Abstract
The basolateral amygdala (BLA) is a critical site for the reconsolidation of labile contextual cocaine memories following retrieval-induced reactivation/destabilization. Here, we examined whether glucocorticoid receptors (GR), which are abundant in the BLA, mediate this phenomenon. Rats were trained to lever press for cocaine reinforcement in a distinct environmental context, followed by extinction training in a different context. Rats were then briefly exposed to the cocaine-paired context (to elicit memory reactivation and reconsolidation) or their home cages (no reactivation control). Exposure to the cocaine-paired context elicited greater serum corticosterone concentrations than home cage stay. Interestingly, the GR antagonist, mifepristone (3–10 ng/hemisphere), administered into the BLA after memory reactivation produced a further, dose-dependent increase in serum corticosterone concentrations during the putative time of cocaine-memory reconsolidation but produced an inverted U-shaped dose-effect curve on subsequent cocaine-seeking behavior 72 h later. This effect was anatomically selective, dependent on memory reactivation (i.e., not observed after home cage exposure), and did not reflect protracted hyperactivity. However, the effect was also observed when mifepristone was administered after novelty stress that mimics drug context-induced hypothalamic-pituitary-adrenal (HPA) axis activation without explicit memory reactivation. Together, these findings suggest that, similar to explicit memory retrieval, a stressful event is sufficient to destabilize cocaine memories and permit their manipulation. Furthermore, BLA GR stimulation exerts inhibitory feedback upon HPA axis activation and thus suppresses cocaine-memory reconsolidation.
Keywords: cocaine seeking, memory reconsolidation, basolateral amygdala, glucocorticoid receptor, corticosterone
Graphical Abstract

1. Introduction
Exposure to cocaine-associated environmental stimuli can trigger intense craving and drug relapse in cocaine users (Childress et al., 1993; Fox et al., 2005; Rohsenow et al., 1990; Sinha et al., 2000) and cocaine-seeking behavior in rodent models even after extended periods of abstinence (Crombag et al., 2008; de Wit and Stewart, 1981; Fuchs et al., 2008). Exposure to a cocaine-paired context results not only in the retrieval, but also in the destabilization, of cocaine-related memories that promote drug-seeking behavior. Once labile, these memories must undergo protein synthesis-dependent reconsolidation into long-term memory stores for updating and maintenance (Fan et al., 2010; Fuchs et al., 2009; Nader, 2015; Sorg et al., 2015; Wells et al., 2011). Successful interference with memory reconsolidation may weaken maladaptive cocaine-associated memories and attenuate cue reactivity in cocaine users (Lonergan et al., 2015; Saladin et al., 2013; Sorg, 2012). Thus, understanding the neurobiological underpinnings of drug memory reconsolidation is important from a treatment perspective.
Adrenally secreted glucocorticoids (cortisol in humans, corticosterone in rats) potently modulate cocaine-induced behaviors, including cocaine self-administration and several forms of cocaine reinstatement (Deroche et al., 1997; Erb et al., 1998; Graf et al., 2013; Mantsch and Goeders, 1999; Stringfield et al., 2016). Moreover, glucocorticoids play an important role in memory storage by regulating intracellular signaling and gene transcription (Bamberger et al., 1996). In support of a role for glucocorticoids in drug memory reconsolidation in particular, social stress manipulation following the initial retrieval of drug-related words disrupts the subsequent recall of these words in heroin users (Zhao et al., 2009). Furthermore, systemic GR antagonism inhibits the reconsolidation of morphine-conditioned place preference memories (Fan et al., 2013), but intra-BLA GR antagonism rescues these memories from the disruptive effects of intense stress on memory reconsolidation (Wang et al., 2008). Importantly, the contributions of GRs to the reconsolidation of cocaine-related memories or of drug memories in an instrumental paradigm have not been investigated.
The BLA is a principal target of glucocorticoids (Joels and Karst, 2012) and a critical brain region for contextual memory reconsolidation that promotes subsequent cocaine-seeking behavior. Within the BLA, inhibition of protein synthesis or of elements of the mitogen activated protein kinase signaling pathway, including protein kinase A and extracellular signal-regulated kinase (ERK), disrupts contextual cocaine-memory reconsolidation and subsequent drug context-induced reinstatement of extinguished cocaine-seeking (Arguello et al., 2014; Fuchs et al., 2009; Wells et al., 2013). Evidence suggests that ERK-induced phosphorylation requires interaction between ERK and activated GRs at least in the hippocampus (Reul, 2014). Hence, we evaluated whether GRs in the BLA contribute to contextual cocaine-memory reconsolidation in an instrumental model of drug relapse. Furthermore, we examined changes in serum corticosterone concentrations after memory reactivation followed by GR antagonist treatment in order to further explore the possible mechanisms of GR-mediated effects.
2. Methods
2.1. Animals
Male Sprague-Dawley rats (Harlan/Envigo, Livermore, CA; 275–300 g) were housed in a humidity-controlled vivarium on a reversed light-dark cycle. Throughout the study, animals received 20–25 grams of rat chow per day, with water available ad libitum. This mild food restriction regimen facilitates the acquisition of operant responding without altering the magnitude of cue-induced reinstatement of cocaine-seeking behavior (Bongiovanni and See, 2008). Protocols followed the Guide for the Care and Use of Laboratory Rats (Institute of Laboratory Animal Resources on Life Sciences, 2011) and were approved by the Institutional Animal Care and Use Committee.
2.2. Food training
To facilitate the acquisition of drug self-administration, rats were trained to lever press during an overnight session. Each response on one (active) lever resulted in food reinforcement (45-mg food pellet; Bio-Serv., Flemington, NJ). Responses on a second (inactive) lever had no programmed consequences. Food training took place in sound-attenuated operant conditioning chambers (26×27×27 cm, Coulbourn Instruments, Allentown, PA) that did not contain the multimodal sensory stimuli used subsequently for contextual conditioning.
