Abstract
Objective
To examine the relationship between alcohol, both the amount and type, and cognitive decline in a cohort of Alzheimer's disease (AD) patients.
Methods
A cohort of 360 patients with early AD in New York, Boston, Baltimore and Paris were followed-up biannually for up to 19.28 years. At each visit, the cognitive profile of the patients was assessed using the modified Mini-Mental State Examination (mMMSE), and patients' alcohol intake, including beverage type, was reported by patients' primary caregivers. General estimating equation analysis was used to determine whether baseline alcohol use was associated with the rate of cognitive decline.
Results
Heavy drinkers (8 or more alcoholic drinks/week) had a faster cognitive decline, deteriorating 1.849 more points on their mMMSE score annually compared to abstainers (P = 0.001), or 2.444 more points compared to mild-moderate drinkers (1-7 alcoholic drinks/week) (P = 0.008). There was no significant difference when comparing mild-moderate drinkers to abstainers. Increasing standard drinks of hard liquor, but not beer or wine, was also associated with a faster rate of cognitive decline (β = -0.117 P = 0.001).
Conclusions
Heavy alcohol consumption and more hard liquor are associated with a faster rate of cognitive decline in AD patients, suggesting that they may hasten the progression of AD. Our results suggest that alcohol drinking habits might alter the course of AD.
Keywords: Alzheimer's disease, Dementia, Risk factors in epidemiology, Disease progression, Cognitive decline, Alcohol, Hard liquor
Introduction
Mild-moderate alcohol intake is widely considered to be associated with a lower risk of Alzheimer's disease (AD) [1-4], while heavy drinking increases the risk [1, 5-7]. Some studies have suggested that this relationship is ‘U shaped’, with no alcohol and heavy drinking conveying an increased risk of developing AD compared to moderate drinking [5, 8]. However, only a few studies [9, 10] have examined the role of alcohol consumption on AD progression. In a study of 38 AD patients, those who had stopped habitually drinking after their diagnosis had a slower decline than individuals who were never regular drinkers [9]. Yet, in another study, a past history of excessive alcohol consumption has been shown to not alter AD progression over a year [10].
There is debate about whether the effects of alcohol on AD are due to ethanol itself or if these results are biased by a specific beverage type. Several studies have found that wine, but not beer or hard liquor, are protective against the development of AD [1, 11-13] but another disagreed with these findings [14]. One study found that mixed drinks are solely beneficial [15], while others found that beer [13], or spirits are associated with worse outcomes [16, 17]. In addition, little is known how each beverage type might affect AD progression.
To our knowledge no study has examined how active alcohol consumption, and which type, may affect cognitive decline in AD patients over an extended period of follow-up.
Material and Methods
Participants
Two cohorts were used in this analysis. The Predictors 1 cohort had 252 subjects from three locations Harvard University (Boston), John Hopkins University (Baltimore) and Columbia University (New York). The Predictors 2 cohort was comprised of 305 subjects from the above sites and also included Hôpital de la Salpêtrière in Paris, France.
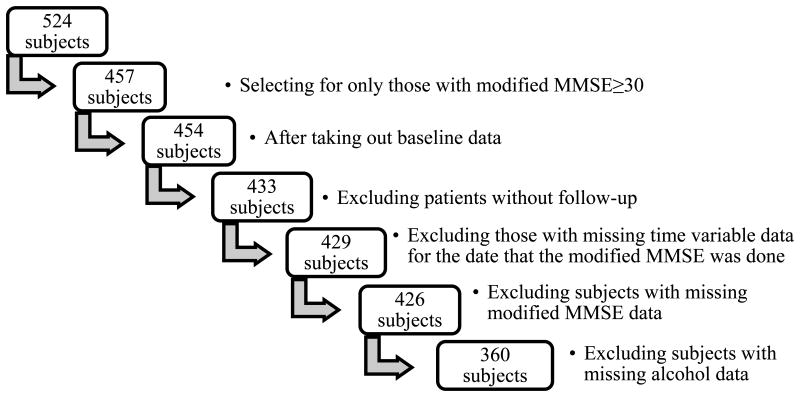
The Predictors Study inclusion criteria, exclusion criteria and evaluation procedures are described elsewhere [18, 19]. In brief, participants enrolled in the study were clinical patients who had already met the Diagnostic and Statistical Manual of Mental Disorders, Revised Fourth Edition criteria for primary degenerative dementia of the Alzheimer type and Related Disorders Association criteria (NINCDS-ADRDA) for probable AD. Inclusion criteria included a modified Mini-Mental State Examination (mMMSE) score of 30 or more, which equates to a Folstein MMSE score of approximately 16 or more [20, 21]. After the initial visit, participants were followed up approximately every six months. Those with missing cognition score data or missing data on alcohol consumption were excluded (Figure 1).
