Abstract
There is considerable interest in maintaining working memory (WM) because it is essential to accomplish most cognitive tasks, and it is correlated with fluid intelligence and ecologically valid measures of daily living. Toward this end, WM training protocols aim to improve WM capacity and extend improvements to unpracticed domains, yet success is limited. One emerging approach is to couple WM training with transcranial direct current stimulation (tDCS). This pairing of WM training with tDCS in lon gitudinal designs promotes behavioral improvement and evidence of transfer of performance gains to untrained WM tasks. However, the mechanism(s) underlying tDCS-linked training benefits remain unclear. Our goal was to gain purchase on this question by recording high-density EEG before and after a weeklong WM training + tDCS study. Participants completed four sessions of frontoparietal tDCS (active anodal or sham) during which they performed a visuospatial WM change detection task. Participants who received active anodal tDCS demonstrated significant improvement on the WM task, unlike those who received sham stimulation. Importantly, this pattern was mirrored by neural correlates in spectral and phase synchrony analyses of the HD-EEG data. Notably, the behavioral interaction was echoed by interactions in frontal-posterior alpha band power, and theta and low alpha oscillations. These findings indicate that one mechanism by which paired tDCS + WM training operates is to enhance cortical efficiency and connectivity in task-relevant networks.
Keywords: working memory training, tDCS, HD-EEG, neural oscillations
1.1 Introduction
Working memory (WM) provides the mental workspace engaged during most cognitive tasks (e.g., Conway, Kane, & Engle, 2003; Kane & Engle, 2002). Unfortunately, WM is generally considered limited in capacity (Cowan, 2001; Eriksson, Vogel, Lansner, Bergstrom, & Nyberg, 2015; Franconeri, Alvarez, & Cavanagh, 2013; Luck & Vogel, 1997; Oberauer, Farrell, Jarrold, & Lewandowsky, 2016; for other factors influencing WM capacity see: Alvarez & Cavanagh, 2004; Brady, Stormer, & Alvarez, 2016; Curby, Glazek, & Gauthier, 2009). Furthermore, there is an active debate regarding whether WM capacity is a discrete resource, accommodating a fixed number of items (Barton, Ester, & Awh, 2009; Ester, Fukuda, May, Vogel, & Awh, 2014; Zhang & Luck, 2011), or a pooled resource permitting flexible allocation across a variable number of items (Bays, Catalao, & Husain, 2009; Ma, Husain, & Bays, 2014; see also: Fukuda, Vogel, Mayr, & Awh, 2010; Wei, Wang, & Wang, 2012). Although these observations point to what remains unclear about WM, it is unquestioned that successful WM is important for everyday tasks. This means that interventions that preserve or enhance WM are important for people in general, and especially for vulnerable populations such as the aging.
A variety of WM training interventions propose that practicing specific WM tasks will generally strengthen WM (reviewed in: Karbach & Verhaeghen, 2014; Morrison & Chein, 2011). Yet, there is marked skepticism regarding the claims of commercial products1 (Chacko, et al., 2013; “A Consensus on the Brain Training Industry from the Scientific Community,” 2014; Steenbergen, et al., 2015), accompanied by limited empirical evidence that WM training provides generalized WM improvement (Klingberg, 2010; Morrison & Chein, 2011; Shipstead, Redick, & Engle, 2012).
Recently, training paired with transcranial direct current stimulation (tDCS) has shown promise in enhancing cognitive task performance. TDCS is a form of non-invasive brain stimulation that applies electrical current (typically 1-2 mA) through scalp-based electrodes to alter the resting state of underlying neuronal populations (Nitsche & Paulus, 2000, 2001; Stagg & Nitsche, 2011). It is well suited for targeting the frontoparietal substrates of WM as it is safe (Nitsche, et al., 2003) and well tolerated (Kessler, Turkeltaub, Benson, & Hamilton, 2012; Poreisz, Boros, Antal, & Paulus, 2007; for recent reviews see: Berryhill, Peterson, Jones, & Stephens, 2014; Bikson, et al., 2016; Parkin, Ekhtiari, & Walsh, 2015; Woods, Bryant, Sacchetti, Gervits, & Hamilton, 2015). Importantly, most studies using longitudinal designs report consistent cognitive benefits across participants in WM (Au, et al., 2016; Jones, Stephens, Alam, Bikson, & Berryhill, 2015; Park, Seo, Kim, & Ko, 2014; Richmond, Wolk, Chein, & Olson, 2014) and other tasks (Choe, Coffman, Bergstedt, Ziegler, & Phillips, 2016; Ditye, Jacobson, Walsh, & Lavidor, 2012; Martin, et al., 2013; Meinzer, et al., 2014; reviewed in: Elmasry, Loo, & Martin, 2015). However, one recent WM training study applying three sessions found that two participants performed worse after the active tDCS protocol (Talsma, Kroese, & Slagter, 2016). This observation echoed some of our previous research showing that individual differences are important in tDCS research. In short, we previously found that only high WM capacity younger adults, or more educated older adults benefited from a single session of tDCS (Berryhill & Jones, 2012; Jones, Gozenman, & Berryhill, 2015; also see: Berryhill, et al., 2014; Hsu, Juan, & Tseng, 2016; Jones, Gozenman, et al., 2015; London & Slagter, 2015). Our data suggest active tDCS may enhance training-related benefits by prolonging improved performance, as our most robust effects were apparent after a month of no contact (Jones, Stephens, et al., 2015; Stephens & Berryhill, 2016). There is consistency across laboratories, protocols, and tasks that stands in marked contrast to the single session tDCS studies which is highly variable that we argue contributes to debates regarding the effectiveness of tDCS when applied to cognitive tasks (see meta-analyses: Horvath, Forte, & Carter, 2015a, 2015b; Jacobson, Koslowsky, & Lavidor, 2012; Mancuso, Ilieva, Hamilton, & Farah, 2016; but see: Antal, Keeser, Priori, Padberg, & Nitsche, 2015; Berryhill & Jones, 2012; Berryhill, et al., 2014; Brunye, et al., 2014; Price & Hamilton, 2015). This controversy is outside the scope of this article, but it is important to reiterate that the consistency of cognitive benefits reported in the small longitudinal tDCS literature is not matched in the single session tDCS literature. This discrepancy will ultimately need to be reconciled.
One primary gap in knowledge exists with regard to the mechanism(s) of longitudinal tDCS-linked WM improvement. At different levels of inquiry, it is very likely to include neuroplasticity via an LTP-like mechanism (reviewed in: Brunoni, et al., 2012; Filmer, Dux, & Mattingley, 2014; Medeiros, et al., 2012; Stagg & Nitsche, 2011), altered resting state connectivity (e.g. Keeser, et al., 2011; Weber, Messing, Rao, Detre, & Thompson-Schill, 2014), and modulated brain perfusion (Nord, Lally, & Charpentier, 2013; Stagg, et al., 2013). One recent review indicated that rather than being a ‘bug’, a ‘feature’ of tDCS is that it provides diffuse stimulation, with effects seeming to alter task relevant networks alone (Filmer, et al., 2014). This is consistent with our observation that stimulating frontoparietal networks via PFC, PPC, or alternating between PFC and PPC sites yielded statistically equivalent behavioral effects on WM performance, although alternating sites had a numerical advantage (Jones, Stephens, et al., 2015), which suggests that stimulating various nodes of this network results in a WM boost.
To increase the explanatory power of the tDCS technique, it is important to isolate candidate neural mechanisms associated with tDCS-linked performance improvements. Several recent experiments paired tDCS with neuroimaging techniques such as functional near-infrared spectroscopy (fNIRS; Ishikuro, et al., 2014; Jones, Gozenman, et al., 2015; Khan, et al., 2013; McKendrick, Parasuraman, & Ayaz, 2015; Merzagora, et al., 2010; Muthalib, Besson, Rothwell, Ward, & Perrey, 2016; Muthalib, Kan, Nosaka, & Perrey, 2013), or functional magnetic resonance imaging (fMRI; Alon, Roys, Gullapalli, & Greenspan, 2011; Antal, Polania, Schmidt-Samoa, Dechent, & Paulus, 2011; Holland, et al., 2011; Kwon & Jang, 2011) to investigate neural changes after tDCS. These data lead to the interpretation that tDCS improves the efficiency of task relevant neural networks. By way of example, four days of flight simulator training paired with dorsolateral prefrontal tDCS enhanced the mid-frontal theta power during both the flight simulation task and an untrained WM n-back task (Choe, et al., 2016).
TDCS may change neural oscillations. This would converge with an established EEG-WM training literature. Alpha (and theta) oscillations support WM maintenance and are linked to individual differences in WM performance (reviewed in: Roux & Uhlhaas, 2014). There are currently two prominent theories of the role of alpha oscillations in WM maintenance. First, the inhibition-timing hypothesis suggests that increased alpha power serves to inhibit task-irrelevant regions to prioritize processing task-relevant information (e.g., Jensen, Gelfand, Kounios, & Lisman, 2002; Jokisch & Jensen, 2007; Kelly, Lalor, Reilly, & Foxe, 2006; Klimesch, Sauseng, & Hanslmayr, 2007). Second, an alternative view is that the delay period alpha during a WM task may reflect the underlying WM maintenance process itself (Herrmann, Senkowski, & Rottger, 2004; Leiberg, Lutzenberger, & Kaiser, 2006; Palva, Kulashekhar, Hamalainen, & Palva, 2011; Sauseng, et al., 2005). Posterior alpha power increases with WM load (e.g., Jensen & Tesche, 2002; Manza, Hau, & Leung, 2014) and may be inhibiting task irrelevant information (Klimesch, Doppelmayr, Schwaiger, Auinger, & Winkler, 1999).
