Abstract
A number of studies have shown that autologous and allogeneic hematopoietic cell transplantation (HCT) contribute to an increased incidence of cardiovascular disease (CVD) and worsening of cardiovascular risk factors (CVRFs) that could contribute to further CVD over time. These observations combined with a notable increase in the number of survivors after HCT in recent years highlight the need for studies aimed at modifying risk or preventing these outcomes by changing specific approaches and/or post-HCT interventions. To address these issues, an international consensus conference on late effects after HCT was held in Washington DC in June 2016. This report summarizes the major gaps in knowledge along with detailed recommendations regarding study priorities from the Cardiovascular Disease and Associated Risk Factors Committee, a multi-disciplinary panel of international experts. The committee calls for specific studies aimed at understanding and preventing arterial disease and cardiac dysfunction (heart failure, valvular disease, and arrhythmias), as well as decreasing cardiovascular risk factors (hypertension, hyperglycemia, dyslipidemia, and sarcopenic obesity) after HCT.
Keywords: NIH consensus, Late Effects, Hematopoietic Cell Transplantation, Cardiovascular disease, Cardiovascular risk factors, Chronic disease, Survivorship
INTRODUCTION
Despite improvements in overall outcomes, HCT survivors continue to have substantially higher mortality rates when compared with the general population.1–3 This risk persists beyond ten years after HCT, a period in time when non-relapse-related mortality rates exceed relapse-related mortality rates.1–3 An important example of this is found in cardiovascular-related mortality which, among HCT survivors, is 2- to 4-fold greater than that expected for the general population, and the incidence increases with time from HCT.4
Mortality attributed to cardiovascular disease (CVD: heart failure, myocardial infarction, stroke) underestimates the true burden of CVD after HCT. HCT survivors have a 4-fold higher risk of developing CVD when compared with the general population,4 adding to the already high burden of chronic health-related conditions in these survivors.5 This increased risk of CVD is multi-factorial, having been associated with pre-HCT therapeutic exposures (e.g. anthracycline chemotherapy, chest radiation), HCT conditioning, and cardiovascular risk factors ([CVRFs], e.g. hypertension, diabetes, dyslipidemia, abnormal body composition).6, 7 The median age at first cardiovascular event such as myocardial infarction in HCT survivors is as low as 53 years (range 35–66 years),8 much lower than would be expected in the general population (67 years).9 The markedly increased CVD risk, coupled with the recognition that these complications develop earlier than would be expected in the general population, has raised the possibility of an accelerated cardiovascular aging phenotype in HCT survivors.
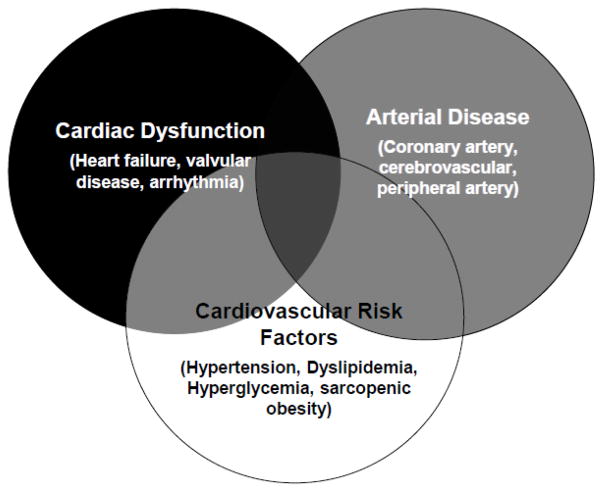
Given current high transplant volumes and improvements in post HCT survival, there is an increasing need for healthcare providers to screen for and initiate preventive measures to reduce the risk of late-occurring CVD in HCT survivors. It therefore timely to review gaps in knowledge with regards to the epidemiology, pathophysiology, as well as appropriate screening and prevention strategies for CVD. To address these gaps, our working group evaluated the current state of science, and highlighted research priorities for future studies aimed at improving the long-term cardiovascular health of the HCT survivor. Recognizing the heterogeneity of risk factors and differences in pathophysiology of disease, the working group and hence the current manuscript was organized around the following overarching topics (Figure 1): arterial disease (e.g. coronary artery, cerebrovascular, peripheral artery), cardiac dysfunction (e.g. heart failure, valvular, arrhythmia), CVRFs (e.g. hypertension, hyperglycemia, dyslipidemia, sarcopenic obesity).
Figure 1.
Health outcomes included in the current initiative, with Venn diagrams representing areas of overlap across diseases
ARTERIAL DISEASE
Arterial diseases (AD), including coronary artery, cerebrovascular and peripheral artery disease have emerged as a leading cause of non-relapse related mortality and morbidity in long-term HCT survivors.6, 7, 10 The cumulative incidence of AD is up to 22% at 20 years after allogeneic-HCT,11, 12 and the risk is twofold higher when compared to the general population.4, 12 This is due in part to the increased incidence of CVRFs including hypertension, dyslipidemia and diabetes, but also to unhealthy lifestyle behaviors (lack of physical activity, physical deconditioning) after HCT.4, 11–15 To date, most studies have been retrospective in nature or have relied on self-reported questionnaires. The lack of standardized definitions used to define AD or CVRFs have limited the generalizability of findings. Additionally, challenges remain with regards to the selection of appropriate control populations. Comparison of long-term HCT survivors with siblings is frequently used, but might represent a bias due to the familial predisposition to AD and CVRF; the use of unrelated donors is an alternative, but remains unrealistic due to the inability to collect relevant data for proper risk assessment. Finally, most studies have focused on clinically overt AD, providing an underestimation of the true burden of AD in HCT survivors.
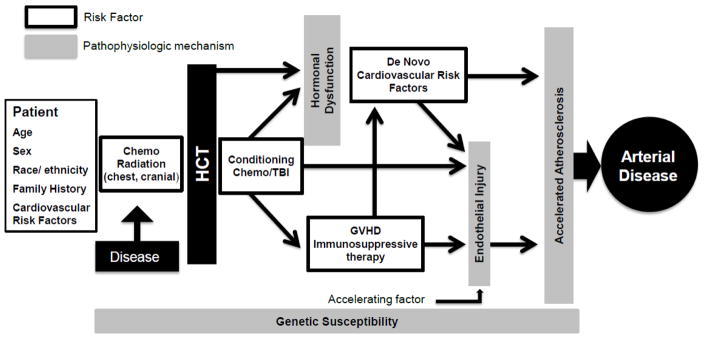
The pathogenesis of post-HCT AD seems to be multifactorial, and CVRFs play an important role in the acceleration of atherogenesis (Figure 2).4, 11–15 Additional modifying factors that have been implicated include: endothelial injury induced by radio-chemotherapy (radiation, alkylating agents, platinum agents, high-dose cyclophosphamide conditioning), graft versus host disease (GVHD), immunosuppressive agents, and other endocrine disorders (e.g. gonadal dysfunction).6, 7 Host genetic polymorphisms may be involved in modulation of AD risk after HCT;16 however, to date no specific genetic variant has been described. There remain gaps in knowledge with regards to ethnic, geographic and socio-demographic influences on post-HCT AD.17 Less is known regarding differences in the epidemiology of AD in survivors of autologous vs. allogeneic HCT, or the effect of reduced intensity conditioning (RIC) on long-term AD risk. This is may be an important area for future investigation, as there are distinct therapeutic exposures and complications (e.g. increased prevalence of chest RT exposure in autologous HCT survivors, higher burden of systemic inflammation or CVRFs in allogeneic HCT survivors) that may differentiate the screening and prevention strategies for each group.8, 11
Figure 2.
