Abstract
Chronic adrenal insufficiency (CAI) is characterized by a lack of glucocorticoid and mineralocorticoid production due to destroyed adrenal cortex cells. However, elevated cortisol secretion is thought to be a central part in a well-orchestrated immune response to stress. This raises the question to what extent lack of cortisol in CAI affects stress-related changes in immune processes.
To address this question, 28 CAI patients (20 females) and 18 healthy individuals (11 females) (age: 44.3 ± 8.4 years) were exposed to a psychosocial stress test (Trier Social Stress Test: TSST). Half the patients received a 0.03 mg/kg body weight injection of hydrocortisone (HC) post-TSST to mimic a healthy cortisol stress response. Catecholamines and immune cell composition were assessed in peripheral blood and free cortisol measured in saliva collected before and repeatedly after TSST.
CAI patients showed norepinephrine (NE) stress responses similar to healthy participants, however, epinephrine (E) as well as cortisol levels were significantly lower. HC treatment post-TSST resulted in cortisol increases comparable to those observed in healthy participants (interaction effects – NE: F = 1.05, p = .41; E: F = 2.56, p = .045; cortisol: F = 13.28, p < .001). Healthy individuals showed the expected pattern of stress-related early lymphocyte increase with subsequent decrease below baseline. The opposite pattern was observed in granulocytes. While exhibiting a similar initial increase, lymphocytes kept increasing over the following 2 h in untreated patients. HC treatment buffered this effect (interaction effects – lymphocyte%: F = 7.31, p < .001; granulocyte%: F = 7.71, p < .001).
Using CAI in humans as a model confirms cortisol’s central involvement in post-stress lymphocyte migration from blood into immune-relevant body compartments. As such, future studies should investigate whether psychosocial stress exposure may put CAI patients at an increased health risk due to attenuated immune responses to pathogens.
Keywords: Cell trafficking, Chronic adrenal insufficiency, Psychosocial stress, Cortisol, Catecholamines
1. Introduction
Elevated cortisol and catecholamine secretion are thought to be a central part in a well-orchestrated immune response to stress (Dhabhar et al., 2012). While studied extensively in animals, it is less clear to what extent the inability to mount a cortisol response affects stress-related changes in immune processes in humans. The current study proposes chronic adrenal insufficiency (CAI) as a model for assessing the role of cortisol in stress-related immune cell trafficking.
1.1. Stress affects immune cell distribution
Psychosocial stress is associated with increased activity of the hypothalamus–pituitary–adrenal (HPA) axis as well as the sympathetic nervous system (SNS) (Dickerson and Kemeny, 2004). The major end products of these systems are the hormones cortisol (HPA axis) and epinephrine and norepinephrine (SNS). Stress-induced release of these mediators has a wide range of effects on somatic systems, which generally are thought of as well-orchestrated and thus primarily protective (Sapolsky et al., 2000). One immune process of particular interest is the migration of immune cells across vascular endothelium (i.e., cell trafficking), for example, from blood or lymph fluid into tissue or a site of inflammation.
Stress interacts with this process on multiple levels. The fast release of catecholamines occurring within seconds after onset of a stressor increases the number of circulating natural killer (NK)-cells and granulocytes. This process is thought to ensure fast transportation of cells central for innate immunity to sites of tissue damage, thereby reducing the risk for infections (Dhabhar et al., 2012; Benschop et al., 1996; Sanders, 2006). Stress-induced changes in glucocorticoids induce a pronounced decrease in lymphocytes, indicating migration of lymphocytes out of blood and into immune compartments or sites of inflammation (Dhabhar et al., 2012). Importantly, cell trafficking is a clinically relevant immune function. For example, changes in immune cell composition patterns observed in response to the stress of undergoing surgery have been shown to predict speed of recovery post-surgery (Rosenberger et al., 2009).
1.2. Methodological considerations
To determine whether cortisol is sufficient or necessary for stress-related cell trafficking patterns, lack of hormone and subsequent hormone replacement is the approach of choice (Sapolsky et al., 2000). Adrenalectomy studies have been helpful in determining the extent to which a lack of cortisol response affects the stress–immune relationship. For example, adrenalectomized animals do not show a stress-induced decline in leukocytes compared to intact animals (Dhabhar and McEwen, 1999), while administering corticosterone to these animals restores the response (Dhabhar et al., 1995). However, adrenalectomy removes the entire adrenal gland, including the medulla. This not only prevents the production of glucocorticoids, but also the local production of catecholamines (Dhabhar and McEwen, 1999), making it difficult to tease apart the effects of a missing cortisol response from altered catecholamine responses. In addition to adrenalectomy studies, glucocorticoid synthesis inhibitors and receptor antagonists have been used to isolate the role of glucocorticoids in stress induced cell trafficking (Glover et al., 2011; Dhabhar et al., 1996). However, these approaches acutely remove glucocorticoids from the system and thus do not separate the impact of missing cortisol responsivity from effects of missing basal glucocorticoid levels. Furthermore, they do not allow for investigation of more health-relevant long-term adaptations to a chronic lack of glucocorticoid increases in response to physical and psychosocial stress.
1.3. The current study: Chronic adrenal insufficiency
The current study aimed at finding a model for studying the role of cortisol in stress-related immune cell trafficking that addresses some of the methodological gaps from alternative approaches outlined above. Investigating patients suffering from chronic adrenal insufficiency (CAI) is proposed as a promising approach.
