Statistics
The upper and lower GI tract sum scores, as described in the Section on Assessment of gastrointestinal symptomatology, represent GI symptomatology. In order to normalize the distribution of the psychophysiological variables, the pNN50 data were root-arcsine transformed and the mean RR data were log transformed. Three variables were analyzed for each psychophysiological endpoint: first baseline, a cold pressor minus baseline change score, and a vibrotactile minus baseline change score. As previously mentioned, the vibrotactile condition was not available for the skin conductance endpoint due to artifact from the vibrotactile stimulator.
The relationships between GI scores and psychophysiological variables, as well as secondary behavioral variables such as ABC and Vineland, were assessed through Pearson partial correlations controlling for age and gender. ANOVA was used to assess differences in GI scores when key comorbidities were reported, and Cohen’s d is reported as a measure of effect size. Behavioral variables and key comorbidities were considered candidate effect modifiers of the associations between GI scores and psychophysiological variables; these relationships were tested using likelihood ratio tests on interaction terms in multiple linear regression.
Nonparametric versions of some of the statistical tests described above and modifications to the GI scoring algorithm were performed in order to assess the sensitivity of our results to model assumptions and missing data in the QPGS Rome III. Modifications to the GI scoring algorithm utilized to account for the effect of missing data included (a) defining the score as the mean of the non-missing items instead of the sum, (b) excluding participants with more than three missing items, (c) a square-root transform of the sum-score, and (d) removal of several large outliers in the mean R-R data. Conclusions did not differ, and results are not reported. Finally, a significance threshold of 0.05 was used to report findings, and the issue of reporting and interpreting P-values in light of the multiple comparisons problem is discussed further in the Discussion section.
Results
Participants
Of the 120 participants, 108 (90%) were male, the average age was 11.8 years (SD 3.8), and the average full scale intelligence quotient (FSIQ) from their AS-ATN data (performed by trained psychometricians at each ATN site, specific IQ tests chosen based on the participant’s verbal ability) was 84.0 (SD 22.6), as observed in Table 1, where average scores on adaptive behavior and aberrant behavior scales are also noted. The most frequent gastrointestinal disorders present in the sample were functional constipation (42.5%), lower abdominal pain associated with irritable bowel symptoms (9.2%) and upper abdominal pain associated with irritable bowel symptoms (7.5%) according to Rome III criteria. Primary analyses therefore focused on lower GI tract symptoms.
Owing to motion artifact at initial baseline ECG or a lack of protocol compliance, 10 participants were excluded from all ANS analyses. For the vibrotactile and cold pressor stimulus conditions, a total of 109 and 106 participants were included in the ECG analyses, respectively. After exclusion for motion artifact on the cold pressor stimulus condition, analysis for skin conductance included a total of 84 participants.
Psychophysiological markers and lower GI tract symptomatology
For lower GI tract score, a significant positive relationship between QPGS Rome III lower GI tract score and the primary parasympathetic marker, baseline pNN50, was observed while controlling for age and gender, P = 0.039, r = 0.20, 95% CI [0.01, 0.37]. A significant negative relationship between QPGS Rome III lower GI tract score and the pNN50 change score for the cold pressor condition was observed, P = 0.015, r = −0.24, 95% CI [−0.41, −0.05]. Thus, subjects with worse lower GI symptoms (a higher score for lower GI) tend to have greater parasympathetic tone at baseline, but lower parasympathetic tone change-score in response to cold pressor stimulation. There was no significant relationship with the change score for the vibrotactile condition The correlations between lower GI tract symptomatology and the mean RR interval and SCL variables were low in magnitude and not statistically significant (Table 2).
Table 2.