WM performance is also associated with frontal oscillations in theta (e.g., Schack, Klimesch, & Sauseng, 2005) and alpha (e.g., Itthipuripat, Wessel, & Aron, 2013). In particular, enhanced phase synchrony between anterior and posterior sites appears to protect items held in WM (Bonnefond & Jensen, 2012). Modulating anterior-posterior phase synchrony via rTMS impairs WM performance suggesting that long-range phase coupling may be the mechanism for top-down modulation between PFC and more posterior cortical areas (Zanto, Rubens, Thangavel, & Gazzaley, 2011). Importantly, both alpha and theta frequency bands (3-15 Hz) show modulation by tDCS (Mangia, Pirini, & Cappello, 2014; Spitoni, Cimmino, Bozzacchi, Pizzamiglio, & Di Russo, 2013). This makes HD-EEG a particularly good tool to study the mechanism(s) underlying tDCS-linked WM improvement.
Here, we investigated this question by pairing a week of WM training with anodal frontoparietal tDCS. High-density EEG (HD-EEG) was collected before and after training to measure neural changes, as EEG is able to record cortical activity with high temporal resolution. Furthermore, EEG will allow for analyses of neural oscillations during the WM change detection task. Participants completed four WM training sessions paired with active anodal or sham tDCS targeting right frontoparietal WM networks (right DLPFC and PPC). Participants performed the same supra-capacity WM change detection task during each session2. During analysis, we focused on alpha and theta frequency bands (3-15Hz) because they are modulated by tDCS (Mangia, et al., 2014; Spitoni, et al., 2013) and involved in WM maintenance (Roux & Uhlhaas, 2014). We tested the prediction that tDCS would benefit WM performance and reveal corresponding neural correlates detectible as decreased alpha, suggesting greater efficiency in WM networks, and increased phase locking, consistent with improved connectivity in WM networks.
2.1 Material and Methods
2.2 Participants
Twenty-four neurotypical right-handed University of Nevada students (mean age: 24.20, standard deviation (SD: 3.81) participated. Participants were randomly assigned to the active tDCS (5 females) or sham tDCS (6 females) groups. Participants were screened for use of neuroleptic, hypnotic, or seizure medications. Participants reported no history of neurological or psychiatric symptoms or head injuries. One participant from the active tDCS group was excluded from all subsequent behavioral and EEG group-level analyses due to excessive noise in the pre-training EEG data. The University of Nevada Institutional Review Board approved all procedures. Participants provided informed consent and were compensated $15/hour ($70 total).
2.3 Experimental Sequence and WM change detection Task
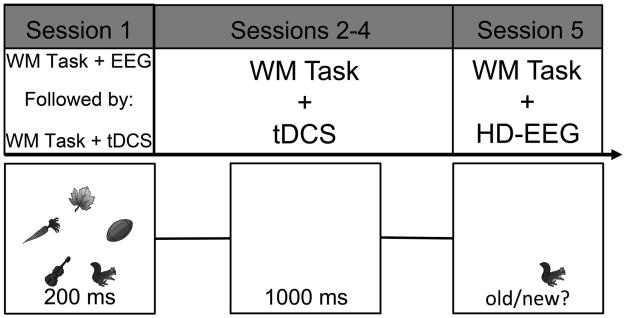
Participants first completed a WM change detection task during HD-EEG recording (on Monday; see section 2.5). Immediately afterward, on the same day the EEG cap was removed from the head and participants received tDCS (session 1: see section 2.4), followed by completion of the same WM change detection task for a second time. During sessions 2-4, on the following three days (Tuesday, Wednesday, Thursday), participants received tDCS prior to completing the WM change detection task. During the final session (session 5, Friday) participants completed the WM change detection task during HD-EEG recording but they did not receive tDCS.
During each trial, participants first were presented with a fixation point at the center of the screen (500 ms), then the participants viewed five gray scale pictures (3.5° × 3.5°) of common objects drawn from a set of 20 items (ant, axe, carrot, chicken, corn, fence, flower, football, eyeglasses, hammer, kettle, kite, leaf, pipe, scissors, snake, squirrel, toothbrush, windmill, violin; 200 ms; Rossion & Pourtois, 2004), followed by a blank delay (1000 ms), and a single-recognition test probe, to which participants made an old/new judgment (3000 ms) indicating whether or not the item was previously seen (Figure 1). Participants completed 432 trials of the task during each HD-EEG and tDCS session. This task was not adaptive in order to maintain a consistent set size between participants. This meant that the WM-linked EEG amplitudes across participants reflected responses to a consistent task. The WM change detection task was controlled and stimulus event onsets were triggered using the Psychophysics Toolbox (Brainard, 1997; Pelli, 1997) for MATLAB (MathWorks Inc., Natick, MA). Participants viewed the stimuli from a distance of ∼57 cm.
Figure 1.
Top) Timeline for the Experiment. Sessions 1 and 2 both took place on the same day (Monday). The first session acted as a baseline for performance while HD-EEG recorded cortical activity prior to the application of tDCS. Sessions 2-4 had the WM change detection task take place following the application of tDCS. Session 5 (Friday) had participants complete the WM change detection task while the HD-EEG recorded cortical activity and there was no tDCS prior to task performance. In total, participants completed the WM change detection task six times between the four tDCS sessions and the two HD-EEG sessions. Bottom) WM change detection task paradigm used in the Experiment. Five grayscale items appeared for 200 ms followed by a 1000 ms delay. Participants were then required to judge the probed item as old or new.
2.4 Transcranial Direct Current Stimulation
Stimulation consisted of a single continuous direct current delivered by a battery-driven continuous stimulator (Eldith MagStim, GmbH, Ilmenau, Germany). Current (1.5 mA, 15 minutes) was delivered through two 5 × 7 cm2 electrodes within saline-soaked sponges. Sham stimulation included 20 seconds of ramping up and down stimulation at the beginning and end of the 15-minute period to give the participant a physical sense of stimulation associated with current change. Participants were randomly assigned group membership: active anodal or sham tDCS. Participants were blinded as to which tDCS condition they received, and the experimenter who conducted the pre- and post-EEG sessions was also blind to the tDCS condition. Participants also completed a post-tDCS questionnaire, in which they indicated any adverse symptoms experienced during stimulation. No participants reported any adverse effects and none indicated they were aware of their stimulation condition. For all participants regardless of stimulation group, the anode alternated between the right PFC (F4) and right PPC (P4) in a counterbalanced order across all four sessions, whereby every participant completed two sessions of anodal PFC (F4) and two sessions of anodal PPC (P4; Jones, Stephens, et al., 2015). We had used this unconventional alternating frontoparietal montage in a previous WM training study in healthy older adults and found that the anode location had equivalent behavioral effects when applied to right prefrontal, right parietal, or alternating between the two, although there was a numerical advantage in the alternating condition (Jones, Stephens, et al., 2015). Thus, we selected to alternate between the two in the current study, to target both ends of the frontoparietal WM network and to increase the likelihood of observing effects in a young adult population. The reference (cathode) electrode was placed on the contralateral cheek, which has been effective in previous research studies (Berryhill & Jones, 2012; Berryhill, Wencil, Coslett, & Olson, 2010; Elmer, Burkard, Renz, Meyer, & Jancke, 2009; Jones, Gozenman, & Berryhill, 2014; Jones, Gozenman, et al., 2015; Jones, Stephens, et al., 2015; Stephens & Berryhill, 2016; Tanoue, Jones, Peterson, & Berryhill, 2013). Participants completed a practice version of the WM change detection task, which consisted of 36 trials of equal difficulty during the 15 minutes of stimulation. Once the 15 minutes of stimulation was completed, the electrodes were removed from the head and the participants completed the experimental WM change detection task. As such, an offline tDCS protocol was used in the current work (reviewed in: Hill, Fitzgerald, & Hoy, 2016).
2.5 HD-EEG
The EEG was recorded in DC mode, at a sampling rate of 1000 Hz with a vertex (Cz) reference from 256 high-impedance electrodes mounted in a HydroCel Geodesic Sensor Net amplified by a Net Amps 300 amplifier and acquired using Net Station 4.5.5 software (Electrical Geodesics Inc., Eugene, OR) running on a 2.7 GHz dual-core Apple Power Mac G5. Electrode impedances were kept below 50 KΩ.
2.6 Analyses
2.6.1 Preprocessing
Data were analyzed using the Fieldtrip software package, a MATLAB-based toolbox (Oostenveld, Fries, Maris, & Schoffelen, 2011). Data were first high-pass filtered at 0.5 Hz, then segmented into epochs covering the time from 1.0 sec before to 3.0 sec after the onset of the sample array in each trial. The data were down-sampled offline to 512 Hz. Independent components analysis (ICA) was performed on the epoched data, and the eye blink component(s) were identified and removed for each participant's data. After eye blink correction, EEG waveforms from frontal electrodes (i.e., E237/E247) were visually inspected to identify voltage fluctuations (i.e., fluctuations greater than 18.75 μV or less than -18.75 μV) typical of horizontal eye movements. Trials containing horizontal or vertical eye movements were rejected entirely. To maintain sufficient statistical power for each session, any participants with fewer than 150 remaining trials artifact rejection and incorrect trial rejection were not included in analyses (n=1). The remaining 23 participants had an average of 281 trials per session (SD=44.7). EEG data were analyzed only for correct trials (de Vries, van Driel, & Olivers, 2017; Active tDCS group pre-EEG clean/correct trials: 275.73 (SD: 28.90), artifact/incorrect trials: 118.36 (22.39); Active tDCS group post-EEG clean/correct: 305.45 (35.99), artifact/incorrect: 99 (23.40); Sham tDCS group pre-EEG clean/correct: 275.25 (54.38); artifact/incorrect: 119 (33.79); Sham tDCS group post-EEG clean/correct: 269.5 (49.85); artifact/incorrect: 113.42 (27.19).
2.6.2 Spectral analysis
Power spectra were calculated using a multitaper time-frequency transformation based on multiplication in the frequency domain from 1 to 30Hz with 0.5Hz increments using a Hanning taper applied to short sliding time windows (Percival & Walden, 1993) every 100 ms. An adaptive time window of five cycles for each frequency (AT = 5/f) was applied. Spectral data were baseline corrected using the fixation period as the baseline time period (i.e., (delay – fixation) / fixation).