Patients with CAI do not produce any cortisol due to destroyed adrenal cortex cells and thus receive glucocorticoid (and mineralocorticoid) replacement (Ten et al., 2001; Betterle et al., 2002; Oelkers, 1996; Arlt and Allolio, 2003). This therapy only substitutes basal cortisol levels, while no provisions are made for additional doses during stress. As a result, CAI patients are specifically lacking the ability to mount a cortisol stress response. The first aim of the current study was therefore to confirm the supposed lack of endocrine stress responses in patients with CAI. The second aim was to investigate the effects of missing cortisol stress responses on immune cell trafficking. To distinguish between sufficient and necessary effects of cortisol, half of the CAI patients were treated with 0.03 mg/kg hydrocortisone i.v. to pharmacologically mimic cortisol stress responses.
2. Methods
2.1. Participants
A total of 36 patients with CAI and 21 age- and gender-matched healthy participants (HP) were investigated. We excluded five participants for missing one or more blood samples (4 CAI patients), two for missing saliva samples (one CAI patient), three for being under-age (two CAI patients), and one patient for abnormally high norepinephrine levels at baseline (>4 SD) and throughout the study protocol (2–3 SD above the mean). Hence, the final sample consisted of 28 individuals with CAI (20 females) and 18 controls (11 females) with a mean age of 44.33 years (SD = 8.36 years). Based on presence of autoantibodies (adrenocortical autoantibodies, steroid cell antibodies) (Betterle et al., 2002; Peterson et al., 2000) or co-morbidities fulfilling criteria for classification to Autoimmune Polyglandular Syndrome (APS) type 1 or type 2 (Neufeld et al., 1981), 19 patients were diagnosed with autoimmune CAI (67.9%). In four patients, the cause for CAI was former Cushing’s disease and five patients did not provide sufficiently detailed information for differential diagnosis.
Half of the CAI patients were randomly assigned to receive 0.03 mg/kg hydrocortisone (HC) i.v. (Sigma, Berlin) after a psychosocial stress test (CAI-HC: n = 14), while the remaining n = 14 patients as well as healthy participants (HP) received an injection of 4 mL saline (NaCl). The CAI-HC and CAI-NaCl groups each consisted of 10 females and 4 males and the gender make-up of the three groups did not significantly differ (χ2 = .53, p = .77). The three groups also did not differ in age (F2,43 = .36, p = .70; CAI-HC: mean = 43.81, SD = 8.5; CAI-NaCl: mean = 44.87, SD = 7.9; HP-NaCl: mean = 45.06, SD = 9.8) or body mass index (BMI: F2,43 = 1.31, p = .28; CAI-HC: mean = 23.89, SD = 4.3; CAI-NaCl: mean = 23.98, SD = 2.6; HP-NaCl: mean = 25.40, SD = 4.6). Lastly, the two patient groups did not differ in etiology of CAI (χ2 = 4.16, p = .25) nor the number of years with the disease (CAI-HC: mean = 7.42 years, SD = 7.22; CAI-NaCl: mean = 10.14 - years, SD = 9.90; χ2 = 19.29, p = .31). The ethics committee of the University of Düsseldorf approved the study protocol.
2.2. Procedures
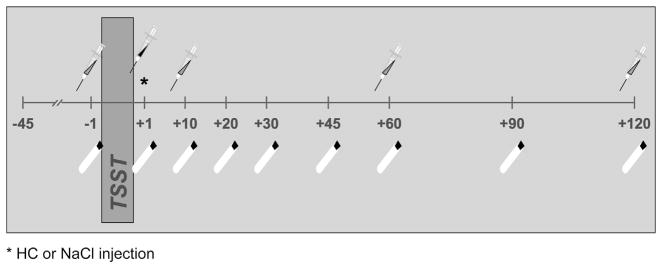
Patients and healthy participants were recruited from across Germany (travel distance: 287 ± 157 km; t = 1.69, p = .20), with patients being referred by the study’s endocrinologist (J.F.). Participants arrived in the laboratory at 1 PM and were examined for past or current health problems by the study’s physician. CAI participants were asked to postpone their second glucocorticoid replacement dose usually taken around 2 PM (mean = 13:49 h, SD = 1h 57 min) to avoid cortisol levels pre-stressor being significantly higher than in healthy participants. After obtaining written consent, a catheter was inserted into participants’ preferred arm. After a 45-min adjustment period allowing stress from catheter insertion to subside, a first blood (2.7 mL and 9 mL EDTA Monovettes, Sarstedt, Nümbrecht, Germany) and saliva (Salivette, Sarstedt, Nümbrecht, Germany) sample was collected. Subsequently, participants were exposed to the Trier Social Stress Test (TSST). Shortly after TSST exposure, participants received either a hydrocortisone or placebo injection in a double-blind design. Additional blood and saliva samples were collected 1, 10, 20, 30, 45, 60, 90, and 120 min after stress exposure (see Fig. 1).
Fig. 1.
Study protocol diagram indicating blood and saliva collection times relative to TSST exposure. *HC or NaCl injection.
2.3. Manipulations
2.3.1. Pharmacological manipulation
To mimic an acute cortisol stress response, half of the CAI patients received a bolus injection of 0.03 mg/kg body weight hydrocortisone via catheter. This dose was found to successfully increase cortisol levels by roughly 15 nmol/L in a pilot study assessing the effects of various doses of hydrocortisone in healthy adults with a wide range in body types. Healthy participants and the remaining patients received a 4 mL saline (NaCl) i.v. bolus injection.