Pearson Partial Correlations Between GI Scores and Endpoints
| Endpoint | Lower GI tract score
|
Upper GI tract score
|
||||
|---|---|---|---|---|---|---|
| Correlation (95% CI) | P-value | N | Correlation (95% CI) | P-value | N | |
| pNN50: First baseline | 0.20 (0.01, 0.37) | 0.0394 | 110 | 0.11 (−0.08, 0.29) | 0.2712 | 110 |
| pNN50: Vibrotactile-baseline | −0.13 (−0.31, 0.07) | 0.1932 | 109 | 0.08 (−0.11, 0.26) | 0.4276 | 109 |
| pNN50: Cold Pressor-baseline | −0.24 (−0.41, −0.05) | 0.0150 | 106 | −0.11 (−0.30, 0.08) | 0.2526 | 106 |
| meanRR: First baseline | −0.01 (−0.20, 0.18) | 0.9292 | 110 | −0.03 (−0.22, 0.16) | 0.7534 | 110 |
| meanRR: Vibrotactile-baseline | 0.04 (−0.15, 0.23) | 0.6966 | 109 | 0.16 (−0.03, 0.34) | 0.0968 | 109 |
| meanRR: Cold pressor-baseline | −0.11 (−0.30, 0.08) | 0.2597 | 106 | −0.01 (−0.20, 0.19) | 0.9407 | 106 |
| Skin conductance: First baseline | 0.15 (20.07, 0.35) | 0.1829 | 84 | 0.12 (−0.10, 0.33) | 0.2798 | 84 |
| Skin conductance: Cold pressor-Baseline | −0.14 (−0.35, 0.09) | 0.2278 | 78 | −0.17 (−0.38, 0.06) | 0.1391 | 78 |
| Vineland socialization score | −0.09 (−0.30, 0.13) | 0.4354 | 83 | −0.07 (−0.29, 0.15) | 0.5163 | 83 |
| Vineland daily living skills score | −0.08 (−0.29, 0.14) | 0.4828 | 83 | −0.08 (−0.29, 0.14) | 0.4661 | 83 |
| Vineland composite score | −0.12 (−0.33, 0.10) | 0.2979 | 83 | −0.12 (−0.33, 0.10) | 0.2959 | 83 |
| Vineland communication score | −0.10 (−0.31, 0.12) | 0.3922 | 83 | −0.16 (−0.37, 0.06) | 0.1500 | 83 |
| FSIQ | −0.00 (−0.20, 0.20) | 0.9870 | 100 | −0.10 (−0.30, 0.10) | 0.3070 | 100 |
| CSHQ total score | 0.37 (0.17, 0.54) | 0.0005 | 86 | 0.33 (0.12, 0.51) | 0.0020 | 86 |
| ABC stereotypy | 0.08 (−0.10, 0.26) | 0.3919 | 117 | 0.02 (−0.17, 0.20) | 0.8582 | 117 |
| ABC lethargy | −0.02 (−0.20, 0.16) | 0.8221 | 117 | 0.15 (−0.04, 0.32) | 0.1163 | 117 |
| ABC irritability | 0.20 (0.01, 0.37) | 0.0346 | 117 | 0.16 (−0.03, 0.33) | 0.0928 | 117 |
| ABC inappropriate speech | 0.00 (−0.18, 0.19) | 0.9694 | 117 | 0.11 (−0.08, 0.28) | 0.2535 | 117 |
| ABC hyperactivity | 0.13 (−0.05, 0.31) | 0.1525 | 117 | 0.14 (−0.04, 0.31) | 0.1360 | 117 |
[Partial Correlations Controlled for Age and Gender pNN50 and meanRR Variables Transformed Prior to Analysis. Vineland Scores are Standard Scores and ABC Scores are Calculated].
pNN50: percentage of normal R-R intervals that differed by 50 milliseconds or more; meanRR: average amount of time between R-R intervals; FSIQ: full-scale intelligence quotient; CSHQ: The Children’s Sleep Habits Questionnaire; ABC: Aberrant Behavior Checklist.
Bold type for P < 0.05, italicized for P < 0.1.
An individual’s degree of reactivity to either the vibrotactile or the cold pressor condition may be highly dependent upon sympathetic and parasympathetic baseline tone. If a participant enters the testing environment already maximally activated, then reactivity to these stimuli may be reduced. In order to assess the potential for this possible dependent relationship, we examined the correlation between baseline pNN50 and pNN50 change score for the cold pressor condition. A significant inverse correlation was observed, P < 0.001, r = −0.49, 95% CI [−0.63, 0.33], consistent with the possibility that reactivity to cold pressor might be dependent on baseline parasympathetic tone.
It is possible that participants taking medications that interact with gut motility, the adrenergic system, or the serotonergic system (i.e., stimulants, alpha-2 agonists, beta-adrenergic antagonists, neuroleptics, antidepressants, antiepileptics, or drugs directly impacting gut motility) could be a potential confound. Thus, participants taking the aforementioned medications were removed, and separate analyses were conducted. After removal of these participants, the relationship between lower GI tract score and pNN50 change score for the cold pressor condition remained significant (P = 0.020, r = −0.35, 95% CI [−0.59, −0.05] with n = 44), and a trend emerged for a relationship with the pNN50 change score for the vibrotactile condition (P = 0.062, r = −0.28, 95% CI [−0.54, 0.02] with n = 45). The relationship with baseline pNN50 was no longer statistically significant (P = 0.131, r = 0.23, 95% CI [−0.07, 0.50] with n = 45) in the reduced sample, however the magnitude of the correlation remained the same.
