Abstract
Background
Maintenance of quality of life is the primary goal during treatment of brain metastases (BM). This is a protocol of an ongoing phase III randomised multicentre study. This study aims to determine the exact additional palliative value of stereotactic radiosurgery (SRS) over whole brain radiotherapy (WBRT) in patients with 4–10 BM.
Methods
The study will include patients with 4–10 BM from solid primary tumours diagnosed on a high-resolution contrast-enhanced MRI scan with a maximum lesional diameter of 2.5 cm in any direction and a maximum cumulative lesional volume of 30 cm3. Patients will be randomised between WBRT in five fractions of 4 Gy to a total dose of 20 Gy (standard arm) and single dose SRS to the BMs (study arm) in the range of 15–24 Gy. The largest BM or a localisation in the brainstem will determine the prescribed SRS dose. The primary endpoint is difference in quality of life (EQ5D EUROQOL score) at 3 months after radiotherapy with regard to baseline. Secondary endpoints are difference in quality of life (EQ5D EUROQOL questionnaire) at 6, 9 and 12 months after radiotherapy with regard to baseline. Other secondary endpoints are at 3, 6, 9 and 12 months after radiotherapy survival, Karnofsky ≥ 70, WHO performance status, steroid use (mg), toxicity according to CTCAE V4.0 including hair loss, fatigue, brain salvage during follow-up, type of salvage, time to salvage after randomisation and Barthel index. Facultative secondary endpoints are neurocognition with the Hopkins verbal learning test revised, quality of life EORTC QLQ-C30, quality of life EORTC BN20 brain module and fatigue scale EORTC QLQ-FA13.
Discussion
Worldwide, most patients with more than 4 BM will be treated with WBRT. Considering the potential advantages of SRS over WBRT, i.e. limiting radiation doses to uninvolved brain and a high rate of local tumour control by just a single treatment with fewer side effects, such as hair loss and fatigue, compared to WBRT, SRS might be a suitable alternative for patients with 4–10 BM.
Trial registration
Trial registration number: NCT02353000, trial registration date 15th January 2015, open for accrual 1st July 2016, nine patients were enrolled in this trial on 14th April 2017.
Keywords: Brain metastases, Stereotactic radiosurgery, Whole brain radiotherapy, Quality of life
Background
In this randomised study, in patients with 4–10 brain metastases (BM), the standard treatment of whole brain radiotherapy (WBRT) is compared to stereotactic radiosurgery (SRS) for all lesions with the primary endpoint of quality of life (QOL) at 3 months after radiotherapy. We hypothesise that SRS provides better QOL than WBRT because of better local tumour control and avoidance of potential side effects of WBRT. Brain metastases are an important cause of morbidity and mortality in patients with metastasised cancer, and therefore, optimal tumour control is essential. Dutch guideline recommends SRS for patients with 1–3 BM and WBRT for patients with 4 or more BM. WBRT has side effects such as hair loss, fatigue and cognitive dysfunction, which may result in decreased QOL that is undesirable in a palliative setting. [1] There are important advantages of SRS over WBRT, i.e., limiting radiation doses to the uninvolved brain and a high rate of local tumour control by just a single treatment compared to WBRT, in which a relatively low palliative radiation dose is delivered to both the brain and the BM (Fig. 1). SRS is widely available in most Dutch radiotherapy centers. Because of recent technical advances, SRS can be delivered in relatively short treatment time in 10–45 min in patients with multiple BM. With SRS, there is a relatively low risk (±5%) of symptomatic radionecrosis: damage of the surrounding brain tissue, which may occur several months after treatment. Radionecrosis may cause neurologic symptoms, often temporary, which are treated with dexamethasone. Moreover, the clinical value of WBRT over the best supportive care is controversial. A recent interim analysis of the QUARTZ study showed equal QOL and survival for patients treated with WBRT versus treatment with steroids alone. [2] A recent (non-randomised) study in a large cohort of patients with BM showed that after SRS, survival of patients with 5–10 BM was comparable to that of patients treated with 2–4 BM. [3] Thus far, WBRT has never been compared directly with SRS in patients with 4–10 BM, and therefore a randomised trial is needed. In the United States, the NAGKC 12–01 (NCT01731704) was initiated in patients with 5 or more brain metastases in which SRS was directly compared with WBRT. However, this study was closed prior to enrolment of patients because of insufficient staff. Many systemic therapies do not have satisfactory tumour control of BM because of poor passage of the blood brain barrier. In the future, SRS may be the optimal treatment choice to control BM in patients with multiple brain metastases to maintain long-term QOL, whereas new innovative systemic therapies may control extracranial disease.
