Abstract
Here we summarize the composition and uses of Schizosaccharomyces pombe media and discuss key issues for consideration in the generation of S. pombe cultures. We discuss the concept of “culture memory,” in which the growth state and stress experienced by a strain during storage, propagation and starter culture preparation can alter experimental outcomes at later stages. We also describe the triggers that are widely used to manipulate signaling through the environment sensing pathways.
INTRODUCTION
S. pombe cells are rod-shaped and grow by tip extension until they attain a critical cell length whereupon growth ceases and mitosis is initiated (Mitchison 1957). This highly reproducible cell size control is just one of the cellular growth and morphogenesis pathways that are regulated by environmental cues. The Sty1/Spc1 MAP kinase stress response and the Target of Rapamycin (TOR) pathways respond to changes in the extracellular environment to modulate functions as diverse as growth, division, the cytoskeleton, protein synthesis, transcription and translation (Shiozaki and Russell 1995; Weisman 2004; Petersen 2009; Loewith and Hall 2011). The choice of media and culture conditions therefore heavily impacts the outcome of studies in virtually every arena of fission yeast biology.
FISSION YEAST GROWTH MEDIA
Fission yeast has the ability to grow on a range of different media. These include complex rich medium, synthetic minimal medium, and different types of “sporulation” media used to promote sexual differentiation. Rich medium, known as Yeast Extract with Supplements (YES) (see Recipes section below), is made with yeast extract and glucose with specific amino acids or nucleobases added as needed. YES supports fast growth rates (Table 1), hence it used for general non-selective growth. YES is also preferable for growth prior to long-term storage at –80°C and for some experimental procedures, including protoplast generation. Synthetic media recipes are based on the original Edinburgh minimal media (EMM) developed by Murdoch Mitchison for his cell cycle analyses (Mitchison 1970). The totally defined nature of synthetic minimal media for logarithmic growth facilitates the reproduction of culture conditions between physiological experiments and laboratories, as well as the selection of a variety of auxotrophic markers on exogenous vectors or integrated DNA fragments. Changes to the nitrogen or carbon composition of synthetic minimal media are commonly used to manipulate growth and cell cycle controls and promote sexual differentiation.
Table 1.
Approximate generation times of healthy haploids according to media and nitrogen source, and cell dimensions according to ploidy.
| Approximate generation times of healthy haploids | ||
|---|---|---|
| Media | Temperature | Generation time |
| YES | 20°C | ~8 hr |
| 25°C | 3 hr | |
| 28°C | 2 hr 45 min | |
| 30°C | 2 hr 30 min | |
| 32°C | 2 hr 10 min | |
| 35°C | 2 hr | |
| EMM2 | 20°C | ~10 hr |
| 25°C | 4 hr 30 min | |
| 28°C | 3 hr 45 min | |
| 30°C | 3 hr | |
| 32°C | 2 hr 30 min | |
| 35°C | 2 hr 20 min | |
| Nitrogen-dependent generation times of healthy haploids | ||
| Nitrogen source | Temperature | Generation time |
| EMM2 (ammonium) | 28°C | 3 hr 45 min |
| EMMG | 28°C | 3 hr 30 min |
| (glutamic acid) | ||
| EMMP (proline) | 28°C | 4 hr 30 min |
| MSL (arginine) | 28°C | 3 hr 30 min |
| YES (complex) | 28°C | 2 hr 45 min |
| Cell dimensions | ||
| Ploidy | Cell length | Cell width (constant) |
| Haploid | 12–14 μm | ~3.5 μm |
| Diploid | 20–24 μm | ~4.5–5 μm |
Carbon Sources in Growth Media
Although glucose is routinely employed as the sole carbon source in growth medium, S. pombe can also use glycerol, sucrose, raffinose and maltose (Schlanderer and Dellweg 1974). When fission yeast is grown on glucose alone, the levels of several respiratory enzymes are reduced (Poole and Lloyd, 1973). Monitoring carbon dioxide production to gauge glycolytic flux has revealed that glycolysis accounts for most of the ATP production during aerobic growth in 3% glucose (Hamburger et al 1977). In contrast, the catabolite repression that arises from propagation in glucose is lost when glycerol is the sole carbon source (Poole and Lloyd 1974), making growth in glucose highly reminiscent of the Warburg effect seen in cancer cells (Upadhyay et al. 2013). Removal of glucose while maintaining the provision of nitrogen can maintain cells in a G0-like state for extended periods; this growth state is used to study S. pombe metabolic controls and ageing (Su et al. 1996).
Nitrogen Sources in Growth Media
Modified Versions of EMM
Several modified versions of EMM can be selected according to the desired source of nitrogen. Each distinct nitrogen source induces signature fluxes through the TOR and Sty1 growth control pathways to alter the architecture and flux of most cellular processes. The most widely used synthetic medium is EMM2 (Nurse 1975; see EMM(X) in Recipes section below), which employs the excellent nitrogen source ammonium chloride. The switch from the sodium acetate and sodium dihydrogen orthophosphate of the original EMM medium to 15 mM potassium hydrogen phthalate and disodium hydrogen orthophosphate was introduced by Paul Nurse to reduce clumping of wee1–50 (also known as cdc9-50) mutant cells (Nurse 1975); however, as cells were generally healthier in EMM2, it became the medium of choice for most studies. EMM2 was further modified by Fantes and Nurse (1977) to assess the impact of nutrient variation upon cell cycle controls, and different nitrogen sources were substituted for ammonium chloride. EMM2 without nitrogen is called EMM-N (see Recipes section below). Replacement of ammonium chloride with glutamic acid, proline, uracil or serine generated the alternative media EMMG, EMMP, EMMUr and EMMSer, respectively (Fantes and Nurse 1977; see EMM(X) in Recipes section below). Of these media, EMMG and EMMP have been the most widely used (Petersen and Nurse 2007). Because glutamic acid is a good nitrogen source and proline a very poor one, a switch between the two reveals the impact of changing the “quality” of the nitrogen source upon the cell cycle, stress and TOR signaling networks (Petersen 2009). EMMP is also useful for cell cycle analysis, because cells are born in G1 phase and initiate growth before committing to the cell cycle at START (Fantes and Nurse 1978). This contrasts with all other media, in which passage of START precedes cytokinesis.