Psychophysiological markers and upper GI tract symptomatology
For upper GI tract symptomatology, no significant relationships were detected with any of the psychophysiological variables. Relationships with baseline pNN50 and the pNN50 cold pressor change score were not significant. Thus, the stress response at baseline and in response to stressors is not related to upper GI tract symptoms in this sample.
GI symptomatology and sleep, adaptive behaviors, and aberrant behaviors
Significant positive relationships were observed between lower GI tract symptomatology and the ABC irritability subscale (P = 0.035, r = 0.20, 95% CI [0.01, 0.37]) and CSHQ total score (P < 0.001, r = 0.37, 95% CI [0.17, 0.54]). These relationships were also observed with the upper GI tract symptomatology (a trend for ABC Irritability: P = 0.093, r = 0.16, 95% CI −0.03, 0.33], and significant for CSHQ total score: P = 0.002, r = 0.33, 95% CI [0.12, 0.51]). ABC hyperactivity significantly modified the relationship between lower GI tract symptomatology and the pNN50 cold pressor change score (P = 0.028 for the interaction term). The negative association between lower GI tract symptomatology and the cold pressor change score is greatest among those with low hyperactivity scores and attenuates as hyperactivity increases, suggesting that hyperactive participants may not be as sensitive to changes in parasympathetic stimulation. A summary of the findings can be found in Table 3. Sleep, adaptive behavior, and other aberrant behavior measures were not found to modify any of the significant relationships between GI symptomatology and the psychophysical markers described above.
Table 3.
Comorbidity Status and Mean GI Scores by Presence/Absence of Comorbidity
| Comorbidity | Frequency | Mean lower GI tract score
|
Mean upper GI tract score
|
||||
|---|---|---|---|---|---|---|---|
| Present | Absent | P-value | Present | Absent | P-value | ||
| Attention deficit hyperactivity disorder | 42.5% | 20.4 | 16.2 | 0.067 | 5.8 | 4.3 | 0.140 |
| Anxiety disorder | 35.8% | 19.7 | 17.0 | 0.261 | 7.1 | 3.7 | <0.001 |
| Depression | 28.3% | 19.2 | 17.5 | 0.495 | 6.6 | 4.2 | 0.027 |
| Loss of skills/regression | 30.8% | 22.8 | 15.8 | 0.003 | 8.2 | 3.4 | <0.001 |
| Seizures | 13.3% | 21.2 | 17.4 | 0.263 | 8.7 | 4.3 | 0.003 |
Presence/Absence Classified as Yes vs. No/Unknown ANOVA P-values.
Bold type for P < 0.05, italicized for P < 0.1.
GI symptoms and co-occurring medical or psychiatric symptoms
Co-occurring diagnoses were documented in the AS-ATN record for most participants. Our interest focused on anxiety symptoms, while also examining ADHD, depression, regression/loss of skills and seizures. Individuals with anxiety disorder (Cohen’s d = 0.66), depression (Cohen’s d = 0.45), loss of skills/regression (Cohen’s d = 0.95), or seizures (Cohen’s d = 0.83) had significantly higher upper GI tract scores, and individuals with loss of skills/regression (Cohen’s d = 0.59) had significantly higher lower GI tract scores. (See Table 3)
Furthermore, presence or absence of anxiety symptoms was found to be a significant effect modifier for the relationship between lower GI tract score and pNN50 Baseline (P = 0.035 for the interaction term). This interaction is illustrated in Figure 2A: the slope of the regression line for lower GI tract score on baseline pNN50 is near zero among individuals without anxiety disorder (slope = 3.80) and relatively steep among those with anxiety disorder (slope = 27.60), suggesting that individuals with anxiety symptoms are at an increased risk for lower GI tract symptoms, particularly in the setting of greater parasympathetic tone. The presence or absence of a history of regression/loss of skills was also found to modify the relationship between lower GI tract score and base-line pNN50 (P = 0.016 for the interaction term, slope in the absence of regression/loss of skills = 2.73, slope in presence of regression/loss of skills = 30.49, see Fig. 2B), suggesting that individuals with regressive autism are at an increased risk for lower GI tract symptoms, particularly in the setting of greater parasympathetic tone.
Figure 2.