Fig. 1.
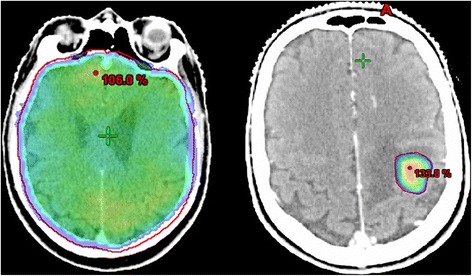
Dose distribution difference between WBRT (left) and SRS (right). A typical dose distribution on a planning-CT of WBRT on the left side and SRS on the right side. With WBRT, the healthy brain tissue receives the same low palliative radiation dose (non-ablative). With SRS, only the metastatic tissue receives a high ablative dose
Methods/design
Design
The study is a randomised phase III study with two study arms. The standard arm is WBRT and the experimental arm is SRS. We hypothesise that SRS provides better QOL than WBRT because of better local tumour control and avoidance of potential side effects of WBRT.
Objectives and endpoints
The primary objective is to determine whether QOL is better preserved after SRS than after WBRT in patients with 4–10 BM. The primary endpoint is difference in QOL (EQ5D EUROQOL score) at 3 months after radiotherapy with regard to baseline. The secondary objective is to determine whether SRS provides better survival and less toxicity than WBRT. [1, 2] Secondary endpoints are difference in QOL (EQ5D EUROQOL questionnaire) at 6, 9 and 12 months after radiotherapy with regard to baseline, survival at 3, 6, 9 and 12 months after radiotherapy, Karnofsky ≥ 70, WHO performance status, steroid use (mg), toxicity according CTCAE V4.0 including hair loss, fatigue, neurocognition and brain salvage during follow-up, type of salvage, time to salvage after randomisation and Barthel index. Facultative secondary endpoints are neurocognition with the Hopkins verbal learning test, quality of life EORTC QLQ-C30, quality of life EORTC BN20 brain module and fatigue scale EORTC QLQ-FA13.
Study population
The study will include patients with 4–10 BM from solid tumours diagnosed on a high resolution contrast-enhanced MRI scan referred for radiotherapy, with a maximum lesional diameter of 2.5 cm. Before randomisation, a new neuronavigation MRI (T1 gadolinium) is made for the definitive evaluation of the inclusion and exclusion criteria. The inclusion criteria are age ≥ 18; minimum of 4 BM up to a maximum of 10 BM on diagnostic MRI scan; maximum diameter of single gross tumour volume (GTV) 2.5 cm; maximum cumulative GTV of 30 cm3; Karnofsky performance status ≥70; any solid primary tumour, and patients’ ability to provide written informed consent. Small cell lung carcinoma, germinoma and lymphoma are excluded. Other exclusion criteria are a contraindication for MRI, prior treatment for BM (i.e. surgery, SRS or WBRT), concurrent use of systemic therapy (systemic therapies should be stopped at least 1 week prior until 1 week after the radiotherapy), maximum cumulative GTV of more than 30 cm3 on planning-MRI, more than 10 BM on planning-MRI, leptomeningeal disease and brainstem metastasis with a PTV of more than 20 cm3. If a patient is not eligible based on the inclusion or exclusion criteria of this study based on the planning-MRI results prior to randomisation (e.g. >10 BM, GTV diameter > 2.5 cm, cumulative GTV > 30 cm3), these non-eligible patients will be replaced by a new patient. These patients are not included in the statistical analysis of the trial.
Study procedures WBRT
On a gadolinium contrast-enhanced (single – triple dose Gd is allowed) MRI (1.0 T–3 T) with a maximal slice thickness of 1.5 mm, the definitive number of BM and the definitive maximum lesion diameter in any direction of the largest BM are determined. Patients randomised for WBRT will be treated with five fractions of 4 Gy up to a total dose of 20 Gy delivered in five consecutive working days. Dose prescription is according to ICRU 50 criteria. [4] The brain is contoured as a clinical target volume (CTV) until the foramen magnum. The CTV is equal to the PTV. To determine the size of the GTVs of the metastases, all BMs and lenses are contoured. Patients are positioned with a mask. The use of a planning CT is mandatory with slice thickness of ≤3 mm. The use of a contrast medium is not obliged. The planning-MRI is co-registered for contouring of the BM. The daily prescription dose will be 4 Gy prescribed at the ICRU reference point, and the 95% isodose must encompass 99% of the planning target volume (PTV); the maximum dose to the PTV should not exceed 107% of the prescribed dose. Generally, two opposed lateral fields are used with shielding of lenses and the pharyngeal space. All techniques that result in the dose requirements being met are allowed.
