Abstract
Objective:
TX-004HR is an investigational, muco-adhesive, vaginal, softgel capsule containing low-dose, solubilized, 17β-estradiol designed to treat postmenopausal vulvar and vaginal atrophy (VVA) and improve user experience without an applicator and less mess.
Methods:
As part of the 12-week, placebo-controlled, double-blind, phase 3 REJOICE trial evaluating the efficacy/safety of 4-, 10-, and 25-μg TX-004HR in 764 postmenopausal women with VVA, a five-question product survey was administered. Pearson correlation coefficients were used to evaluate correlations between clinical endpoints (vaginal physiology, dyspareunia, and vaginal dryness) and patient acceptability and satisfaction.
Results:
Majority of the women receiving TX-004HR or placebo reported that the product was easy to use (85.4%-92.1%) and rated ease of capsule insertion as “good” to “excellent” (75.0%-82.6%). A significantly greater percentage of women reported being “very satisfied” or “satisfied” with TX-004HR (68.6%-76.3%) than with placebo (56.8%, P < 0.05 for all). A greater percentage of women “somewhat” or “very much” preferred TX-004HR over their previous treatment versus those taking placebo (P < 0.05). Significantly more women receiving TX-004HR (72.8%-80.5%) versus placebo (62.5%, P < 0.05) would “probably” or “definitely” consider using the product again. Dyspareunia and vaginal dryness reductions were correlated with higher product satisfaction and the percentage of women who would consider re-using TX-004HR.
Conclusions:
TX-004HR had a high level of product acceptability, and more women were satisfied with TX-004HR, preferred it over their previous treatment, and would consider using it again versus placebo. Women may find TX-004HR to be a more acceptable product than current options to treat their dyspareunia associated with postmenopausal VVA.
Keywords: Acceptability, Estradiol, Genitourinary syndrome, Menopause, Satisfaction, Vulvar and vaginal atrophy
Postmenopausal vulvar and vaginal atrophy (VVA) often results from a decline in circulating estrogen levels experienced at the onset of menopause.1,2 VVA is characterized by a constellation of symptoms, including dyspareunia; vaginal dryness, irritation, soreness, stinging pain, and discharge; and dysuria.1,2 In 2014, the International Society for the Study of Women's Sexual Health and The North American Menopause Society adopted genitourinary syndrome of menopause as a medically more accurate and all-encompassing term for describing VVA and its urogenitary symptoms3; however, this term has yet to be adopted by the United States Food and Drug Administration.
The North American Menopause Society recommends nonhormonal lubricants and vaginal moisturizers as first-line therapies for women with symptomatic VVA and low-dose vaginal estrogens for those with moderate-to-severe VVA or who have failed to respond to lubricants and moisturizers,4 which do not address the underlying physiological condition. The vaginal route of administration allows for localized treatment with low-dose estrogens to relieve symptoms with little to no systemic estrogen exposure.4,5 Potential benefits of vaginal versus oral administration are avoidance of gastrointestinal absorption and hepatic first-pass effects, which allows for lower dosing and lower systemic exposure, and potentially a lower incidence of adverse effects.5,6
Despite being proven as clinically efficacious and safe as indicated, use of many vaginal estrogen treatments has been hindered by issues related to route of administration, convenience, and safety concerns with long-term use and systemic exposure.7-10 Respondents in the Real Women's Views of Treatment Options for Menopausal Vaginal Changes (REVIVE) survey frequently reported messiness, difficult administration, inconvenience, and insufficient symptomatic relief as limitations of over-the-counter vaginal moisturizers or lubricants and prescription vaginal products.7 Women using vaginal creams also expressed difficulties with administering the correct dosage,9 whereas those using vaginal rings experienced difficulties with insertion and removal as well as concern related to potential dislodging of the ring and hygiene.1,4,8