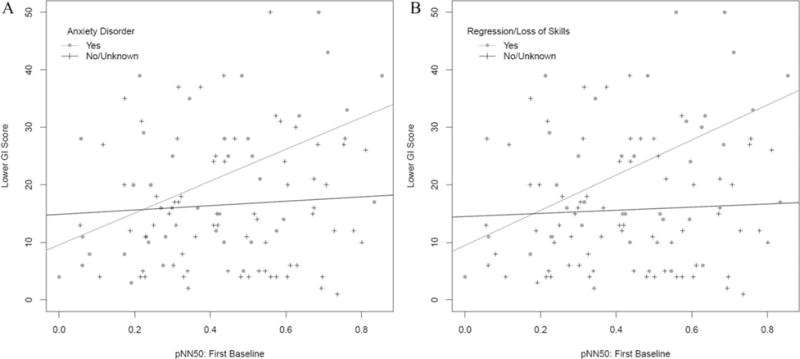
Impact of effect modifiers on the ANS- gastrointestinal symptomatology relationships. (A) Effect of presence or absence of anxiety on the relationship between lower GI tract scores and pNN50 baseline. (B) Effect of presence or absence of history of regression/loss of skills on the relationship between lower GI tract scores and pNN50 baseline.
Discussion
This is the first study to explore the relationship between gastrointestinal symptoms and ANS functioning in individuals with ASD. We observed a significant correlation between lower GI tract symptoms and both the pNN50 from the ECG data at rest and the change in pNN50 with cold pressor stimulation. These results were interrelated, however, with the cold pressor findings possibly driven by the variation in baseline pNN50. However, with strict exclusion of potential confounding medications, the cold pressor change in pNN50 relationship remained significant while the baseline pNN50 was no longer significant with this smaller sample, suggesting that reactivity to stress may be an independent factor of relevance in this effect. As the pNN50 is a marker of parasympathetic function, this supports the hypothesis that lower GI tract symptoms are related to parasympathetic activity in the ASD population. The vast majority of lower GI tract symptoms reported in the population studied herein were constipation symptoms, consistent with previous work [Gorrindo et al., 2012; McElhanon et al., 2014], suggesting a relationship between constipation and parasympathetic tone in ASD. Furthermore, this finding is in agreement with previous research suggesting impaired parasympathetic functioning and sympathovagal balance in those with irritable bowel syndrome without ASD, although for constipation-predominant irritable bowel syndrome, vagal dysfunction had been most prominent [Liu, Wang, Yan, & Chen, 2013]. Future studies should explore whether the relationship between autonomic functioning and lower GI tract symptoms differs in those with ASD as compared to those without ASD, to determine whether this relationship is generalized to all participants with lower GI tract symptoms.
Given the correlational nature of this study, it is not possible to assess the causality of this association. It is tempting to believe that parasympathetic tone affects lower GI tract symptoms, as has been suggested in gastrointestinal disorders in those without ASD [Pellissier et al., 2010], but it is also possible that feedback from a constipated GI tract could affect parasympathetic tone in ASD. One opportunity to evaluate a possible causal relationship between parasympathetic tone and lower GI tract symptoms would be exploring the relationship between parasympathetic tone and response to standard treatment for constipation. If successful treatment of constipation leads to diminished parasympathetic tone, feedback from the gut is likely affecting this element of the ANS. By contrast, parasympathetic tone could predict who will and will not respond to standard constipation treatment, which might serve as an important biomarker and guide treatment selection.
For upper GI tract symptomatology, no significant relationships were observed with the psychophysiological variables; although one contributing factor could be the small number of participants with upper GI tract disorders (19.2%). Future studies targeting greater numbers of patients with upper GI tract problems would be needed in order to more conclusively address a relationship between ANS variables and upper GI tract symptomatology.
A relationship between gastrointestinal disturbances and irritability and sleep problems in ASD is not entirely surprising, as pain resulting from constipation or other abdominal distress would likely affect behavior and sleep [Buie et al., 2010]. In the exploration of these relationships in our study, upper and lower GI tract symptomatology were both independently found to relate to both irritability and sleep. Other co-occurring symptoms have been previously associated with GI disorders in ASD [Peters et al., 2014; Mazurek et al., 2013]. Physiological hyperarousal has been shown in anxiety disorders, such as elevated heart rate and reduced respiratory sinus arrhythmia (a measure of parasympathetic tone) at baseline [Thayer, Friedman, & Borkovec, 1996], and failure to reduce sympathetic tone as evidenced by reduced declines skin conductance level during the daytime and in bed at night [Roth et al., 2008]. Other studies found similar cardiac findings in those under stress, suggesting decreased parasympathetic control [Brosschot, Gerin, & Thayer, 2006]. Furthermore, anxiety disorders commonly co-occur in ASD [van Steensel, Bögels, & Perrin, 2011], and studies have shown similar physiological alterations such as elevated basal heart rate [Kushki et al., 2013], and autonomic hyperarousal at baseline and in response to social anxiety and social cognition [Kushki, Brian, Dupuis, & Anagnostou, 2014]. In the present study, an association with anxiety was identified and found to represent a significant modifier of the association between pNN50 and lower GI tract scores. This suggests that individuals with ASD and anxiety disorders may be at an increased risk of lower GI problems, and that the mechanism by which this occurs is an enhanced stress response. Previous research has found anxiety to be associated with a range of gastrointestinal problems in ASD including constipation [Mazurek et al., 2013]. In our study, specifically, base-line pNN50 was strongly related to lower GI tract scores only in participants whose caretakers reported the presence of an anxiety disorder (Fig. 2A).