2.3. Surgery
Twenty-four hours after the food training session, rats were anesthetized with a ketamine-xylazine cocktail (100.0/5.0 mg/kg, respectively, i.p.). Back-mounted intravenous catheters were constructed in-house and implanted into the right jugular vein, as described previously (Fuchs et al., 2007). Using standard stereotaxic procedures, stainless-steel guide cannulae (Plastics One, Roanoke, VA) were then aimed 2 mm above the BLA (−2.7 mm AP, 5.0 mm ML, −6.7 mm DV, relative to bregma) or the dorsally adjacent pCPu (−2.7 mm AP, 5.0 mm ML, −4.7 mm DV). Tygon tubing and stylets (Plastics One) covered the tips of the catheter and guide cannulae, respectively. The catheters were flushed daily with 0.1 mL of cefazolin (1.0 mg/10 mL, Henry Schein Animal Health, Tualatin, OR; dissolved in 70-U/mL heparinized saline, Patterson Veterinary Supply, Sterling, MA) followed by 0.1 mL of 70-U/mL heparinized saline. Animals received 5 days of post-surgical recovery before drug self-administration training. Catheter patency was verified using propofol (10 mg/0.1 mL, Henry Schein), as needed.
2.4. Cocaine self-administration training
Animals were randomly assigned to self-administer cocaine in operant conditioning chambers arranged to form one of two distinct contexts. Context 1 contained a red house light, intermittent pure tone (80-dB, 1-kHz, 2 sec on, 2 sec off), pine-scented air freshener (Car Freshener Corp., Watertown, NY), and wire mesh flooring. Context 2 contained an intermittent white stimulus light (2 sec on, 2 sec off) located above the inactive lever, continuous pure tone (75-db, 2.5-kHz), vanilla-scented air freshener (Scopus Products, Moorpark, CA), and a ceramic tile bisecting a bar floor. At the start of the session, the rats’ jugular catheters were connected to an infusion pump (Coulbourn) via polyethylene 20 tubing and liquid swivels (Instech, Plymouth Meeting, PA). Active lever presses resulted in unsignaled cocaine infusions (0.15 mg/0.05 mL over 2 sec, i.v.; NIDA Drug Supply Program, Research Triangle Park, NC) under a fixed ratio 1 schedule. Each cocaine infusion was followed by an unsignaled 20-sec timeout period. Active lever presses during the timeout period and inactive lever presses during the session had no programmed consequences. Reinforcer delivery and data collection were controlled using Graphic State Notation software 4.1.04 (Coulbourn). Training continued for two hours daily until rats reached the acquisition criterion (≥10 infusions/session on 10 days).
2.5. Extinction training
Rats that self-administered cocaine in Context 1 were placed in Context 2, and vice versa, for daily 2-h extinction training sessions. During these sessions, active and inactive lever presses had no programmed consequences. Immediately after extinction session 4, rats were acclimated to the microinfusion procedure. Injector cannulae (33-Ga, Plastics One) were inserted 2 mm past the tip of the guide cannulae and remained in place for 4 min while the rats were gently held by the experimenters. All rats received seven extinction sessions in order to keep memory age, a boundary condition of memory reconsolidation (Frankland et al., 2006; Tronson and Taylor, 2007; Wichert et al., 2011), constant at the time of the experimental manipulation.
2.6. Experiment 1: Effects of mifepristone treatment on cocaine-memory reconsolidation
On post-cocaine day 8, rats were re-exposed to the previously cocaine-paired context for 15 min (in order to reactivate contextual cocaine memories) or remained in their home cages (no memory reactivation control) (see experimental timeline in Fig 2). This session length is sufficient to destabilize instrumental cocaine memories without producing appreciable behavioral extinction (Fuchs et al., 2009). During the session, active and inactive lever presses had no programmed consequences. Immediately after the session or following removal from the home cage, rats received bilateral microinfusions of the GR antagonist, mifepristone (RU38486; 3 or 10 ng/0.5 μL/hemisphere; Sigma-Aldrich, St. Louis, MO), or vehicle (2% ethanol/phosphate buffered saline; 0.5 μL/hemisphere) over 2 min. Intra-BLA mifepristone doses were selected based on their effective ability to inhibit memory consolidation (Roozendaal and McGaugh, 1997) and reconsolidation (Jin et al., 2007) in other models. Anatomical control groups received 3 ng of mifepristone or vehicle into the pCPu after re-exposure to the cocaine-paired context. Injection cannulae were left in place for 1 min before and after infusion, and rats were then returned into their home cages in the colony room. Rats received a minimum of 2 daily extinction training sessions in the extinction context until they reached the extinction criterion (≤25 active lever presses/session on two consecutive days). The mean number of days needed to reach this criterion (+ SEM) was 2.15 ± 0.12, given the prior extinction experience of the animals. Accordingly, testing began ~72 h after memory reactivation. At test, the rats were exposed to the previously cocaine-paired context for 2 h during which lever responses had no programmed consequences. Reinstatement of drug-seeking behavior was defined as a significant increase in active lever responding during the test session relative to the last extinction session.
2.7. Experiment 2: Effects of cocaine context re-exposure and mifepristone on serum corticosterone concentrations
Rats underwent self-administration training and extinction training as in experiment 1 (see experimental timeline in Fig 3). Rats were acclimated to blood sample collection via tail nick (~200 μL/sample) before and after extinction session 6. To minimize stress effects on memory reactivation, pre-session blood samples were collected before extinction session 7, 24 h before the memory reactivation session. Additional blood samples were collected immediately after exposure to the extinction context (session 7) and immediately after exposure to the cocaine-paired context (15-min memory reactivation session) or the home cage on post-cocaine day 8. Finally, blood samples were collected 30, 60, and 90 min after intra-BLA mifepristone (3 or 10 ng/0.5 μL/hemisphere) or vehicle administration. Blood samples were centrifuged at 4°C. Serum was stored at −20°C until assayed in duplicate using the MP Biomedicals Corticosterone RIA kit for rats and mice (Solon, OH). The assay provided an intra-assay coefficient of variation of 1.77%, with a lower limit of detectability of 25 ng/mL.
2.8. Experiment 3: Effects of novelty stress and mifepristone treatment on subsequent cocaine seeking
Rats received cocaine self-administration training and extinction training as in experiment 1 (see experimental timeline in Fig 2). However, on post-cocaine day 8, they were exposed to a novel context for 15 min in order to simulate the effects of the cocaine-paired context on HPA axis activity (Seggie and Brown, 1975) without explicit memory reactivation. The novel context contained continuous white stimulus lights above each lever, continuous complex tone (80-dB, alternating between 1, 1.5, and 2.5 kHz, at 2 s intervals), citrus-scented air freshener (Car Freshener Corp., Watertown, NY), and ceramic tile flooring, and it was located in a different testing room than the cocaine-paired context. Immediately after the session, rats received bilateral microinfusions of 3 ng of mifepristone or vehicle. The procedures for subsequent extinction training and testing were the same as in experiment 1.