2.3.2. Trier Social Stress Test (TSST)
The TSST is a widely used acute laboratory stress test with a strong social-evaluative component. It consists of a three-minute preparation period followed by a five-minute speech task and a five-minute mental arithmetic task in front of a two-person panel. It has been shown to reliably elicit both catecholamine and cortisol responses. For more details, see Kirschbaum et al. (1993).
2.4. Biochemical assays
2.4.1. Salivary cortisol
Saliva samples were stored at −30 °C until analysis. Upon completion of the study, samples were thawed, centrifuged, and free cortisol levels in saliva were measured using a commercially available chemiluminescence assay (IBL, Hamburg, Germany). Samples were measured in duplicates and averaged for subsequent statistical analyses. Inter- and intra-assay CVs were below 8%.
2.4.2. Plasma catecholamines
Immediately after collection, plasma was separated at 4 °C, 1600×g and stored at −80 °C. Plasma concentrations of norepinephrine and epinephrine were determined by high-performance liquid chromatography with electrochemical detection (Smedes et al., 1982). Epinephrine levels under detection limit were labeled 5 pg/mL representing half of the lowest standard.
2.4.3. Immune cell composition
Differential blood counts were performed on blood samples collected pre-TSST as well as 10, 60, and 120 min post-TSST. Specifically, after 45 min of incubation to allow for EDTA sample equilibration, each sample was measured five times using an AcT Diff Cell counter (Beckman-Coulter, Krefeld, Germany) and average numbers as well as average percentages of monocytes, lymphocytes, and granulocytes were computed for each sample.
2.5. Statistical analyses
All analyses controlled for age and sex. To assess baseline differences in cortisol, catecholamines, and cell counts and percentages, ANCOVAs were computed. Repeated-measures ANCOVAs were used to evaluate changes in the same measures in response to the TSST. Where indicated, Greenhouse–Geisser corrected values are given. Change scores were computed to capture responses to stress as well as post-stress recovery. More specifically, cortisol and norepinephrine increase indices were computed by subtracting the first sample (pre-TSST baseline) from the peak value (individual maximum of the sample taken immediately following the TSST, 10 min, or 20 min post-TSST). Decrease indices were calculated by subtracting individual minimum value (from sample taken 30, 45, 60, 90, or 120 min post-TSST) from the peak value. Cell number and cell percentage response indices were computed by subtracting values at time 1 (pre-stress baseline) from values at time 2 (10 min post-stress) for each type of cell (lymphocytes, granulocytes, and monocytes). Recovery following stress was computed by subtracting values at time 2 from values at time 3 (60 min post-stress). As for the two-phased endocrine responses, the labels “response” and “recovery” are used to distinguish initial baseline to post-TSST changes from subsequent changes in cell numbers or percentages. Depending on the cell type, both response and recovery can be a positive change (increase) or negative change (decrease). Partial correlations controlling for age and sex were computed between the above indices to test for relationships between endocrine and immune responses.
3. Results
3.1. Baseline group comparisons
Baseline cortisol levels were significantly lower in CAI patients than healthy participants (F1,42 = 11.00, p = .002). While baseline norepinephrine levels were comparable between groups (F1,42 = 2.06, p = .16), epinephrine levels in CAI patients were either significantly lower than those observed in healthy participants or below detection limit (F1,42 = 20.02, p < .001). Lastly, patients and healthy participants did not differ in terms of baseline lymphocyte, granulocyte, or monocyte cell counts or percentages (all p > .37).
3.2. Stress responses
3.2.1. Cortisol and catecholamine responses to stress
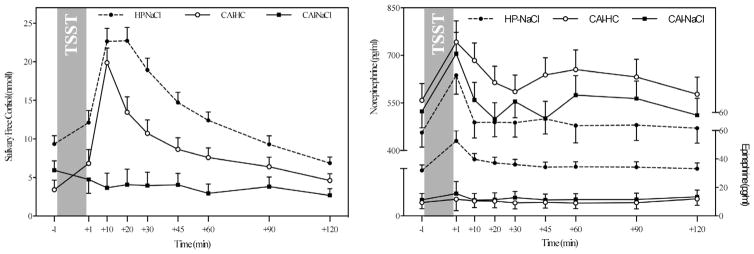
Significant group differences in cortisol responses (F = 13.28, p < .001; see Table 1 for more details) indicated that untreated CAI participants did not show an increase in cortisol following stress exposure, while cortisol concentrations in healthy participants and CAI patients treated with hydrocortisone peaked 10–20 min post-TSST and subsequently decreased to baseline levels (see Fig. 2, left). Contrarily, epinephrine increased in response to stress in healthy participants only, while both patient groups had very low levels of epinephrine throughout the study (F = 2.56, p = .045; see Fig. 2, right). No significant differences in norepinephrine stress responses between the three groups were observed (p = .41).
Table 1.
