Abstract
The relationship between late-life body mass index (BMI) and Alzheimer’s disease (AD) is poorly understood due to the lack of research in samples with autopsy-confirmed AD neuropathology (ADNP). The role of cerebrovascular disease (CVD) in the interplay between late-life BMI and ADNP is unclear. We conducted a retrospective longitudinal investigation and used joint modeling of linear mixed effects to investigate causal relationships among repeated antemortem BMI measurements, CVD (quantified neuropathologically), and ADNP in an autopsy sample of subjects across the AD clinical continuum. The sample included 1,421 subjects from the National Alzheimer’s Coordinating Center’s Uniform Data Set and Neuropathology Data Set with diagnoses of normal cognition (NC; n=234), mild cognitive impairment (MCI; n=201), or AD dementia (n=986). ADNP was defined as moderate to frequent neuritic plaques and Braak stage III-VI. Ischemic Injury Scale (IIS) operationalized CVD. Joint modeling examined relationships among BMI, IIS, and ADNP in the overall sample, and stratified by initial visit Clinical Dementia Rating (CDR) score. Subject-specific random intercept for BMI was the predictor for ADNP due to minimal BMI change (p=0.3028). Analyses controlling for demographic variables and APOE ε4 showed lower late-life BMI predicted increased odds of ADNP in the overall sample (p<0.001), and in subjects with CDR of 0 (p=0.0021) and 0.5 (p=0.0012), but not ≥1.0 (p=0.2012). Although higher IIS predicted greater odds of ADNP (p<.0001), BMI did not predict IIS (p = 0.2814). The current findings confirm lower late-life BMI confers increased odds for ADNP. Lower late-life BMI may be a preclinical indicator of underlying ADNP.
Keywords: Alzheimer’s disease, body mass index, cerebrovascular disease, neuropathology, obesity
INTRODUCTION
A definitive diagnosis of Alzheimer’s disease (AD) requires neuropathological examination and histopathological evidence for amyloid-β plaques and neurofibrillary tangles [1]. AD can now be reliably detected and diagnosed during life due to validated in vivo biomarkers, such as volumetric magnetic resonance imaging (MRI), amyloid positron emission tomography (PET) imaging, and/or cerebrospinal fluid (CSF) protein markers of neurodegeneration [2–5]. In addition to disease detection, these biomarker tools play an important role in determining the efficacy of new therapeutic agents currently being examined in AD clinical trials (e.g., Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease [A4]). Advanced neuroimaging modalities and CSF analysis can be time-intensive, expensive, invasive, and typically only administered after initial risk screening methods. There is a need, therefore, to identify brief and easily assessed risk factors for AD that can assist in disease detection and facilitate early intervention and treatment planning [6].
Obesity is one modifiable risk factor for AD that has been the target of much research given that, in 2015, 30.2% of adult Americans age 20 and over were obese, and this pattern is expected to exponentially grow over the next 15 years [7, 8]. Large prospective cohort (e.g., 1,500 to >10,000 subjects) and meta-analytic studies support elevated BMI at mid-life as a robust risk factor for the later clinical diagnosis of AD [6, 9–13]. Recent work also shows that more than half of 171 young to middle-aged participants with severe obesity met criteria for mild cognitive impairment [14]. Elevated mid-life BMI predicts AD-related brain changes on neuroimaging, including hippocampal atrophy [15]. BMI may confer increased risk for AD dementia through its association with cerebrovascular disease (CVD), given a higher BMI increases risk for CVD (e.g., stroke) [16] and CVD risk factors (e.g., heart disease) [17], and CVD contributes to the pathogenesis of AD [18–29] possibly through blood brain barrier dysfunction and oligaemia that trigger the amyloid-beta cascade of events that underpin AD-related neurodegeneration [30].
The relationship between BMI and risk for clinical AD becomes complex with increasing age [12]. Higher late-life BMI has consistently been associated with no, or even lower risk for AD across the clinical continuum [10, 12, 31, 32]. Longitudinal research shows that decreases in BMI from mid- to late-life, as well as over time throughout late-life, corresponds with greater risk for clinically diagnosed incident AD (e.g., mild cognitive impairment [MCI], dementia) and faster disease progression [12, 33–41]. The age-related change in directionality between BMI and AD risk may be related to the natural declines in BMI that accompany older age [42], and/or the fluctuations in BMI that occur throughout the clinical course of AD [41, 43]. Lower later-life BMI and increased risk for AD has been speculated to involve age- and/or pathophysiological alterations to brain regions that modulate weight control, appetite, and taste [33, 34, 43–46]. A potential survival bias effect may also be present in the context of the later-life BMI and AD risk paradox, as is observed in survival studies among clinical populations (e.g., heart failure), where higher BMI is associated with lower mortality [47].
A majority of the research examining BMI and AD risk has been without neuropathological confirmation of AD. It is possible that one of the reasons for the complicated relationship between BMI and AD risk over the lifespan is that the clinical samples in previous studies included subjects with non-AD pathologies and the absence of AD neuropathology (ADNP). Samples with autopsy-confirmed ADNP can thus elucidate and validate the relationship between late-life BMI and AD. Only four studies to date examined the association between BMI and ADNP. Mrak et al. [48] found that expression of tau and amyloid precursor protein was higher in 12 cognitively normal morbidly obese deceased subjects ranging in age at death from 21–70 compared to 10 non-obese controls and three subjects with AD. Neuropathological changes found in some subjects approached the level found in AD. Recent findings from a population-based cohort demonstrated that a one-unit increase in mid-life BMI corresponded with earlier onset of AD dementia by 6.7 months, and a higher mid-life BMI predicted a higher Braak score [49]. In an autopsy sample of 298 Catholic clergy members with and without dementia, increased ADNP was correlated with decreased late-life BMI proximate to death [31]. A recent study among 193 subjects with normal cognition (NC) from the National Alzheimer’s Coordinating Center (NACC) Neuropathology Data Set (NDS) found that lower later-life baseline BMI and annual increases in BMI over time both predicted greater odds of ADNP [50].
The existing neuropathological studies failed to examine the association between BMI and ADNP in subjects that spanned the clinical continuum during life (cognitively normal to MCI to AD dementia). The role of CVD in the relationship between late-life BMI and ADNP has also not been tested. Two of the studies examined change in BMI over time and its relationship with ADNP [31, 50], but BMI change was quantified through the crude computation of the average across visits. In the present study, we utilized advanced statistical joint model of linear mixed effects, multivariable and logistic regression [51] to investigate the causal relationships among BMI (including antemortem change in BMI), CVD (quantified neuropathologically), and ADNP, in an autopsy sample of >1,400 subjects with antemortem diagnoses of NC, MCI, or AD dementia from the NACC Uniform Data Set (UDS) and NDS. Relative to using average BMI across visits as a predictor to longitudinally investigate BMI change and ADNP, the joint modeling approach provides more efficient and unbiased estimates, while accounting for between-subject variability [51].
MATERIALS AND METHODS
Subjects
The sample included 1,421 deceased subjects from the NACC-UDS and NDS. Based on clinical diagnoses at initial visit, the sample included 234 subjects with NC, 201 with MCI, and 986 with AD dementia (see Table 1 for demographic and clinical characteristics by initial diagnostic status). The NACC was established in 1999 by the National Institute on Aging (NIA) to promote AD research. It is a publicly available database of clinical and neuropathological data gathered from approximately 30 NIA-funded Alzheimer’s Disease Centers (ADCs) across the United States. Since 2005, ADCs have contributed standardized cognitive, behavioral, and functional subject data each year to a common database, known as the NACC-UDS. A full description of NACC-UDS is provided elsewhere [52–54]. Briefly, each ADC longitudinally follows community-dwelling elderly with and without cognitive impairment. Recruitment methods varies across the ADC sites, and includes recruitment from population-based samples and clinics, as well as through public recruitment efforts, subject referrals, and other ongoing studies. Due to variation in recruitment methods, the NACC UDS dataset is often considered to consist of a clinical case series of patients from each ADC site. At each ADC, subjects complete annual medical, neuropsychological, neurological, and psychiatric evaluations. A subset of UDS subjects also agree to post-mortem brain donation and neuropathological examination to form the NACC-NDS [50, 52]. Research using the NACC database was approved by the University of Washington Institutional Review Board, and informed consent from subjects that are part of the NACC datasets was obtained at the individual ADCs.
Table 1.
