Abstract
Purpose of review
T-cell checkpoint blockade has become a dynamic immunotherapy for bladder cancer. In 2016, atezolizumab, an immune checkpoint inhibitor, became the first new drug approved in metastatic urothelial carcinoma (mUC) in over 30 years. In 2017, nivolumab was also approved for the same indication. This overview of checkpoint inhibitors in clinical trials focuses on novel immunotherapy combinations, predictive biomarkers including mutational load and neoantigen identification, and an evaluation of the future of bladder cancer immunotherapy.
Recent findings
Programed cell death protein 1/programed death-ligand 1 (PD-1/PD-L1) checkpoint inhibitors have achieved durable clinical responses in a subset of previously treated and treatment-naïve patients with mUC. The combination of PD-1 and cytotoxic T-lymphocyte antigen 4 (CTLA-4) has successfully improved response rates in multiple malignancies, and combination studies are underway in many tumor types, including bladder cancer, combining T-cell checkpoint blockade with other checkpoint agents and immunomodulatory therapies. Strong tumor responses to checkpoint blockade have been reported to be positively associated with expression of PD-L1 on tumor and tumor-infiltrating immune cells and with increased mutation-associated neoantigen load, which may lead to the development of predictive biomarkers.
Summary
Recent clinical evidence suggests that mUC is susceptible to T-cell checkpoint blockade. A global effort is underway to achieve higher response rates and more durable remissions, accelerate the development of immunotherapies, employ combination therapies, and test novel immune targets.
Keywords: biomarker, bladder cancer, combination immunotherapy, immune checkpoint, mutational burden, neoantigen, urothelial carcinoma
INTRODUCTION
The current burden of urothelial carcinoma
Bladder cancer, or urothelial carcinoma, is the fourth most common cancer in men and the eighth most common cancer in women in the United States [1]. The American Cancer Society estimates 79 030 new cases and 16 870 deaths from urothelial carcinoma in 2017 [2]. Clinically, urothelial carcinoma can be divided into nonmuscle-invasive bladder cancer (NMIBC), muscle-invasive bladder cancer (MIBC), and metastatic urothelial carcinoma (mUC).
Although most patients present with NMIBC (T0–T1), approximately 30–40% of patients have muscle-invasive disease (T2–T4a) at time of diagnosis. Muscle invasion is a poor prognostic factor, presumably due to occult metastasis at the time of diagnosis. The standard of care for MIBC is neoadjuvant cisplatin-based combination chemotherapy followed by radical cystectomy [3]. Patients presenting de novo with mUC, or developing visceral metastatic disease after local treatment, are incurable with currently available therapeutic modalities. Cisplatin-based combination systemic therapy with either dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin or gemcitabine and cisplatin are considered the standard of care for mUC throughout the United States, Europe, Canada, and Japan [4]. With combination chemotherapy, median survival time for mUC is approximately 1 year compared with less than 6 months without treatment; however, long-term survival remains rare. Unfortunately, a large number of patients are cisplatin-ineligible at time of diagnosis, most commonly from renal insufficiency. Carboplatin and gemcitabine is frequently an alternative regimen for these patients [5]. Until the recent approval of atezolizumab and now nivolumab, both checkpoint inhibitors, in the second-line setting for mUC, no new therapy had been approved for mUC in over 30 years.
Box 1.

no caption available
Urothelial carcinoma is immune-responsive
Treatment of NMIBC by intravesical Bacillus Calmette–Guérin (BCG) instillation, originally reported by Morales et al.[6,7] in 1976 and approved by the US Food and Drug Administration (FDA) in 1990, constituted the first FDA-approved immunotherapy and the first demonstration that urothelial carcinoma is immune-responsive [8]. Intravesical instillation of BCG activates innate and adaptive immunity, resulting in infiltration of cytotoxic T lymphocytes (CTLs) and cell-mediated cytotoxicity against bladder tumors [9]. BCG-refractory disease, which occurs in approximately 40% of NMIBC patients [10], is thought to be mediated by complex mechanisms of immune escape, including overexpression of programed death-ligand 1 (PD-L1) and inactivation of cytotoxic T cells.
For BCG-refractory disease and de novo MIBC and mUC, currently accepted salvage and chemotherapeutic agents often fail to achieve cure or prolonged remission. Even fewer treatment options exist for patients with recurrent or progressive disease following failure of platinum-based chemotherapy. In May 2016, the anti-PD-L1 antibody atezolizumab was approved by the FDA for second-line use in platinum-refractory mUC on the basis of results from the phase II IMvigor 210 trial [11▪▪,12]. Atezolizumab is the first anti-PD-L1 antibody to achieve regulatory approval for any indication. In February 2017, the FDA granted accelerated approval to nivolumab, an antiprogrammed cell death protein 1 (anti-PD-1) antibody also for second-line use in platinum-refractory mUC, based on the results of a single-arm phase II study (CheckMate 275) [13]. Evidence that inhibition of the PD-1/PD-L1 pathway has clinical activity in patients with mUC opened the door to the investigation of additional immune therapies, either as single agents or in combination with a broad array of agents, in an effort to increase the number of patients who respond to T-cell checkpoint blockade. This review looks at the biology of immune destruction of bladder tumor cells, emerging immunotherapy treatments for bladder cancer in various stages of clinical development as monotherapy and in combination with other immune therapies, chemotherapy, tyrosine kinase inhibitors, cytokines, vaccines, adoptive cell therapies, and the development of novel immune-based biomarkers.
The biology of immune destruction of bladder tumor cells and the development of novel biomarkers
Bladder cancer, like many other cancers, can evade the immune system by downregulating tumor-antigen presentation, inactivating cytotoxic T cells, upregulating immune checkpoints, and maintaining an immunosuppressive microenvironment. Immune therapies for bladder cancer aim to target one or more of these steps in the immune cascade to induce the production of CD8+ cytotoxic T cells and natural killer (NK) effector cells, thereby propagating an effective antitumor response [14].