Confusingly, the name EMMG was later used to describe an EMM2 variant in which the ammonium is replaced with 5.91 mM glutamate to support sporulation of diploids (Moreno et al. 1991). This led to the use of the name PMG (Pombe Minimal Glutamate) to describe the medium in which the ammonium of EMM2 is replaced with 20 mM glutamic acid (Sabatinos and Forsburg 2010). Throughout this manual, we return to the original nomenclature of EMMG for medium with glutamic acid. There is no mention in this collection of the medium with 5.91 mM glutamate described by Moreno et al. (1991). Wherever EMMG is used, it refers to medium with 20 mM glutamic acid.
EMMG is often a medium of choice when propagating certain auxotrophs. There appear to be two transporters for basic amino acids, of which one is inhibited by ammonium ions (Fantes and Creanor 1984). Growth on EMMG therefore has the advantage that both transporters can be used for uptake of basic amino acids. Empirical observations of a set of aromatic auxotrophic mutants show that EMMG supports much better growth than EMM2 in these mutants as well (Strauss 1979). For most researchers, however, EMMG is used because ura4-d18 mutants are fitter when grown with EMMG than with EMM2, presumably because uracil uptake exploits the same channels. This negative impact of ammonium ions upon uptake of basic amino acids becomes a major issue when performing mass spectrometric analyses using SILAC (stable isotope labeling by amino acids in cell culture), in which strains are engineered to rely entirely upon the import of exogenous arginine and lysine that incorporate heavy carbon or nitrogen isotopes at key positions. Critically, the addition of lysine inhibits arginine uptake by the ammonium insensitive channel. As both lysine and arginine must be imported from the medium for SILAC to be successful, the high levels of ammonium in EMM2 either block growth altogether or generate an excessive lag phase before growth takes off. One solution is to reduce the ammonium levels in the EMM2 used for SILAC from 96 to 24 mM (Bicho et al. 2010); another is to switch to EMMG (Carpy et al. 2015).
Sporulation Media
Minimal Sporulating Liquid or Agar (MSL/MSA) medium (see Recipes section below) was developed by Richard Egel et al. (1994) to facilitate the efficient induction of sexual differentiation in fission yeast. Like EMM-based media, it supports logarithmic growth; however, it employs arginine as its sole nitrogen source. Egel selected arginine because a switch from MSL containing arginine to MSL without it induced sexual differentiation far more rapidly and efficiently than a similar switch from EMM to EMM-N. Furthermore, when the background fluorescence of a fluorescent fusion protein obscures the specific signal in EMM2 medium, a switch from EMM2 to MSL often abolishes background fluorescence to reveal the “true” location of fusion-protein. A further reduction in background fluorescence in live cell imaging can be achieved by using a filter rather than autoclaving to sterilize medium. MSL and MSA are also useful for hygromycin and G418/kanamycin selection, which cannot be applied in EMM2. In contrast, Sporulation Agar (SPA) medium (see Recipes section below), in which no nitrogen is present, invokes an abrupt nitrogen starvation shock for cells (Leupold 1970). Cells plated on SPA either mate or arrest division, whereas they undergo one or two rounds of division when plated on MSA. MSA therefore produces efficient mating of most strains. However, spontaneous ascus breakdown is faster and more synchronous following mating on SPA than MSA, making SPA a medium of choice for mating strains for tetrad analysis.
Supplementation of Minimal Media with Amino Acids and Nucleobases
For growth on solid media, 200 mg/l of the relevant supplements (e.g., leucine, uracil, histidine, adenine, lysine, arginine, or histidine) can be added. Note that amino acid supplements are often added to a final concentration of 225 or 250 mg/l; however, this high level of amino acids stems from Paul Nurse’s observation that survival of a mutant in cdc2 required addition of 250 mg/l leucine. Consequently, 250 mg/l supplements are commonly used, although 150 mg/l is sufficient. It is notable that variance in size at division (i.e., variance in passage through the G2 phase of the cell cycle) is lowest at 150 mg/l, rather than 250 mg/l (J. Petersen and P. Russell, unpubl.).
Amino acid stock solutions are often prepared as 7.5 mg/ml, however, both adenine and uracil have solubility issues. Adenine should be made as a 5-mg/ml stock solution and uracil solutions will need to be heated (e.g., by microwave) to dissolve the solid before adding to medium. The inconvenience and evaporation arising from repeated heating of the stock solution has prompted many to use the much more expensive uridine for ad hoc supplementation of media at the bench and only use uracil when making up large batches of medium.