2.6.3 Phase-Locking Value Analysis
To investigate phase synchrony we applied a method termed phase-locking value (PLV; Lachaux, Rodriguez, Martinerie, & Varela, 1999). PLVs represent the phase covariance between two signals that are close in time. Unlike the potentially more familiar method of spectral coherence, PLVs separate the phase and amplitude components. The advantage of this is that it makes PLV less susceptible to the amplitude of the signal, and this means they can be directly interpreted in the framework of neural integration (Lachaux, et al., 1999). Phase-locking between two signals ( and ) was quantified, from the unaveraged signals, using wavelet analysis (Lachaux, et al., 1999). A complex representation of the phase for trial i at time t and frequency f0 is given by the convolution of a Morlet wavelet, , and the signal normalized by the amplitude, thus:
The width of the wavelet m=f 0/σf was 7 (Grossmann, Kronland-Martinet, & Morlet, 1989); where σf = 1/2πσt. The PLVs over N trials between signals and are defined as
PLV ranges from 0 to 1, which estimates the variability of phase differences between two signals across trials. If the phase difference varies little across trials, PLV is close to 1; with large variability in the phase difference it is close to 0. For all PLV calculations, we selected right frontal electrode E224 as the seed electrode because it corresponds to one of the tDCS stimulation sites (F4) used during training.
2.6.4 Statistical Analysis
To test for significant differences between stimulation groups and pre- and post-training time points, and to correct for multiple comparisons, we subjected spectral analyses and PLV measurements to nonparametric randomization tests (Maris & Oostenveld, 2007; Nichols & Holmes, 2002). This procedure controls for Type I error by calculating the cluster-level statistics by randomizing trial labels at each iteration. First, spectral data from each of the 256 electrodes across the scalp were averaged over the time period of interest, which was the delay period (i.e., 0.2 – 1.2 seconds after the onset of the sample), but we excluded the first 500ms of the Delay period because this time period likely contained sensory-evoked response activity from the cue stimuli (e.g., van Gerven, et al., 2009; also see Bastiaansen, Mazaheri, & Jensen, 2012). Next, a t-value was calculated at each electrode. For each iteration randomizing trial labels, clusters of electrodes where the alpha-level was <0.05 were identified, and their t-values were summed. The largest sum of t-values was used as a t-statistic. This procedure was repeated 5000 times to create the null distribution. The p-value was estimated according to the proportion of the null distributions exceeding the observed cluster-level t-statistic. We focused our analyses on the theta and alpha frequency ranges, given previous work showing the involvement of oscillations in this range for WM performance (for a review see: Roux & Uhlhaas, 2014). Thus, we used nonparametric randomization tests to determine at what specific frequency bins our effects were present from 3-15 Hz at 0.5 Hz intervals.
To compare PLVs, we used nonparametric randomization tests, similar to that described above for the spectral power analysis. For PLV analyses, we also investigated the theta and alpha frequency ranges by examining the 3-15 Hz range at 0.5 Hz intervals. As with the spectral data, phase synchrony in both the alpha and theta ranges has been shown to be involved in WM maintenance and sensitive to load (for a review see: Roux & Uhlhaas, 2014). Specifically, PLVs for each group and time point were averaged across the delay period. A t-value was then calculated for each electrode across the scalp (except the seed), with trial labels randomized. For each iteration, clusters of electrodes where the alpha-level was <0.05 were identified, and their t-values were summed. The largest sum of t-values was used as a t-statistic. This procedure was repeated 5000 times to create the null distribution. The p-value for a cluster with correct trial labels was then estimated according to the proportion of the null distributions exceeding the observed cluster-level t-statistic.
For both spectral power and PLV data, we were most interested in testing for a tDCS group (active, sham) x session (pre-, post-training) interaction within the entire frequency range of interest. This was done by first calculating the difference between post – pre values and then testing for stimulation group differences. We used nonparametric randomization tests to control for multiple comparisons across this entire range of frequency bins (3-15Hz) in order to remain agnostic about where within this range the effects may occur. We then followed up on any significant interaction effects by doing direct within and between group contrasts.
3.1 Results
A
3.1.1 Behavioral Results
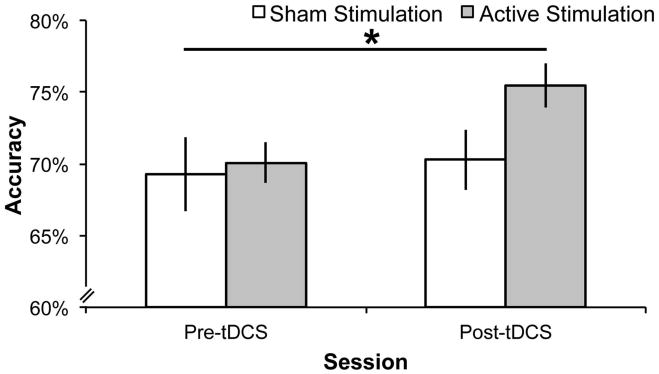
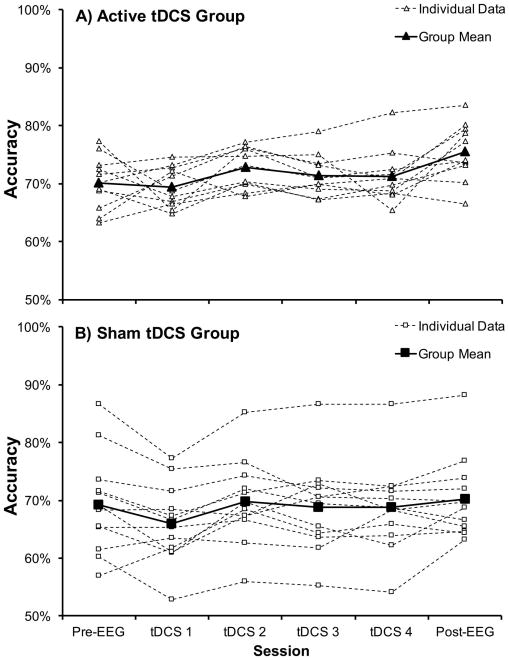
A priori, we selected a focused analysis approach in which we compared improvement in performance between pre-EEG and post-EEG sessions (before/after training). This directly addressed our hypothesis, and followed statistical methods similar to our previous tDCS + WM training study tDCS (Jones, Stephens, et al., 2015), as opposed to conducting an ANOVA that compared performance between the groups across each of the individual WM training session. To determine if tDCS promoted greater WM-related improvement, the accuracy data (proportion of correct trials) were subjected to a mixed ANOVA including the within-subjects factor of session (pre-, post-training) and the between-subjects factor of tDCS group (Sham, Active). There was a significant main effect of session (F1, 21 = 9.46, p = .006, partial η2 = .31, Greenhouse-Geisser corrected) indicating that accuracy improved over the training period. There was no main effect of tDCS group (F1, 21 = 1.42, p = .25). Crucially, there was a significant session x tDCS group interaction (F1, 21 = 4.35, p = .049, partial η2 = .17, Greenhouse-Geisser corrected). To characterize this interaction, we conducted follow-up independent-samples t-tests comparing WM performance between the two tDCS groups during both the pre-EEG session and the post-EEG session. There was no difference in WM performance between the two tDCS groups during the pre-EEG session (Active Mpre = .70 (SD = .05), Sham Mpre = .69 (SD = .09); t(17.19) = .29, p = .77, equal variances not assumed). However, after WM training, a significant difference was evident during the post-EEG session, such that the active tDCS group (Active Mpost = .76, SD = .05), outperformed the Sham tDCS group (Sham Mpost = .70, SD = .07), t(19.76) = 2.07, p = .05, equal variances not assumed). Pairing active tDCS with WM training improved WM performance to a greater extent than WM training alone (see Figure 2; Active Group session means (SD): Pre-EEG: .70 (05), tDCS 1: .69 (.03), tDCS 2: .73 (.04), tDCS 3: .71 (.04), tDCS 4: .71 (.05), Post-EEG: .76 (.05); Sham Group session means (SD): Pre-EEG: .69 (.09), tDCS 1: .66 (.07), tDCS 2: .70 (.07), tDCS 3: .70 (.08), tDCS 4: .69 (.08), Post-EEG: .70 (.08)). To further demonstrate the lack of behavioral improvement for the Sham tDCS group, we conducted a t-test comparing WM performance between the pre-EEG and post-EEG sessions for the Sham tDCS group and found no significant improvement (t(11) = .85, p = .41). In contrast, there was a significant improvement in the Active tDCS group (t(10) = 3.12, p = .01). Active Group had 9/11 (82%) participants improve behavioral performance between pre-EEG and post-EEG sessions and the Sham group had 7/12 (58%) participants improve. This pattern replicates the observation that not everyone may benefit from multiple sessions (Talsma, et al., 2016). In this study, 13/15 (87%) of the anodal group improved following verbal WM training.
Figure 2.
Accuracy results for the WM change detection task during sessions that took place before and after tDCS. The active stimulation group significantly improved performance following tDCS and five training sessions on the WM recognition task. The sham group showed no improvement as compared to the first pre-tDCS session after five sessions of training on the WM change detection task. The asterisk represents a statistically significant difference (p < .05) in the improvement from baseline between tDCS groups.