Study procedures of SRS
Only single fraction treatments are allowed within this protocol. For any given patient, all brain metastases will be treated with the same dose, which will be determined by the PTV of the largest BM or brainstem location in the range of 15–24 Gy. (Table 1) The dose gradient outside the PTV will be as steep as possible to spare healthy brain tissue. Within the PTV, there will be considerable dose inhomogeneity, with a maximum allowed dose within the PTV of 140% of the prescribed dose. The GTV is defined by contouring the outer contrast-enhancing border of the BM on T1 gadolinium-weighted MRI images. BM are named GTVp1, GTVp2, GTVp3, from the cranial to the caudal side. Organs at risk (brainstem, optic nerves, chiasma, pituary gland, cochleae, and lenses) are contoured according to Scoccianti et al. [5]. The PTV is defined by a 0–2 mm isotropic expansion of the GTV, according to institutional standards for SRS. If a BM is within or adjacent to the brainstem, the PTV margin will be 0 mm. If in an institution, a smaller GTV to PTV margin is used when lesions are treated using multiple isocentres, then this technique is to be considered to reduce the V12Gy of the largest BM if it would otherwise be more than 10 cm3.
Table 1.
Risk adapted SRS dose prescription volume and location based
| PTV of the largest brain metastasis | Doses in each PTV | BM in brainstem (GTV = PTV) |
|---|---|---|
| <1 cm3 | 1 × 24 Gy | 1× 16Gy |
| 1–10 cm3 | 1 × 21 Gy | 1 x 16Gy |
| 10–20 cm3 | 1 × 18 Gy | 1 x 16Gy |
| 20–65 cm3 | 1 × 15 Gy | No SRS |
All BMs are dosed equally in the same patient. If the V12 Gy exceeds 10 cm3 of the healthy brain tissue nearby the largest brain metastasis, it is allowed to lower the fraction dose to a single dose of 21, 18 or 15 Gy
Patients will be immobilised in a supine position within a thermoplastic mask or stereotactic noninvasive frame, with or without bite block and/or other fixation, according to institutional standards for SRS. The accuracy of the stereotactic fixation system should be good enough to justify the CTV to PTV margin used. This means the intrafraction motion should at least be within the CTV-PTV margin used. If a margin of 0 mm is used, the maximum intrafraction motion should be <0.5 mm, with the SD being less than 0.25 mm. A planning CT scan with ≤2 mm thick contiguous slices (preferable CT slice thickness = 1 mm) will be fused to a contrast-enhanced stereotactic MRI scan. The interval between the planning-MRI and actual SRS treatment is a maximum of 3 weeks. Single or multiple isocentres are allowed for delivering SRS according to the preference of treatment center. Tissue density inhomogeneity correction will be used. Positional verification and correction prior to (and/or during) radiation should be executed according to the institutional protocol for stereotactic radiotherapy and should be in accordance with the CTV-PTV margin used. All techniques that result in the dose requirements being met are allowed. Participating institutes will have to define their radiation delivery treatment prior to the initiation of the study. Techniques that have a shorter treatment time duration are preferred as this is more comfortable for the patient and might prevent an increase in the intrafraction displacement over the treatment time. All vendors are allowed to deliver SRS, such as a linear accelerator, Gamma Knife and CyberKnife.
Number of patients and recruitment
Questionnaires measuring QOL with the EQ5D EUROQOL questionnaire are collected from patients with multiple BM treated with WBRT or SRS at baseline and at 3 months after treatment. For this phase III trial, sample size calculation is based on the clinically relevant difference of 0.10 points of the EQ5D-5 L index value (range 0–1) at 3 months after treatment with regard to baseline, with a standard deviation of 0.25 points. For every patient this difference in EQ5D-5 L QOL score is calculated (score at 3 months minus score at baseline). The average score of all patients in the SRS group is calculated and this average score is compared to the calculated average score of all patients in the WBRT group. This is accordingly the method described by Pickard. [6] Sample size calculation is performed for a comparison of means with two-sided alpha 0.05 and power of 0.80. This leads to a sample size per group of 100 patients. To account for drop out, the sample size for this study will be increased by 15% to 230 patients (115 per group). After 86 patients treated, an interim analysis is performed to monitor the safety of the trial regarding the experimental SRS arm.