Mutations in ade6+
Mutations in the ade6+ gene are widely used in S. pombe genetics because a precursor in the adenine biosynthesis pathway, P-ribosylaminoimidazole, accumulates in the mutants and oxidizes to a red pigment (Reaume and Tatum 1949). Consequently, supplementation with a limiting level of adenine (10 μg/ml) supports growth yet reveals the red pigmentation (Schar and Kohli 1994). ade6-M210 colonies appear dark red while ade6-M216 colonies appear pink (Schar and Kohli 1994). Colonies harboring the ade6-705 nonsense mutation are a deep crimson when not complemented by the sup3-5 tRNASer mutation, but appear white when the opal suppressor allows reading through the stop mutation to produce a functional phosphor-ribosylaminoimidazole carboxylase enzyme (Hofer et al. 1979).
This color difference has been used for colony color/sectoring assays in genetic screens, for example, to detect the loss of a non-essential mini-chromosome. The suppression of ade6+ mutations by mini-chromosome-borne sequences, which generates white colonies, is lost with the mini-chromosome. This produces neighboring red colonies or reveals a red sector within a colony when plates are supplemented with 10 μg/ml adenine (Niwa et al. 1986; Niwa et al. 1989; Takahashi et al. 1994; Allshire et al. 1995).
Phloxin B
Phloxin B (2′,4′,5′,7′-Tetrabromo-4,5,6,7-tetrachlorofluorescein disodium salt or cyanosine) is a red dye that is used as vital stain when added to YES. Dead cells are unable to exclude phloxin B and thus appear red. It is especially useful for identifying colonies consisting of haploid or diploid cells, as the latter are more likely to die and therefore stain more darkly with phloxin B (Tange and Niwa 1995); thus, the overall colony will be a darker pink. Phloxin B can also be useful for identifying colonies of temperature-sensitive lethal mutants because of increased cell death at the restrictive temperature.
5-FOA selection
The orotidine 5′-phosphate decarboxylase encoded by the ura4 gene decarboxylate 5-fluororotic-acid (5-FOA) to generate 5-fluorouracil that blocks DNA replication. Therefore strains from which the ura4+ gene has been deleted or mutated are resistant to 5-fluororotic-acid (5-FOA) making it an excellent reagent for positive selection for the loss of ura4+ marker.
STRAIN HUSBANDRY
Storage
The best method for long-term storage of fission yeast is in 25% glycerol at −80°C. After 2 days of growth in 10 ml of YES to generate a dense culture, cells are resuspended in 1 ml of YES containing 25% glycerol and transferred to a cryotube. If a plasmid that relies upon the selection of an auxotrophic marker must be maintained during storage, the strain should be grown with appropriate selection in minimal medium before resuspension in YES with 25% glycerol for storage at −80°C. To re-isolate strains from −80°C, a sterile pipette tip is used to transfer a small lump of the frozen cell suspension to a “spot” on a plate (while taking care not to let the stock thaw). The cells are streaked out to generate single colonies whose genotypes should be checked before use.
For short-term storage, cells can be maintained at 4°C as patches on agar plates wrapped in plastic film for 2–3 weeks. However, storage at 4°C can increase the frequency of diploidization and the emergence of phenotype-reversing mutations. It is therefore essential to re-isolate the stored strain to a single colony and check the relevant genotypes (see below) each time it is used from a patch at 4°C.
Checking Genotypes by Replica Plating
Replica plating is an efficient way to determine genotypes after storage. The plate containing the cells is inverted onto velvet or filter paper stretched over a replica plating block, and a low, even pressure is applied to ensure that a portion of each colony is retained on the paper after the plate is removed. The procedure is then repeated with dry blank plates to transfer cells from each colony on the paper to each blank plate. The addition or omission of appropriate supplements or drugs in these replica copies is used to reveal the genotypeof each colony. Cells with a ura4− genotype divide several times after replica plating onto plates lacking uracil, making it necessary to sequentially replicate these colonies on 2 consecutive days to clearly differentiate uracil prototrophic from auxotrophic colonies.
GROWTH STATE
Controlling cell physiology lies at the core of successful, reproducible experimentation with fission yeast. There are five principal impacts upon growth state: (1) changes in amino acid provision or requirement, (2) cell density, (3) the status of the starter culture used to inoculate the main culture, (4) the timing of inoculation and (5) the level of aeration.
Auxotrophic/Prototrophic Status and Amino Acid Provision
Auxotroph markers are invaluable tools for selecting for the maintenance of vectors or integration of foreign DNA sequences into the genome. When auxotrophs are propagated, supplements must be added to the medium to support cell growth. The addition of certain amino acids alone can radically change cell physiology (Weisman et al. 2005; Bonfils et al. 2012; Han et al. 2012; Kim et al. 2013; Xu et al. 2013). For example, leucine addition transiently blocks mitotic commitment (Hartmuth and Petersen 2009) and alters cell length at division during steady-state growth (Fig. 1A).
Figure 1.
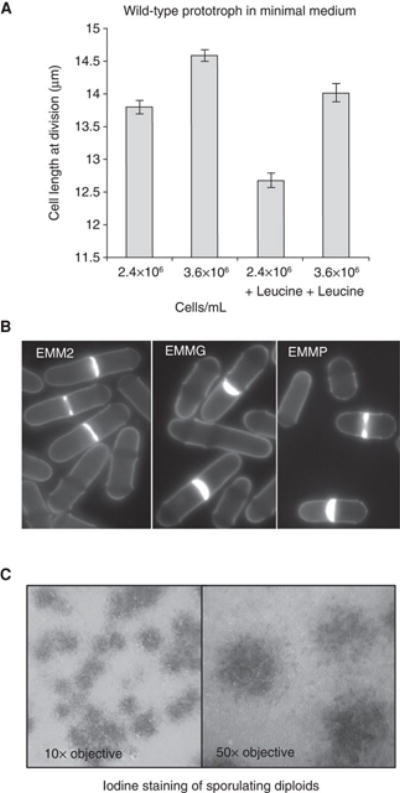
A) Wild type cells grown in EMMG with or without 150 mg/l leucine added. Cell lengths at division (± SEM, 200 cells) are shown. B) Calcofluor staining of wild type cells grown in medium indicated to a cell density of 1.5 × 106 cell/ml. C) Pheromone secretion by wild type h− cells. A diploid tester strain was mixed 100:1 with haploid h− cells on MSA. Iodine staining was used to visualize the halo of sporulating diploid cells around the pheromone-producing haploid cells.