Mechanism of action of checkpoint inhibitors
Ipilimumab, a first-in-class immune checkpoint monoclonal antibody (mAb), was approved by the FDA in 2011. Ipilimumab is directed at CTL antigen 4 (CTLA-4), which is expressed on the surface of conventional CD4+ and CD8+ T cells and regulatory T cells. CTLA-4 is in the same superfamily as the T-cell costimulatory molecule CD28, with which it competes for binding to their shared ligands B7-1 and B7-2 on antigen-presenting cells. T-cell immunity is regulated by a balance of stimulatory and inhibitory signals mediated by costimulatory and coinhibitory signaling pathways. This balance is dysregulated in the tumor microenvironment, in which expression of immune checkpoints and coinhibitory signaling predominate. Ipilimumab antagonizes coinhibitory signals, allowing costimulatory signaling to predominate, resulting in improved antitumor immunity.
In 2014, pembrolizumab and nivolumab, two antibodies that target PD-1, an immune checkpoint discovered in 2000, were FDA-approved for unresectable metastatic melanoma. PD-1 limits activated T-cell response and has two ligands, PD-L1 and PD-L2. PD-1 interferes with T-cell antigen receptor signaling when it is bound to its ligands, which can be expressed on immune cells and on tumor cells. Upon ligation to PD-L1/2, PD-1 suppresses downstream PI3K and Akt signaling. This mechanism differs from CTLA-4 signaling, which inhibits Akt independently from PI3K. Thus, antibodies targeting CTLA-4 and PD-1/PD-L1 can be cooperative and, as described below, are being developed in combination therapy protocols. Atezolizumab was the first FDA-approved antibody that targets the ligand in the PD-1/PD-L1 immune checkpoint.
Emerging immunotherapy treatments for bladder cancer in various stages of clinical development
Inhibition of immune escape mechanisms of the PD-1/PD-L1 pathway has demonstrated rapid, durable responses in multiple tumor types, including advanced urothelial carcinoma [14]. Five checkpoint inhibitors (atezolizumab, pembrolizumab, avelumab, nivolumab, and durvalumab) have demonstrated clinical efficacy in patients with mUC in the second-line setting, with comparable objective response rates (ORRs – 15–20%) (Table 1). Two of these agents, atezolizumab and pembrolizumab, have also been tested in the first-line setting in cisplatin-ineligible patients, again showing similar outcomes. Here, we summarize the results of checkpoint clinical trials in mUC and discuss the numerous immunotherapy single-agent (Fig. 1) and combination (Fig. 2) clinical trials at various stages of development for the entire spectrum of disease states in urothelial carcinoma that have stemmed from the results of the initial clinical trials.
Table 1.
Clinical studies of programed cell death protein 1/programed death-ligand 1 inhibitors reported in urothelial carcinoma
| Metastatic second-line | Metastatic first-line cisplatin-ineligible | |||||||||
| Pembrolizumab | Atezolizumab | Nivolumab | Durvalumab | Avelumab | Atezolizumab | Pembrolizumab | ||||
| Reference | Plimack et al. [15] | Bellmunt [16▪] | Powles et al. [12] | Rosenberg et al. [11▪▪] | Sharma et al. [17▪▪] | Galsky et al. [13] | Massard et al. [18] | Apolo et al. [19▪▪] | Balar et al. [20▪▪] | Balar et al. [21▪] |
| FDA approval | May 2016 | February 2017 | ||||||||
| Phase | Ib | III | I | II | I/II | II | I/II | I | II | II |
| n | 27 | 542 | 100 | 331 | 78 | 265 | 42 | 44 | 119 | 100 |
| ORR (%) | 26 (PD-L1+) | Pembro: 21.1; Chemo: 11.4 | 21 | 15 | 24.4 | 19.6 | 31 | 18.2 | 23 | 24 |
| ORR PD-L1+ (%) | 26 | Pembro: 21.6; Chemo: 6.7 | 43.3 | 26 | 24.0 | 28.4 | 46.4 | 53.8 | 28 | 37 |
| PR (%) | 15 | Pembro: 14.1; Chemo: 8.1 | NR | 10 | 18 | 17.4 | NR | 6.8 | 13 | 18 |
| CR (%) | 11 | Pembro: 7; Chemo: 3.3 | 7 (PD-L1+) | 5 | 6.4 | 2.3 | NR | 11.4 | 9 | 6 |
| PD-L1+ prevalence (%) | 53 | Pembro: 27.4; Chemo: 33.1 | 27 | 32.2 | 32 | 30.5 | 65.6 | 29.5 | 27 | 30 |
| PFS (months) | 2 | Pembro: 2.1; Chemo: 3.3 | 6 (PD-L1+); 1 (PD-L1−) | 2.1 | 2.8 | 2.0 | NR | 2.9 | 2.7 | NR |
| OS (months) | 13 | Pembro: 10.3; Chemo: 7.4 | NR (PD-L1+); 8 (PD-L1−) | 11.4 | 9.7 | 8.7 | NR | 13.7 | 15.9 | NR |
| Grade 3/4; AEs (%) | 15 | Pembro: 15; Chemo: 49.4 | 4.4 | 16 | 22 | 17.8 | 4.9 | 6.8 | 16 | 16 |
AEs, adverse events; Chemo, chemotherapy; CR, complete response; FDA, Food and Drug Administration; IHC, expression; NR, not reported; ORR, objective response rate; OS, overall survival; Pembro, Pembrolizumab; PD-L1+, programed cell death ligand 1-positive; PFS, progression-free survival; PR, partial response.
FIGURE 1.
