Abstract
Sensory stimulation leads to structural changes within the CNS (Central Nervous System), thus providing the fundamental mechanism for learning and memory. The olfactory circuit offers a unique model for studying experience-dependent plasticity, partly due to a continuous supply of integrating adult born neurons. Our lab has recently implemented an olfactory cued learning paradigm in which specific odor pairs are coupled to either a reward or punishment to study downstream circuit changes. The following protocol outlines the basic set up for our learning paradigm. Here, we describe the equipment setup, programming of software, and method of behavioral training.
Keywords: Olfactory, Circuit, Learning, Synaptic, Plasticity, Go/No-Go, Behavior
Background
The adult brain features ongoing experience-dependent structural changes. Within the rodent olfactory bulb (OB) where odor information is first processed, a continuous supply of adult born interneurons (granule cells) either integrates into the olfactory circuitry or undergoes apoptosis (Petreanu and Alvarez-Buylla, 2002; Carleton et al., 2003 ; Lledo et al., 2006 ; Sakamoto et al., 2014 ). This choice between survival or death is greatly influenced by sensory stimulus and olfactory cued learning ( Rochefort et al., 2002 ; Alonso et al., 2006 ). Moreover, younger granule cells also undergo experience-dependent synaptic changes within a critical time window (Yamaguchi and Mori, 2005). To examine how sensory experience affects synaptic plasticity in OB circuits, our lab has successfully implemented a Go/No-Go olfactory cued learning task ( Huang et al., 2016 ; Quast et al., 2016 ). Mice are trained to associate a ‘Go Odor’ with a water reward and a separate ‘No-Go Odor’ with a punishment (trial timeout) (Figure 1). Upon completion of training, mice will be able to distinguish the two odors by performing the associated task with greater than 85% accuracy (Supplemental Video 1).
Figure 1. Go/No-Go task.
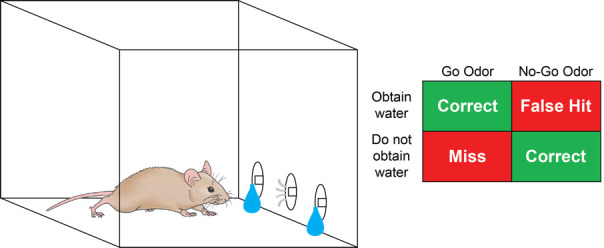
Trained, water-deprived mice will first poke their nose into the central odor port to initiate odor delivery. Subsequently, either a Go or No-Go odor is delivered at random. If the Go Odor is delivered, trained mice will move to either of the two side ports to collect the water reward. If the No-Go odor is delivered, trained mice will refrain from seeking water and re-poke into the odor port.
Materials and Reagents
Distinct pair of odorants selected by experimenter to represent the ‘Go’ or S+ stimulus and ‘No-Go’ or S- stimulus. Example: 1-butanol and propionic acid (Sigma-Aldrich, catalog numbers: 437603 and 402907, respectively)–diluted to 10% in mineral oil (Alfa Aesar, catalog number: 31911) (500 µl odorant in 5 ml of mineral oil)
Qorpak borosilicate glass vial with Green Polypropylene Hole Cap (Qorpak, catalog number: GLC-01016)
Nalgene silicone tubing (Thermo Fisher Scientific, Thermo ScientificTM, catalog number: 8600-0030)
BD PrecisionGlideTM 18 gauge beveled needles (BD, catalog number: 305196)
Adult mice (> 6 weeks old, our lab uses on average 12 to 19 weeks old mice with average body weights of 19 g for females and 23 g for males)
Equipment
Extra Wide Modular Test Chamber-Mouse (Med Associates, catalog number: ENV-307W)
Stainless Steel Grid Floor (Med Associates, catalog number: ENV-307W-GFW)
Illuminated Nose Poke Response for Wide Mouse Modular Chamber (Med Associates, catalog number: ENV-313W) (x2)
Two Channel Olfactory Stimulus (Med Associates, catalog number: ENV-275)
Illuminated Nose Poke Response with Olfactory Ports for Mouse (Med Associates, catalog number: ENV-375W-NPP)
Stand Alone USB Interface, 4 In/8 out Compatible with 32bit OS only (Med Associates, catalog number: DIG-703A-USB)
Standard desktop computer with Windows 2000, XP, Vista, or 7 operating system
-
VWR flow meter, Acrylic (VWR, catalog number: 97004-952)
Figure 2. Go/No-Go equipment.