Therefore, it is essential to ensure that all strains in a given experiment have identical auxotrophic genotypes or, preferably, are all prototrophs. Amino acid dropout mixes that are commonly used for culturing Saccharomyces cerevisiae strains in synthetic growth media are not typically used for fission yeast, although they might be useful for isolating and culturing mutants that have caused unexpected auxotrophic phenotypes. If cell length at division is to be measured and the provision of amino acids cannot be avoided, supplementation with 150 mg/l of the relevant amino acid is recommended, as the standard deviation of cell length at division is lowest at this concentration of supplements (J. Petersen and P. Russell, unpubl.).
Starvation-induced Memory from Starters to Main Cultures
A widespread assumption, which may stem from work with budding yeast, is that because a new yeast cell is generated in one generation, a culture at any cell density will be the same irrespective of when it was inoculated. An extension of this misconception is the assumption that diluting a culture from a saturated stationary phase culture into early log phase the evening before or the morning of an experiment will generate a culture from which meaningful conclusions can be drawn. However, this is not always the case. There is clear evidence of “culture memory” in S. pombe.
At the crudest level, the behaviour of the spindle pole body shows that cells take at least two cell cycles to fully mature (Grallert et al. 2004). More generally, drop/spot test assays derived from cultures that were not in exponential phase can often produce misleading or irreproducible results. The ability to synchronize cultures by elutriation can be influenced by culture density. Inoculation from a dense culture the evening before elutriation often generates a size-selected population whose synchrony declines rapidly after the first division. Finally, the dynamic properties of microtubules vary according to culture age, time of inoculation and age of pre-culture (A. Grallert, pers. comm.).
Changes in the heterochromatin that alter the global transcription landscape account for culture memory (Yamanaka et al. 2013). Alarmingly, once these modifications are established they may be stably inherited for many generations over several weeks of continual culture. There can be no stronger warning for taking extreme care to follow good practice in strain maintenance, propagation and culture than this clear demonstration of culture memory.
Physiological Experiments Using Liquid Cultures
The ability to grow S. pombe in defined minimal medium has been a cornerstone of fission yeast research. Importantly, culture history (discussed above) and the density that a correctly inoculated culture achieves at the point of analysis are equally important to achieving reproducible results. For most studies, growth until early to mid-log phase is recommended: 1 × 106 – 5 × 106 cells/ml in EMM2 and 1 × 106 – 1 × 107 cells/ml in YES. Cell physiologies change significantly if cultures are grown beyond the upper ranges of these cell densities. For cell cycle analyses, comparisons should be made between cultures with identical biomass rather than cell densities. The optical density (OD) at 595 nm is a useful indirect measure of culture biomass (mass accumulation). Each spectrophotometer should be calibrated with cell number determined using a hemocytometer, but typically for wild type haploid strains, OD595 = 0.1 equates to approximately 1.5 – 2 × 106 cells/ml.
Culture Setup
The physiological state of a culture is influenced by the level of oxygenation; therefore, best practice dictates that a freshly isolated colony is inoculated into a 50-ml conical flask containing 5–10 ml of the same medium that will be used for the main culture. However, for particularly sick mutants, inoculating the starter culture in YES can be preferable. Following overnight shaking at the appropriate temperature, the starter culture should be diluted to early-exponential phase (~1 × 106 cells/ml) if needed, and left to grow exponentially during the day. Alternatively, a freshly isolated colony can be inoculated into a non-shaking pre-culture which will then need to be grown with aeration and no starvation for at least 30 hours prior to the experiment. The main culture should be inoculated from the exponentially grown culture into a flask large enough to ensure the liquid is below the first line of graduation. Furthermore, when comparing two cultures, it is important to standardize aeration speed as well as culture and flask volume.
The following formula can be used to calculate the amount of starter culture needed to inoculate the final overnight culture.
where X = number of generations = number of hours/generation time1
1If the starter culture is not in exponential phase, one generation is subtracted to account for the time required for cells to reach optimum growth rate.
Generation Time
The generation times for haploid wild type S. pombe strains are listed in Table 1. Diploid strains have similar generation times. If the generation time of a mutant strain is unknown, it can be easily calculated from 2 cell number measurements (cells/ml) within exponential growth phase (1 × 106 – 5 × 106 cells/ml in minimal medium)
*For wild type and mutant strains with normal coupling of growth and cell division, cells/ml at Time 2 (T2) and Time 1 (T1) can be replaced by OD595 measurements at T2 and T1 as follows.
Temperature
Wild type fission yeast cells can grow between 18–37°C, although growth at 37°C or below 20°C is far from ideal. However, this breadth of temperature range has been invaluably exploited in the use of temperature-sensitive mutations that maintain function at 25°C but not 35–37°C, or cold-sensitive mutations that maintain function above 30°C but not below 20°C.