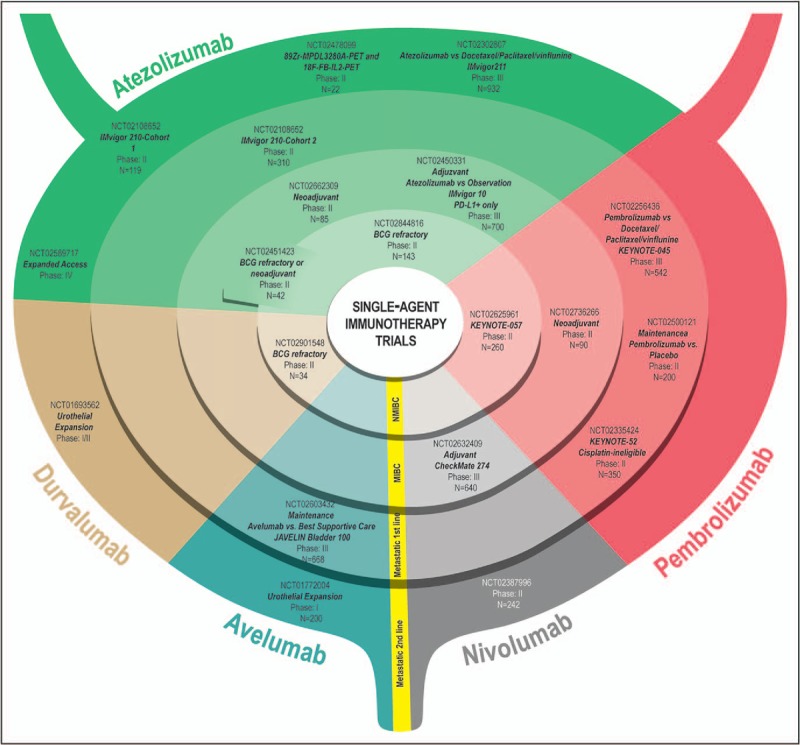
Immunotherapy Single-agent Clinical Trials of atezolizumab, nivolumab, pembrolizumab, avelumab, and durvalumab in various stages of development further subdivided by disease state, into nonmuscle-invasive bladder cancer, muscle-invasive bladder cancer, metastatic first line, and metastatic second line.
FIGURE 2.
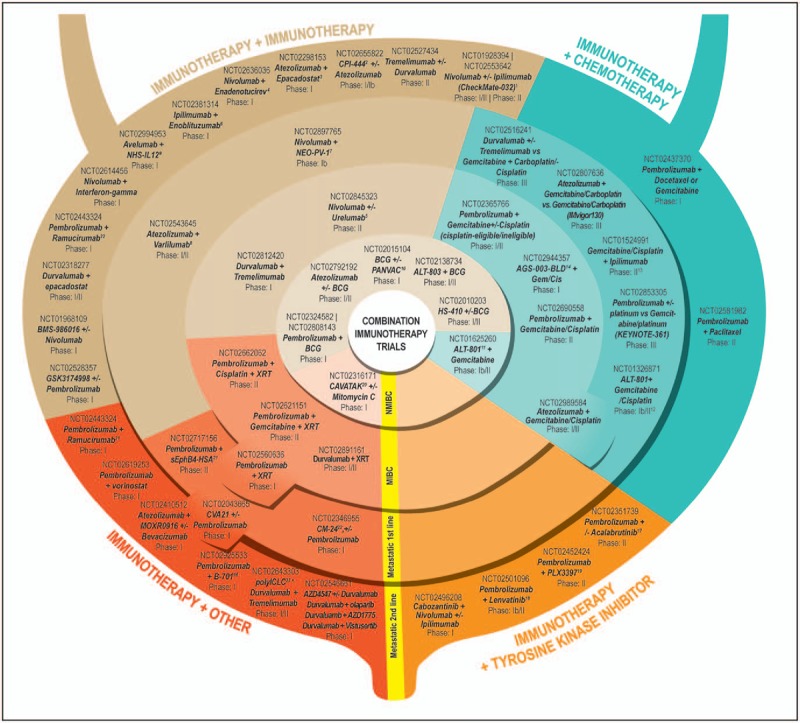
Immunotherapy Combination Clinical Trials in various stages of development utilizing immune therapies in combination with other immune therapies, chemotherapy, tyrosine kinase inhibitors, and other agents. Trials are further subdivided by disease state, into nonmuscle-invasive bladder cancer, muscle-invasive bladder cancer, metastatic first line, and metastatic second line. 1Objective response rate 38% for Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg (n = 26; complete response 3.8%). Objective response rate 26% for Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg (n = 104; complete response 2.9%). SITC 2016. 2Immune checkpoint inhibitor of the adenosine A2A receptor. 3Oral inhibitor of indoleamine 2,3-dioxygenase 1. 4Oncolytic group B adenovirus vaccine. 5mAb against CD137 receptor. 6mAb against B7-H3. 7Personalized cancer vaccine. 8mAb against CD27. 9Antibody-cytokine conjugate consisting of two heterodimers of IL-12, fused to a human mAb that has affinity for both single-stranded and double-stranded DNA. 10Poxvirus-based vector vaccine that induces tumor-specific immune response in T cells. 11IL-2 genetically fused to a humanized soluble T-cell receptor directed against the p53-derived peptides expressed on tumor cells. 12Objective response rate in five chemo-naïve patients 100% (two complete responses and three partial responses) and in five previously treated patients 60% (one complete response and two partial responses), for an overall objective response rate of 80% (three complete responses, five partial responses, 1 stable disease, and 1 progressive disease). Fishman et al.[22]. 13Objective response rate 64% (partial response 50%; complete response 14%); Galsky et al.[23]. 14Autologous dendritic cell-based immunotherapy. 15mAb against FGFR3. 16Second-generation Bruton's tyrosine kinase inhibitor. 17Tyrosine kinase inhibitor of VEGFR2. 18Tyrosine kinase inhibitor of KIT, CSF1R, and FLT3. 19Formulation of coxsackievirus type A21. 20Recombinant protein inhibitor of tumor growth and angiogenesis, complete response in one of three patients in higher dose cohort. https://www.viralytics.com/wp-content/uploads/2014/05/160315-EAU-CANON.pdf. 21mAb against VEGFR2. 22Humanized IgG4 (kappa) isotype mAb against CEACAM1. 23TLR3 agonist. BCG, Bacillus Calmette–Guérin; MIBC, muscle-invasive bladder cancer; NMIBC, nonmuscle-invasive bladder cancer; UC, urothelial carcinoma.
