Abstract
Atherosclerotic cardiovascular disease still represents the leading cause of death in Western countries. A wealth of scientific evidence demonstrates that increased blood cholesterol levels have a major impact on the outbreak and progression of atherosclerotic plaques. Moreover, several cholesterol-lowering pharmacological agents, including statins and ezetimibe, have proved effective in improving clinical outcomes. This document focuses on the clinical management of hypercholesterolaemia and has been conceived by 16 Italian medical associations with the support of the Italian National Institute of Health. The authors discuss in detail the role of hypercholesterolaemia in the genesis of atherosclerotic cardiovascular disease. In addition, the implications for high cholesterol levels in the definition of the individual cardiovascular risk profile have been carefully analysed, while all available therapeutic options for blood cholesterol reduction and cardiovascular risk mitigation have been explored. Finally, this document outlines the diagnostic and therapeutic pathways for the clinical management of patients with hypercholesterolaemia.
Keywords: Atherosclerosis, Diagnostic and therapeutic pathways, Hypercholesterolaemia, PCSK9 inhibitors, Statins, Sustainable health care
Document Revisors: Antonio Francesco Amico, Gianfranco Alunni, Pasquale Caldarola, Roberto Caporale, Giancarlo Casolo, Giacomo Chiarandà, Giuseppe Di Tano, Domenico Gabrielli, Giovanna Geraci, Giovanni Gregorio, Gian Francesco Mureddu, Guerrino Zuin, Francesco Barillà, Mauro Borzi, Paolo Guido Camici, Livio Dei Cas, Matteo Di Biase, Francesco Fedele, Ciro Indolfi, Giuseppe Mercuro, Vincenzo Montemurro, Luigi Padeletti, Carmine Dario Vizza, Carlo Bruno Giorda, Calogero Calcullo, Leonardo Costa, Ettore Antoncecchi, Achille Giuseppe Dato, Giuseppe Augello, Salvatore Lenti, Alberto Mazza, Roberto Franco Enrico Pedretti, Alfredo Marchese, Roberta Rossini, Pasquale Guarini, Aldo Clerico, Martina Zaninotto, Giovanni Annuzzi, Carla Giordano, Francesco Purrello, Gian Alfonso Cibinel, Francesco Rocco Pugliese, Francesco Cipollone, Pietro Amedeo Modesti, and Sebastiano Calandra
Table of contents
Hypercholesterolaemia and cardiovascular risk
The fundamentals of prevention
The lipid target value in the management of cardiovascular risk
The role of lifestyle in the approach to patients with dyslipidaemia
Diet and cholesterol
Exercise and cholesterol
Other interventions
Lipid-lowering therapy individualized according to the cardiovascular risk level: the indications of the Regulatory Authorities in Italy
Treatment target value and drug choice
Patients receiving secondary cardiovascular prevention interventions
Patients with diabetes mellitus
Patients with chronic kidney disease
Patients with familial dyslipidaemia
Patients receiving primary cardiovascular prevention interventions
General principles of therapy with statins
Peculiarities of diabetic dyslipidaemia
Clinical relevance
Atherogenic dyslipidaemia in diabetes
Management of diabetic dyslipidaemia
Statins
Ezetimibe
Resins
Fibrates
Niacin
PCSK9 inhibitors
Considerations for diabetic patients
The role of dietary supplements in the treatment of dyslipidaemia
Nutraceuticals and dyslipidaemia
Polyunsaturated omega-3 fatty acids
Management of high cardiovascular risk patients with hypercholesterolaemia
Why AIFA [Italian Medicines Agency] note no. 13 must be abolished
Clinical diagnosis and familial dyslipidaemia: AIFA note no. 13 and clinical algorithms
Hypercholesterolaemia
Prevalence and diagnosis of familial hypercholesterolaemia in Italy: sensitivity and specificity of AIFA’s diagnostic algorithm 20S
Clinical epidemiology of heterozygous familial hypercholesterolaemia in Italy
Critical criteria for screening
Role of genetic testing
Guidance for laboratory reporting of lipid parameters
Guidance for the reporting of lipid profile parameters
Presentation of the results
Reference system
Reporting
Unmet clinical needs in the management of hypercholesterolaemia
Statin intolerance
International definitions
Operative synopsis for clinical practice
Failure to meet therapeutic goals: the lower, the better, the sooner, the better
Non-compliance with treatment prescriptions
Extent of the phenomenon
Clinical assessment
Intervention to improve compliance
The proposals in ANMCO consensus documents
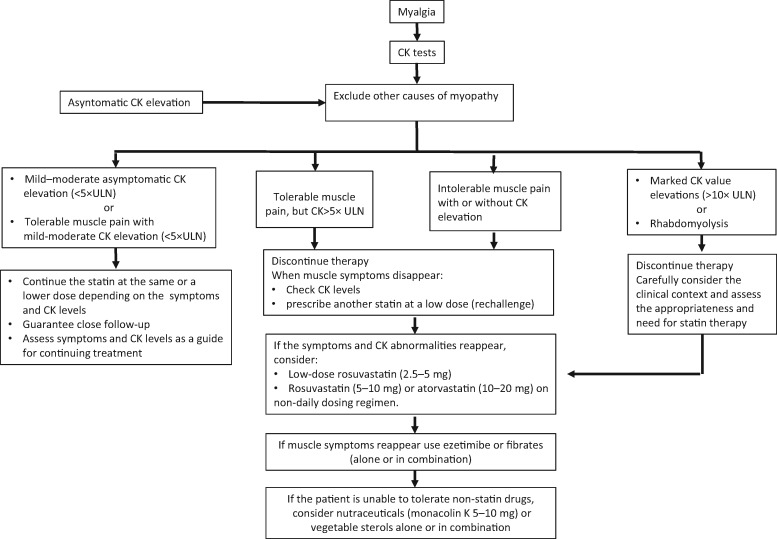
Diagnostic and therapeutic protocols in patients with statin-induced myalgia
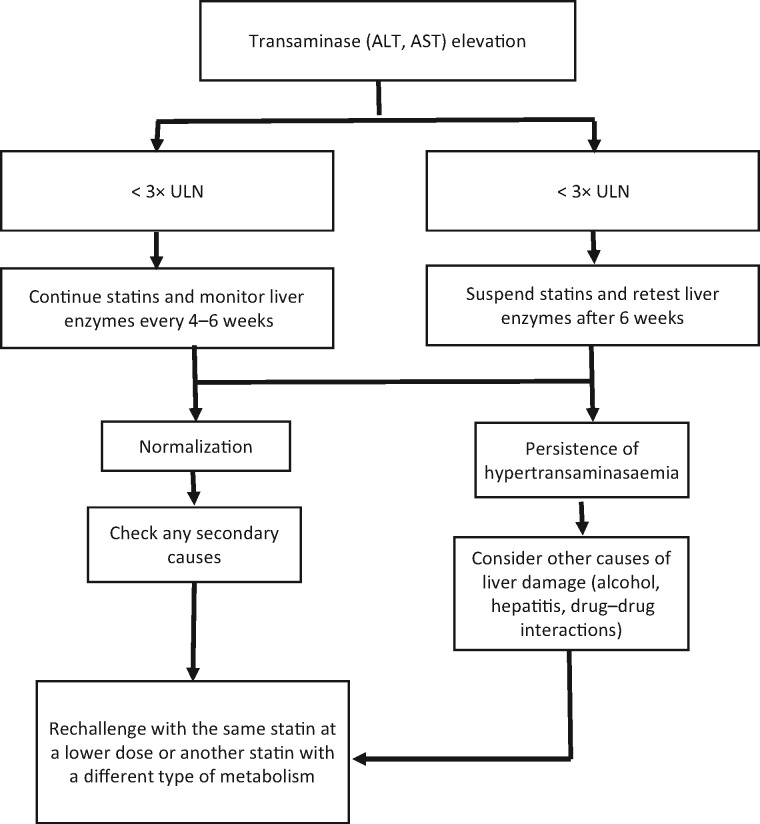
Diagnostic and therapeutic protocols in patients with statin-induced liver injury
New drugs for the treatment of dyslipidaemia
Introduction
Drugs for the treatment of severe genetic dyslipidaemia
PCSK9 inhibitors
Alirocumab: the pharmacodynamic and pharmacokinetic aspects
Clinical studies on alirocumab
Tolerability of alirocumab
Evolocumab: pharmacodynamic and pharmacokinetic aspects
Clinical studies on evolocumab
Tolerability of evolocumab
Other PCSK9 inhibitors
Conclusions
Eligibility for treatment with PCSK9 inhibitors
How to identify the patients to be treated
Possible criteria for National Health Service reimbursement of PCSK9 inhibitors
Assessment of the co-morbidities: the internal medicine patient
Introduction
Co-morbidity and polymorbidity
Functional dependence
Fragility
The definition of complexity
Overdiagnosis and the increase in co-morbidities
Co-morbidity assessment: common clinical cases
Atherogenic dyslipidaemia
The hypercholesterolaemic patient with neurological co-morbidities
The hypercholesterolaemic patient with thrombophilic status
The role of statin therapy in elderly patients
The diabetic patient
Clinical and laboratory criteria for prescriptive appropriateness pending outcome data
Heterozygous familial hypercholesterolaemia
Patients with atherosclerotic cardiovascular disease and diabetes mellitus
Closing comments
The emerging ‘real-world’ data regarding the use of cholesterol-lowering agents in patients with recent cardiovascular events in Italy
Introduction
Analysis methodology
The cohort analysed
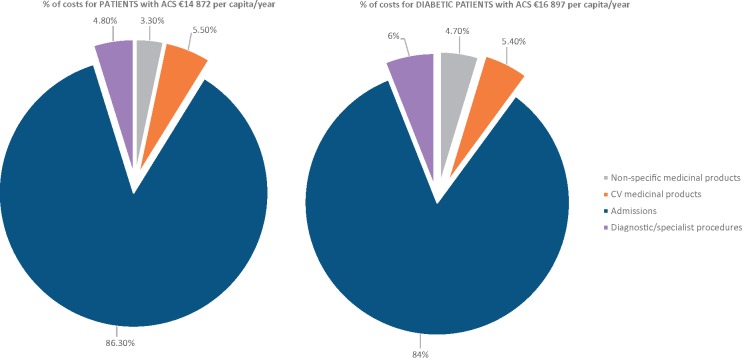
Statin use and cost assessment
Results
Statin use
Compliance to treatment with statins
New admissions
Cost analysis
Discussion and conclusions
From efficacy and safety to clinical effectiveness: the challenge of monoclonal antibodies in sustainable cardiovascular prevention: the reuse of savings in hospitalizations
References
Hypercholesterolaemia and cardiovascular risk
The fundamentals of prevention
Despite the progressive reduction in cardiovascular mortality observed in the industrialized world, the conditions resulting from atherosclerosis and thrombosis (especially ischaemic cardiomyopathy, ischaemic stroke, and peripheral arterial disease) are still extremely common and among the principal causes of early death and permanent disability in the European population.1 These diseases have multifactorial causes, some of which can be modified by lifestyle intervention, such as lack of exercise, smoking, and unhealthy diet. Other diseases, such as dyslipidaemias, arterial hypertension, and diabetes, can be modified by pharmacological treatment. Lipid metabolism disorders may be related to other diseases (secondary forms) or genetic abnormalities (familial forms) or may be due to the interaction between predisposing and environmental factors. These may modify the levels and the function of plasma lipoproteins, which may in turn facilitate the development of cardiovascular diseases when combined with other risk factors. The plasmatic cholesterol and triglycerides (TG) bind different apoproteins to form lipoproteins that are classified as: HDL with antiatherogenic properties; LDL, which transport the majority of plasma cholesterol and are atherogenic; and chylomicrons and VLDL, which are rich in TGs and not atherogenic but may cause pancreatitis when present in high concentrations.
Both genetic and anatomopathological observational and interventional studies have shown that dyslipidaemias and hypercholesterolaemia, in particular, play a crucial role in the development of cardiovascular disease. Large-scale epidemiological studies conducted in the latter half of the last century, such as the Multiple Risk Factor Intervention Trial (MRFIT)2 and the Framingham Study,3 which was characterized by an extremely long observational period, unequivocally demonstrated a strong and linear relationship between cholesterolaemia, mortality, and incidence of cardiovascular diseases, especially ischaemic cardiomyopathy, a relationship that decreases in relative terms with age.4 The strong relationship was observed in both subjects with and without a prior history of cardiovascular disease. In addition, there is also a relationship between LDL cholesterol (C-LDL) and cardiovascular risk.2 Indeed, high levels of C-LDL are one of the most important modifiable cardiovascular risk factors.5
Dyslipidaemias prevention and treatment must be considered an essential part of individual interventions aimed at reducing the burden of cardiovascular disease. These interventions should primarily address subjects who are at the highest risk and who will benefit the most from them. Therefore, identifying the subjects at highest cardiovascular risk represents the starting point for the implementation of measures aimed at reducing risk factors through the modification of unhealthy lifestyles and the introduction of pharmacological intervention.