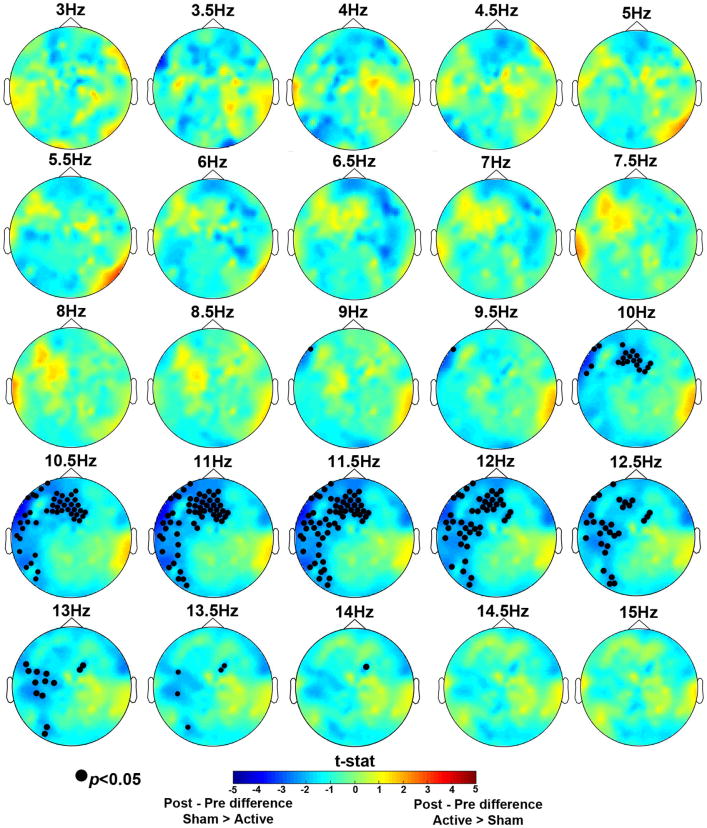
3.1.2 Spectral Alpha Power
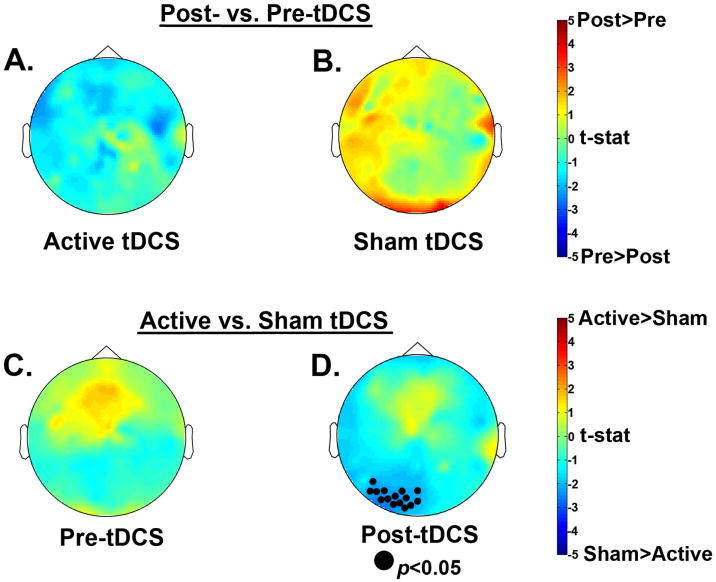
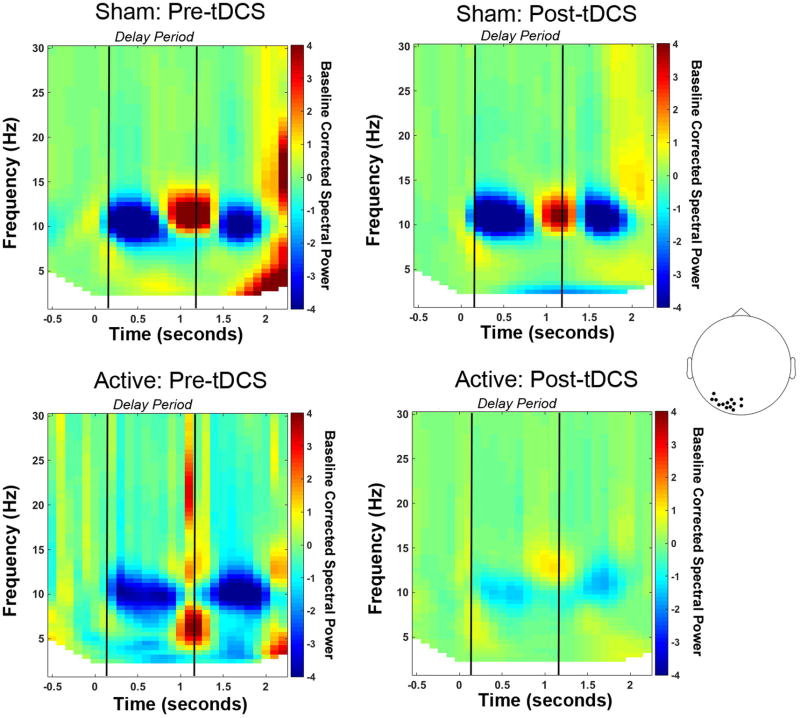
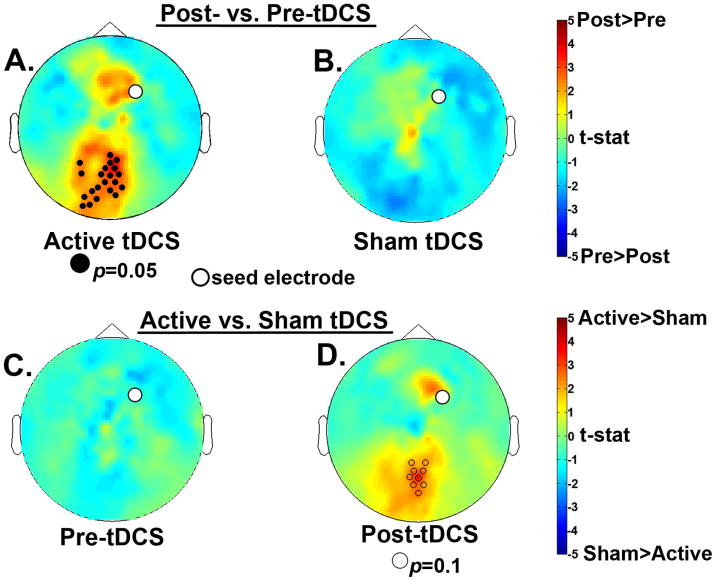
To identify neural correlates of the behavioral interaction, we first sought a group x session interaction across all frequency bins from 3-15 Hz during the delay period (Figure 4). In the alpha (9-14 Hz) range, there was a significant session x group interaction, (p<0.05). The effect was robust across frontal and left lateralized electrode sites, with little variability in the topography over this range. To follow-up on this interaction we focused on the alpha 9-14 Hz frequency range and examined spectral power differences in each tDCS group. For spectral analysis, we averaged across the entire significant frequency range of 9-14 Hz for the follow-up tests. The Active tDCS group showed a numerical decrease in alpha power after training, whereas the Sham tDCS group showed an increase in alpha power (Figure 5A, B). However, these effects did not reach significance. We also looked for significant group differences before and after training and found that, in line with the behavioral data, prior to training there were no differences between the two groups; see Figure 5C. Importantly, post-training there was a cluster of posterior electrodes that showed significantly greater alpha power for the Sham group compared to the Active group (p < 0.05); see Figure 5D. Because posterior alpha power increases with WM load (Jensen, et al., 2002; Lenartowicz, et al., 2014; Sauseng, et al., 2009), these results suggest that after training the Active group more efficiently maintained items in WM (Figure 6).
Figure 4.
Nonparametric randomization test results for the delay period for 3-15Hz frequency bins. The topographic maps represent group x session interaction test results for each frequency bin. Electrodes marked with a closed black circle represent electrodes that showed a significant interaction, p<0.05. The interaction was significant in the 9-14Hz range. Cooler colors indicate a greater difference in spectral power between post- and pre-sessions for the Sham group compared to the Active group. Warmer colors indicate a greater difference in spectral power between post- and pre-sessions for the Active group compared to the Sham group.
Figure 5.
Follow-up nonparametric randomization test results for delay period alpha power (9-14Hz). Comparing the data by group, there was no significant difference between post- and pre-tDCS sessions for A) the Active tDCS group or B) the Sham tDCS group. Comparing the data by time point, C) there was no difference between groups prior to tDCS, but D) after tDCS the Active tDCS group showed significantly less alpha power in a cluster of posterior electrodes compared to the Sham tDCS group, which suggests greater neural efficiency after training for the Active group.
Figure 6.
Time-frequency representations for each group and session separately. Spectral power is shown for the group of left posterior electrodes, marked in the topoplot, that showed a significant difference between groups after tDCS. Power is baseline corrected using the fixation period as the baseline period (-0.5 to 0 seconds).
3.1.3 Low Frequency Phase Synchrony
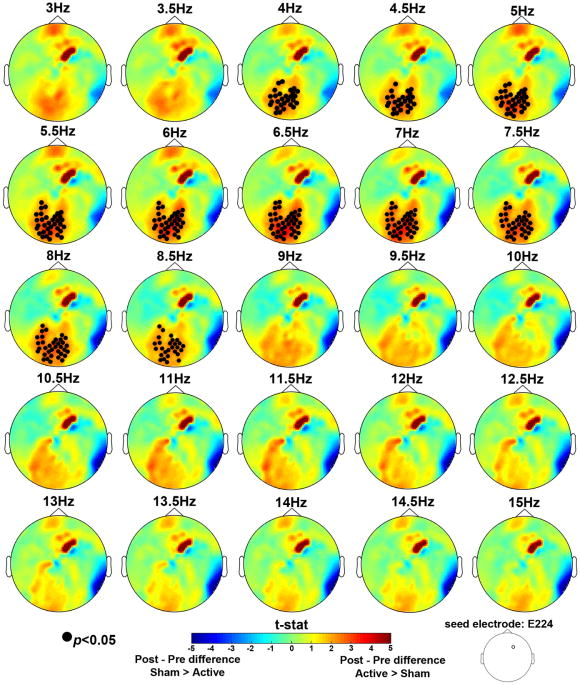
The phase locking value (PLV) data were subjected to the same analyses described for the spectral data. Recall that the right frontal electrode corresponding to the tDCS stimulation site (i.e., E224) served as the seed electrode. We first tested a group x session interaction on delay period PLV for 3-15 Hz. A significant interaction emerged between the right frontal seed and a consistent cluster of posterior electrodes in the 4-8.5 Hz range, p<0.05 (Figure 7). This frequency range encompasses the theta band and low alpha. This pattern of connectivity was present throughout the entire alpha band, but did not reach significance beyond 8.5 Hz (Figure 7).
