Abstract
Despite significant efforts to reduce preventable adverse events in medical processes, such events continue to occur at unacceptable rates. This paper describes a computer science approach that uses formal process modeling to provide situationally aware monitoring and management support to medical professionals performing complex processes. These process models represent both normative and non-normative situations, and are validated by rigorous automated techniques such as model checking and fault tree analysis, in addition to careful review by experts. Context-aware Smart Checklists are then generated from the models, providing cognitive support during high-consequence surgical episodes. The approach is illustrated with a case study in cardiovascular surgery.
Keywords: process modeling, checklists, situation management
I. Introduction
Health systems are struggling to find new approaches to reduce treatment variability and iatrogenic harm. More than 234 million surgical procedures are performed worldwide each year and the operating room (OR) is the most common site for adverse events in the hospital [4]. There is widespread consensus that overall surgical quality, reflected by measures of clinical outcomes and patient safety, needs to improve. However, despite the introduction of mandatory pre-operative safety checklists, preventable adverse events in surgery continue to occur at a rate between 3% and 17% [5]. Cardiovascular Surgery (CVS) is a high-consequence surgical subspecialty that relies on clear communication and timely coordination to achieve high-quality clinical outcomes but remains intrinsically error-prone. Unfortunately, communication-related hazards are still prevalent in the CVS OR, resulting in widespread and substantial risk to patient safety. High cognitive and physical workloads required during life-critical maneuvers can impact the surgical team’s performance and ultimately patient safety. The CVS OR is an advanced technological environment but lacks sophisticated cognitive aids to support surgical teams’ situation management during complex surgery. Thus, we need tools to help surgical teams reach desired goal states within predefined quality, safety, resource, and time constraints.
One of the most devastating consequences of poor performance in CVS is embolic cerebral stroke associated with manipulation of the ascending aorta, required for aortic cannulation and cross-clamping. To mitigate the risk of stroke, current multi-society guidelines recommend routine performance of epiaortic ultrasound (EAU) to assess the quality of the ascending aorta [6]. These guidelines, like many others, lack specifics to guide the surgical team in selecting among multiple alternatives, including alternatives for normative (i.e., expected or typical) situations as well as alternatives for non-normative (i.e., problematic or unusual) situations where issues that need special handling (e.g., severely calcified aorta) have arisen.
Thus we applied a computer science approach to define precise and detailed goal-directed process models for aortic cannulation/clamping and to provide timely situation awareness, monitoring, and management tools to support the surgical team during high consequence episodes, reducing the risk of devastating CVS complications. Our approach deploys cognitive support tools only when most needed, to reduce unnecessary surgical workflow disruptions.
II. Approach
In recent years, significant efforts have been made to introduce intra-operative checklists to standardize surgical procedures and team communication, but such checklists have difficulty capturing the dynamic nature of complex episodes of care in surgery. We have begun to build and validate detailed, formal process models for critical episodes of commonly performed CVSs such as aortic valve replacement (AVR) and coronary artery bypass grafting (CABG). We have focused on one high-consequence episode, aortic cannulation/clamping using EAU to mitigate the risk of stroke. We then use the validated process models to generate dynamic, context-aware Smart Checklists to guide process performers during actual CVS processes.
For this work, the CVS process models specify the recommended ways to perform the processes based on best-practice, guidelines, and domain expert interviews. The process models hierarchically decompose the episodes of care into the individual steps to be completed by the process performers (e.g., medical professionals or medical devices). The “perform aortic cannulation/clamping assessment” process involves the surgical, anesthesiology, nursing, and perfusion teams. The process model specifies which steps are to be performed concurrently by different teams (e.g., step “perform EAU” is performed by the surgical/nursing team while the step “perform TEE” is performed by the anesthesiology team) and the data to be passed between these teams. Additionally, the process model specifies how to first recognize non-normative situations (e.g., by identifying problems such as target therapeutic activated clotting time was not achieved) and then how to recover from them (e.g., administer additional anticoagulant) and return to the normative process.
Our surgical process models are specified in the Little-JIL process modeling language [3], which is capable of precisely defining such process intricacies as concurrency, non-normative situations, resources, and data passing. Little-JIL has been used for many years to define complex processes in health care (e.g., [8]) as well as in other domains such as software development and elections. This experience suggests that it is expressive enough to capture the complexity of CVS processes. Additionally, Little-JIL has formally defined semantics that support formal analysis and execution of the process models.