Between 2008 and 2012, a survey was conducted on the adult general population, known as the Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey (OEC/HES), as part of the National Health Institute (ISS), Associazione Nazionale Medici Cardiology Ospedalieri (ANMCO), and Heart Care Foundation (HCF) partnership agreement. This survey, which involved a representative sample of 23 municipalities, at least one for each region of Italy, examined 7912 adults aged 35 to 74 years, plus a subsample of 802 elderly people (aged 75–79 years) and another of 397 young people (aged 25–34 years). In the adult sample, the prevalence of hypercholesterolaemia (defined as ≥240 mg/dL or lower when being treated with lipid-lowering drugs), measured on serum after 12 h fasting in the same laboratory, was approximately 34% [confidence interval (CI) 33–36] in men and 36% (CI 35–38) in women, with a 39% increase in men and 33% increase in women when compared with the previous survey conducted in 1998–2002 (in the 1998–2002 survey, the prevalence was 21%, CI 20–22, in men and 25%, CI 23–26, in women). Low-density lipoprotein cholesterolaemia was calculated by applying Friedewald’s formula, excluding subjects with triglyceridaemia >400 mg/dL. Twenty-six per cent of men and 27% of women had a value ≥155 mg/dL. Of those with dyslipidaemia, approximately 40% did not know they had this disorder, and more than 35% were aware that they had the disorder but did not follow any diet or specific therapy.6,7 Those on lipid-lowering therapy had dietary habits very similar to those of the general population (11.8% of saturated fats and 328 mg/day of dietary cholesterol consumption) and in excess of the guidelines of the European Society of Cardiology and the European Atherosclerosis Society (ESC/EAS),8 which recommend that saturated fats consumption should not exceed 7% and dietary cholesterol should not exceed 300 mg.
Recent longitudinal studies conducted in the USA [MRFIT, Coronary Artery Risk Development in (Young) Adults (CARDIA), and Chicago Heart Association Detection Project in Industry (CHA)] showed that, in the general population, those with a favourable risk profile (arterial pressure <120/80 mmHg, total cholesterol (TC) <200 mg/dL without specific therapy, body mass index <25 kg/m2, non-smokers, and no diabetes) are those who present the lowest cardiovascular and all-cause mortality, have the best quality of life in old age, and cost less in terms of health care.2,9–12 This led to a new direction in cardiovascular prevention aimed not merely at identifying and guiding those with a high cardiovascular risk but also at keeping risk low during the course of life by means of healthy lifestyle choices.13 The lower risk of a major cardiovascular event (myocardial infarction or stroke) in people with a favourable risk profile was also shown after 10 years of follow-up in the cohorts of the CUORE project.14,15
Patients who have already had an acute coronary syndrome (ACS) or a stroke are at higher risk of future events and automatically qualify as deserving of a thorough assessment and intensive treatment for all their risk factors, including high C-LDL level. Conventionally, the term used to indicate the intervention undertaken to reduce the risk of future events in these patients is secondary prevention, whereas the term primary prevention refers to the same intervention undertaken for subjects with no prior history of cardiovascular events. However, those individuals with a combination of many risk factors may have unexpectedly high cardiovascular risk, which may be similar to, or even higher than, the risk of those subjects who have had a previous cardiovascular event. All the current guidelines on the prevention of cardiovascular disease in clinical practice recommend global risk factors assessment.5,8,16 Indeed, in most individuals, atherosclerosis and thrombosis are the product of an interaction among different risk factors. Based on observational studies, several global risk assessment algorithms are available, including the American Framingham Study,3 the European Systematic Coronary Risk Evaluation (SCORE) for the regions of Europe at high and low risk, and the Italian risk charts of the CUORE project, all of which include cholesterolaemia as a risk factor. The purpose of the risk charts is to facilitate the estimation of the probability of a fatal/non-fatal major cardiovascular event (myocardial infarction, ACS, and stroke) in apparently healthy individuals without signs and symptoms of disease. The European SCORE system, in particular, estimates the risk at 10 years of a first fatal atherosclerotic event (acute myocardial infarction, acute coronary syndrome, stroke, peripheral arterial disease, and sudden death). On the basis of this risk, subjects are classified as low, medium, high, or very high risk (Table 1).5 In Italy, the ISS performed a comparison between the CUORE project charts, which were previously used in Italy, and the European SCORE charts and found the CUORE charts to yield similar results to the corresponding charts of the SCORE project (Table 2).17,18 The SCORE charts, despite following European guidelines, are not advantageous in terms of ease of use for global risk calculation when compared with the CUORE algorithm; furthermore, it is important to remember that one general criterion in the choice of the instrument used to estimate the risk is that it must have been developed in the population in which it will effectively be used.
Table 1.
LDL cholesterol target according to risk conditions
| Risk | Conditions | Target C-LDL |
|---|---|---|
| Low | Rating according to the SCORE risk charts <1%. | <115 mg/dL |
| Medium | Rating according to the SCORE risk charts ≥ 1% and <5%. | <115 mg/dL |
| High | Patients with familial dyslipidaemia or severe hypertension. Patients with diabetes, without other cardiovascular risk factors or organ damage. Patients with moderate chronic kidney disease (GFR 30–59 mL/min/1.73 m2). | <100 mg/dL |
| Rating according to the SCORE risk charts ≥5% and <10%. | ||
| Very high | Patients with documented cardiovascular disease (per coronary angiogram, stress echocardiography, radionuclide imaging, and ultrasound evidence of carotid plaques), prior myocardial infarction, prior ACS, prior coronary revascularization intervention (with CABG or PCI) or peripheral revascularization, prior ischaemic stroke and peripheral arterial disease, diabetics with one or more cardiovascular risk factors, and/or organ damage markers (e.g. microalbuminuria) and with severe kidney disease (GFR <30 mL/min/1.73 m2). | <70 mg/dL |
| Rating according to the SCORE risk charts >10%. |
CABG, coronary artery bypass grafting; C-LDL, LDL cholesterol; GFR, glomerular filtration rate; PCI, percutaneous coronary intervention; ACS, acute coronary syndrome.
Readapted from Perk et al.5
Table 2.
Risk level correspondence between SCORE and CUORE risk charts.
| SCORE | CUORE |
|---|---|
| <1 | <5 |
| 1 | 5–10 |
| 2 | 10–14 |
| 3–4 | 15–20 |
| 5–9 | 20–30 |
| ≥ 10 | >30 |
Readapted from Donfrancesco et al.18
Otherwise European guidelines suggest using algorithms based on local data, where available.
Total risk assessment using risk charts, regardless of the type, do not have to be utilized in patients with familial hypercholesterolaemia (FH), a condition caused by LDL receptor (LDLR) mutation, because the levels of C-LDL >240 mg/dL or of TC >320 mg/dL place these patients, by definition, in a condition of high risk. Similarly, patients with several other clinical conditions are considered, according to the ESC guidelines, to be at high and very high risk and therefore do not satisfy the criteria for SCORE chart application (Table 1).
The lipid target value in the management of cardiovascular risk
In the OEC/HES survey, the prevalence of high risk (≥5% SCORE and ≥20% CUORE) was seen to be 8.5%in men aged 35–69 years and 1.1% in women. Of note, it should be taken into account that subjects on treatment with cholesterol-lowering medication appear to be at lower risk as neither the SCORE nor the CUORE algorithm considers the concomitant treatment with specific therapy for dyslipidaemia. In the same survey, 53% men and 83% women appear to be at low risk, with the remaining part of the population at medium risk.
Regarding the C-LDL targets, it is important to remember that the prevalence of non-optimal C-LDL (≥ 115 mg/dL) in the same general OEC/HES population examined in 2008–2012 was greater than 65% and more than 25% had high C-LDL values (≥ 155 mg/dL). A frequency this high in the general population indicates that inadequate action is addressed to high-risk subjects. More incisive community lifestyle intervention is required, which is aimed at improving eating habits, moderating alcohol consumption, reducing smoking (which still involves over 20% of the adult population), and increasing physical activity (40% of adult women and 32% of males do not practice daily physical activity). Lifestyle changes, which, as recommended in all guidelines, should always accompany pharmacological therapy, are often considered by the subject/patient as deprivation rather than a way to return to a more favourable risk profile (lower risk) and keep fit, because the benefits are not obvious in the short-term though they are important for the long-term prevention of all chronic degenerative diseases.
International guidelines indicate C-LDL reduction as one of the most important interventions in terms of reducing the risk of premature cardiovascular events. High TC and C-LDL plasmatic levels can be reduced by lifestyle changes or with pharmacological therapy. Unfortunately, lifestyle changes alone have not proved sufficient to significantly reduce these levels and many of the drugs developed in the past were not effective and safe enough. By inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase, statins have been shown to significantly reduce C-LDL in the absence of significant adverse events or, in any case, with a completely favourable risk–benefit ratio and therefore represent a milestone in cardiovascular prevention. There is validated evidence that these agents reduce the risk of myocardial infarction and stroke, in both primary and secondary prevention intervention. As far back as the 1990s, it was shown19,20 that statin therapy reduces mortality and the recurrence of ischaemic events. The efficacy of an intensive therapy (with C-LDL <70 mg/dL as target) in ischaemic cardiomyopathy was subsequently proved.21,22 The benefits of treatment with statins has been confirmed in real-world population registries23 and subsequent meta-analysis of numerous randomized controlled trials (RCTs), including over 170 000 patients in the Cholesterol Treatment Trialists’ Collaboration.24,25 It has been observed that a 1 mmol/L (approximately 38 mg/dL) reduction in C-LDL is associated with a 20–25% decrease in the relative risk of new major cardiovascular events (cardiovascular mortality and non-fatal infarction). It is likely that this treatment has made a significant contribution to the considerable reduction in the age-standardized cardiovascular mortality rates observed in recent years (from 62/100 000 male inhabitants in 1980 to 19 in 2008 and from 13/100 000 female inhabitants to 4 in 2008), without affecting the high total mortality for cardiovascular causes in the population.1
Unlike relative risk, whose reduction is the same for each starting condition, the absolute benefit of treatment is, however, greater the higher the patients’ basic absolute risk and C-LDL levels. On the basis of this evidence, the most recent European guidelines, as indicated previously, clearly indicate that there are different cardiovascular risk categories and that each one must be matched with a certain C-LDL target, which should be lower the higher the risk. The targets for the treatment of dyslipidaemia are based, above all, on the results of clinical studies aimed at reducing lipids, in the majority of which the C-LDL levels are used as indicators of response to therapy; C-LDL, therefore, remains the main target of the strategies for dyslipidaemia management.
By extrapolating the available data, the absolute reduction in C-LDL below 70 mg/dL or a relative reduction of at least 50% provide the best benefits in terms of reduction in cardiovascular disease; therefore, for patients with a very high cardiovascular risk, the target is <70 mg/dL or a more than 50% reduction in baseline C-LDL.5,8 In subjects with high or moderate risk, a C-LDL target of <100 and 115 mg/dL, respectively, should be taken into consideration. In asymptomatic individuals, the first step consists of assessing cardiovascular risk and identifying any modifiable elements. The assessment should be repeated at 5-year intervals if the absolute cardiovascular risk is low and there are no significant variations in the recommended values of the main risk factors.8 Clinicians should obviously use their judgement to avoid premature or unnecessary implementation of lipid-lowering therapy. Indeed, lifestyle intervention can have an important long-term impact on health, whereas the long-term effects of pharmacological therapy must be weighed against potential side effects and polymorbidity, a condition that, of course, increases with age.
On both sides of the Atlantic, controversy has recently developed between the high-dose/high-efficacy statin-based strategy and the target-based strategy. Indeed, the very recent US guidelines criticized the benefit of reaching specific C-LDL targets and have even disputed the appropriateness of their use in clinical practice, proposing instead an appropriate intensity of treatment with statins over all other cholesterol-lowering agents in order to reduce cardiovascular risk in those subjects who are most likely to benefit, such as those with coronary disease.16 In these patients, the guidelines suggest high-intensity statins (approximately 50% reduction in C-LDL) if <75 years and moderate-intensity statins (30–50% reduction in C-LDL) in those over 75 years of age.16 This strategy has been vehemently disputed.26 Indeed, treatment compliance is significantly greater in patients with a strategy addressing a specific target than with the ‘fire-and-forget’ approach that is recommended in the USA. In addition, the benefit of obtaining C-LDL levels that are well below 70 mg/dL, even with agents other than statins, was demonstrated by the Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT)27 study, which assessed the effect of ezetimibe in combination with simvastatin compared with simvastatin monotherapy in patients with stable ischaemic cardiomyopathy with the levels of C-LDL that were low at the outset. The results of the IMPROVE-IT study would therefore appear to support the opinion of European experts.