Nutrient Availability and Cell Physiology
Starvation arrests cell cycle progression to initiate a differentiation program that improves viability (Egel et al. 1990). When cultures reach stationary phase in most media, depletion of glucose will become the limiting factor and cells will arrest in G2. In contrast, cells arrest in G1 when specifically starved of nitrogen (a response that prepares them for sexual differentiation). When the nitrogen source is changed, proliferation continues but at a rate that is specific for each nitrogen source (Fantes and Nurse 1977) (Table 1; Fig. 1B). This distinction in the physiological response to growth on different nitrogen sources makes it difficult to compare experiments performed in different media (Table 1). For example, the plo1.S402E mutation has no impact upon cell length at division (a measure of the duration of G2 phase) in EMM2, but decreases it by 1.1 μm in EMMG (Petersen and Hagan 2005; Petersen and Nurse 2007).
Changes in the flux through the “stress response” Sty1/Spc1 MAP kinase cascade are revealed by changes in activating phosphorylation of the Sty1/Spc1 by the MAPK kinase Wis1, or in the nuclear import of Sty1-GFP (Shiozaki and Russell 1995; Gaits et al. 1998; Toone and Jones 2004). Importantly, the steady state “non-stressed” Sty1/Spc1 and TOR activities differ depending on the medium of choice (Shiozaki and Russell 1995; Petersen and Nurse 2007). A final point that is often ignored is that the slight differences in cell density arising from the gradual depletion of nutrient supply during growth alter cell physiology. Consequently, cell length at division of wild type prototrophs increases as cell density/biomass increases (Fig. 1A). It is therefore essential to compare length at division at identical biomass (OD measurement) rather than cell number.
Sources of Unwanted Stress in Liquid Cultures
Most studies inadvertently induce stress by harvesting cells through centrifugation. Exposure to the 800g routinely used to harvest cells in a microfuge enhances Sty1/Spc1 signaling (Shiozaki et al. 1998), making cell isolation by filtration the only option for some experiments. However, it is important to ensure that filters are not overloaded or dried out during filtration, and that the conical flask and medium receiving the filtered cells are pre-warmed to the appropriate temperature.
GROWTH RATE MEASUREMENTS
Monitoring Optical Density
Monitoring OD at 595 nm provides a measurement of growth rate or biomass accumulation. A parallel estimate of cell numbers over time can be used to determine whether the exponential increase in cell growth and cell division displays the linear correlation of wild type strains under steady state growth conditions. Importantly, in wild type cells this coupling between cell growth and division is altered by environmental changes including starvation. Cell counting with a hemocytometer is a simple way to monitor cell number increases, although more accurate measurements can be attained using a Coulter counter.
Assessing Fitness with Spot/Drop Tests
Testing the ability of 10-fold serial dilutions of cultures to form colonies on agar plates provides a powerful assessment of gene function and the impact of multiple genetic interactions upon cell viability. It is particularly useful for detecting subtle differences in growth rate or viability. It is essential that the cultures used to inoculate such spot/drop tests have been grown under identical physiological conditions (in the same way and to the same cell density), and are diluted 10-fold without any prior centrifugation or filtration. While spot/drop tests have traditionally been end-point analyses that catalogue colony formation after 2–3 days, technologies developed for genome-wide analyses can be used to monitor colony formation by time-lapse microscopy (Banks et al. 2012). This new approach has proven highly informative, indicating that checking plates on a daily basis reveals early differences that are lost when colonies saturate towards the end of growth.
NITROGEN STARVATION
Nitrogen starvation can be used to arrest the cell cycle in G1 (Egel 1971; Nurse 1975); however it is important to remember that cell metabolism is altered by this starvation. If both mating types are present, nitrogen starvation will induce sexual differentiation. If sexual differentiation is to be monitored in liquid cultures, increasing aeration through the use of baffled conical flasks will considerably enhance the efficiency of differentiation. (The expression of agglutinins ensures cells aggregate for mating in the most turbulent of conditions).
Nitrogen Starvation to Enhance Phenotype Penetrance in Deletion Spores
Heterozygous diploids, in which one copy of a gene of interest has been replaced with a selectable marker, can be sporulated by switching a log-phase culture into nitrogen-free medium. Preferential growth of the deletant spores over the non-deletants can be ensured when the spore mix is inoculated into liquid cultures by selecting for or against the gene encoded in the deletion cassette. Although such spores will have low levels of the protein encoded by the deleted gene, some protein and mRNAs may be inherited from the parental zygote to confound phenotype analysis. This residual protein can be eliminated by germination in nitrogen-free EMM2 (EMM2-N). The lack of nitrogen prompts autophagy to scavenge nitrogen for protein synthesis by turning over existing proteins, including any residual parental protein. Thus, the germinating spore depletes the protein of interest so that the subsequent addition of nitrogen provides true insight into the “null” phenotype.
Starvation-induced Pseudo-hyphal Growth
When nitrogen deprivation is combined with a good carbon source at a remote location, pseudo-hyphal growth produces elaborately branched pseudo-hyphae that invade deep into solid medium to scavenge for nutrients (Amoah-Buahin et al. 2005).
HALO ASSAYS
Mating pheromones are secreted by fission yeast upon nitrogen starvation. Egel et al. (1994) developed a semi-quantitative plate assay for monitoring pheromone secretion. A diploid strain that only sporulates upon exposure to exogenous pheromone is used to monitor the gradient of pheromone produced by the strain of interest. Iodine staining reveals a halo of dark asci around individual cells (Fig. 1C). The diameter of the halo is indicative of the level of pheromone secreted. In principle, halo assays—in in which drug-soaked filter papers are placed on lawns of cells—should be as effective in fission yeast as they are in budding yeast for the assessment of cell growth in response to small molecules. If a small molecule blocks growth, a clearing zone of no growth will be observed (Gassner et al. 2007).
