Abstract
We recently reported the generation and initial characterization of the first direct model of human Fibrous Dysplasia (OMIM #174800), obtained through the constitutive systemic expression of one of the disease causing mutations, GsαR201C, in the mouse. To define the specific pathogenetic role(s) of individual cell types within the stromal/osteogenic system in FD, we generated mice expressing GsαR201C selectively in mature osteoblasts using the 2.3kb Col1a1 promoter. We show here that this results in a striking high bone mass phenotype, but not in a mimicry of human FD. The high bone mass phenotype involves specifically a deforming excess of cortical bone and prolonged and ectopic cortical bone remodeling. Expression of genes characteristic of late stages of bone cell differentiation/maturation is profoundly altered as a result of expression of GsαR201C in osteoblasts, and expression of the Wnt inhibitor, Sost, is reduced. While high bone mass is in fact a feature of some types/stages of FD lesions in humans, it is marrow fibrosis, localized loss of adipocytes and hematopoietic tissue, osteomalacia and osteolytic changes that together represent the characteristic pathological profile of FD, as well as the sources of specific morbidity. None of these features are reproduced in mice with osteoblast-specific expression of GsαR201C. We further show that hematopoietic progenitor/stem cells, as well as more mature cell compartments, and adipocyte development are normal in these mice. These data demonstrate that effects of Gsα mutations underpinning FD-defining tissue changes and morbidity do not reflect the effects of the mutations on osteoblasts proper.
Keywords: Fibrous Dysplasia, mouse models, Gsα, GNAS, osteoblasts, bone marrow, hematopoietic niche
Introduction
Fibrous dysplasia of bone (FD, OMIM #174800) is a crippling disease with serious adverse consequences in its severe forms, including deformity, fracture, severe bone pain, confinement to a wheelchair and major sensorial damage (1). All clinical forms of the disease (monostotic, polyostotic, polyostotic with endocrinopathies/McCune-Albright syndrome, or mono-polyostotic associated with myxoma of muscle, otherwise known as Mazabraud’s syndrome) are caused by activating missense mutations in the GNAS locus, encoding the α subunit of the stimulatory G protein, Gs [(2,3), and reviewed in (1)]. Gsα-activating mutations induce a 30-fold reduction in the GTPase activity of Gsα, which as a consequence, remains in a prolonged state of activation, causing excess production of cAMP (4). In FD bone lesions, bone trabeculae are abnormal in architecture, structure, and mineral content, while the intertrabecular bone marrow space is depleted of both hematopoietic tissue and adipocytes, and filled with a fibrotic tissue (5–8). Over the past 20 years, studies focusing on skeletal stem/progenitor cells (also known as bone marrow-derived mesenchymal stem cells), used either in vitro (9–11) or in in vivo transplantation assays (12,13), have been employed to reveal the pathogenic effects of these mutations on cells of the osteogenic lineage, and the contribution of the latter to the generation of tissue changes characteristic of FD and of the associated morbidity (1,14). We have recently shown that constitutive systemic expression of one of the FD-causing mutations (GsαR201C) generates an exact replica of the bone pathology seen in human FD, thus providing the long-missed mouse model of the disease (15). Such a model is necessary for improved understanding of crucial aspects of the natural history of the disease, and for direct testing of therapeutic strategies, whether existing or novel, pharmacological or based on more innovative approaches, such as cell or gene therapy. However, precise elucidation of the relative role of individual cells types in bone and bone marrow in vivo remains of crucial importance. The so-called stromal system (16) includes multiple lineages (chondrogenic, osteogenic, adipogenic) emanating from a common postnatal progenitor (17,18). Within the osteogenic lineage proper, definition of discrete differentiation steps has been elusive. Nonetheless, advances in that direction have included the identification of surface markers that are robustly expressed in early stromal progenitors and downregulated in mature bone cells [e.g., CD146 in humans (19)], as well as of transcription factors that are expressed and activated either across the osteogenic lineage, such as Runx2 (20), or in cell compartments that include mature osteoblasts and possibly committed progenitors thereof, such as Osx (21). The functional state of mature, bone-depositing osteoblasts is however precisely defined by the use of a specific Col1a1 promoter element, which is not activated either in progenitor cells or in other lineages within the stromal system (22,23), and represents a major tool for investigating the function of individual gene products in osteoblasts proper (24). As a natural next step towards the precise definition of the effects of Gsα in each of the members of the stromal/osteogenic system, we have therefore generated mice with osteoblast-specific expression of GsαR201C utilizing the 2.3kb Col1a1 promoter. We show here that, unlike mice with constitutive systemic expression of GsαR201C (15), Col1a1-GsαR201C expressing mice develop a striking skeletal phenotype characterized by deforming excess of bone. However, defining features of human FD that emanate from changes in the bone marrow are not reproduced in this model, which points to cells other than mature osteoblasts in the bone environment as being responsible for the generation of the FD phenotype.
Materials and Methods
Col1a1-GsαR201C construct
A 2.3kb fragment of the mouse Col1a1 collagen promoter from the pJ251 vector (kind gift from B. de Crombrugghe, Anderson Cancer Center, Houston, TX, USA), was cloned into pBluescript SK+ (Stratagene, La Jolla, CA USA). The intermediate pKO-Col1a1-SV40 plasmid was generated by inserting the Col1a1 fragment into pKO-SV40, in which a 200 bpSV40 polyA fragment from the pKN185 plasmid was previously inserted. The final construct Col1a1-GsαR201C was assembled by insertion of a rat GsαR201CcDNA from the ATCC #63317 clone (GenBank M12673; ATCC, Manassas, VA) into the pKO-Col1a1-SV40 construct.
Col1a1-GsαR201Ctransgenic mice
The Col1a1-GsαR201C vector was injected into the pronucleus of one-cell stage FVB embryos and these were subsequently implanted into pseudo-pregnant CD-1 mice (Harlan, Italy) (25). Founders were serially backcrossed with wild type FVB mice (Harlan) and two transgenic lines (D7 and E8) were selected for long-term maintenance and analysis. Animals were maintained under standard environmental conditions (temperature 22°C–25°C, humidity 40%–70%, 12:12 dark-light photoperiod), and food and water were provided ad libitum. All experiments involving animals were performed according to the relevant Italian laws and Institutional guidelines and all procedures were IACUC approved. All studies on the characterization of the skeletal phenotype of TG mice were conducted both in male and female mice.
PCR and qPCR
Genotyping was performed on gDNA as described (15) by using the primers listed in Suppl. Table 1. RNA was extracted from frozen tissues and cell cultures with TRIzol (Invitrogen, Carlsbad, CA, USA) as per the manufacturer’s instructions and reverse transcribed as already described(26). Col1a1-GsαR201C transgene expression was analyzed by standard PCR reaction using the primers listed in Table 1. qPCR analysis on cultured cells was performed by Q-PCR using the TaqMan Gene Assays listed in Suppl. Table 2.
Cell cultures and in vitro differentiation
Osteogenic cultures were established from collagenase-treated bone fragments from calvariae or vertebrae and grown as described (15,27). Bone marrow stromal cell cultures (BMSCs, skeletal stem/progenitor cells) were established from mouse long bones and grown or induced to osteoblastic differentiation as described (26,28). Differentiated cultures were either stained with Von Kossa or harvested for RNA extraction.
cAMP assay
cAMP was measured by using the cAMP Direct Biotrak™ EIA kit (GE Healthcare, Buckinghamshire, UK, Europe GmbH) according to the manufacturer’s instructions (29) in the presence of 3-isobutyl-1-methylxanthine (IBMX, Sigma, MO, USA).
X-ray analysis
Radiograms were taken using a Faxitron MX-20 Specimen Radiography System (Faxitron X-ray Corp., Wheeling, IL) at 25 kV for 8 sec. The images (1 to 5x projective magnification) were captured with Medical Imaging Film HM Plus (Ferrania USA, Inc., Woodbury, MN).
X-ray Microtomography (XMT)
Samples were scanned at Queen Mary University of London by using the in-house designed “MuCat 2” X-ray microtomography scanner. This uses a time-delay integration readout CCD camera to provide high quality images, similar to the original scanner (30), but having a larger, fibre-optically coupled detector (6 cm2). The X-ray generator was set to 40 kV and 405 μA; beam hardening correction was applied to give an equivalent monochromatic energy of 25 keV (31). The voxel size was set to 12.5 μm and 951 projections were acquired over 360° rotation. 3D data sets were rendered using Drishti software (Australian National University, Canberra).