The role of lifestyle in the approach to patients with dyslipidaemia
The reduction in age-standardized cardiovascular mortality rates observed in all industrialized countries in recent decades is to a great extent related to lifestyle changes that must proceed and accompany any pharmacological approach to dyslipidaemia control.28 The study conducted by Palmieri et al.29 examined the relative weight of the reduction in risk factors compared with acute-phase intervention in explaining the reduction in mortality rates between 1980 and 2000 in Italy. The reduction in mortality for ischaemic cardiomyopathy between 1980 and 2000 was due to the effects derived from treatments and risk factor reduction. Approximately 23 660 (55%) fewer deaths from ischaemic cardiomyopathy were attributable to changes in the risk factors in the population (range: 20 260–28 455). In particular, the reduction in TC (−0.35 mmol/L) prevented or delayed 10 045 deaths (23%) from coronary causes. The reduction, albeit limited, of 10% in sedentary lifestyle prevented or delayed approximately 2490 deaths. On the contrary, the 0.1% increase in the prevalence of diabetes caused approximately 945 additional deaths, whereas the increase, albeit limited, in obesity (increase in body mass index) is thought to have caused approximately 245 additional deaths.
Diet and cholesterol
There is a recognized relationship between dietary cholesterol and cardiovascular mortality.8,30,31 More specifically, of the various diet-related factors, saturated fatty acids have the greatest impact on C-LDL. It is calculated that for every 1% increase in saturated fatty acid intake, there is a 0.8–1.6 mg/dL increase in C-LDL.32 Industrial-processed partially hydrogenated fatty acids represent the greatest source of trans-unsaturated fats in the diet, accounting for between 2% and 5% of the daily dietary intake in Western countries. Their effect on C-LDL values is similar to that of saturated fatty acids.33
It has been calculated that if 1% of the dietary intake of saturated fatty acids was substituted by monounsaturated fatty acids, polyunsaturated fatty acids (PUFAs) n-6, and carbohydrates, C-LDL values could drop by 1.6, 2.0 and 1.2 mg/dL, respectively.32
Polyunsaturated fatty acids n-3 do not have any direct cholesterol-lowering effect, and indeed, their TG-reducing effect could lead to a slight increase in C-LDL, when calculated using Friedewald’s formula. The protective cardiovascular effect of a diet rich in fish, which contains these substances, is exercised by means of other mechanisms. The GISSI-Prevenzione (Italian Group for Myocardial Infarction Survival) study,34 which studied, among other things, the effects of an extra 1 g of PUFA n-3 per day, in fact showed a significant reduction in sudden death for arrhythmia in over 11 000 patients who had had myocardial infarction.
Carbohydrates also have no impact on C-LDL and therefore replacing saturated fats with carbohydrates constitutes an advantageous option.28 A diet rich in fibre, legumes, fruits, vegetables, and wholegrain cereals has a direct cholesterol-lowering effect and should, therefore, be encouraged so that by replacing saturated fatty acids it is possible to optimize the effects of diet on C-LDL and the potentially unfavourable effects of carbohydrates on the other lipoproteins are minimized.35
The evidence derived from RCTs on the benefit of diet in terms of a reduction in cardiovascular risk and of the type of diet to be adopted, primarily concerns the Dietary Approaches to Stop Hypertension (DASH) diet and the Mediterranean diet.36–39 Both have shown efficacy in reducing cardiovascular risk,30 and the Mediterranean diet, in particular, has been shown to reduce cardiovascular risk in both primary and secondary prevention.
The Mediterranean diet as described by Ancel Keys and Margaret Keys40 in their book published in 1975 indicated the Mediterranean diet of those years as being ‘…a large bowl of pasta and beans, a lot of bread, without any added spreadable fat, large amounts of fresh vegetables, a modest portion of meat or fish twice a week, wine…; and fresh fruit only for dessert… For the prevention of coronary heart disease, it would be difficult to find something better than the daily diet of the population of Naples in the early 1950s’. During the same years, Fidanza et al.41 reported a 7% saturated fat consumption in their survey of eating habits in Nicotera. This average consumption of saturated fats in the populations with the longest life expectancy is also qualitatively indicated in the DASH study diet.42
The fact that European guidelines recommend a consumption not exceeding 10% should not be misleading: Italy is considered a country at low coronary risk, thanks to the benefits of our diet, and the Italian diet model suggests (and recommends) consumption closer to Mediterranean cultural characteristics than those recorded in the rest of Europe. Unfortunately, the recent OEC/HES survey showed that the average fat consumption levels in the adult population have changed since the 1960s, more specifically, saturated fat consumption now accounts for 12% of all calories, mean cholesterol consumption is >350 mg/day (compared with a recommended intake of less than 300 mg) and the consumption of fibre is low (less than 20 g/day compared with a recommended intake of 30–45 g).43
One interesting recently published editorial44 considers the ‘weak points’ of the Mediterranean diet (high consumption of added salt for the preservation and preparation of food, high consumption of extra virgin olive oil and wine, both of which have a high calorie content and the use of refined cereals). Indeed, in Italy, the prevalence of obesity and overweightness among adults exceeds 70% of the population43 and moderate use must be made of high-calorie foods that are rich in saturated fatty acid and cholesterol and have a high salt content.
The guidance provided by the ESC/EAS guidelines on diet are summarized in Table 3.
Table 3.
The recommendations of the ESC/EAS diet guidelines8
| Reduce saturated fatty acids to less than 10% of the entire calorie intake by replacing them with polyunsaturated fatty acids. |
| Transunsaturated fatty acids: eliminate or minimize consumption of those of industrial origin and restrict to less than 1% those of a natural origin. |
| Salt <5 g. |
| 30–45 g of fibre a day |
| 200 g of fruit per day |
| 200 g of vegetables a day |
| Fish at least twice a week |
| Reduce alcohol consumption: do not exceed two glasses of wine a day for men and one glass for women. |
Exercise and cholesterol
A correct lifestyle must combine the positive effects of diet with those of exercise, which is able to improve the cardiovascular risk profile by reducing lipid, glycaemia, and blood pressure values.45–47
Regular exercise increases HDL cholesterol (C-HDL) and reduces TG, with consequent improvements in C-LDL and TC levels, albeit to a lesser extent. Moderate exercise causes a 4–43% increase in C-HDL levels. Athletes who practice endurance sports have 40–50% higher HDL levels and 20% lower TG levels than a corresponding sedentary population. The mechanism by which exercise determines these effects would appear to be associated with an increase in lipoproteinase and a reduction in hepatic lipase, which leads to TG catabolism and an increase in C-HDL. In addition, even without any change in C-LDL, an increase in the size of LDL particles has been observed, with an obvious reduction in small, dense LDL particles, which are notoriously more atherogenic.
The improvement in C-HDL does not appear to be related to the type of exercise, rather there is a dose–response relationship: moderate exercise, performed at a heart rate of between 40% and 60% of maximum heart rate, for 30–40 min, 5 times a week, or better still every day, has the effects on the lipid profile indicated above. Exercise has benefits in both men and women, albeit to a lesser extent in the latter, especially in the postmenopausal period. Other mechanisms, such as an improvement in endothelial function, reduced oxygen consumption, and inflammatory profile modulation contribute to a protective action, even in patients with a positive history of prior cardiovascular events.
Therefore, regular exercise has a protective role with regard to cardiovascular diseases, in both primary and secondary prevention, and is one of the types of lifestyle intervention with the greatest impact on the cardiovascular risk profile (Table 4).
Table 4.
Recommendations for physical exercise
| Exercise improves the lipid profile. |
| Encourage all adults to do moderate exercise which achieves a heart rate of between 40% and 60% of maximum heart rate for 30–40 min 5 times a week. |
| Encourage sedentary patients to start exercising. |
| Physical exercise is also strongly recommended in patients with a history of cardiovascular events, angina, infarction, percutaneous or surgical revascularisation procedures, and heart failure. The intensity of the exercise and the way in which it is practised should be adjusted to suit the individual characteristics of the patient and defined after cardiological assessment or an adequate period of cardiovascular rehabilitation. |
Other interventions
Certainly efficacious, but to a relatively lesser extent, is the effect of weight loss: loss of approximately 10 kg reduces C-LDL by approximately 8 mg/dL. Greater benefit is achieved with weight loss obtained by means of a low-fat diet.47,48
Moderate alcohol consumption (no more than 20–30 g/dL in men and 10–20 mg/dL in women), in subjects who do not have hypertriglyceridaemia, may be acceptable.
Smoking can affect the lipid profile by causing the oxidation of small and dense LDL particles, which are more atherogenic. In addition to having a series of beneficial effects on cardiovascular risk, stopping smoking is one of the measures recommended in order to improve lifestyle.8
Lipid-lowering therapy individualized according to cardiovascular risk: the indications of the Regulatory Authority in Italy
Although they are not guidelines, the notes produced by the Italian Medicines Agency (AIFA) listing the reimbursement criteria for certain medications must be known and considered for expenditure management. AIFA note number 13, which regulates anti-dyslipidaemia drug reimbursement, has been considered one of the most complex regulations, because it considers many clinical situations and has been subject to numerous changes throughout the years.
Indeed, cardiovascular risk has been calculated in diverse ways throughout the years with the Framingham charts being the first point of reference, followed by the CUORE project charts, and lastly the European SCORE charts. The use of anti-dyslipidaemia drugs, and statins, in particular, pertains to patients, in both primary and secondary prevention interventions, who have a moderate, high, or very high cardiovascular risk and often present with organ damage or other cardiovascular conditions such as diabetes mellitus, kidney disease, or genetic disease.
Therapeutic target and drug choice
To decide how to treat a patient with hypercholesterolaemia, physicians must ask certain questions (Figure 1):
Figure 1.
Algorithms for National Health Service prescription of statins in compliance with AIFA note number 13.
What are the patient’s clinical conditions?
What is the patient’s overall cardiovascular risk?
What is his/her C-LDL target?
Which drugs can be used to achieve it?
In light of these questions, physicians should be familiar with the patient’s personal and family history; consider gender, age, weight, smoking habits, body mass index; and blood pressure; and know glycaemia, total cholesterolaemia and cholesterol fractions, triglyceridaemia values, creatininaemia, and creatinine clearance. Physicians must assess the presence of organ damage as microalbuminuria, left ventricular hypertrophy, and atheromatous plaques.
Thereafter, the presence of one or more of the following conditions must be identified:
secondary cardiovascular prevention;
diabetes mellitus;
Stage III or IV chronic kidney disease [glomerular filtration rate (GFR) <60 mL/min];
familial dyslipidaemia; and
primary cardiovascular prevention with moderate or high cardiovascular risk.
If the patient simultaneously presents more than one of the above conditions, treatment must be chosen according to the condition for which the overall cardiovascular risk is greater.
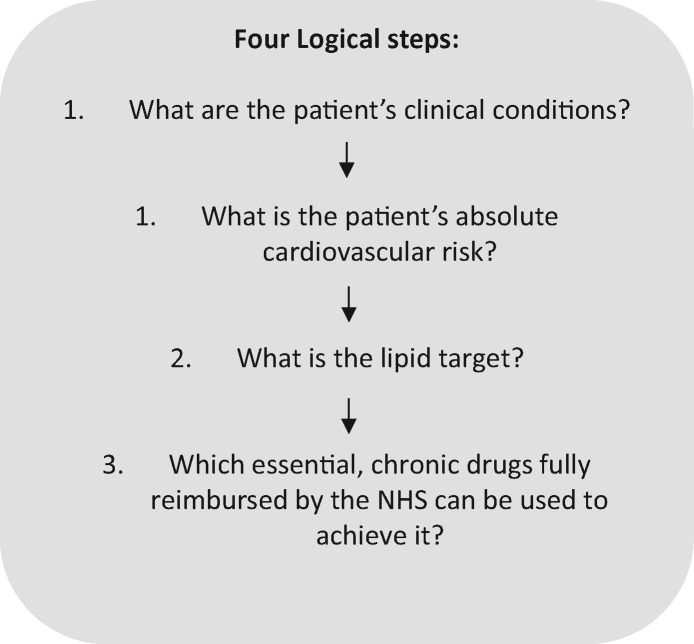
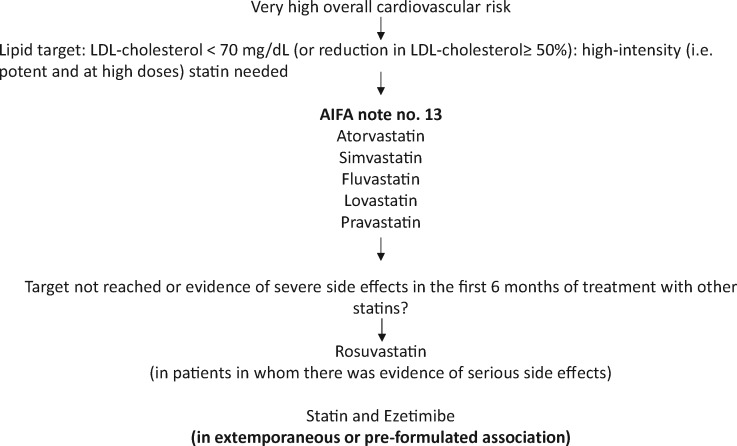
Patients receiving secondary cardiovascular prevention interventions
Clinical documentation should be collected regarding previously diagnosed atherosclerotic disease (stable chronic angina, prior myocardial infarction with or without ST-segment elevation, and unstable angina), coronary revascularization procedures (coronary artery bypass grafting and angioplasty), ischaemic stroke, and peripheral atherosclerotic arterial disease (Figure 2).
Figure 2.