2.9. Protracted effects of mifepristone on motor activity
Intracranial manipulations can affect lever pressing performance by altering general motor activity. To evaluate this possibility, 12 BLA-cannulated rats from experiment 1 were assigned to receive bilateral intra-BLA microinfusions of each dose of mifepristone and vehicle using a counterbalanced within-subjects testing procedure that commenced 2–7 days after the test of cocaine-seeking behavior. Locomotor activity was assessed 72 h after each treatment to match the treatment-to-testing interval in experiment 1. The sessions were spaced at least 48 h apart. Testing was conducted in Plexiglas activity chambers (42×20×20 cm) equipped with eight light sources and photodetectors. Photobeam breaks were measured for 1 h by a computerized system (San Diego Instruments, San Diego, CA).
2.10. Histology
Rats were euthanized by ketamine/xylazine overdose (300/15 mg/kg, i.p.) or rapid decapitation. The brains were removed, flash frozen in isopentane, and stored at −80°C. Forty μm brain sections were stained with cresyl violet (Kodak, Rochester, NY, USA) to visualize cannula placement.
2.11. Data Analysis
Separate analyses of variance (ANOVAs) or t-tests were used to evaluate group differences with treatment (mifepristone doses, vehicle), memory reactivation (memory reactivation, home cage stay), or context (extinction, cocaine-paired) as between-subjects factors and treatment (mifepristone doses, vehicle) or time (days, 20-min intervals, or time points) as the within-subject factor, where appropriate. Tukey’s post hoc tests were used to further investigate significant ANOVA effects, when appropriate. Only significant pair-wise comparisons are reported. Alpha was set at 0.05.
3. Results
3.1. Cannula placement
Light microscopy of the brain sections did not indicate excessive gliosis or other abnormalities at the infusion sites. Cannula placements were located bilaterally in the lateral or basal nuclei of the amygdala or the overlying pCPu (Fig 1). Data from rats with incorrect cannula placements were excluded from statistical analyses. The resulting sample sizes are reported in the figures and figure captions.
Figure 1.
Representative photomicrograph illustrating cannula placement in the basolateral amygdala (BLA) and schematics of cannula placements in Experiments 1–3. Symbols represent the most ventral point of injection cannula tracts for rats that had received mifepristone (Mif) or vehicle microinfusions into the BLA or posterior caudate-putamen (pCPu) immediately after memory reactivation (M-REACT), home cage stay (HC), or novel context exposure (NOVEL). Numbers represent distance from bregma in mm according to the rat brain atlas (Paxinos and Watson, 1997).
3.2. Behavioral history
There were no preexisting differences among the BLA-cannulated or pCPu-cannulated groups in cocaine intake and lever responding during drug self-administration, extinction training, and memory reactivation, where applicable. Inactive lever presses remained low at test. Descriptive statistics for these measures are reported in Table S1, Fig 2, and Fig 3.
3.3. Mifepristone treatment after cocaine context re-exposure produces an inverted U-shaped dose effect curve for subsequent drug context-induced cocaine seeking
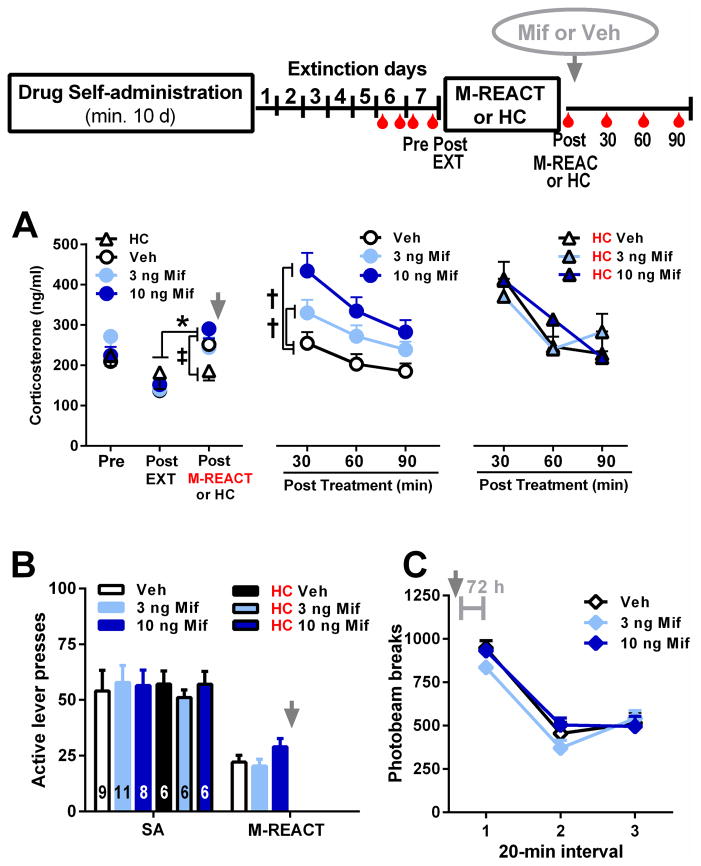
Intra-BLA mifepristone administered after cocaine-memory reactivation (i.e., at the time of memory reconsolidation) altered subsequent cocaine-seeking behavior 72 h later (Fig 2A). The three groups did not differ in active lever responding in the extinction context and exhibited more active lever responding in the cocaine-paired context at test than on the preceding day in the extinction context (treatment x context interaction: F1,22=3.57, P=0.04; context main effect: F1,22=62.39, P=0.0001; treatment main effect, F2,22=4.34, P=0.03). Furthermore, the 3-ng mifepristone group exhibited more active lever responding in the cocaine-paired context relative to the vehicle group (Tukey’s test, P<0.05), while the 10-ng mifepristone group did not. The time course analysis (Fig 2B; treatment x time interaction: F10,110=2.02, P=0.04; treatment main effect: F2,22=3.86, P=0.04; time main effect: F5,110=25.58, P=0.0001) indicated that the 3-ng mifepristone group exhibited more active lever responding than the vehicle group during the first 40 min of testing (Tukey’s tests, P<0.05).
Figure 2.