Demographics and clinical features of the sample by diagnostic status
| Characteristicsa | Diagnosis at Initial Visit | p-valuec | |||
|---|---|---|---|---|---|
| NC | MCI | AD dementia | MCI vs. NC | AD vs NC | |
| Number of subjects, n | 234 | 201 | 986 | NA | NA |
| Age at initial visit (years), mean (SD) | 81.6 (9.5) | 77.4 (11.1) | 76.1 (10.8) | <.0001 | <.0001 |
| Age at death (years), mean (SD) | 86.1 (9.5) | 81.6 (11.1) | 79.7 (10.9) | <.0001 | <.0001 |
| Male, n (%) | 96 (41.0%) | 122 (60.7%) | 547 (55.5%) | <.0001 | <.0001 |
| Education (years), mean (SD) | 15.6 (2.7) | 15.5 (3.2) | 14.9 (3.3) | 0.75 | 0.002 |
| Non-white race, n (%) | 12 (5.2%) | 6 (3.0%) | 75 (7.7%) | 0.27 | 0.19 |
| Number of UDS visits, mean (SD) | 3.9 (2.0) | 3.4 (1.8) | 2.8 (1.7) | 0.003 | <0.0001 |
| Global CDR initial visit, mean (SD) | 0.07 (0.17) | 0.48 (0.16) | 1.49 (0.88) | <.0001 | <.0001 |
| CDR-SB at initial visit, mean (SD) | 0.2 (0.4) | 1.7 (1.2) | 8.7 (5.1) | 0.0002 | <.0001 |
| Diagnosis via consensus panel, initial visit, n (%) | 139 (59.4%) | 159 (79.1%) | 755 (76.6%) | <.0001 | <.0001 |
| ≥ 1 APOE e4 allele, n (%) | 53 (24.4%) | 65 (37.8%) | 504 (59.0%) | 0.005 | <.0001 |
| BMI (kg/m2) at initial visit, mean (SD) | 25.7 (4.6) | 25.9 (4.9) | 25.4 (4.5) | 0.56 | 0.48 |
| BMI (kg/m2) at initial visit, categorized, n (%) | |||||
| <18.5 | 3 (1.3%) | 4 (2.0%) | 38 (3.9%) | 0.68 | 0.48 |
| 18.5 to 24.9 | 104(44.4%) | 86 (42.8%) | 406 (41.2%) | ||
| 25–29.9 | 86 (36.8%) | 70 (34.8%) | 390 (39.6%) | ||
| 30 or higher | 41 (17.5%) | 41 (20.4%) | 152 (15.4%) | ||
| BMI (kg/m2) at most recent visit, mean (SD)b | 24.6 (5.2) | 25.7 (5.0) | 25.4 (4.6) | 0.06 | 0.08 |
| BMI (kg/m2) at most recent visit, categorized, n (%)b | |||||
| <18.5 | 17 (11.2%) | 6 (4.8%) | 19 (4.6%) | 0.06 | 0.08 |
| 18.5 to 24.9 | 60 (39.5%) | 45 (36.0%) | 164 (39.4%) | ||
| 25–29.9 | 52 (34.2%) | 52 (41.6%) | 170 (40.9%) | ||
| 30 or higher | 23 (15.1%) | 22 (17.6%) | 63 (15.1%) | ||
| Systolic blood pressure at initial visit, mean (SD) | 135.6(20.5) | 133.5(18.9) | 131.9 (19.4) | 0.29 | 0.01 |
| Diastolic blood pressure at initial visit, mean (SD) | 71.8 (10.9) | 73.0 (10.0) | 73.1 (10.5) | 0.26 | 0.10 |
| Hypertension, n (%) | 166(70.9%) | 126(62.7%) | 534 (54.2%) | 0.07 | <.0001 |
| Diabetes, n (%) | 29 (12.4%) | 24 (11.9%) | 107 (10.9%) | 0.89 | 0.50 |
| Hypercholesterolemia, n (%) | 140(59.8%) | 112(56.3%) | 506 (51.5%) | 0.46 | 0.02 |
| Stroke, n (%) | 27 (11.5%) | 31 (15.4%) | 115 (11.8%) | 0.24 | 0.93 |
| Cardiovascular disease, n (%) | 109(46.6%) | 75 (37.3%) | 279 (28.4%) | 0.05 | <.0001 |
| Thyroid disease, n (%) | 71 (30.5%) | 53 (26.4%) | 199 (20.2%) | 0.34 | 0.001 |
| Atrial fibrillation, n (%) | 52 (22.3%) | 31 (15.4%) | 124 (12.6%) | 0.07 | 0.0002 |
| Smoking history, n (%) | 116(49.6%) | 107(53.2%) | 441 (44.7%) | 0.45 | 0.18 |
| Antihypertensive use at any visit, n (%) | 182(77.8%) | 135(67.2%) | 532 (54.0%) | 0.01 | <.0001 |
| Antilipid medication at any visit, n (%) | 117(50.0%) | 105(52.2%) | 435 (44.1%) | 0.64 | 0.10 |
Abbreviations: NA = Not applicable; NC = normal cognition; MCI = mild cognitive impairment; AD = Alzheimer’s disease; UDS = Uniform Data Set; CDR-SB = Clinical Dementia Rating Sum of Boxes; APOE = apolipoprotein E;
Number missing data: Education (NC, n=4; MCI, n=1; AD, n=10); Race (NC, n=1; MCI, n=1; AD, n=7); APOE genotype (NC, n=17; MCI, n=29; AD, n=132); diabetes (NC, n = 0; MCI, n = 0; AD, n = 1); hypercholesterolemia (NC, n = 0; MCI, n = 2; AD, n=4); thyroid disease (NC, n = 1; MCI, n = 0; AD, n =2); stroke (NC, n = 0; MCI, n = 0; AD, n = 7); cardiovascular disease (NC, n = 0; MCI, n = 0; AD, n =3); atrial fibrillation (NC, n = 1; MCI, n = 0; AD, n = 0); systolic blood pressure (NC, n = 2; MCI, n = 3; AD, n = 29); diastolic blood pressure (NC, n = 2; MCI, n = 3; AD, n = 29);
Among the 152 NC, 125 MCI, and 416 AD subjects with longitudinal BMI measurements;
Unadjusted logistic or linear regression
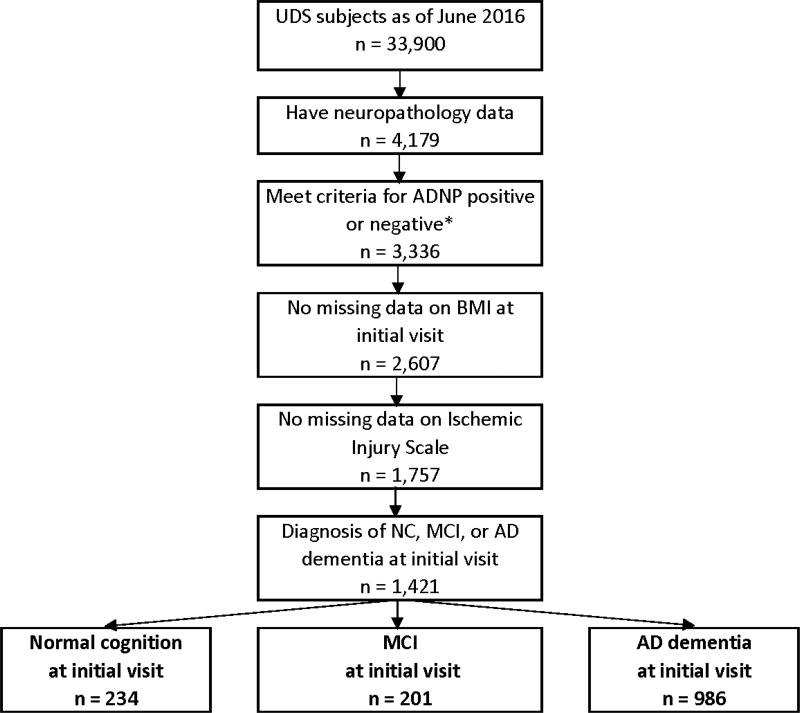
A formal data request to NACC for this study was approved (proposal ID: 608) and data were provided on June 15, 2016. The NACC-UDS and NDS data that were queried are described below and presented in Tables 1, 2, 3, and4. Figure 1 presents the systematic derivation of the current sample. The sample was restricted to all UDS subjects with an initial clinical diagnosis of NC, MCI, or AD dementia who had neuropathological data as of the June 2016 data freeze, and were without missing data on primary study variables (e.g., CERAD, Braak stage, BMI, CVD neuropathology variables, clinical diagnostic status, Clinical Dementia Rating [CDR] score). Only subjects with an initial clinical diagnosis of NC, MCI, or AD dementia were included to ascertain an autopsy sample of subjects that spanned the AD clinical continuum during life, facilitate generalizability to the target population being studied (i.e., AD), and allow for examination of the relationship between BMI and ADNP by clinical status. Of note, although UDS subjects complete annual evaluations, clinical diagnoses and CDR scores from the initial visit were used to be consistent with baseline BMI as a primary predictor in the statistical model (see Statistical Analyses), and to maximize the sample size. Only those who were ADNP positive or negative were included (see Neuropathology section for more details). As shown in Table 5, there were no significant differences between the analytic sample and subjects excluded for key demographic and clinical features, with the exception of age; however, the mean difference in age was trivial.