Collectively, these issues with currently available products often result in poor patient satisfaction and ultimately poor adherence to the prescribed dosing regimen and longer-term treatment. About one-quarter of the respondents in the Women's EMPOWER survey reported being not very and not at all satisfied (21%-50%) with currently available vaginal-based hormone therapies for VVA, with those using a vaginal ring being the least satisfied (50%).11 Poor adherence was demonstrated by a survey (n = 200) that found many of the postmenopausal vaginal cream users deliberately did not adhere to the prescribed dosage, with some applying larger amounts to achieve greater efficacy or faster therapeutic response, and others applying a smaller amount to avoid messiness or leakage.9 Furthermore, a retrospective analysis of prescription renewals reported that the majority of American women discontinued the use of vaginal creams after an average of 45 days and vaginal tablets after 103 days.12 Similarly, 38% of women who had ever used prescription products in the Respondents in the REVIVE survey chose not to refill their vaginal estrogen therapy because of concerns related to safety or adverse effects, including long-term safety, administration, messiness, and overall treatment efficacy.10
TX-004HR (TherapeuticsMD, Inc, Boca Raton, FL) is an investigational, applicator-free, muco-adhesive, vaginal, softgel capsule containing low-dose solubilized 17β-estradiol designed to provide relief from symptoms of postmenopausal VVA.13 TX-004HR at doses of 4, 10, and 25 μg significantly and rapidly improved the percentages of superficial and parabasal cells, vaginal pH, and severity of dyspareunia and vaginal dryness in postmenopausal women with VVA during the 12-week study period,13 with negligible to very low systemic absorption of estradiol.14
The present study evaluated the product acceptability and patient satisfaction with applicator-free TX-004HR vaginal softgel capsules, as assessed by a survey as part of the phase 3, placebo-controlled REJOICE pivotal trial.
METHODS
Study design
The 12-week phase 3, multicenter, randomized, double-blind, placebo-controlled REJOICE trial evaluated the efficacy and safety of TX-004HR at doses of 4, 10, and 25 μg in postmenopausal women diagnosed with VVA and self-reported most bothersome symptom of moderate-to-severe dyspareunia (NCT02253173). Complete details regarding the study design for the REJOICE trial have been previously reported.13 Briefly, women were randomized 1:1:1:1 to 4-, 10-, or 25-μg TX-004HR or matching placebo, using a computer-generated randomization schedule.13 All study medication looked the same, and study staff and participants were not aware of the treatment they dispensed or received, respectively.13 Women self-administered one capsule per day intravaginally for 2 weeks, after biweekly dosing (3-4 days apart) for 10 weeks.13 Co-primary endpoints were changes from baseline to week 12 in the percentages of superficial and parabasal cells, vaginal pH, and severity of dyspareunia.13 Secondary outcome measures included changes from baseline to weeks 2, 6, and 8 in the same endpoints and vaginal dryness and vulvar and/or vaginal itching and irritation at all time points, as well as a product acceptability questionnaire administered at week 12.13 Dyspareunia and vaginal dryness were assessed with the VVA Symptom Self-Assessment Questionnaire (scoring: 0 = none, 1 = mild, 2 = moderate, 3 = severe) at each timepoint.
The study was conducted in accordance with applicable laws and regulations including, but not limited to, the International Conference on Harmonization Guideline for Good Clinical Practice and the ethical principles that have their origins in the Declaration of Helsinki. The study protocol, including the informed consent form, was approved by an independent institutional review board.
Study participants
Complete details regarding the inclusion/exclusion criteria for the REJOICE trial have been previously published.13 Postmenopausal women 40 to 75 years of age were enrolled if they had ≤5% superficial cells on vaginal cytological smear; vaginal pH >5.0, most bothersome symptom of moderate-to-severe vaginal pain associated with sexual activity (dyspareunia), onset of moderate-to-severe dyspareunia after menopause, body mass index ≤38 kg/m2, and were sexually active (with vaginal penetration) and anticipated having sexual activity during the trial period. Participants with an intact uterus must have had a screening endometrial biopsy sample sufficient for analysis.
Acceptability questionnaire
The acceptability questionnaire consisted of five questions and was administered to study participants at the end of their study participation. The questions were related to ease of product use, ease of capsule insertion, level of product satisfaction, the likelihood of reusing this form of treatment, and the preference of the study medication to previous medications (Table 1).
TABLE 1.