The behavior system contains 6 key components: Behavior chamber, water reservoirs, air adjustment, USB interface system, odor delivery module, and a personalized computer (A). Room air is first relayed from the air adjustment to the input air manifold and subsequently diverged to the two odor-containing glass vials and the center valve (B). Odorized air outputs are controlled by two solenoids (C), which can be programmed by the Schedule Manager software. An odor intake line, a vacuum line, and two water dispensers are connected to the behavior chamber (D). Each odor vial is paired with its own two silicone tubes fitted with 18 gauge needles to prevent contamination of odors (E).
Software
-
MED-PC IV behavioral software suite (Med Associates, SOF-735)
Note: This version of the software has now been replaced with Med-PC V.
MPC2XL-Data Transfer Utility for all MED-PC Users (Med Associates, SOF-731), required for data reformatting to Excel
Schedule Manager Software (Med Associates, catalog number: DIG-703A-USB), required for programming training stages
Procedure
-
Establishing behavioral chamber and programmed olfactory cue and reward delivery
-
Obtain and set up operant conditioning behavioral chamber for mice
Set up must include modular odorant and water delivery ports.
Programming training stages for associative learning requires a computer that can run Med-PC IV software.
-
Med-PC IV software utilizes a proprietary programming language that the experimenter must learn to program training stages. Specific requirements for each stage of training include:
For all stages: Trials are blocked into 20 trials per block to compute per-block performance of each mouse.
Stage 1: The program must specify that the mouse receives a water reward when its nose pokes into the center water delivery port. Output parameters for this stage are: time duration of trial and number of rewards received.
Stage 2: The program must specify that the mouse receives water reward from two side ports only after it pokes its nose into the odor delivery port (center port). Water should be dispensed immediately after center poke even if the mouse does not go to the side ports for the reward. Output parameters: time duration of trial, number of trials initiated and number of timely rewards (received within 5 sec of a nose poke in the center port).
Stage 3: The program must specify the delivery of the Go (S+) odor upon nose poke to the center port. The water reward should be only dispensed if mouse nose pokes the side ports within 5 sec of odor delivery. The program must also automatically shape the behavior of mice with a 50 msec step-wise increase (from 100-400 msec) in the length of time required for a center port nose poke to yield a reward. If within a block of 20 trials the mouse pokes in the center port long enough 80% of the time, the program should increase the required time by 50 msec. Output parameters are identical to those in stage 2 plus number of rewards received and percent correct response in all trials.
Stage 3: Pseudo-training: This program is utilized for control groups of mice that undergo training without association of Go or No-Go (S-) odors to either reward or punishment. All parameters are the same as normal stage 3 except S+ odor will not be introduced.
Stage 4A: At this stage, the program must start with all trials delivering Go/S+ odorant like stage 3. When mice complete 1-2 blocks of Go/S+ trials with > 85% accuracy, the experimenter can program randomized delivery of the S-/Go odor for half of the trials. 2 sec ‘time out’ punishment will be given if the mouse inaccurately seeks a water reward after the No-Go cue. To help mice distinguish between Go and No-Go trials, the experimenter can program the side lights on the chamber near the ports to be on during Go trials and off during No-Go trials. Output parameters are: Time duration of trial, Number of trials initiated (Number of center port nose pokes), Number of trials completed (Number of center port nose pokes longer than 400 msec), % correct response, Number of rewards received.
Stage 4B: The program must increase the ‘time out’ punishment for mice to 4 sec. Output parameters are identical to those in stage 4A.
Stage 4: Pseudo-training: Same as stage 4B except reward and punishment are assigned randomly to Go/S+ and No-Go/S- odors.