Table 2.
Demographics and clinical features of ADNP positive and ADNP negative subjects
| Characteristicsa | ADNP+b | ADNP− | p-valuec |
|---|---|---|---|
| Number of subjects, n | 1132 | 289 | NA |
| Age at initial visit (years), mean (SD) | 77.1 (10.6) | 77.5 (11.7) | 0.55 |
| Age at death (years), mean (SD) | 80.9 (10.8) | 81.2 (11.8) | 0.70 |
| Male, n (%) | 610 (53.9%) | 155 (53.6%) | 0.94 |
| Education (years), mean (SD) | 15.1 (3.2) | 15.1 (3.3) | 0.69 |
| Non-white race, n (%) | 74 (6.6%) | 19 (6.6%) | 0.97 |
| Number of UDS visits, mean (SD) | 3.1 (1.8) | 3.1 (1.9) | 0.86 |
| Global CDR initial visit, mean (SD) | 1.26 (0.93) | 0.53 (0.70) | <0.0001 |
| CDR-SB at initial visit, mean (SD) | 7.2 (5.6) | 2.8 (4.2) | <0.0001 |
| Diagnosis at Initial Visit, n (%) | |||
| Normal cognition | 106 (9.4%) | 128 (44.3%) | Ref. |
| MCI | 128 (11.3%) | 73 (25.3%) | 0.0001 |
| AD dementia | 898 (79.3%) | 88 (30.5%) | <0.0001 |
| Diagnosis via consensus panel, initial visit, n (%) | 862 (76.2%) | 191 (66.1%) | 0.0005 |
| ≥ 1 APOE e4 allele, n (%) | 582 (58.9%) | 40 (15.7%) | <0.0001 |
Abbreviations: NA = Not applicable; NC = normal cognition; MCI = mild cognitive impairment; AD = Alzheimer’s disease; UDS = Uniform Data Set; CDR-SB = Clinical Dementia Rating Sum of Boxes; APOE = apolipoprotein E;
Number missing data: Education (ADNP+, n=13; ADNP-, n=2); Race (ADNP+, n=6; ADNP-, n=3); APOE genotype (ADNP+, n=144; ADNP-, n=34);
Moderate to frequent neuritic plaques and Braak Stage III-VI;
Unadjusted logistic regression
Table 3.
Neuropathological characteristics of ADNP positive and ADNP negative subjects
| Neuropathology characteristica | ADNP+b | ADNP‒ | p-valuec |
|---|---|---|---|
| Months between last visit and autopsy, mean (SD) | 18.0 (17.5) | 15.3 (14.5) | 0.02 |
| Ischemic Injury Scale (IIS), mean (SD) | 4.7 (2.2) | 3.7 (2.3) | <0.0001 |
| Presence of individual IIS pathologies, n (%) | |||
| Hippocampal sclerosis | 126 (11.1%) | 37 (12.8%) | 0.43 |
| Infarct or lacune | 269 (23.8%) | 100 (34.6%) | 0.0002 |
| Microinfarct | 238 (21.0%) | 73 (25.3%) | 0.12 |
| Arteriolosclerosis | |||
| None | 225 (19.9%) | 65 (22.5%) | 0.003 |
| Mild | 393 (34.7%) | 120 (41.5%) | |
| Moderate | 342 (30.2%) | 80 (27.7%) | |
| Severe | 172 (15.2%) | 24 (8.3%) | |
| Atherosclerosis | |||
| None | 198 (17.5%) | 57 (19.7%) | 0.58 |
| Mild | 479 (42.3%) | 125 (43.3%) | |
| Moderate | 330 (29.2%) | 69 (23.9%) | |
| Severe | 125 (11.0%) | 38 (13.2%) | |
| Amyloid angiopathy | |||
| None | 262 (23.1%) | 205 (70.9%) | <0.0001 |
| Mild | 384 (33.9%) | 52 (18.0%) | |
| Moderate | 318 (28.1%) | 23 (8.0%) | |
| Severe | 168 (14.8%) | 9 (3.1%) | |
| Laminar necrosis | 16 (1.4%) | 4 (1.4%) | 0.97 |
| Lewy body disease, any, n (%) | 415 (36.8%) | 55 (19.4%) | <0.0001 |
Abbreviations: NC = normal cognition; MCI = mild cognitive impairment; AD = Alzheimer’s disease; IIS = Ischemic Injury Scale; FTLD = frontotemporal lobar degeneration;
Number missing data: Lewy body disease (ADNP+, n=3; ADNP-, n =5);
Moderate to frequent neuritic plaques and Braak Stage III-VI;
Unadjusted logistic regression
Table 4.
Medical status of ADNP positive and ADNP negative subjects
| Characteristica | ADNP+b | ADNP− | p-valued |
|---|---|---|---|
| BMI (kg/m2) at initial visit, mean (SD) | 25.3 (4.4) | 26.6 (5.0) | <0.0001 |
| BMI (kg/m2) at initial visit, categorized, n (%) | |||
| <18.5 | 39 (3.5%) | 6 (2.1%) | 0.0001 |
| 18.5 to 24.9 | 495 (43.7%) | 101 (35.0%) | |
| 25–29.9 | 431 (38.1%) | 115 (39.8%) | |
| 30 or higher | 167 (14.8%) | 67 (23.2%) | |
| BMI (kg/m2) at most recent visitc, mean (SD) | 24.9 (4.5) | 26.6 (5.5) | 0.0001 |
| BMI (kg/m2) at most recent visitc, categorized, n (%) | |||
| <18.5 | 34 (6.3%) | 8 (5.2%) | 0.002 |
| 18.5 to 24.9 | 222 (41.3%) | 47 (30.3%) | |
| 25–29.9 | 210 (39.0%) | 64 (41.3%) | |
| 30 or higher | 72 (13.4%) | 36 (23.2%) | |
| Systolic blood pressure at initial visit, mean (SD) | 132.5 (19.1) | 133.8 (21.2) | 0.31 |
| Diastolic blood pressure at initial visit, mean (SD) | 72.8 (10.5) | 72.9 (10.5) | 0.93 |
| Hypertension, n (%) | 642 (56.7%) | 184 (63.7%) | 0.03 |
| Diabetes, n (%) | 112 (9.9%) | 48 (16.6%) | 0.002 |
| Hypercholesterolemia, n (%) | 600 (53.2%) | 158 (54.9%) | 0.62 |
| Stroke, n (%) | 133 (11.8%) | 40 (13.8%) | 0.35 |
| Cardiovascular disease, n (%) | 352 (31.2%) | 111 (38.4%) | 0.02 |
| Thyroid disease, n (%) | 249 (22.0%) | 74 (25.7%) | 0.19 |
| Atrial fibrillation, n (%) | 160 (14.1%) | 47 (16.3%) | 0.35 |
| Smoking history, n (%) | 521 (46.0%) | 143 (49.5%) | 0.29 |
| Antihypertensive use at any visit, n (%) | 657 (58.0%) | 192 (66.4%) | 0.01 |
| Antilipid medication at any visit, n (%) | 523 (46.2%) | 134 (46.4%) | 0.96 |
Abbreviations: NC = normal cognition; MCI = mild cognitive impairment; AD = Alzheimer’s disease; UDS = Uniform Data Set; CDR-SB = Clinical Dementia Rating Sum of Boxes; APOE = apolipoprotein E
Number missing data: diabetes (ADNP+, n = 1; ADNP-, n = 0); hypercholesterolemia (ADNP+, n = 5; ADNP-, n=1); thyroid disease (ADNP+, n = 2; ADNP-, n =1); stroke (ADNP+, n = 7; ADNP-, n = 0); cardiovascular disease (ADNP+, n = 3; ADNP-, n =0); atrial fibrillation (ADNP+, n = 0; ADNP-, n = 1); systolic blood pressure (ADNP+, n = 27; ADNP-, n = 7); diastolic blood pressure (ADNP+, n = 27; ADNP-, n = 7)
Moderate to frequent neuritic plaques and Braak Stage III-VI
Among the 538 ADNP+ and 155 ADNP- subjects with longitudinal BMI measurements
Unadjusted logistic regression
Figure 1.