Ipilimumab
Ipilimumab can produce durable long-term responses in patients with advanced melanoma but has a higher rate of immune-related toxicities than PD-1/PD-L1 inhibitors, most involving the skin and gastrointestinal tract [24]. In a phase Ib study of 15 patients, ipilimumab (3 or 10 mg/kg) as neoadjuvant monotherapy for patients with MIBC undergoing surgical resection appeared to have a tolerable immune-related adverse event profile, with the skin and gastrointestinal tract being the most commonly affected organs [25]. Early findings from a phase II clinical trial in mUC combining four doses of ipilimumab with gemcitabine and cisplatin in the first-line metastatic setting showed promising results. In 36 evaluable patients, the ORR was 64%, with five patients (14%) achieving a radiologic complete response (CR). However, the study did not meet its primary endpoint of improved 1-year overall survival (OS). The addition of ipilimumab to chemotherapy increased levels of circulating CD4+ and CD8+ cells, although not necessarily ICOS+ cells, and induced a potentially more immunostimulatory environment [23,26]. Further studies of ipilimumab are currently in progress in combination with nivolumab (NCT01928394 and NCT02553642), nivolumab and cabozantinib (NCT02496208), and enoblituzumab, an mAb targeting B7-H3 (NCT02381314).
Nivolumab
Nivolumab is an mAb to PD-1 approved for the treatment of advanced melanoma, advanced NSCLC, advanced renal cell carcinoma, classical Hodgkin lymphoma, and advanced squamous cell carcinoma of the head and neck [27–29]. In a multicenter phase I/II trial (CheckMate 032) of 78 mUC patients completed in 2015, single-agent nivolumab showed a favorable ORR of 24.4% [95% confidence interval (CI) 15–35%] [17▪▪]. Grades 3–4 treatment-related adverse events occurred in 22% of patients, slightly higher than the 5–15% reported in other tumor studies. The most common adverse events were laboratory abnormalities (elevated lipase and amylase) in addition to fatigue, maculopapular rash, dyspnea, decreased lymphocyte count, and decreased neutrophil count [17▪▪]. Two (3%) patients discontinued therapy because of treatment-related adverse events (grade 4 pneumonitis and grade 4 thrombocytopenia) and subsequently died [17▪▪].
In February 2017, nivolumab was FDA-approved for patients with advanced mUC refractory to platinum-based therapy [30]. Approval was based on a single-arm study treating 270 patients with locally advanced or mUC who progressed during or following platinum-containing chemotherapy or progressed within 12 months of neoadjuvant or adjuvant treatment with platinum-containing chemotherapy. Patients received nivolumab 3 mg/kg every 2 weeks until disease progression or unacceptable toxicity. ORR was 19.6% (53/270; 95% CI: 15.1, 24.9). Seven patients had CRs, and 46 had partial responses (PRs) [13].
Nivolumab is currently being evaluated in urothelial carcinoma and other solid tumors in the metastatic second-line setting in combination with ipilimumab (NCT02553642 and NCT01928394), and with ipilimumab and cabozantinib (NCT02496208). Preliminary data presented at the SITC 2016 conference (Checkmate 023) showed a promising ORR of 38% for nivolumab 1 mg/kg + ipilimumab 3 mg/kg (n = 26, CR = 3.8%) and an ORR of 26% for nivolumab 3 mg/kg + ipilimumab 1 mg/kg (n = 104; CR = 2.9%) [31]. Further trials in urothelial carcinoma with nivolumab are currently in progress in combination with the oncolytic group B adenovirus vaccine enadenotucirev (NCT02636036); the personalized cancer vaccine NEO-PV-1 (NCT02897765); urelumab, a fully humanized IgG4 mAb against CD137, a tumor necrosis factor family receptor expressed primarily on activated T cells and activated NK cells (NCT02845323); and interferon-gamma (NCT02614456).
Pembrolizumab
Pembrolizumab is a humanized IgG4 mAb targeting PD-1 that is currently FDA-approved for the treatment of PD-L1+ metastatic melanoma [32] and metastatic NSCLC [patients whose tumors have high PD-L1 expression (Tumor Proportion Score ≥50%)] [33]. Based on data from the KEYNOTE-012 study in all solid tumors, mUC patients selected for tumors with at least 1% PD-L1+ status showed an ORR of 26%. The phase II KEYNOTE-052 study, the preliminary data of which for the first 100 patients were presented at ESMO 2016, evaluated pembrolizumab as first-line treatment in PD-L1-unselected, cisplatin-ineligible mUC patients, revealing an ORR of 24% (CR 6%; PR 18%) [21▪]. Preliminary data from the phase III KEYNOTE-045 trial presented at the SITC 2016 conference demonstrated that pembrolizumab is the first immunotherapy agent to show improved OS over chemotherapy in patients with advanced urothelial carcinoma following progression after first-line platinum-based therapy [16▪]. This trial compared single-agent pembrolizumab with the physician's choice of chemotherapy (paclitaxel, docetaxel, or vinflunine), producing an ORR of 21.1 versus 11.4% (P = 0.0011) and a median OS of 10.3 versus 7.4 months (hazard ratio 0.73; P = 0.0022), respectively [16▪]. Median duration of response had not been reached at time of presentation, and there was a lower incidence of treatment-related adverse events of any grade (61% with pembrolizumab versus 90% with chemotherapy) [16▪]. Pembrolizumab benefit was observed regardless of PD-L1 expression, and immune-related adverse events were consistent with previous experience (1–10% of patients) [16▪]. Given pembrolizumab's promising antitumor activity and manageable toxicity profile, over a dozen combination trials are currently in progress, including with BCG, cytotoxic therapy, tyrosine kinase inhibitors, and novel agents (refer to Fig. 2 for further information).