Histology, histomorphometry, SEM and qBSE imaging
Tissue samples were processed as described (7,15). Paraffin sections were used for hematoxylin and eosin (H&E) staining, Sirius red staining, Giemsa staining for transmitted light and fluorescence microscopy (32) and immunohistochemistry. Three-micron-thick sections were cut from GMA and PMMA embedded tissues and used for histomorphometry following von Kossa/methylene blue staining (7). Enzyme cytochemical reaction for alkaline phosphatase (Alp) and tartrate resistant acid phosphatase (Trap) were performed on undecalcified GMA embedded tissues as described (33). Immunolocalization of Sp7/Osterix was performed using the rabbit anti mouse Sp7/Osterix antiserum (#ab22552, ABCAM, Cambridge, UK), applied at 1:200 dilution, overnight at 4°C. A standard immunoperoxidase (DAB) reaction was used to reveal binding sites. Scanning Electron Microscopy (SEM) and Quantitative Back-Scattered Electrom (qBSE) imaging were performed as described (34,35,36–38).
Serum Fgf-23 measurement
Blood samples were collected from the jugular vein. The serum Fgf-23 levels were measured using a mouse Fgf-23 (C-Term) ELISA Kit (Immutopics Inc, San Clemente, CA) according to the manufacturer’s instructions.
Analysis of the hematopoietic compartment
Femora and tibiae were isolated from 6 month old mice and flushed with Hanks’ Balanced Salt Solution containing 1% FBS. The marrow cell suspension was then stained and analyzed by flow cytometry. The following antibodies were used: anti-CD43-PE (BD Pharmingen, San Jose, CA), anti-c-Kit-APCCy7 (BD Pharmingen), anti-Sca-1-FITC (BD Pharmingen), anti-B220-FITC (BD Pharmingen), anti-IL-7rα PECy7 (eBioscience, San Diego, CA, USA), anti-CD19-APCCy7 (BD Pharmingen), anti-IgM-APC (BD Pharmingen), anti-CD34-APC (BD Pharmingen). To analyze Lin− cells, total bone marrow cells were stained with a biotinylated Lin+ antibody cocktail of anti-CD4, anti-CD8, anti-NK1.1, anti-CD3, anti-CD11c, anti-B220, anti-CD19, anti-Gr1, anti-CD11b and anti-Ter119 (BD Pharmingen), followed by streptavidin-Pacific Blue (BD Pharmingen). FACS analyses were performed on a FACS Canto II running with the FACSDiva software (Becton Dickinson, Franklin Lakes, NJ, USA). In all analyses, propidium iodide was used to exclude dead cells. FACS analysis was done using the FlowJo (Treestar Inc., Ashland, OR, USA) software.
Statistical analyses
Two-tailed t-test with Welch’s correction for unpaired samples was performed using GraphPad Prism 5. Comparisons were deemed statistically significant when P <0.05.
Results
Osteoblast-specific expression of GsαR201C results in high bone mass
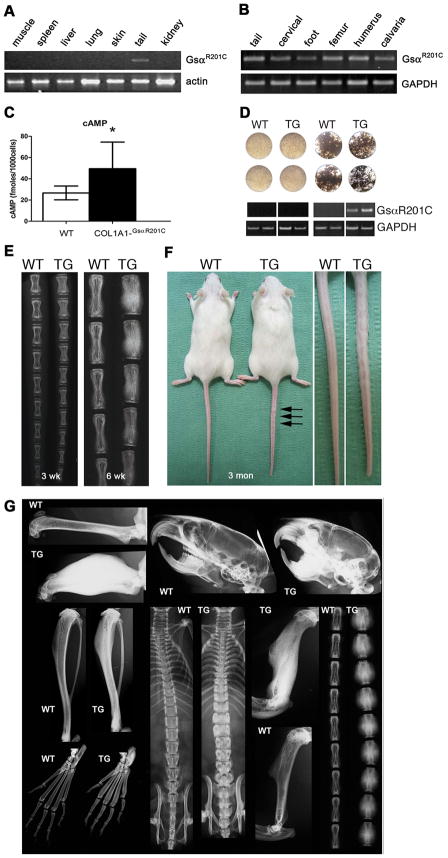
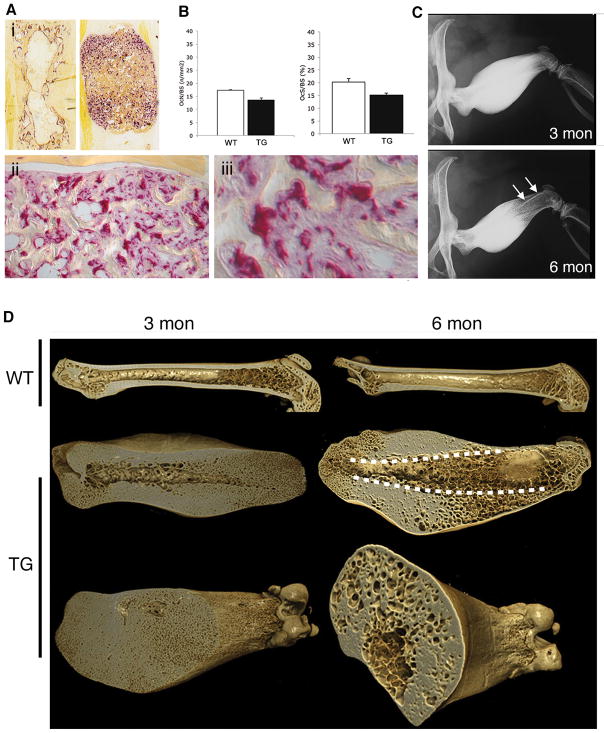
Mice with osteoblast-specific expression of GsαR201C exhibited normal viability and fertility. Expression of the transgene was restricted to bone (Fig. 1A), uniform across the skeleton (Fig. 1B), and sustained throughout life. Excess production of cAMP was easily demonstrated in cultures of mature osteogenic cells derived either from calvariae or from trabecular bone (spine and tail vertebrae) (Fig. 1C). Expression of the transgene was not observed in cultures of BMSCs unless they were induced to differentiate into osteoblasts (Fig. 1D), confirming specific targeting of GsαR201C expression to mature cells within the osteogenic lineage. Transgenic mice were born free of skeletal abnormalities detectable by X-ray or histological analyses. However, a prominent skeletal phenotype developed in the postnatal life. The phenotype was identical in every respect in male and female mice. The earliest signs of a skeletal phenotype became obvious at around 3 weeks as an increased radiographic density in tail vertebrae, which progressed over time to a deforming excess of bone mass, radiographically detectable at 6 weeks (Fig. 1E). At 3 months, deformity of tail vertebrae was grossly apparent (“bumpy tail” phenotype, Fig. 1F) and the excess of bone was obvious in all skeletal segments (Fig. 1G), but with variable severity, symmetry and uniformity in individual mice (Suppl. Fig. 1).
Fig. 1.
Expression of GsαR201C is restricted to bone and mature osteoblasts in Col1a1-GsαR201C mice. (A) RT-PCR analysis of skeletal (tail) vs.extraskeletal tissues, showing no expression of the transgene in extraskeletal tissues. (B) RT-PCR analysis of mRNA extracted from different bones, showing uniform TG expression. (C) Cyclic AMP production in osteogenic cells from tail vertebrae. (D) RT-PCR analysis of non-differentiated or osteoblast-differentiated cultures of bone marrow stromal cells from WT and TG mice. mRNA was extracted from confluent cultures of WT and TG BMSCs, either non-differentiated or induced to osteoblastic differentiation and mineralization. Transgene expression was restricted to differentiated cultures of TG BMSCs. Data are representative of multiple replicates. (E) X-ray analysis of tail vertebrae at 3 and 6 weeks. Note the increased density with no gross deformity at 3 weeks, and the partial “bumping” of vertebrae at 6 weeks. (F) Gross phentoype of Col1a1-GSαR201C mice at 3 months. Note the shorter snout and tail, and the “bumpy” tail. (G) Generalized high bone mass phenotype at 3 months. All bones show a marked increase in bone density. The cranial base, tibiae and tail vertebrae are shorter compared to WT; a specific thickening of cortical bone can be easily appreciated in metatarsal bones. Femora and humeri are deformed, due to a marked increase in bone mass at mid-diaphyseal regions, whereas epiphyses are spared.