Sample size flow chart
Abbreviations: UDS = Uniform Data Set; ADNP = Alzheimer’s disease neuropathology; BMI = body mass index; NC = normal cognition; MCI = mild cognitive impairment; AD = Alzheimer’s disease * ADNP positive = moderate to frequent neuritic plaques and Braak stage III-VI; ADNP negative = none or sparse neuritic plaques and Braak stage 0-II
Table 5.
Comparison of excluded subjects to analytic sample
| Characteristic | Analytic sample |
Total excluded subjectsa |
Subjects with autopsy data who were excluded due to missing the followingc: | |||
|---|---|---|---|---|---|---|
| Missing BMI data at IV |
Missing IIS | No NC, MCI, or AD dementia at IV |
Not ADNP+ or ADNP-b |
|||
| Sample size, n | 1,421 | 2758 | 966 | 1420 | 900 | 843 |
| Age (years), mean (SD) | 77.1 (10.8) | 75.6 (11.6)* | 77.3 (12.0) | 75.5 (10.8)* | 68.8 (10.7)* | 79.9 (11.1)* |
| Male, n (%) | 765 (53.8%) | 1518 (55.0%) | 482 (49.9%) | 801 (56.4%) | 553 (61.4%)* | 449 (53.3%) |
| Diagnosis at IV, n (%) | ||||||
| Normal cognition | 234 (16.5%) | 467 (16.9%) | 163 (16.9%) | 270 (19.0%) | 0 (0.0%) | 259 (30.7%) |
| Impaired not MCI | 0 (0.0%) | 75 (2.7%) | 10 (1.0%) | 19 (1.3%) | 75 (8.3%) | 24 (2.9%) |
| MCI | 201 (14.1%) | 353 (12.8%) | 110 (11.4%) | 223 (15.7%) | 0 (0.0%) | 153 (18.2%) |
| AD dementia | 986 (69.4%) | 1038 (37.6%) | 431 (44.6%) | 647 (45.6%) | 0 (0.0%) | 219 (26.0%) |
| Non-AD dementia | 0 (0.0%) | 825 (29.9%) | 252 (26.1%) | 261 (18.4%) | 825 (91.7%) | 188 (22.3%) |
| Global CDR at IV, mean (SD) | 1.11 (0.94) | 1.16 (1.01) | 1.43 (1.14)* | 1.02 (0.93)* | 1.44 (0.95)* | 0.83 (0.91)* |
| BMI (kg/m2) at IV, mean (SD) | 25.5 (4.6) | 25.6 (4.7) | N/A | 25.5 (4.6) | 26.1 (5.0)* | 25.3 (4.4) |
| BMI (kg/m2) at most recent visit, mean (SD) | 25.2 (4.7) | 24.9 (4.8) | N/A | 24.9 (4.9) | 25.4 (5.1) | 24.6 (4.6)* |
Abbreviations: NC = normal cognition; MCI = mild cognitive impairment; AD = Alzheimer’s disease; BMI = body mass index; kg = kilogram; m = meter; CDR = Clinical Dementia Rating; IV = initial visit; N/A = not applicable; IIS = Ischemic Injury Scale
Missing BMI at initial visit, missing IIS; not diagnosed with normal cognition, mild cognitive impairment, or AD dementia at the initial visit, and/or did not meet ADNP+ or ADNP- definition;
Had moderate to frequent neuritic plaques and Braak stage 0-II, or none or sparse neuritic plaques and Braak stage III-VI;
Could be missing one or all four
Statistically significant difference between excluded group and analytic sample at p<0.05 (tested using unadjusted logistic or linear regression; diagnosis at IV was not tested due to empty cells in analytic sample and group missing NC, MCI, or AD dementia diagnosis at IV)
Body Mass Index
BMI at each visit was incorporated into one statistical model to investigate change in BMI over time. As part of the annual UDS evaluations, height and weight are measured and used to calculate body mass index (BMI) according to the standard formula [(weight in pounds*703)/(height in inches2)].
Neuropathology
AD Neuropathology
Neuropathological data are collected via a standardized Neuropathology Form and Coding Guidebook. An AD neuropathological diagnosis is based on the current National Institute on Aging-Alzheimer’s Association criteria (NIA-AA). Alzheimer’s disease neuropathologic change (ADNC) (Thal stages for amyloid deposition) was added to the NACC’s neuropathology form in January 2014, but was not included in the current study due to the small sample size with ADNC data collected to date. We computed a binary ADNP variable that indicated the presence or absence of ADNP. The presence of ADNP was defined as moderate to frequent neuritic plaques and Braak stage III-VI. The absence of ADNP included none to sparse neuritic plaques and Braak stage 0-II. Similar operationalization of ADNP has been previously used [50, 55], including a recent study that examined vascular risk factors and ADNP from the NACC-NDS [50]. Subjects with moderate to frequent neuritic plaques and Braak stage 0-II, or none or sparse neuritic plaques and Braak stage III-VI were not included in this study in order to limit ambiguity regarding the presence of ADNP. These excluded subjects were statistically different from the analytic sample in terms of age, initial visit CDR, and most recent visit BMI, however, the effect sizes were small and clinically insignificant (Table 5).
CVD—Ischemic Injury Scale (IIS)
CVD neuropathology was scored using an adapted version of the Ischemic Injury Scale (IIS) developed by Au et al [56]. The IIS is a summary composite of vascular and microvascular lesions. The original IIS included items related to white matter disease, but such data are not available in the NACC-NDS and was thus not included as part of the current IIS. The present IIS included a summary composite of the following: hippocampal sclerosis, infarct or lacune, microinfarct, and laminar necrosis (0=No, 1=Yes), and degree of arteriosclerosis, atherosclerosis of Circle of Willis, and amyloid angiopathy (0=None, 1=mild, 2=moderate, 3=severe). Scores range from 0 to 13, with 13 reflecting worse CVD pathology.
Clinical Status
The global rating from the CDR scale from the initial UDS visit characterized clinical function in the sample [57–59]. The CDR is a widely-used tool for staging dementia severity, and assesses function in six domains: memory, orientation, judgment/problem-solving, community affairs, home and hobbies, and personal care. Each domain is assigned a score ranging from 0 to 3, with higher scores representing greater impairment. Using an algorithm, a Global rating of impairment severity is designated: 0 (no dementia), 0.5 (questionable dementia), 1.0 (mild dementia), 2.0 (moderate dementia), or 3.0 (severe dementia). Typically, a score of 0.5 is given to individuals with a diagnosis of MCI [57].
Demographic, Clinical, and Genotype Characteristics
Annual UDS clinical evaluations ascertain a range of demographic and clinical characteristics. Samples are collected by the ADCs to determine apolipoprotein E (APOE) ε4 allele status.
Statistical Analysis
Unadjusted regression analyses (linear or logistic, when appropriate) were conducted to examine demographic, clinical, and neuropathological differences across the clinical diagnostic groups (NC, MCI, AD dementia) (Table 1), and across the ADNP positive and negative groups (Table 2–Table 4). A joint model of linear mixed effects, multivariable and logistic regression [51] examined the causal relationships among repeated measures of BMI, the IIS, and ADNP in the overall sample, and then stratified by CDR rating; that is, subjects with a CDR = 0 (n = 226), 0.5 (n = 376), and ≥1.0 (n = 819). The joint model involved three models that were performed simultaneously (the model being tested is presented in Figure 2). This joint model approach allowed for unbiased and simultaneous examination of the direct effect of late-life BMI on odds of having ADNP, and whether this relationship was mediated by the indirect effect of IIS. Testing of indirect effects follows the basic principles of simple mediation [60]. The first-level mixed effect model examined change in BMI between subjects across all NACC-UDS visits, estimating the baseline level (random intercepts) and change (random slope for time) in BMI for each subject. This model showed that, in the overall sample, the random intercept for BMI (i.e., baseline BMI) was significant (Chi-square (1) = 143.2, p-value < 0.0001), but the effect of random slopes (i.e., change) was not statistically significant (Chi-square (1) < 0.01, p-value = 1.00). Consequently, only the random intercept was used as the predictor variable in subsequent analyses; as described previously, the reason for examining initial visit clinical diagnoses and CDR scores was, in part, to be consistent with the statistical prediction model that used baseline BMI. Using the random intercept from the BMI longitudinal model, a second-level linear regression examined the relationship between BMI and the IIS. Lastly, the random intercepts from the longitudinal BMI model and the IIS were included as predictors in a third-level logistic regression to determine their relationship with odds of having ADNP (absence or presence). All three levels were jointly estimated using PROC NLMIXED in SAS 9.3. All analyses controlled for age (at initial visit for the longitudinal model examining BMI change, and age at death for analyses examining neuropathological outcomes), education, race, sex, and presence of the APOE ε4 allele.