Atezolizumab
Atezolizumab, a humanized IgG1 mAb targeting PD-L1 on tumor cells and immune cells, was granted FDA approval in May 2016 for platinum-resistant mUC based on results of the phase II IMvigor 210 trial [11▪▪]. The study reported a 15% ORR overall and 27% ORR in patients with higher PD-L1 immunohistochemistry (IHC) expression (IC2/3 defined as ≥5% of cells). The median OS was 11.9 months for those with higher PD-L1 expression, with median duration of response not reached at time of reporting, and a favorable safety profile (4% of patients with grades 3–4 immune-related adverse events) [11▪▪].
As anticipated with the high somatic mutational burden of urothelial carcinoma, the Cancer Genome Atlas mutation type and load were shown to be independent predictive factors for response to immune checkpoint blockade [11▪▪]. Cohort 2 of the IMvigor 210 trial enrolled 123 treatment-naïve patients, among whom 119 received at least one dose of therapy. Interestingly, responses occurred regardless of PD-L1 status and across poor prognostic factor subgroups, but there was an association between tumor mutational load and response. Based on these positive results, many studies of atezolizumab are underway in urothelial carcinoma. In the IMvigor 010 trial, atezolizumab is in a phase III study versus observation as adjuvant therapy in patients with PD-L1+, high-risk MIBC after cystectomy. Atezolizumab is also in a phase II international study as neoadjuvant therapy in operable MIBC.
Combination studies include platinum-based chemotherapy (NCT02807636 and NCT02989584); novel immunotherapy agents, including CPI-444 [immune checkpoint inhibitor of the adenosine A2A receptor (ADORA2A) NCT02655822]; epacadostat [oral inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1), NCT02298153]; varlilumab (mAb against CD27, NCT02543645); BCG (NCT02792192) for NMIBC; and versus physician's choice of chemotherapy for mUC (IMvigor 211; NCT02302807).
Durvalumab
In February 2016, durvalumab, another PD-L1 inhibitor, was granted breakthrough therapy designation by the FDA for mUC, based on the findings of a phase I/II study in all solid tumors presented at ASCO 2016 [34]. The study revealed an encouraging ORR of 46.4% in patients with PD-L1+ tumors with advanced urothelial carcinoma [18]. Interestingly, no patients expressed a response unless they exhibited an increased PD-L1 expression of at least 25% in either immune or tumor cells. Consequently, current trials with durvalumab are in combination with immunomodulatory agents, including tremelimumab in mUC (NCT02527434; allows for addition of durvalumab following disease progression); tremelimumab and polyICLC (a Toll-like receptor 3 agonist) for mUC (NCT02643303); tremelimumab in MIBC (NCT02812420); and in combination with radiotherapy for MIBC (NCT02891161, DUART).
Avelumab
Avelumab is an anti-PD-L1 mAb that has been shown to induce antibody-dependent cell-mediated cytotoxicity of tumor cells in preclinical studies [35]. Avelumab demonstrated an ORR of 18.2% in platinum-refractory mUC patients unselected for PD-L1+ tumors and had an acceptable safety profile in the phase Ib JAVELIN solid tumor trial [36]. There was a trend toward higher ORR and prolonged progression-free survival at 12 weeks in patients with PD-L1+ mUC [using a ≥5% cutoff, ORR was 53.8% in PD-L1+ patients versus 9% in PD-L1− patients (2/22; P = 0.060)] [36]. A pooled analysis of the initial 44 patients and a larger cohort of 197 mUC patients reported an ORR of 17.6% [25% for PD-L1+ and 14.7% for PD-L1− tumors (P = 0.178)]. In this larger cohort, PD-L1 tumor IHC seemed less relevant [37]. A phase III trial of avelumab and best supportive care versus best supportive care as a maintenance therapy in patients with advanced urothelial carcinoma not progressing after first-line platinum-based therapy is in progress (JAVELIN Bladder 100; NCT02603432). In addition, a phase Ib trial (NCT02994953) will be looking at immune subsets in all solid tumor types following combination treatment with avelumab and the novel immunotherapy agent NHS-IL12, a fusion protein consisting of the heavy chains of antibody NHS76, raised against necrotic tumor cell DNA, and genetically modified human IL-12, an interleukin with immunostimulatory and antiangiogenic activity, that plays a critical role in regulating the transition from innate to adaptive immunity.
NEW IMMUNE TARGETS
Many new immune-based targets are being explored in urothelial carcinoma clinical trials, either as monotherapy or in combination with other immune-targeted or nonimmune-targeted therapies.
Small molecules
CPI-444 (Corvus Pharmaceuticals, Burlingame, CA, USA) is an oral small molecule inhibitor that blocks binding of adenosine to the A2A receptor on T cells and other immune cells and suppresses antitumor activity. It is in a phase I/Ib study as monotherapy and in combination with atezolizumab in advanced cancers, including bladder cancer (NCT02655822).
Adoptive cell therapy
Solid tumors including bladder cancer are being studied in a phase I trial of adoptive transfer of autologous T cells engineered to recognize NY-ESO-1, MAGE-A4, PRAME, survivin, and SSX (NCT02239861).
Oncolytic viruses
CAVATAK (Viralytics, Sydney, New South Wales, Australia) is a proprietary formulation of the common cold coxsackievirus A21. CAVATAK has been reported to bind to specific receptors highly expressed on a range of cancer cells. Through tumor cell lysis, CAVATAK induces changes in the tumor microenvironment that promote antitumor immunity. CAVATAK is being evaluated in a two-part study in NMIBC. The first part will examine the safety and tolerability of intravesical instillation in NMIBC patients scheduled to undergo transurethral resection. The second part will assess the safety and tolerability of CAVATAK in sequential combination with low-dose mitomycin C.
Enadenotucirev (PsiOxus Therapeutics, Abingdon, Oxfordshire, UK) is a nonnaturally occurring Group B adenovirus developed by directed evolution to generate optimal anticancer activity. It was selected to retain cancer-killing activity in human blood and thus can be administered intravenously. It is in a phase I study in patients with advanced or metastatic epithelial tumors not responding to standard therapy, including urothelial carcinoma (NCT02636036).