Product acceptability questionnaire administered as part of the REJOICE trial
| Questions | Choices |
| 1. Was the product easy to use? | Yes, or no |
| 2. How would you rate the ease of insertion of the capsule? | Excellent, good, fair, or poor |
| 3. Level of satisfaction with the product | Very satisfied, satisfied, unsure, dissatisfied, or very dissatisfied |
| 4. How do you compare the treatment you received in this study to previous medication or therapies for your vulvar and vaginal atrophy symptoms? | Very much prefer present treatment, somewhat prefer present treatment, no preference, somewhat prefer previous treatment, very much prefer previous treatment, or previously not used treatment |
| 5. Would you consider using this form of treatment again? | Definitely, probably, unsure, probably not, or definitely not |
Statistical analyses
Responses to the acceptability questionnaire were analyzed for the intent-to-treat population, with each TX-004HR dose group being compared with placebo using either Fisher's exact test or the Cochran-Mantel-Haenszel test. Pearson correlation coefficients were calculated and used to assess the relationship between product acceptability and each of the four co-primary endpoints (changes from baseline to week 12 in percentage of superficial cells, percentage of parabasal cells, vaginal pH, and severity of dyspareunia) and the secondary endpoint of change from baseline to week 12 in vaginal dryness evaluated as part of the primary REJOICE trial. Correlations were based on individual level paired data. Change in dyspareunia and vaginal dryness severity ranged from −3 to +3, ease of insertion ranged from 1 (poor) to 4 (excellent), level of satisfaction ranged from −2 (very dissatisfied) to 2 (very satisfied), and whether women would consider using the product again ranged from −2 (definitely no) to 2 (definitely yes). Associations between ease of product use (binary variable: yes or no) and mean changes in dyspareunia and vaginal dryness were analyzed using t tests. P values <0.05 were considered statistically significant. No Bonferroni adjustments were made for simultaneous multiple inferences.
RESULTS
Participant disposition and baseline characteristics
Of 2,183 women screened, 764 satisfied eligibility criteria and were randomized to TX-004HR 4 μg (n = 191), 10 μg (n = 191), or 25 μg (n = 190), or placebo (n = 192). Ninety-two percent (704/764) of the women completed the study, and 96% (731/764) provided answers to the questionnaire. Participant demographics and baseline characteristics were similar between the four groups, with women having a mean age of 59, and the majority being white (Table 2). At baseline, approximately 32% of women had not used any product to treat their VVA symptoms (Table 2).
TABLE 2.
Patient demographics and baseline characteristics of ITT population
| TX-004HR (4 μg) | TX-004HR (10 μg) | TX-004HR (25 μg) | Placebo | |
| N | 191 | 191 | 190 | 192 |
| Age, y | ||||
| Mean ± SD | 59.8 ± 5.9 | 58.5 ± 6.3 | 58.9 ± 6.3 | 59.3 ± 6.1 |
| Race, n (%) | ||||
| White | 167 (87.4) | 168 (88.0) | 165 (86.8) | 162 (84.4) |
| Black or African American | 20 (10.5) | 21 (11.0) | 24 (12.6) | 24 (12.5) |
| Asian | 3 (1.6) | 2 (1.0) | 1 (0.5) | 1 (0.5) |
| Other | 1 (0.5) | 0 (0.0) | 0 (0.0) | 5 (2.6) |
| BMI, kg/m2 | ||||
| Mean ± SD | 26.5 ± 4.9 | 26.8 ± 4.7 | 26.7 ± 4.8 | 26.7 ± 4.6 |
| Never used VVA treatment, n (%) | 62 (32.5) | 68 (35.6) | 56 (29.5) | 61 (31.8) |
BMI, body mass index; ITT, intent-to-treat; VVA, vulvar and vaginal atrophy; SD, standard deviation.
Product administration
The majority of women taking either TX-004HR or placebo (85.4%-92.1%) found the product easy to use. Similarly, the majority of all women (75.0%-82.6%) rated the ease of capsule insertion as “good” or “excellent.” Statistically significant differences between 10-μg (90.1%) and 25-μg (92.1%) TX-004HR with placebo (85.4%, P < 0.05) were found for ease of use but not for the 4-μg dose (89.5%, P > 0.05). Statistically significant differences were also found for ease of insertion for all three TX-004HR doses compared with placebo (4 μg [81.7%], 10 μg [81.2%], and 25 μg [82.6%] vs placebo [75.0%], P < 0.05).