Repeated-measures ANCOVA results assessing changes in cortisol, catecholamines, cell numbers, and cell percentages in response to stress.
| df | F | P | Partial η2 | ||
|---|---|---|---|---|---|
| Cortisol | Group | 2, 41 | 19.57 | <.001 | .49 |
| Time | 3.34, 136.74 | 1.57 | .20 | .04 | |
| Group-by-time | 6.67, 136.74 | 13.28 | <.001 | .39 | |
| Norepinephrine | Group | 2, 41 | 1.92 | .16 | .09 |
| Time | 5.12, 210.42 | .29 | .92 | .01 | |
| Group-by-time | 10.26, 210.42 | 1.05 | .41 | .05 | |
| Epinephrine | Group | 2, 41 | 13.67 | <.001 | .40 |
| Time | 1.96, 80.20 | .52 | .59 | .01 | |
| Group-by-time | 3.91, 80.20 | 2.56 | .045 | .11 | |
| LY# | Group | 2, 41 | 1.17 | .32 | .05 |
| Time | 2.08, 85.41 | .49 | .62 | .01 | |
| Group-by-time | 4.17, 85.41 | 5.17 | .001 | .20 | |
| GR# | Group | 2, 41 | 3.09 | .056 | .08 |
| Time | 1.60, 65.59 | 3.49 | .046 | .13 | |
| Group-by-time | 3.20, 65.59 | 3.31 | .047 | .14 | |
| MO# | Group | 2, 41 | .34 | .72 | .02 |
| Time | 2.33, 95.56 | .30 | .78 | .01 | |
| Group-by-time | 4.66, 95.56 | .35 | .87 | .02 | |
| LY% | Group | 2, 41 | 1.76 | .19 | .08 |
| Time | 1.75, 71.55 | .94 | .39 | .02 | |
| Group-by-time | 3.50, 71.55 | 7.31 | <.001 | .26 | |
| GR% | Group | 2, 41 | 1.75 | .19 | .08 |
| Time | 1.73, 70.73 | 1.69 | .20 | .04 | |
| Group-by-time | 3.45, 70.73 | 7.71 | <.001 | .27 | |
| MO% | Group | 2, 41 | .20 | .82 | .01 |
| Time | 2.42, 99.13 | 1.45 | .24 | .03 | |
| Group-by-time | 4.84, 99.13 | .30 | .91 | .02 |
Note: LY = Lymphocytes; GR = Granulocytes; MO = Monocytes; # = Number; % = Percentage.
Fig. 2.
Comparisons of cortisol and catecholamine stress responses across the three study groups. Note: HP-NaCl = placebo-treated healthy participants; CAI-HC = CAI patients treated with hydrocortisone; CAI-NaCl = placebo-treated CAI patients.
3.2.2. Cell trafficking in response to stress
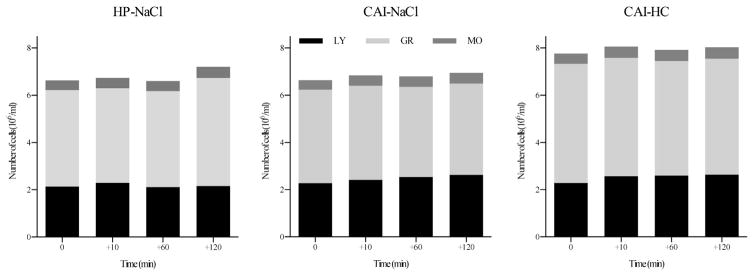
Group comparisons of cell count changes in response to stress are depicted in Fig. 3 and statistical values are listed in Table 1. Assessing the numbers of immune cell subtypes present in peripheral blood revealed that all participants showed comparable increases in lymphocyte numbers in response to stress, however, placebo-treated CAI patients exhibited lower numbers of lymphocytes overall (F = 5.17, p = .001). While overall granulocyte numbers were higher in HC-treated CAI, only healthy participants showed an increase in granulocyte numbers 2 h after stress (F = 3.31, p = .047). Monocyte cell counts were not significantly different between groups or over time (all p > .71).
Fig. 3.
Changes in cell counts in response to stress for each of the study groups. Note: HP-NaCl = placebo-treated healthy participants; CAI-HC = CAI patients treated with hydrocortisone; CAI-NaCl = placebo-treated CAI patients.
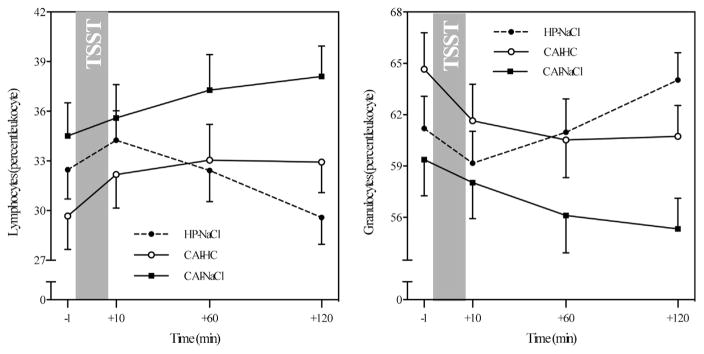
In terms of immune cell composition in peripheral blood, the three participants groups showed significantly different response patterns in lymphocyte percentages (F = 7.31, p < .001). Specifically, all groups showed an initial increase in response to the stressor. After a peak 10 min post-stressor, percentage of lymphocytes declined in healthy participants (see Fig. 4, left). Contrarily, lymphocyte percentages continued to increase over the following 2 h in placebo-treated CAI patients, while the percentage of lymphocytes in HC-treated CAI patients leveled out. Granulocyte percentages showed the opposite patterns, such that subsequent to an initial decrease observed in all participants, percentage of granulocytes increased after stress in healthy participants, leveled off in HC-treated CAI patients, and continued to decrease in placebo-treated CAI patients (F = 7.71, p < .001; see Fig. 4, right). Monocyte proportion did neither change in response to stress nor did they differ between groups (all p > .23).
Fig. 4.