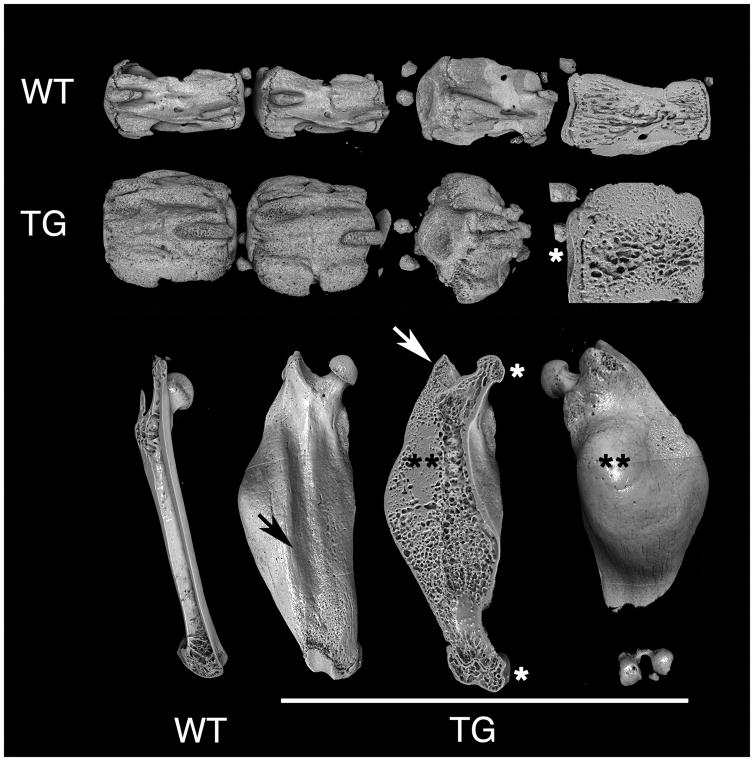
X-ray and XMT analyses also demonstrated that the excess bone mass was not uniformly distributed in individual bones either, but affected or spared specific anatomical features, resulting in regular patterns of deformity in individual bones. In vertebrae (Fig. 2, top two rows), for example, endplates were normally configured and vestiges of the spinous and transverse processes were moderately affected, while zygapophyses were markedly deformed. In femora (Fig. 2, bottom row), femoral heads and condyles were neither misshapen nor deformed, and trochanters were only moderately affected. Over the extensor surface, overgrowth of bone spared the region in contact with the quadriceps muscle, resulting in the appearance of a groove. Most of the excess bone mass formed conspicuous lateral and posterior bulges in the subtrochanteric regions. Although an increase in bone mass was obvious in both cortical and cancellous regions, involvement of cortical bone was striking (Suppl. Movie 1, Suppl. Fig. 2). The excess cortical bone was permeated by a system of interconnected micropores, extending from the endosteal to the subperiosteal surface (Suppl. Movie 1). SEM and XMT analyses clearly demonstrated their connection to vascular and bone marrow spaces (Suppl. Movie 1, Suppl. Fig. 2)
Fig. 2.
XMT analysis of tail vertebrae and femora at 6 months. The increase in bone mass is non-uniform in individual skeletal segments: endplates in vertebrae, femoral heads and condyles are essentially unchanged in size or shape (*), and the greater trochanters are also not majorly deformed (white arrow). In femora, the mid-portion of the anterior surface, which is in direct contact with the quadriceps muscle, is spared, resulting in a longitudinal groove (black arrow), while massive bulges are obvious in the subtrochanteric, posterior and lateral aspects (**). Both in vertebrae and femora, the marrow space is occupied by an excess of cancellous bone, and cortical bone is specifically increased in mass.
Osteoblast-specific expression of GsαR201C results in abnormal bone formation
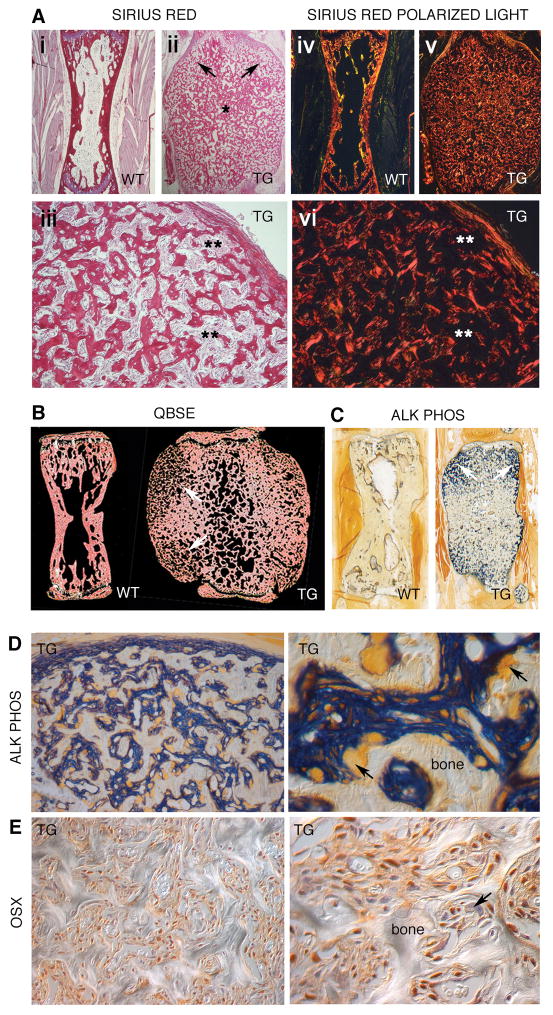
The very shape of the massive bones of Col1a1-GsαR201C mice per se implied an excess of periosteal bone formation, which was easily confirmed by histological studies using different techniques (Fig. 3, Suppl. Fig. 3). In tail vertebrae from 2–6 month old mice, sites of neo-osteogenesis were localized to metaphyseal and subperiosteal, circumferential regions (Fig. 3A-ii and 3A-v, 3B, right). These regions extended up to the lateral aspect of the growth plates and endplates (a site equivalent, in tail vertebrae, to Ranvier’s grooves of long bones), where they formed “shoulders” of nascent periosteal bone, and included the complex system of metaphyseal apophyses characteristic of tail vertebrae (vestiges of transverse processes, zygapophyses). Nascent bone trabeculae were highly irregular in profile (Fig. 3A-iii) and woven in character (Fig 3A-vi, Suppl. Fig. 3D), and thus vaguely reminiscent of fibrous dysplastic bone in humans. However, the densely cellular osteogenic tissue giving rise to nascent bone (Suppl. 3B and C) did not include bona fide fibrotic tissue, as revealed by Sirius red-polarized light analysis (Fig. 3A-vi, Suppl. Fig. 3D) and its collagen content was limited to isolated fibers directly continuous with periosteal collagen. By quantitative backscattered electron (QBSE) microscopy, there was no overall reduction in bone mineral content in TG bone compared to WT (Fig. 3B). Lack of mineralized cartilage remnants in QBSE images (which would be seen as white) further denoted the non-endochondral origin of the nascent, excess cortical bone. Excess membranous ossification in the periosteum led to the generation of a system of nascent bone trabeculae, separated by a highly cellular tissue, directly continuous with the osteogenic periosteum (Fig. 3C, Suppl. Fig. 3B) and comprised of tightly packed cells with strong Alp activity at the plasma membrane (Fig. 3D) and expressing Osx (Fig. 3E) in the nucleus. Towards the mid-diaphysis, maturation of nascent bone into thicker and more interconnected bone trabeculae was noted. Not surprisingly, bone formation activity measured as ObS/BS % was significantly higher in TG mice compared to WT; dual calcein/alizarin red fluorescent labeling resulted in intense and ubiquitous labeling that could not be reliably measured as per standard histomorphometric techniques (not shown).
Fig. 3.