Effects of intra-BLA or intra-pCPu mifepristone administration on cocaine-memory reconsolidation as indicated by drug-seeking behavior in the cocaine-paired context (COC-paired) 72 h later. Top panel: Experimental timeline for experiments 1 and 3. (A) Active lever presses (mean ± SEM) during cocaine self-administration (SA, mean of last three 2-h sessions), during the memory reactivation session (M-REACT, 15-min session), in the extinction context (EXT, last 2-h session), and in the cocaine-paired context (2-h session) in groups that received intra-BLA administration of mifepristone or vehicle immediately after the memory reactivation session (grey arrow). The numbers in the bars indicate sample sizes. (B) Time course of active lever presses (mean/20 min ± SEM) during the test session in the cocaine-paired context (n=6–9/group, as in panel A). (C) Active lever presses (mean ± SEM) during each experimental phase in the no memory reactivation control groups that stayed in the home cage (HC) immediately prior to intracranial treatment. (D) Active lever presses (mean ± SEM) during each experimental phase in the anatomical control groups that received intracranial treatment into the pCPu immediately after the cocaine-memory reactivation session. (E) Active lever presses (mean ± SEM) during each experimental phase in the control groups that were exposed to a novel context prior to intracranial treatment. (F) Time course of active lever presses (mean/20 min ± SEM) by the novel context control groups during the test session in the cocaine-paired context (n=7–8/group, as in panel E). Symbols represent difference from responding in the extinction context (*, A and E: Tukey’s tests, P<0.05; C and D: ANOVA context main effect, P=0.0001) or from the vehicle control group (†, A, B, and E: Tukey’s tests, P<0.05; F: ANOVA treatment main effect, P=0.005).
3.4. Low dose mifepristone treatment after home cage stay fails to alter subsequent drug context-induced cocaine seeking
Intra-BLA mifepristone administered in the absence of explicit memory reactivation (i.e., following home cage stay) failed to alter cocaine-seeking behavior 72 h later (Fig 2C). Both the vehicle and 3-ng groups exhibited more active lever responding in the cocaine-paired context than in the extinction context (ANOVA context main effect: F1,12=103.96, P=0.0001) with no difference in active lever responding between the groups in either context (treatment main and interaction effects, NS). Furthermore, a time course analysis indicated that responding declined in both groups during the session (time main effect: F5,60=27.32, P=0.0001; treatment main and interaction effects, NS; data not shown).
3.5. Low dose mifepristone treatment in the pCPu after cocaine context re-exposure fails to alter subsequent drug context-induced cocaine seeking
Intra-pCPu administration of the 3-ng dose of mifepristone (behaviorally effective BLA dose) after memory reactivation failed to alter cocaine-seeking behavior 72 h later relative to vehicle (Fig 2D; context main effect: F1,11=29.39, P=0.0001; treatment main and interaction effects, NS). Both groups exhibited more active lever responding in the cocaine-paired context than in the extinction context.
3.6. Low dose mifepristone treatment after novel context exposure potentiates subsequent drug context-induced cocaine seeking
Intra-BLA mifepristone administered after novel context exposure without explicit memory reactivation increased cocaine-seeking behavior 72 h later relative to vehicle (Fig 2E). The vehicle and 3-ng groups did not differ in active lever responding in the extinction context and exhibited more active lever responding in the cocaine-paired context than in the extinction context (ANOVA treatment x context interaction: F1,13=10.18, P=0.007; context main effect: F1,13=40.30, P=0.0001; treatment main effect, F1,13=11.40, P=0.005). Furthermore, the 3-ng mifepristone group exhibited more active lever responding in the cocaine-paired context relative to the vehicle group (Tukey’s test, P<0.05). A time course analysis confirmed this effect (Fig 2F; treatment main effect: F1,13 =11.07, P=0.005; treatment x time interaction, NS) and indicated that responding declined in both groups during the session (time main effect: F5,60=24.15, P=0.0001).
3.7. Cocaine context re-exposure increases serum corticosterone concentrations
There was no preexisting difference between the groups in pre-session serum corticosterone concentrations (Fig 3A left panel, F3,28=0.84, NS) or in their behavioral history during cocaine self-administration training (Fig 3B, F3,28=0.05, NS) or during the cocaine-memory reactivation session (F2,28=2.50, NS, where applicable). Conversely, post-session serum corticosterone concentrations were significantly higher following cocaine-memory reactivation (Fig 3A left panel, ANOVA group x day interaction: F1,30=5.33, P=0.03, Tukey’s test, P<0.05; day main effect: F1,30=6.26, P=0.02; group main effect: F1,30=0.15, NS), but not after home cage exposure, relative to extinction context exposure.
Figure 3.
Effects of cocaine-paired context re-exposure and intra-BLA mifepristone administration on serum corticosterone concentrations. Top panel: Experimental timeline for experiment 2. Rats were repeatedly adapted to the tail nick procedure (red symbols). (A) Serum corticosterone concentrations (mean ng/ml ± SEM) pre-session (Pre; samples collected 24 h before memory reactivation or home cage stay), immediately following exposure to the extinction context (Post Ext), the cocaine-paired context (Post M-REACT), or the home cage (Post HC), and at 30, 60, and 90 min after intracranial treatment (n=6–11/group, as in panel B). (B) Active lever presses (mean ± SEM) during cocaine self-administration (SA, mean of last three 2-h sessions) and during the memory reactivation session (15-min session, where appropriate) in the groups that received intra-BLA administration of mifepristone or vehicle immediately after the memory reactivation session or home cage exposure. The numbers in the bars indicate sample sizes. Symbols represent difference relative to corticosterone concentrations observed after exposure to the extinction context (*, Tukey’s test, P<0.05), after home cage stay (‡, Tukey’s test, P<0.05), or in the vehicle group (†, ANOVA treatment simple main effect, Tukey’s test, P<0.05). (C) Locomotor activity (mean photobeam breaks/20 min ± SEM) 72 h after intracranial treatment (n=12, within-subjects design).
3.8. Mifepristone treatment after cocaine context re-exposure dose-dependently increases serum corticosterone concentrations
Intra-BLA mifepristone administration after cocaine-memory reactivation dose-dependently increased serum corticosterone concentrations 30 min post infusion (Fig 3A middle panel, ANOVA treatment x time interaction: F3,28=4.09, P=0.02; time main effect: F1,28=24.83, P=0.0001; treatment main effect: F3,28=1.42, NS). Specifically, vehicle or 3 ng of mifepristone infusion did not alter corticosterone concentrations relative to the post-memory reactivation baseline. In contrast, infusion of 10 ng of mifepristone increased corticosterone concentrations relative to the baseline (Tukey’s test, P<0.01) and relative to vehicle infusion (Tukey’s test, P<0.05). During the first 90 min following mifepristone or vehicle infusion into the BLA, serum corticosterone concentrations gradually decreased in all groups (time main effect, F2,56=24.57, P=0.0001; 30-min > 60-min > 90-min time point, Tukey’s tests, P<0.05). However, corticosterone concentrations were consistently higher following 3 or 10 ng of mifepristone administration relative to vehicle (treatment main effect: F3,28=2.92, P=0.05, Tukey’s tests, P<0.05; treatment x time interaction, F6,56=1.36, NS).