Changes in dyspareunia severity were significantly associated with ease of product use (P = 0.0002) and correlated with an inverse relationship with ease of capsule insertion (r = −0.217, P < 0.0001). Changes in vaginal dryness severity were also significantly associated with ease of product use (P < 0.0001) and correlated with an inverse relationship with ease of capsule insertion (r = −0.236, P < 0.0001).
Product satisfaction and treatment preference
A significantly greater percentage of women reported being “satisfied” or “very satisfied” with TX-004HR 4 μg (68.6%, P = 0.0009), 10 μg (72.8%, P < 0.0001), and 25 μg (76.3%, P < 0.0001) versus placebo (56.8%; Fig. 1). Correlations with an inverse relationship were observed between the level of product satisfaction and changes in dyspareunia (r = −0.551, P < 0.0001) as well as changes in vaginal dryness (r = −0.533, P < 0.0001). Level of product satisfaction was also correlated with changes in the percentage of superficial cells (r = 0.142, P = 0.0002) and inversely correlated with changes in vaginal pH (r = −0.159, P < 0.0001).
FIG. 1.
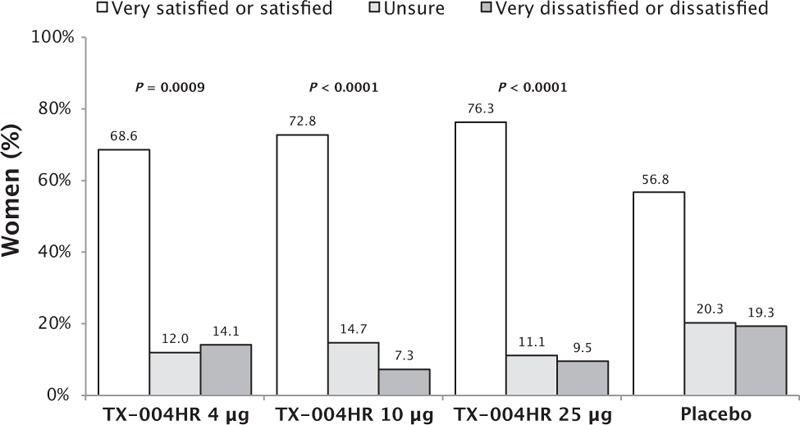
Women's satisfaction with TX-004HR. P value versus placebo.
Of the women who had previously used a product to treat their VVA, significantly more preferred TX-004HR 4 μg (68.2%, P = 0.0010), 10 μg (61.8%, P = 0.0212), and 25 μg (70.9%, P = 0.0003) over a previously used treatment than the ones who used placebo (51.9%; Fig. 2). A significantly greater percentage of women from the TX-004HR groups (72.8%-80.5%) would “probably” or “definitely” consider using the product again relative to placebo (62.5%; P = 0.0015 for 4 and 10 μg, P < 0.0001 for 25 μg; Fig. 3). Changes in dyspareunia (r = −0.433, P < 0.0001) and vaginal dryness (r = −0.410, P < 0.0001) severity were inversely correlated with the likelihood that women would consider using the study treatment again.
FIG. 2.
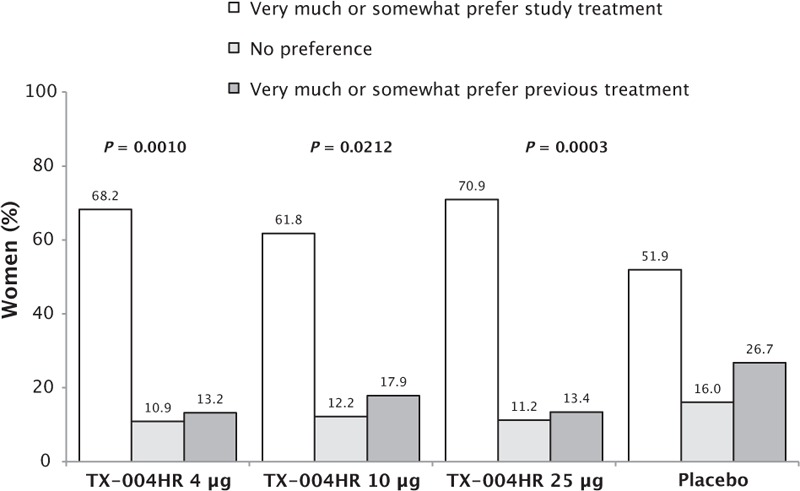
Treatment preference over previously used vulvar and vaginal atrophy treatments. P value versus placebo.