Group comparisons of stress-related changes in lymphocyte percentages (left) and granulocyte percentages (right). Note: HP-NaCl = placebo-treated healthy participants; CAI-HC = CAI patients treated with hydrocortisone; CAI-NaCl = placebo-treated CAI patients.
3.3. Endocrine-immune associations
Results of partial correlations (controlling for age and sex) between endocrine change indices and cell number and percentage change indices are summarized in Table 2. Lymphocyte% recovery was negatively correlated with both the magnitude of the cortisol increase following stress (r = −.40, p = .007) and the steepness of the subsequent slope (r = −.42, p = .004), such that a stronger cortisol stress response and slower cortisol clearance were linked to faster migration of lymphocytes back into tissue. Associations between cortisol indices and granulocyte percentage recovery showed the opposite pattern, with granulocyte percentage recovery being positively correlated with both cortisol indices, indicating stronger cortisol stress responses and slower cortisol clearance being linked to a larger proportion of granulocytes being present in peripheral blood 2 h after stress (r = .40, p = .007; r = .45, p = .002, respectively). For norepinephrine, neither increase nor recovery was associated with change indices in immune cell subtype compositions (all p > .40).
Table 2.
Partial correlations between response and recovery indices for cortisol, norepinephrine, cell numbers and cell percentages.
| Diff | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Cortisol increase | 1 | – | .93** | .09 | .08 | .09 | −.40* | −.12 | .40* | .05 | −.12 |
| 2. | Cortisol decrease | 1 | .93** | – | .02 | −.01 | .14 | −.42* | −.11 | .45* | −.08 | −.18 |
| 3. | NE increase | 3 | .09 | .02 | – | .66** | .13 | −.01 | −.12 | −.01 | −.04 | .09 |
| 4. | NE decrease | 3 | .08 | −.01 | .66** | – | .10 | .11 | −.09 | −.11 | −.03 | .02 |
| 5. | LY% response | 3 | .09 | .14 | .13 | .10 | – | .14 | −.91** | −.21 | −.28^ | .19 |
| 6. | LY% recovery | 2 | −.40* | −.42* | −.01 | .11 | .14 | – | −.15 | −.94** | −.002 | .03 |
| 7. | GR% response | 3 | −.12 | −.11 | −.12 | −.09 | −.91** | −.15 | – | .17 | −.15 | −.08 |
| 8. | GR% recovery | 2 | .40* | .45* | −.01 | −.11 | −.21 | −.94** | .17 | – | .09 | −.35* |
| 9. | MO% response | 3 | .05 | −.08 | −.04 | −.03 | −.28^ | −.002 | −.15 | .09 | – | −.27^ |
| 10. | MO% recovery | 3 | −.12 | −.18 | .09 | .02 | .19 | .03 | −.08 | −.35* | −.27^ | – |
Note: NE = Norepinephrine; Diff: MANOVA post-hoc Tukey test: 1: (HP-NaCl = CAI-HC) ≠ CAI-NaCl; 2: HP-NaCl ≠ (CAI-HC = CAI-NaCl); 3: HP-NaCl = CAI-HC = CAI-NaCl.
p < .05.
p < .001.
p < .10.
Exploratory analyses confirmed that controlling for disease etiology (e.g., autoimmune CAI, Cushing’s disease) and disease duration did not change the pattern of findings within patients. The same was true for therapy-related variables, such as daily replacement dosage, medication type (hydrocortisone versus cortisone acetate), or medication regimen (number of doses per day).
4. Discussion
The current study utilized CAI as a model for studying the role of cortisol in stress-related immune cell trafficking. As expected, CAI patients demonstrated a lack of cortisol as well as epinephrine increases in response to stress exposure. In terms of immune cell composition changes, untreated CAI patients showed an initial increase in lymphocytes similar to healthy participants, while lymphocytes kept increasing over the following 2 h. The opposite pattern was observed in granulocytes. Treatment with 0.03 mg/kg hydrocortisone resulted in cortisol increases comparable to those observed in healthy participants. Interestingly, hydrocortisone treatment further buffered the CAI-related immune effects. Regardless of treatment, all participants responded to stress exposure with significant increases in norepinephrine, while epinephrine levels were very low in both groups of CAI participants. Lastly, strong associations in the expected directions between cortisol responses and subsequent clearance rates and lymphocyte and granulocyte recoveries were observed.
4.1. Stress responses in healthy participants
Similar to previous reports, the TSST was successful in inducing increases in catecholamines as well as salivary free cortisol in healthy participants in the current study (Dickerson and Kemeny, 2004). Furthermore, stress-related changes in lymphocyte and granulocyte proportions in peripheral blood are in line with previous findings linking endocrine stress responses to peripheral immune cell subtype composition changes (Dhabhar et al., 1995, 2012; Benschop et al., 1996; Dhabhar and McEwen, 1999; Schedlowski et al., 1996).