3.9. Mifepristone treatment after home cage stay fails to alter serum orticosterone concentrations
Serum corticosterone concentrations rose 30 min after treatment relative to the post home cage stay baseline (Fig 3A right panel, time main effect: F2,15=49.91, P=0.0001; treatment x time interaction: F2,15=1.38, NS; treatment main effect: F2,15=0.36, NS), possibly reflecting anticipation of, or stress associated with deviation from, the daily routine involving placement into an operant chamber. However, during the first 90 min following mifepristone or vehicle infusion into the BLA, serum corticosterone concentrations declined (time main effect: F2,30=29.42, P=0.0001), and neither 3 nor 10 ng of mifepristone altered serum corticosterone concentrations relative to vehicle (treatment x time interaction: F4,30=2.20, NS; treatment main effect: F2,15=0.11, NS).
3.10. Mifepristone fails to alter general activity 72 h post treatment
Locomotor activity decreased gradually during each 1-h session (Fig 3C, ANOVA time main effect: F2,22=109.50, P=0.0001). Furthermore, neither 3 nor 10 ng of mifepristone altered locomotor activity did not differ 72 h later, relative to vehicle (treatment x time interaction: F4,44=1.56, NS; treatment main effect: F2,22=1.86, NS).
4. Discussion
In the present study, mifepristone, a GR and progesterone receptor antagonist (Cadepond et al., 1997), was used to probe BLA GR contributions to cocaine-memory reconsolidation. Mifepristone-induced selective manipulation of GRs was possible because progesterone receptors are not expressed in the BLA of rats (Forbes-Lorman et al., 2014; Quadros et al., 2007). The main findings of the present study were that either cocaine context re-exposure or novel context exposure was sufficient to make cocaine memories vulnerable to manipulation by mifepristone administered during the putative time of cocaine-memory reconsolidation (Fig 2). Furthermore, intra-BLA mifepristone administration produced a dose-dependent increase in serum corticosterone concentrations during the putative time of memory reconsolidation (Fig 3) but an inverted U-shaped dose effect curve on subsequent cocaine-seeking behavior (Fig 2), consistent with effects on cocaine memory strength or salience.
4.1. HPA axis activation and memory reconsolidation
Previous findings regarding the contributions of BLA GRs to memory reconsolidation have been inconsistent. For instance, intra-BLA mifepristone administration disrupts auditory fear memory reconsolidation (Jin et al., 2007) following re-exposure to a shock-predictive cue that elicits modest HPA axis activation (Kiyokawa et al., 2015). Conversely, intra-BLA mifepristone administration restores Pavlovian morphine memory and object recognition memory reconsolidation after impairment produced by forced cold water swim and novel elevated platform stressors (Maroun and Akirav, 2008; Wang et al., 2008) that prompt robust HPA axis activation (Caudal et al., 2014; Jain et al., 1996). In the present study, chronic mild food restriction (20–25 g of chow per day) may have slightly elevated baseline corticosterone levels, as such effects have been reported with more severe food restriction (12 g of chow/day) (Stamp et al., 2008). Nevertheless, exposure to the cocaine-paired context modestly raised blood serum corticosterone concentrations, similar to mild stressors (Kiyokawa et al., 2015), also concurring with reports of cue-induced HPA axis activation in cocaine users (Berger et al., 1996). Specifically, following the 15-min cocaine-memory reactivation session serum corticosterone concentrations were significantly higher relative to those observed (a) following exposure to the extinction context in the same animals and (b) following exposure to the home cage in a different group of animals at the same stage of adaptation to handling and to the tail nick procedure (Fig 3A left panel). However, unlike in previous studies, intra-BLA administration of 3 ng of mifepristone following cocaine-memory reactivation enhanced memory strength or salience and thus subsequent drug context-induced cocaine-seeking behavior, whereas 10 ng of mifepristone appeared to block memory enhancement without impairing memory reconsolidation relative to vehicle (Fig 2A–B). Together the findings suggest that the effects of BLA GR stimulation on memory reconsolidation may be determined by the degree of HPA axis activation and/or by other paradigm-specific variables as described below.
4.2. Mifepristone-induced memory enhancement following explicit memory reactivation
We postulate that mifepristone administered into the BLA at the lower dose (3 ng/hemisphere) elicited a reactivation-dependent enhancement of memory strength or salience in the course of memory reconsolidation, based on the results of the control experiments. Specifically, mifepristone administered without explicit memory reactivation (i.e., following home cage stay) failed to facilitate subsequent cocaine-seeking behavior (Fig 2C), suggesting that mifepristone-induced memory enhancement required memory destabilization. It is unlikely that the increase in cocaine-seeking behavior at test reflected a mifepristone-induced inhibition of extinction learning. Responding at test typically approximates the magnitude of responding during cocaine self-administration training in this paradigm, and responding in the 3-ng mifepristone group significantly exceeded the self-administration baseline (Fig 2A) and was also more robust than responding in the vehicle control group at the onset of the test session (Fig 2B). The effects of mifepristone were anatomically selective to the BLA in that mifepristone infusion into the pCPu after memory reactivation failed to enhance cocaine-seeking behavior (Fig 2D). Finally, the increase in cocaine-seeking behavior did not reflect mifepristone-induced protracted hyperactivity since mifepristone did not increase inactive lever responding in the cocaine-paired context (Table S1) or locomotion in a novel context (Fig 2F) 72 h post administration, relative to vehicle.