Go/No-Go: This stage represents ‘testing’ phase and can be performed on a novel pair of odorants to test how accurately odor associations are made for experimental versus control mice. Output parameters are identical to those in stage 4A. If side lights were programmed in stage 4A, the program should now eliminate these side light cues.
-
-
Associative learning (Go/No-Go) task
-
Preparation of mice [3 days]
-
3 days prior to preliminary training, water is restricted to 40 ml/kg/day and mice are habituated to the testing environment and chamber.
Preliminary trials of water restriction on control animals may be performed to determine the minimum volume of water provided to maintain performance in the behavioral task without surpassing humane endpoints (National Research Council 2003) as determined by the experimenter’s IACUC guidelines. In our hands, 40 ml/kg/day per mouse sufficed to maintain performance while avoiding bodyweight loss in excess of 15% of each animal’s baseline. This endpoint was established due to the ability of rodents to compensate to a gradual water restriction paradigm, and sustain losses up to 15% of their baseline body weight, without displaying indicators of distress (Rowland, 2007)
During water restriction, mice must be weighed daily to ensure that they are above 85% of baseline mass. If mice fall below this threshold, they are removed from the study and provided free water.
Note that as with other behavioral paradigms, results can vary exquisitely with small changes in procedure. Therefore, before the start of training, ensure that all environmental factors (temperature, noise, stray odors) remain constant throughout the protocol and that the experimental observers do not introduce random variation (e.g., the scent of perfumes or irregular changes in observers/handling procedure).
-
-
Preliminary training [8-24 days]
-
Mice are divided into control, pseudo-trained, or trained groups.
Control mice receive no further treatment.
Pseudo-trained mice are treated similarly to the trained group, except each trial yields reward and punishment at equal probability regardless of the odor presented.
Trained mice are subjected to a Go/No-Go olfactory associative learning paradigm.
Configure the behavioral chamber to have only one central port available to deliver water. (Figure 3A)
-
Pseudo-trained and trained mice are subjected to the following phases of preliminary training.
-
Stage 1 [1-3 days, 60 min/mouse/day]: mouse learns to associate the chamber with the presence of water.
Allow each mouse to explore the chamber and discover that the port delivers water.
Completion criteria: 100 trials within 60 min.
Notes:
The sound of the water valve may startle mice, and they might stop performing. If mice are too afraid to explore, ‘free’ water can be offered. This is done by using the timed output option on the software to deliver ~200 msec worth of water into the water delivery port.
If a mouse freezes or stops exploring despite free water delivery as above, allow the mouse to stay in the chamber until the end of 60 min. Remove and repeat this stage on the following day for that mouse.
Empty any solid waste from the mice between trials. Spray and wipe the chamber with 70% ethanol between cages of mice to prevent odor distraction.
Even within groups, mice display natural variability in performance. Thus, mice can be promoted to stage 2 as a group when the average performance is within a reasonable range of the standard above (100 completed trials within 60 min).
-
Stage 2 [1-5 days, 60 min/mouse/day]: Mice are taught to nose poke into the center port for an automatic water reward that will be dispensed from both side ports.
Configure the chamber to have one central port for odor delivery and two side ports for water delivery (Figure 3B). This will be the chamber set up for the remainder of the experiment.
Completion criteria: 40-50 + trials in 60 min, with at least 25-50% timely rewards. Timely rewards are those received within 5 sec of a nose poke in the center port.
-
Stage 3 [1-3 days, 60 min/mouse/day]: Mice must first poke into the center port then the side port within 5 sec to get a reward. Further, mice must nose-poke in the center for longer and longer times (up to 400 msec) before seeking water at either of the side ports for a dispensed reward. This stage also introduces the Go/S+ odor paired with a water reward.
Completion criteria: > 60 rewards within 60 min.
Apply stage 3 pseudo-training to the pseudo-trained control group, but do not introduce the S+/Go odor.
-
Stage 4A [1-2 days, 60 min/mouse/day]: This stage trains mice on the Go vs. No-Go task and introduces the No-Go/S- odor, along with a 2 sec time out punishment for the mouse seeking a water reward on the No-Go cue. The program will start with all Go/S+ odor presentation. Parameters can be switched to 50% Go/S+ and 50% No-Go/S- trial after the mouse performs well on 1-2 blocks of all Go/S+ trials.