Figure 1.
Inclusion and exclusion criteria breakdown.
Standard Protocol Approvals, Registrations, and Patient Consents
The local institutional review boards authorized this study and all subjects provided written informed consent.
Evaluation measures
Demographic information (age, gender, ethnicity and years of education) and mMMSE score were obtained at the initial visit [19]. Apolipoprotein E (ApoE) was measured using the method of Hixson [22]. Patients were categorized into 2 groups, based on having at least one ApoE ε4 allele and those possessing none (as the reference).
Questions about alcohol intake were asked to the patient's primary caregiver, the spouse or less commonly the patient's children, on the first visit and at follow-up visits. The questions included asking about number of 12 oz. beer bottles per week, number of 4 oz. glasses of wine per week, and number of 1 oz. jiggers of hard liquor per week. Predictors 1 had an additional question about total number of alcohol drinks per week, while the total alcohol per week was calculated for the Predictors 2 data by summing the intake of the different alcoholic beverages together. The total alcohol intake (see description before) was categorized into three groups; abstainers, mild-moderate drinkers (1-7 alcoholic drinks per week) and heavy drinkers (8 or more alcoholic drinks per week). These were chosen based on what is commonly used in the literature [4, 8]. Because of the tendency of subjects underreporting alcohol use and the equivalent validity of collaterally attained and self-reported alcohol data [23,24], the alcohol category attributed to each patient was calculated from the maximum number of drinks recorded over the first two visits, that is, in the first six months from entry into the study. This was used as baseline alcohol intake. As such, the progression analysis started from the second visit and the cognitive measurements from the first visit were excluded from the analysis (Figure 1). A sensitivity analysis was done by grouping those who drank 1 to 14 drinks per week as the mild-moderate group and 15 or more drinks as the heavy drinking group. We also performed a sensitivity analysis using only the alcohol data reported at the initial visit, as opposed to the maximum amount reported in the first 6 months.
We also examined the effect of beer, wine and hard liquor on the cognitive decline in AD. Like the total alcohol variable, we used the maximum weekly consumption of these different beverages over the first two visits. Due to the relatively small sample of heavy drinkers within each alcoholic beverage group (6 in the heavy drinking beer group, 12 in the wine and 10 in the hard liquor), the amount of each beverage consumed per week was used as a continuous variable.
At the initial and follow-up visits, patients' caregivers were asked about a history of hypertension, diabetes and chronic alcohol use. Subjects were considered having the condition if they reported having the condition at any visit. Subjects were also asked biannually whether they have been diagnosed or been treated for depression. Similarly, the subject was considered as having depression if at any time they answered yes to these questions.
The mMMSE was used as the cognitive measurement. It was assessed in English in the United States, and in French in Paris [19]. The mMMSE is a 57 point version of the Folstein MMSE and includes as well two calculation items, forward and backward digit span [25], confrontation naming of 10 items from the Boston Naming Test [26] and recall of the current country's president and 4 previous presidents.
Statistical Analysis
Baseline characteristics of those who were included in the study and those who were excluded due to missing data were compared using t tests for continuous variables and Chi-Square Tests for categorical variables. When examining the demographic information of the 3 alcohol groups, one-way ANOVA and Post Hoc Tests were used for continuous variables and Chi-Square Tests for categorical variables.
General estimating equation (GEE) analysis was then used to determine whether alcohol use was associated with the rate of cognitive decline. The duration (in years) between the dates of the first mMMSE (i.e. the first mMMSE after the collection of the baseline alcohol data) and the dates of the follow-up mMMSEs was used as the time variable in the GEE models. A significant interaction between the time variable and alcohol groups indicates that the rate of cognitive decline varies across the alcohol groups, with negative and positive β values indicating faster and slower declines respectively. In order to examine how the mild-moderate drinking group compared to the abstainer and the heavy drinking groups and the potential presence of a ‘U shaped’ relationship, two analyses were done when examining the effect of total alcohol use on AD. One used abstainers as the reference group and the other used heavy drinkers as the reference group.