Proposed multi-factorial mechanism for accelerated atherogenesis and arterial disease in HCT survivors.
Screening for subclinical AD in HCT survivors is limited by the lack of standardized and reproducible methods for early screening in the general population. As such, most recommendations pertaining to HCT survivors have advocated early screening for CVRFs, providing opportunities to implement interventions to prevent or delay the occurrence of AD.18–21 Additionally, screening should include assessment of high-risk lifestyle behaviors such as: smoking, physical inactivity, unhealthy diet, and being overweight/obese.18–21 There is general consensus that screening should begin 1 year after HCT, and that existing population-based guidelines do not accurately predict risk of AD in long-term HCT survivors.20–22 Moreover, there is discordance among existing long-term follow-up guidelines with regards to the frequency and duration of screening. Screening for asymptomatic vascular disease using imaging studies (e.g. coronary artery calcium scoring, vascular intima-media thickness), or blood biomarkers of endothelial injury is not routinely indicated in HCT survivors; this remains an active area of investigation.
It remains to be seen whether existing prevention recommendations for the general population (e.g. aggressive management of CVRFs, adoption of healthy lifestyle)23, 24 can result in the same beneficial effect in HCT survivors. Additionally, there are gaps in knowledge with regards to the role of primary prevention (e.g. selection of reduced-intensity conditioning vs. myeloablative conditioning) for AD risk reduction. The increasing trends of HCT for higher risk patients with older age and/or more comorbidities mandate continued research in this area. Future studies aimed at prevention of AD could achieve endothelial protection and attenuation of subclinical and/or clinical disease in survivors at highest risk for clinically significant disease.
Research priorities: Arterial disease (AD).
Describe the incidence of AD in HCT survivors using standardized definitions (American College of Cardiology/American Heart Association) for cardiovascular outcomes and for CVRFs; identify representative non-HCT comparison controls.
Evaluate the effect of other metabolic derangements (e.g. gonadal dysfunction after HCT) on long-term AD risk.
Examine the latency of AD with regards to HCT-specific exposures (e.g. donor source, conditioning intensity, radiation); identify populations at highest risk.
Clarify fundamental pathways (e.g., inflammation, endothelial damage, role of endothelial progenitor cells, angiogenesis) in the development of post- HCT arterial complications.
Evaluate allo-effect and GVHD presentations on post-HCT AD risk; this knowledge would be particularly relevant for patients who have undergone RIC.
Develop studies to examine the utility of imaging approaches (e.g. coronary artery calcium scoring, vascular intima-media thickness) and blood biomarkers (e.g. endothelial injury, metabolomics) for detection of atherosclerosis in HCT survivors.
Develop HCT-specific risk prediction models for AD and other cardiovascular complications.
Test the efficacy of lifestyle modification and/or prophylactic pharmacotherapy (e.g. use of lipid lowering agents) on AD risk reduction in HCT survivors.
CARDIAC DYSFUNCTION
Heart Failure (HF)
HF is a well-described occurrence during the immediate post-HCT period.25–27 Mortality attributed to early HF ranges from 1% to 9%, whereas morbidity as defined by reduction in cardiac output is considerably higher (5% to 43%).25–27 Risk factors for early HF include reduced pre-HCT ejection fraction (EF), conditioning with high-dose cyclophosphamide (HD-CY) and total body irradiation (TBI).25–28 The incidence of late-occurring HF (1+ year post-HCT) appears to be especially high for autologous HCT recipients – cumulative incidence estimated at 5% at 5 years, approaching 10% at 15-years post-HCT.29 The risk of late-occurring HF is primarily attributable to pre-HCT anthracycline exposure, in a dose-response manner.29, 30 HF risk increases significantly among those who also have conventional CVRFs such as hypertension and/or diabetes.4, 13, 29
The vast majority of studies examining HF risk in HCT survivors have relied on retrospective cohort or population-based registry data, potentially representing the most severe phenotypes of HF in this population. Few have compared the expected incidence and/or prevalence of HF in the HCT population to that in the general population, and none have examined the contribution of other pre-HCT organ function impairments (e.g. pulmonary dysfunction, musculoskeletal impairment, arterial disease) on long-term cardiac health.
The American College of Cardiology/American Heart Association (ACC/AHA) classifies HF as a progressive disorder with a variable period of asymptomatic cardiac dysfunction that precedes clinically overt disease.31 Asymptomatic patients can present with systolic (e.g. low EF) or diastolic (e.g. prolonged mitral annular velocity) dysfunction, typically identified on routine screening resting echocardiography. 31 However, few studies have characterized the prevalence and magnitude of risk of asymptomatic HF in HCT patients, and none have described the latency of disease or trajectory of change between the two (asymptomatic, symptomatic) health states. There is very little known regarding the structural changes in cardiac function (e.g. systolic vs. diastolic impairment, or both) that precede symptomatic HF in HCT survivors, or the role existing chronic health conditions (e.g. iron overload, hypertension, microvascular disease, endothelial dysfunction, renal dysfunction) play in chronic cardiac injury after HCT.
Early screening for asymptomatic disease may provide opportunities for implementation of interventions to reduce the risk of clinically overt disease. In this context, an effective population-based strategy is one where asymptomatic disease can be detected before irreversible impairment has occurred, utilizes widely available techniques that are preferably non-invasive, inexpensive, reproducible, highly predictive of clinically significant disease, and actionable in guiding therapy. While echocardiography has been advocated for screening of asymptomatic disease, there is little information regarding the cost-effectiveness of routine surveillance post-HCT. This may be due to poor reproducibility of certain echocardiographic parameters, or lack of their predictability of clinically significant disease.32
Outcome following post-HCT HF is poor, with less than 50% surviving five years,29, 30 emphasizing the need for preventive strategies to decrease the risk of HF. Given the growing body of literature on the association between cardiovascular risk factors and HF in HCT survivors, future efforts may need to examine the utility of aggressive management of CVRFs as a means of reducing HF risk. Ongoing studies33, 34 in other cancer populations are examining the role of secondary prevention (e.g. use of beta-blockers, angiotensin-converting enzyme inhibitors, or lifestyle modification) in asymptomatic patients at high risk for clinically overt HF. Such a strategy may be considered as a means to disrupt the neurohormonal dysregulation that precedes irreversible cardiac injury.