GROWTH IN RESPONSE TO STRESS AGENTS
Although the Sty1/Spc1 MAPK pathway is activated in response to stress (Toone and Jones 2004), it is rarely appreciated that a low level activation of the Sty1/Spc1 pathway under steady-state, or “non-stressed,” conditions makes a significant contribution to the timing of cell division (Shiozaki and Russell 1995). Thus, it may be more appropriate to consider the Sty1/Spc1 pathway as a homeostasis, rather than stress, pathway. Cellular stresses simply enhance the signaling through the pathway, which is already setting a level of response for a particular steady state. Stimulation of the Sty1/Spc1 pathway by a wide range of agents promotes a remarkably sophisticated set of changes to help the cell deal with each individual insult with a specific response.
Osmotic Stress
Either 1.2 M sorbitol or 0.65–1.0 M KCl is added to plates to invoke an osmotic stress response; however it is important to note that, unlike sorbitol, KCl also induces salt ion stress. For osmotic stress in liquid cultures, 0.6–0.9 M KCl results in progressively increased activation of Spc1/Sty1.
Temperature
Survival after brief exposure to higher temperatures (42°C) is commonly used to assess tolerance to heat stress. Furthermore, steady-state growth at 37°C is stressful, and mutants in the Sty1/Spc1 signaling pathway cannot grow at this temperature.
Nutrients
The reduction in the quality of nutrient supply in a shift from EMMG to EMMP stimulates Sty1/Spc1 signaling (Fantes and Nurse 1977; Hartmuth and Petersen 2009). The generation time in medium using proline as a nitrogen source is longer compared to medium exploiting glutamic acid (Table 1), and cells are born in the G1 rather than G2 phase of the cell cycle.
Heavy Metals
Exposure to heavy metals, including copper and cadmium, stimulates Sty1/Spc1 signaling to promote survival. CdCI2 or CdSO4 is added to medium at a final concentration of 0.2 mM (Ortiz et al. 1992), and CuCl2 or CuSO4 to a concentration of 16 nM (Labbe et al. 1997). Metalloid arsenite (0.5 M) also activates Spc1/Sty1 (Rodriguez-Gabriel and Russell 2005)
Oxidative Stress
Oxidative stress can be generated with several different chemicals. The most commonly used oxidative stress agent is hydrogen peroxide (H2O2). Hydrogen peroxide is highly unstable; therefore it is best to freeze small aliquots from a new bottle and discard these approximately one week after thawing. The concentration of hydrogen peroxide can be modified to suit the experimental goals, but as a general guideline a final concentration of 0.2 mM H2O2 is sufficient to cause rapid and robust activation of Sty1/Spc1. The growth of wild type cells is inhibited by 0.5–1.0 mM H2O2 in plates containing YES and lower concentrations in plates containing EMM2. Tert-butyl hydroperoxide, also known as t-BuOOH or under the trade name Luperox, can also be used at concentrations of ~1 mM to cause oxidative stress.
DNA Damage
Fission yeast has been an excellent model organism for studying cellular responses to DNA damage and replication inhibition. A large variety of DNA damaging agents have been used for these studies. Ionizing radiation (IR) is often used to cause double-strand breaks (DSBs), which are amongst the most lethal forms of DNA damage. The exact protocols vary depending on the specific type of gamma irradiator. For labs that do not have access to a gamma irradiator, the glycopeptide antibiotic bleomycin (~5 mU/ml) and the related compound phleomycin (~10 μg/ml) are effective substitutes for IR. The precise mechanism of action for these genotoxins is unknown, but it is believed to involve generation of free radicals in close proximity to DNA. The quinolone alkaloid camptothecin (CPT), which is a DNA topoisomerase I (topo I) inhibitor, can also be used to create DSBs. In this case, DSBs arise when replication forks encounter cleavage complexes formed by DNA, CPT and topo I, thereby converting single-strand DNA breaks into one-ended DSBs. CPT is typically used at concentrations between 0.1 and 10 μM. The DNA alkylating agent methyl methanesulfonate (MMS) is used in agar plates at concentrations between 0.001 and 0.01%. Toxicity from methylated DNA primarily arises from replication fork stalling and collapse. Ultraviolet (UV) radiation causes several types of DNA damage, most notably cyclobutane-pyrimidine dimers (CPDs) and 6–4 photoproducts (6–4PPs). UV exposure can be performed with a UV cross-linker (254 nm), originally designed for fixing purified nucleic acids to membranes. In this event, plates must be dry and cells present in a monolayer. Hydroxyurea (HU), an inhibitor of ribonucleotide reductase, causes replications forks to stall by depleting deoxyribonucleotides. HU is used at concentrations in the range of 0.1 to 10 mM and upon extended exposure can lead to DNA strand breaks.
GROWTH CONDITIONS FOR EFFICIENT MATING
Malt Extract Agar (MEA) medium (see Recipes section below) and EMM-N can be used to invoke sexual differentiation, but SPA and MSL both support more efficient and more rapid mating (Gutz and Doe 1973). Zygotes form after two days on EMM2-N but overnight on SPA. When setting up crosses, there are a number of points to bear in mind. It is important to remember that S. pombe can recycle the nitrogen of added amino acids to use as a nitrogen source for the synthesis of all amino acids. Thus, when mating auxotrophs, it is important to supply only the amino acids required to support growth of the two parental strains (i.e., do not simply use a mixture of the 5 most commonly required amino acids) and to supply them at a minimal level (5 – 40 μg/ml). The nitrogen starvation response and, consequently, mating efficiency declines with every microgram of amino acid supplied. Although wild type cells can mate from colonies or patches on plates stored at 4°C, it is important to remember that all discussions around the benefits of working with healthy cells for proliferating cultures apply to the optimization of mating efficiency. Cells entering the stationary phase invoked by nutrient exhaustion on old plates mate poorly, while those entering from actively growing, fresh, healthy colonies are much more efficient. When a mating persistently fails it can be useful to change to another mating medium. If this is still unsuccessful, then mixing equal numbers of cells from mid-log phase liquid cultures and washing them in nitrogen-free medium before plating them on the surface of the mating plate can persuade the most reluctant of partners to form zygotes. A final point to remember is that mating is blocked above 33°C and probably most efficient at 30°C.