The GEE analysis was adjusted for baseline mMMSE score and demographics (age, gender, ethnicity, years of education) (model 1). We then additionally adjusted for a history of hypertension, diabetes, chronic alcohol use and depression (model 2). In the GEE models examining the individual effects of wine, beer and hard liquor, the alcoholic groupings (abstainer, mild-moderate and heavy drinkers) and maximum weekly intake of other beverage types within the first six months of the study were also accounted for in all three models.
We performed a few sensitivity analyses. We used the alcohol consumption from their first visit only. We also used the 1-14 and 15+ groupings to define mild-moderate and heavy drinking. Finally, we additionally adjusted for occupational type, smoking status, ApoE genotype, as well as the alcohol groupings based on the data from the first visit only.
Results
A total of 360 patients with AD were included in the study (Figure 1). Ninety-four people were excluded due to missing follow up or missing data on the date the mMMSE was performed, the recorded mMMSE score, and alcohol data. There were no significant differences in age, gender, race, or educational level between this group with missing data and the 360 people included in the study (Table 1).
Table 1.
Demographic and clinical characteristics of the three alcohol groups, as well as the included and excluded participants.
| Abstainers | Mild-Mod* | Heavy | P value | Included - mean | Excluded - mean | P value | ||
|---|---|---|---|---|---|---|---|---|
| Number of Subjects | 252 | 78 | 30 | 360 | 94 | |||
| Gender | Male |
94 (62.7%) |
38 (25.3%) |
18 (12.0%) |
0.021 | 150 (42%) |
40 (43%) |
0.877 |
| Female |
158 (75.2%) |
40 (19.0%) |
12 (5.7%) |
210 (58%) |
54 (57%) |
|||
| Race | White | 229 (68.8%) |
74 (22.2%) |
30 (9.0%) |
0.05 | 333 | 88 | 0.606 |
| Black | 22 (91.7%) |
2 (8.3%) |
0 | 24 | 5 | |||
| Other | 1 (33.3%) |
2 (66.7%) |
0 | 3 | 0 | |||
| Diagnosed Diabetes Mellitus | 7/158 (4.4%) |
1/44 (2.3%) |
2/19 (5.3%) |
0.342 | 10/222 (4.5%) |
2/46 (4.3%) |
0.963 | |
| Diagnosed Hypertension | 19/158 (12.0%) |
6/45 (13.3%) |
1/19 (5.2%) |
0.640 | 26/222 (11.7%) |
15/46 (32.6%) |
0.000 | |
| Age at Baseline |
Mean (SD) |
74.87 (8.897) |
72.72 (7.809) |
72.10 (6.418) |
0.058 | 74.17 (8.539) |
75.33 (9.007) |
0.247 |
| Years of Education |
Mean (SD) |
13.17 (3.332) |
15.03 (3.564) |
13.80 (4.514) |
<0.0001 | 13.62 | 14.31 | 0.094 |
| Baseline mMMSE Score |
Mean (SD) |
38.16 (5.629) |
38.33 (5.332) |
39.53 (6.213) |
0.451 | N/A | N/A | |
| Length of Follow-up (years) |
Mean (SD) |
4.36 (3.08) |
4.86 (3.57) |
5.02 (3.62) |
0.366 | N/A | N/A |
Mild to moderate alcohol use was defined as 1-7 alcoholic drinks per week.
Heavy use was defined as 8 or more drinks per week.
SD= standard deviation
Most of the patients included in the study were from the United States. 142 patients were from New York (39.4%), 108 were from Baltimore (30.0%), 94 were from Boston (26.1%), and 16 patients were from Paris (4.4%). The average mMMSE at the time of recruitment was 38.7 (SD=5.61). Subjects were followed on average 4.5 years (SD=3.28, range 0.26 and 19.28 years). During this time there were 2776 mMMSE assessments.