Valvular heart disease (VHD)
Clinically significant VHD is an uncommon complication of HCT. Risk factors for VHD include pre-HCT anthracycline chemotherapy and/or chest/mediastinal radiation therapy (RT), older age at HCT, and younger age at radiation exposure. Survivors of autologous HCT appear to be at heightened risk for VHD, due in large part to pre-HCT therapeutic exposures such as chest RT.35, 36 Long-term follow-up guidelines recommend extended cardiac screening with echocardiography for those at high risk for VHD based on age at treatment, cumulative radiation and anthracycline doses, and underlying diagnosis for HCT.20, 37 Existing AHA/ACC guidelines for the management of VHD provide specific recommendations for the management of VHD, and may be applicable to HCT patients.38
Cardiac arrhythmias
Cardiac arrhythmias can have serious health implications in HCT survivors, but often cause no symptoms. The focus of long-term care is to identify and treat arrhythmias that may eventually result in symptomatic disease, other cardiovascular complications such as stroke, or hemodynamic collapse and death. This list includes, but is not limited to: atrial and ventricular fibrillation, QTc prolongation, and heart block. The incidence of arrhythmia is greatest in older populations and increases over time after HCT, reflecting the known association of older age with rhythm disturbance.4, 6 The reported incidence of cardiac arrhythmia in HCT survivors ranges between 2% and 10%, and is largely limited to lymphoma and breast cancer autologous HCT survivors treated with chest RT.11, 36, 39 The impact of allogeneic HCT on the development of arrhythmias is not clear.14
There are no established strategies for screening and prevention of arrhythmias following HCT. Existing HCT consensus guidelines20, 37 do not recommend routine screening electrocardiogram or Holter monitoring in patients in the absence of symptoms or concerning family history, though consideration for screening in those with history of chest/mediastinal radiation is suggested. Despite common clinical practice of obtaining post-HCT electrocardiograms, there are no clear guidelines for frequency and duration of electrocardiograms in asymptomatic survivors on medications known to cause QTc prolongation.
Research priorities: Cardiac dysfunction.
Define the relative contribution of pre-HCT therapeutic exposures on long-term cardiac dysfunction risk.
Examine the role pre-HCT comorbidities (e.g. iron overload, vascular disease, renal dysfunction, physical deconditioning) play in the development of chronic cardiac injury after HCT.
Examine the mechanisms of enhanced cardiotoxicity observed in HCT survivors with comorbidities such as hypertension and diabetes.
Describe the prevalence and risk factors for asymptomatic cardiac dysfunction; evaluate whether the pathophysiologic changes in HCT survivors differ from those seen in other high-risk cancer and non-cancer populations.
Evaluate the utility of novel imaging approaches (e.g. cardiac strain, cardiac MRI), as well as blood biomarkers for detection of asymptomatic cardiac dysfunction.
Describe the optimal timing, frequency, and duration of screening that would result in long-term cardiac disease risk reduction.
Develop primary and secondary interventions aimed at long-term cardiac disease risk reduction that are informed by epidemiologic studies in high risk populations.
CARDIOVASCULAR RISK FACTORS
Hypertension
Studies have consistently reported a higher prevalence of hypertension in HCT survivors when compared to the general population.4, 11, 13, 14, 40–42 Reported rates of hypertension have ranged from 10–50%, with the variation due to differences in population composition, follow-up length, and assessment method. Risk factors for hypertension among HCT survivors include allogeneic versus autologous HCT, increasing age, and the presence of obesity and other CVRFs.40, 43 While immunosuppressive medications used to treat GVHD often are associated with acute hypertension,40 evidence for acute or chronic GVHD as a risk factor for persistent hypertension once survivors are off immunosuppression has been mixed.4, 13–15 Similarly, TBI, kidney injury, and male sex while postulated to be potential risk factors have generally not been consistently found to be independent risk factors.42, 44
Limitations of the current literature include a reliance on self-report and/or medication status to define hypertension, particularly among larger studies. This likely misclassifies undiagnosed patients as well as those receiving blood pressure (BP) medications for other indications (e.g., cardiomyopathy, chronic kidney disease). Self-report also limits information on the severity of one’s hypertension, with strong evidence in the general population that risk increases with greater BP values.45, 46 Furthermore, not all survivors receiving hypertension treatment have their hypertension well-controlled. In contrast, studies based on in-person assessment may over-estimate prevalence of hypertension if ascertainment is based on only a single BP measurement versus alternative measurement modalities such as 24-hour ambulatory monitoring.45, 46
The increased prevalence of hypertension after HCT is thought to contribute to the accelerated development of other cardiovascular diseases post-transplant.6, 7 Pre-transplant and HCT-specific treatment exposures such as radiotherapy and select antineoplastic agents (e.g., anthracyclines, some tyrosine kinase inhibitors) may cause both vascular and/or direct cardiac injury. 6, 7 Some studies have shown that radiation may exert adverse effects on endothelial function even in regions distant from direct radiation exposure.47 GVHD and its associated inflammatory state, while not consistently implicated as an epidemiologic risk factor for hypertension, may plausibly still contribute to the development of premature cardiovascular disease and hypertension among HCT survivors.48 In studies that have examined the timing of hypertension, 1-year allogeneic survivors had much shorter onset versus 1-year autologous survivors (0.2 versus 3.7 years).13 An important caveat to this observation, however, is that a proportion of allogeneic patients with hypertension at 1-year post-transplant will have their condition resolve with further follow-up; therefore some of these effects can be temporary.13, 40
BP can be easily measured in most clinical environments. Current AHA and American Academy of Pediatrics guidelines49 and the BMT International LTFU Consensus guidelines20 recommend that BP be checked at every clinic visit and that any concern for hypertension (i.e., if values are high on ≥2 visits more than one week apart) be worked up and managed. While no HCT-specific data exist, data from the general population suggest that ambulatory and even home BP monitoring are likely to be superior to office-based measurements for confirming the diagnosis.45, 46 BP thresholds used to confirm a hypertension diagnosis are slightly lower for 24-hour ambulatory BP values.45, 46
Across numerous studies of both HCT and non-HCT cancer survivors, hypertension has been identified as one of the most important (and potentially modifiable) risk factors associated with subsequent more severe cardiovascular outcomes.11–13, 29, 50–52 For example, pre-transplant hypertension and hypertension persisting ≥2-years post-HCT was associated with a 3–5 times increased hazard ratio of late ischemic heart disease, heart failure, and stroke among ≥2-year HCT survivors.50 Among survivors with cancer treatment exposures known to increase cardiovascular disease risk (i.e., anthracyclines, chest radiation), hypertension appears to act synergistically to further increase risk.29, 51 As such, better control of hypertension offers the promise of potentially ameliorating a significant burden of late serious cardiovascular disease among HCT survivors.43
Treatment goals are typically to reduce BP values to <140/<90 mmHg. However, there is evidence in the general population that lower targets in the 120–129 mmHg systolic range may be even more beneficial.53 Treatment should be two-pronged, with an emphasis on lifestyle modifications (weight reduction, diet modification, increased physical activity) for those in the pre-hypertensive range, and adding in pharmacologic therapy for those meeting hypertension thresholds.54 What remains unknown is whether improved control of hypertension among HCT survivors will reduce their subsequent cardiovascular disease risk to the same degree seen in the general population.
Research priorities: Hypertension.
There are limited to no longitudinal data on the development and progression of hypertension as well as vascular and endothelial dysfunction among HCT survivors. Such data would be informative in guiding the timing of potential interventions to improve the cardiovascular health of these individuals. This would include a better understanding of the magnitude of under-treatment, and determination of clinician- and patient-specific barriers to hypertension control, including adherence and medication burden.