FIG. 3.
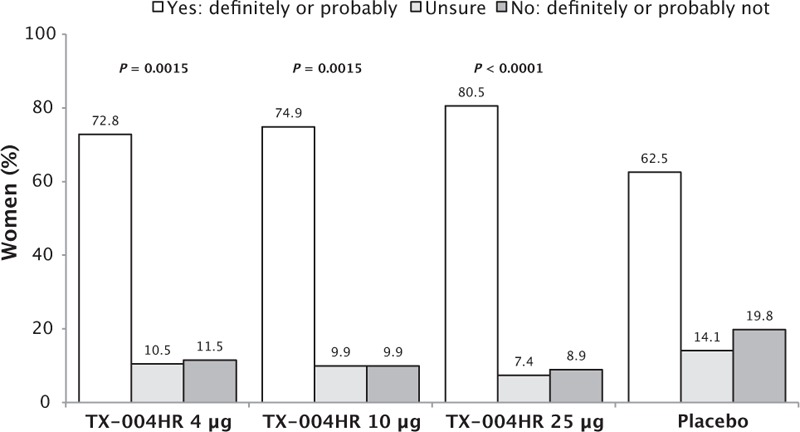
Likelihood of using TX-004HR again. P value versus placebo.
DISCUSSION
This product acceptability questionnaire, administered as part of the phase 3 REJOICE trial, revealed that approximately 90% of the women found the product easy to use, with most rating the ease of capsule insertion as “good” or “excellent.” The majority of women reported being “satisfied” to “very satisfied” with TX-004HR (68.6%-76.3% vs 56.8% for placebo) and would “definitely” or “probably use” TX-004HR again (72.8%-80.5% vs 62.5% for placebo), with statistically significant differences between the three TX-004HR doses and placebo. Importantly, most women reported that they preferred TX-004HR over their previous treatment. As expected, correlation analyses found that as severity of dyspareunia and vaginal dryness decreased, ease of product use, ease of capsule insertion, patient satisfaction, and the likelihood of using the study treatment again increased. In addition, an increase in the percentage of superficial cells and a decrease in vaginal pH were associated with higher patient satisfaction.
Data from this study are consistent with previously presented data from a phase 2 trial evaluating TX-004HR 10 μg (N = 49).15 Overall, 96% of the women participating in the study reported that the capsule was easy to insert, and 66% stated that they would “probably” or “definitely” consider using the capsule again.15 More women reported being “satisfied” or “very satisfied” with TX-004HR 10 μg (63%) versus 36% with placebo.15
Patient satisfaction with all three doses of TX-004HR (68.6%-76.3%) was considerably higher than that reported in separate studies for currently available vaginal products; in the Women's EMPOWER survey, the percentage of women who were very to extremely satisfied with their vaginal products did not exceed 51%.11 Furthermore, TX-004HR is the only vaginal product to have satisfaction rates that approach (68.5% [4 μg]) or meet (72.8% [10 μg] and 76.3% [25 μg]) the benchmark for acceptable patient satisfaction of greater than 70%.16
In this study, patient satisfaction was correlated, to a varying extent, with improvements in dyspareunia and vaginal dryness severity, percentage of superficial cells, and vaginal pH. Because all four treatments were identical in appearance, patient satisfaction with TX-004HR is likely due to the demonstrated therapeutic efficacy of TX-004HR.13 The higher than expected satisfaction rate with placebo (56.8%) is likely due to a large placebo response likely resulting from the use of Miglyol, a fractionated coconut oil with potential lubricating properties, in the formulation of the vaginal capsule.13,17 Reductions in the severity of dyspareunia and vaginal dryness being correlated with higher ease of product use and capsule insertion suggest that using the product becomes easier as vaginal physiology improved and severity of dyspareunia and vaginal dryness decreased with TX-004HR.