Figure 7.
Nonparametric randomization test results for the delay period PLV data for 3-15Hz frequency bins. The topographic maps represent group x session interaction test results for each frequency bin. Electrode E224, which corresponded to the right frontal tDCS stimulation site was used as the seed electrode. Electrodes marked with a closed black circle represent electrodes that showed a significant interaction, p<0.05. The interaction was significant in the 4-8.5Hz range. Cooler colors indicate a greater difference in PLV between the seed and the marked electrodes between post- and pre-sessions for the Sham group compared to the Active group. Warmer colors indicate a greater difference in PLV between post- and pre-sessions for the Active group compared to the Sham group.
To follow-up on this interaction we had to choose a specific frequency bin because PLV are calculated at a specific frequency, which does not allow us to average across frequency bins as we did above for the spectral analysis follow-up tests. Therefore, we chose the 7 Hz frequency bin, which had the most robust interaction p-value (p=0.01) and examined PLV differences by tDCS group. These analyses revealed that the Active group alone showed significantly greater frontal-posterior phase synchrony post- compared to pre-tDCS (p = 0.05); whereas the Sham group showed no difference (Figure 8A, B). Further, the pre-training data confirmed that there was no initial difference between groups (Figure 8C). After training, there was a trend toward greater frontal-posterior phase synchrony for the Active compared to the Sham group (p = 0.1; Figure 8D). We interpret these data as evidence that tDCS + WM training enhanced the oscillatory phase synchrony between frontal and posterior brain regions in the theta and low alpha frequency range. Although we cannot localize the posterior cluster of electrodes to a specific cortical population, they are over the PPC stimulation site. Given that this effect was only present in the Active tDCS group, this may reflect the mechanism by which tDCS-linked training enhanced performance benefits in the WM change detection task.
Figure 8.
Nonparametric randomization test results for delay period 7Hz PLVs with a right frontal seed electrode shown as a white circle on all topomaps. Comparing each group separately, A) the Active tDCS group showed significantly more frontal-posterior phase synchrony after training compared to before training; and B) the Sham tDCS group showed no significant difference. Comparing sessions separately, C) the pre-tDCS session showed no significant difference between groups but, D) in the post-tDCS session the Active group showed marginally more frontal-posterior phase synchrony after training compared to the Sham group.
One common concern regarding PLV data is the possibility of volume conduction overinflating estimates of phase synchrony. Fortunately, although volume conduction can elicit artificially high PLVs for short-range synchronies, the PLV results presented here represent long-range synchronization (i.e., between frontal and posterior regions), which cannot be explained readily by volume conduction (Lachaux, et al., 1999).
4.1 Discussion
Many training regimens target WM for improvement because it is important for most cognitive tasks. Pairing WM training with tDCS can be successful under certain circumstances, but the mechanism of tDCS-linked improvement is not well understood. To address this gap, young adult participants received active or sham, offline tDCS targeting right frontal and parietal sites (in alternation) during four sessions of training in a WM change detection task. The Active tDCS group demonstrated significantly greater gains in WM accuracy compared to the Sham tDCS group, which showed no significant improvement. This finding is consistent with previous verbal and visuospatial WM training studies showing that tDCS strengthens WM training benefits (Jones, Stephens, et al., 2015; Park, et al., 2014; Richmond, et al., 2014; Stephens & Berryhill, 2016). It is important to note that the behavioral difference between the Active and Sham tDCS group reached significance at the final post-EEG session. This is consistent with research showing that the effects of tDCS can follow non-linear time courses (e.g., Au, et al., 2016; Jones, Stephens, et al., 2015; Stephens & Berryhill, 2016). Additionally, the results suggest that tDCS provides sufficient neuromodulation to elicit WM performance benefits.
To better understand the neural mechanisms underlying the behavioral effect we measured HD-EEG before and after the paired tDCS-WM training sessions. The spectral data revealed that after training there was decreased posterior alpha power for the Active tDCS group compared to the Sham tDCS group. Our interpretation is that these data are evidence of superior efficiency at WM maintenance given that posterior alpha power typically increases with WM load (e.g., Jensen & Tesche, 2002). The phase synchrony data told a complementary story. There was significantly more frontal-posterior phase synchrony in the theta and low alpha range after training in the Active tDCS group as compared to the Sham tDCS group. These modulations in neural activity were not due to WM training alone, as they were not evident in the Sham tDCS group. In short, in young adults anodal tDCS paired with WM training enhanced frontoparietal connectivity and improved performance on a WM change detection task repeatedly administered over the course of a single week.
In addition to clarifying the mechanism by which tDCS-linked WM training operates, the current data provide an additional example of tDCS-related WM benefits to young adults (Richmond, et al., 2014; Snowball, et al., 2013; reviewed in: Elmasry, et al., 2015). These data have translational potential in developing cognitive interventions that could potentially benefit a variety of participant populations. Indeed, one topic of interest is the observation that training studies using multiple tDCS sessions observe consistent benefits across healthy older adult participants (Jones, Stephens, et al., 2015; Stephens & Berryhill, 2016). However, single sessions of tDCS produced some cases of equal and opposite results predicted by factors such as education or independent measures of WM capacity (Berryhill & Jones, 2012). It will be important to determine how individual or group differences predict benefits in single sessions of a particular tDCS protocol and in training studies involving multiple sessions. Identifying who will benefit and under what parameter settings will be important for tDCS to achieve translational value. Furthermore, factors such as session number should be considered given recent critical meta-analyses characterizing thefcognitive applications of tDCS as ineffectual (Horvath, et al., 2015a, 2015b), as well as those that support the effectiveness of tDCS to varying degrees (see these recent reviews for more nuanced interpretations: Dedoncker, Brunoni, Baeken, & Vanderhasselt, 2016; Hill, et al., 2016; Jacobson, et al., 2012; Mancuso, et al., 2016). To optimize all future designs involving tDCS, it will be important to customize the number of training sessions to reap maximal benefit – in terms of performance gains and durability of effects.
4.2 Neural Mechanisms
To clarify the neural mechanism underlying the behavioral effects we subjected the HD-EEG data to a data-driven series of analyses. These data elucidate several mechanisms by which WM benefits are instantiated after frontoparietal tDCS and they make contact with the existing WM-oscillation literature. Previous studies show that posterior alpha power increases with WM load (Jensen, et al., 2002; Jensen & Tesche, 2002; Sauseng, et al., 2009), reflecting increased top-down control (Herrmann, et al., 2004; Leiberg, et al., 2006; Palva, et al., 2011; Sauseng, et al., 2005) and/or inhibition of task-irrelevant information (Jensen, et al., 2002; Jokisch & Jensen, 2007; Medendorp, et al., 2007; Sauseng, et al., 2009). Both of which are crucial when WM capacity is reached or exceeded (Klimesch, et al., 1999). This previous work guides us to the following interpretation of the current data: the Active tDCS group more efficiently suppressed distracting information and/or controlled task-relevant information during WM maintenance as evidenced by a decrease in posterior alpha power after training. The PLV data revealed greater phase synchrony between frontal and posterior sites after training paired with Active tDCS. TDCS enhances WM processes by modulating underlying frontoparietal network connectivity and that a week of training is sufficient to detect these changes in young adults.
The current findings complement previous work identifying disrupted WM after rTMS over the inferior frontal junction (Zanto, et al., 2011). rTMS prior to the WM task disrupted connectivity between frontal and posterior scalp sites during encoding and predicted declines in WM performance. In addition, the same study found evidence that broad alpha band phase synchrony (i.e., 7-14 Hz) supported top-down modulation within the frontoparietal network. Here, the paired tDCS-training paradigm likely benefited WM performance by improving connectivity between regions critical for top-down control (e.g., frontal sites corresponding to PFC) and those involved in early attention and encoding during the WM process (e.g., posterior sites corresponding to PPC and visual cortex: (Berryhill & Olson, 2008a, 2008b; Harrison & Tong, 2009; Olson & Berryhill, 2009; Serences, Ester, Vogel, & Awh, 2009). Additionally, the alternating right anterior (i.e., over PFC)-right posterior (i.e., over PPC) anodal tDCS montage may have strengthened frontoparietal connectivity between regions that were conceivably active during both stimulation (i.e., during the practice WM change detection task) and immediately following stimulation (i.e., during the actual WM change detection task) Moreover, the lack of evidence for connectivity changes in the Sham group supports the view that anodal tDCS strengthened frontoparietal connectivity whereas WM training alone did not.
4.3 Future Directions
To increase the benefit offered by tDCS-linked WM training, other factors should be considered including the nature of the training and transfer tasks, the strategy employed while learning (see also: von Bastian & Oberauer, 2014), and individual difference factors (e.g., age, genetics, motivation level, personality, initial WM capacity). Beyond performance benefits observed for the trained task, to improve translational value, there should be significant transfer to untrained tasks. Although no transfer effects were examined in the current work, we contribute to the training literature by identifying modulation of frontoparietal activity as a potential mechanism underlying observations of tDCS-linked WM training benefits. While the current task was not adaptive by design, some research groups argue that training must be adaptive to promote transfer and improvement in fluid intelligence (Brehmer, Westerberg, & Backman, 2012; Jaeggi, Buschkuehl, Jonides, & Perrig, 2008; Jaeggi, Buschkuehl, Jonides, & Shah, 2011; Karbach, Strobach, & Schubert, 2015; further reviewed in: Au, et al., 2015; Klingberg, 2010). However, other researchers have challenged the reliability and necessity of adaptive training paradigms (reviewed in: von Bastian & Eschen, 2016). Importantly, previous research indicates that WM training paired with tDCS improves performance in both adaptive (Au, et al., 2016; Richmond, et al., 2014) and non-adaptive WM training paradigms (Jones, Stephens, et al., 2015; Park, et al., 2014; (Stephens & Berryhill, 2016). Future research will need to continue to provide converging evidence derived from multiple techniques to better understand the differences between these two training paradigms and their potentially unique influence on putative neural mechanisms when paired with tDCS.