Patients receiving secondary cardiovascular prevention interventions.
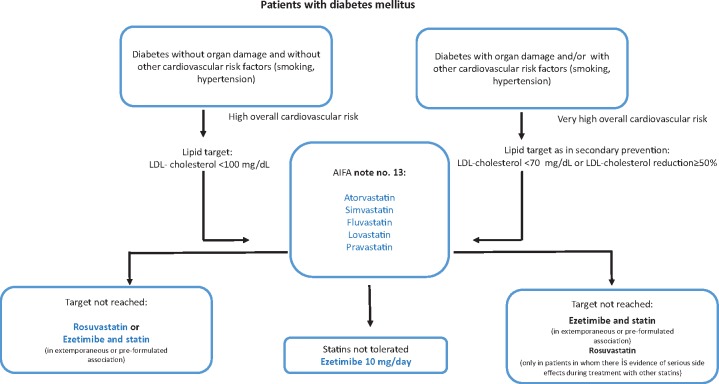
Patients with diabetes mellitus
The presence of diabetes mellitus represents a condition of high cardiovascular risk, which nevertheless differs according to the presence or absence of other cardiovascular risk factors, organ damage, or the presence of other diseases. These variables can help determine the LDL target to achieve (Figure 3).
Figure 3.
Patients with diabetes mellitus.
This topic is dealt with in greater depth in the ‘Peculiarities of diabetic dyslipidaemia’ section.
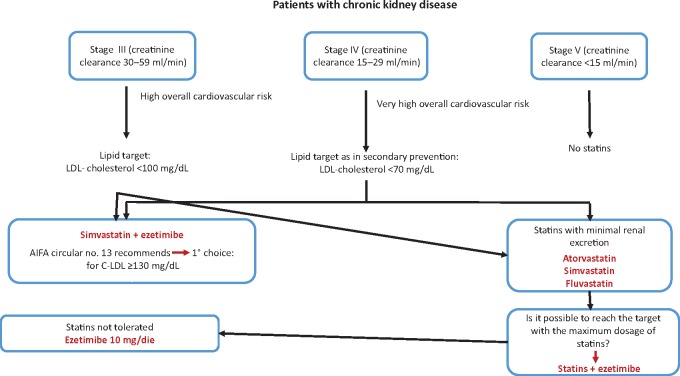
Patients with chronic kidney disease
The prevalence of this condition is far greater than commonly thought. As shown by the Health Search data, an average of 10% of all patients of a general practitioner (GP) have a GFR <60 mL/min, and therefore with a kidney disease Stage III or higher, according to the National Kidney Foundation Disease Outcomes Quality Initiative guidelines. If the subpopulation of diabetic and hypertensive subjects over 65 years of age is considered separately, the percentage of subjects with kidney disease reaches 33–35%. These subjects have a high cardiovascular risk, which must be appropriately treated.
The Document Panel believes that, in conflict with the recommendations of AIFA note number 13, the possibility of the statin intervention, even in the early stages of chronic kidney disease, should be assessed in order to obtain effective cardiovascular prevention even if the treatment does not slow progression of the kidney disease (Figure 4).
Figure 4.
Patients with chronic kidney disease.
Patients with familial dyslipidaemia
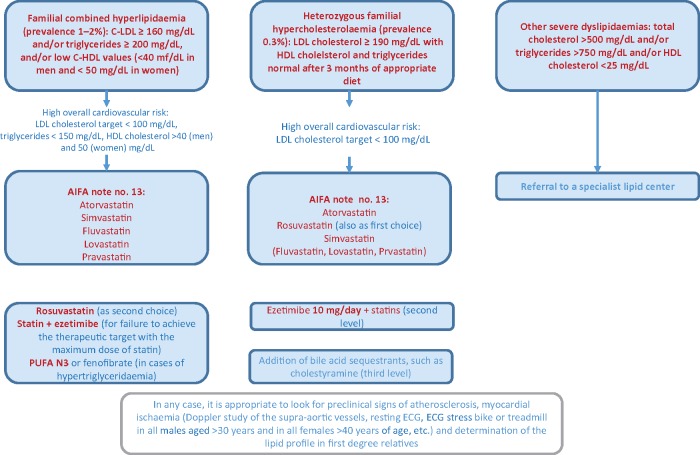
Familial dyslipidaemias are genetic conditions characterized by high plasma lipid fraction levels and an often early occurrence of cardiovascular events. Because of the severe complications that patients with familial dyslipidaemia can have, the presence of familial dyslipidaemia must be identified and adequately treated.
Monogenic familial hypercholesterolaemia has a prevalence between 1:200 and 1:500. In the absence of a molecular analysis, which is not always easy to perform, the monogenic familial hypercholesterolaemia can be strongly suspected by the presence of C-LDL >190 mg/dL and a first-degree relative with these biochemical alterations as well as by a family history of coronary heart disease (CHD) at a young age (<55 years in men and <65 years for women). A finding of tendon xanthomas constitutes further confirmation.
Familial combined hyperlipidaemia (FCHL) has a prevalence of 1–2:100; therefore, each physician should expect to have a number of subjects with FCHL among his/her patients. It is characterized by phenotypical variability that often presents with an alteration in the laboratory tests as prevalent hypertriglyceridaemia (Frederickson type IV) or prevalent hypercholesterolaemia (Frederickson type IIB).
Once again, in the absence of a genetic diagnosis, suspicion is strong when C-LDL >160 mg/dL and/or TG >200 mg/dL with vertical transmission of the same lipid disorders and in the presence of atherosclerotic disease in the family history (Figure 5).
Figure 5.
Patients with familial dyslipidaemia.
Interventions for primary cardiovascular prevention
In a patient with hypercholesterolaemia without prior events or known atherosclerotic conditions, who is not diabetic and whose kidney function is normal, without any suspicion of familial dyslipidaemia or secondary hypercholesterolaemia, the cardiovascular risk is moderate or high if associated with other risk factors or organ and should be assessed (in agreement with note number 13) with the European SCORE rating system. After an initial phase of non-pharmacological measures such as an intense lifestyle intervention lasting for at least 3 months, if C-LDL values >139 mg/dL for subjects at medium risk and >100 mg/dL for subjects at high risk, appropriate pharmacological therapy must be started.
General principles of therapy with statins
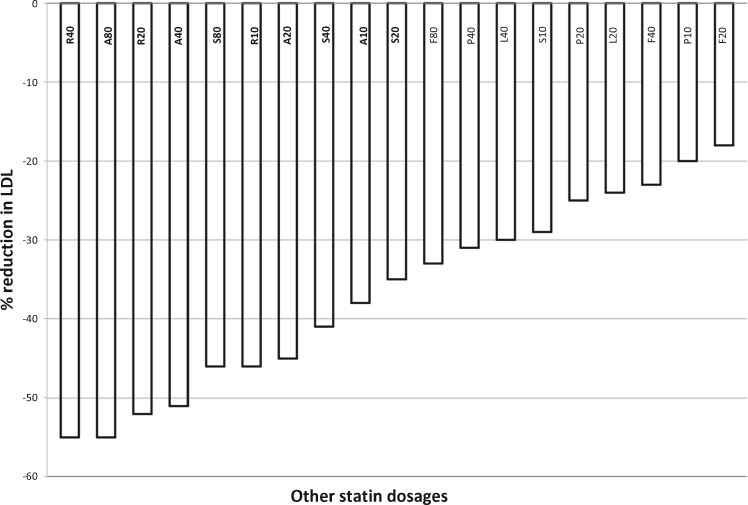
Statins are not all the same. Figure 6 shows a diagram with the comparative assessment of the C-LDL reduction rate for each statin.49
Figure 6.
Diagram for the comparative assessment of the LDL cholesterol reduction rate. Adapted from NHS Foundation Trust.49
In order to reduce cardiovascular risk, there is a class effect of all statins, related to C-LDL the reduction. Most of the clinically detectable effects of long-term statin use on either cardiovascular or general mortality and morbidity are related to the reduction in C-LDL.
Statins differ in pharmacokinetic characteristics (absorption, plasma protein binding, metabolism, and solubility) and in their interaction with other medications, which must be known in order to individualize therapy. Usually, by doubling the dose of a statin, there is a further 4–7% reduction in C-LDL, whereas by combining the statin with 10 mg of ezetimibe, it is possible to obtain a further reduction (at least 15%), with a better possibility of achieving the set target.
In patients with severe side effects or statin toxicity, the prescription of 10 mg of ezetimibe may represent a therapeutic option that is undeniably less efficacious than statins but that has a lower side effect incidence; in these cases, it will be more difficult to reach the therapeutic target. Each case should be evaluated in terms of global risk–benefit ratio, patient’s quality of life and expectations, clinical complexity and the expected adherence.
The incidence of side effects (myopathy and transaminase elevation) increases considerably with an increase in the dose of each statin. High-dose statins are associated with a slight but statistically significant increase (mean 9%; CI 2–17%) in the incidence of new cases of type 2 diabetes mellitus, especially in subjects with risk factors for diabetes (family history, obesity, metabolic syndrome, sedentary lifestyle, and age).
Peculiarities of diabetic dyslipidaemia
Clinical relevance
Diabetes mellitus incidence and prevalence are increasing significantly around the world. In Italy, there are currently 4 million people with diabetes.50 Cardiovascular disease is the main cause of morbidity and mortality among patients with diabetes, with a risk 1–3 times higher in men and 2–5 times higher in women compared with the non-diabetic population.51–53 It has been estimated that diabetic patients have a risk of cardiovascular events equal to that of the non-diabetic population with ischaemic cardiopathy, although some evidence is discordant on this point.52,54
The high cardiovascular risk is due to several risk factors such as obesity, dyslipidaemia, hypertension, and hyperglycaemia, all of which interact synergistically.
Atherogenic dyslipidaemia in diabetes
Patients with type 2 diabetes are characterized by lipid profile alterations that constitute a substantial part of the disease: hypertriglyceridaemia, reduction in C-HDL, increase in VLDL and LDL, and postprandial increase in TG-rich lipoproteins.55 The combination of these alterations constitutes the condition known as ‘atherogenic dyslipidaemia in diabetes’, which contributes to the higher cardiovascular risk of diabetic patients. Unlike other lipid disorders, the increase in C-LDL is not strictly related to the presence of diabetes, though it constitutes the main lipid factor of cardiovascular risk in these patients. In an attempt to take into account both C-LDL and the other lipid alterations typical of diabetes so as to better define the cardiovascular risk of the disease, other indices have been proposed, such as non-HDL cholesterol and the apolipoprotein B/apolipoprotein A1 ratio (ApoB/ApoA1).56
Management of diabetic dyslipidaemia
The reduction of plasma lipid levels, especially using statins, has been shown to reduce the risk of cardiovascular events in patients with diabetes.57 In diabetic patients, the reduction of C-LDL causes a decrease in all-cause and cardiovascular mortality and in cardiovascular events at least equal to that obtained in non-diabetics. This reduction does not depend on the initial C-LDL levels and is present in both primary and secondary prevention.
All the current guidelines for the treatment of dyslipidaemia highlight that patients with diabetes benefit from a reduction in C-LDL, though they differ with regard to the C-LDL targets and about the need to guide the treatment on the basis of lipid targets. The recent American guidelines recommend using medium-/high-intensity statins in patients with diabetes, regardless of the C-LDL goal,16 whereas the European and Italian guidelines (SID/AMD) recommend lowering LDL cholesterol to precise levels.8,58 Non-HDL cholesterol can be used as a secondary target (30 mg more than C-LDL values), especially in patients with TG >200 mg/dL. The ApoB/ApoA1 ratio may constitute another index of cardiovascular risk in diabetic subjects (high-risk values: in men >0.9 and in women >0.8).
In diabetes with dyslipidaemia, both lifestyle changes (reducing saturated fats and cholesterol, increasing fibre intake, and exercise) and the correction of all cardiovascular risk factors (optimization of glycaemic compensation and blood pressure and smoking cessation) are fundamental. Statin therapy should be the first-choice treatment for patients with type 1 and type 2 diabetes, with C-LDL levels that are off-target with non-pharmacological intervention (recommended target <100 mg/dL). In patients with cardiovascular disease and/or multiple non-modifiable cardiovascular risk factors, the therapeutic target is C-LDL <70 mg/dL.
In individuals that do not achieve C-LDL targets, despite the use of statin therapy, a combination of statins plus second-line agents, such as ezetimibe in particular, may help to obtain the set target. In mixed dyslipidaemia, the combination of statins with fibrates can be considered, avoiding gemfibrozil. In the case of statin intolerance (SI), ezetimibe, resins, or statin at the minimum-tolerated dose plus ezetimibe can be used.
In subjects with TG >500 mg/dL, a fibrate should be used in order to reduce the risk of pancreatitis.
Pharmacological therapy for dyslipidaemia is more effective if associated with an optimal control of glycaemia and lifestyle intervention, optimal nutritional strategies, and a reduction in alcohol consumption.