The formulation of the capsule may address key limitations of currently available VVA products and, in turn, may contribute to the high rates of satisfaction observed with TX-004HR. Women using vaginal creams reported missing doses because of messiness when filling and inserting the applicator, general unpleasantness of the cream, and leakage.8 Additionally, significant safety concerns associated with underdosing or overdosing were reported to cause many women to switch from a vaginal cream to a vaginal tablet.8 Women using vaginal rings were found to delay refilling their prescription because they had difficulty removing or inserting the ring; they or their partner could feel the ring; they were concerned about vaginal infection, hygiene, and cleanliness; or because the ring was not the lowest effective dose of estrogen that could be prescribed.8 The softgel capsule formulation of TX-004HR may circumvent many of these issues by allowing for accurate dosing and easy administration, which may be more hygienic as an applicator is not necessary. TX-004HR has been designed to rapidly dissolve, with no vaginal secretions required for activation, and therefore, should minimize vaginal discharge, resulting in less messiness.
A separate analysis of 13,074 American women found that women initiating vaginal tablets versus vaginal cream were more likely to continue therapy for a longer period and exhibited greater adherence to medication, suggested by the authors to be due to less issues with messiness and fixed dosing.18 An online survey of 200 American women reported that 45% of women who use vaginal creams for VVA deliberately used less than the recommended dose to avoid messiness or leakage.9 Furthermore, significantly more women who used vaginal creams reported delaying or not using the medication because of messiness compared with users of vaginal tablets (67% vs 17%).9 Another online survey of 423 Swedish women reported that women would be willing to pay more for vaginal tablets than vaginal creams to avoid smudges/leakage.19 Thus, women prescribed TX-004HR may be more likely to adhere to treatment due to the demonstrated efficacy of TX-004HR for treating postmenopausal VVA and overall ease of use of the product and inserting the capsule, as well as patient satisfaction.
CONCLUSIONS
TX-004HR, an investigational, applicator-free, muco-adhesive, vaginal, softgel capsule of low-dose, solubilized, 17β-estradiol designed to treat postmenopausal VVA provided an improved user experience compared with other currently used vaginal treatments for VVA. The majority of postmenopausal women participating in the REJOICE trial rated using the product and inserting the applicator-free capsule as easy, and when compared with placebo, were more satisfied with TX-004HR, preferred it over their previous VVA treatment, and would consider using TX-004HR again. Together with previously reported improvements in vaginal physiology and severity of dyspareunia, these survey results suggest women prescribed TX-004HR will be satisfied with their vaginal therapy, which may enhance treatment adherence for their postmenopausal VVA and moderate-to-severe dyspareunia.
Acknowledgments
The authors would like to thank the investigators of the REJOICE trial who participated in the collection of the acceptability data. The authors also thank Disha Patel, PhD, of Precise Publications, LLC, for medical writing support.
Footnotes
Funding/support: TherapeuticsMD sponsored the REJOICE study and funded the medical writing support provided by Disha Patel, PhD, of Precise Publications, LLC.
Financial disclosure/conflicts of interest: S.A.K. has served as a consultant for Acerus Pharmaceuticals, Bayer Healthcare, Emotional Brain, Materna, Novo Nordisk, Nuelle, Palatin Technologies, Pfizer, Sermonix Pharmaceuticals, Shionogi Inc, Sprout Pharmaceuticals, TherapeuticsMD, and Valeant Pharmaceuticals. R.K. has received research support or consulting fees from AbbVie, Actavis, Bayer Healthcare, Endoceutics, Palatin Technologies, Shionogi Inc, Teva Pharmaceuticals, TherapeuticsMD, and Trimel. I.G. has received research support or consulting fees from Emotional Brain, The Female Condom Company, Nuelle, Palatin Technologies, Shionogi Inc, Strategic Science & Technologies, and TherapeuticsMD. G.D.C. consults to multiple pharmaceutical companies, including but not limited to TherapeuticsMD and has stock options with TherapeuticsMD. H.K. consults to pharmaceutical companies including but not limited to TherapeuticsMD. B.B. is a board member and an employee of TherapeuticsMD with stock/stock options. S.G. and S.M. are employees of TherapeuticsMD with stock/stock options.