Acknowledgments
We thank K. Kowalczyk for technical assistance with the Halo assay. J. Petersen is supported by Manchester and Flinders University and a Cancer Research UK Senior Fellowship [C10888/A11178] and P. Russell is supported by grants from the National Institutes of Health [GM059447, CA077325, CA117638, and P42ES010337).
References
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G. Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev. 1995;9:218–233. doi: 10.1101/gad.9.2.218. [DOI] [PubMed] [Google Scholar]
- Amoah-Buahin E, Bone N, Armstrong J. Hyphal growth in the fission yeast Schizosaccharomyces pombe. Eukaryot Cell. 2005;4:1287–1297. doi: 10.1128/EC.4.7.1287-1297.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks AP, Lawless C, Lydall DA. A quantitative fitness analysis workflow. J Vis Exp. 2012 doi: 10.3791/4018. pii: 4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Bicho CC, de Lima Alves F, Chen ZA, Rappsilber J, Sawin KE. A genetic engineering solution to the “arginine conversion problem” in stable isotope labeling by amino acids in cell culture (SILAC) Mol Cell Proteomics. 2010;9:1567–1577. doi: 10.1074/mcp.M110.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell. 2012;46:105–110. doi: 10.1016/j.molcel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Carpy A, Patel A, Tay YD, Hagan IM, Macek B. Nic1 inactivation enables stable isotope labeling with 13C615N4-arginine in Schizosaccharomyces pombe. Mol Cell Proteomics. 2015;14:243–250. doi: 10.1074/mcp.O114.045302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R. Physiological aspects of conjugation in fission yeast. Planta. 1971;98:89–96. doi: 10.1007/BF00387025. [DOI] [PubMed] [Google Scholar]
- Egel R, Nielsen O, Weilguny D. Sexual differentiation in fission yeast. Trends Genet. 1990;6:369–373. doi: 10.1016/0168-9525(90)90279-f. [DOI] [PubMed] [Google Scholar]
- Egel R, Willer M, Kjaerulff S, Davey J, Nielsen O. Assessment of pheromone production and response in fission yeast by a halo test of induced sporulation. Yeast. 1994;10:1347–1354. doi: 10.1002/yea.320101012. [DOI] [PubMed] [Google Scholar]
- Fantes PA, Creanor J. Canavanine resistance and the mechanism of arginine uptake in the fission yeast Schizosaccharomyces pombe. J Gen Microbiol. 1984;130:3265–3273. doi: 10.1099/00221287-130-12-3265. [DOI] [PubMed] [Google Scholar]
- Fantes P, Nurse P. Control of cell size at divsion in fission yeast by growth-modulated size control over nuclear division. Exp Cell Res. 1977;107:377–386. doi: 10.1016/0014-4827(77)90359-7. [DOI] [PubMed] [Google Scholar]
- Fantes PA, Nurse P. Control of the timing of cell division in fission yeast. Cell size mutants reveal a second control pathway. Exp Cell Res. 1978;115:317–329. doi: 10.1016/0014-4827(78)90286-0. [DOI] [PubMed] [Google Scholar]
- Gaits F, Degols G, Shiozaki K, Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassner NC, Tamble CM, Bock JE, Cotton N, White KN, Tenney K, St Onge RP, Proctor MJ, Giaever G, Nislow C, et al. Accelerating the discovery of biologically active small molecules using a high-throughput yeast halo assay. J Nat Prod. 2007;70:383–390. doi: 10.1021/np060555t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grallert A, Krapp A, Bagley S, Simanis V, Hagan IM. Recruitment of NIMA kinase shows that maturation of the S. pombe spindle-pole body occurs over consecutive cell cycles and reveals a role for NIMA in modulating SIN activity. Genes Dev. 2004;18:1007–1021. doi: 10.1101/gad.296204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz H, Doe FJ. Two different h mating types in Schizosaccharomyces pombe. Genetics. 1973;74:563–569. doi: 10.1093/genetics/74.4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Hartmuth S, Petersen J. Fission yeast Tor1 functions as part of TORC1 to control mitotic entry through the stress MAPK pathway following nutrient stress. J Cell Sci. 2009;122:1737–1746. doi: 10.1242/jcs.049387. [DOI] [PubMed] [Google Scholar]
- Hofer F, Hollenstein H, Janner F, Minet M, Thuriaux P, Leupold U. The genetic fine structure of nonsense suppressors in Schizosaccharomyces pombe I. sup3 and sup9. Curr Genet. 1979;1:45–61. doi: 10.1007/BF00413306. [DOI] [PubMed] [Google Scholar]
- Kim J, Song G, Wu G, Gao H, Johnson GA, Bazer FW. Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod. 2013;88:113. doi: 10.1095/biolreprod.112.105080. [DOI] [PubMed] [Google Scholar]
- Labbe S, Zhu Z, Thiele DJ. Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem. 1997;272:15951–15958. doi: 10.1074/jbc.272.25.15951. [DOI] [PubMed] [Google Scholar]
- Leupold U. Genetical methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:169–177. [Google Scholar]
- Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM. The growth of single cells. I. Schizosaccharomyces pombe. Exp Cell Res. 1957;13:244–262. doi: 10.1016/0014-4827(57)90005-8. [DOI] [PubMed] [Google Scholar]