In addition, greater upper GI tract scores were associated with history of regression, and seizures, while lower GI tract scores were also associated with regression history. An association between gastrointestinal disturbances in general and seizures has also been previously observed in a recent electronic health record time-series analysis [Doshi-Velez et al., 2014]. The observed relationship with a history of regression is of some interest. The relationship between pNN50 and lower GI tract symptomatology was significantly stronger in participants with a reported history of regression or loss of skills (Fig. 2B). The etiology of regression and loss of skills in ASD remains very poorly understood. One previous study had reported abnormal stool patterns in individuals with ASD with regression [Valicenti-McDermott et al., 2008]. These results suggest further exploration of the autonomic and gastrointestinal systems is needed in children with a history of regression.
There are several important limitations in this study. First, few participants had upper GI tract diagnoses, and many participants scored a “0” for upper GI tract symptomatology on our quantitative measure derived from the QPGS Rome III, limiting the conclusions that can be drawn regarding upper GI tract symptoms. A future study may need to specifically recruit participants with upper GI tract symptomatology to address this challenge. Second, the use of the QPGS Rome III as a continuous measure allowed us to evaluate correlations with ANS biomarkers and co-occurring symptoms, but this differs from its designed use to determine if an individual meets criteria for functional GI disorders. Another potential limitation is the fact that GI symptoms were assessed by self- or parent-report on the QPGS Rome III. This may be problematic for individuals with ASD who have limited expressive language and whose parents may not be aware of their child’s GI pain or potential discomfort. However, previous research has demonstrated a direct correlation between parent report and true GI symptoms [Gorrindo et al., 2012]. Alternative approaches to supplement this type of information with direct assessment by a gastroenterologist should be considered in future work. Regardless, use of the QPGS Rome III in this study is a strength given that the measure is standardized and reliable [Van Tilburg et al., 2013] and Rome III criteria display construct validity [Saps et al., 2014]. It is also possible that dietary restrictions, food preferences, or utilization of complementary alternative treatments, which were not assessed, could also have impacted the results. Finally, the sample size is modest and we chose to present uncorrected P-values given the exploratory nature of the research. The significant findings in this study may be most useful as hypotheses for future studies, including studies exploring whether these findings serve as markers that predict response to standard treatment, and for studies examining the co-occurring disorders as they may also have important implications for treatment of specific ASD subgroups.
A final but important limitation in the present study is that the enteric nervous system was not directly studied. The activity of the gut is modulated by both preganglionic sympathetic and parasympathetic neurons. While generally speaking, stimulation of sympathetic neurons inhibits gut motility, whereas stimulation of parasympathetic neurons allows digestive activities, there are extensive, bidirectional connections between the CNS and the ENS, providing multiple potential pathways for an interaction between stress, the brain, and the gut. As such, future research should examine ENS and CNS-ENS interactions and their influences on constipation in ASD.
Despite these limitations, the primary finding of a relationship between parasympathetic psychophysical markers and lower GI tract symptoms, moderated by anxiety in the ASD population, may lead to better understanding of why constipation is so problematic in these individuals. Follow-up studies could establish the directionality of this association by assessing whether treatment of constipation results in a change in parasympathetic tone. Likewise, GI symptoms could be reevaluated after successful treatment of anxiety in the ASD population. Future work will be necessary to see how these factors relate to regression and loss of skills. Targeted work in individuals with ASD and upper GI tract symptoms will be necessary to address these questions more robustly; although the low rate of these problems in our population may suggest that they are not enriched in the ASD population. Subsequent work could also explore how these findings in the ASD population compare to findings in individuals without ASD.