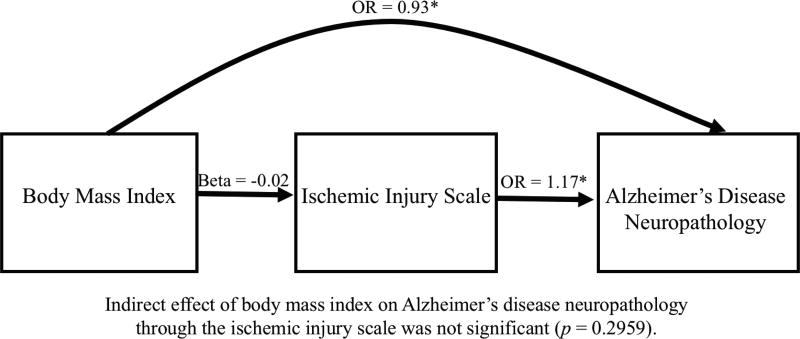
Figure 2.
Joint Modeling of the Relationships Among BMI, Cerebrovascular Disease, and Alzheimer’s Disease Neuropathology in 1,421 Subject’s from the NACC Neuropathology Data Set. Figure shows that lower BMI significantly predicted higher odds of having Alzheimer’s disease neuropathology. BMI was not associated with the Ischemic Injury Scale, however, higher scores on the Ischemic Injury Scale (worse cerebrovascular disease) predicted greater odds of having Alzheimer’s disease neuropathology. All analyses were controlled for age at death, education, race, sex, and presence of the APOE ε4 allele.
RESULTS
Clinical and Neuropathological Characteristics
The mean number of UDS visits for subjects with NC was 3.9 (SD = 2.0), 3.4 (SD = 1.8) visits for MCI subjects, and 2.8 (1.7) visits for AD dementia subjects. As expected, Global CDR rating was highest in subjects with AD dementia, followed by MCI, and then NC. Based on diagnosis at the initial visit, ADNP was present in 91.1% of subjects with AD dementia, 63.7% of MCI subjects, and 45.3% of subjects with NC. Subjects with AD dementia at the initial visit had more CVD neuropathology (as operationalized by mean IIS score) relative to subjects with NC (p = 0.004), but there was no difference between MCI and NC (p = 0.67). As shown in Table 2, the number of UDS visits was nearly identical for the ADNP positive and negative subjects. The ADNP positive subjects were more likely to be diagnosed with MCI (p = 0.0001) and AD dementia (p < 0.0001), and have greater CVD neuropathology (p < 0.0001), when compared to the ADNP negative subjects.
BMI Change
There were no significant differences in BMI between the clinical diagnostic groups at initial visit (Table 1). The ADNP positive subjects had a lower initial (p < 0.0001) and most recent visit BMI (p = 0.0001), relative to the ADNP negative subjects (Table 4). In the joint mixed effect model, there was no significant effect for change in BMI in the overall sample (beta = 0.06, SE = 0.06), t (1234) = 3.03, p = 0.3028) with little between-subject variability. That is, BMI remained relatively stable over time, and there were minimal differences in change in BMI across subjects. As previously mentioned, it is for this reason that baseline BMI served as the predictor variable in the joint models, and not change in BMI.
BMI, IIS, and ADNP
See Table 6 for a summary of the results examining the relationships among BMI, IIS, and ADNP. Figure 2 displays the relationships among BMI, IIS, and ADNP in the overall sample. There was a direct effect for BMI on ADNP in the overall sample (OR = 0.93, CI = 0.90–0.97, p < 0.001), and in subjects with a CDR score of 0 (OR = 0.87, CI = 0.79–0.95, p = 0.0021), and a CDR score of 0.5 (OR = 0.89, CI = 0.83–0.96, p = 0.0012). In each case, a lower BMI corresponded with greater odds of having ADNP. There was no relationship between BMI and ADNP in subjects with an initial visit CDR score ≥ 1.0 (p = 0.2012).
Table 6.
Joint mixed effect models examining body mass index, cerebrovascular disease, and ADNP
| OUTCOME: ADNP (Absence or Presence) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall Sample | CDR = 0 | CDR = 0.5 | CDR ≥ 1 | |||||||||
| BMI Direct Effect |
IIS Indirect Effect |
Total BMI Effect |
BMI Direct Effect |
IIS Indirect Effect |
Total BMI Effect |
BMI Direct Effect |
IIS Indirect Effect |
Total BMI Effect |
BMI Direct Effect |
IIS Indirect Effect |
Total BMI Effect |
|
| Estimate | −0.07 | 0.00 | −0.07 | −0.14 | 0.00 | −0.15 | −0.12 | 0.00 | −0.12 | −0.04 | 0.00 | −0.05 |
| Standard Error | 0.02 | 0.00 | 0.02 | 0.05 | 0.01 | 0.05 | 0.04 | 0.00 | 0.04 | 0.03 | 0.00 | 0.03 |
| Odds Ratio | 0.93 | 1.00 | 0.93 | 0.87 | 1.00 | 0.86 | 0.89 | 1.00 | 0.89 | 0.96 | 1.00 | 0.96 |
| t-value | −3.90 | −1.05 | −4.00 | −3.12 | −0.15 | −3.07 | −3.27 | 0.41 | −3.24 | −1.28 | −1.18 | −1.39 |
| p-value | <0.0001 | 0.2959 | <0.001 | 0.0021 | 0.8788 | 0.0024 | 0.0012 | 0.6794 | 0.0013 | 0.2012 | 0.2375 | 0.1653 |
Note. Mixed effect model examined between subject change in BMI across each NACC-UDS visit, estimating the baseline level (random intercepts) and change (random slope for time) in BMI for each subject. Because BMI remained relatively stable over time and change in BMI was similar across subjects, the random intercept effect (i.e., baseline BMI) was used as the predictor variable in analyses. BMI direct effect examines the relationship between the random intercept estimate of BMI and ADNP. IIS indirect effect tested IIS as a mediator of the relationship between BMI (random intercept estimate) and ADNP. All analyses controlled for age (at initial visit for longitudinal model examining only BMI change, and age at death for analyses examining neuropathological outcomes), education, race, sex, and presence of the APOE ε4 allele. For the full sample and those with a CDR < 1.0, a lower BMI corresponded with greater odds of having ADNP. Greater CVD neuropathology predicted increased odds of having ADNP, but BMI was not associated with CVD, and in-turn, CVD neuropathology did not mediate the relationship between BMI and ADNP.
Abbreviations: BMI = body mass index; ADNP = Alzheimer’s disease neuropathology; IIS = Ischemic Injury Scale
The IIS significantly predicted odds of having ADNP in the overall sample (OR = 1.17, 95% CI = 1.09–1.25, p < 0.0001). Greater CVD neuropathology (i.e., higher scores on the IIS) predicted increased odds of having ADNP. BMI was not associated with the IIS (beta = −0.02, SE = 0.02, t(1234) = −1.1, p = 0.2814), and the relationship between BMI and ADNP was not mediated by the IIS (p = 0.2959). Overall, the results show that late-life BMI was not associated with CVD neuropathology, and in-turn, CVD neuropathology did not mediate the relationship between BMI and ADNP. Consequently, we did not explore CVD as a mediator of BMI and ADNP in the CDR groups.
DISCUSSION
In this autopsy sample of more than 1,400 subjects, lower late-life BMI predicted greater odds of having ADNP. This relationship was evident only in subjects with an antemortem global CDR score < 1.0, that is, without dementia. Later-life BMI was not associated with CVD neuropathology, and CVD did not affect the association between late-life BMI and ADNP. Consistent with the extant literature, greater CVD predicted higher odds of having ADNP. This is the largest neuropathological study to date to examine the relationship between late-life BMI and ADNP, and the first to do so using longitudinal BMI in subjects diagnosed with NC, MCI, and AD during life. Although change in BMI was minimal across the UDS visits and the subject-specific random intercept was used as the predictor variable in the model, the longitudinal design still accounted for the effects of time, providing more accurate estimates on the effect of BMI and ADNP compared to a cross-sectional design. The results from this study confirm and validate the association between lower late-life BMI and higher odds of having ADNP. The results from this study confirm and validate the association between lower late-life BMI and higher odds of having ADNP. That said, sample selection bias is an important limitation of autopsy investigations, in general, and the current study examining BMI and CVD risk, in particular. Those with CVD are more prone to a premature death and therefore subjects of this sample were likely among the healthiest of their peers and/or included those with a life history of a lower BMI. A further discussion on sample selection, as well as survival bias, and how they relate to the present findings is provided below.