Interestingly, intra-BLA administration of mifepristone dose-dependently increased serum corticosterone concentrations relative to vehicle during the putative time of memory reconsolidation (Fig 3A middle panel). This effect likely reflected a mifepristone-induced disinhibition of drug context-induced HPA axis activation and a consequent increase in corticosterone concentrations (Tasker and Herman, 2011), because mifepristone alone (i.e., following home cage exposure) failed to alter serum corticosterone levels (Fig 3A right panel), relative to vehicle. Accordingly, we hypothesize that enhanced HPA axis activity during memory reconsolidation is related to the formation of stronger cocaine memories during reconsolidation. The exact mechanisms by which HPA axis activation mediates this effect (e.g., corticotropin-releasing hormone, adrenocorticotropic hormone, glucocorticoids) have yet to be determined. One possible mechanism supported by existing literature is that HPA axis activation amplifies norepinephrine release and consequently ERK activation in the BLA (Nathan et al., 2004; Roozendaal et al., 2009). Beta-adrenergic receptor stimulation and ERK activation in the BLA are required for cocaine-memory reconsolidation (Otis et al., 2013; Wells et al., 2013) and, at supraphysiological levels (i.e., following mifepristone administration), these mechanisms may produce cocaine-memory enhancement.
While corticosterone has inhibitory feedback function at the level of the hypothalamus and the anterior pituitary, corticosterone in the BLA may enhance memory strength by prolonging excitatory neurotransmission in the BLA and thus lengthening the time window of memory encoding (Karst et al., 2010; Karst et al., 2002; Milton et al., 2008). This possibility is supported by the observation that corticosterone elicits rapid increases in BLA principal neuronal excitability through mineralocorticoid receptor-mediated (MR) mechanisms, whereas it elicits enduring increases in neuronal excitability (Karst et al., 2010) and facilitates long-term potentiation (Sarabdjitsingh et al., 2012) through GR-mediated mechanisms. Since BLA MR are not saturated under baseline conditions (Karst et al., 2010), moderate corticosterone concentrations may preferentially increase MR stimulation in the BLA based on the higher binding affinity of corticosterone for MRs than for GRs (Reul, 2014). However, following drug context-induced HPA axis activation and mifepristone, the associated higher corticosterone concentrations increase BLA GR stimulation, produce enduring enhancement in BLA excitability (Karst et al., 2010), and thus boost memory strength and subsequent drug context-induced cocaine-seeking behavior. Importantly, both doses of mifepristone fully antagonize cytoplasmic GRs in the BLA based on the higher affinity of mifepristone for these GRs (KD = 0.4 nM, IC50 = 2 nM) relative to corticosterone’s affinity for the same receptors (KD = 2.5–5 nM) (Heikinheimo et al., 1987; Reul, 2014; Svec, 1985). However, mifepristone does not antagonize membrane-bound GRs (Di et al., 2003). Therefore, increased membrane-bound GR stimulation may contribute to memory enhancement following mifepristone administration by facilitating endogenous cannabinoid synthesis and subsequent cannabinoid receptor 1 stimulation (Di et al., 2003; Tasker and Herman, 2011), predominantly on GABAergic terminals within the BLA (Katona et al., 2001).
In summary, the present findings suggest that cytoplasmic GR stimulation in the BLA exerts an inhibitory influence on HPA axis activation, drug-memory reconsolidation, and subsequent contextual control over drug-seeking behavior. In support of this conclusion, low-dose mifepristone treatment, which appeared to disinhibit drug context-induced HPA activation, potentiated drug context-induced cocaine-seeking behavior. Cocaine-context exposure plus high-dose mifepristone (10 ng/hemisphere) treatment failed to potentiate drug context-induced cocaine-seeking behavior (Fig 2A–B), despite further increasing HPA axis activation, as indicated by an increase in serum corticosterone concentrations (Fig 3B middle panel). This may reflect that HPA axis activity must fall within a specific range to permit cocaine-memory strengthening during reconsolidation (Meir Drexler and Wolf, 2016), similar to what has been theorized for memory consolidation (de Quervain et al., 2009).
4.3. Memory enhancement following stress-induced memory destabilization
Remarkably, mifepristone administration after novel context exposure increased subsequent cocaine-seeking behavior (Fig 2E–F), similar to mifepristone administration after explicit cocaine-memory reactivation through cocaine-paired context exposure. This effect did not simply arise from generalization between the novel and cocaine contexts and subsequent cocaine memory retrieval and destabilization. In strong support of this, we have shown that manipulations that reliably disrupt the reconsolidation of labile cocaine memories fail to alter cocaine-seeking behavior when administered after exposure to the same novel context (Fuchs et al., 2009; Ramirez et al., 2009; Wells et al., 2013). Furthermore, the novel context control group (Fig 2E) did not exhibit greater drug context-induced cocaine-seeking behavior, and thus evidence of recent cocaine-memory reconsolidation and memory enhancement, relative to the home cage control group (Fig 2A).
Extant literature indicates that novel context exposure can trigger similar elevations in serum corticosterone levels (~270 ng/ml; (Seggie and Brown, 1975) as cocaine-paired context exposure did in the present study (Fig 3A left panel). Notably, it is improbable that strong HPA axis activation following novel context exposure plus mifepristone treatment potentiated the expression of cocaine-seeking behavior, independent of effects on cocaine memories. Stress-induced reinstatement of drug-seeking behavior is highly context-specific and time-dependent (Shalev et al., 2000), and stress-induced augmentation of reinstatement requires presentation of the stressor in the drug-paired context during the test session. Similarly, the results of drug discrimination studies fail to support the idea that HPA axis activation or high corticosterone concentrations could have destabilized cocaine memories by inducing a cocaine-like interoceptive state (Filip et al., 2000). One possible explanation, however, is that robust HPA axis activation destabilizes, and thus triggers the reconsolidation and strengthening of, long-term memories in a nonspecific manner. In support of this intriguing hypothesis, Ježek and colleagues (2010) have demonstrated that a more robust stressor, forced swim stress (corticosterone concentrations ~450 ng/ml; Connor et al., 1997), alone can enhance the subsequent recall of unrelated appetitive or aversive memories. Moreover, swim stress effects on recall could be inhibited by standard reconsolidation inhibitor manipulations (i.e., electroconvulsive shock or propranolol) administered immediately, but not 5 hours, post swim. Future research will need to ascertain whether the effects of novelty stress plus mifepristone on memory reconsolidation are indeed nonspecific in the drug context-induced reinstatement model.
4.4. Implications for maladaptive memories
The findings from the present study suggest that exposure to a cocaine-paired context, unrelated stressful events, or HPA axis dysfunction can increase the vulnerability of cocaine memories to enhancement. Importantly, these mechanisms may contribute to the development of pathologically strong and intrusive cocaine memories in substance abusers. Future studies will need to explore the circuitry and signaling events involved in this form for memory enhancement. It will also be interesting to evaluate the effects of GR manipulations on the incubation of craving (i.e., time-dependent increase in the magnitude of cocaine-seeking behavior) in rats (Grimm et al., 2001; Tran-Nguyen et al., 1998), a phenomenon that may be a manifestation of memory enhancement during abstinence (Wells et al., 2011). The present findings suggest that preempting the enhancement of memory strength drug memory reconsolidation may be a useful adjunct to other approaches for drug relapse prevention.