Acknowledgments
This research was supported by a grant given to the Autism Treatment Network, Autism Intervention Research Network on Physical Health by the Health Resources Services Administration (HRSA Grant# UA3MC11054). We would like to sincerely thank the participants, their families, and all study team members for their time and efforts associated with this study. This project was supported by the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) under cooperative agreement UA3 MC11054 – Autism Intervention Research Network on Physical Health. This information or content and conclusions are those of the author and should not be construed as the official position or policy of, nor should any endorsements be inferred by HRSA, HHS or the U.S. Government. This work was conducted through the Autism Speaks Autism Treatment Network serving as the Autism Intervention Research Network on Physical Health.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Supporting Information
References
- Aman MG, Singh NN, Stewart AW, Fields CJ. The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency. 1985;89:485–491. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th edition-text revision) (DSM-IV-TR) Washington: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington: American Psychiatric Association; 2013. [Google Scholar]
- Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559–578. doi: 10.1016/s0016-5085(98)70540-2. [DOI] [PubMed] [Google Scholar]
- Barry RJ, James AL. Coding of stimulus parameters in autistic, retarded, and normal children: evidence for a two-factor theory of autism. International Journal of Psychophysiology. 1988;6:139–149. doi: 10.1016/0167-8760(88)90045-1. [DOI] [PubMed] [Google Scholar]
- Bauman ML. Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics. 2010;7:320–327. doi: 10.1016/j.nurt.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger JT, Kleiger RE, Fleiss JL, Rolnitzky LM, Steinman RC, Miller JP. Components of heart rate variability measured during healing of acute myocardial infarction. The American Journal of Cardiology. 1988;61:208–215. doi: 10.1016/0002-9149(88)90917-4. [DOI] [PubMed] [Google Scholar]
- Bouchoucha M, Hejnar M, Devroede G, Babba T, Bon C, Benamouzig R. Anxiety and depression as markers of multiplicity of sites of functional gastrointestinal disorders: a gender issue? Clinics and Research in Hepatology & Gastroenterology. 2013;37:422–430. doi: 10.1016/j.clinre.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal Activity. 2nd. New York: Springer; 2012. [Google Scholar]
- Bresnahan M, Hornig M, Schultz AF, Gunnes N, Hirtz D, Lie KK, et al. Association of maternal report of infant and toddler gastrointestinal symptoms with autism: evidence from a prospective birth cohort. JAMA Psychiatry. 2015;72:466–474. doi: 10.1001/jamapsychiatry.2014.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60:113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Buie T, Campbell DB, Fuchs GJ, III, Furuta GT, Levy J, Van de Water J, et al. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: A consensus report. Pediatrics. 2010;125(S1):S1–18. doi: 10.1542/peds.2009-1878C. [DOI] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, Hertz-Picciotto I. Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism & Developmental Disorders. 2014;44:1117–1127. doi: 10.1007/s10803-013-1973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S, Carcani-Rathwell I, Charman T, Pickles A, Loucas T, Meldrum D. Parent-reported gastrointestinal symptoms in children with autism spectrum disorders. Journal of Autism & Developmental Disorders. 2013;43:2737–2747. doi: 10.1007/s10803-013-1768-0. [DOI] [PubMed] [Google Scholar]
- Collins SM, Surette M, Bercik P. The interplay between the intestinal microbiota and the brain. Nature Reviews Microbiology. 2012;10:735–742. doi: 10.1038/nrmicro2876. [DOI] [PubMed] [Google Scholar]
- Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. 2014;133:e54–e63. doi: 10.1542/peds.2013-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress system-organization, physiology, and immunoregulation. Neuroimmunomodulation. 2006;13:257–267. doi: 10.1159/000104853. [DOI] [PubMed] [Google Scholar]
- Foster PS, Hubbard T, Yung RC, Ferguson BJ, Drago V, Harrison DW. Cerebral asymmetry in the control of cardiovascular functioning: Evidence from fine motor control. Laterality. 2013;18:108–119. doi: 10.1080/1357650X.2011.631545. [DOI] [PubMed] [Google Scholar]
- Gillis RA, Quest JA, Pagini FD, Norman WP. Control centers in the central nervous system for regulating gastrointestinal motility. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology. New York: Oxford University; 1989. pp. 621–683. (Section 6. The Gastrointestinal System). [Google Scholar]