Specifically, acute time-limited increases in catecholamines have been shown to mobilize lymphocytes, specifically natural killer (NK) cells, and granulocytes from depots (Dhabhar et al., 2012; Benschop et al., 1996; Schedlowski et al., 1996). The modulation of NK-cell trafficking thereby appears to be beta2-adrenergic receptor (AR)-dependent; whereas the transient delayed increase in granulocytes involves alpha-AR stimulation (Benschop et al., 1996). In terms of glucocorticoid effects on immune cell trafficking, previous research has demonstrated that stress-induced changes in glucocorticoids as well as pharmacological glucocorticoid application induce a pronounced decrease in lymphocyte, monocyte, and eosinophil numbers in the blood (Fauci and Dale, 1974). These effects are interpreted as cells migrating out of circulation into other body compartments as a result of glucocorticoid receptor-mediated (Dhabhar et al., 1996) alterations in cell adhesion molecules (Cronstein et al., 1992; Pitzalis et al., 2002). Together, stress-induced increases in catecholamines and cortisol may thus help reducing the risk for infections by first mobilizing cells of innate immunity, as indicated by increased numbers of circulating lymphocytes and granulocytes (Benschop et al., 1996), followed by cortisol-mediated migration of cells back into immune compartments or sites of inflammation (Dhabhar et al., 2012; Dhabhar and McEwen, 1999).
4.2. Stress responses in CAI patients
To confirm the involvement of cortisol in the psychosocial stress-induced cell distribution effects, CAI patients were exposed to the same study protocol. As expected, patients with CAI were not able to elicit a cortisol stress response, confirming reports from studies investigating the effects of adrenalectomy on corticosteroid stress response (Desser-Wiest, 1976). Furthermore, the present findings regarding normal to slightly elevated norepinephrine levels in combination with very low epinephrine levels confirm earlier observations in patients with CAI (Bornstein et al., 1995), experimental animals with reduced glucocorticoid synthesis (Wurtman, 2002), and adrenalectomy (Eisenhofer et al., 1995; Merke et al., 2000). One study additionally included stress stimulation in patients with isolated glucocorticoid deficiency (Zuckerman-Levin et al., 2001) and found a minimal stress response in epinephrine and slightly increased norepinephrine responses compared to healthy subjects. Based on the data outlined above and on findings of decreased norepinephrine in 21-hydroxylase deficient mice (Bornstein et al., 1999), the elevated norepinephrine levels observed in CAI have been repeatedly suggested to be an intra-adrenal effect. Specifically, they point towards the role of glucocorticoids in normal functioning of chromaffin cells and their capacity at the transcriptional level to express phenylethanolamine N-methyltransferase (PNMT), the enzyme that converts norepinephrine to epinephrine (Wong et al., 1995). In other words, lack of cortisol within adrenal tissue results in reduced PNMT levels, and consequently, less conversion from norepinephrine to epinephrine.
These findings show for the first time the dysregulated endocrine response pattern to psychosocial stress expected in patients without endogenous cortisol production. These endocrine response dysregulations were accompanied by distinct stress response patterns in the peripheral composition of immune cell subsets. Specifically, CAI patients showed decreases in lymphocytes accompanied by increases in granulocytes in the first hour following stress, similar to healthy control participants. While the cell subtype composition changed back to pre-stress ratios in healthy participants over the subsequent hour (i.e., from 1 to 2 h post-TSST), lymphocyte percentages continued to decrease and granulocyte percentages continued to increase in CAI patients. These patterns suggest that cortisol is not directly involved in the early processes, but that particularly NK-cell mobilization from depots are mainly catecholamine-mediated (Dhabhar et al., 2012; Benschop et al., 1996; Schedlowski et al., 1996). It also implies that norepinephrine is able to make up for the missing epinephrine effects, as NK-cell trafficking is mainly mediated by activation of an adrenergic receptor with high affinity for epinephrine (i.e., beta2-AR). Conversely, cortisol appears to be involved in the later cell distribution effects described above for healthy participants (Dhabhar et al., 1996; Fauci and Dale, 1974), such that a lack of cortisol stress responses was accompanied by a lack of redistribution of lymphocytes and granulocytes. CAI patients did not show the late increase in granulocytes observed in healthy participants. This is surprising given that this effect is thought to be mainly mediated by alpha-AR activation (Kavelaars, 2002), i.e., an adrenergic receptor subtype with high affinity for norepinephrine (Kvetnansky et al., 1993). However, cortisol has been suggested to be involved in this late granulocyte distribution effect as well (Fauci and Dale, 1974), an observation supported by the present findings.
4.3. Stress responses in hydrocortisone-treated CAI patients
To determine to what extent cortisol is not only involved but necessary for the observed stress-related changes in cell composition, CAI patients were treated with 0.03 mg/kg hydrocortisone. This treatment was successful in producing cortisol increases comparable to cortisol stress responses seen in healthy participants. Furthermore, while lymphocytes continued to increase over the entire study period in untreated CAI patients, lymphocytes in hydrocortisone-treated CAI leveled out after 1 h. Thus, although still statistically different from a healthy pattern, hydrocortisone treatment appeared to buffer CAI patients to some extent from a prolonged increase in lymphocytes and as such, partially restored stress effects on cell distribution.
One reason why hydrocortisone treatment did not fully restore the healthy cell trafficking pattern may be the dose of 0.03 mg/kg being injected as a bolus. Although this treatment resulted in salivary free cortisol levels comparable to those seen in healthy participants immediately after stress, hydrocortisone-treated patients showed faster clearance of cortisol levels thereafter. Thus, cortisol effects may have been terminated earlier in patients than in healthy participants. Shorter cortisol exposure could then, in turn, also explain the lack of late granulocyte increases. Similarly, it has been suggested that cortisol must be present at high doses within the adrenals to restore the PNMT-dependent effect on norepinephrine conversion to epinephrine (Wong et al., 1995). Consequently, increasing cortisol levels acutely and systemically was insufficient to restore a healthy catecholamine balance. Furthermore, patients in the current study took a total of 24.77 mg hydrocortisone or 41.67 mg cortisone acetate per day in two to three doses. Being exposed to such high concentrations of cortisol several times a day may alter the sensitivity of lymphocytes to glucocorticoid regulation of cell adhesion molecule expression, presenting an alternative explanation for the partial effect of hydrocortisone treatment.