Excess bone mass and excess bone formation in tail vertebrae. (A-i-iii) Sirius red staining and illumination with polarized light (A-iv–vi) of tail vertebrae from 2-month old mice, demonstrating a massive excess of woven bone with a highly irregular trabecular architecture. Bone trabeculae are thinner and more widely separated from one another in superiosteal metaphyseal regions (arrows), and more confluent in mid-diaphyseal regions (*). Intertrabecular space is occupied by a densely cellular tissue, which does not contain significant amounts of Sirius red-staining birefringent collagen (fibrosis) (**). (B) Quantitative Back-Scattered Electron microscopy (QBSE) images of tail vertebrae from 6 month old mice. There is no overall reduction in bone mineral concentrationin TG bone compared to WT. The metaphyseal subperiosteal regions of the TG contain thinner trabeculae (arrows). (C) Alkaline phosphatase cytochemistry on undecalcified, low temperature processed, GMA-embedded TG samples, submacroscopic view. Note the intense staining of the superiosteal metaphyseal regions [arrows, compare with (A) and (B)]. (D) Details of the subperiosteal, metaphyseal regions of TG tail vertebrae. The osteogenic periosteum is intensely stained (in sharp contrast with the adjacent non-osteogenic soft tissues) and fully continuous with a highly cellular, Alp-positive tissue separating nascent bone trabeculae from one another. Nascent trabeculae have a highly irregular profile, and undergo extensive remodeling. Osteoclasts stand out as large, ALP-negative cells (arrows). (E) Osx immunolabeling of the superiosteal metaphyseal region of a tail vertebra from a 6 monthold TG. The Alp-positive highly cellular tissue that separates nascent bone trabeculae from one another is enriched in cells with nuclear labeling for Osx. Note that the labeling is not restricted to osteoblasts on bone surfaces. Osteoclasts are negative (arrow), as are single cells interspersed among the Osx-positive osteogenic cells.
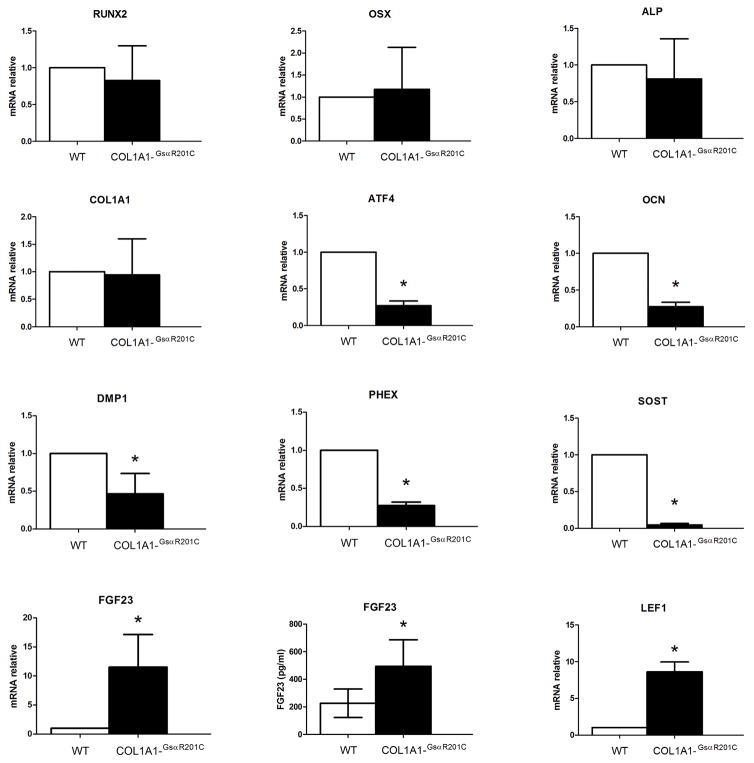
Consistent with an overtly abnormal osteoblast function in vivo, in vitro analysis of gene expression by differentiated BMSCs from TG and WT mice revealed a profound dysregulation of multiple markers and regulators of osteoblastic function (Fig. 4). Messenger RNA levels of Runx2, Osx, Alp and Col1a1 were normal in TG cells compared with WT cells. On the other hand, mRNA levels for Atf4 [a regulator of osteogenic genes (39)], and multiple markers of very late stages of osteogenic differentiation such as Ocn, Dmp-1, Phex and Sost were reduced in TG cells compared with WT. In contrast, mRNA levels for Fgf23, also thought to be predominantly expressed in late stages of osteogenic differentiation, were higher in TG osteoblastic cells compared with WT, and this finding was reflected in higher levels of total (C-term and intact) serum Fgf23 in TG mice compared with WT. Lastly, consistent with a reduced expression of the Wnt repressor, Sost, mRNA levels for the Wnt target gene, Lef-1, were increased in TG cells compared to WT, suggesting a functional link between the high bone mass phenotype and enhanced Wnt signaling in Col1a1-GsαR201Cmice.
Fig. 4.
In vitro expression of osteogenic markers by differentiated bone marrow stromal cells. Confluent bone marrow stromal stem cells from Col1a1-GsαR201C and control mice were switched to osteogenic medium and RNA was isolated after 28 days of induction. Relative expression of different osteogenic markers, all normalized to GAPDH mRNA expression, were analyzed by qRT-PCR (n=4/group). The data are reported as the expression level relative to WT. qPCR analysis revealed a significant reduction in expression of the osteogenic regulator Atf4, and of late osteoblast specific markers Ocn, Dmp1, Phex and Sost in transgenic cells. qPCR and serum analysis show significantly higher levels of Fgf23 in transgenic cells and mice, respectively, relative to WT. Consistent with the downregulation of Sost, persistence of the Wnt signaling pathway was marked by upregulation of Lef1
Osteoblast-specific expression of Gsα results in excess, ectopic bone remodeling
Tartrate-resistant acid phosphatase staining of tail vertebrae from 3–6 months old TG mice demonstrated high osteoclastic resorption activity (Fig. 5A-i–iii), which grossly co-distributed with new bone formation activity as mapped by Alp staining (Suppl. Fig. 4, compare B and C). A wealth of mononuclear Trap-positive cells interspersed among the Alp-positive osteogenic cells documented high levels of osteoclastogenesis, in addition to obviously high numbers of osteoclasts. Interestingly, in spite of this, measurements of osteoclast numbers and surfaces per bone surface units failed to reveal differences between WT and TG mice (Fig. 5B). While counterintuitive, this finding was easily accounted for by the morphology of the thin, curvilinear bone trabeculae in TG mice, noted for a high surface complexity and as a result, surface length. Taken together, these data suggested that high bone formation activity was coupled with high resorption activity in cortical bone of TG mice. Serial X-ray analyses of individual mice at 3 and 6 months demonstrated that the deforming excess in bone mass peaked at 3 months and progressively declined thereafter (Fig. 5C), consistent with sustained levels of continued bone remodeling within the excess cortical bone. In the femur, the massive density seen in the distal half at 3 months was replaced, in the same individual bones, by patterns of cancellous bone and peculiar “bone-in-bone” (cortex-in-cortex) features (Fig. 5C, bottom panel). XMT analysis of femora dissected from 3 and 6 month old animals documented that these radiographic features arose from the replacement of previously massive cortical bone by cancellous bone; this ex-massive, newly cancellous bone merged directly, at the midshaft, with a system of lacunar spaces interspersed within massive cortical bone, and widely interconnected (Fig. 5D). SEM analysis of the walls delimiting such lacunar spaces directly documented the origin of such space from intracortical remodeling, by demonstrating a wealth of resorption bays over their surfaces (Suppl. Fig. 5A–D). Giemsa-stained sections (and QBSE images, not shown) demonstrated the aberrant presence of pseudo-Haversian systems [cortical bone does not normally include secondary osteonal Haversian systems in the mouse (Suppl. Fig. 6A)], further documenting the occurrence of extensive, protracted and ectopic intracortical bone remodeling in TG mice (Suppl. Fig. 6B–D).
Fig. 5.