Before using the surgical process models to generate Smart Checklists to guide process performers, we validated that these models accurately reflect the best-practices. We applied both manual validation techniques, such as reviews by domain experts, and automated validation techniques, such as model checking and fault tree analysis [1]. We used the FLAVERS model checker to verify that all potiential executions of the process model satisfy given safety properties. FLAVERS has also been used successfully for many years to verify both processes and computer code, proving that they adhere to rigorously-defined desired properties. FLAVERS assumes that the process steps are performed correctly but checks whether the process allows steps to be performed in an order that would violate key safety properties (e.g., could the cannula be inserted before the EAU). To complement the FLAVERS analysis, we also apply fault tree analysis using a tool that has seen considerable use on processes from other domains. This tool determines if combinations of incorrectly performed steps (e.g. a step that produces the wrong EAU findings) could cause the occurrence of a rigorously-defined hazard (e.g. that the wrong cannula is used). Mertens et al. [8] showed a roughly 70% reduction in chemotherapy errors reaching the patient after applying these process modeling and analysis techniques.
The Smart Checklist framework [2] takes as input a model for the process, including the activities of all process performers, and generates Smart Checklists to guide those performers. These Smart Checklists show the steps that have already been performed, the steps currently being performed, and the steps still to be performed. This framework assumes there is a mechanism for monitoring the real-world process and capturing such process execution events as completion of a step by either a human or a medical device. Based on the process model and the process execution events, the framework dynamically updates the Smart Checklists. In the next two sections, we first provide an illustrative example of a Smart Checklist to be used by a single team (e.g., surgical) and then discuss possible directions for future work to support multiple team collaboration.
III. Example
We use our validated CVS process model to automatically generate Smart Checklists for highly consequential CVS episodes. Figure 1 shows a generated Smart Checklist to guide a surgeon, Jane Smith, through the aortic cannulation/clamping assessment using EAU for patient Margaret Geary. This Smart Checklist shows the medical team member or team information (in the top right corner), some patient-specific information (in the top half), and dynamic, context-aware process information (in the bottom half).
Fig. 1.

Generated Smart Checklist guiding the surgical team through the cannulation/clamping assessment
The patient-specific information includes patient identifiers such as the patient’s photo, full name, and date of birth (on the top left) and some patient’s physiological data such as heartrate and temperature (on the top right). This data is provided by integration with the Open Integrated Clinical Environment [7]. The patient-specific information can be customized based on the medical team’s preferences, the patient, and the process.
The dynamic, context-aware process information includes the process header (the green rectangle with “PERFORM AORTIC CANNULATION…”) and the dynamic listing of the process steps (shown in the remainder of the bottom half). The process header shows the process name (the capitalized text), the process execution status (“In progress”), and the general notes button (the yellow icon at the far right) that the surgeon clicks to document the process execution state. This listing of process steps shows that the immediate previous step (shown by the gray background) is “assess aortic cannulation site” was completed successfully, that the current step (shown by the green background), “choose aortic cannulation site”, is in progress, and that the first substep “perform and choose aortic cannulation using standard cannula” is being performed.
Based on the EAU and TEE Katz scores (e.g., 0 = no intraluminal atheroma, 5 = mobile protruding atheroma), the surgical team decides whether using a standard cannula is appropriate. If so, the surgeon (or another team member responsible for the surgical documentation) clicks the green button with the white checkmark. If not, the surgeon clicks the red button with the white X. In addition, the surgeon may click the notes button (on the far right) to document this decision. Based on the process model and the button clicked, the Smart Checklist updates the dynamic listing of process steps by updating the current step’s execution status and adds the next steps that are to be executed. Figure 2 shows the process steps generated if the surgical team decided against using the standard cannula.
Fig. 2.
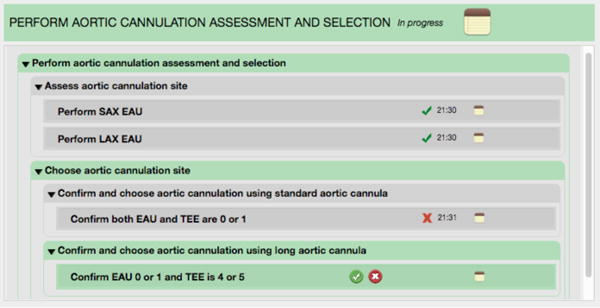
Updated Smart Checklist after the surgical team decides against using the standard cannula
In Figure 2, the substep “confirm and choose aortic cannulation using standard cannula” was updated to show that it was completed (shown by the gray backgound and the timestamp), and that the surgical team decided against using the standard cannula (indicated by the red X). The next substep “confirm and choose aortic cannulation using long cannula” was added and shown to be in progress.
IV. Discussion
The surgical process model implemented in Little-JIL acts as a high-level decision framework for cognitive support during the aortic cannulation/clamping assessment used by both CABG and AVR. This process model defines an evidence-based best practice aimed at increasing patient safety by mitigating the risk of stroke arising from finger palpation to ascertain the location and extent of calcification in the ascending aorta.