Our findings in hydrocortisone-treated patients as well as the strong associations between cortisol and cell percentage changes confirm a crucial role of cortisol in stress-induced changes in peripheral cell composition. As such, the current findings raise the question whether CAI patients would benefit from additional doses of hydrocortisone during times of stress. However, besides differences in pharmacological dynamics of injected versus orally taken hydrocortisone, increasing evidence points to the fact that patients generally tend to be over-treated (Lovas and Husebye, 2003; Peacey et al., 1997; Howlett, 1997). Chronic over-treatment, in turn, has repeatedly been linked to increased risks of impaired glucose tolerance, obesity, and osteoporosis (al-Shoumer et al., 1995; Zelissen et al., 1994; Florkowski et al., 1994). Hence, benefits of adding glucocorticoid doses during times of stress have to be weighted against a patient’s individual glucocorticoid replacement regimen in order to avoid increasing the risk for over-treatment related morbidities.
4.4. Limitations
The current study has several limitations. First and foremost, the group sizes were relatively small; however, the manipulations appeared to be strong enough to produce all predicted effects, reducing the concerns of potentially missing smaller effects. Furthermore, hydrocortisone and placebo treatment was randomly assigned to patients. Nevertheless, hydrocortisone-treated patients showed overall higher peripheral leukocyte numbers, mainly due to elevated granulocytes. This elevated state of immune activation may be linked to the fact that although not statistically different, the hydrocortisone-treated group consisted of almost twice as many patients with an autoimmune disease etiology than the placebo-treated patient group. Although controlling for CAI subtype also did not change the findings presented, future studies may benefit from a more in-depth investigation of CAI etiology on immune cell trafficking.
4.5. Summary and outlook
Chronic adrenal insufficiency provides a human model for investigating the effects of permanently and context-independently altered stress response patterns on bodily systems. Studying such effects in patients with CAI may provide valuable insights into the interplay of endocrine stress systems in the human whole organism and the extent to which it is able to compensate for dysregulations in these systems. This knowledge may help to further our understanding of how altered stress response patterns are associated with physiological diseases and psychological disorders.
In terms of effects of stress systems on immune functions, missing lymphocyte migration out of blood and into other immune compartments, as found in patients with CAI, appeared to be mediated by missing cortisol increases to psychosocial stress. As such, psychosocial stress exposure may put CAI patients as well as other patient groups with reduced glucocorticoid production or sensitivity at an increased health risk due to an attenuated ability of the immune system to mount a timely response to pathogens.
Acknowledgments
This work was supported by the German Research Foundation (DFG; KI 537/18; C.K.), by an NIGMS “Brain–Body–Behavior Interface in Learning and Development Across the Lifespan” training grant T32GM084907 (A.M.G.), and by a Department of Defense SMART fellowship (K.P.P.).
Footnotes
Conflict of interest
The authors declare no conflict of interests.
References
- al-Shoumer KA, Beshyah SA, Niththyananthan R, Johnston DG. Effect of glucocorticoid replacement therapy on glucose tolerance and intermediary metabolites in hypopituitary adults. Clin Endocrinol (Oxf) 1995;42(1):85–90. doi: 10.1111/j.1365-2265.1995.tb02602.x. [DOI] [PubMed] [Google Scholar]
- Arlt W, Allolio B. Adrenal insufficiency. Lancet. 2003;361(9372):1881–1893. doi: 10.1016/S0140-6736(03)13492-7. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10(2):77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23(3):327–364. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Breidert M, Ehrhart-Bornstein M, Kloos B, Scherbaum WA. Plasma catecholamines in patients with Addison’s disease. Clin Endocrinol (Oxf) 1995;42(2):215–218. doi: 10.1111/j.1365-2265.1995.tb01866.x. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Tajima T, Eisenhofer G, Haidan A, Aguilera G. Adrenomedullary function is severely impaired in 21-hydroxylase-deficient mice. FASEB J. 1999;13(10):1185–1194. doi: 10.1096/fasebj.13.10.1185. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial–leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1992;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desser-Wiest L. Corticosterone in serum of adrenalectomized male rats. Osterr Z Onkol. 1976;3(3):70–72. [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci USA. 1999;96(3):1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Effects of stress on immune cell distribution. Dynamics and hormonal mechanisms. J Immunol. 1995;154(10):5511–5527. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol. 1996;157(4):1638–1644. [PubMed] [Google Scholar]