The inverse association between late-life BMI and ADNP is similar to findings from two other post-mortem studies that have examined this relationship [31, 50], as well as in vivo research linking lower late-life BMI with higher cortical amyloid burden [61]. These findings may involve the strategic location of ADNP in brain regions that modulate appetite, olfaction, taste, and weight regulation. The neuropathological progression of AD involves neurofibrillary degeneration first in the medial temporal lobes (MTL), including the entorhinal/perirhinal cortex and hippocampal subfields, followed by the association cortex, and finally the primary neocortex [1, 62]. ADNP can be present long before symptom onset [63], and weight loss is a common preclinical sequela of AD [33, 35, 36, 39, 44–46, 64]. We only found a relationship between BMI and ADNP in subjects who were not demented at the time of their initial visit. The early deposition of ADNP in the MTL could explain preclinical or early stage weight changes in AD. Atrophy of the MTL correlates with low body weight in AD due to disturbance in functional neuroanatomical connections that modulate body weight regulation [44]. The anterior MTL (i.e., amygdala and hippocampus) has recently been proposed to play a role in food intake and weight regulation [65]. Animal research shows the hippocampus has direct connections to hypothalamic nuclei and other brain networks that underpin energy regulation [66]. Disturbances of the MTL can also lead to neuropsychiatric symptoms common in AD, such as depression or apathy [67, 68], which could lead to weight loss, particularly in the early stages of disease when insight is still intact. In addition to the MTL, the olfactory bulbs are an early target of ADNP [69] that, when impacted, may interfere with food intake [70]. Lastly, lower baseline BMI and increases in BMI over time during late-life corresponded to greater odds of ADNP in an autopsy sample of subjects with antemortem normal cognition [50]. Individuals clinically unaffected by ADNP in late-life and who do not lose weight, but experience weight gain, may still be at risk for the presence of ADNP due to the deleterious effects of increased adiposity.
An additional explanation for our findings is survival bias. Higher mid-life BMI is associated with increased risk for clinical AD and ADNP [48, 49], presumably due to CVD secondary to coronary artery disease, diabetes, and hypertension, which typically accompany a higher BMI [17, 71] and can cause ischemic brain alterations. As supported by our findings, CVD plays a critical role in the pathogenesis of AD, and has recently been proposed to be a core feature of AD [21]. Those with a high BMI have increased risk for premature death [72] and thus may become excluded in the examination of BMI and AD at older ages, especially in autopsy samples, leading to diminished risk effects of BMI on ADNP. In other words, individuals that live to older ages are more likely to have a BMI that falls within the normal range and is unaccompanied by CVD; or, those with high BMI who survived to old age are self-selected to be the healthiest among their peers. Buchman et al. [31] indeed found that BMI in later-life (mean age at death of 85) was unrelated to cerebral infarctions in an autopsy sample of 298 subjects from the Religious Orders Study, where the presence of obesity was low (average BMI of 26). We also found that late-life BMI was not associated with CVD neuropathology, potentially because the weight status of the sample was relatively normal, the prevalence of CVD risk factors such as diabetes and stroke were low, and the severity of CVD neuropathology was mild overall. According to principles of simple mediation [60], the presence, strength, and significance of an indirect effect is assessed through the cross product of the coefficient of the independent variable (i.e., BMI) on the mediator (i.e., CVD) with the coefficient of the mediator (i.e., CVD) on the outcome (i.e., ADNP). Therefore, the lack of association between BMI and CVD precluded significant indirect effects of BMI on ADNP through CVD. Notably, CVD risk factors were significantly more common in the controls of this sample, compared to subjects with AD dementia. These findings support that the AD dementia subjects of this sample were among the healthiest of their peers, as those with CVD were likely more prone to a premature death before reaching AD dementia status. Alternatively, the subjects with AD dementia in this sample may have their CVD risk factors properly managed due to increased medical care and clinical monitoring. The higher prevalence of CVD risk factors in controls, in conjunction with BMI only predicting ADNP for subjects with a CDR < 1.0, also provides additional evidence that the association between BMI and ADNP may be independent of CVD.
The finding that lower later-life BMI and higher odds of having ADNP emerged only in non-demented subjects (CDR < 1.0) is similar to past work [50]. Baseline BMI and one-year weight change in late life are early markers of amnestic MCI and early-stage AD [41]. A longitudinal study that mapped weight change throughout the clinical course of AD from three cohort studies (Washington Heights and Inwood Columbia Aging Project, Predictors Study, and National Alzheimer’s Coordinating Center) provides robust evidence that BMI declines before clinical AD onset, stabilizes after clinical AD onset, and then potentially increases or remains stable in AD dementia [43]; although, accelerated declines in BMI with dementia onset has been reported [39]. Large-scale prospective and epidemiological studies [39, 45], and research from the Washington University School of Medicine Alzheimer’s Disease Research Center [36] also provide evidence for weight loss preceding cognitive impairment. Subjects with NC in the present sample experienced the largest, but still not significant, declines in BMI over time. Although the subjects with NC in this sample had significantly more years of follow-up compared to subjects with MCI and AD dementia, many of the subjects with NC could potentially be in the preclinical phase of AD, when declines in BMI may be most evident. The average age of the subjects with NC at the initial visit was also >80 years (compared to late 70s for subjects with MCI and AD dementia), and longitudinal research that has examined BMI across the adult lifespan among >6,000 subjects from two substudies of the Swedish Twin Registry (with up to 5 and 12 assessment time points) found that BMI increased from early adulthood to age 65, stabilized, and then declined after 80 years old [42]. Because the subjects with NC were only followed for an average of four years, the trajectory of their BMI is likely to continue to decline with continued follow-up, perhaps until they clinically convert to AD dementia [43]. It is plausible that subjects with AD dementia experience increases in BMI due to receiving healthcare or personal attention by care partners that may improve weight status, such as monitoring of weight and diet [43]. More severe functional impairment, increased sedentary behaviors, abulia, dysphagia, and medication-related weight gains in AD dementia may also be potential explanation for increases in BMI at this stage of the disease. The differential findings of weight gain/loss across the clinical AD continuum may also be dependent on the individuals initial or lifetime BMI status, as overweight/obese persons are more susceptible to weight loss, whereas normal and underweight individuals have a greater opportunity for weight gain. This is particularly evident following an AD diagnosis when the weight and dietary goals of care are tailored to the needs of the individual.
The current findings are limited in several ways. Most importantly, BMI is a coarse measure to operationalize adiposity. The accuracy of BMI in older adults can be affected by age-related loss of lean body mass with increases in adiposity, possibly from conditions like sarcopenia. That said, BMI is a practical measure and future studies should examine other antemortem body composition metrics (e.g., waist circumference, waist-to-hip ratio) and postmortem ADNP. We were restricted to data available from the NACC-NDS and neuropathological items related to white matter disease were not factored into the IIS, potentially contributing to the null effects between BMI and CVD. Although clinical cardiovascular variables could have been used to operationalize CVD and may have increased the clinical utility of our findings, this methodological approach is less optimal to the direct quantification of CVD via neuropathological examination. Despite the large sample size, potential sample selection bias is critical to address. The current sample consisted of relatively healthy older adults who agreed to brain donation, representing a highly selective sample and thus limiting external validity. In general, interpretation of postmortem findings can be problematic due to potential sample selection bias from distinct mechanisms that drive an observed autopsy sample [73, 74]. Notably, past work that has examined the influence of sample selection bias on neuropathological risk factors for dementia in the Adult Changes in Thought (ACT) study found that adjustment for selection bias actually strengthens the magnitude of associations [73]. The present sample may have been further biased through exclusion of subjects due to missing data, or those that did not meet ADNP criteria (see Methods). Concern for bias from exclusion of these subjects is attenuated due to the large sample size, and the lack of meaningful differences between subjects included and excluded (Table 5). Overall, the benefits of neuropathological data to greatly improve understanding on risk factors and mechanisms of neurodegenerative diseases outweighs the limitations from sample selection.