Supplementary Material
Highlights.
Increased HPA axis activation within a certain range is sufficient to strengthen labile context-cocaine associative memories during the time window of memory reconsolidation.
GR receptor stimulation in the BLA exerts inhibitory control over HPA axis activation and this phenomenon.
Thus, HPA axis dysfunction may contribute to the development of pathologically salient or intrusive drug memories.
Acknowledgments
This work was supported by the National Institute on Drug Abuse [R01 DA025646 to R.F.L.] and the National Institute of Neurological Disorders and Stroke [T32 NS007431 to S. J. S.]. The authors thank Dr. Kelley Harmon, Dr. Amy Arguello, Carey Lyons, and Nicole Jones for technical assistance with data collection.
Footnotes
CONFLICT OF INTEREST
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arguello AA, Hodges MA, Wells AM, Lara H, 3rd, Xie X, Fuchs RA. Involvement of amygdalar protein kinase a, but not calcium/calmodulin-dependent protein kinase ii, in the reconsolidation of cocaine-related contextual memories in rats. Psychopharmacology (Berl) 2014;231:55–65. doi: 10.1007/s00213-013-3203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- Berger SP, Hall S, Mickalian JD, Reid MS, Crawford CA, Delucchi K, Carr K, Hall S. Haloperidol antagonism of cue-elicited cocaine craving. Lancet. 1996;347:504–508. doi: 10.1016/s0140-6736(96)91139-3. [DOI] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacol Biochem Behav. 2008;89:227–233. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): Mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129–156. doi: 10.1146/annurev.med.48.1.129. [DOI] [PubMed] [Google Scholar]
- Caudal D, Jay TM, Godsil BP. Behavioral stress induces regionally-distinct shifts of brain mineralocorticoid and glucocorticoid receptor levels. Front Behav Neurosci. 2014;8:19. doi: 10.3389/fnbeh.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Hole AV, Ehrman RN, Robbins SJ, McLellan AT, O’Brien CP. Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr. 1993;137:73–95. [PubMed] [Google Scholar]
- Connor TJ, Kelly JP, Leonard BE. Forced swim test-induced neurochemical endocrine, and immune changes in the rat. Pharmacol Biochem Behav. 1997;58:961–967. doi: 10.1016/s0091-3057(97)00028-2. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Review. Context-induced relapse to drug seeking: A review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJ, Aerni A, Schelling G, Roozendaal B. Glucocorticoids and the regulation of memory in health and disease. Front Neuroendocrinol. 2009;30:358–370. doi: 10.1016/j.yfrne.2009.03.002. [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Le Moal M, Piazza PV. Glucocorticoids and behavioral effects of psychostimulants. Ii: Cocaine intravenous self-administration and reinstatement depend on glucocorticoid levels. J Pharmacol Exp Ther. 1997;281:1401–1407. [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan HY, Cherng CG, Yang FY, Cheng LY, Tsai CJ, Lin LC, Yu L. Systemic treatment with protein synthesis inhibitors attenuates the expression of cocaine memory. Behav Brain Res. 2010;208:522–527. doi: 10.1016/j.bbr.2009.12.034. [DOI] [PubMed] [Google Scholar]
- Fan YD, Niu HC, Huma T, Li L, Wang GM, Xu LQ, Ren H, Ma YY, Yu HL. Blockage of glucocorticoid receptors during memory acquisition, retrieval and reconsolidation prevents the expression of morphine-induced conditioned place preferences in mice. Dongwuxue Yanjiu. 2013;34:E26–34. doi: 10.3724/SP.J.1141.2013.E01E26. [DOI] [PubMed] [Google Scholar]
- Filip M, Nowak E, Siwanowicz J, Przegalinski E. Effects of corticosterone and its synthesis blockade on the cocaine-induced discriminative stimulus effects in rats. Pol J Pharmacol. 2000;52:411–421. [PubMed] [Google Scholar]
- Forbes-Lorman R, Auger AP, Auger CJ. Neonatal ru-486 (mifepristone) exposure increases androgen receptor immunoreactivity and sexual behavior in male rats. Brain Res. 2014;1543:143–150. doi: 10.1016/j.brainres.2013.11.012. [DOI] [PubMed] [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects drug craving and associated responses to stress and drug-related cues. Psychoneuroendocrinology. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Ding HK, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learn Mem. 2006;13:451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Bell GH, Ramirez DR, Eaddy JL, Su ZI. Basolateral amygdala involvement in memory reconsolidation processes that facilitate drug context-induced cocaine seeking. Eur J Neurosci. 2009;30:889–900. doi: 10.1111/j.1460-9568.2009.06888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Eaddy JL, Su ZI, Bell GH. Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur J Neurosci. 2007;26:487–498. doi: 10.1111/j.1460-9568.2007.05674.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: The role of environmental stimuli. Drug Discov Today Dis Models. 2008;5:251–258. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR, Robble MA, Vranjkovic O, Wheeler DS, Mantsch JR, Gasser PJ. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci. 2013;33:11800–11810. doi: 10.1523/JNEUROSCI.1969-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo O, Kontula K, Croxatto H, Spitz I, Luukkainen T, Lahteenmaki P. Plasma concentrations and receptor binding of ru 486 and its metabolites in humans. J Steroid Biochem. 1987;26:279–284. doi: 10.1016/0022-4731(87)90083-5. [DOI] [PubMed] [Google Scholar]
- Jain S, Bruot BC, Stevenson JR. Cold swim stress leads to enhanced splenocyte responsiveness to concanavalin a, decreased serum testosterone, and increased serum corticosterone, glucose, and protein. Life Sci. 1996;59:209–218. doi: 10.1016/0024-3205(96)00286-x. [DOI] [PubMed] [Google Scholar]
- Jin XC, Lu YF, Yang XF, Ma L, Li BM. Glucocorticoid receptors in the basolateral nucleus of amygdala are required for postreactivation reconsolidation of auditory fear memory. Eur J Neurosci. 2007;25:3702–3712. doi: 10.1111/j.1460-9568.2007.05621.x. [DOI] [PubMed] [Google Scholar]