The current data replicate findings showing benefits of tDCS-linked WM training. This suggests that a fruitful approach for future investigation will be to pair tDCS with cognitive training paradigms that successfully elicit transfer and to assess other relevant factors (e.g., task type, individual differences). For instance, anodal tDCS to the intraparietal sulcus increases local glutamate concentration differently across participants and the extent of change predicts connectivity during resting state (Hunter, et al., 2015). Further advances, such as those combining tDCS with graph theory (Luft, Pereda, Banissy, & Bhattaacharya, 2014) and neural dynamics (Wokke, Talsma, & Vissers, 2015) are needed to refine tDCS protocols. These previous studies reviewed the possibilities of pairing neurostimulation with other neuroimaging techniques and the cost associated with shifting the balance between competing neural networks. Specifically, the PLV findings discussed above support the theory that excitation in one network likely changes connectivity throughout the cortex (Wokke, et al., 2015). Continuing to pair neuroimaging methodologies with tDCS will help further the understanding of the mechanism at work behind behavioral improvements following neurostimulation.
4.3.1 Limitations
Several limitations deserve mention. First, we tested one population: healthy young adults. There is considerable interest in identifying cognitive interventions for at risk populations, such as the aging or those with dementia, instead of healthy young adults. Our previous work indicates that healthy older adults benefit when tDCS is linked to a WM training regimen, with the greatest effects being observed at a one-month follow-up session (Jones, Stephens, et al., 2015; (Stephens & Berryhill, 2016). However, the underlying mechanisms of tDCS-induced benefits may be different in an aging population due to differences in patterns of cortical activity (e.g., Cabeza, Anderson, Locantore, & McIntosh, 2002; Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008). Second, our participants received just four sessions of WM training conducted on sequential days. It would be valuable to refine the current paradigm including optimizing the number and spacing of WM training sessions (reviewed in: Karbach & Verhaeghen, 2014; see also Au, et al., 2016). The WM effects may be optimized with fewer sessions spaced farther apart, or there may be a benefit of more sessions over a longer period of time. Furthermore, we do not know anything about the longevity of the behavioral improvement. While there is a growing understanding of mechanistic changes induced by tDCS at the network level, the cellular and molecular changes associated with each task paradigm are not clear (reviewed in: Filmer, et al., 2014). In other words, tDCS remains a frontier. In addition, we acknowledge that group sizes of 11-12 participants raises concerns regarding power. It is important to make note that our EEG data were only analyzed for correct trials, whereas the behavioral results investigated the proportion of correct vs incorrect trials (accuracy). Future work is needed to replicate and extend these findings.
In closing, the current findings identify several underlying mechanisms associated with tDCS and WM training-related improvements to WM performance. Namely, reduced alpha power after tDCS paired with WM training suggests that tDCS paired with WM training reduces the amount of neural resources required for maintaining items in WM. Moreover, tDCS and WM training can facilitate performance by synchronizing activity within frontoparietal networks involved in WM. Future investigations are now needed to clarify the duration of these benefits, and whether the same mechanisms persist across other populations and for other cognitive domains.
Figure 3.
A) Anodal tDCS Group individual participant data on the WM change detection task across all sessions. The bold line represents the group mean on each session. B) The individual group data for the Sham tDCS Group across all sessions. The bold line represents the group mean on each session.
Highlights.
Anodal tDCS paired with WM-training led to significant behavioral improvement.
WM-training alone did not lead to significant behavioral improvements.
The behavioral improvements also resulted in a reduction of posterior alpha power.
Active tDCS was also associated with greater WM network phase synchrony
WM training paired with tDCS can enhance cortical efficiency and connectivity.
Acknowledgments
We would like to thank Gabriella Dimotsantos for her assistance with data collection. K.T.J., D.J.P., and M.E.B. conceived and designed the experiment. K.T.J. and D.J.P. collected behavioral-tDCS and HD-EEG data and analyzed behavioral data. K.J.B. analyzed the HD-EEG data. K.T.J., D.J.P., K.J.B., and M.E.B. contributed to writing and revising the manuscript. This work was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH P20GM103650 (Project Leader MB), NEI R15EY022775 (to MB), NSF OIA 1632849 and NSF OIA 1632738.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
“A Consensus on the Brain Training Industry from the Scientific Community,” Max Planck Institute for Human Development and Stanford Center on Longevity, accessed February 3rd, 2016, http://longevity3.stanford.edu/blog/2014/10/15/the-consensus-on-the-brain-training-industry-from-the-scientific-community/
The terms working memory and short-term memory are used interchangeably in the existing literature. In the strictest sense the current WM change detection task measures visual short-term memory. However, we have opted to use the term working memory to stay consistent with the broader literature.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alon G, Roys SR, Gullapalli RP, Greenspan JD. Non-invasive electrical stimulation of the brain (ESB) modifies the resting-state network connectivity of the primary motor cortex: a proof of concept fMRI study. Brain Research. 2011;1403:37–44. doi: 10.1016/j.brainres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Alvarez GA, Cavanagh P. The capacity of visual short-term memory is set both by visual information load and by number of objects. Psychological Science. 2004;15:106–111. doi: 10.1111/j.0963-7214.2004.01502006.x. [DOI] [PubMed] [Google Scholar]
- Antal A, Keeser D, Priori A, Padberg F, Nitsche MA. Conceptual and Procedural Shortcomings of the Systematic Review “Evidence That Transcranial Direct Current Stimulation (tDCS) Generates Little-to-no Reliable Neurophysiologic Effect Beyond MEP Amplitude Modulation in Health y Human Subjects: A Systematic Review” by Horvath and Co-workers. Brin Stimul. 2015;8:846–849. doi: 10.1016/j.brs.2015.05.010. [DOI] [PubMed] [Google Scholar]
- Antal A, Polania R, Schmidt-Samoa C, Dechent P, Paulus W. Transcranial direct current stimulation over the primary motor cortex during fMRI. Neuroimage. 2011;55:590–596. doi: 10.1016/j.neuroimage.2010.11.085. [DOI] [PubMed] [Google Scholar]
- Au J, Katz B, Buschkuehl M, Bunarjo K, Senger T, Zabel C, Jaeggi SM, Jonides J. Enhancing Working Memory Training with Transcranial Direct Current Stimulation. J Cogn Neurosci. 2016:1–14. doi: 10.1162/jocn_a_00979. [DOI] [PubMed] [Google Scholar]
- Au J, Sheehan E, Tsai N, Duncan GJ, Buschkuehl M, Jaeggi SM. Improving fluid intelligence with training on working memory: a meta-analysis. Psychon Bull Rev. 2015;22:366–377. doi: 10.3758/s13423-014-0699-x. [DOI] [PubMed] [Google Scholar]
- Barton B, Ester EF, Awh E. Discrete resource allocation in visual working memory. J Exp Psychol Hum Percept Perform. 2009;35:1359–1367. doi: 10.1037/a0015792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen M, Mazaheri A, Jensen O. Beyond ERPs: Oscillatory neuronal dynamics. New York: Oxford University Press; 2012. p. 1. [Google Scholar]
- Bays PM, Catalao RFG, Husain M. The precision of visual working memory is set by allocation of a shared resource. Journal of Vision. 2009;9 doi: 10.1167/9.10.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Jones KT. tDCS selectively improves working memory in older adults with more education. Neurosci Lett. 2012;521:148–151. doi: 10.1016/j.neulet.2012.05.074. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. Is the posterior parietal lobe involved in working memory retrieval? Evidence from patients with bilateral parietal lobe damage. Neuropsychologia. 2008a;46:1775–1786. doi: 10.1016/j.neuropsychologia.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Olson IR. The right parietal lobe is critical for visual working memory. Neuropsychologia. 2008b;46:1767–1774. doi: 10.1016/j.neuropsychologia.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Peterson DJ, Jones KT, Stephens JA. Hits and misses: leveraging tDCS to advance cognitive research. Front Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill ME, Wencil EB, Coslett BH, Olson IR. A selective working memory impairment after transcranial direct current stimulation to the right parietal lobe. Neurosci Lett. 2010;479:312–316. doi: 10.1016/j.neulet.2010.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, Mourdoukoutas AP, Kronberg G, Truong D, Boggio P, Brunoni AR, Charvet L, Fregni F, Fritsch B, Gillick B, Hamilton RH, Hampstead BM, Jankord R, Kirton A, Knotkova H, Liebetanz D, Liu A, Loo C, Nitsche MA, Reis J, Richardson JD, Rotenberg A, Turkeltaub PE, Woods AJ. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefond M, Jensen O. Alpha Oscillations Serve to Protect Working Memory Maintenance against Anticipated Distracters. Current Biology. 2012;22:1969–1974. doi: 10.1016/j.cub.2012.08.029. [DOI] [PubMed] [Google Scholar]