CONCLUSIONS
In the largest study to date examining late-life BMI and ADNP, our findings confirm that lower late-life BMI confers increased odds of ADNP. Lower BMI in older adults may reflect underlying ADNP. Future work that monitors individuals from mid-life until autopsy is ultimately needed to better understand the relationships among BMI, CVD, and ADNP across the lifespan.
Acknowledgments
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI David Teplow, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P50 AG005136 (PI Thomas Montine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), and P50 AG005681 (PI John Morris, MD).
This work was supported by grants from the NIH (P30 AG013846; R01 NS078337; R56 9500304025; U01 NS093334, U01NS086659-01). This publication was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through BU-CTSI Grant Number 1UL1TR001430. Michael L. Alosco and research reported in this publication is supported by the National Institutes of Health under grant number 1F32NS096803-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Jonathan Duskin was also supported by the Boston University School of Medicine Medical Student Summer Research Program (MSSRP).
Robert A. Stern has received research funding from Avid Radiopharmaceuticals, Inc. (Philadelphia, PA, USA). He is a member of the Mackey-White Committee of the NFL Players Association. He is a paid consultant to Avanir Pharmaceuticals, Inc. (Aliso Viejo, CA), Biogen (Cambridge, MA), and Eli Lilly (Indianapolis, IN), and he receives compensation as a member of the Medical Science Committee for the NCAA Student-Athlete Concussion Injury Litigation.. He receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA). Ann C. McKee has received funding from the NFL, WWE, and is a member of the Mackey-White Committee of the NFL Players Association.
Footnotes
CONFLICTS OF INTEREST
For the remaining authors, there are no conflicts of interest to declare.
References
- 1.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack CR, Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–47. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan TK, Alkon DL. Alzheimer’s Disease Cerebrospinal Fluid and Neuroimaging Biomarkers: Diagnostic Accuracy and Relationship to Drug Efficacy. J Alzheimers Dis. 2015;46:817–36. doi: 10.3233/JAD-150238. [DOI] [PubMed] [Google Scholar]
- 4.Lautner R, Palmqvist S, Mattsson N, Andreasson U, Wallin A, Palsson E, Jakobsson J, Herukka SK, Owenius R, Olsson B, Hampel H, Rujescu D, Ewers M, Landen M, Minthon L, Blennow K, Zetterberg H, Hansson O Alzheimer’s Disease Neuroimaging I. Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease. JAMA Psychiatry. 2014;71:1183–91. doi: 10.1001/jamapsychiatry.2014.1060. [DOI] [PubMed] [Google Scholar]
- 5.Palmqvist S, Zetterberg H, Mattsson N, Johansson P, Alzheimer’s Disease Neuroimaging I. Minthon L, Blennow K, Olsson M, Hansson O, Swedish Bio FSG Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology. 2015;85:1240–9. doi: 10.1212/WNL.0000000000001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu W, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Zhao QF, Li JQ, Wang J, Yu JT. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2015;86:1299–306. doi: 10.1136/jnnp-2015-310548. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–30. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 8.Clark TCWB, Freeman G, Schiller JS. Early release of selected estimates based on data from the January-March 2015 National Health Interview Survey. National Center for Health Statistics; 2015. [Available from: http://www.cdc.gov/nchs/nhis.htm. [Google Scholar]
- 9.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev. 2011;12:e426–37. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–42. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 12.Tolppanen AM, Ngandu T, Kareholt I, Laatikainen T, Rusanen M, Soininen H, Kivipelto M. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. J Alzheimers Dis. 2014;38:201–9. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 13.Whitmer RA, Gunderson EP, Quesenberry CP, Jr, Zhou J, Yaffe K. Body mass index in midlife and risk of Alzheimer disease and vascular dementia. Curr Alzheimer Res. 2007;4:103–9. doi: 10.2174/156720507780362047. [DOI] [PubMed] [Google Scholar]
- 14.Rochette AD, Spitznagel MB, Strain G, Devlin M, Crosby RD, Mitchell JE, Courcoulas A, Gunstad J. Mild cognitive impairment is prevalent in persons with severe obesity. Obesity (Silver Spring) 2016;24:1427–9. doi: 10.1002/oby.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherbuin N, Sargent-Cox K, Fraser M, Sachdev P, Anstey KJ. Being overweight is associated with hippocampal atrophy: the PATH Through Life Study. Int J Obes (Lond) 2015;39:1509–14. doi: 10.1038/ijo.2015.106. [DOI] [PubMed] [Google Scholar]
- 16.Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418–26. doi: 10.1161/STROKEAHA.109.576967. [DOI] [PubMed] [Google Scholar]
- 17.Mongraw-Chaffin ML, Peters SA, Huxley RR, Woodward M. The sex-specific association between BMI and coronary heart disease: a systematic review and meta-analysis of 95 cohorts with 1.2 million participants. Lancet Diabetes Endocrinol. 2015;3:437–49. doi: 10.1016/S2213-8587(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolan H, Crain B, Troncoso J, Resnick SM, Zonderman AB, Obrien RJ. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol. 2010;68:231–40. doi: 10.1002/ana.22055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jefferson AL, Beiser AS, Himali JJ, Seshadri S, O’Donnell CJ, Manning WJ, Wolf PA, Au R, Benjamin EJ. Low cardiac index is associated with incident dementia and Alzheimer disease: the Framingham Heart Study. Circulation. 2015;131:1333–9. doi: 10.1161/CIRCULATIONAHA.114.012438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferson AL, Hohman TJ, Liu D, Haj-Hassan S, Gifford KA, Benson EM, Skinner JS, Lu Z, Sparling J, Sumner EC, Bell S, Ruberg FL. Adverse vascular risk is related to cognitive decline in older adults. J Alzheimers Dis. 2015;44:1361–73. doi: 10.3233/JAD-141812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S, Viqar F, Zimmerman ME, Narkhede A, Tosto G, Benzinger TL, Marcus DS, Fagan AM, Goate A, Fox NC, Cairns NJ, Holtzman DM, Buckles V, Ghetti B, McDade E, Martins RN, Saykin AJ, Masters CL, Ringman JM, Ryan NS, Forster S, Laske C, Schofield PR, Sperling RA, Salloway S, Correia S, Jack C, Jr, Weiner M, Bateman RJ, Morris JC, Mayeux R, Brickman AM Dominantly Inherited Alzheimer N. White matter hyperintensities are a core feature of Alzheimer’s disease: Evidence from the dominantly inherited Alzheimer network. Ann Neurol. 2016;79:929–39. doi: 10.1002/ana.24647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marnane M, Al-Jawadi OO, Mortazavi S, Pogorzelec KJ, Wang BW, Feldman HH, Hsiung GY Alzheimer’s Disease Neuroimaging I. Periventricular hyperintensities are associated with elevated cerebral amyloid. Neurology. 2016;86:535–43. doi: 10.1212/WNL.0000000000002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mortimer JA. The Nun Study: risk factors for pathology and clinical-pathologic correlations. Curr Alzheimer Res. 2012;9:621–7. doi: 10.2174/156720512801322546. [DOI] [PubMed] [Google Scholar]
- 24.Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Quinn JF, Galasko DR. Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology. 2013;81:2024–7. doi: 10.1212/01.wnl.0000436935.47657.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nation DA, Edmonds EC, Bangen KJ, Delano-Wood L, Scanlon BK, Han SD, Edland SD, Salmon DP, Galasko DR, Bondi MW Alzheimer’s Disease Neuroimaging Initiative I. Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol. 2015;72:546–53. doi: 10.1001/jamaneurol.2014.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed BR, Marchant NL, Jagust WJ, DeCarli CC, Mack W, Chui HC. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging. 2012;33:1979–87. doi: 10.1016/j.neurobiolaging.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Starks EJ, Patrick O’Grady J, Hoscheidt SM, Racine AM, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Puglielli L, Asthana S, Dowling NM, Gleason CE, Anderson RM, Davenport-Sis NJ, DeRungs LM, Sager MA, Johnson SC, Bendlin BB. Insulin Resistance is Associated with Higher Cerebrospinal Fluid Tau Levels in Asymptomatic APOEvarepsilon4 Carriers. J Alzheimers Dis. 2015;46:525–33. doi: 10.3233/JAD-150072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, Monsell SE, Kukull WA, Trojanowski JQ. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136:2697–706. doi: 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Midlife vascular risk factors and Alzheimer’s disease: evidence from epidemiological studies. J Alzheimers Dis. 2012;32:531–40. doi: 10.3233/JAD-2012-120802. [DOI] [PubMed] [Google Scholar]