Investigators should determine whether a properly powered study with sufficient follow-up focused on BP control and other potentially modifiable traits in relation to serious and life-threatening cardiac endpoints (e.g., ischemic heart disease, cardiomyopathy) is feasible. If not feasible, then what surrogate measures of vascular health may serve as appropriate surrogate end points?
Hyperglycemia
Hyperglycemia is frequently observed during or shortly after HCT as a side effect of immunosuppressive drugs (glucocorticoids, tacrolimus, cyclosporin and sirolimus), transplant factors and/or acute complications of HCT (infection, inflammation, total parenteral nutrition and GvHD).55 Dysregulated glucose homeostasis may persist beyond this first phase or emerge during long-term follow-up in the form of 3 related entities: 1) Type 2 Diabetes Mellitus (T2DM), encompassing conditions characterized by hyperglycemia due to various degrees of impairment of insulin secretion or action;56 2) pre-diabetic states (PDS) where fasting or post-prandial glucose levels are chronically above the normal range without reaching those diagnostic of DM;57, 58 and 3) Insulin resistance (IR), a metabolic trait that predisposes to PDS and type 2 DM, the most prevalent form of DM in the general adult population.56
The prevalence of post- HCT T2DM ranges between 3.3–17%,13–15, 40, 43, 59, 60 rates that are higher than those observed in siblings (7.6% vs. 3.1%)14 or age/gender matched adults from the general population (14.3% vs. 11.7%, p=0.04).43 Diabetes also occurred sooner than expected in HCT survivors when compared to matched controls from the general population.4 The latency period between HCT and DM is highly variable. A trend towards worsening glucose tolerance, as evidenced by decreased glucose clearance in response to IV challenge testing, was observed as early as 6 months after HCT;61 median time to onset of post HCT of DM was 1.9 years (range 0–11 years) in a retrospective study of 1,885 HCT recipients.13 The natural history of glucose metabolism dysregulation post HCT is not well understood, given the cross-sectional nature of most studies, the complexity and heterogeneity of methods used to assess glucose tolerance (fasting labs or oral or IV glucose challenge), as well as variations in the methods of reporting DM (patient report, medication use or direct assessment) across studies.
TBI is the most consistent treatment-related risk factor for T2DM and IR post HCT.13, 14, 62, 63 Additional risk factors include allogeneic HCT,13, 14 grades II–IV acute GvHD,13 cumulative prednisone dose,40 unfavorable dietary habits,43 lower physical activity43 and family history of T2DM.59 The contributions of therapeutic exposures prior to HCT64 and acute phase hyperglycemia during HCT40 to post-HCT risk of glucose metabolism dysregulation are not known. Patients treated with HCT and who developed DM, PDS or IR were more likely to experience changes in body composition and fat distribution rather than obesity based on body mass index (BMI).50, 62, 63, 65, 66 Such differences in the phenotypic presentation are suggestive of a distinct pathophysiology. A combination of IR and relatively impaired insulin secretion61, 67 with decreased pancreatic volume and beta-cell reserve68 has been shown in patients treated with TBI and this may be a potential mechanism for the observed glucose metabolism dysregulation. Decreased insulin reserve conjugated with IR and a decrease in lean mass (sarcopenia) naturally occur with older age,69 and abnormal glucose tolerance may be among the symptoms of accelerated aging and frailty following exposure to cancer treatments.70
The Children’s Oncology Group long-term follow-Up guidelines for survivors of childhood, adolescent and young adult cancers (www.survivorshipguidelines.org) recommend measuring fasting glucose or HbA1c every 2 years or more frequently in case of impairment in patients treated with TBI.37 Screening for abnormal glucose in the general population is recommended by the United States Preventive Services Task Force (USPSTF) in overweight or obese adults aged 40–70 years.71 It is not clear whether these recommendations are appropriate for patients treated with HCT, a population at high risk for CVD, irrespective of excess adiposity.
Higher exercise capacity may reduce the risk of cardiovascular events post-HCT.72–74 A variety of oral glucose lowering medications (for e.g., metformin, insulin and acarbose) have been tested to delay or reduce the progression from PDS to DM and/or decrease CVD risk in the general population; however, metformin has been the only agent recommended by the ADA.58 No studies have tested pharmacologic interventions in HCT survivors, thus, it remains unknown whether diagnosing PDS early and treating these aggressively may improve CVD risk in this population.
Research priorities: Hyperglycemia.
Examine the role of non-BMI measures of body composition (e.g. waist/abdominal circumference) in the study of glucose metabolism in HCT recipients.
Investigate whether changes in glucose tolerance, insulin sensitivity and secretion are, along with body composition changes, part of a wider phenomenon of accelerated aging and increased CVD risk post HCT.
Assess the effects of metabolic status and toxicity from therapy give prior to HCT
Identify the ideal time interval for screening using fasting glucose levels.
Describe the utility of glucose challenge tests in comparison to fasting labs for screening for abnormal glucose metabolism.
Study the effect of diet and physical activity interventions with or without metformin on HCT patients with PDS on delaying the onset of DM and CVD.
Dyslipidemia
Dyslipidemia is a strong and targetable risk factor for coronary heart disease and ischemic stroke in the general population75 and its relevance has also been recognized in HCT survivors over the last decade.6, 7, 19 The high age-related background prevalence of dyslipidemia in the general population,76, 77 however, complicates direct comparison.
The prevalence of dyslipidemia is 2 to 3-fold higher in allo- versus auto-HCT survivors. In a retrospective cohort study, allo-HCT recipients had significantly higher risk of new-onset dyslipidemia (RR: 2.31; 95% CI, 1.15 to 4.65) compared to auto-HCT recipients.11 In the Bone Marrow Transplant Survivor Study (BMTSS), the prevalence estimates at baseline, 1 and 5 years were 22.8%, 31.3% and 38.8% for auto-HCT survivors versus 12.5%, 36.6% and 45.0% for allo-HCT survivors.14 The time to onset of dyslipidemia was also significantly shorter for allo-HCT recipients (0.2 years vs 1.6 years, P < .01).14 Once established, dyslipidemia tends to be durable. Similar trends have been reported in pediatrics, where the risk of de novo dyslipidemia is ~2 to 3-fold higher in allo- when compared to auto-HCT recipients (RR: 2.31; 95% CI, 1.15 to 4.65; P =.036). In addition, qualitative differences in pediatric dyslipidemia, such as disproportionate elevations of triglycerides, have been observed.42, 78–80
Clinical factors associated with post-HCT dyslipidemia include family history, obesity, high-dose TBI, acute GVHD, chronic GVHD, and chronic liver disease.42, 79, 81 There appear to be several mediators of risk, including: inflammation use of immunosuppressive medications endocrinopathies, or renal disease.19
Postulated links between GVHD and vascular damage include inflammatory cytokines (TNF-α, IL-6), as well as prolonged calcineurin inhibitors and steroids.6, 7 Severe and chronic liver GVHD may induce dyslipidemia by intrahepatic cholestasis, bile lipoprotein reflux into the circulation and subsequent formation of lipoprotein X.80, 82 However, overt clinical chronic GVHD as a risk factor has been disputed,11, 44 suggesting instead that other inflammatory mechanisms may be involved. Immunosuppressive drugs such as sirolimus, calcineurin inhibitors and glucocorticoids not only increase lipid levels but also lead to significant drug-drug interactions with 3-hydroxy-3-methyl-gutaryl (HMG)-CoA reductase inhibitors (statins) via the cytochrome p450 pathway.19 Other complications and comorbidities in HCT survivors such as DM, hypothyroidism, growth hormone deficiency, metabolic syndrome, insulin/leptin resistance, hypogonadism, as well as renal disease such as nephrotic syndrome may exacerbate the overall risk of dyslipidemia in HCT survivors.