Statins
By inhibiting the synthesis of intracellular cholesterol, primarily in the liver, and reducing cholesterol deposits, statins cause an increased expression of hepatic LDLRs. This causes increased endocytosis of the circulating LDL particles, with a consequent reduction in the C-LDL of 30% to over 50%. Both the Heart Protection Study (HPS)59 and the Collaborative Atorvastatin Diabetes Study (CARDS)60 provided convincing evidence for supporting the use of statins in diabetic patients over 40 years of age. During the 5 years of the HPS study, simvastatin (40 mg) reduced C-LDL by 1 mmol/L (39 mg/dL), cardiovascular events by 27%, and stroke by 25%.59 The risk reduction was present regardless of the type of diabetes, levels of glycosylated haemoglobin, or baseline levels of C-LDL. The CARDS study was interrupted because of the obvious benefits achieved in the treated group.60 After an average follow-up of 3.9 years, atorvastatin (10 mg) reduced the risk of a first cardiovascular event by 37% and of stroke by 48%. The Cholesterol Treatment Trialists’ Collaboration (CTTC) meta-analysis of 14 RCTs of statin therapy involving 18 686 people with diabetes (1466 with type 1 and 17 220 with type 2 diabetes) revealed, during an average treatment period of 4.3 years, a 9% reduction in mortality and a 21% decrease in myocardial infarction, coronary death, coronary revascularization, and stroke for every 1.0 mmol/L (39 mg/dL) reduction in C-LDL.57
These results support the guideline recommendations that state that patients with diabetes are a high-risk group and receive substancial benefit from statin treatment.
The very clear benefit of statins is tempered by concerns regarding the adverse events, even if these are relatively mild in most patients.61 Recent RCT meta-analyses seem to indicate an increased risk of new-onset diabetes associated with statin treatment.62,63 However, this risk is small, age related, and more consistent with higher doses of statins. Indeed, it seems to accelerate by a few months the clinical expression of diabetes in predisposed patients, i.e. those who a have metabolic syndrome, for whom the increased risk of developing diabetes is greatly outweighed by the benefit of the reduction in cardiovascular events.63
Ezetimibe
When a higher dose of statins is unable to achieve the target C-LDL values, simultaneous treatment with ezetimibe should be considered. Ezetimibe reduces cholesterol absorption by blocking the Niemann-Pick C1-Like Protein 1 sterol transport protein. The IMPROVE-IT study conducted on patients with recent ACS showed that the combination of ezetimibe with a statin caused a further reduction in LDL cholesterol compared with statins alone (54 vs. 70 mg/dL) and was associated with a small but significant improvement in the primary endpoint composite of cardiovascular death, non-fatal infarction, unstable angina requiring hospitalization, coronary revascularization 30 days from randomization or non-fatal stroke (32.7% in the simvastatin–ezetimibe group vs. 34.7% in the simvastatin monotherapy group; hazard ratio 0.936; 95% CI 0.89–0.99; P = 0.016).27 This result is in line with the findings of the CTTC meta-analysis. Twenty-seven per cent of the patients included in the study had diabetes (n = 4933). It is interesting to note that the only subgroup analysis that showed a statistically significant effect was the one that compared the presence of diabetes [relative risk (RR) 0.86, 95% CI 0.78–0.94] vs. absence of diabetes (RR 0.98, CI 95% 0.92–1.04). One possible mechanism underlying the greater efficacy in diabetic patients could be related to the effects of ezetimibe on the atherogenic potential of the fasting and postprandial lipoprotein profile in these patients.64
Resins
Resins are another class of lipid-lowering drugs that act on the intestinal level, albeit in a different site respect to ezetimibe (terminal ileum vs. duodenum/jejunum for ezetimibe) and through a different mechanism (inhibition of the enterohepatic circulation of bile acids). The Lipid Research Clinics Coronary Primary Prevention Trial study showed that the reduction in C-LDL obtained with cholestyramine (∼20%) was associated with an approximately 20% reduction in cardiovascular events.65 A systematic review of 36 studies showed that low-intensity statins plus resins reduced C-LDL levels by up to 14% more than medium-intensity statin monotherapy.66 Bile acid sequestrants can be useful in diabetes because they improve glycaemic control, probably through an incretin-like effect.67 Therefore, despite the poor compliance observed with cholestyramine and its side effects, such as constipation, increase in plasma triglycerides, and reduction in the absorption of drugs, including statins, resins represent an additional therapy that could be useful for reducing C-LDL in patients with diabetes, although an RCT is still needed to determine the net effects of resins on cardiovascular outcomes in diabetic patients.
Fibrates
Some decades ago, RCTs such as the Helsinki Heart Study (HHS)68 and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT)69 showed a reduction in cardiovascular risk with fibrates compared with a placebo. However, since statins became the standard of care for individuals at high cardiovascular risk, RCTs with fibrates have been unable to avoid the simultaneous use of statins, such as in the Bezafibrate Infarction Prevention registry70 and Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study.71 These studies did not show any benefit to therapy with fibrates compared with placebo groups in which statins were largely used. The same observation was found in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study72 when fenofibrate was added to statins.
However, the advantages of fibrates should be reconsidered, firstly due to the positive effects observed in the older HHS and VA-HIT studies, which make its use possible in patients who are unable to take statins, but also because, in meta-analyses of the most important studies using fibrates, the subgroup of patients with high TG (>2.3 mmol/L) and low C-HDL (<0.9 mmol/L—35 mg/dL) showed a reduction in cardiovascular risk of up to 35%, regardless of the background therapy with statins.73,74 Fibrates would therefore appear to be ideal for the dyslipidaemia profile commonly observed in patients with diabetes. This type of patient has been routinely excluded from trials such as the FIELD and ACCORD studies, and, therefore, it would be appropriate to conduct a trial with fibrates in patients with high TG and low C-HDL. In the meantime, fibrates are still recommended in patients with very high TG levels (>10 mmol/L—387 mg/dL) for pancreatitis prophylaxis.75 In addition, fibrates appear to be associated with reduced retinopathy progression, regardless of their effects on lipids76; however, this unexpected effect on microvascular disease warrants confirmation in ad hoc studies.
Niacin
Niacin, which is currently not available in Italy, has a positive effect on the lipid profile of diabetic patients.77 Forty years ago, the Coronary Drug Project showed a reduction in cardiovascular events and mortality with short-acting niacin compared with placebo.78 This beneficial effect was not observed when sustained-release niacin was added to background therapy with statins in either the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) study79 or the Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study,80 in which a third of participants had diabetes (8299 of 25 673). In this latter study, in order to reduce hot flushes, niacin was co-administered with laropiprant, a prostaglandin receptor antagonist, which may have inhibited some of the beneficial effects of niacin.81 The niacin–laropiprant combination was also associated with a greater incidence of perturbations in diabetes control (absolute excess compared with placebo, 3.7%; P < 0.001) and with a higher incidence of diabetes diagnoses (absolute excess 1.3%; P < 0.001).
It has been said that the duration of the AIM-HIGH study was too short and that the sample was too small to reveal an effect and that we still need to see the analysis of the subgroups of the AIM-HIGH and HPS2-THRIVE studies before we set niacin aside for good.82 Moreover, as niacin lowers C-LDL by 20–30%, it could be beneficial in monotherapy; however, this must be weighed against its tolerability and side effects, especially in patients with diabetes. Lastly, niacin may help reduce TG and could, therefore, be useful in people with high TG levels in order to reduce the risk of acute pancreatitis, as already discussed for fibrates. It is important to remember the effect of niacin on glycaemia, which is increased after treatment.
PCSK9 inhibitors
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are a new class of drugs approved in Europe for the reduction of C-LDL in high-risk patients. Although there are no available studies conducted exclusively on patients with diabetes, one meta-analysis based on three studies showed that evolocumab caused an average reduction in C-LDL of 60% in 413 patients with type 2 diabetes vs. placebo and of 39% vs. ezetimibe, and of 66% in 2119 non-diabetic patients vs. placebo and of 40% vs. ezetimibe. In diabetic patients, evolocumab was associated with a decrease in non-C-HDL (55% vs. placebo and 34% vs. ezetimibe), and lipoprotein(a) [Lp(a); 31% vs. placebo and 26% vs. ezetimibe], and an increase in C-HDL (7% vs. placebo and 8% vs. ezetimibe). The results were irrespective of glycaemia, insulin use, renal function, and degree of cardiovascular disease.83 Side effects had the same frequency as among the non-diabetics and consisted primarily of neurocognitive events (∼1% vs. 0.5% with placebo, regardless of the C-LDL levels achieved), injection site reactions (∼6% vs. 4%), myalgia (6% vs. 3%), and ophthalmological events (∼3 vs. 2%).
Two studies that enrolled a relatively high number of subjects with diabetes provided very similar results in terms of reduction in cholesterol and cardiovascular events, without the differences between subjects with and without diabetes.84,85 In the Long-term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM) study, which randomized 2341 patients at high risk of cardiovascular disease, of whom 818 had diabetes (34.9% of the study sample), to alirocumab 150 mg or placebo administered subcutaneously every other week for a period of 78 weeks, the decrease in C-LDL observed with alirocumab was 62%.84 The incidence of cardiovascular events (death from coronary disease, non-fatal myocardial infarction, fatal or non-fatal ischaemic stroke, or unstable angina requiring hospitalization) was low but significantly lower with alirocumab than with placebo (1.7 vs. 3.3%; P = 0.02). In the Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER)-1 and -2 studies combined, in 4802 patients at high risk of cardiovascular disease, of whom 802 had diabetes (17.7% of the study sample), the addition of evolocumab reduced C-LDL by 61% compared with the standard therapy alone.85 The effect on C-LDL, non-C-HDL, Lp(a), and TG was similar to that in non-diabetic subjects, and it was not influenced by gender, type of statin used, insulin treatment, presence of cardiovascular disease, GFR, or degree of glycaemic compensation. The incidence of cardiovascular events at 1 year was significantly lower in the evolocumab group (0.95 vs. 2.18%; P = 0.003).
Considerations for diabetic patients
Patients with diabetes have a high risk of cardiovascular disease. Statins remain a fundamental therapy for the prevention of cardiovascular events in these patients, according to a number of RCTs conducted specifically on patients with diabetes and meta-analyses on a large number of subjects with diabetes enrolled in many randomized studies involving statins. The aforementioned analysis of the IMPROVE-IT study86 suggests that adding ezetimibe could be particularly advantageous for high-risk diabetic patients. A lower degree of evidence suggests that fibrates could reduce cardiovascular risk in subgroups of patients with high TG and low C-HDL. Of the various emerging treatments, PCSK9 inhibitors have demonstrated to be very significantly effective in lowering C-LDL. Until the results of studies conducted directly on diabetic patients87 are available, it can be presumed that PCSK9 inhibitors can be used in diabetic patients with certain characteristics, such as, e.g. concomitant familial dyslipidaemia, recurrent episodes of cardiovascular events, persistence of high C-LDL levels despite high doses of statins, or SI.
The role of dietary supplements in the treatment of dyslipidaemia
Nutraceuticals and dyslipidaemia
A nutraceutical (a neologism coined in 1989, by uniting the terms nutritional and pharmaceutical) is, according to its original definition, a food product or part of a food product whose sole purpose is to maintain good health. Nutraceuticals comprise a great number of compounds, including dietary supplements and functional foods (food products enriched with ingredients with specific protective functions), and preparations containing medicinal plants. Most nutraceuticals are of plant origin; however, some substances are animal derivatives (e.g. fish oil). From a regulatory standpoint, marketing a product as a medicinal product is significantly different from marketing a dietary supplement. This is clearly demonstrated, e.g. by the information provided on the label: the only products that can boast therapeutic or preventative properties are medicinal products and medical devices. Supplements, according to Italian regulations (assimilating [EC] Regulation no. 1924/2006 issued by the European Parliament and European Council on 20 December 2006), have the sole purpose of maintaining a good state of health, with an exclusively nutritional or physiological effect. For this reason, medicinal products have to follow a far more complex regulatory procedure in order to ensure patients and doctors that the information provided concerning their therapeutic efficacy has been proved by specific clinical studies. The clinical studies (together with all the documentation constituting the marketing authorization dossier) are submitted to regulatory authorities who, after approving them, authorize marketing for those indications for which the medicinal product has shown a favourable risk–benefit assessment.
Nutraceuticals are almost always used in the absence of valid clinical studies documenting their efficacy and safety. Indeed, the natural derivation of a nutraceutical does not represent a guarantee that it is harmless; in addition, the absence of post-marketing monitoring does not make it possible to evaluate the onset of adverse effects related to the use of these products. Medicinal products must be manufactured in sites that have been approved by AIFA and must follow standards of good manufacturing practice (GMP), which involve, in addition to hygiene and sanitary inspections, also stringent technical assessments such as the guarantee of batch reproducibility, assessment of the incoming starting materials, and validation of all manufacturing processes. The manufacture of a supplement, on the other hand, may take place in a site that does not have to comply with GMP, merely with Hazard Analysis and Critical Control Points, a set of procedures only aimed at preventing food contamination hazards and therefore essentially a hygiene and sanitary assessment similar to that performed on food.