Excess remodeling in cortical bone of Col1a1-GsαR201C mice. (A) Trap staining on undecalcified, low-temperature processed tail vertebrae. Note the concentration of Trap activity in the superiosteal metaphyseal regions (Ai), and the irregular profile of bone trabeculae (Aii), resulting in an increase in bone surfaces, and the crowding of mononuclear Trap-positive cells in the intertrabecular regions (Aiii) (compare with mononuclear Osx-negative cells in Fig. 3E). (B) Histomorphometric analysis of bone resorption parameters. (C) Serial radiographic images from the same TG mouse at 3 and 6 months. Note the marked reduction in bone mass at 6 months compared to 3 months, particularly obvious in the cortex of the distal region. The reduced bone mass gives rise to a “cortex in cortex” feature in the distal metaphysis (arrows, 6 months). (E) XMT analysis of massively hyperostotic femurs in 3 and 6 months old mice. In WT mice, a marked reduction in the amount of cancellous bone in the distal metaphysis is obvious at 6 months compared to 3 months. In 3 month old TG mice, the marrow cavity is markedly narrowed in the distal femur, and its endosteal wall has wide vascular openings. The cortex is diffusely microporous in the distal end, both internally and at the surface. In 6 month old TG mice, most of the cortical bone in the distal region has a cancellous architecture, which explains the radiographic appearance including the origin of the “cortex-in-cortex” feature (dotted lines) from the projection of the cortex onto the trabecularized cortical bone in plain radiographs. The cortical cancellous bone of the distal half of the femur merges cranially with a system of intracortical lacunar spaces within the dense cortical bone.
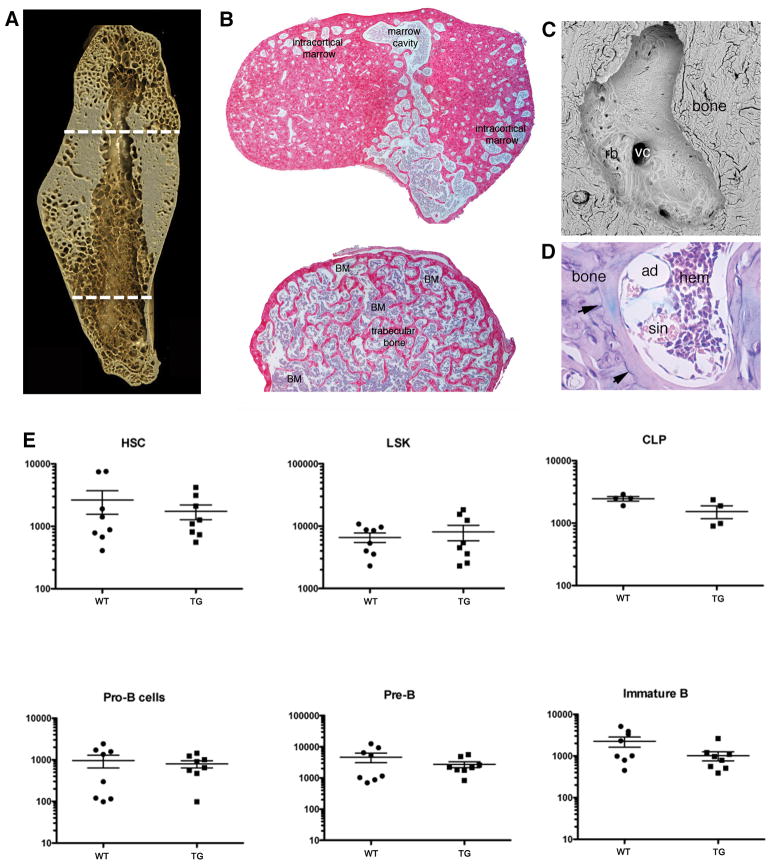
Osteoblast-specific expression of GsαR201C has no effect on bone marrow per se
In the femur, histological analysis demonstrated that the intracortical space, and spaces within the newly formed cancellous bone that, over time, replaced large portions of the massive cortical bone were, like the marrow cavity of long bones, occupied by hematopoietic (red) marrow (Fig. 6A and B). The aberrant, excessive, and ectopic remodeling of the cortical bone of Col1a1-GsαR201C mice (Fig. 6C) thus represented, in fact, the remodeling of bone into bone marrow space. Bone marrow tissue, however, was entirely free of fibrosis (Fig 6D), which characterizes fibrous dysplastic bone lesions in humans. All hematopoietic lineages were normally represented therein. Analysis of hematopoietic progenitor cell compartments in red marrow (from long bones of 6-months old Col1a1-GsαR201C mice) also failed to reveal changes. The HSC-containing fraction (Lin-/Sca-1+/c-kit+, LSK) was unchanged in frequency and absolute numbers. LSK/CD34− cells were also similar to wild-type mice. Numbers of CLP (Lin-/IL-7Ra+/Sca-1+/−/c-kit+/−), Pro-B (CD19+/CD43+/IgM-), Pre-B (CD19+/IgM-/CD43-) and immature B (CD19+/IgM+) cells were not significantly different from controls (Fig. 6E). Thus, while osteoblast-specific expression of GsαR201C did affect the gross distribution of hematopoietic bone marrow, it did not affect either its structure (lack of fibrosis) or its function, including the size of stem cell and progenitor compartments.
Fig. 6.
Remodeling captures intracortical space accommodating normal red marrow in long bones of Col1a1-GsαR201C mice. (A) XMT image providing localization of regions chosen for histological analysis of bone and bone marrow, and (B) Sirius red-stained cross sections of the femur, demonstrating that intracortical space and the medullary cavity are occupied by red marrow, with no fibrosis. (C) SEM image of a single intracortical space, demonstrating the occurrence of remodeling over its wall (rb, resorption bays) and the opening of vascular channels (vc). (D) A single intracortical spaceas seen in a Giemsa stained section. The lacuna is occupied by hematopoietic marrow, including sinusoids (sin) and adipocytes (ad), with no fibrosis. Note the system of cement lines marking remodeling events (arrows). E) Analysis of hematopoietic progenitors in the bone marrow of 6 month old WT and TG mice. The graphs show the number of the cells in the respective hematopoietic progenitor compartment in 106 viable cells. The HSC-containing compartment was identified as Lin-/Sca-1+/c-kit+ (LSK); hematopoietic stem cells were identified as Lin-/Sca-1+/c-kit+/CD34+; CLPs as Lin-/IL-7Ra+/Sca-1+/−/c-kit+/−; Pro-Bs as CD19+/CD43+/IgM-; Pre-Bs as CD19+/IgM-/CD43− and immature B as CD19+/IgM+ cells. Neither frequencies nor absolute numbers were significantly altered.
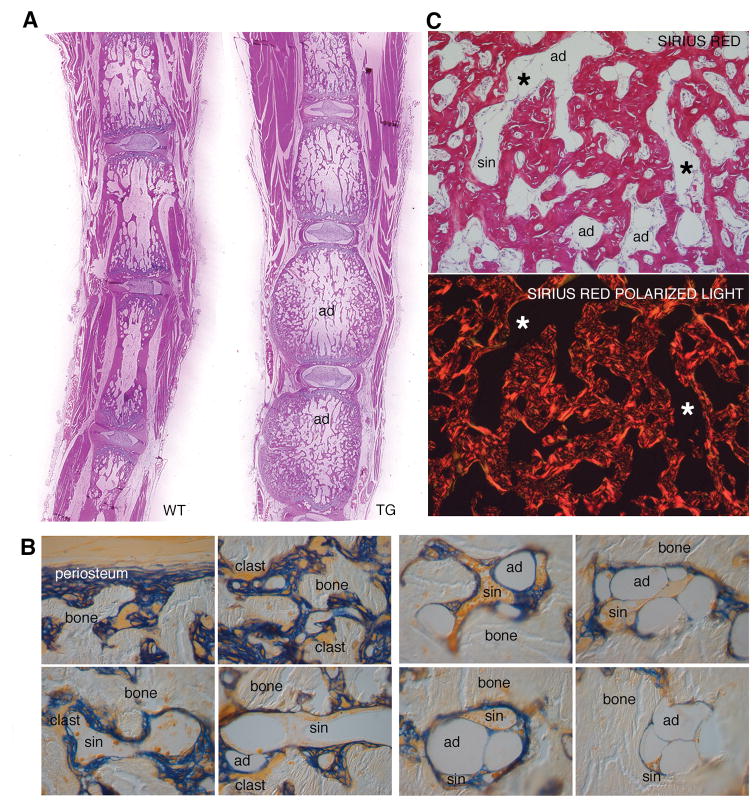
Different regions of the skeleton are normally occupied by either red (hematopoietically active) or yellow (adipose) marrow (40). While long bones including the femur are typical red marrow sites in the mouse, the tail vertebrae are typical sites of yellow (adipose) marrow, whose development begins postnatally and is completed within 3 weeks (data not shown). Just as continued remodeling of cortical bone expanded the space available for red marrow in long bones over time, continued remodeling in tail vertebrae gained space for yellow marrow (Fig. 7). While the excess bone formation impinged on bone marrow space in tail vertebrae, development of adipocytes from a local progenitor was not per se impaired. Development of adipocytes around sinusoids could actually be pinpointed in the inner parts of tail vertebrae at 3 months, and full-blown yellow marrow was clearly discernible at 6 months (Fig. 7A). Thus, in tail vertebrae, which are normally filled with yellow marrow, expression of the constitutively active GsαR201C did not impact on the type of marrow that formed. While osteoblasts and adipocytes have been claimed to represent positive (41) and negative (42) regulators of the hematopoietic stem cell niche and hematopoiesis, respectively, the change in their local balance in the tail vertebrae did not turn yellow marrow into red marrow; i.e., it did not establish ectopic hematopoietic activity in the tail at any time. Aside from the progressive increase in ectopic marrow space within cortical bone, no gross osteolysis was ever observed in TG mice of any age (Fig 7B). At any site, bone marrow tissue, whether red or yellow, whether located in the marrow cavity or in the newly established intracortical marrow space, did not show any signs of fibrosis (Fig. 7C). While marrow fibrosis, lack of adipocytes and local depletion of hematopoietic tissue are hallmark effects of Gsα activating mutations in human FD, none of these changes were reproduced by targeting GsαR201C expression to mouse osteoblasts.