- 30.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–38. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–54. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 32.Cova I, Clerici F, Maggiore L, Pomati S, Cucumo V, Ghiretti R, Galimberti D, Scarpini E, Mariani C, Caracciolo B. Body Mass Index Predicts Progression of Mild Cognitive Impairment to Dementia. Dement Geriatr Cogn Disord. 2016;41:172–80. doi: 10.1159/000444216. [DOI] [PubMed] [Google Scholar]
- 33.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J Am Geriatr Soc. 2008;56:111–6. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 34.Cronk BB, Johnson DK, Burns JM Alzheimer’s Disease Neuroimaging I. Body mass index and cognitive decline in mild cognitive impairment. Alzheimer Dis Assoc Disord. 2010;24:126–30. doi: 10.1097/WAD.0b013e3181a6bf3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72:1741–6. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63:1312–7. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 37.Luchsinger JA, Mayeux R. Adiposity and Alzheimer’s disease. Curr Alzheimer Res. 2007;4:127–34. doi: 10.2174/156720507780362100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power BD, Alfonso H, Flicker L, Hankey GJ, Yeap BB, Almeida OP. Changes in body mass in later life and incident dementia. Int Psychogeriatr. 2013;25:467–78. doi: 10.1017/S1041610212001834. [DOI] [PubMed] [Google Scholar]
- 39.Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, Launer LJ. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62:55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 40.Ye BS, Jang EY, Kim SY, Kim EJ, Park SA, Lee Y, Hong CH, Choi SH, Yoon B, Yoon SJ, Na HR, Lee JH, Jeong JH, Kim HJ, Na DL, Seo SW. Unstable Body Mass Index and Progression to Probable Alzheimer’s Disease Dementia in Patients with Amnestic Mild Cognitive Impairment. J Alzheimers Dis. 2015;49:483–91. doi: 10.3233/JAD-150556. [DOI] [PubMed] [Google Scholar]
- 41.Besser LM, Gill DP, Monsell SE, Brenowitz W, Meranus DH, Kukull W, Gustafson DR. Body mass index, weight change, and clinical progression in mild cognitive impairment and Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:36–43. doi: 10.1097/WAD.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dahl AK, Reynolds CA, Fall T, Magnusson PK, Pedersen NL. Multifactorial analysis of changes in body mass index across the adult life course: a study with 65 years of follow-up. Int J Obes (Lond) 2014;38:1133–41. doi: 10.1038/ijo.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu Y, Scarmeas N, Cosentino S, Brandt J, Albert M, Blacker D, Dubois B, Stern Y. Change in body mass index before and after Alzheimer’s disease onset. Curr Alzheimer Res. 2014;11:349–56. doi: 10.2174/1567205010666131120110930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grundman M, Corey-Bloom J, Jernigan T, Archibald S, Thal LJ. Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology. 1996;46:1585–91. doi: 10.1212/wnl.46.6.1585. [DOI] [PubMed] [Google Scholar]
- 45.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69:739–46. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 46.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65:892–7. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 47.Tobias DK, Hu FB. Does being overweight really reduce mortality? Obesity (Silver Spring) 2013;21:1746–9. doi: 10.1002/oby.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mrak RE. Alzheimer-type neuropathological changes in morbidly obese elderly individuals. Clin Neuropathol. 2009;28:40–5. doi: 10.5414/npp28040. [DOI] [PubMed] [Google Scholar]
- 49.Chuang YF, An Y, Bilgel M, Wong DF, Troncoso JC, O’Brien RJ, Breitner JC, Ferruci L, Resnick SM, Thambisetty M. Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatry. 2016;21:910–5. doi: 10.1038/mp.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Besser LM, Alosco ML, Ramirez Gomez L, Zhou XH, McKee AC, Stern RA, Gunstad J, Schneider JA, Chui H, Kukull WA. Late-Life Vascular Risk Factors and Alzheimer Disease Neuropathology in Individuals with Normal Cognition. J Neuropathol Exp Neurol. 2016 doi: 10.1093/jnen/nlw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albert PS. A linear mixed model for predicting a binary event from longitudinal data under random effects misspecification. Stat Med. 2012;31:143–54. doi: 10.1002/sim.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beekly DL, Ramos EM, van Belle G, Deitrich W, Clark AD, Jacka ME, Kukull WA, Centers NI-AsD. The National Alzheimer’s Coordinating Center (NACC) Database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270–7. [PubMed] [Google Scholar]
- 53.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–6. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 54.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, Peskind E, Dietrich W, Beekly DL, Kukull WA, Morris JC. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dugger BN, Serrano GE, Sue LI, Walker DG, Adler CH, Shill HA, Sabbagh MN, Caviness JN, Hidalgo J, Saxon-Labelle M, Chiarolanza G, Mariner M, Henry-Watson J, Beach TG the Arizona Parkinson’s Disease C. Presence of Striatal Amyloid Plaques in Parkinson’s Disease Dementia Predicts Concomitant Alzheimer’s Disease: Usefulness for Amyloid Imaging. J Parkinsons Dis. 2012;2:57–65. doi: 10.3233/JPD-2012-11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Au R, Seshadri S, Knox K, Beiser A, Himali JJ, Cabral HJ, Auerbach S, Green RC, Wolf PA, McKee AC. The Framingham Brain Donation Program: neuropathology along the cognitive continuum. Curr Alzheimer Res. 2012;9:673–86. doi: 10.2174/156720512801322609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–72. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 58.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 59.Schafer KA, Tractenberg RE, Sano M, Mackell JA, Thomas RG, Gamst A, Thal LJ, Morris JC Alzheimer’s Disease Cooperative S. Reliability of monitoring the clinical dementia rating in multicenter clinical trials. Alzheimer Dis Assoc Disord. 2004;18:219–22. [PMC free article] [PubMed] [Google Scholar]
- 60.Preacher KJ, Rucker DD, Hayes AF. Addressing Moderated Mediation Hypotheses: Theory, Methods, and Prescriptions. Multivariate Behav Res. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- 61.Hsu DC, Mormino EC, Schultz AP, Amariglio RE, Donovan NJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA Harvard Aging Brain S. Lower Late-Life Body-Mass Index is Associated with Higher Cortical Amyloid Burden in Clinically Normal Elderly. J Alzheimers Dis. 2016;53:1097–105. doi: 10.3233/JAD-150987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barrett-Connor E, Edelstein S, Corey-Bloom J, Wiederholt W. Weight loss precedes dementia in community-dwelling older adults. J Nutr Health Aging. 1998;2:113–4. [PubMed] [Google Scholar]
- 65.Coppin G. The anterior medial temporal lobes: Their role in food intake and body weight regulation. Physiol Behav. 2016 doi: 10.1016/j.physbeh.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 66.Cenquizca LA, Swanson LW. Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. J Comp Neurol. 2006;497:101–14. doi: 10.1002/cne.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chi S, Yu JT, Tan MS, Tan L. Depression in Alzheimer’s disease: epidemiology, mechanisms, and management. J Alzheimers Dis. 2014;42:739–55. doi: 10.3233/JAD-140324. [DOI] [PubMed] [Google Scholar]
- 68.Landes AM, Sperry SD, Strauss ME, Geldmacher DS. Apathy in Alzheimer’s disease. J Am Geriatr Soc. 2001;49:1700–7. doi: 10.1046/j.1532-5415.2001.49282.x. [DOI] [PubMed] [Google Scholar]
- 69.Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. Neuroreport. 2001;12:285–8. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- 70.Tamura BK, Masaki KH, Blanchette P. Weight loss in patients with Alzheimer’s disease. J Nutr Elder. 2007;26:21–38. doi: 10.1300/j052v26n03_02. [DOI] [PubMed] [Google Scholar]
- 71.Labounty TM, Gomez MJ, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Chow B, Cury R, Delago A, Dunning A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann P, Kim YJ, Leipsic J, Lin FY, Maffei E, Raff G, Shaw LJ, Villines TC, Min JK. Body mass index and the prevalence, severity, and risk of coronary artery disease: an international multicentre study of 13,874 patients. Eur Heart J Cardiovasc Imaging. 2013;14:456–63. doi: 10.1093/ehjci/jes179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collaboration GBM. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–86. doi: 10.1016/S0140-6736(16)30175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haneuse S, Schildcrout J, Crane P, Sonnen J, Breitner J, Larson E. Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology. 2009;32:229–39. doi: 10.1159/000197389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaccai J, Ince P, Brayne C. Population-based neuropathological studies of dementia: design, methods and areas of investigation--a systematic review. BMC Neurol. 2006;6:2. doi: 10.1186/1471-2377-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]