CG0070 (Cold Genesys, Santa Ana, CA, USA) is an oncolytic adenovirus that expresses the immune stimulatory cytokine GM-CSF. It is being administered intravesically as a single-arm intervention in an open-label, phase III study (goal n = 122) of safety and efficacy in patients with NMIBC who have progressed on BCG therapy and refused cystectomy (NCT02365818).
New checkpoint target
AMG 228 (Amgen, Thousand Oaks, CA, USA) is an antibody to glucocorticoid-induced tumor necrosis factor receptor-related protein, a costimulatory immune checkpoint molecule that is increased by T-cell activation and is reported to inhibit the suppressive activity of regulatory T cells and extend the survival of effector T cells. It is in clinical trials in multiple solid tumor indications, including bladder cancer.
Checkpoint combination and new target
A new phase I/II study will evaluate in situ vaccination with the anti-CTLA-4 antibody tremelimumab and durvalumab in combination with the tumor microenvironment modulator polyICLC, a Toll-like receptor 3 agonist that plays a fundamental role in activating innate immunity in patients with advanced, measurable, biopsy-accessible cancers, including bladder cancer (NCT02643303).
mAbs not directed at immune checkpoints
Several nonimmunotherapy mAbs are also being investigated in multiple studies of solid tumors, including bladder cancer: anti-CEA antibody MK-6018 in advanced or recurrent cancers including bladder cancer (NCT02346955); antibody-drug conjugate HuMax targeting tissue factor (NCT02552121); anti-FGFR3 antibody B-701 (NCT02401542); and ramucirumab (Eli Lilly, Indianapolis, IN, USA), a VEGFR2 mAb, and chemotherapy (NCT02426125).
New antibody strategies
B7-H3, a member of the B7 family of immunoregulatory proteins, is overexpressed in a variety of cancers and cancer stem cells, as well as in tumor stroma, including tumor vasculature. Inhibition of several members of the B7 family has been shown to have powerful antitumor effects in several solid tumor types. MGD009 (Macrogenics, Rockville, MD, USA) is a humanized B7-H3 × CD3 dual affinity retargeting protein that acts primarily by redirecting T cells via their expression of CD3 to kill B7-H3-expressing cells. It is in a phase I trial in patients with unresectable or metastatic B7-H3-expressing neoplasms, including bladder cancer (NCT02628535).
ALT-801 (Altor Bioscience, Miramar, FL, USA), a fusion of IL-2 and an antibody directed to tumor cell surface peptides, is in a phase I/II trial in combination with gemcitabine (both agents administered intravenously) in patients with NMIBC who have progressed on BCG (NCT01625260).
Development of biomarkers
Many immune-based biomarkers are being studied in urothelial carcinoma, including predictive biomarkers for patient selection, pharmacodynamic markers of target engagement, and identification and standardization of surrogate markers/endpoints in the development of immunotherapy.
Programed death-ligand 1 immunohistochemistry
PD-L1 is highly expressed in urothelial carcinoma and has been correlated with pathologic stage [38] and OS [39]. The rapid development of PD-1/PD-L1 immune checkpoint inhibitors has created an urgent need for predictive biomarkers to aid in the selection of patients most likely to respond to therapy [40]. Many clinical trials, particularly of monotherapies, have suggested that patients with PD-L1-expressing tumors or tumor-infiltrating immune cells have a greater response to PD-1/PD-L1 inhibition [12,18,19▪▪]. However, a fairly large proportion of patients with PD-L1− tumors also benefit from PD-1/PD-L1 inhibition. Also, baseline PD-L1 status seems to be less relevant in combination studies. If PD-L1 expression correlates with higher responses to PD-1/PD-L1 inhibitors, there is a rationale for potentially inducing higher responses by priming the tumor and immune system with immunomodulatory agents that increase the expression of PD-L1 in tumors. This is the leading rationale for combination immunotherapy. There are two major issues with using PD-L1 as a predictive biomarker in checkpoint-inhibitor clinical trials. First, PD-L1 status is dynamic. Therefore, a biopsy at one time point, such as baseline, may not accurately reflect the tumor microenvironment. Second, PD-L1 antibody assays and interpretation of IHC staining are not standardized and thus are highly variable. Of the four common PD-L1 antibody assays (SP142, 22C3, 28-8, and 5H1), some measure PD-L1 in the tumor, some measure PD-L1 in immune-infiltrating cells, and some measure both [40]. The assays use different cutoffs for positivity, including 1%, 5%, and an IHC score based on a sliding range. This lack of standardized PD-L1 testing is a major obstacle to comparing the strength of PD-L1 as a predictive biomarker across trials.
Tumor samples from the phase II IMvigor 210 trial of atezolizumab in locally advanced or mUC patients who progressed following platinum-based chemotherapy were analyzed for response on the basis of expression of PD-L1 on tumor-infiltrating immune cells [11▪▪]. Among all patients, 14.8% had some tumor shrinkage that lasted from 2.1 to more than 13.8 months at the time of response analysis. Among patients classified as positive for PD-L1 expression on infiltrating immune cells, 26% had tumor shrinkage versus 9.5% classified as negative for infiltrating immune-cell PD-L1 expression. These data suggested that, in this setting, PD-L1 expression on infiltrating immune cells may predict which patients would most benefit from this therapy. Therefore, the FDA also approved a complementary (but not mandatory) diagnostic for atezolizumab, the Ventana PD-L1 (SP142) assay, to quantify PD-L1 expression on tumor-infiltrating immune cells [41].