Precise mechanisms are unknown and likely to be multifactorial with an emphasis on inflammation, endothelial injury and immunosuppressive agents. The impact of chronic GVHD is controversial. The intermediary role of the liver is poorly described except in instances of severe liver damage by GVHD.
Current guidelines for HCT recipients20 recommend similar screening practices as those used for dyslipidemia amongst the general population, starting at 1 year post HCT or earlier for those with known vulnerabilities. The USPSTF strongly recommends screening for lipid disorders every 5 years in men ≥35 years, women ≥45 years, and persons ≥20 years at increased risk for CHD. The National Heart, Lung, and Blood Institute (NHLBI) recommends first screening in children between the ages of 9–11 years or earlier in those with family history.
A recent international position statement83 focused on the metabolic syndrome advances the start of initial monitoring and recommends an initial lipid profile 3 months after HCT. High-risk patients who have ongoing risk factors (e.g. those on sirolimus, calcineurin inhibitors, or corticosteroids), repeat evaluations should occur every 3–6 months.83 However, the optimal frequency of monitoring is unknown in HCT patients, and vulnerable subgroups deserving special monitoring have yet to be delineated; screening frequency in long term (>5 to 10 years) survivors are not established. Moreover, screening guidelines that trigger lipid-lowering therapy are typically based upon guidelines (e.g. Framingham score) developed from the general population that may be relatively insensitive in the HCT survivor population. Standard fasting lipid panels may fail to detect pathobiologically relevant lipoprotein profiles in this population.
Non-pharmacologic lifestyle modifications and correction of any existing endocrinopathy are the first step. Statins can be effective for lowering lipid levels.84 There may be differences in the propensity of various immunosuppressants (e.g. calcineurin inhibitors vs. Sirolimus) to cause dyslipidemia. As such, modification of ongoing immunosuppressive therapy may be a viable option for these patients. Future studies to evaluate impact and efficacy of statins/fibrates, PCSK9 inhibitors, novel weight loss agents, and bariatric surgery are required. Pediatrics is a special population with distinct safety considerations as the AHA recommends considering drug therapy for high-risk lipid abnormalities in boys ≥10 years of age and after onset of menses in girls, preferably after a 6 to 12 month trial of fat- and cholesterol-restricted dietary management.85
The measurable impact of aggressive management of elevated lipids on long-term CVD risk in HCT survivors is unknown. This is particularly relevant to the pediatric population in whom long-term safety considerations are paramount.
Research priorities: Dyslipidemia.
Define subgroups at greatest risk for developing dyslipidemia so that strategies can be implemented to uncover mechanisms of disease and to optimize treatment.
Investigate the association between dyslipidemia and inflammation; in this context, measure immunomodulatory aspects of lipid-lowering medications (eg. statins)
Investigate lipoprotein profiles in HCT patients. Specifically examine if: 1) there is a distinct dyslipidemic profile in HCT patients that puts them at increased CHD risk 2) there are population differences between children and adults, or those with renal injury, 3) advanced lipid testing, such as HDL and LDL subparticle distribution by nuclear magnetic resonance (NMR) spectroscopy could be useful in the diagnosis of patients at risk and or to manage their lipid lowering therapy, 4) non-fasting triglycerides or post-prandial triglyceride-rich particles are better predictors of risk than lipoprotein profiles analyzed on fasting samples, 5) functional assays of lipoprotein function, such as HDL-mediated cholesterol efflux, predict CHD risk, and 6) there are functional defects in HDL that may contribute to increase CVD risk.
Investigate effectiveness of lifestyle and lipid-lowering medications, such as statins, on reducing CVD risk in HCT patients.
Do other alternative lipid-lowering medication, such as fibrates, peroxisome proliferator-activated receptor (PPAR) agonists, niacin, and ezetimide, have value?
Explore the interaction between lipid-lowering drugs and various immunosuppressant therapies in terms of dyslipidemia and in preventing GVHD.
SARCOPENIC OBESITY
Sarcopenic obesity is a condition whereby an individual has both sarcopenia and obesity. Sarcopenia is an age related quantitative and qualitative change in skeletal muscle in which both strength and mass are decreased. Two International bodies have published formal definitions of sarcopenia. The European working group on sarcopenia in older people (EWGSOP) defines sarcopenia as a generalized decrease in muscle mass and strength,86 while the International working group on sarcopenia defines it as a decrease in muscle mass and decrease in walking speed.87 The diagnosis of sarcopenia is typically confirmed by muscle mass measurement with dual-energy X-ray absorptiometry (DEXA), bioelectrical impedance, computerized tomography, or magnetic resonance imaging, depending on local availability and purpose (research or clinical) of the diagnosis. Obesity is frequently defined in terms of the BMI, although there is increased recognition that distribution of fat (e.g. abdominal adiposity or ectopic fat) is relevant to morbidity even in the setting of a normal BMI.88–90 There is a paucity of information on the incidence of sarcopenic obesity in HCT survivors. That said, sarcopenic obesity is a topic of increasing interest because: 1) there is evidence that it is more closely linked to CVD risk than obesity alone,91–93 2) large epidemiological studies in non-HCT populations have highlighted the predictive role of indirect measures of sarcopenia (e.g. grip strength) on all-cause mortality and cardiovascular mortality.94–96
While there is clear evidence that survivors of pediatric ALL are at increased risk for obesity after therapy,97 less is known about the risk of obesity following HCT. In the childhood cancer survivor study (CCSS), TBI and by implication HCT was a risk factor for being underweight in adulthood.98 That said, while BMI may be normal or reduced after HCT, patients may nonetheless have evidence of increase in body fat or abdominal obesity.60, 99, 100 In a retrospective study which investigated body composition post HCT in 82 recipients of allogeneic HCT, there was evidence of a reduction in lean body mass and increase in fat percentage compared to normal controls 2–3 years post transplant.101 In a subsequent study by Slater and colleagues,72 119 children and young adult HCT survivors (mean age at HCT 12.2y) were demonstrated to have a higher percent fat mass and lower lean mass with higher fasting insulin compared to sibling controls. Notably, in this latter study BMI and waist circumference were not significantly higher than the control group.
There are some pathogenic factors overlapping between sarcopenia and obesity and it has been suggested that these two phenomena are not independent, but instead, they are functionally linked. Obesity may drive sarcopenia through the release of inflammatory molecules (e.g. TNF-alpha and IL-6) from adipose tissue, resulting in decreased muscle protein synthesis and increased myofibrillar protein degradation.102, 103 The interplay between inflammation, obesity, and sarcopenia may be especially relevant for allogeneic HCT survivors with GVHD, potentially resulting in a disproportionately higher risk of sarcopenic obesity when compared to other HCT populations.