Patient accrual was started on 1st July, 2016. Up to 14th April, 2017, nine patients were randomised. With expected participation of 12 centres, it is estimated that patient’s accrual can be completed within 2 years with an expected accrual of 10–15 patients each year per centre.
For this phase III study, a comparison of the above described difference in EQ5D score between SRS and WBRT group will be performed using an independent samples Student’s t-test with two-sided significance level alpha set at 0.05. A clinical significant difference in EQ5D is determined at 0.10 points in the index score. [4] For the total patient cohort, a multivariate analysis is performed to identify prognostic factors for a difference in EQ5D score at 3 months with regard to baseline. Differences in secondary endpoints that repeat in time are analysed with Kaplan Meyer curves including log-rank test or ANOVA test. Time-to-event data (e.g. overall survival) will be compared using Kaplan–Meier curves and log-rank test. Means will be compared using independent samples Student’s t-tests. Frequencies (e.g. WHO-PFS) will be compared using Chi-square test.
Discussion
A typical SRS treatment requires a high-resolution contrast-enhanced planning-MRI, frameless mask, a planning-CT, a treatment plan and quality assurance. Generally, all preparations will take a maximum of 2 weeks. Duration of SRS delivery depends on the number of isocentres, arcs and dose rate, but usually in the range of several minutes up to 45 min. With WBRT, all other preparation steps are required, but treatment planning and quality assurance are less complex. WBRT treatment is delivered in five fractions compared to a single treatment fraction with SRS. Questionnaires will be assessed at baseline, 3 months after treatment and every 3 months thereafter until 1 year after treatment. Questionnaires can be assessed by telephone, or during scheduled outpatient clinic visits. Not all hospitals have the logistic capacity to perform neurocognitive tests and extensive QOL assessment; hence, neurocognition (Hopkins Verbal Learning Test) and more extensive QOL (EORTC QLQ-C30, EORTC BN20, and EORTC QLQ-FA13) will be facultative endpoints and will only be performed at baseline and at 3 months after radiotherapy at the outpatient clinic in centres willing to participate. Although these secondary endpoints are facultative, these endpoints will also provide valuable information, and participating centres are recommended to monitor them.
Acknowledgements
MAASTRO clinic: Rody Zuidema, Anita Botterweck, Kim Smits, and Andre Dekker for their support and collaboration in this trial. Ruud Houben for advice considering statistical analysis.
VUmc Amsterdam: Frank Lagerwaard for commenting on the study protocol.
All patients willing to participate, all referring physicians supporting this trial and co-investigators of other hospitals who participate in this multicentre study.
Funding
According to the research agreement of MAASTRO clinic with Varian Medical Systems, this trial is mentioned and financially supported. Varian Medical Systems is not involved in the design of the study, and collection/storage/analysis of the data gathered in this study.
Availability of data and materials
The study coordinator (JZ) has full access to the original data, the sequence of authors has been determined upfront, and all authors will read the final report before publication. The first author on papers with results of the study will be JZ (Jaap Doeke Zindler), second author AB (Anne Marie Bruynzeel), third author DE (Danielle Eekers), fourth author CH (Coen Hurkmans), fifth author Ans Swinnen and the last author will be PL (Philippe Lambin). The same policy will be applied to side results. Everyone who further contributed, such as investigators or participating centres, will also be considered as co-authors. The sequence co-authorship of participating investigators will be determined by the number of patients included in the study. Persons who contributed in a minor way to a study may be considered for the acknowledgments section. Results will be published unreservedly regardless of their nature in accordance with the CCMO statement on publication policy.
Abbreviations
- BM
brain metastases
- CT
computed tomography
- CTCAE
common criteria of adverse events
- GTV
gross tumour volume
- Gy
Gray
- ICRU
International Commission on Radiation Units & Measurements
- MRI
magnetic resonance imaging
- PTV
planning target volume
- QOL
quality of life
- SRS
stereotactic radiosurgery
- WBRT
whole brain radiotherapy
Authors contributions
JZ has written the study protocol in close cooperation with AB and PL. CH, DE, and AS also commented on the content of the study protocol. All authors have read and approved this manuscript.