The three alcohol groups did not significantly differ with regards to their age or baseline cognitive scores. However, the mild-moderate drinkers had more years of education compared to the abstainers. The mild-moderate and heavy drinking groups had greater proportions of males compared to the abstainer group. There was a borderline significant difference in ethnicity between the three groups. All the heavy drinkers were Caucasian and a larger proportion of those who identified as Black were in the abstainer group (Table 1).
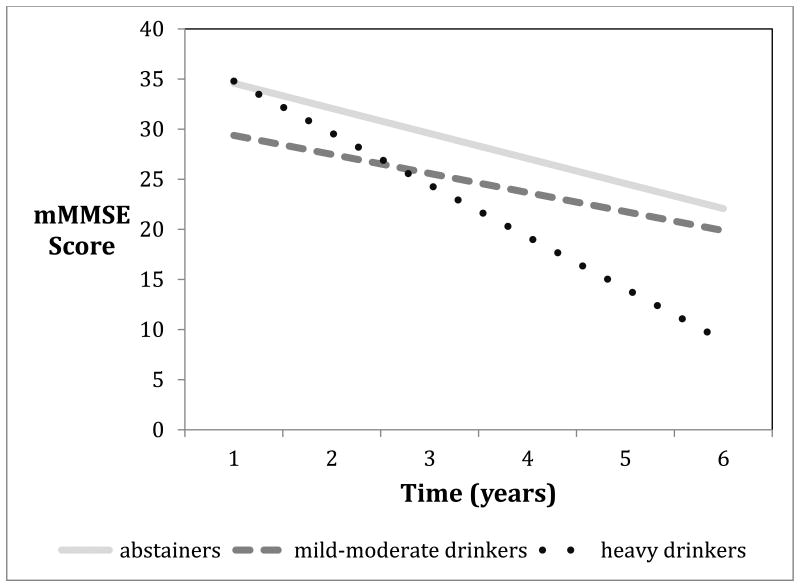
When using abstainers as the reference group, the GEE analysis found that heavy drinkers had a significantly faster cognitive decline. Compared to the heavy drinking group, both abstainers and mild-moderate drinkers had a significantly slower decline, with mild-moderate drinkers showing the slowest decline (Table 2, Figure 2). However, there was no significant difference in the decline when mild-moderate drinkers were compared to abstainers. Results remained the same when adjusted for covariates.
Table 2.
Progression of cognitive decline in abstainers and mild-moderate drinkers compared to heavy drinkers, and in mild-moderate drinkers compared to abstainers.
| Abstainers compared to heavy drinkers | Mild-moderate drinkers compared to heavy drinkers | Mild-moderate drinkers compared to abstainers | |||||
|---|---|---|---|---|---|---|---|
| Number of subjects | B | P | β | P | β | P | |
| Unadjusted | 360 | 1.420 | 0.012 | 2.484 | 0.006 | 1.064 | 0.179 |
| Model 1a | 359 | 1.594 | 0.001 | 1.930 | 0.020 | 0.336 | 0.658 |
| Model 2b | 221 | 1.849 | 0.001 | 2.444 | 0.008 | 0.596 | 0.475 |
Adjusted for demographics (age, gender, race, years of education) and baseline cognitive score.
Adjusted for demographics, baseline cognitive score, diabetes, hypertension and past history of chronic alcohol use or depression.
Figure 2.
The rates of cognitive decline among AD patients with different alcohol drinking habits.
When the type of alcoholic beverage was analyzed we found that increasing standard drinks of hard liquor per week, but not beer or wine, were associated with a faster decline in mMMSE scores in adjusted models (Table 3).
Table 3.
Progression of cognitive decline with increasing standard drinks of hard liquor, beer and wine.
| Hard liquor | Beer | Wine | |||||
|---|---|---|---|---|---|---|---|
| Number of subjects | B | P | β | P | β | P | |
| Unadjusted | 359-360 | -0.076 | 0.069 | -0.089 | 0.251 | 0.015 | 0.826 |
| Model 1a | 358 | -0.106 | 0.001 | -0.075 | 0.208 | -0.037 | 0.402 |
| Model 2b | 221 | -0.117 | 0.001 | -0.081 | 0.209 | -0.035 | 0.714 |
Adjusted for demographics (age, gender, race, years of education), maximum consumption of other alcoholic beverages, alcohol grouping, and baseline cognitive score.