A great many functional foods or dietary supplements are promoted as benefits for subjects with dyslipidaemia or to reduce cardiovascular risk. Although some of these products appear to have potential functional effects, they have never been studied in long-term clinical studies and should therefore only be used if clinical evidence is available that clearly shows their safety and positive effects on plasma lipids. Generally speaking, they should only be used in those subjects whose overall cardiovascular risk does not justify the use of medicinal products.
We must also remember that, in general, an individual who eats a healthy and balanced diet does not need to add any dietary supplements and that the use of these types of products must not substitute consumption of ‘real’ foods through a suitable diet.
Polyunsaturated omega-3 fatty acids
Omega-3s are a category of essential fatty acids known as polyunsaturates because their chain comprises a number of double bonds. The term omega-3 derives from the position of the first double bond, which is the third from the terminal carbon atom (carbon ω).
The three main PUFA n-3s, alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA), differ in the length of their chain (comprising 18, 20, and 22 carbon atoms, respectively) and in the number of double bonds present (3, 5, and 6, respectively).
Omega-3s can be derived directly from food sources or synthesized by chain elongation and anaerobic desaturation using ALA. This acid is present in certain seeds (flax seeds), in walnuts and in vegetable oils, whereas the main sources of EPA and DHA come from the sea (mainly certain types of fish, such as blue fish, tuna, and salmon).88
A number of epidemiological studies have confirmed the relationship between a high dietary intake of omega-3 and a reduction in cardiovascular risk. The American Heart Association (AHA) recommends including at least two portions of fish a week in the diet in order to reduce cardiovascular events and reduce the progression of atherosclerosis in patients with coronary disease.89
The GISSI-Prevenzione study34 showed that the administration of a pharmaceutical formulation containing a highly purified concentration of omega-3 (ethyl esters) equal to at least 85% significantly and clinically relevantly reduces the incidence of mortality for cardiovascular causes. However, this effect cannot be attributed to an effect on the lipid profile, rather, presumably, to an antifibrillatory action, documented by the significant reduction in the events validated in the trial as ‘sudden death’. In actual fact, no significant changes in the lipid profile were observed compared with the baseline, with the exception of a modest (−3.4%) but significant reduction in the concentration of TG in the group treated with omega-3. The use of pharmacological doses of >2 g of omega-3 a day reduces TG levels. The average reduction in TG with a dose of 2–4 g/day is approximately 30% and the benefit would appear to be dose dependent; the reduction in TG is approximately 45% in subjects with baseline values >500 mg/dL.90
Management of high cardiovascular risk patients with hypercholesterolaemia
In managing patients at high and very high cardiovascular risk in general medicine, a number of preliminary considerations must be made.5,8,16,91–100 First and foremost, the patient’s clinical condition must be clearly defined. In the vast majority of cases, these patients are on polytherapy and have co-morbidities. The main problems posed are the need for transverse clinical monitoring for all the conditions present and of the choice and management of the pharmacological treatments, in particular with regard to their efficacy, but also considering adverse reactions, drug–drug interactions, and treatment compliance. The targets to be met must be considered in the light of the many clinical, anagraphical, cultural, and motivational variables that each subject presents. In light of this managerial complexity and the great interindividual variability associated with the conditions indicated above, the physician must evaluate, on a case-by-case basis, whether to set ideal targets, or rather targets that are ‘realistic’ in real life. How should one behave in the choice of medicinal products and in the complex management of therapeutic strategies (polytherapy, doses, interference, and motivational counselling)? We believe that the answer is to find the right balance between the guidelines/evidence-based medicine, regulatory standards such as those proposed in AIFA notes, and good clinical practice, i.e. patient-centred medicine.
On the one hand, we possess data that show how very low C-LDL levels correlate with significant reductions in cardiovascular events, and, on the other hand, we are aware of the great difficulty experienced by most patients in reaching these C-LDL levels. Attempting to reach targets is, however, just part of the management of patients at high cardiovascular risk: lifestyle monitoring is extremely important and, as regards pharmacological therapy, it is essential to monitor, when indicated, antiplatelet and/or antiaggregant therapy, inotrope, anti-arrhythmia, and diuretic therapy. If we then consider the patient from the complex management standpoint, caring for one aspect or reaching a target is a mere part of a whole; it is part of the protocol, but it is not the centre of the protocol.
Why AIFA [Italian Medicines Agency] note number 13 must be abolished
AIFA note number 13 has been the subject of great debate, as shown by the three very different versions issued in a relatively short amount of time.
The last version of circular 13 takes the form of a guideline for the treatment of dyslipidaemia that is to a large extent repetitive in certain parts and with certain specific issues with regard to consistency that have already been appropriately summarized in documents issued by the Italian Society for the Study of Atherosclerosis (SISA), Italian Society of General Medicine (SIMG), and Emilia-Romagna Regional Authority published in literature.101–103
Indeed, the aims of regulatory notes are very different from those of the guidelines. Guidelines are the result of an in-depth assessment of existing evidence; they provide a useful foundation on which to base the clinical decisions and are not binding. On the other hand, being a regulatory note, the AIFA note number 13 does have a binding nature, with the main aim of rationalizing, especially from an economic point of view, prescriptions for the correction of dyslipidaemia. Although this type of rationalisation may have been indispensable a few years ago when the economic commitment of the NHS relating to the prescription of statins was very great, in the current context, characterized by the availability of a great many equivalent medicinal products, this type of rationale undoubtedly has much less reason to exist. In other words, the guidelines should be more than sufficient for guiding the clinical decisions of a prescribing physician, without having a significant impact, from an economic point of view, on NHS costs.
Diagnosis of familial dyslipidaemia: AIFA note number 13 and clinical algorithms
Dyslipidaemia is a clinical condition in which there are qualitative and quantitative alterations in plasma lipids and lipoproteins. The increase in lipid levels may be absolute and indicative of the presence of a primary (genetic) dyslipidaemia or a secondary dyslipidaemia related to other conditions. However, the concentration of plasma lipids may also be relatively high in relation to the overall cardiovascular risk of a specific patient.
Familial dyslipidaemias comprise a large group of lipid metabolism alterations, and those that are the most important because they are the most common are polygenic hypercholesterolaemia, FH and FCHL.
Hypercholesterolaemia
Polygenic hypercholesterolaemia is the most common cause of an increase in cholesterolaemia. The increase in C-LDL is moderate, whereas triglyceridaemia usually is in the normal range. Most patients with polygenic hypercholesterolaemia present an LDL clearance alteration. There is an underlying genetic predisposition associated with the presence of numerous allele variants with a cholesterol-raising effect in genes able to influence plasma levels of C-LDL. This genetic predisposition is, in many cases, worsened by environmental factors such as a diet rich in saturated fats and a sedentary lifestyle. Total cholesterolaemia is usually between 240 and 350 mg/dL and its familial transmission does not present the characteristics of monogenic diseases (i.e. family members present either very high or absolutely normal levels of C-LDL). For this condition, there are no specific diagnostic criteria.
FH is a monogenic disease caused by a defect in the function of the LDLRs, with consequent absent or slowed removal of these lipoproteins from the plasma and an increase in the blood levels of C-LDL. FH is transmitted as a co-dominant trait, therefore expressing with both a heterozygous phenotype (HeFH) and a homozygous phenotype (HoFH). FH is caused by several different gene mutations. Mutations may affect the gene encoding for the LDLR (LDLR), or for ApoB (APOB), a specific ligand of the LDLR, or that encoding for protein PCSK9 (PCSK9), which regulates the amount of receptors present on the cell surface or the gene encoding for LDLR adaptor protein 1 (LDLRAP1), which is essential for the correct LDLR function. Mutations of the LDLR gene are known to be the most common cause of FH (accounting for approximately 90% of cases).
In the heterozygous form, LDLR activity is only partly compromised (by about 50%). It therefore manifests with C-LDL values between 200 and 350 mg/dL. In addition, patients may have tendon xanthomas, especially of the Achilles’ tendon and extensor tendons of the hands. Patients with HeFH often experience premature coronary events (<55 years) and severe hypercholesterolaemia is common in first-degree relatives.
A diagnosis of HeFH can be suspected on the basis of high TC and C-LDL levels according to the following values:
in adults: C-LDL ≥ 190 mg/dL;
in pre-puberty: C-LDL ≥ 160 mg/dL.
In addition, at least one of the following criteria must be satisfied for the diagnosis:
presence of hypercholesterolaemia in a first-degree relative (parents, siblings, and offspring);
presence of tendon xanthomas; and
presence of CHD in the patient or in a first-degree relative before the age of 55 years in men and 65 years in women.
This is the set of criteria proposed by the Simon Broome Register and adopted by AIFA note number 13.93 There is another algorithm for diagnosing FH that would appear to be more accurate and, indeed, it has been adopted by several international guidelines. This is the score algorithm based on the criteria of the Dutch Lipid Clinic Network (DLCN) (Table 5).104
Table 5.
Dutch Lipid Clinic Network criteria for the diagnosis of familial hypercholesterolaemia in adults
| Score | |
|---|---|
| Family history | |
| First-degree relatives with premature CHD (<55 years in men; <60 years in women)or first-degree relatives with cholesterol >8 mmol/L (≥310 mg/dL) (or > 95° percentile) | 1 |
| First-degree relatives with tendon xanthomas and/or arcus senilis or offspring <18 years with cholesterol >6 mmol/L (≥ 230 mg/dL) (or > 95° percentile) | 2 |
| Patient history | |
| Subject with premature CHD (<55 years in men; <60 years in women) | 2 |
| Subject with premature cerebral or peripheral vascular disease (<55 years in men; <60 years in women) | 1 |
| Physical examination | |
| Tendon xanthomas | 6 |
| Arcus senilis in a subject <45 years | 4 |
| LDL cholesterol plasmatic levels | |
| >325 mg/dL | 8 |
| 251–325 mg/dL | 5 |
| 191–250 mg/dL | 3 |
| 155–190 mg/dL | 1 |
| Known causal gene mutation | 8 |
| Stratification | Total score |
| Certain diagnosis of FH | ≥ 8 |
| Probable diagnosis of FH | 6-7 |
| Possible diagnosis of FH | 3-5 |
| Unlikely diagnosis of FH | 0-2 |
CHD, coronary heart disease; FH, familial hypercholesterolaemia; LDL, low-density lipoprotein.
In the rarer homozygous form, there is an almost total absence of receptor activity and TC is particularly high, reaching values as high as 500–1200 mg/dL. In this case, a diagnosis is usually made in the paediatric age, and tendon and/or skin xanthomas, signs of severe cardiovascular system impairment, are present before the age of 10. The criteria for the diagnosis of homozygous FH (Table 6) are those suggested by the recent EAS document.104
Table 6.
Diagnostic criteria of homozygous familial hypercholesterolaemia
| LDL cholesterol levels ≥ 500 mg/dL.a |
| Childhood presentations including premature coronary heart disease and aortic valve disease. |
| Xanthomas in the tendons of the hand and Achilles’ tendon. |
aHomozygous familial hypercholesterolaemia can also be observed in the presence of lower levels of LDL cholesterol, considering the recent recognition of the clinical and genetic heterogeneity of familial hypercholesterolaemia.
The HoFH group may also include a severe form of FH with recessive transmission (the parents of affected patients have normal or slightly high cholesterol values) known as ‘autosomal recessive hypercholesterolaemia’. This form is caused by the presence of homozygous mutations in the LDLRAP1 gene and it is extremely rare. Studies have shown that it is particularly common in Sardinia.
Prevalence and diagnosis of familial hypercholesterolaemia in Italy: sensitivity and specificity of AIFA’s diagnostic algorithm in general medicine
When diagnosing familial dyslipidaemia, general practitioners (GPs) are faced with several critical issues:
the need for a low level of ‘diagnostic suspicion’ for certain borderline forms of familial dyslipidaemia;
objective difficulties in identifying, in certain cases, the presence of premature hypercholesterolaemia and/or ischaemic cardiomyopathy in the patient’s family;
the non-univocal and/or complex and/or often contradictory diagnostic criteria and/or different C-LDL/TC threshold values adopted by the various algorithms, meaning that application in the general medicine setting is not practical or immediate;
the absence, in some cases, of a specific International Classification of Diseases–Ninth Revision (ICD9) classification of the different forms of familial dyslipidaemia and, in particular, of FH. Indeed, in this last case, the code (272.0) is common to other, less severe, forms of hypercholesterolaemia (e.g. the polygenic form). This causes a diagnostic overlap and makes it impossible to calculate with precision the true prevalence of the disease and, therefore, to assess diagnostic appropriateness;
the non-systematic and non-widespread use of Friedewald’s formula to calculate LDL; and
the variations in reporting the lipid profile by the different laboratories;
The main consequences of these issues are:
possible presence of undiagnosed cases with familial dyslipidaemia (under-diagnosis);
over-recording of diagnoses of heart failure (HF) (due to the absence of aspecific ICD9 code, which, as mentioned previously, also includes other forms of hypercholesterolaemia such as the polygenic form);
possible inappropriate diagnoses of familial dyslipidaemia.