- Dhabhar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells – from barracks to boulevards to battlefields: a tale of three hormones – Curt Richter Award winner. Psychoneuroendocrinology. 2012;37(9):1345–1368. doi: 10.1016/j.psyneuen.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Friberg P, Pacak K, Goldstein DS, Murphy DL, Tsigos C, et al. Plasma metadrenalines: do they provide useful information about sympatho-adrenal function and catecholamine metabolism? Clin Sci (Lond) 1995;88(5):533–542. doi: 10.1042/cs0880533. [DOI] [PubMed] [Google Scholar]
- Fauci AS, Dale DC. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florkowski CM, Holmes SJ, Elliot JR, Donald RA, Espiner EA. Bone mineral density is reduced in female but not male subjects with Addison’s disease. N Z Med J. 1994;107(972):52–53. [PubMed] [Google Scholar]
- Glover M, Cheng B, Deng X, Pruett S. The role of glucocorticoids in the immediate vs. delayed effects of acute ethanol exposure on cytokine production in a binge drinking model. Int Immunopharmacol. 2011;11(6):755–761. doi: 10.1016/j.intimp.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett TA. An assessment of optimal hydrocortisone replacement therapy. Clin Endocrinol (Oxf) 1997;46(3):263–268. doi: 10.1046/j.1365-2265.1997.1340955.x. [DOI] [PubMed] [Google Scholar]
- Kavelaars A. Regulated expression of alpha-1 adrenergic receptors in the immune system. Brain Behav Immun. 2002;16(6):799–807. doi: 10.1016/s0889-1591(02)00033-8. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Fukuhara K, Pacak K, Cizza G, Goldstein DS, Kopin IJ. Endogenous glucocorticoids restrain catecholamine synthesis and release at rest and during immobilization stress in rats. Endocrinology. 1993;133(3):1411– 1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]
- Lovas K, Husebye ES. Replacement therapy in Addison’s disease. Expert Opin Pharmacother. 2003;4(12):2145–2149. doi: 10.1517/14656566.4.12.2145. [DOI] [PubMed] [Google Scholar]
- Merke DP, Chrousos GP, Eisenhofer G, Weise M, Keil MF, Rogol AD, et al. Adrenomedullary dysplasia and hypofunction in patients with classic 21-hydroxylase deficiency. N Engl J Med. 2000;343(19):1362–1368. doi: 10.1056/NEJM200011093431903. [DOI] [PubMed] [Google Scholar]
- Neufeld M, Maclaren NK, Blizzard RM. Two types of autoimmune Addison’s disease associated with different polyglandular autoimmune (PGA) syndromes. Medicine (Baltimore) 1981;60(5):355–362. doi: 10.1097/00005792-198109000-00003. [DOI] [PubMed] [Google Scholar]
- Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335(16):1206–1212. doi: 10.1056/NEJM199610173351607. [DOI] [PubMed] [Google Scholar]
- Peacey SR, Guo CY, Robinson AM, Price A, Giles MA, Eastell R, et al. Glucocorticoid replacement therapy: are patients over treated and does it matter? Clin Endocrinol (Oxf) 1997;46(3):255–261. doi: 10.1046/j.1365-2265.1997.780907.x. [DOI] [PubMed] [Google Scholar]
- Peterson P, Uibo R, Krohn KJ. Adrenal autoimmunity: results and developments. Trends Endocrinol Metab. 2000;11(7):285–290. doi: 10.1016/s1043-2760(00)00283-6. [DOI] [PubMed] [Google Scholar]
- Pitzalis C, Pipitone N, Perretti M. Regulation of leukocyte–endothelial interactions by glucocorticoids. Ann NY Acad Sci. 2002;966:108–118. doi: 10.1111/j.1749-6632.2002.tb04208.x. [DOI] [PubMed] [Google Scholar]
- Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP, et al. Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. A prospective study. J Bone Joint Surg Am. 2009;91(12):2783–2794. doi: 10.2106/JBJS.H.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VM. Interdisciplinary research: noradrenergic regulation of adaptive immunity. Brain Behav Immun. 2006;20(1):1–8. doi: 10.1016/j.bbi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schedlowski M, Hosch W, Oberbeck R, Benschop RJ, Jacobs R, Raab HR, et al. Catecholamines modulate human NK cell circulation and function via spleen-independent beta 2-adrenergic mechanisms. J Immunol. 1996;156(1):93–99. [PubMed] [Google Scholar]
- Smedes F, Kraak JC, Poppe H. Simple and fast solvent extraction system for selective and quantitative isolation of adrenaline, noradrenaline and dopamine from plasma and urine. J Chromatogr. 1982;231(1):25–39. doi: 10.1016/s0378-4347(00)80506-x. [DOI] [PubMed] [Google Scholar]
- Ten S, New M, Maclaren N. Clinical review 130: Addison’s disease 2001. J Clin Endocrinol Metab. 2001;86(7):2909–2922. doi: 10.1210/jcem.86.7.7636. [DOI] [PubMed] [Google Scholar]
- Wong DL, Siddall B, Wang W. Hormonal control of rat adrenal phenylethanolamine N-methyltransferase. Enzyme activity, the final critical pathway. Neuropsychopharmacology. 1995;13(3):223–234. doi: 10.1016/0893-133X(95)00066-M. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ. Stress and the adrenocortical control of epinephrine synthesis. Metabolism. 2002;51(6 Suppl 1):11–14. doi: 10.1053/meta.2002.33185. [DOI] [PubMed] [Google Scholar]
- Zelissen PM, Croughs RJ, van Rijk PP, Raymakers JA. Effect of glucocorticoid replacement therapy on bone mineral density in patients with Addison disease. Ann Intern Med. 1994;120(3):207–210. doi: 10.7326/0003-4819-120-3-199402010-00005. [DOI] [PubMed] [Google Scholar]
- Zuckerman-Levin N, Tiosano D, Eisenhofer G, Bornstein S, Hochberg Z. The importance of adrenocortical glucocorticoids for adrenomedullary and physiological response to stress: a study in isolated glucocorticoid deficiency. J Clin Endocrinol Metab. 2001;86(12):5920–5924. doi: 10.1210/jcem.86.12.8106. [DOI] [PubMed] [Google Scholar]