Fig. 7.
Remodeling captures space accommodating normal yellow marrow in tail vertebrae of Col1a1-GsαR201C mice. (A) Submacroscopic view of tail sections from 6 months old WT and TG mice. Marrow space is occupied by adipocytes (ad) regardless of genotype. (B) Detail of the marrow space in a tail vertebra of a 6 month old TG mouse. Trabeculae are rimmed with Alp-positive cells and osteoclasts (clasts), while intertrabecular spaces are occupied by typical yellow marrow, with sinusoids (sin), and adipocytes (ad). (C) Sirius red staining demonstrates the highly irregular trabecular architecture and woven bone texture, interspersed with sinusoids (sin), adipocytes (ad), but with no fibrosis (*, polarized light image).
Discussion
We recently showed that constitutive, global expression of an FD-causing mutation results in a direct replica of human FD in the mouse (15), with gross, microscopic and even subtle features of FD pathology in humans. Data from this study demonstrate directly the effects of osteoblast-specific expression of GsαR201C. These effects, we have shown, fail to reproduce the defining features of human FD; i.e., marrow fibrosis, osteolysis, fracture, osteomalacia, and local disappearance of both marrow adipocytes and hematopoietic tissues from bone marrow. None of these effects, observed both in the human disease and in its recently developed mouse model (15), can thus be attributed to osteoblasts. As FD emanates from effects of the mutation on the osteogenic lineage, all of these effects must be attributed to cells in the stromal/osteogenic system distinct from the mature osteoblast, including osteogenic progenitors, as previously postulated based on in vivo models employing local transplantation of mutated human skeletal stem/osteoprogenitor cells (12). Of note, the characteristics of the human disease that are not reproduced by osteoblast-specific Gsα mutations in mice (notably, marrow fibrosis, local loss of hematopoietic tissue, local loss of adipocytes) are centered on the function of bone marrow stromal stem/progenitors rather than mature osteoblasts. Consistent with the lack of conspicuous structural or architectural changes in the bone marrow proper in our Col1a1-GsαR201C mice, we observed normal compartments of hematopoietic stem and progenitor cells, in the presence of normal skeletal progenitors and mutation-expressing osteoblasts. This is of specific interest in view of recent attention paid to osteoblasts as potential regulators of both the HSC niche (43–45) and B-lymphopoiesis (46), and of a putative role in this respect for Gsα expressed in osteoblasts proper (46). Our data, rather, lend support to the view that osteoprogenitors, but not mature osteoblasts, are the osteogenic cells more directly involved with hematopoietic progenitor regulation (47).
From our data, the sole effect of overactivity of Gsα in osteoblasts appears to be a marked increase in bone mass. This by default reflects in an altered shaping of marrow space during skeletal growth. The boundaries and relative volumes of bone and bone marrow do change as a result of osteoblast-specific expression of activating mutation of Gsα, but bone marrow structure and cellular composition do not. Furthermore, ectopic perivascular remodeling within cortical bone wins, over time, perivascular space for hematopoiesis, while the anatomical mapping of yellow and red bone marrow across the skeleton remain unchanged. Excess bone mass is a feature of FD of bone in humans, and specifically contributes to facial deformity, for example (1). However, excess bone mass is not an obligate feature of individual FD lesions, and never occurs in isolation in the context of FD (1). Interestingly, osteoblast-specific expression of GsαR201C, while failing to mimic the characteristic changes of FD and its overall pathology in the mouse, mimics human high bone mass phenotypes and disorders that are unrelated to Gsα and FD, such as sclerosteosis (48) and Van Buchem’s disease (49). These disorders represent the effects of loss-of-function mutations in SOST (50,51), a known Wnt inhibitor expressed in late stages of bone cell differentiation (52–55), which is otherwise known to be downregulated by agonists of the Gsα signaling pathway such as PTH (56–60). Consistent with these known effects of excess cAMP and with the excess of cAMP in osteoblastic cells of the Col1a1-GsαR201C mice, we observed a reduced expression of Sost and increased expression of the Wnt target gene, Lef1, in late stages of osteogenic cultures from Col1a1-GsαR201C mice. These results mirror precisely the opposite results obtained by using similar cultures from Gsα conditionally-deficient specifically in osteoblasts (46,61) and support the suggestion made by these authors that Gsα can act as a brake in late stages of osteoblastic differentiation via its effect on Wnt signaling (46). Likewise, the reduced expression of markers commonly regarded as “osteocytic” in our late stage cultures might mirror a delayed or disturbed osteoblast-to-osteocyte transition, even if in the context of an in vivo excess of woven bone, known to be enriched in osteocytes, but indeed, specifically in immature osteocytes. Overall, when in vitro data are matched to the overt in vivo phenotype of our mice, it is clear that these effects on very late stages of maturation of osteogenic cells are globally superseded by (and in fact contribute to), in vivo, a pronounced anabolic net effect of Gsα activity on bone formation and bone mass. A pronounced increase in bone mass was observed previously in other unrelated models in which excess Gsα signaling and cAMP production (in the presence of WT Gsα) take place, and can be predicted based on the nature of the transgene, residing upstream [a constitutively active PTH1R (41), and an engineered, overactive G protein coupled receptor (62)] or downstream [PKA (63)] of Gsα in the signaling pathway. A direct comparison of our model with mice of the same background (FVB/N) in which a constitutively active PTH1R was specifically expressed in osteoblasts by the same promoter we used (2.3kb Col1a1) (41) (Suppl. Fig. 7) reveals that the peculiar cortical bone phenotype induced by expressing an activating mutation of Gsα in osteoblasts is not mirrored by expression, in osteoblasts, of a constitutively active mutant of PTH1R, which predicts a similarly enhanced cAMP response (Suppl. Fig. 7). A divergent response of cortical and trabecular [actually, intramedullary (28)] bone in mice expressing a constitutively active PTH1R in osteoblasts had been noted previously (41). Our data now also reveal a divergent response of cortical bone itself to constitutively active Gsα vs. the Gsα-activating, constitutively active PTH1R and may suggest a specific, distinct, possibly PTH1R-independent role for Gsα in cortical bone.
Lastly, the deforming nature of the excess bone mass in our mice per se denotes a non-uniform effect of the transgene on cognate bone-forming cells existing at different anatomical sites (e.g., in tail vertebrae, endplates vs. main body; in femora, articular regions vs. midshaft). Specific regions undergo the most profound changes in individual bones in Col1a1-GsαR201Cmice, resulting in consistent patterns of deformity (bumpy tails, subtrochanteric bulges). Non-cell autonomous specifiers of the response of osteoblasts to the transgene may determine or contribute to these localized effects of the transgene. While PTH is commonly seen as the main agonist of the Gsα signaling pathway in bone, additional players such as β-adrenergic signals (known to operate in bone (64–69)) should not be overlooked. Elucidating further which ligands, in which physiological context, and through which downstream mediators [e.g., PKA-I and II, known to be differentially activated by different ligands and cognate receptors activating the cAMP pathway (70)] are the dominant triggers of activation of Gsα in different anatomical compartments of bone, in the osteogenic lineage, and in the stromal system thus appears to be an important step to undertake. This will improve our understanding of the physiology of Gsα signaling in the bone-bone marrow organ and of its derangements leading to development of FD lesions. These endeavors can be facilitated by the models that we have reported previously and herein.