We asked domain experts to validate our model by manually reviewing it. We also applied model checking to verify that this model satisfies a key set of properties. For example, we verified that the model assures that EAU is always used before aortic cannulation and that the standard cannula is only used when Katz scores support that use. We also automatically constructed a fault tree for the hazard “wrong cannula selected for aortic cannulation.” The fault tree shows how incorrectly acquiring, communicating, and using Katz scores can cause that hazard. By validating the process model as described, we assure that checklists generated from this model will provide CVS teams with guidance that conforms to evidence-based best practices. Such guidance should be of particular value when process performers are working under conditions of high cognitive load.
The Smart Checklists are context-aware along several dimensions including the team, patient, and process. As can be seen from the example, the context-aware process information presented in the checklist depends on the model’s structure, which reflects such features as step hierarchy and non-normative situations. Annotations can be added to customize what process information is displayed and how that information is displayed. Our current Smart Checklist framework can be configured to generate a shared checklist for multiple teams (e.g., surgical, perfusion, anesthesiology, nursing) or an individual checklist for each single team (e.g., surgical) as shown in the previous section. To support multiple team collaboration, we are currently modifying a team’s individual checklist to provide information about where communication with other teams is needed. We are also investigating a shared dashboard to inform each team about the progress of the other teams through the process. For instance, it should be helpful to show whether each team is actively performing some of their steps or waiting for another team to perform needed steps.
V. Conclusion
Procedural standardization and routine implementation of evidence-based safe practices have been recommended to improve patient safety in surgery. This paper presents a high-level decision support framework that uses current contexts and step execution history to help teams determine what steps to perform next in order to adhere to defined best practices. In future work, we will model other key subprocesses in the AVR and CABG processes. For training purposes, we plan to use our approach in a simulated setting to guide CVS teams through both normative and non-normative situations. To mitigate risk during actual surgeries, we will investigate various techniques for clearly presenting checklist information to CVS teams.
Acknowledgments
This material is based upon work supported by the National Science Foundation under awards IIS-1239334 and CMMI-1234070 and the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under award 1R01HL126896-01A1. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Contributor Information
Heather M. Conboy, College of Information and Computer Sciences, University of Massachusetts, Amherst, MA, USA
George S. Avrunin, College of Information and Computer Sciences, University of Massachusetts, Amherst, MA, USA
Lori A. Clarke, College of Information and Computer Sciences, University of Massachusetts, Amherst, MA, USA
Leon J. Osterweil, College of Information and Computer Sciences, University of Massachusetts, Amherst, MA, USA
Stefan C. Christov, Department of Engineering, Quinnipiac University, Hamden, CT, USA
Julian M. Goldman, Anesthesiology, MGH, Harvard Medical School, Boston, MA, USA
Steven J. Yule, STRATUS Simulation Center, BWH, and Harvard Medical School, Boston, MA, USA
Marco A. Zenati, BWH, and Division of Cardiac Surgery, VABHCS, Harvard Medical School, Boston, MA, USA
References
- 1.Avrunin G, Clarke LA, Osterweil LJ, et al. Experience modeling and analyzing medical processes: UMass/Baystate medical safety project overview. 1st ACM Int Health Informatics Symp. 2010:316–325. [Google Scholar]
- 2.Avrunin GS, Clarke LA, Osterweil LJ, Goldman JM, Rausch T. Smart Checklists for human-intensive medical systems. Workshop on Open Resilient Human-Aware Cyberphysical Systems. 2012:1–6. [Google Scholar]
- 3.Cass AG, Staudt Lerner B, Sutton SM, Jr, et al. Little-JIL/Juliette: a process definition language and interpreter. 22nd Int Conf on Software Engineering. 2000:754–757. [Google Scholar]
- 4.Wahr JA, Prager RL, Abernathy JH. Patient safety in the operating room: human factors and teamwork. A scientific statement from the American Heart Association. Circulation. 2013;128:1139–1169. doi: 10.1161/CIR.0b013e3182a38efa. [DOI] [PubMed] [Google Scholar]
- 5.Finks JF, Osborne NH. Trends in hospital volume and operative mortality for high-risk surgery. New England Journal of Medicine. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillis LD, Smith PK, Anderson JL, et al. ACCF/AHA guideline for coronary artery bypass graft surgery: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124(23):2610–2642. doi: 10.1161/CIR.0b013e31823b5fee. [DOI] [PubMed] [Google Scholar]
- 7.MD PnP Program. Open Integrated Clinical Environment (Open ICE) www.openice.info.
- 8.Mertens W, Christov SC, Avrunin GS, et al. Using process elicitation and validation to understand and improve chemotherapy ordering and delivery. The Joint Commission Journal on Quality and Patient Safety. 2012;38(11):497–505. doi: 10.1016/s1553-7250(12)38066-5. 497. [DOI] [PubMed] [Google Scholar]