- Mitchison JM. Physiological and cytological methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:131–165. [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Niwa O, Matsumoto T, Yanagida M. Construction of a mini-chromosome by deletion and its mitotic and meiotic behavior in fission yeast. Mol Gen Genet. 1986;203:397–405. [Google Scholar]
- Niwa O, Matsumoto T, Chikashige Y, Yanagida M. Characterization of Schizosaccharomyces pombe minichromosome deletion derivatives and a functional allocation of their centromere. EMBO J. 1989;8:3045–3052. doi: 10.1002/j.1460-2075.1989.tb08455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Ortiz DF, Kreppel L, Speiser DM, Scheel G, McDonald G, Ow DW. Heavy metal tolerance in the fission yeast requires an ATP-binding cassette-type vacuolar membrane transporter. EMBO J. 1992;11:3491–3499. doi: 10.1002/j.1460-2075.1992.tb05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J, Hagan IM. Polo kinase links the stress pathway to cell cycle control and tip growth in fission yeast. Nature. 2005;435:507–512. doi: 10.1038/nature03590. [DOI] [PubMed] [Google Scholar]
- Petersen J, Nurse P. TOR signalling regulates mitotic commitment through the stress MAP kinase pathway and the Polo and Cdc2 kinases. Nat Cell Biol. 2007;9:1263–1272. doi: 10.1038/ncb1646. [DOI] [PubMed] [Google Scholar]
- Petersen J. TOR signalling regulates mitotic commitment through stress-activated MAPK and Polo kinase in response to nutrient stress. Biochem Soc Trans. 2009;37:273–277. doi: 10.1042/BST0370273. [DOI] [PubMed] [Google Scholar]
- Reaume SE, Tatum EL. Spontaneous and nitrogen mustard-induced nutritional deficiencies in Saccharomyces cerevisiae. Arch Biochem. 1949;22:331–338. [PubMed] [Google Scholar]
- Rodriguez-Gabriel MA, Russell P. Distinct signaling pathways respond to arsenite and reactive oxygen species in Schizosaccharomyces pombe. Eukaryot Cell. 2005;4:1396–402. doi: 10.1128/EC.4.8.1396-1402.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinos SA, Forsburg SL. Molecular genetics with Schizosaccharoymces pombe. Methods Enzymol. 2010;470:759–795. doi: 10.1016/S0076-6879(10)70032-X. [DOI] [PubMed] [Google Scholar]
- Schar P, Kohli J. Preferential strand transfer and hybrid DNA formation at the recombination hotspot ade6-M26 of Schizosaccharomyces pombe. EMBO J. 1994;13:5212–5219. doi: 10.1002/j.1460-2075.1994.tb06852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to extracellular environment by map kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism. Mol Biol Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss A. Isolation and genetical classification of aromatic amino acid auxotrophic mutants in Schizosaccharomyces pombe. J Gen Microbiol. 1979;113:173–176. [Google Scholar]
- Su SS, Tanaka Y, Samejima I, Tanaka K, Yanagida M. A nitrogen starvation-induced dormant G0 state in fission yeast: the establishment from uncommitted G1 state and its delay for return to proliferation. J Cell Sci. 1996;109:1347–1357. doi: 10.1242/jcs.109.6.1347. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamada H, Yanagida M. Fission yeast minichromosome loss mutants mis cause lethal aneuploidy and replication abnormality. Mol Biol Cell. 1994;5:1145–1158. doi: 10.1091/mbc.5.10.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tange Y, Niwa O. A selection system for diploid and against haploid cells in Schizosaccharomyces pombe. Mol Gen Genet. 1995;248:644–648. doi: 10.1007/BF02191703. [DOI] [PubMed] [Google Scholar]
- Toone WM, Jones N. Stress responses in S pombe. In: Egel R, editor. The molecular biology of Schizosaccaromyces pombe. Springer-Verlag; Berlin Heidelberg: 2004. pp. 57–72. [Google Scholar]
- Upadhyay M, Samal J, Kandpal M, Singh OV, Vivekanandan P. The Warburg effect: Insights from the past decade. Pharmacol Ther. 2013;137:318–330. doi: 10.1016/j.pharmthera.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Weisman R. The fission yeast TOR proteins and the rapamycin response: An unexpected tale. Curr Top Microbiol Immunol. 2004;279:85–95. doi: 10.1007/978-3-642-18930-2_6. [DOI] [PubMed] [Google Scholar]
- Weisman R, Roitburg I, Nahari T, Kupiec M. Regulation of leucine uptake by tor1+ in Schizosaccharomyces pombe is sensitive to rapamycin. Genetics. 2005;169:539–550. doi: 10.1534/genetics.104.034983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Lee KK, Zhang L, Gerton JL. Stimulation of mTORC1 with L-leucine rescues defects associated with Roberts syndrome. PLoS Genet. 2013;9:e1003857. doi: 10.1371/journal.pgen.1003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, Mehta S, Reyes-Turcu FE, Zhuang F, Fuchs RT, Rong Y, Robb GB, Grewal SI. RNAi triggered by specialized machinery silences developmental genes and retrotransposons. Nature. 2013;493:557–560. doi: 10.1038/nature11716. [DOI] [PMC free article] [PubMed] [Google Scholar]