Therefore, the problem has been investigated by evaluating the sensitivity and specificity of the diagnostic algorithms most commonly used by GPs for the diagnosis of FH and calculating more precisely the prevalence of FH in Italy. Three algorithms were tested:
Algorithm 1: ICD9CM-based algorithm. This algorithm adopts the specific ICD9CM code (ICD9CM subcode: 272.0/09 or 272.0/10, or 272.0 adding the comment ‘familial’), which is available in the modified version of the Health Search ICD9CM thesaurus.
Algorithm 2: SIMG/SISA. This algorithm was developed by the SIMG and the SISA to provide GPs with a straight-forward tool to use in clinical practice. A case of FH is identified by a value of C-LDL ≥190 mg/dL associated with a history of premature (<55 for men and <60 for women) cardiovascular events (coronary, cerebral, or peripheral vascular event) or the presence of xanthoma or early coronary events in the patient’s family (with the same age limits indicated above). It is important to remember that this algorithm also corresponds with the diagnostic criteria recommended by AIFA note number 13.93
Algorithm 3: SIMG/SISA algorithm plus familial history of premature events. This algorithm defines a case of FH using the same criteria as Algorithm 2, but by identifying family cases of premature cardiovascular events by searching medical records for the following free-format words: ‘premature’, ‘juvenile’, ‘under 60’, associated with the words ‘cardio’, ‘cerebro’, and ‘vascular’. However, the cases were identified manually one by one. The results of this algorithm are identical to those obtained for Algorithm 2.
Of the sample of 1 240 000 subjects present in the Health Search database on 31 December 2014, all patients with the following characteristics were recruited:
presence of C-LDLa recorded in 2014 but without a prescription for statins and/or ezetimibe;
presence of C-LDLa in the year prior to the prescription of statins and/or ezetimibe.
aNB: the C-LDL could be recorded or calculated using Friedewald’s formula if the data were available.
The prevalence of FH obtained with the algorithms and their sensitivity and specificity were evaluated using as a landmark and gold standard the modified DLCN score104 (Table 7), so that it could be applied to the data available in the Health Search database. This algorithm defined as certain cases those with a score >8, as probable cases those with a score of 6–8, and as possible cases those with a score of 3–5.
Table 7.
Dutch Lipid Clinic Network criteria modified by the application of the data available in the Health Search database
| Score | |
|---|---|
| Family history | |
| First-degree relatives with premature CHD (<55 in men; <60 in women) (ICD9CM:410a–414a) and/or premature cerebrovascular disease (ICD9CM: 342a, 433a–436a, 438a), or peripheral vascular disease (ICD9CM: 093.0, 440a, 443.1–443.9, 447.1, V43.4). | 1 |
| Patient history | |
| Patient with premature CHD (<55 years in men; <60 in women) (ICD9CM: 410a–414a). | 2 |
| Patient (<55 years in men; <60 years in women) with premature cerebrovascular disease (ICD9CM: 342a, 433a–436a, 438a) or peripheral vascular disease (ICD9CM: 093.0, 440a, 443.1–443.9, 447.1, V43.4). | 1 |
| Physical examination | |
| Tendon xanthoma (ICD9CM: 272.7 associated with ‘xanthomaa’ in the description of the code or in free-format words; ICD9CM: 374.51). | 6 |
| Arcus senilis (ICD9CM: 371.0a; to be verified in the description of the code) in a subject <45 years | 4 |
| Laboratory tests | |
| C-LDL >320 mg/dL | 8 |
| C-LDL 250–319 mg/dL | 5 |
| C-LDL 193–249 mg/dL | 3 |
| C-LDL 155–192 mg/dL | 1 |
| Diagnosis of FH | Total score |
| Certain | >8 |
| Probable | 6–8 |
| Possible | 3–5 |
CHD, coronary heart disease; C-LDL, LDL cholesterol; FH, familial hypercholesterolaemia.
aIndicates all codes starting with the number reported.
With the DLCN score, the ‘certain’ cases (n = 99) had a prevalence of 0.01%. The addition of ‘probable’ and ‘possible’ cases increased the prevalence to 0.18 and 1.48%, respectively. Algorithms 1 and 2, on the other hand, gave a prevalence of 0.9 and 0.13%, respectively. Although there are no substantial differences, females usually have a higher prevalence than males, and the highest prevalence was observed in the middle age subgroup (Table 8).
Table 8.
Prevalence of FH based on the Dutch Lipid Clinic Network (DLCN) score and tested algorithms
| DLCN score |
Tested algorithms |
||||
|---|---|---|---|---|---|
| Certain | Certain/probable | Certain/probable/possible | Algorithm 1 | Algorithm 2 | |
| Sex | |||||
| Male | 37 (0.01) | 591 (0.11) | 6485 (1.21) | 3770 (0.7) | 551 (0.1) |
| Female | 62 (0.01) | 1452 (0.25) | 10 002 (1.73) | 6272 (1.09) | 917 (0.16) |
| Age range (years) | |||||
| 15–24 | 0 (0) | 22 (0.02) | 66 (0.06) | 156 (0.14) | 2 (0) |
| 25–34 | 0 (0) | 67 (0.04) | 260 (0.17) | 382 (0.25) | 11 (0.01) |
| 35–44 | 6 (0) | 237 (0.12) | 1076 (0.55) | 1005 (0.52) | 64 (0.03) |
| 45–54 | 26 (0.01) | 521 (0.25) | 3225 (1.56) | 2117 (1.02) | 262 (0.13) |
| 55–64 | 35 (0.02) | 618 (0.37) | 4881 (2.94) | 2743 (1.65) | 500 (0.3) |
| 65–74 | 24 (0.02) | 382 (0.28) | 4099 (2.96) | 2216 (1.6) | 413 (0.3) |
| 75–84 | 7 (0.01) | 149 (0.15) | 2275 (2.28) | 1145 (1.15) | 190 (0.19) |
| ≥85 | 1 (0) | 47 (0.11) | 605 (1.43) | 278 (0.66) | 26 (0.06) |
| Total | 99 (0.01) | 2043 (0.18) | 16 487 (1.48) | 10 042 (0.9) | 1468 (0.13) |
Data are expressed as n (%).
Considering the ‘certain’ cases, Algorithm 1 showed a sensitivity of 10.10% (95% CI 5–17.8%) and a specificity of 99.10% (95% CI 99.10–99.10%). Otherwise, Algorithm 2 better identifies the true positives [sensitivity 85.90% [95% CI 77.40–92.00%)] while maintaining the same specificity as Algorithm 1 (99.9%). By including the ‘probable’ or ‘possible’ cases, the sensitivity of both algorithms was significantly reduced to 5.90% (95% CI 5.50–6.20%) and 8.20% (95% CI 7.80–8.60%), respectively (Table 9).
Table 9.
Accuracy of the tested algorithms in relation to certain and total cases identified by the Dutch Lipid Clinic Network score
| Algorithm 1 |
Algorithm 2 |
|||
|---|---|---|---|---|
| Certain cases (DLCN) | All cases (DLCN) | Certain cases (DLCN) | All cases (DLCN) | |
| Sensitivity | 10.10 (95% CI 5.00–17.80) | 5.90 (95% CI 5.50–6.20) | 85.90 (95% CI 77.40–92.00) | 8.20 (95% CI 7.80–8.60) |
| Specificity | 99.10 | 99.20 | 99.90 | 100 |
| PPV | 0.10 (95% CI 0.00–0.20) | 9.60 (95% CI 9.10–10.20) | 5.80 (95% CI 4.70–7.10) | 91.90 (95% CI 90.40–93.20) |
| NPV | 100 | 98.60 | 100 | 98.60 (95% CI 98.60-98.70) |
| AUC | 0.55 (95% CI 0.52–0.58) | 0.53 (95% CI 0.52–0.53) | 0.93 (95% CI 0.89–0.96) | 0.54 |
The CIs are not indicated if the upper or lower limit correspond with the proportion.
AUC, area under the curve; DLCN, Dutch Lipid Clinic Network; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value.
The results obtained with Algorithm 2 were more or less in line with published literature where the prevalence reported is 1:200–1:500, as observed in studies in which the genetic test was also used. Indeed, Algorithm 2 is significantly more efficacious in identifying ‘certain’ cases. Despite the fact that specificity is also high for Algorithm 1, this method produces a lot of false negatives.
These results show that the ICD9CM for FH is probably often used to indicate ‘suspected’ cases requiring further diagnostic investigation. On the contrary, Algorithm 2, which includes more specific criteria that are more similar to the DLCN score, appears to be more precise for the diagnosis (almost 90% sensitivity). This algorithm also showed better accuracy in identifying probable and possible cases that require further investigation.
The results of the Health Search survey lead to the conclusion that:
the systematic application of the DLCN score using of a score of ≥ 4 for FH diagnosis (as recommended in the SISA-SIMG consensus96) would hypothetically lead to a rate prevalence of FH in Italy greater than 1%. However, this rate also includes probable and possible cases and therefore, particularly in those cases with the lowest scores (e.g. between 4 and 6), the GP should seek diagnostic confirmation with a genetic test referring the patient to a second-level centre;
the prevalence of FH according to the ICD9 classification (Algorithm 1) performed by GPs applying mainly the AIFA criteria and, to a lesser extent the other algorithms, is 0.9%, very close to the rate previously reported and therefore the same operating considerations are valid;
the very low prevalence of FH (0.13%) provided by the automatic application of Algorithm 2 and AIFA criteria is likely due to the test’s low sensitivity and to the aforementioned difficulties in obtaining information on the clinical and biochemical data of the patient’s family;
both algorithms guarantee high specificity, indeed they exclude almost all true negatives, whereas a high sensitivity in the identification of true positives was only shown for ‘certain’ cases using Algorithm 2, while the sensitivity remains low for all other situations. This means that, with the exception of a few certain cases, in all other cases, there is a high possibility of identifying cases that are merely ‘suspected’ and therefore require further tests to confirm the diagnosis.
Epidemiology of heterozygous familial hypercholesterolaemia in Italy
HeFH is a frequent genetic cause of premature CHD, i.e. myocardial infarction and angina pectoris, due to lifelong exposure to high C-LDL levels.105,106 If not treated, men and women with HeFH with cholesterol levels of 8–15 mmol/L (310–580 mg/dL) develop CHD before 55 and 60 years of age, respectively; whereas those with the homozygous form, with cholesterol levels of 12–30 mmol/L (460–1160 mg/dL) develop CHD at a lower age and, unless treated, die before the age of 20 (60%). However, once they have been identified, patients with the heterozygous form can be efficaciously treated with cholesterol-lowering agents, attenuating the development of atherosclerosis and preventing CHD. Indeed, if the HeFH (usually indicated using simply FH) is diagnosed relatively early in life and the patients are treated efficaciously with statins, their risk of myocardial infarction comes close to that of the general population.107
Among the Caucasian population, the prevalence of HeFHis is estimated to be 1/500 and of HoFH 1/1 000 000105,106; however, in most countries these individuals are not identified.108 Therefore, this theoretically estimated prevalence is likely to be underestimated, as it is based on the prevalence rates in hospitalized patient and disease-register samples, furthermore, it is biased by premature death of patients with FH. Indeed, many individuals and families with FH are not identified and are consequently underdiagnosed and therefore under-treated.109 Recent data indicate a prevalence of 1/250 for HeFH and 1/160 00–-1/360 000 for HoFH.110–112
The degree of underestimation and under-treatment of individuals with FH in the general population is largely unknown. The recent OEC/HES survey 2008–20127 (see Section 1 for a description of the OEC/HES) listed the prevalence of the different plasmatic levels of LDL in the population examined (35–79 years). LDL cholesterolaemia was calculated using Friedewald’s formula and excluding subjects with triglyceridaemia >400 mg/dL on a serum sample collected after 12 h fasting and processed by the same laboratory. Sixty men and 10 women, corresponding to 0.08% of the total sample, were excluded from the statistical analysis due to excessively high values of TGs. Table 10 indicates prevalence according to the LDL value classes. Twenty-five per cent of men and 27% of women were seen to have high C-LDL values (≥155 mg/dL).
Table 10.
OEC/HES Osservatorio Epidemiologico Cardiovascolare/Health Examination 2008–2012
| LDL (mg/dL) | Men (n = 4555) |
Women (n = 4556) |
||
|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |
| ≤70 | 5.0 | 4.3–5.6 | 3.1 | 2.6–3.6 |
| 71–100 | 16.4 | 15.3–17.5 | 15.7 | 14.6–16.7 |
| 101–114 | 12.5 | 11.5–13.5 | 13.0 | 12.1–14.0 |
| 115–130 | 16.7 | 15.6–17.8 | 17.3 | 16.2–18.4 |
| 131–154 | 23.4 | 22.2–24.7 | 23.4 | 22.2–24.7 |
| 155–190 | 19.2 | 18.0–20.4 | 20.2 | 19.1–21.4 |
| 191–250 | 6.7 | 6.0–7.4 | 6.7 | 6.0–7.5 |
| 251–325 | 0.2 | 0.1–0.3 | 0.4 | 0.2–0.6 |
| >325 | 0.0 | — | 0.02 | 0.00–0.1 |
| ≥155 | 26.1 | 24.8–27.4 | 27.4 | 26.1–28.7 |
Prevalence of people with different LDL cholesterolaemia levels.
CI, confidence interval.