Author information
JZ: radiation oncologist, MAASTRO clinic Maastricht, the Netherlands.
AB: radiation oncologist, VU university medical centre, the Netherlands.
DE: radiation oncologist, MAASTRO clinic Maastricht, the Netherlands.
CH: clinical physicist radiation oncology, Catharina Hospital, Eindhoven, the Netherlands.
AS: clinical physicist radiation oncology, MAASTRO clinic, Maastricht, the Netherlands.
PL: radiation oncologist and research professor, MAASTRO clinic, Maastricht, the Netherlands.
Ethics approval and consent to participate
This study was approved by the ethics committee of the following hospital: the Maastricht University medical center (Mumc), Maastricht, the Netherlands: reference number protocol NL53852.068.15/METC153053. The responsible investigator will ensure that this study is conducted in agreement with the Declaration of Helsinki (Brazil, October 2013) and in accordance with the Medical Research Involving Human Subjects Act (WMO). The protocol has been written, and the study will be conducted according to the ICH Harmonized Tripartite Guideline for Good Clinical Practice (ref: http://www.ifpma.org/pdfifpma/e6.pdf). All patients will be informed about the aims of the study, the possible adverse events, the procedures and possible hazards to which they will be exposed. They will be informed about the strict confidentiality of their data, and that their medical records may be reviewed for trial purposes by authorised individuals other than their treating physician. Information will be given in both spoken and written form as given in the Patient Information text. The Patient Informed consent statement and the Patient Information text are given as an appendix to this protocol. It will be emphasised that the participation is completely voluntary, and the patient does not need to give any further explanation for not participating. The patient is allowed to refuse further participation in the protocol whenever he wants. This will not prejudice the patient’s subsequent care. Documented informed consent must be obtained for all patients included in the study before they are registered in the study.
Consent for publication
All patients have provided written informed consent for participation in this trial, and publication of the results gathered in this trial. The publication does not contain recognisable individual patient data, but an analysis of the results of the whole study population (n = 230).
Competing interests
The department of radiotherapy of MAASTRO clinic has a research agreement with Varian Medical Systems, Palo Alto USA. Varian Medical Systems is not involved in the design of the study, and collection/storage/analysis of the data gathered in this study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaap D. Zindler, Phone: 0031 88 44 55 666, Email: jaap.zindler@maastro.nl
Anna M. E. Bruynzeel, Email: AME.Bruynzeel@vumc.nl
Daniëlle B. P. Eekers, Email: danielle.eekers@maastro.nl
Coen W. Hurkmans, Email: coen.hurkmans@catharinaziekenhuis.nl
Ans Swinnen, Email: ans.swinnen@maastro.nl.
Philippe Lambin, Email: philippe.lambin@maastro.nl.
References
- 1.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316(4):401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016;388(10055):2004–2014. doi: 10.1016/S0140-6736(16)30825-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 4.Chavaudra J, Bridier A. Definition of volumes in external radiotherapy: ICRU reports 50 and 62. Cancer Radiother. 2001;5(5):472–478. doi: 10.1016/S1278-3218(01)00117-2. [DOI] [PubMed] [Google Scholar]
- 5.Scoccianti S, Detti B, Gadda D, et al. Organs at risk in the brain and their dose-constraints in adults and in children: a radiation oncologist's guide for delineation in everyday practice. Radiother Oncol. 2015;114(2):230–238. doi: 10.1016/j.radonc.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Pickard, A Simon, Maureen P Neary, and David Cella. “Estimation of Minimally Important Differences in EQ-5D Utility and VAS Scores in Cancer.” Health Qual Life Outcomes 5 (2007): 70. PMC. Web. 23 Feb. 2015. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The study coordinator (JZ) has full access to the original data, the sequence of authors has been determined upfront, and all authors will read the final report before publication. The first author on papers with results of the study will be JZ (Jaap Doeke Zindler), second author AB (Anne Marie Bruynzeel), third author DE (Danielle Eekers), fourth author CH (Coen Hurkmans), fifth author Ans Swinnen and the last author will be PL (Philippe Lambin). The same policy will be applied to side results. Everyone who further contributed, such as investigators or participating centres, will also be considered as co-authors. The sequence co-authorship of participating investigators will be determined by the number of patients included in the study. Persons who contributed in a minor way to a study may be considered for the acknowledgments section. Results will be published unreservedly regardless of their nature in accordance with the CCMO statement on publication policy.