In contrast, in a study of atezolizumab in cisplatin-ineligible locally advanced or mUC patients, which was designed to test the association of response and PD-L1+ infiltrating immune cells, there was no significant enrichment of response by PD-L1 expression. This will be further analyzed in two phase III studies, IMvigor 130 in treatment-naïve patients (NCT02807636) and IMvigor 211 in platinum-treated patients (NCT02302807) [20▪▪]. It is interesting that mUC patients with tumors that had high PD-L1+ infiltrating immune cells had higher responses to monotherapy with atezolizumab if they had been previously treated with chemotherapy. This was not true for chemotherapy-naïve patients. Chemotherapy may be acting as an immunomodulator that increases PD-L1 expression in tumor immune-infiltrating cells. This was demonstrated in a retrospective analysis by IHC staining for PD-L1 in matched samples from 40 patients with MIBC before and after neoadjuvant chemotherapy [42]. The study showed that PD-L1 tumor expression was significantly higher after neoadjuvant chemotherapy compared with baseline (P = 0.0235), indicating that adaptive regulation of the immune response by PD-L1 can occur in patients with MIBC treated with neoadjuvant chemotherapy.
Neoantigens
Increased mutational load has been associated with enhanced checkpoint inhibitor responsiveness. Diaz et al.[43] compared response with pembrolizumab in patients with progressive metastatic colorectal cancer with mismatch repair deficiency to patients with mismatch repair-replete tumors. The correlation of higher mutational load and response to checkpoint inhibitor therapy has also been shown with CTLA-4 blockade in melanoma [43,44]. Furthermore, Swanton et al. have shown that in advanced melanoma and NSCLC patients treated with anti-CTLA-4 or anti-PD-1, a high tumor burden of clonal neoantigens correlated with higher levels of tumor-infiltrating lymphocytes and improved survival. When they analyzed the clonality of tumor neoantigen expression, almost every tumor with a high mutational load and low neoantigen subclonal fraction (<5% subclonal) demonstrated durable clinical benefit from anti-PD-1 therapy [45].
These data suggest that mutational burden and neoantigen expression may be predictive of response to immune checkpoint therapy in bladder cancer patients. The research of Swanton et al. further suggests that tumors should be more extensively analyzed for neoantigen clonality and that therapeutic development should be targeted at clonal neoantigens. The Parker Institute for Cancer Immunotherapy and the Cancer Research Institute have announced that they will be leading a new global collaboration of 30 cancer neoantigen research groups [46]. This collaboration, the Tumor neoantigEn SeLection Alliance (TESLA), is designed to accelerate the discovery of personalized cancer immunotherapies based on refining bioinformatics for analysis of tumor DNA and RNA sequences to predict neoantigens most likely to be present on each patient's cancer and most visible to the immune system. Thus, neoantigen burden and clonality are potentially predictive of response to immune-targeted therapy, and patient tumor-specific neoantigen sequences may potentially form the basis of personalized, targeted cancer immunotherapy.
CONCLUSION
The current review discusses recent advances in immunotherapy for urothelial carcinoma, highlighting the development of novel biomarkers and emerging immunotherapies in various stages of clinical development. An ongoing question in immunotherapy clinical trials is whether we can enhance response to checkpoint inhibitors with combination therapy. The ideal biomarker to predict response to treatment is still a matter of intense research. PD-L1 expression appears to predict better response to monotherapy only in patients previously treated with chemotherapy, and results have been inconsistent across mUC trials. Assays need to be compared and standardized before they can be used to select patients for therapy. Furthermore, biomarker evaluation of tumor samples shows that immune response is dynamic, and that PD-L1 expression at a single time point may not reflect an evolving immune response in the blood or tumor microenvironment [47,48]. Finally, combining checkpoint inhibition with other active agents that enhance immune response may increase responsiveness in urothelial carcinoma and other solid tumors. Many clinical trials are in development to further investigate this approach.
Acknowledgements
The authors thank Bonnie L. Casey for editorial assistance in the production of this article, and Erina He and Alan Hoofring for the illustration of the figures.
Financial support and sponsorship
The work was supported by the Intramural Research Program, Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 2.American Cancer Society, American Cancer Society. Key statistics for bladder cancer. 2017; Available from: https://www.cancer.org/cancer/bladder-cancer/about/key-statistics.html. [Accessed 17 February 2017]. [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003; 349:859–866. [DOI] [PubMed] [Google Scholar]
- 4.Witjes JA, Comperat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol 2014; 65:778–792. [DOI] [PubMed] [Google Scholar]
- 5.De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012; 30:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette–Guerin in the treatment of superficial bladder tumors. J Urol 1976; 116:180–183. [DOI] [PubMed] [Google Scholar]
- 7.Morales A. Treatment of carcinoma in situ of the bladder with BCG. Cancer Immunol Immunother 1980; 9:69–72. [Google Scholar]
- 8.Brandau S, Suttmann H. Thirty years of BCG immunotherapy for nonmuscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother 2007; 61:299–305. [DOI] [PubMed] [Google Scholar]
- 9.Kitamura H, Tsukamoto T. Immunotherapy for urothelial carcinoma: current status and perspectives. Cancers (Basel) 2011; 3:3055–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin FM, Kamat AM. Definition and management of patients with bladder cancer who fail BCG therapy. Expert Rev Anticancer Ther 2009; 9:815–820. [DOI] [PubMed] [Google Scholar]
- 11▪▪.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016; 387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]; Largest single-arm phase II study of a PD-L1 checkpoint inhibitor in patients with refractory or recurrent advanced metastatic urothelial carcinoma (mUC). This study led to US Food and Drug Administration approval.
- 12.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515:558–562. [DOI] [PubMed] [Google Scholar]
- 13.Galsky MD, Retz MM, Siefker-Radtke A, et al. Efficacy and safety of nivolumab monotherapy in patients with metastatic urothelial cancer (mUC) who have received prior treatment: results from the phase II CheckMate-275 study. Presented at: 2016 ESMO Congress; 7–11 October 2016. Copenhagen, Denmark; 2016:abstr LBA31. [Google Scholar]
- 14.Kim J, Tomita Y, Trepel J, Apolo AB. Emerging immunotherapies for bladder cancer. Curr Opin Oncol 2015; 27:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomized, open-label, phase 1b study. Lancet Oncol 2017; 18:212–220. [DOI] [PubMed] [Google Scholar]
- 16▪.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. NEJM 2017; Feb 17. doi: 10.1056/NEJMoa1613683. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Only randomized phase III study reported to date of a treatment with pembrolizumab, a checkpoint inhibitor, in patients with refractory or recurrent mUC.