Supplementary Material
Acknowledgments
This work was supported by grants from Telethon (Grant GGP09227), Fondazione Institut Pasteur-Cenci Bolognetti, MIUR, Ministry of Health, Sapienza University and EU (PluriMes consortium) to PB; Fondazione Roma to PB and MR; and by the DIR, NIDCR, of the IRP, NIH, DHHS (PGR, KH).
Footnotes
Authors’ contribution: CR, SM, KH, IS construct design and transgenesis; CR, ADC, SC, ES, BS, genotyping, mouse breeding, X-ray analysis, histology, cytochemistry, immunocytochemistry; CR, cell cultures, in vitro assays, QPCR; AB, SEM, QBSE, XMT; GD, XMT analysis; AC, bone marrow studies; CR, IS, AB, KH, PGR, MR, PB, experiment planning and data analysis and interpretation, writing and editing of manuscript; PB, project oversight.
References
- 1.Bianco P, Wientroub S. Fibrous Dysplasia. In: Glorieux FH, Pettifor JM, Juppner H, editors. Pediatric Bone: Biology and Disease. 2. Elsevier; Ney York: 2012. pp. 589–624. [Google Scholar]
- 2.Weinstein LS, Shenker A, Gejman PV, Merino MJ, Friedman E, Spiegel AM. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N Engl J Med. 1991;325(24):1688–1695. doi: 10.1056/NEJM199112123252403. [DOI] [PubMed] [Google Scholar]
- 3.Shenker A, Weinstein LS, Sweet DE, Spiegel AM. An activating Gs alpha mutation is present in fibrous dysplasia of bone in the McCune-Albright syndrome. J Clin Endocrinol Metab. 1994;79(3):750–755. doi: 10.1210/jcem.79.3.8077356. [DOI] [PubMed] [Google Scholar]
- 4.Lania A, Persani L, Ballare E, Mantovani S, Losa M, Spada A. Constitutively active Gs alpha is associated with an increased phosphodiesterase activity in human growth hormone-secreting adenomas. J Clin Endocrinol Metab. 1998;83(5):1624–1628. doi: 10.1210/jcem.83.5.4814. [DOI] [PubMed] [Google Scholar]
- 5.Riminucci M, Fisher LW, Shenker A, Spiegel AM, Bianco P, Gehron Robey P. Fibrous dysplasia of bone in the McCune-Albright syndrome: abnormalities in bone formation. Am J Pathol. 1997;151:1587–1600. [PMC free article] [PubMed] [Google Scholar]
- 6.Riminucci M, Liu B, Corsi A, Shenker A, Spiegel AM, Gehron Robey P, Bianco P. The histopathology of fibrous dysplasia of bone in patients with activating mutations of the Gs alpha gene: site-specific patterns and recurrent histological hallmarks. J Pathol. 1999;187:249–258. doi: 10.1002/(SICI)1096-9896(199901)187:2<249::AID-PATH222>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 7.Corsi A, Collins MT, Riminucci M, Howell PG, Boyde A, Robey PG, Bianco P. Osteomalacic and hyperparathyroid changes in fibrous dysplasia of bone: core biopsy studies and clinical correlations. J Bone Miner Res. 2003;18(7):1235–1246. doi: 10.1359/jbmr.2003.18.7.1235. [DOI] [PubMed] [Google Scholar]
- 8.Siegal G, Bianco P, PDC . Fibrous Dysplasia. In: Fletcher CDMBJHP, Mertens F, editors. WHO Classification of bone and soft tissue tumors pathology and genetics. 4. IARC; Lyon: 2013. pp. 353–353. [Google Scholar]
- 9.Riminucci M, Kuznetsov SA, Cherman N, Corsi A, Bianco P, Gehron Robey P. Osteoclastogenesis in fibrous dysplasia of bone: in situ and in vitro analysis of IL-6 expression. Bone. 2003;33(3):434–442. doi: 10.1016/s8756-3282(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 10.Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, Waguespack S, Gupta A, Hannon T, Econs MJ, Bianco P, Gehron Robey P. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112(5):683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karadag A, Riminucci M, Bianco P, Cherman N, Kuznetsov SA, Nguyen N, Collins MT, Robey PG, Fisher LW. A novel technique based on a PNA hybridization probe and FRET principle for quantification of mutant genotype in fibrous dysplasia/McCune-Albright syndrome. Nucleic Acids Res. 2004;32(7):e63. doi: 10.1093/nar/gnh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco P, Kuznetsov S, Riminucci M, Fisher LW, Spiegel AM, Gehron Robey P. Reproduction of human fibrous dysplasia of bone in immunocompromised mice by transplanted mosaics of normal and Gs-alpha mutated skeletal progenitor cells. J Clin Invest. 1998;101:1737–1744. doi: 10.1172/JCI2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bianco P, Riminucci M, Majolagbe A, Kuznetsov SA, Collins MT, Mankani MH, Corsi A, Bone HG, Wientroub S, Spiegel AM, Fisher LW, Robey PG. Mutations of the GNAS1 gene, stromal cell dysfunction, and osteomalacic changes in non-McCune-Albright fibrous dysplasia of bone. J Bone Miner Res. 2000;15(1):120–128. doi: 10.1359/jbmr.2000.15.1.120. [DOI] [PubMed] [Google Scholar]
- 14.Riminucci M, Gehron Robey P, Saggio I, Bianco P. Skeletal progenitors and the GNAS gene: fibrous dysplasia of bone read through stem cells. J Mol Endocrinol. 2010;45(6):355–364. doi: 10.1677/JME-10-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saggio I, Remoli C, Spica E, Cersosimo S, Sacchetti B, Robey PG, Holmbeck K, Cumano A, Boyde A, Bianco P, Riminucci M. Constitutive Expression of Gsalpha(R201C) in Mice Produces a Heritable, Direct Replica of Human Fibrous Dysplasia Bone Pathology and Demonstrates Its Natural History. J Bone Miner Res. 2014;29(11):2357–2368. doi: 10.1002/jbmr.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Foundation symposium. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 17.Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105(12):1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell stem cell. 2008;2(4):313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 21.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 22.Rossert J, Eberspaecher H, de Crombrugghe B. Separate cis-acting DNA elements of the mouse pro-alpha 1(I) collagen promoter direct expression of reporter genes to different type I collagen-producing cells in transgenic mice. J Cell Biol. 1995;129(5):1421–1432. doi: 10.1083/jcb.129.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dacquin R, Starbuck M, Schinke T, Karsenty G. Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Developmental dynamics : an official publication of the American Association of Anatomists. 2002;224(2):245–251. doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 24.Lyons KM. Animal models: genetic manipulation. In: CJR, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. John Wiley and Sons, Inc; Oxford, UK: 2013. pp. 69–75. [Google Scholar]
- 25.Nagy A, Gertsenstein M, Vintersten K. Press CSHL, editor. Manipulating the Mouse Embryo: A Laboratory Manual. Inglis J; Woodbury NY USA: 2003. [Google Scholar]
- 26.Piersanti S, Remoli C, Saggio I, Funari A, Michienzi S, Sacchetti B, Robey PG, Riminucci M, Bianco P. Transfer, analysis, and reversion of the fibrous dysplasia cellular phenotype in human skeletal progenitors. J Bone Miner Res. 2010;25(5):1103–1116. doi: 10.1359/jbmr.091036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robey P, Termine J. Human bone cells in vitro. Calcif Tissue Int. 1985;37(5):453–460. [PubMed] [Google Scholar]
- 28.Kuznetsov SA, Riminucci M, Ziran N, Tsutsui TW, Corsi A, Calvi L, Kronenberg HM, Schipani E, Robey PG, Bianco P. The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow. J Cell Biol. 2004;167(6):1113–1122. doi: 10.1083/jcb.200408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Maciejewski BS, Lee N, Silbert O, McKnight NL, Frangos JA, Sanchez-Esteban J. Strain-induced fetal type II epithelial cell differentiation is mediated via cAMP-PKA-dependent signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2006;291(4):L820–827. doi: 10.1152/ajplung.00068.2006. [DOI] [PubMed] [Google Scholar]
- 30.Davis GR, Elliott JC. High definition X-ray microtomography using a conventional impact X-ray source. J Phys IV France. 2003;104:131–134. [Google Scholar]
- 31.Davis G, Jain B, Elliott J. A modelling approach to beam hardening correction. Proc of SPIE. 2008:7078. [Google Scholar]
- 32.Bradbeer JN, Riminucci M, Bianco P. Giemsa as a fluorescent stain for mineralized bone. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1994;42(5):677–680. doi: 10.1177/42.5.7512588. [DOI] [PubMed] [Google Scholar]