- Gorrindo P, Williams KC, Lee EB, Walker LS, McGrew SG, Levitt P. Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Research. 2012;5:101–108. doi: 10.1002/aur.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, McKirdy HC. A nervous mechanism for descending inhibition in guinea-pig small intestine. The Journal of Physiology. 1974;238:129–143. doi: 10.1113/jphysiol.1974.sp010514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirstein W, Iversen P, Ramachandran VS. Autonomic responses of autistic children to people and objects. Proceedings of Biological Science. 2001;268:1883–1888. doi: 10.1098/rspb.2001.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovarth K, Perman JA. Autism and gastrointestinal symptoms. Current Gastrointestinal Reports. 2002a;4:251–258. doi: 10.1007/s11894-002-0071-6. [DOI] [PubMed] [Google Scholar]
- Hovarth K, Perman JA. Autistic disorder and gastrointestinal disease. Current Opinion in Pediatrics. 2002b;14:583–587. doi: 10.1097/00008480-200210000-00004. [DOI] [PubMed] [Google Scholar]
- Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: A population-based study. Pediatrics. 2009;124:680–686. doi: 10.1542/peds.2008-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Ehrman K, McNally R, Keehn B. Affective response to eye contact and face recognition ability in children with ASD. Journal of the International Neuropsychological Society. 2008;14:947–955. doi: 10.1017/S1355617708081344. [DOI] [PubMed] [Google Scholar]
- Katona PG, Poitras JW, Barnett GO, Terry BS. Cardiac vagal efferent activity and heart period in the carotid sinus reflex. American Journal of Physiology. 1970;218:1030–1037. doi: 10.1152/ajplegacy.1970.218.4.1030. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Stein PK, Bigger JT. Heart rate variability: Measurement and clinical utility. Annals of Noninvasive Electrocardiology. 2005;10:88–101. doi: 10.1111/j.1542-474X.2005.10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki A, Drumm E, Pla Mobarak M, Tanel N, Dupuis A, Chau T, Anagnostou E. Investigating the autonomic nervous system response to anxiety in children with autism spectrum disorders. PLoS ONE. 2013;8(4):e59730. doi: 10.1371/journal.pone.0059730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushki A, Brian J, Dupuis A, Anagnostou E. Functional autonomic nervous system profile in children with autism spectrum disorder. Molecular Autism. 2014;5:39. doi: 10.1186/2040-2392-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kylliäinen A, Hietanen JK. Skin conductance responses to another person’s gaze in children with autism. Journal of Autism & Developmental Disorders. 2006;36:517–525. doi: 10.1007/s10803-006-0091-4. [DOI] [PubMed] [Google Scholar]
- Lidberg L, Wallin G. Sympathetic skin nerve discharges in relation to amplitude of skin resistance responses. Psychophysiology. 1981;18:268–270. doi: 10.1111/j.1469-8986.1981.tb03033.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wang EM, Yan XJ, Chen SL. Autonomic functioning in irritable bowel syndrome measured by heart rate variability: A meta-analysis. Journal of Digestive Diseases. 2013;14:638–646. doi: 10.1111/1751-2980.12092. [DOI] [PubMed] [Google Scholar]
- Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterology and Motility. 2010;22:7–18. doi: 10.1111/j.1365-2982.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, et al. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. Journal of Autism & Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lyte M, Freestone PP, Neal CP, Olson BA, Haigh RD, Bayston R, Williams PH. Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet. 2003;361:130–135. doi: 10.1016/S0140-6736(03)12231-3. [DOI] [PubMed] [Google Scholar]
- Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa – bacteria interactions. Cell Tissue Research. 2011;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- Martinez-Martinez LA, Mora T, Vargas A, Fuentes-Iniestra M, Martinez-Lavin M. Sympathetic nervous system dysfunction in fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, and interstitial cystitis: a review of case-control studies. Journal of Clinical Rheumatology. 2014;20:146–150. doi: 10.1097/RHU.0000000000000089. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: The emerging biology of gutbrain communication. Nature Reviews Neuroscience. 2011;12:453–458. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur M, Fugala A, Jablonski K, Mach T, Thor P. Autonomic nervous system activity in constipation-predominant irritable bowel syndrome patients. Medical Science Monitor. 2012;18:493–499. doi: 10.12659/MSM.883269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurak N, Seredyuk N, Sauer H, Teufel M, Enck P. Heart rate variability in the irritable bowel syndrome: a review of the literature. Neurogastroenetrology & Motility. 2012;24:206–216. doi: 10.1111/j.1365-2982.2011.01866.x. [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Vasa RA, Kalb LG, Kanne SM, Rosenberg D, Keefer A, Murray DS, Freedman B, Lowery LA. Anxiety, sensory over-responsivity, and gastrointestinal problems in children with autism spectrum disorders. Journal of Abnormal Child Psychology. 2013;41:165–176. doi: 10.1007/s10802-012-9668-x. [DOI] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: A meta-analysis. Pediatrics. 2014;133:872–883. doi: 10.1542/peds.2013-3995. [DOI] [PubMed] [Google Scholar]