There currently are no screening recommendations for sarcopenic obesity for HCT survivors, nor are there recommendations for the general population. There remain important gaps in knowledge with regards to: 1) characterizing the incidence and risk factors for sarcopenia and/or sarcopenic obesity following HCT; 2) describing pre-HCT, conditioning, and post-HCT risk factors; 3) delineating biologic (e.g. interruption of the growth hormone-insulin growth factor [GH-IGF] axis) drivers of sarcopenic obesity risk;60, 104, 105 4) defining the impact of decreased lean muscle mass and increased percent body fat on all-cause and CVD-related mortality; and 5) understanding the role of interventions such as resistance training,106, 107 diet modification (e.g. increased protein intake), and pharmacologic (e.g. metformin) approaches to attenuate lean muscle mass loss after HCT.108
Sarcopenic obesity: research priorities.
Describe longitudinal changes in body composition for adults and children undergoing HCT; describing % body fat, BMI, as well as measures of muscle mass and strength
Examine the utility and feasibility of advanced imaging (e.g. MRI) for assessment of visceral vs. subcutaneous vs. ectopic fat
Investigate the association between changes in body composition and HCT-related outcome (overall survival, relapse- and non-relapse-related outcomes)
Investigate the association between therapeutic exposures (e.g. radiation, chemotherapy, other medications) and post-HCT sarcopenia
Examine whether interventions such as exercise (resistance and/or aerobic), or dietary modification (e.g. protein intake) can influence fat/muscle mass following HCT
Conclusion
Investigations performed to date have shown in a compelling fashion that patients both young and old are at significant risk for development of overt CVD in the first decade after HCT, along with worsening of CVRFs that will likely result in more significant CVD as more time passes after their HCT. Because non-HCT studies of intervention for CVRF have shown clear benefits in CVD risk, and there are a number of proven and emerging methods of observing or treating CVRFs, there is a compelling case to fund and carry out studies that will improve the long term cardiovascular health of HCT survivors. The defined research gaps and future study priorities outlined in this document should serve as a starting point for researchers and funding agencies to begin to address this critical need.
Acknowledgments
We thank André Tichelli and Bipin Savani for critically appraising the recommendations and the manuscript as external reviewers. In addition, we thank the following members of the National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative for their coordination and guidance through this initiative: Nonniekaye Shelburne, Steve Pavletic, Shahrukh Hashmi, and Navneet Majhail. This initiative was sponsored jointly by the National Heart, Lung and Blood Institute (NHLBI) and National Cancer Institute (NCI).
Footnotes
Disclosures
The opinions expressed here are those of the authors and do not represent the official position of the NIH or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Annals of internal medicine. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Sun CL, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant. 2013;19:1073–1080. doi: 10.1016/j.bbmt.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armenian SH, Chow EJ. Cardiovascular disease in survivors of hematopoietic cell transplantation. Cancer. 2014;120:469–479. doi: 10.1002/cncr.28444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rovo A, Tichelli A. Cardiovascular complications in long-term survivors after allogeneic hematopoietic stem cell transplantation. Seminars in hematology. 2012;49:25–34. doi: 10.1053/j.seminhematol.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Syrjala KL, Martin PJ, Lee SJ. Delivering care to long-term adult survivors of hematopoietic cell transplantation. J Clin Oncol. 2012;30:3746–3751. doi: 10.1200/JCO.2012.42.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 12.Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 13.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow EJ, Baker KS, Flowers ME, et al. Influence of metabolic traits and lifestyle factors on cardiovascular disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:S226–227. [Google Scholar]
- 16.Musunuru K, Hickey KT, Al-Khatib SM, et al. Basic concepts and potential applications of genetics and genomics for cardiovascular and stroke clinicians: a scientific statement from the American Heart Association. Circulation. Cardiovascular genetics. 2015;8:216–242. doi: 10.1161/HCG.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havranek EP, Mujahid MS, Barr DA, et al. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2015;132:873–898. doi: 10.1161/CIR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 18.Passweg JR, Halter J, Bucher C, et al. Hematopoietic stem cell transplantation: a review and recommendations for follow-up care for the general practitioner. Swiss medical weekly. 2012;142:w13696. doi: 10.4414/smw.2012.13696. [DOI] [PubMed] [Google Scholar]
- 19.Griffith ML, Savani BN, Boord JB. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: evaluation and management. Blood. 2011;116:1197–1204. doi: 10.1182/blood-2010-03-276576. [DOI] [PubMed] [Google Scholar]
- 20.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savani BN, Griffith ML, Jagasia S, Lee SJ. How I treat late effects in adults after allogeneic stem cell transplantation. Blood. 2011;117:3002–3009. doi: 10.1182/blood-2010-10-263095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nieder ML, McDonald GB, Kida A, et al. National Cancer Institute-National Heart, Lung and Blood Institute/pediatric Blood and Marrow Transplant Consortium First International Consensus Conference on late effects after pediatric hematopoietic cell transplantation: long-term organ damage and dysfunction. Biol Blood Marrow Transplant. 2011;17:1573–1584. doi: 10.1016/j.bbmt.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alagona P, Jr, Ahmad TA. Cardiovascular Disease Risk Assessment and Prevention: Current Guidelines and Limitations. The Medical clinics of North America. 2015;99:711–731. doi: 10.1016/j.mcna.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Pasternak R, Greenland P, Smith S, Jr, Fuster V. AHA/ACC scientific statement: Assessment of cardiovascular risk by use of multiple-risk-factor assessment equations: a statement for healthcare professionals from the American Heart Association and the American College of Cardiology. Journal of the American College of Cardiology. 1999;34:1348–1359. doi: 10.1016/s0735-1097(99)00387-3. [DOI] [PubMed] [Google Scholar]
- 25.Hertenstein B, Stefanic M, Schmeiser T, et al. Cardiac toxicity of bone marrow transplantation: predictive value of cardiologic evaluation before transplant. J Clin Oncol. 1994;12:998–1004. doi: 10.1200/JCO.1994.12.5.998. [DOI] [PubMed] [Google Scholar]
- 26.Fujimaki K, Maruta A, Yoshida M, et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone marrow transplantation. 2001;27:307–310. doi: 10.1038/sj.bmt.1702783. [DOI] [PubMed] [Google Scholar]
- 27.Murdych T, Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone marrow transplantation. 2001;28:283–287. doi: 10.1038/sj.bmt.1703133. [DOI] [PubMed] [Google Scholar]
- 28.Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol. 1991;9:1215–1223. doi: 10.1200/JCO.1991.9.7.1215. [DOI] [PubMed] [Google Scholar]