Completion criteria: 40 trials with > 60% correct responses.
Apply stage 4 pseudo-training to the pseudo-trained control group.
-
Stage 4B [5-11 days, 60 min/mouse/day]: this stage increases punishment time to 4 sec for mice incorrectly seeking rewards on a No-Go/S- trial or failing to seek a water reward on a Go/S+ trial.
Completion criteria: > 100 trials within 60 min at > 85% accuracy.
Notes:
-
It is helpful to keep daily track of not only the total % correct, but also the max % and min % correct for each session, to monitor each mouse’s progress during this stage.
In our hands, 85-90% of control mice reach the completion criteria. If the study requires that the mice continue into the Go/No Go testing phase, we recommend exclusion of mice who do not meet these criteria moving forward, as they have not demonstrated adequate ability to perform the task.
The experimenter may wish to program a ‘reversal’ training trial at the end of this stage, wherein the Go and No-Go odors are swapped in their position on the odor vial platform. This is to ensure that the mice are not cueing to another stimulus (e.g., the sound of a specific valve associated with one or the other side port).
-
-
Go/No-Go trial [1 day, ~20 min/mouse/day]
Mice promoted beyond stage 4B of training have now sufficiently learned the behavioral task and can be exposed to novel odor pairs to test their ability to discriminate between new odors (e.g., stereoisomers such as + and - limonene).
The parameters of Go/No-Go trials are like those of stage 4B, with the exception that a 300 msec nose poke in the center port is sufficient for mice to execute either the Go or No-Go action.
Enter experimental details into the program. Load each mouse into the chamber and select the correct program to run from the on-screen interface.
The endpoints or variables of statistical analysis are determined by the experimenter, but in our hands, control mice will often display accurate performance with new odor pairs (> 85% correct responses) after approximately 200-400 trials or 10-20 blocks.
-
Figure 3. Port configurations in behavioral chamber.
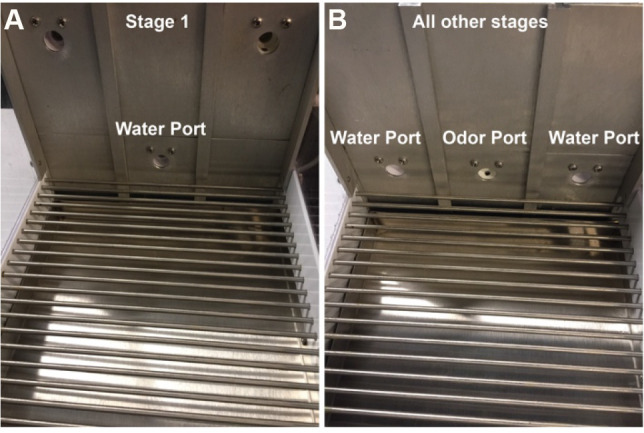
Stage 1 contains a center water port (A) with the side ports inaccessible. All other stages including the Go/No-Go testing stage are performed with a central odor port and two water side ports (B).
Data analysis
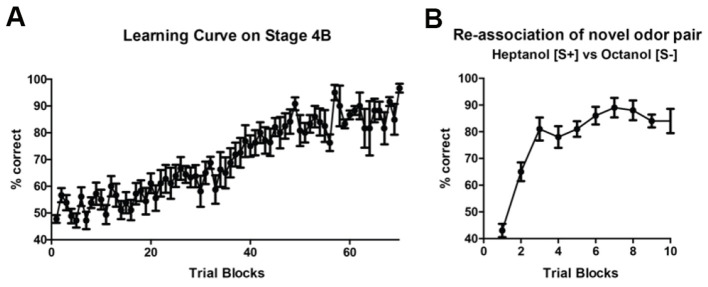
In the trained group, only mice which perform the Go/No-Go trial at > 85% accuracy was utilized in our circuit mapping experiments ( Huang et al., 2016 ). Mice that did not meet the criteria are not included in further studies. Furthermore, olfactory learning progression can be displayed for both the initial two associated odors used in training (Figure 4A), as well as for any subsequent re-associated odors (Figure 4B). Any statistical package may be used to analyze data, with specific tests depending on the experimenter’s needs. In Huang et al. (2016) , connectivity patterns in the olfactory bulb among trained, pseudotrained and control groups were compared by two-way ANOVA with repeated measures.