- Ming X, JuLu POO, Brimacombe M, Conner S, Daniels ML. Reduced cardiac parasympathetic activity in children with autism. Brain & Development. 2005;27:509–516. doi: 10.1016/j.braindev.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Mouridsen SE, Rich B, Isager T. A longitudinal study of gastrointestinal diseases in individuals diagnosed with infantile autism as children. Child Care Health and Development. 2010;36:437–443. doi: 10.1111/j.1365-2214.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- Neuhaus E, Bernier R, Beauchaine TP. Brief report: social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. Journal of Autism and Developmental Disorders. 2014;44:730–737. doi: 10.1007/s10803-013-1923-7. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1–9. [PubMed] [Google Scholar]
- Pellissier S, Dantzer C, Canini F, Mathieu N, Bonaz B. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology. 2010;35:653–662. doi: 10.1016/j.psyneuen.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Perlman SE, Friedman S, Galea S, Nair HP, Erős-Sarnyai M, Stellman SD, Hon J, Greene CM. Short-term and medium-term health effects of 9/11. The Lancet. 2011;378:925–934. doi: 10.1016/S0140-6736(11)60967-7. [DOI] [PubMed] [Google Scholar]
- Peters B, Williams KC, Gorrindo P, Rosenberg D, Lee EB, Levitt P, Veenstra-VanderWeele J. Rigid-compulsive behaviors are associated with mixed bowel symptoms in autism spectrum disorder. Journal of Autism and Developmental Disorders. 2014;44:1425–1432. doi: 10.1007/s10803-013-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT, Doberenz S, Dietel A, Conrad A, Mueller A, Wollburg E, Meuret AE, Taylor CB, Kim S. Sympathetic activation in broadly defined generalized anxiety disorder. Journal of Psychiatry Research. 2008;42:205–212. doi: 10.1016/j.jpsychires.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saps M, Nichols-Vinueza DX, Mintjens S, Pusatcioglu CK, Cenk K, Velasco-Benitez CA. Construct validity of the pediatric Rome III criteria. Journal of Pediatric Gastroenterology and Nutrition. 2014;59:577–581. doi: 10.1097/MPG.0000000000000482. [DOI] [PubMed] [Google Scholar]
- Schoen S, Miller LJ, Green K. Pilot study of the sensory over-responsivity scales: assessment and inventory. The American Journal of Occupational Therapy. 2008;62:393–406. doi: 10.5014/ajot.62.4.393. [DOI] [PubMed] [Google Scholar]
- Scott LV, Clarke G, Dinan TG. The brain-gut axis: a target for treating stress-related disorders. Modern Trends in Pharmacopsychiatry. 2013;28:90–99. doi: 10.1159/000343971. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, Balla DA. The Vineland-II Adaptive Behavior Scales. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Stasi C, Orlandelli E. Role of the brain-gut axis in the pathophysiology of Crohn’s disease. Digestive Diseases. 2008;26:156–166. doi: 10.1159/000116774. [DOI] [PubMed] [Google Scholar]
- Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-aho PO, Karjalainen PA. Kubios HRV – Heart rate variability analysis software. Computer Methods and Programs in Biomedicine. 2014;113:210–220. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Task Force. Heart Rate Variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kamio Y. Paradoxical autonomic response to mental tasks in autism. Journal of Autism and Developmental Disorders. 2003;33:417–426. doi: 10.1023/a:1025062812374. [DOI] [PubMed] [Google Scholar]
- Valicenti-McDermott MD, McVicar K, Cohen HJ, Wershil BK, Shinnar S. Gastrointestinal symptoms in children with an autism spectrum disorder and language regression. Pediatric Neurology. 2008;39:392–398. doi: 10.1016/j.pediatrneurol.2008.07.019. [DOI] [PubMed] [Google Scholar]
- van Engeland H. The electrodermal orienting response to auditive stimuli in autistic children, normal children, mentally retarded children, and child psychiatric patients. Journal of Autism and Developmental Disorders. 1984;14:261–279. doi: 10.1007/BF02409578. [DOI] [PubMed] [Google Scholar]
- van Steensel FJ, Bögels SM, Perrin S. Anxiety disorders in children and adolescents with autistic spectrum disorders: a meta-analysis. Clinical Child and Family Psychology Review. 2011;14:302–317. doi: 10.1007/s10567-011-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilburg MAL, Squires M, Blois-Martin N, Leiby A, Langseder A. Test of the child/adolescent Rome III criteria: agreement with physician diagnosis and daily symptoms. Neurogastroenterology & Motility. 2013;25:302–e246. doi: 10.1111/nmo.12056. [DOI] [PubMed] [Google Scholar]
- Walker LS, Caplan A, Rasquin A. Manual for the Questionnaire on Pediatric Gastrointestinal Symptoms. Nashville, TN: Department of Pediatrics, Vanderbilt University Medical Center; 2000. [Google Scholar]
- Zvan B, Zaletel M, Pretnar J, Pogacnik T, Kiauta T. Influence of the cold pressor test on the middle cerebral arterial circulation. Journal of the Autonomic Nervous System. 1998;74:175–178. doi: 10.1016/s0165-1838(98)00163-5. [DOI] [PubMed] [Google Scholar]