Adjusted for demographics, maximum consumption of other alcoholic beverages, alcohol grouping, baseline cognitive score, diabetes, hypertension and past history of chronic alcohol use or depression.
Sensitivity analysis using the alcohol consumption from their first visit only showed similar results (data not shown). When using the 1-14 and 15+ groupings as well as adjusting for ApoE genotype the results were also similar (data not shown). Additional adjustment for occupational type or smoking did not change the results either (data not shown). Compared to non-drinking AD patients, AD patients with mild-moderate drinking showed a non-significantly positive effect, while those with heavy drinking showed a faster cognitive decline.
Discussion
Results from this study indicate a relationship between heavy alcohol use, as well as hard liquor, and a faster cognitive decline in AD. Mild-moderate drinkers had slower cognitive decline than the abstainer group, when compared to heavy drinkers. Heavy drinkers declined an extra 2.444 points per year on their mMMSE compared to mild-moderate drinkers, but only 1.849 points more than abstainers. This may be indicative of the ‘U’ shaped curve seen in the discourse of the development of AD, with mild-moderate drinking acting as a protective factor in the development of AD and heavy drinking as a risk factor [5, 8]. However, while the mild-moderate drinking group had a less steep slope in cognitive decline than abstainers (Figure 2), when comparing these two groups, mild-moderate drinkers showed no significant beneficial effect. It is also possible that some subjects with a more aggressive form of AD may lose control of their alcohol consumption. Therefore heavy drinking may be merely a marker of this steeper decline rather than causing it.
The non-significant effect of mild-moderate alcohol use when compared to abstainers suggests that the beneficial effect of mild-moderate alcohol use in AD patients may be less than that found in healthy individuals. Moderate alcohol consumption has been shown to be protective in decreasing the risk of developing AD [1-4]. Moderate Ethanol Preconditioning (MEP) is thought to best explain the beneficial effects of moderate subtoxic alcohol usage in preventing AD by promoting prosurvival pathways and decreasing neuroinflammation [27-29]. A sensor-transducer–effector model was proposed as the mechanism for this, which involves N-methyl-D-aspartate (NMDA) receptors as the sensor, multiple protein kinase Cs and focal adhesion kinases as the transducer and heat shock proteins 70 and 27 as the effector [27, 30, 31]. We hypothesize that the pathophysiology of AD impairs the upregulation of prosurvival and anti-inflammatory mechanisms, seen by Collins et al. [27], such that moderate alcohol use does not hinder the disease process enough to lead to a significant slower cognitive decline. This is an area that requires further research.
The harmful effects of heavy alcohol consumption, shown by this study, are consistent with the reported detrimental effects of heavy alcohol consumption on cognition among non-demented subjects [32-34]. Heavy alcohol use can cause brain atrophy and related neurological impairments in executive function, working memory, visuospatial abilities, cognitive processing of emotional signs, gait and balance. This cognitive impairment is separate from other alcohol related disorders such as Werrnicke's encephalopathy, pellagra and hepatic encephalopathy [34]. Heavy alcohol use is also thought to exacerbate the pathology of AD. For example, heavy alcohol use has profound effects on the cholinergic system, which plays a large role in AD pathology seen by the widespread use of cholinesterase inhibitors to slow AD progression [36, 37]. Heavy alcohol consumption leads to the destruction of cholinergic neurons and cortical muscarinic receptors, as well as decreases acetylcholine release and choline acetyltransferase activity [38, 39]. Furthermore, heavy alcohol use may also have a direct role in AD's pathophysiology by enhancing tau accumulation, delaying its clearance and thereby increasing cell death [40].
Our analysis with regards to the effect of different types of alcoholic beverages showed that an increasing amount of standard drinks of hard liquor was associated with faster cognitive decline in AD patients. Hard liquor has been associated in some studies with an increased risk of AD [16, 17], but others failed to show this [11, 13]. While our study did not show a beneficial effect of wine, it is hypothesized that wine may engender extra protective effects compared to other beverages, because polyphenols, which are found in wine, both have antioxidant properties and also inhibit the aggregation of beta amyloid [41,42]. To our knowledge this is the first study to look at how specific types of alcoholic beverages affect AD progression, and the first that has shown hard liquor to be associated with faster cognitive decline in AD patients. The mechanism by which hard liquor exerts this harmful effect on AD patients, may be due to the increased likelihood of spirits leading to binge drinking [43], which is associated with a cognitive impairment [5]. This is an area for future research.