- 33.Bianco P, Bonucci E. Endosteal surfaces in hyperparathyroidism: an enzyme cytochemical study on low-temperature-processed, glycol-methacrylate-embedded bone biopsies. Virchows Arch A Pathol Anat Histopathol. 1991;419(5):425–431. doi: 10.1007/BF01605077. [DOI] [PubMed] [Google Scholar]
- 34.Boyde A, Travers R, Glorieux F, Jones S. The mineralization density of iliac crest bone from children with osteogenesis imperfecta. Calcified Tissue Int. 1999;64(3):185–190. doi: 10.1007/s002239900600. [DOI] [PubMed] [Google Scholar]
- 35.Boyde A, Firth EC. Musculoskeletal responses of 2-year-old Thoroughbred horses to early training. 8. Quantitative back-scattered electron scanning electron microscopy and confocal fluorescence microscopy of the epiphysis of the third metacarpal bone. New Zealand veterinary journal. 2005;53(2):123–132. doi: 10.1080/00480169.2005.36489. [DOI] [PubMed] [Google Scholar]
- 36.Boyde A. Improved digital SEM of cancellous bone: scanning direction of detection, through focus for in-focus and sample orientation. Journal of anatomy. 2003;202(2):183–194. doi: 10.1046/j.1469-7580.2003.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyde A. Scanning electron microscopy of bone. Methods in molecular biology. 2012;816:365–400. doi: 10.1007/978-1-61779-415-5_24. [DOI] [PubMed] [Google Scholar]
- 38.Boyde A. Staining plastic blocks with triiodide to image cells and soft tissues in backscattered electron SEM of skeletal and dental tissues. European cells & materials. 2012;24:154–160. doi: 10.22203/ecm.v024a11. discussion 160–151. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, Li L, Brancorsini S, Sassone-Corsi P, Townes TM, Hanauer A, Karsenty G. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry Syndrome. Cell. 2004;117(3):387–398. doi: 10.1016/s0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 40.Bianco P, Riminucci M. The bone marrow stroma in vivo: ontogeny, structure, cellular composition and changes in disease. In: Beresford JNOM, editor. Marrow stromal cell cultures. Cambridge University Press; Cambridge UK: 1998. pp. 10–25. [Google Scholar]
- 41.Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107(3):277–286. doi: 10.1172/JCI11296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 44.Arai F, Hirao A, Suda T. Regulation of hematopoiesis and its interaction with stem cell niches. International journal of hematology. 2005;82(5):371–376. doi: 10.1532/IJH97.05100. [DOI] [PubMed] [Google Scholar]
- 45.Wu JY, Scadden DT, Kronenberg HM. Role of the osteoblast lineage in the bone marrow hematopoietic niches. J Bone Miner Res. 2009;24(5):759–764. doi: 10.1359/jbmr.090225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105(44):16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117(20):5281–5288. doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- 48.Truswell AS. Osteopetrosis with syndactyly; a morphological variant of Albers-Schonberg’s disease. The Journal of bone and joint surgery British volume. 1958;40-B(2):209–218. [PubMed] [Google Scholar]
- 49.Van Buchem FS, Hadders HN, Hansen JF, Woldring MG. Hyperostosis corticalis generalisata. Report of seven cases. The American journal of medicine. 1962;33:387–397. doi: 10.1016/0002-9343(62)90235-8. [DOI] [PubMed] [Google Scholar]
- 50.Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Human molecular genetics. 2001;10(5):537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 51.Balemans W, Van Hul W. Identification of the disease-causing gene in sclerosteosis--discovery of a novel bone anabolic target? Journal of musculoskeletal & neuronal interactions. 2004;4(2):139–142. [PubMed] [Google Scholar]
- 52.van Bezooijen RL, ten Dijke P, Papapoulos SE, Lowik CW. SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine & growth factor reviews. 2005;16(3):319–327. doi: 10.1016/j.cytogfr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 53.van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Lowik CW. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. The Journal of experimental medicine. 2004;199(6):805–814. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Atkins GJ, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM. Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res. 2011;26(7):1425–1436. doi: 10.1002/jbmr.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baron R, Rawadi G, Roman-Roman S. Wnt signaling: a key regulator of bone mass. Current topics in developmental biology. 2006;76:103–127. doi: 10.1016/S0070-2153(06)76004-5. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PloS one. 2008;3(8):e2942. doi: 10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37(2):148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 58.Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H. Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res. 2007;22(12):1957–1967. doi: 10.1359/jbmr.070804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellido T. Downregulation of SOST/sclerostin by PTH: a novel mechanism of hormonal control of bone formation mediated by osteocytes. Journal of musculoskeletal & neuronal interactions. 2006;6(4):358–359. [PubMed] [Google Scholar]
- 60.Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146(11):4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- 61.Wu JY, Aarnisalo P, Bastepe M, Sinha P, Fulzele K, Selig MK, Chen M, Poulton IJ, Purton LE, Sims NA, Weinstein LS, Kronenberg HM. Gsalpha enhances commitment of mesenchymal progenitors to the osteoblast lineage but restrains osteoblast differentiation in mice. J Clin Invest. 2011;121(9):3492–3504. doi: 10.1172/JCI46406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsiao EC, Millard SM, Louie A, Huang Y, Conklin BR, Nissenson RA. Ligand-mediated activation of an engineered gs g protein-coupled receptor in osteoblasts increases trabecular bone formation. Mol Endocrinol. 2010;24(3):621–631. doi: 10.1210/me.2009-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsang K, Starost M, Nesterova M, Boikos S, Watkins T, Almeida M, Harran M, Li A, Collins M, Cheadle C, Mertz E, Leikin S, Kirschner L, Robey P, Stratakis C. Alternate protein kinase A activity identifies a unique population of stromal cells in adult bone. Proc Natl Acad Sci U S A. 2010;107(19):8683–8688. doi: 10.1073/pnas.1003680107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 65.Nagao M, Feinstein TN, Ezura Y, Hayata T, Notomi T, Saita Y, Hanyu R, Hemmi H, Izu Y, Takeda S, Wang K, Rittling S, Nakamoto T, Kaneko K, Kurosawa H, Karsenty G, Denhardt DT, Vilardaga JP, Noda M. Sympathetic control of bone mass regulated by osteopontin. Proc Natl Acad Sci U S A. 2011;108(43):17767–17772. doi: 10.1073/pnas.1109402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanyu R, Wehbi VL, Hayata T, Moriya S, Feinstein TN, Ezura Y, Nagao M, Saita Y, Hemmi H, Notomi T, Nakamoto T, Schipani E, Takeda S, Kaneko K, Kurosawa H, Karsenty G, Kronenberg HM, Vilardaga JP, Noda M. Anabolic action of parathyroid hormone regulated by the beta2-adrenergic receptor. Proc Natl Acad Sci U S A. 2012;109(19):7433–7438. doi: 10.1073/pnas.1109036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 68.Karsenty G, Ducy P. The hypothalamic control of bone mass, implication for the treatment of osteoporosis. Annales d’endocrinologie. 2006;67(2):123. doi: 10.1016/s0003-4266(06)72566-5. [DOI] [PubMed] [Google Scholar]
- 69.Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, Guo XE, Karsenty G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. The Journal of experimental medicine. 2011;208(4):841–851. doi: 10.1084/jem.20102608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Livesey SA, Kemp BE, Re CA, Partridge NC, Martin TJ. Selective hormonal activation of cyclic AMP-dependent protein kinase isoenzymes in normal and malignant osteoblasts. The Journal of biological chemistry. 1982;257(24):14983–14987. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.