- 29.Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 32.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. Journal of the American College of Cardiology. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 33.Pituskin E, Haykowsky M, Mackey JR, et al. Rationale and design of the Multidisciplinary Approach to Novel Therapies in Cardiology Oncology Research Trial (MANTICORE 101--Breast): a randomized, placebo-controlled trial to determine if conventional heart failure pharmacotherapy can prevent trastuzumab-mediated left ventricular remodeling among patients with HER2+ early breast cancer using cardiac MRI. BMC cancer. 2011;11:318. doi: 10.1186/1471-2407-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heck SL, Gulati G, Ree AH, et al. Rationale and design of the prevention of cardiac dysfunction during an Adjuvant Breast Cancer Therapy (PRADA) Trial. Cardiology. 2012;123:240–247. doi: 10.1159/000343622. [DOI] [PubMed] [Google Scholar]
- 35.Murbraech K, Wethal T, Smeland KB, et al. Valvular Dysfunction in Lymphoma Survivors Treated With Autologous Stem Cell Transplantation: A National Cross-Sectional Study. JACC. Cardiovascular imaging. 2016;9:230–239. doi: 10.1016/j.jcmg.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Nieto Y, Cagnoni PJ, Bearman SI, Shpall EJ, Matthes S, Jones RB. Cardiac toxicity following high-dose cyclophosphamide, cisplatin, and BCNU (STAMP-I) for breast cancer. Biol Blood Marrow Transplant. 2000;6:198–203. doi: 10.1016/s1083-8791(00)70043-7. [DOI] [PubMed] [Google Scholar]
- 37.Chow EJ, Anderson L, Baker KS, et al. Late Effects Surveillance Recommendations among Survivors of Childhood Hematopoietic Cell Transplantation: A Children’s Oncology Group Report. Biol Blood Marrow Transplant. 2016;22:782–795. doi: 10.1016/j.bbmt.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. The Journal of thoracic and cardiovascular surgery. 2014;148:e1–e132. doi: 10.1016/j.jtcvs.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Majhail NS, Ness KK, Burns LJ, et al. Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Biol Blood Marrow Transplant. 2007;13:1153–1159. doi: 10.1016/j.bbmt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majhail NS, Challa TR, Mulrooney DA, Baker KS, Burns LJ. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1100–1107. doi: 10.1016/j.bbmt.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Rovo A, Daikeler T, Halter J, et al. Late altered organ function in very long-term survivors after allogeneic hematopoietic stem cell transplantation: a paired comparison with their HLA-identical sibling donor. Haematologica. 2011;96:150–155. doi: 10.3324/haematol.2010.030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oudin C, Simeoni MC, Sirvent N, et al. Prevalence and risk factors of the metabolic syndrome in adult survivors of childhood leukemia. Blood. 2011;117:4442–4448. doi: 10.1182/blood-2010-09-304899. [DOI] [PubMed] [Google Scholar]
- 43.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–198. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pophali PA, Klotz JK, Ito S, et al. Male survivors of allogeneic hematopoietic stem cell transplantation have a long term persisting risk of cardiovascular events. Experimental hematology. 2014;42:83–89. doi: 10.1016/j.exphem.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 46.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) European heart journal. 2013;34:2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 47.Brouwer CA, Postma A, Hooimeijer HL, et al. Endothelial damage in long-term survivors of childhood cancer. J Clin Oncol. 2013;31:3906–3913. doi: 10.1200/JCO.2012.46.6086. [DOI] [PubMed] [Google Scholar]
- 48.Biedermann BC, Sahner S, Gregor M, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078–2083. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]
- 49.Kavey RE, Allada V, Daniels SR, et al. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114:2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 50.Chow EJ, Wong K, Lee SJ, et al. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:794–800. doi: 10.1016/j.bbmt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. Journal of the American Heart Association. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Group SR, Wright JT, Jr, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) Jama. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 55.Fuji S, Rovo A, Ohashi K, et al. How do I manage hyperglycemia/post-transplant diabetes mellitus after allogeneic HSCT. Bone marrow transplantation. 2016 doi: 10.1038/bmt.2016.81. [DOI] [PubMed] [Google Scholar]
- 56.Diagnosis and classification of diabetes mellitus. Diabetes care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 57.Diagnosis and classification of diabetes mellitus. Diabetes care. 2009;32(Suppl 1):S62–67. doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nathan DM, Davidson MB, DeFronzo RA, et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 59.Hoffmeister PA, Storer BE, Sanders JE. Diabetes mellitus in long-term survivors of pediatric hematopoietic cell transplantation. J Pediatr Hematol Oncol. 2004;26:81–90. doi: 10.1097/00043426-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 60.Frisk P, Rossner SM, Norgren S, Arvidson J, Gustafsson J. Glucose metabolism and body composition in young adults treated with TBI during childhood. Bone marrow transplantation. 2011;46:1303–1308. doi: 10.1038/bmt.2010.307. [DOI] [PubMed] [Google Scholar]
- 61.Smedmyr B, Wibell L, Simonsson B, Oberg G. Impaired glucose tolerance after autologous bone marrow transplantation. Bone marrow transplantation. 1990;6:89–92. [PubMed] [Google Scholar]
- 62.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer. Increased risk associated with radiation therapy: a report for the childhood cancer survivor study. Archives of internal medicine. 2009;169:1381–1388. doi: 10.1001/archinternmed.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei C, Thyagiarajan MS, Hunt LP, Shield JP, Stevens MC, Crowne EC. Reduced insulin sensitivity in childhood survivors of haematopoietic stem cell transplantation is associated with lipodystropic and sarcopenic phenotypes. Pediatric blood & cancer. 2015;62:1992–1999. doi: 10.1002/pbc.25601. [DOI] [PubMed] [Google Scholar]
- 64.Yeshayahu Y, Koltin D, Hamilton J, Nathan PC, Urbach S. Medication-induced diabetes during induction treatment for ALL, an early marker for future metabolic risk? Pediatric diabetes. 2015;16:104–108. doi: 10.1111/pedi.12138. [DOI] [PubMed] [Google Scholar]
- 65.Mostoufi-Moab S, Ginsberg JP, Bunin N, et al. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. The Journal of pediatrics. 2012;160:122–128. doi: 10.1016/j.jpeds.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. The Journal of clinical endocrinology and metabolism. 2006;91:4401–4407. doi: 10.1210/jc.2006-0128. [DOI] [PubMed] [Google Scholar]
- 67.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone marrow transplantation. 2008;41:797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 68.Wei C, Thyagiarajan M, Hunt L, et al. Reduced beta-cell reserve and pancreatic volume in survivors of childhood acute lymphoblastic leukaemia treated with bone marrow transplantation and total body irradiation. Clinical endocrinology. 2015;82:59–67. doi: 10.1111/cen.12575. [DOI] [PubMed] [Google Scholar]
- 69.Kalyani RR, Egan JM. Diabetes and altered glucose metabolism with aging. Endocrinology and metabolism clinics of North America. 2013;42:333–347. doi: 10.1016/j.ecl.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arora M, Sun CL, Ness KK, et al. Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients: Results From the Bone Marrow Transplant Survivor Study. JAMA oncology. 2016 doi: 10.1001/jamaoncol.2016.0855. [DOI] [PMC free article] [PubMed]