This study has limitations. Alcohol consumption in this study was reported by the patients' primary caregivers. Gathering collateral information has shown to be of equivalent legitimacy to self-reporting [24] and therefore may be subject to the same risks of under reporting. However, considering the patients' memories are intrinsically compromised by the disease process, there may be no simple way to examine alcohol use in a longitudinal study of AD patients that is more valid. Another problem is that we measured alcohol use only in the first six months from entry and therefore did not take into account intrapersonal variation in alcohol use during the follow-up period or earlier in life. Although we controlled for multiple confounding factors, we could not completely rule out the possibility of residual confounding factors such as nutritional factors and exercise. Unfortunately, we do not have dietary or exercise information in this study population so we cannot adjust for these factors. We also did not inquire into the pattern of use, including binge drinking, which is an independent risk factor of AD [5]. Additionally, because we used observational data, we were not able to account for unmeasured confounders and therefore cannot infer causality.
In general, our confidence in our findings is strengthened by several factors. To our knowledge, this is the largest study of its kind to look at the relationship between AD progression and alcohol. The frequent and thorough follow-up allowed for there to be enough power to detect and estimate the effects of alcohol and control for confounders. The longitudinal data allowed the construction of a statistical model that could examine the temporal sequencing of alcohol as harmful or beneficial to AD progression. Contributing to the sensitivity of the analysis was the careful diagnosis, which was based on widely approved criteria and included a consensus diagnostic conference. The study was also done in university hospitals with staff who had expertise and experience in the area. As a testament to this, 93% of post mortem analyses showed AD pathological features [19]. As alcohol consumption alone does not significantly increase the accumulation of amyloid beta or hyperphosphorylated tau, this minimizes the possibility of patients with cognitive decline caused by heavy alcohol use being misclassified as AD [44]. The cohort's baseline mMMSE of more than 30 ensured that the patients had relatively mild AD at the beginning of the study and therefore we were able to see the full effects of alcohol use over the course of the disease. Our results are also supported by sensitivity analysis, with the results still significant at a lower power.
Conclusion
Heavy drinking (8 or more standard alcoholic drinks per week) and drinking larger amounts of hard liquor are associated with a faster cognitive decline in AD. The importance of this study lies in that it is the only study to examine the effects of alcohol intake on the rate of cognitive decline in AD patients. The findings give support for further investigation into whether modifying alcohol drinking habits could alter the course of AD. Slowing the cognitive decline of AD through simple modifiable risk factors is of great clinical importance to both clinicians and patients alike.
Acknowledgments
Study funding: The study's design and conduct, as well as the data collection, management, analysis, and interpretation and the manuscript's preparation, review, and approval were supported by federal grants R01AG07370 and R00AG042483. The funding organizations played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, approval of the manuscript; and the decision to submit the manuscript for publication.
List of Abbreviations
- AD
Alzheimer's disease
- ApoE
Apolipoprotein E
- GEE
General estimating equation
- MCI
Mild cognitive impairment
- MEP
Moderate ethanol preconditioning
- mMMSE
Modified Mini-Mental State Examination
- MMSE
Mini–mental state examination
- NMDA
N-methyl-D-aspartate
Footnotes
Conflict of interest: All authors report no relevant disclosures. There are no conflicts of interest with the funding source of this study.