REFERENCES
- 1.Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc 2010; 85:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lev-Sagie A. Vulvar and vaginal atrophy: physiology, clinical presentation, and treatment considerations. Clin Obstet Gynecol 2015; 58:476–491. [DOI] [PubMed] [Google Scholar]
- 3.Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and the North American Menopause Society. Menopause 2014; 21:1063–1068. [DOI] [PubMed] [Google Scholar]
- 4.North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20:888–902. [DOI] [PubMed] [Google Scholar]
- 5.Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertil Steril 2004; 82:1–12. [DOI] [PubMed] [Google Scholar]
- 6.Vermani K, Garg S. The scope and potential of vaginal drug delivery. Pharm Sci Technol Today 2000; 3:359–364. [DOI] [PubMed] [Google Scholar]
- 7.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (Real Women's Views of Treatment Options for Menopausal Vaginal Changes) survey. J Sex Med 2013; 10:1790–1799. [DOI] [PubMed] [Google Scholar]
- 8.Minkin MJ, Maamari R, Reiter S. Improved compliance and patient satisfaction with estradiol vaginal tablets in postmenopausal women previously treated with another local estrogen therapy. Int J Womens Health 2013; 5:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minkin MJ, Maamari R, Reiter S. Postmenopausal vaginal atrophy: evaluation of treatment with local estrogen therapy. Int J Womens Health 2014; 6:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wysocki S, Kingsberg SA, Krychman M. Management of vaginal atrophy: implications from the REVIVE study. Clin Med Insights Reprod Health 2014; 8:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kingsberg SA, Krychman M, Graham S, Mirkin S, Constantine GD. The Women's EMPOWER survey: identifying women's perceptions on vulvar and vaginal atrophy (VVA) and treatment. Presented at the 27th Annual Meeting of The North American Menopause Society October 5-8, 2016, Orlando, FL (S-10). Menopause 2016; 23:1363–1407. [Google Scholar]
- 12.Portman D, Shulman L, Yeaw J, et al. One-year treatment persistence with local estrogen therapy in postmenopausal women diagnosed as having vaginal atrophy. Menopause 2015; 22:1197–1203. [DOI] [PubMed] [Google Scholar]
- 13.Constantine GD, Simon JA, Pickar JH, et al. REJOICE Study Group. The REJOICE trial: a phase 3 randomized, controlled trial evaluating the safety and efficacy of a novel vaginal estradiol soft-gel capsule for symptomatic vulvar and vaginal atrophy. Menopause 2017; 24:409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Archer DF, Constantine GD, Simon JA, et al. REJOICE Study Group. TX-004HR vaginal estradiol has negligible to very low systemic absorption of estradiol. Menopause 2017; 24:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingsberg SA, Amadio J, Graham S, Bernick B, Mirkin S. Patient experience with solubilized estradiol given vaginally in a novel softgel capsule (VagiCap™). ISSWSH Annual Meeting February 19-22, 2015, Austin, TX. Available at: http://www.isswshmeeting.org/images/ISSWSH.Annual.Meeting.2015.Program.Book.pdf Accessed February 19, 2015. [Google Scholar]
- 16.B2B International. Customer satisfaction surveys & research: how to measure. CSAT. Available at: https://www.b2binternational.com/publications/customer-satisfaction-survey/ Accessed September 8, 2016. [Google Scholar]
- 17.Kingsberg SA, Derogatis L, Simon JA, et al. TX-004HR improves sexual function as measured by the female sexual function index in postmenopausal women with vulvovaginal vulvar and vaginal atrophy: the REJOICE Trial. J Sex Med 2016; 13:1930–1937. [DOI] [PubMed] [Google Scholar]
- 18.Shulman LP, Portman DJ, Lee WC, et al. A retrospective managed care claims data analysis of medication adherence to vaginal estrogen therapy: implications for clinical practice. J Womens Health (Larchmt) 2008; 17:569–578. [DOI] [PubMed] [Google Scholar]
- 19.Mattsson LA, Ericsson A, Bogelund M, Maamari R. Women's preferences toward attributes of local estrogen therapy for the treatment of vaginal atrophy. Maturitas 2013; 74:259–263. [DOI] [PubMed] [Google Scholar]