- 71.Siu AL. Screening for Abnormal Blood Glucose and Type 2 Diabetes Mellitus: U.S. Preventive Services Task Force Recommendation Statement. Annals of internal medicine. 2015;163:861–868. doi: 10.7326/M15-2345. [DOI] [PubMed] [Google Scholar]
- 72.Slater ME, Steinberger J, Ross JA, et al. Physical Activity, Fitness, and Cardiometabolic Risk Factors in Adult Survivors of Childhood Cancer with a History of Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:1278–1283. doi: 10.1016/j.bbmt.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelsey CR, Scott JM, Lane A, et al. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone marrow transplantation. 2014;49:1330–1336. doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wood WA, Deal AM, Reeve BB, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone marrow transplantation. 2013;48:1342–1349. doi: 10.1038/bmt.2013.58. [DOI] [PubMed] [Google Scholar]
- 75.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 76.Scheidt-Nave C, Du Y, Knopf H, et al. Prevalence of dyslipidemia among adults in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS 1) Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2013;56:661–667. doi: 10.1007/s00103-013-1670-0. [DOI] [PubMed] [Google Scholar]
- 77.Gonzalez-Juanatey JR, Millan J, Alegria E, Guijarro C, Lozano JV, Vitale GC. Prevalence and characteristics of lipid abnormalities in patients treated with statins in primary and secondary prevention in Spain. DYSIS-Spain Study. Revista espanola de cardiologia. 2011;64:286–294. doi: 10.1016/j.recesp.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 78.Gurney JG, Ness KK, Sibley SD, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006;107:1303–1312. doi: 10.1002/cncr.22120. [DOI] [PubMed] [Google Scholar]
- 79.Paris C, Yates L, Lama P, Zepeda AJ, Gutierrez D, Palma J. Evaluation of metabolic syndrome after hematopoietic stem cell transplantation in children and adolescents. Pediatric blood & cancer. 2012;59:306–310. doi: 10.1002/pbc.24104. [DOI] [PubMed] [Google Scholar]
- 80.Turchin A, Wiebe DA, Seely EW, Graham T, Longo W, Soiffer R. Severe hypercholesterolemia mediated by lipoprotein X in patients with chronic graft-versus-host disease of the liver. Bone marrow transplantation. 2005;35:85–89. doi: 10.1038/sj.bmt.1704739. [DOI] [PubMed] [Google Scholar]
- 81.Kagoya Y, Seo S, Nannya Y, Kurokawa M. Hyperlipidemia after allogeneic stem cell transplantation: prevalence, risk factors, and impact on prognosis. Clinical transplantation. 2012;26:E168–175. doi: 10.1111/j.1399-0012.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 82.Zidan H, Lo S, Wiebe D, Talano J, Alemzadeh R. Severe hypercholesterolemia mediated by lipoprotein X in a pediatric patient with chronic graft-versus-host disease of the liver. Pediatric blood & cancer. 2008;50:1280–1281. doi: 10.1002/pbc.21522. [DOI] [PubMed] [Google Scholar]
- 83.DeFilipp Z, Duarte RF, Snowden JA, et al. Metabolic syndrome and cardiovascular disease following hematopoietic cell transplantation: screening and preventive practice recommendations from CIBMTR and EBMT. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blaser BW, Kim HT, Alyea EP, 3rd, et al. Hyperlipidemia and statin use after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:575–583. doi: 10.1016/j.bbmt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCrindle BW, Urbina EM, Dennison BA, et al. Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation. 2007;115:1948–1967. doi: 10.1161/CIRCULATIONAHA.107.181946. [DOI] [PubMed] [Google Scholar]
- 86.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. Journal of the American Medical Directors Association. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 89.Consitt LA, Bell JA, Houmard JA. Intramuscular lipid metabolism, insulin action, and obesity. IUBMB life. 2009;61:47–55. doi: 10.1002/iub.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122–127. doi: 10.1136/gut.2003.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lu CW, Yang KC, Chang HH, Lee LT, Chen CY, Huang KC. Sarcopenic obesity is closely associated with metabolic syndrome. Obesity research & clinical practice. 2013;7:e301–307. doi: 10.1016/j.orcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 92.Ma J, Hwang SJ, McMahon GM, et al. Mid-adulthood cardiometabolic risk factor profiles of sarcopenic obesity. Obesity (Silver Spring) 2016;24:526–534. doi: 10.1002/oby.21356. [DOI] [PubMed] [Google Scholar]
- 93.Lim S, Kim JH, Yoon JW, et al. Sarcopenic obesity: prevalence and association with metabolic syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA) Diabetes care. 2010;33:1652–1654. doi: 10.2337/dc10-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leong DP, Teo KK, Rangarajan S, et al. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386:266–273. doi: 10.1016/S0140-6736(14)62000-6. [DOI] [PubMed] [Google Scholar]
- 95.Rantanen T, Masaki K, He Q, Ross GW, Willcox BJ, White L. Midlife muscle strength and human longevity up to age 100 years: a 44-year prospective study among a decedent cohort. Age (Dordr) 2012;34:563–570. doi: 10.1007/s11357-011-9256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ortega FB, Silventoinen K, Tynelius P, Rasmussen F. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ. 2012;345:e7279. doi: 10.1136/bmj.e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang FF, Kelly MJ, Saltzman E, Must A, Roberts SB, Parsons SK. Obesity in pediatric ALL survivors: a meta-analysis. Pediatrics. 2014;133:e704–715. doi: 10.1542/peds.2013-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170–181. doi: 10.1158/1055-9965.EPI-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000;356:993–997. doi: 10.1016/S0140-6736(00)02717-3. [DOI] [PubMed] [Google Scholar]
- 100.Ruble K, Hayat M, Stewart KJ, Chen A. Body composition after bone marrow transplantation in childhood. Oncology nursing forum. 2012;39:186–192. doi: 10.1188/12.ONF.186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kyle UG, Chalandon Y, Miralbell R, et al. Longitudinal follow-up of body composition in hematopoietic stem cell transplant patients. Bone marrow transplantation. 2005;35:1171–1177. doi: 10.1038/sj.bmt.1704996. [DOI] [PubMed] [Google Scholar]
- 102.Pasini E, Aquilani R, Dioguardi FS, D’Antona G, Gheorghiade M, Taegtmeyer H. Hypercatabolic syndrome: molecular basis and effects of nutritional supplements with amino acids. The American journal of cardiology. 2008;101:11E–15E. doi: 10.1016/j.amjcard.2008.02.074. [DOI] [PubMed] [Google Scholar]
- 103.Lang CH, Frost RA, Nairn AC, MacLean DA, Vary TC. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. American journal of physiology. Endocrinology and metabolism. 2002;282:E336–347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 104.Ogilvy-Stuart AL, Clark DJ, Wallace WH, et al. Endocrine deficit after fractionated total body irradiation. Archives of disease in childhood. 1992;67:1107–1110. doi: 10.1136/adc.67.9.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Clement-De Boers A, Oostdijk W, Van Weel-Sipman MH, Van den Broeck J, Wit JM, Vossen JM. Final height and hormonal function after bone marrow transplantation in children. The Journal of pediatrics. 1996;129:544–550. doi: 10.1016/s0022-3476(96)70119-1. [DOI] [PubMed] [Google Scholar]
- 106.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. Jama. 1990;263:3029–3034. [PubMed] [Google Scholar]
- 107.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. The New England journal of medicine. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 108.Lee CG, Boyko EJ, Barrett-Connor E, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes care. 2011;34:2381–2386. doi: 10.2337/dc11-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]