- Joels M, Karst H. Corticosteroid effects on calcium signaling in limbic neurons. Cell Calcium. 2012;51:277–283. doi: 10.1016/j.ceca.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci U S A. 2010;107:14449–14454. doi: 10.1073/pnas.0914381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Nair S, Velzing E, Rumpff-van Essen L, Slagter E, Shinnick-Gallagher P, Joels M. Glucocorticoids alter calcium conductances and calcium channel subunit expression in basolateral amygdala neurons. Eur J Neurosci. 2002;16:1083–1089. doi: 10.1046/j.1460-9568.2002.02172.x. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–18. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa Y, Mikami K, Mikamura Y, Ishii A, Takeuchi Y, Mori Y. The 3-second auditory conditioned stimulus is a more effective stressor than the 20-second auditory conditioned stimulus in male rats. Neuroscience. 2015;299:79–87. doi: 10.1016/j.neuroscience.2015.04.055. [DOI] [PubMed] [Google Scholar]
- Lonergan M, Saumier D, Tremblay J, Kieffer B, Brown TG, Brunet A. Reactivating addiction-related memories under propranolol to reduce craving: A pilot randomized controlled trial. J Behav Ther Exp Psychiatry. 2015;50:245–249. doi: 10.1016/j.jbtep.2015.09.012. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Ketoconazole blocks the stress-induced reinstatement of cocaine-seeking behavior in rats: Relationship to the discriminative stimulus effects of cocaine. Psychopharmacology (Berl) 1999;142:399–407. doi: 10.1007/s002130050905. [DOI] [PubMed] [Google Scholar]
- Maroun M, Akirav I. Arousal and stress effects on consolidation and reconsolidation of recognition memory. Neuropsychopharmacology. 2008;33:394–405. doi: 10.1038/sj.npp.1301401. [DOI] [PubMed] [Google Scholar]
- Meir Drexler S, Wolf OT. The role of glucocorticoids in emotional memory reconsolidation. Neurobiol Learn Mem. 2016 doi: 10.1016/j.nlm.2016.11.008. pii: S1074–7427(16)30315-X Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of nmda receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K. Reconsolidation and the dynamic nature of memory. Cold Spring Harb Perspect Biol. 2015;7(10):a021782. doi: 10.1101/cshperspect.a021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan SV, Griffith QK, McReynolds JR, Hahn EL, Roozendaal B. Basolateral amygdala interacts with other brain regions in regulating glucocorticoid effects on different memory functions. Ann N Y Acad Sci. 2004;1032:179–182. doi: 10.1196/annals.1314.015. [DOI] [PubMed] [Google Scholar]
- Otis JM, Dashew KB, Mueller D. Neurobiological dissociation of retrieval and reconsolidation of cocaine-associated memory. J Neurosci. 2013;33:1271–1281a. doi: 10.1523/JNEUROSCI.3463-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Wagner CK. Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol. 2007;504:42–56. doi: 10.1002/cne.21427. [DOI] [PubMed] [Google Scholar]
- Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:901–912. doi: 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM. Making memories of stressful events: A journey along epigenetic, gene transcription, and signaling pathways. Front Psychiatry. 2014;5:5. doi: 10.3389/fpsyt.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Niaura RS, Childress AR, Abrams DB, Monti PM. Cue reactivity in addictive behaviors: Theoretical and treatment implications. Int J Addict. 1990;25:957–993. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol Learn Mem. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, Van der Zee EA, Lee S, McGaugh JL, McIntyre CK. Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J Neurosci. 2009;29:14299–14308. doi: 10.1523/JNEUROSCI.3626-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Gray KM, McRae-Clark AL, Larowe SD, Yeatts SD, Baker NL, Hartwell KJ, Brady KT. A double blind, placebo-controlled study of the effects of post-retrieval propranolol on reconsolidation of memory for craving and cue reactivity in cocaine dependent humans. Psychopharmacology (Berl) 2013;226:721–737. doi: 10.1007/s00213-013-3039-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabdjitsingh RA, Kofink D, Karst H, de Kloet ER, Joels M. Stress-induced enhancement of mouse amygdalar synaptic plasticity depends on glucocorticoid and ss-adrenergic activity. PLoS One. 2012;7:e42143. doi: 10.1371/journal.pone.0042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggie JA, Brown GM. Stress response patterns of plasma corticosterone, prolactin, and growth hormone in the rat, following handling or exposure to novel environment. Can J Physiol Pharmacol. 1975;53:629–637. doi: 10.1139/y75-087. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: Studies on the generality of the effect. Psychopharmacology (Berl) 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- Sorg BA. Reconsolidation of drug memories. Neurosci Biobehav Rev. 2012;36:1400–1417. doi: 10.1016/j.neubiorev.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Todd RP, Slaker M, Churchill L. Anisomycin in the medial prefrontal cortex reduces reconsolidation of cocaine-associated memories in the rat self-administration model. Neuropharmacology. 2015;92:25–33. doi: 10.1016/j.neuropharm.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and deltafosb expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Stringfield SJ, Higginbotham JA, Fuchs RA. Requisite role of basolateral amygdala glucocorticoid receptor stimulation in drug context-induced cocaine-seeking behavior. Int J Neuropsychopharmacol. 2016;19(12) doi: 10.1093/ijnp/pyw073. pii: pyw073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svec F. Biopotency of corticosterone and dexamethasone in causing glucocorticoid receptor downregulation. J Steroid Biochem. 1985;23:669–671. doi: 10.1016/0022-4731(85)90020-2. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L. Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci. 2008;28:5602–5610. doi: 10.1523/JNEUROSCI.0750-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Arguello AA, Xie X, Blanton MA, Lasseter HC, Reittinger AM, Fuchs RA. Extracellular signal-regulated kinase in the basolateral amygdala, but not the nucleus accumbens core, is critical for context-response-cocaine memory reconsolidation in rats. Neuropsychopharmacology. 2013;38:753–762. doi: 10.1038/npp.2012.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichert S, Wolf OT, Schwabe L. Reactivation, interference, and reconsolidation: Are recent and remote memories likewise susceptible? Behav Neurosci. 2011;125:699–704. doi: 10.1037/a0025235. [DOI] [PubMed] [Google Scholar]
- Zhao LY, Zhang XL, Shi J, Epstein DH, Lu L. Psychosocial stress after reactivation of drug-related memory impairs later recall in abstinent heroin addicts. Psychopharmacology (Berl) 2009;203:599–608. doi: 10.1007/s00213-008-1406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.