Authors' contributions: DH contributed to the conception and design of the study, carried out the study, data analyses and writing of the manuscript. YS contributed to the conception and design of the study, revision of the manuscript, and obtained funding. SC contributed to the interpretation of the data and critical revision of the manuscript. OTN and JND contributed to the data collection. YG contributed to the conception and design of the study, data analysis, interpretation of the data, and revision of the manuscript. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Neafsey EJ, Collins MA. Moderate alcohol consumption and cognitive risk. Neuropsychiatric Disease and Treatment. 2011;7:465–484. doi: 10.2147/NDT.S23159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17:542–555. doi: 10.1097/JGP.0b013e3181a2fd07. [DOI] [PubMed] [Google Scholar]
- 3.Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- 4.Solfrizzi V, D'Introno A, Colacicco AM, et al. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology. 2007;68:1790–1799. doi: 10.1212/01.wnl.0000262035.87304.89. [DOI] [PubMed] [Google Scholar]
- 5.Virta JJ, Järvenpää T, Heikkilä K, et al. Midlife alcohol consumption and later risk of cognitive impairment: a twin follow-up study. J Alzheimers Dis. 2010;22:939–948. doi: 10.3233/JAD-2010-100870. [DOI] [PubMed] [Google Scholar]
- 6.Fratiglioni L, Ahlbom A, Viitanen M, Winblad B. Risk factors for late-onset Alzheimer's disease: a population-based, case-control study. Ann Neurol. 1993;33 doi: 10.1002/ana.410330306. [DOI] [PubMed] [Google Scholar]
- 7.Anttila T, Helkala EL, Viitanen M, et al. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ. 2004;329:539–542. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289:1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- 9.Toda A, Tagata Y, Nakada T, Komatsu M, Shibata N, Arai H. Changes in Mini-Mental State Examination score in Alzheimer's disease patients after stopping habitual drinking. Psychogeriatrics. 2013;13:94–98. doi: 10.1111/psyg.12008. [DOI] [PubMed] [Google Scholar]
- 10.Rosen J, Colantonio A, Becker JT, Lopez OL, DeKosky ST, Moss HB. Effects of a history of heavy alcohol consumption on Alzheimer's disease. The British journal of psychiatry : the journal of mental science. 1993;163:358–363. doi: 10.1192/bjp.163.3.358. [DOI] [PubMed] [Google Scholar]
- 11.Luchsinger JA, Tang MX, Siddiqui M, et al. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay J, Laurin D, Verreault R, et al. Risk Factors for Alzheimer's Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am J Epidemiol. 2002;56:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 13.Truelsen T, Thudium D, Grønbaek M Copenhagen City Heart Study. Amount and type of alcohol and risk of dementia: the Copenhagen City Heart Study. Neurology. 2002;59:1313–1319. doi: 10.1212/01.wnl.0000031421.50369.e7. [DOI] [PubMed] [Google Scholar]
- 14.Ruitenberg A, van Swieten JC, Witteman JC, et al. Alcohol consumption and risk of dementia: the Rotterdam Study. Lancet. 2002;359:281–286. doi: 10.1016/S0140-6736(02)07493-7. [DOI] [PubMed] [Google Scholar]
- 15.Weyerer S, Schäufele M, Wiese B, et al. Current alcohol consumption and its relationship to incident dementia: results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age and Ageing. 2011;40:456–463. doi: 10.1093/ageing/afr007. [DOI] [PubMed] [Google Scholar]
- 16.Sabia S, Elbaz A, Britton A, et al. Alcohol consumption and cognitive decline in early old age. Neurology. 2014;82:332–339. doi: 10.1212/WNL.0000000000000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehlig K, Skoog I, Guo X, et al. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in Goteborg. American journal of epidemiology. 2008;167:684–691. doi: 10.1093/aje/kwm366. [DOI] [PubMed] [Google Scholar]
- 18.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). I. Study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord. 1993;7:3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, et al. Motor signs during the course of Alzheimer disease. Neurology. 2004;63:975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-Mental State Examination: validity and reliability. Neurology. 1987;37(suppl 1):179. [Google Scholar]
- 22.Hixson JE. Apolipoprotein E polymorphisms affect atherosclerosis in young males. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb. 1991;11:1237–1244. doi: 10.1161/01.atv.11.5.1237. [DOI] [PubMed] [Google Scholar]