Figure 4. Learning curve.
In stage 4B, mice are presented both Go and No-Go trials at random. Average percent correct choice for sequential trial blocks (20 trials/block) are shown for a group of 5 mice (A). Error bars display standard error for the same block across all animals within one group. Re-association of novel odorants once mice are successfully trained can also be graphed similarly (B).
Notes
To reduce variability between experiments, we recommend distributing a controlled mix of age and sex of experimental animals among all groups tested.
Be certain that all tubing and fittings on the behavioral chamber are connected tightly to their bases to prevent odorant leakage.
Set up the behavior box in a low traffic, dimly lit area to prevent distractions to the mice.
Keep house air flow at ~3-5 L/min and adjust vacuum suction in the center port to avoid lingering odors between trials.
Use odor-specific tubes to connect odorant vials to the behavioral chamber. This will eliminate cross-contamination of odorants.
Clean the box thoroughly with 70% EtOH between cages/sets of mice.
Recipes
-
Odor preparation
Add 500 μl of odor with 4.5 ml of mineral oil into borosilicate glass vial
Prepare two 1 foot length silicon tubings and fit one end of each into separate 18 gauge needles. Both needles will be placed into the odor vial (Supplemental Video 2) and the tube ends will be fitted either to the air intake or the air outlet to the odor box
Always prepare new odors on the same day. Odors are remade weekly
Acknowledgments
This protocol is adapted from previous work within our lab ( Huang et al., 2016 ). It is supported by the McNair Medical Institute, NINDS grant R01NS078294 to B.R.A., and NIH IDDRC grant U54HD083092. The authors declare no conflicts of interest and no competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Alonso M., Viollet C., Gabellec M. M., Meas-Yedid V., Olivo-Marin J. C. and Lledo P. M.(2006). Olfactory discrimination learning increases the survival of adult-born neurons in the olfactory bulb. J Neurosci 26(41): 10508-10513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carleton A., Petreanu L. T., Lansford R., Alvarez-Buylla A. and Lledo P. M.(2003). Becoming a new neuron in the adult olfactory bulb. Nat Neurosci 6(5): 507-518. [DOI] [PubMed] [Google Scholar]
- 3.Huang L., Ung K., Garcia I., Quast K. B., Cordiner K., Saggau P. and Arenkiel B. R.(2016). Task learning promotes plasticity of interneuron connectivity maps in the olfactory bulb. J Neurosci 36(34): 8856-8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lledo P. M., Alonso M. and Grubb M. S.(2006). Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci 7(3): 179-193. [DOI] [PubMed] [Google Scholar]
- 5.National Research Council(2003). Food and Fluid Regulation. Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. The National Academies Press, pp: 49-61.
- 6.Petreanu L. and Alvarez-Buylla A.(2002). Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci 22(14): 6106-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quast K. B., Ung K., Froudarakis E., Huang L., Herman I., Addison A. P., Ortiz-Guzman J., Cordiner K., Saggau P., Tolias A. S. and Arenkiel B. R.(2016). Developmental broadening of inhibitory sensory maps. Nat Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochefort C., Gheusi G., Vincent J. D. and Lledo P. M.(2002). Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci 22(7): 2679-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowland N. E.(2007). Food or fluid restriction in common laboratory animals: Balancing welfare considerations with scientific inquiry. Comp Med 57:149-160. [PubMed] [Google Scholar]
- 10.Sakamoto M., Kageyama R. and Imayoshi I.(2014). The functional significance of newly born neurons integrated into olfactory bulb circuits. Front Neurosci 8: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi M. and Mori K.(2005). Critical period for sensory experience-dependent survival of newly generated granule cells in the adult mouse olfactory bulb. Proc Natl Acad Sci U S A 102(27): 9697-9702. [DOI] [PMC free article] [PubMed] [Google Scholar]