- Brady TF, Stormer VS, Alvarez GA. Working memory is not fixed- capacity: More active storage capacity for real-world objects than for simple stimuli. Proc Natl Acad Sci U S A. 2016;113:7459–7464. doi: 10.1073/pnas.1520027113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Brehmer Y, Westerberg H, Backman L. Working-memory training in younger and older adults: training gains, transfer, and maintenance. Frontiers in Human Neuroscience. 2012;6:63. doi: 10.3389/fnhum.2012.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabre A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunye TT, Holmes A, Cantelon J, Eddy MD, Gardony AL, Mahoney CR, Taylor HA. Direct current brain stimulation enhances navigation efficiency in individuals with low spatial sense of direction. Neuroreport. 2014;25:1175–1179. doi: 10.1097/WNR.0000000000000214. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Chacko A, Feirsen N, Bedard AC, Marks D, Uderman JZ, Chimiklis A. Cogmed Working Memory Training for youth with ADHD: a closer examination of efficacy utilizing evidence-based criteria. J Clin Child Adolesc Psychol. 2013;42:769–783. doi: 10.1080/15374416.2013.787622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe J, Coffman BA, Bergstedt DT, Ziegler MD, Phillips ME. Transcranial Direct Current Stimulation Modulates Neuronal Activity and Learning in Pilot Training. Frontiers in Human Neuroscience. 2016;10:34. doi: 10.3389/fnhum.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A Consensus on the Brain Training Industry from the Scientific Community. Max Planck Institute for Human Development and Stanford Center on Longevity; 2014. [Google Scholar]
- Conway AR, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci. 2003;7:547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: A reconsideration of mental storage capacity (vol 23, pg 87, 2001) Behavioral and Brain Sciences. 2001;24 doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Curby KM, Glazek K, Gauthier I. A visual short-term memory advantage for objects of expertise. J Exp Psychol Hum Percept Perform. 2009;35:94–107. doi: 10.1037/0096-1523.35.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries IE, van Driel J, Olivers CN. Posterior alpha EEG Dynamics Dissociate Current from Future Goals in Working Memory-Guided Visual Search. J Neurosci. 2017;37:1591–1603. doi: 10.1523/JNEUROSCI.2945-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedoncker J, Brunoni AR, Baeken C, Vanderhasselt MA. A Systematic Review and Meta-Analysis of the Effects of Transcranial Direct Current Stimulation (tDCS) Over the Dorsolateral Prefrontal Cortex in Healthy and Neuropsychiatric Samples: Influence of Stimulation Parameters. Brain Stimul. 2016;9:501–517. doi: 10.1016/j.brs.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Ditye T, Jacobson L, Walsh V, Lavidor M. Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res. 2012;219:363–368. doi: 10.1007/s00221-012-3098-4. [DOI] [PubMed] [Google Scholar]
- Elmasry J, Loo C, Martin D. A systematic review of transcranial electrical stimulation combined with cognitive training. Restor Neurol Neurosci. 2015;33:263–278. doi: 10.3233/RNN-140473. [DOI] [PubMed] [Google Scholar]
- Elmer S, Burkard M, Renz B, Meyer M, Jancke L. Direct current induced short-term modulation of the left dorsolateral prefrontal cortex while le a rning auditory presented nouns. Behav Brain Funct. 2009;5:29. doi: 10.1186/1744-9081-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J, Vogel EK, Lansner A, Bergstrom F, Nyberg L. Neurocognitive Architecture of Working Memory. Neuron. 2015;88:33–46. doi: 10.1016/j.neuron.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ester EF, Fukuda K, May LM, Vogel EK, Awh E. Evidence for a fixed capacity limit in attending multiple locations. Cogn Affect Behav Neurosci. 2014;14:62–77. doi: 10.3758/s13415-013-0222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci. 2014;37:742–753. doi: 10.1016/j.tins.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Franconeri SL, Alvarez GA, Cavanagh P. Flexible cognitive resources: competitive content maps for attention and memory. Trends Cogn Sci. 2013;17:134–141. doi: 10.1016/j.tics.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Vogel E, Mayr U, Awh E. Quantity, not quality: The relationship between fluid intelligence and working memory capacity. Psychonomic Bulletin & Review. 2010;17:673–679. doi: 10.3758/17.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann A, Kronland-Martinet R, Morlet J. Reading and Understanding Continuous Wavelet Transforms. Springer; Berlin Heidelberg: 1989. [Google Scholar]
- Harrison SA, Tong F. Decoding reveals the contents of visual working memory in early visual areas. Nature. 2009;458:632–635. doi: 10.1038/nature07832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Senkowski D, Rottger S. Phase-locking and amplitude modulations of EEG alpha: Two measures reflect different cognitive processes in a working memory task. Exp Psychol. 2004;51:311–318. doi: 10.1027/1618-3169.51.4.311. [DOI] [PubMed] [Google Scholar]
- Hill AT, Fitzgerald PB, Hoy KE. Effects of Anodal Transcranial Direct Current Stimulation on Working Memory: A Systematic Review and Meta- Analysis of Findings From Healthy and Neuropsychiatric Populations. Brain Stimul. 2016;9:197–208. doi: 10.1016/j.brs.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, Rothwell JC, Crinion J. Speech facilitation by left inferior frontal cortex stimulation. Current Biology. 2011;21:1403–1407. doi: 10.1016/j.cub.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O. Evidence that transcranial direct current stimulation (tDCS) generates little-to-no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: A systematic review. Neuropsychologia. 2015a;66:213–236. doi: 10.1016/j.neuropsychologia.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS) Brain Stimul. 2015b;8:535–550. doi: 10.1016/j.brs.2015.01.400. [DOI] [PubMed] [Google Scholar]
- Hsu TY, Juan CH, Tseng P. Individual Differences and State-Dependent Responses in Transcranial Direct Current Stimulation. Frontiers in Human Neuroscience. 2016;10:643. doi: 10.3389/fnhum.2016.00643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MA, Coffman BA, Gasparovic C, Calhoun VD, Trumbo MC, Clark VP. Baseline effects of transcranial direct current stimulation on glutamatergic neurotransmission and large-scale network connectivity. Brain Research. 2015;1594:92–107. doi: 10.1016/j.brainres.2014.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikuro K, Urakawa S, Takamoto K, Ishikawa A, Ono T, Nishijo H. Cerebral functional imaging using near-infrared spectroscopy during repeated performances of motor rehabilitation tasks tested on healthy subjects. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itthipuripat S, Wessel JR, Aron AR. Frontal theta is a signature of successful working memory manipulation. Exp Brain Res. 2013;224:255–262. doi: 10.1007/s00221-012-3305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216:1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proc Natl Acad Sci U S A. 2011;108:10081–10086. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Gelfand J, Kounios J, Lisman JE. Oscillations in the alpha band (9-12 Hz) increase with memory load during retention in a short-term memory task. Cereb Cortex. 2002;12:877–882. doi: 10.1093/cercor/12.8.877. [DOI] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci. 2007;27:3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Gozenman F, Berryhill ME. Enhanced long-term memory encoding after parietal neurostimulation. Exp Brain Res. 2014;232:4043–4054. doi: 10.1007/s00221-014-4090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Gozenman F, Berryhill ME. The strategy and motivational influences on the beneficial effect of neurostimulation: a tDCS and fNIRS study. Neuroimage. 2015;105:238–247. doi: 10.1016/j.neuroimage.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KT, Stephens JA, Alam M, Bikson M, Berryhill ME. Longitudinal neurostimulation in older adults improves working memory. Plos One. 2015;10:e0121904. doi: 10.1371/journal.pone.0121904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Karbach J, Strobach T, Schubert T. Adaptive working-memory training benefits reading, but not mathematics in middle childhood. Child Neuropsychol. 2015;21:285–301. doi: 10.1080/09297049.2014.899336. [DOI] [PubMed] [Google Scholar]
- Karbach J, Verhaeghen P. Making Working Memory Work: A Meta-Analysis of Executive-Control and Working Memory Training in Older Adults. Psychological Science. 2014;25:2027–2037. doi: 10.1177/0956797614548725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeser D, Padberg F, Reisinger E, Pogarell O, Kirsch V, Palm U, Karch S, Moller HJ, Nitsche MA, Mulert C. Prefrontal direct current stimulation modulates resting EEG and event-related potentials in healthy subjects: a standardized low resolution tomography (sLORETA) study. Neuroimage. 2011;55:644–657. doi: 10.1016/j.neuroimage.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95:3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Di fferences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5:155–162. doi: 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan B, Hodics T, Hervey N, Kondraske G, Stowe AM, Alexandrakis G. Functional near-infrared spectroscopy maps cortical plasticity underlying altered motor performance induced by transcranial direct current stimulation. J Biomed Opt. 2013;18:116003. doi: 10.1117/1.JBO.18.11.116003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T. ‘Paradoxical’ alpha synchronization in a memory task. Brain Res Cogn Brain Res. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53:63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14:317–324. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Kwon YH, Jang SH. The enhanced cortical activation induced by transcranial direct current stimulation during hand movements. Neurosci Lett. 2011;492:105–108. doi: 10.1016/j.neulet.2011.01.066. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiberg S, Lutzenberger W, Kaiser J. Effects of memory load on cortical oscillatory activity during auditory pattern working memory. Brain Research. 2006;1120:131–140. doi: 10.1016/j.brainres.2006.08.066. [DOI] [PubMed] [Google Scholar]
- Lenartowicz A, Delorme A, Walshaw PD, Cho AL, Bilder RM, McGough JJ, McCracken JT, Makeig S, Loo SK. Electroencephalography correlates of spatial working memory deficits in attention-deficit/hyperactivity disorder: vigilance, encoding, and maintenance. J Neurosci. 2014;34:1171–1182. doi: 10.1523/JNEUROSCI.1765-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London RE, Slagter HA. Effects of Transcranial Direct Current Stimulation over Left Dorsolateral pFC on the Attentional Blink Depend on Individual Baseline Performance. J Cogn Neurosci. 2015;27:2382–2393. doi: 10.1162/jocn_a_00867. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Luft C, Pereda E, Banissy MJ, Bhattaacharya J. Best of both worlds: promise of combining brain stimulation and brain connectome. Front Syst Neurosci. 2014;8 doi: 10.3389/fnsys.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WJ, Husain M, Bays PM. Changing concepts of working memory. Nature Neuroscience. 2014;17:347–356. doi: 10.1038/nn.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso LE, Ilieva IP, Hamilton RH, Farah MJ. Does Transcranial Direct Current Stimulation Improve Healthy Working Memory?: A Meta-analytic Review. Journal of Cognitive Neuroscience. 2016;28:1063–1089. doi: 10.1162/jocn_a_00956. [DOI] [PubMed] [Google Scholar]