- 17▪▪.Sharma P, Callahan MK, Bono P, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open-label, two-stage, multiarm, phase 1/2 trial. Lancet Oncol 2016; 17:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]; First published study of nivolumab, a PD-1 checkpoint agent, in refractory or recurrent mUC.
- 18.Massard C, Gordon MS, Sharma S, et al. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016; 34:3119–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪▪.Apolo AB, Infante J, Balmanoukian A, et al. Avelumab, an anti-PD-L1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase 1b study. J Clin Oncol 2017; [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]; First published study of avelumab, a PD-L1 checkpoint agent, in refractory or recurrent mUC.
- 20▪▪.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]; First reported study of a PD-L1 checkpoint agent, atezolizumab, as first-line therapy for patients with mUC.
- 21▪.Balar AV, O’Donnell PH, Vuky J, et al. Pembrolizumab (pembro) as first-line therapy for advanced/unresectable or metastatic urothelial cancer: Preliminary results from the phase 2 KEYNOTE-052 study. ESMO Congress Meeting; Abstracts 2016:abstr LBA32_PR. [Google Scholar]; Study of pembrolizumab as first-line therapy for patients with mUC.
- 22.Fishman M, Hadjenberg J, Kuzel T, et al. Phase I/II clinical trial of ALT-801, a T-cell receptor/IL-2 fusion protein, plus gemcitabine and cisplatin in urothelial cancer. J Clin Oncol 2013; 31 (6_suppl):271–271. [Google Scholar]
- 23.Galsky MD, Hahn N, Albany C, et al. Phase II trial of gemcitabine + cisplatin + ipilimumab in patients with metastatic urothelial cancer. J Clin Oncol 2016; 34 suppl 2S:abstr 357. [Google Scholar]
- 24.Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med 2015; 13:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carthon BC, Wolchok JD, Yuan J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res 2010; 16:2861–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galsky MD, Hahn N, Albany C, et al. Impact of gemcitabine + cisplatin + ipilimumab on circulating immune cells in patients (pts) with metastatic urothelial cancer (mUC). J Clin Oncol 2015; 33 suppl:abstr 4586. [Google Scholar]
- 27.Modification of the dosage regimen for nivolumab. U.S. Food & Drug Administration. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm520871.htm. [Accessed 15 February 2017]. [Google Scholar]
- 28.Nivolumab (Opdivo) for hodgkin lymphoma. U.S. Food & Drug Administration. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm501412.htm. [Accessed 15 February 2017]. [Google Scholar]
- 29.Nivolumab for SCCHN. U.S. Food & Drug Administration. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm528920.htm. [Accessed 15 February 2017]. [Google Scholar]
- 30.Nivolumab for treatment of urothelial carcinoma. U.S. Food & Drug Administration. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm539646.htm. [Accessed 15 February 2017]. [Google Scholar]
- 31.SITC 2016: immune checkpoint inhibitors shrink tumors in some patients with metastatic bladder cancer. The ASCO Post. Available from: http://www.ascopost.com/News/44121. [Accessed 17 February 2017]. [Google Scholar]
- 32.Pembrolizumab label updated with new clinical trial information. U.S. Food & Drug Administration. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm478493.htm. [Accessed 17 February 2017]. [Google Scholar]
- 33.Pembrolizumab (KEYTRUDA) checkpoint inhibitor. U.S. Food & Drug Administration. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm526430.htm. [Accessed 17 February 2017]. [Google Scholar]
- 34.Durvalumab granted breakthrough therapy designation by U.S. FDA for treatment of patients with PD-L1 positive urothelial bladder cancer. Drugs.com; 2016. Available from: https://www.drugs.com/clinical_trials/durvalumab-granted-breakthrough-therapy-designation-u-s-fda-patients-pd-l1-positive-urothelial-17029.html. [Google Scholar]
- 35.Boyerinas B, Jochems C, Fantini M, et al. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res 2015; 3:1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apolo AB, Infante J, Hamid O, et al. Safety, clinical activity, and PD-L1 expression of avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase Ib trial. J Clin Oncol 2016; 34 suppl 2S:abstr 367. [Google Scholar]
- 37.Patel M, Ellerton J, Agrawal M, et al. 777PD – Avelumab (MSB0010718C; anti-PD-L1) in patients with metastatic urothelial carcinoma progressed after platinum-based therapy or platinum ineligible. Annn Oncol 2016; 27:266–295. [Google Scholar]
- 38.Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Cancer 2007; 109:1499–1505. [DOI] [PubMed] [Google Scholar]
- 39.Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 2008; 14:4800–4808. [DOI] [PubMed] [Google Scholar]
- 40.Apolo AB. PDL1: the illusion of an ideal biomarker. Eur Urol Focus 2016; 1:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FDA approves new, targeted treatment for bladder cancer. U.S. Food & Drug Administration. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm501762.htm. [Accessed 12 February 2017]. [Google Scholar]
- 42.McDaniel A, Alva A, Zhan T, et al. Expression of PDL1 (B7-H1) before and after neoadjuvant chemotherapy in urothelial carcinoma. Eur Urol Focus 2016; 1:265–268. [DOI] [PubMed] [Google Scholar]
- 43.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014; 371:2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351:1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parker Institute for Cancer Immunotherapy and Cancer Research Institute Launch Collaboration on cancer neoantigens: Parker Institute for Cancer Immunotherapy. Available from: http://www.parkerici.org/media/2016/parker-institute-for-cancer-immunotherapy-cri-neoantigen-alliance. [Accessed 12 February 2017]. [Google Scholar]
- 47.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Callea M, Genega E, Gupta M, et al. PD-L1 expression in primary clear cell renal cell carcinomas (ccRCCs) and their metastases. J Clin Oncol 2014; 32 suppl 4:abstr 467. [Google Scholar]