- 23.Stockwell T, Donath S, Cooper-Stanbury M, Chikritzhs T, Catalano P, Mateo C. Under-reporting of alcohol consumption in household surveys: a comparison of quantity-frequency, graduated-frequency and recent recall. Addiction. 2004;99:1024–1033. doi: 10.1111/j.1360-0443.2004.00815.x. [DOI] [PubMed] [Google Scholar]
- 24.Midanik LT. Validity of self-reported alcohol use: a literature review and assessment. Br J Addict. 1988;83:1019–1030. doi: 10.1111/j.1360-0443.1988.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 25.Wechsler D. Wechler Adult Intelligence Scale Revised. New York, NY: Psychological Corp; 1981. [Google Scholar]
- 26.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, Pa: Lea & Febiger; 1983. [Google Scholar]
- 27.Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate Ethanol Preconditioning of Rat Brain Cultures Engenders Neuroprotection Against Dementia-Inducing Neuroinflammatory Proteins: Possible Signaling Mechanisms. Mol Neurobiol. 2010;41:420–425. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minagar A, Shapshak P, Fujimura R, Ownbyc R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. Neurological Sciences. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 29.Belmadani A, Zou JY, Schipma MJ, Neafsey EJ, Collins MA. Ethanol pre-exposure suppresses HIV-1 glycoprotein 120-induced neuronal degeneration by abrogating endogenous glutamate/Ca2+-mediated neurotoxicity. Neuroscience. 2011;104:769–781. doi: 10.1016/s0306-4522(01)00139-7. [DOI] [PubMed] [Google Scholar]
- 30.Kalev-Zylinska ML, During MJ. Paradoxical facilitatory effect of low-dose alcohol consumption on memory mediated by NMDA receptors. J Neurosci. 2007;27:10456–10467. doi: 10.1523/JNEUROSCI.2789-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belmadani A, Kumar S, Schipma M, Collins MA, Neafsey EJ. Inhibition of amyloid-b-induced neurotoxicity and apoptosis by moderate ethanol preconditioning. NeuroReport. 2004;15:2093–2096. doi: 10.1097/00001756-200409150-00019. [DOI] [PubMed] [Google Scholar]
- 32.Cargiulo T. Understanding the health impact of alcohol dependence. Am J Health Syst Pharm. 2007;64:S5–11. doi: 10.2146/ajhp060647. [DOI] [PubMed] [Google Scholar]
- 33.Paul CA, Au R, Fredman L, et al. Association of alcohol consumption with brain volume in the Framingham study. Arch Neurol. 2008;65:1363–1367. doi: 10.1001/archneur.65.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013 Mar;18(2):297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 36.Kar S, Slowikowski SP, Westaway D, Mount HT. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer's disease. J Psychiatry Neurosci. 2004;29:427–441. [PMC free article] [PubMed] [Google Scholar]
- 37.Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. 2006;25 doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyas SL. Alcohol use and the risk of developing Alzheimer's disease. Alcohol Res Health. 2001;25:299–306. [PMC free article] [PubMed] [Google Scholar]
- 39.Casamenti F, Scali C, Vannucchi MG, Bartolini L, Pepeu G. Long-term ethanol consumption by rats: effect on acetylcholine release in vivo, choline acetyltransferase activity, and behavior. Neuroscience. 1993;56:465–471. doi: 10.1016/0306-4522(93)90346-h. [DOI] [PubMed] [Google Scholar]
- 40.Gendron TF, McCartney S, Causevic E, Ko LW, Yen SH. Ethanol enhances tau accumulation in neuroblastoma cells that inducibly express tau. Neurosci Lett. 2008;443:67–71. doi: 10.1016/j.neulet.2008.07.052. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasinetti GM. Novel role of red wine-derived polyphenols in the prevention of Alzheimer's disease dementia and brain pathology: experimental approaches and clinical implications. Planta Med. 2012;78(15):1614–9. doi: 10.1055/s-0032-1315377. [DOI] [PubMed] [Google Scholar]
- 42.Valaasani KR, Sun Q, Hu G, Li J, Du F, Guo Y, et al. Identification of human ABAD inhibitors for rescuing Aβ-mediated mitochondrial dysfunction. Curr Alzheimer Res. 2014 Feb;11(2):128–36. doi: 10.2174/1567205011666140130150108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell MC, Jr, Teigen EL, Ramchandani VA. Absorption and peak blood alcohol concentration after drinking beer, wine, or spirits. Alcoholism, clinical and experimental research. 2014;38:1200–1204. doi: 10.1111/acer.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aho L, Karkola K, Juusela J, Alafuzoff I. Heavy alcohol consumption and neuropathological lesions: a post-mortem human study. J Neurosci Res. 2009;87(12):2786–92. doi: 10.1002/jnr.22091. [DOI] [PubMed] [Google Scholar]