- Mangia AL, Pirini M, Cappello A. Transcranial direct current stimulation and power spectral parameters: a tDCS/EEG co-registration study. Frontiers in Human Neuroscience. 2014;8:601. doi: 10.3389/fnhum.2014.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manza P, Hau CL, Leung HC. Alpha power gates relevant information during working memory updating. J Neurosci. 2014;34:5998–6002. doi: 10.1523/JNEUROSCI.4641-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG- data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- Martin DM, Liu R, Alonzo A, Green M, Player MJ, Sachdev P, Loo CK. Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. Int J Neuropsychopharmacol. 2013;16:1927–1936. doi: 10.1017/S1461145713000539. [DOI] [PubMed] [Google Scholar]
- McKendrick R, Parasuraman R, Ayaz H. Wearable functional near infrared spectroscopy (fNIRS) and transcranial direct current stimulation (tDCS): expanding vistas for neurocognitive augmentation. Front Syst Neurosci. 2015;9:27. doi: 10.3389/fnsys.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros LF, de Souza IC, Vidor LP, de Souza A, Deitos A, Volz MS, Fregni F, Caumo W, Torres IL. Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry. 2012;3:110. doi: 10.3389/fpsyt.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medendorp WP, Kramer GF, Jensen O, Oostenveld R, Schoffelen JM, Fries P. Oscillatory activity in human parietal and occipital cortex shows hemispheric lateralization and memory effects in a delayed double-step saccade task. Cereb Cortex. 2007;17:2364–2374. doi: 10.1093/cercor/bhl145. [DOI] [PubMed] [Google Scholar]
- Meinzer M, Jahnigen S, Copland DA, Darkow R, Grittner U, Avirame K, Rodriguez AD, Lindenberg R, Floel A. Transcranial direct current stimulation over multiple days improves learning and maintenance of a novel vocabulary. Cortex. 2014;50:137–147. doi: 10.1016/j.cortex.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Merzagora AC, Foffani G, Panyavin I, Mordillo-Mateos L, Aguilar J, Onaral B, Oliviero A. Prefrontal hemodynamic changes produced by anodal direct current stimulation. Neuroimage. 2010;49:2304–2310. doi: 10.1016/j.neuroimage.2009.10.044. [DOI] [PubMed] [Google Scholar]
- Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychonomic Bulletin & Review. 2011;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- Muthalib M, Besson P, Rothwell J, Ward T, Perrey S. Effects of Anodal High-Definition Transcranial Direct Current Stimulation on Bilateral Sensorimotor Cortex Activation During Sequential Finger Movements: An fNIRS Study. Adv Exp Med Biol. 2016;876:351–359. doi: 10.1007/978-1-4939-3023-4_44. [DOI] [PubMed] [Google Scholar]
- Muthalib M, Kan B, Nosaka K, Perrey S. Effects of transcranial direct current stimulation of the motor cortex on prefrontal cortex activation during a neuromuscular fatigue task: an fNIRS study. Adv Exp Med Biol. 2013;789:73–79. doi: 10.1007/978-1-4614-7411-1_11. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114 doi: 10.1016/s1388-2457(03)00235-9. author reply 2222-2223. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology-London. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nord CL, Lally N, Charpentier CJ. Harnessing electric potential: DLPFC tDCS induces widespread brain perfusion changes. Front Syst Neurosci. 2013;7:99. doi: 10.3389/fnsys.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberauer K, Farrell S, Jarrold C, Lewandowsky S. What limits working memory capacity? Psychological Bulletin. 2016;142:758–799. doi: 10.1037/bul0000046. [DOI] [PubMed] [Google Scholar]
- Olson IR, Berryhill M. Some surprising findings on the involvement of the parietal lobe in human memory. Neurobiol Learn Mem. 2009;91:155–165. doi: 10.1016/j.nlm.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Com put Intell Neurosci. 2011;2011:156869. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S, Kulashekhar S, Hamalainen M, Palva JM. Localization of cortical phase and amplitude dynamics during visual working memory encoding and retention. J Neurosci. 2011;31:5013–5025. doi: 10.1523/JNEUROSCI.5592-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Seo JH, Kim YH, Ko MH. Long-term effects of transcranial direct current stimulation combined with computer-assisted cognitive training in healthy older adults. Neuroreport. 2014;25:122–126. doi: 10.1097/WNR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- Parkin BL, Ekhtiari H, Walsh VF. Non-invasive Human Brain Stimulation in Cognitive Neuroscience: A Primer. Neuron. 2015;87:932–945. doi: 10.1016/j.neuron.2015.07.032. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Percival DB, Walden AT. Spectral Analysis for Physical Applications Cambridge. United Kingdom: Cambridge University Press; 1993. p. 1. [Google Scholar]
- Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Price AR, Hamilton RH. A Re-evaluation of the Cognitive Effects From Single-session Transcranial Direct Current Stimulation. Brain Stimul. 2015;8:663–665. doi: 10.1016/j.brs.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Richmond LL, Wolk D, Chein J, Olson IR. Transcranial Direct Current 2 Stimulation Enhances Verbal Working Memory Training Performance over Time and Near Transfer Outcomes. Journal of Cognitive Neuroscience. 2014;26:2443–2454. doi: 10.1162/jocn_a_00657. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Roux F, Uhlhaas PJ. Working memory and neural oscillations: alpha-gamma versus theta-gamma codes for distinct WM information? Trends Cogn Sci. 2014;18:16–25. doi: 10.1016/j.tics.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, Glennon M, Gerloff C, Birbaumer N, Hummel FC. Brain oscillatory substrates of visual short-term memory capacity. Current Biology. 2009;19:1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. European Journal of Neuroscience. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Schack B, Klimesch W, Sauseng P. Phase synchronization between theta and upper alpha oscillations in a working memory task. International Journal of Psychophysiology. 2005;57:105–114. doi: 10.1016/j.ijpsycho.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Serences JT, Ester EF, Vogel EK, Awh E. Stimulus-specific delay activity in human primary visual cortex. Psychological Science. 2009;20:7–214. doi: 10.1111/j.1467-9280.2009.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipstead Z, Redick TS, Engle RW. Is Working Memory Training Effective? Psychological Bulletin. 2012;138:628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- Snowball A, Tachtsidis I, Popescu T, Thompson J, Delazer M, Zamarian L, Zhu TT, Kadosh RC. Long-Term Enhancement of Brain Function and Cognition Using Cognitive Training and Brain Stimulation. Current Biology. 2013;23:987–992. doi: 10.1016/j.cub.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitoni GF, Cimmino RL, Bozzacchi C, Pizzamiglio L, Di Russo F. Modulation of spontaneous alpha brain rhythms using low-intensity transcranial direct-current stimulation. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Lin RL, Mezue M, Segerdahl A, Kong Y, Xie J, Tracey I. Widespread modulation of cerebral perfusion induced during and after transcranial direct current stimulation applied to the left dorsolateral prefrontal cortex. J Neurosci. 2013;33:11425–11431. doi: 10.1523/JNEUROSCI.3887-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Steenbergen L, Sellaro R, Hommel B, Lindenberger U, Kuhn S, Colzato LS. Unfocus on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res. 2015 doi: 10.1007/s00221-015-4391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JA, Berryhill ME. Older Adults Improve on Everyday Tasks after Working Memory Training and Neurostimulation. Brain Stimul. 2016 doi: 10.1016/j.brs.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsma LJ, Kroese HA, Slagter HA. Boosting Cognition: Effects of Multiple Session Transcranial Direct Current Stimulation on Working Memory. J Cogn Neurosci. 2016:1–14. doi: 10.1162/jocn_a_01077. [DOI] [PubMed] [Google Scholar]
- Tanoue RT, Jones KT, Peterson DJ, Berryhill ME. Differential frontal involvement in shifts of internal and perceptual attention. Brain Stimul. 2013;6:675–682. doi: 10.1016/j.brs.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerven M, Farquhar J, Schaefer R, Vlek R, Geuze J, Nijholt A, Ramsey N, Haselager P, Vuurpijl L, Gielen S, Desain P. The brain-computer interface cycle. J Neural Eng. 2009;6:041001. doi: 10.1088/1741-2560/6/4/041001. [DOI] [PubMed] [Google Scholar]
- von Bastian CC, Eschen A. Does working memory training have to be adaptive? Psychol Res. 2016;80:181–194. doi: 10.1007/s00426-015-0655-z. [DOI] [PubMed] [Google Scholar]
- von Bastian CC, Oberauer K. Effects and mechanisms of working memory training: a review. Psychol Res. 2014;78:803–820. doi: 10.1007/s00426-013-0524-6. [DOI] [PubMed] [Google Scholar]
- Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: a tDCS-fMRI study. Hum Brain Mapp. 2014;35:3673–3686. doi: 10.1002/hbm.22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Wang XJ, Wang DH. From distributed resources to limited slots in multiple-item working memory: a spiking network model with normalization. J Neurosci. 2012;32:11228–11240. doi: 10.1523/JNEUROSCI.0735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wokke ME, Talsma LJ, Vissers ME. Biasing neural network dynamics using non-invasive brain stimulation. Front Syst Neurosci. 2015;8 doi: 10.3389/fnsys.2014.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Bryant V, Sacchetti D, Gervits F, Hamilton R. Effects of Electrode Drift in Transcranial Direct Current Stimulation. Brain Stimul. 2015;8:515–519. doi: 10.1016/j.brs.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature Neuroscience. 2011;14:656–U156. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WW, Luck SJ. The Number and Quality of Representations in Working Memory. Psychological Science. 2011;22:1434–1441. doi: 10.1177/0956797611417006. [DOI] [PMC free article] [PubMed] [Google Scholar]