In the same population sample, a family history of coronary events (angina pectoris, myocardial infarction, coronary artery bypass grafting, or angioplasty) at a young age (<55 years) in first-degree relatives (parents, siblings, or offspring) was reported in 8.2% (95% CI 7.4–9.0) of men and 10.7 (95% CI 9.8–11.6) of women. The average C-LDL value in those who had a family history positive for premature CHD was 130 mg/dL in men and 130 mg/dL in women and was not significantly different to that found in those without a positive family history (131 mg/dL in men and 134 mg/dL in women, respectively). If the diagnosis of HeFH depended on the contemporaneous presence of three conditions: a family history of premature CHD, a declared family history of hypercholesterolaemia, and/or hypertriglyceridaemia in first-degree relatives and a LDL value >190 mg/dL, the HeFH prevalence dropped to 3.2% in men (95% CI 2.7–3.7) and 3.6% (95% CI 3.1–4.1) in women.
We therefore applied the DLCN criteria for the diagnosis of familial dyslipidaemia (as indicated in Section 3 regarding the Health Search data); however, in our analysis, we could not use the presence of tendon xanthomas and arcus senilis as diagnostic criteria because these data were not available in our study. Of the 70 people excluded for superseding LDL cholesterolaemia values, 6 men and 1 woman had a family history of a premature coronary event. The results of our analysis are provided in Table 11. The sample of the Italian population between 35 and 79 years analysed in this study included 16 734 434 men and 17 856 380 women (ISTAT 2010 census).
Table 11.
OEC/HES Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey 2008–2012: men and women aged 35–79 years
| Men (n = 4555) |
Women (n = 4556) |
|||
|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |
| LDL >190 mg/dL and: | ||||
| Family history of CHD and family history of hypercholesterolaemia/hypertriglyceridaemia | 3.2 | 2.7–3.7 | 3.6 | 3.1–4.1 |
| Familial hypercholesterolaemia—DLCN score | ||||
| <3 unlikely diagnosis | 93.3 | 92.6-94.0 | 92.9 | 92.2–93.7 |
| 3–5 possible diagnosis | 6.5 | 5.8–7.3 | 6.8 | 6.1–7.5 |
| 6–8 probable diagnosis | 0.2 | 0.0–0.3 | 0.2 | 0.1–0.4 |
| >8 certain diagnosis | 0.0 | — | 0.02 | 0.00–0.1 |
Familial dyslipidaemia. The two criteria for FH diagnosis reported are: first criterion (LDL >190 mg/dL associated with a family history of premature coronary heart disease (<55 years), or family history of hypercholesterolaemia; second criterion: Dutch Lipid Clinic Network score >6 (probable diagnosis and certain diagnosis).
CHD, coronary heart disease; DLCN, Dutch Lipid Clinic Network.
The different number obtained from the OEC/HES and Health Search estimates can be attributed to the difference in the age of the sample considered (the Health Search contains data for over 15 years) and the difficulties in applying comparable standardized measurements and criteria.
Clinical criteria for screening
FH screening in children, adults, and families is recommended according to the following criteria:
– presence of FH among family members;
–plasma cholesterol ≥8 mmol/L (≥310 mg/dL) in an adult subject or in an adult family member (or > 95° age- and sex-specific percentile);
– plasma cholesterol ≥6 mmol/L (≥230 mg/dL) in a child or in a child in the family (or > 95° age- and sex-specific percentile);
–premature CHD in the subject or a member of the family;
– tendon xanthomas in the subject or a member of the family; and
– premature sudden cardiac death in a member of the family.
There is a higher likelihood of diagnosing FH when there are very high levels of C-LDL, tendon xanthomas, and/or premature CHD in a member of the family. It is essential to draw up a family tree in order to evaluate the probability of FH as indicated in Table 5. In the presence of probable or certain FH, C-LDL tests must be carried out in the family and the subject. If available, a genetic test should be performed, with subsequent cascade tests in the family if a causal mutation is observed. The family members to evaluate are mainly the first-degree biological relatives, i.e. parents, siblings, and offspring. However, second-degree biological relatives should also be considered, namely grandparents, aunts and uncles, nieces and nephews, grandchildren, and half-brothers and half-sisters.
Role of genetic testing
The utility of genetic tests in the diagnosis of HeFH is still a matter for debate. In the vast majority of cases, a genetic diagnosis is not believed to be useful unless in cases of uncertain clinical diagnosis. The genetic variability of FH (approximately 1700 mutations have been observed113) means that the systematic application of genetic analysis is still not cost-effective, despite the fact that recent developments have allowed savings in terms of time and costs. In a certain proportion of cases, a polygenic aetiology underlying the FH clinical phenotype has been observed.114 Furthermore, since one of the main determinants of cardiovascular risk in FH is the biochemical phenotype (high C-LDL levels from birth) and not the genotype, C-LDL level-based screening is considered cost-effective, considering the employment of genetic analyses only in borderline cases.115
It should also be highlighted that there is a stronger agreement between clinical diagnoses using DLCN criteria104and genetic diagnoses when the DLCN score is ≥8 (mutation present in 80% of cases); whereas with a score >5, the mutation was present in 50% of cases.
The panel unanimously decided to adopt the DLCN score for the diagnosis of FH with a cut-off of ≥6, reserving molecular diagnosis for those with a score of <6 only.
FCHL is most likely the result of a combination of different genetic abnormalities. It is primarily caused by an increase in the hepatic synthesis of ApoB, whose plasma levels are constantly high, and with the consequent increase in VLDL secretion by the liver. In patients with HeFH, dyslipidaemia does not usually present before the subject reaches adulthood. It is also known as dyslipidaemia with variable phenotype to reflect the presence of different lipoprotein phenotypes in the same individual and in affected family members.
A diagnosis can be made with the simultaneous presence of:
a dyslipidaemia with a variable phenotype, i.e. a patient that presents with variations in TC and TG levels over time, with the same clinical conditions (thus excluding the diagnosis of pure hypercholesterolaemia or pure hypertriglyceridaemia that have constant plasmatic values over time) and signs of vascular damage (e.g. asymptomatic carotid atherosclerosis) and
at least one family member presenting a dyslipidaemia phenotype that differs from that of the patient (see above) and/or at least one family member with premature cardiovascular events (including peripheral atherosclerotic disease and revascularization procedures).
These criteria are taken into account by AIFA note number 13.
Guidance for laboratory reporting of lipid parameters
Guidance for the reporting of lipid profile parameters
Reporting is the final step in the laboratory data production process and has the purpose, in addition to providing the results of the tests performed, of providing tools for their interpretation. Lab reports consist of three parts: the first is the presentation of the results (type of material analysed, name of the analyte, and unit of measurement)116, the second part regards the comparison system to allow a correct interpretation of the results provided,117 and the third part provides for an interpretative comment to aid the assessment of the data.
Presentation of the results
Table 12 provides an example of a report with parameters that must necessarily be present in a lab report: type of material analysed, name of the analyte, and unit of measurement.
Table 12.
Method for presenting the results of lipid profile tests (example)
| System | Component | Unit of measurement Traditional | Unit of measurement SI unit |
|---|---|---|---|
| S-(serum) | Total cholesterol | 195 mg/dL | 5.05 mmol/L |
| P-(plasma) | |||
| S-(serum) | LDL cholesterol | 100 mg/dL | 2.59 mmol/L |
| P-(plasma) | |||
| S-(serum) | Non-HDL cholesterol | 135 mg/dL | 3.50 mmol/L |
| P-(plasma) | |||
| S-(serum) | HDL cholesterol | 60 mg/dL | 1.55 mmol/L |
| P-(plasma) | |||
| S-(serum) | Triglycerides | 75 mg/dL | 0.84 mmol/L |
| P-(plasma) | |||
| S-(serum) | Apolipoprotein A-I | 150 mg/dL | 1.50 g/L |
| P-(plasma) | |||
| S-(serum) | Apolipoprotein B | 90 mg/dL | 0.90 g/L |
| P-(plasma) |
To convert values expressed in mmol/L into mg/dL, multiply by 38.6 (total, HDL and LDL cholesterol) or by 88.8 (triglycerides).
Heparinized serum or plasma are equivalent materials on which these analytes can be measured. The units of measurement chosen (traditional or S.I.) depend on the cultural and organizational models adopted in the individual laboratories. There are three significant figures (two decimals in the case of SI units and an integer for the traditional units) except for HDL cholesterol, triglycerides and apolipoprotein B (traditional units).
Reference system
The criteria used for the interpretation of the analytic results are:
comparison with normal value ranges,
comparison with clinically significant values (decision-making values),
comparison with previous values for the same subject (critical difference), and
comparison with alert values (panic values or critical values).
Reference values make it possible to compare a measured value with those obtained in a control population, to which the subject belongs.118 It is not advisable to adopt this criterion for the evaluation of a lipid parameter because it would not be possible to estimate the level of risk the individual is subject to.119
The decision-making values, as the name suggests, are those values on the basis of which clinical decisions are made.117 With dyslipidaemias, these values define the entity of the cardiovascular risk associated with a given concentration. They represent a ‘desirable’ value that should be achieved or not exceeded in order to keep cardiovascular risk within acceptable limits. These values have been established by the US120 and European5,8 guidelines and recommendations issued over time, but they are not uniform for all subjects and/or patients as they depend on the clinical characteristics of the individual (primary prevention, presence of co-morbidities such as diabetes, hypertension, etc.) and the presence of other risk factors (family history, smoking, sedentary lifestyle, etc.). The lab that processes the tests and writes the report does not usually have access to this information. Moreover, the most recent US guidelines have removed the C-LDL values as treatment targets.121 In light of this, it is very difficult to indicate decision-making values on the report. Consequently, we believe it appropriate to recommend a simplified method of reporting, based on the desirable values as defined by the European guidelines. It is also appropriate that this reporting method is accompanied by an explanatory note clarifying how the desirable values indicated refer to low/moderate risk subjects/patients and that they should be even lower in very high-risk patients. This approach is in line with the recent recommendations of the European Federation of Clinical Chemistry and Laboratory Medicine/EAS,122 which uses values obtained in epidemiological studies conducted on European populations—therefore with characteristics similar to that of Italy—and suggests a simple, uniform method of reporting that can be adopted nationwide. For the paediatric population, the reference standards available are comprised of the US recommendations.123 The values are presented in Table 13.
Table 13.
Desirable values and corresponding comment to be included in the report according to the European guidelines for adults5,8,122 and according to the indications of the US National Heart, Lung, and Blood Institute for children and adolescents123
| Desirable value | Adults |
Children and adolescents |
||||
|---|---|---|---|---|---|---|
| mg/dL | mmol/L | g/L | mg/dL | mmol/L | g/L | |
| Total cholesterol | ≤190 | ≤5.00 | ≤170 | ≤4.40 | ||
| LDL cholesterol | ≤115 | ≤3.00 | ≤110 | ≤2.85 | ||
| Non-HDL cholesterol | ≤145 | ≤3.80 | ≤120 | ≤3.10 | ||
| HDL cholesterol | ≥45 | ≥1.15 | ||||
| Males | ≥40 | ≥1.00 | ||||
| Females | ≥45 | ≥1.20 | ||||
| Triglycerides | ≤150 | ≤1.70 | ≤75 | |||
| 0–9 years | ≤90 | ≤0.84 | ||||
| 10–19 years | ≤1.01 | |||||
| Apolipoprotein A-I | ≥125 | ≥1.25 | ≥120 | ≥1.20 | ||
| Apolipoprotein B | ≤100 | ≤1.00 | ≤90 | ≤0.90 | ||
The desirable values indicated refer to subjects at low/moderate cardiovascular risk. For subjects at high or very high risk, the desirable values should be lower.
In the same subject, the comparison with the previous values measured serves to check whether the therapeutic goal is achieved due to pharmaceutical intervention or lifestyle changes.117 Only if the difference between the observed value and the previous value exceeds a critical value124 could the two values be considered to differ from one another (with a 95% probability). This reporting method is usually used for tumour markers, with advantages for the clinical management of patients125; however, it has not been confirmed whether this approach is useful also with lipid parameters. Consequently, the decision to include the critical difference values in the report is left to the individual laboratory. In this sense, it would be preferable for the report to include at least the two previous results.
Critical values are unexpected results that should be promptly brought to the clinician’s attention because they require an immediate intervention.126 They are usually used for lab tests (e.g. such as glucose, potassium, haemoglobin, and cardiac troponins), for which considerable variations may constitute an immediate threat to the patient’s health. In dyslipidaemia, the concept of rapid notification may apply to TC and C-LDL values that are indicative of familial hypercholesterolaemia [in adults ≥310 mg/dL (≥8.00 mmol/L) and ≥190 mg/dL (≥4.90 mmol/L), respectively]5,112,127 and TG values indicative of a risk of acute pancreatitis ≥880 mg/dL (≥10.0 mmol/L).75 In the paediatric age, a value of TC ≥230 mg/dL (6.00 mmol/L) requires further evaluation. These values must be indicated on the report in an appropriate manner, with a specific note, if necessary, and, if possible, the treating clinician should be informed.
Reporting
The Panel suggests expressing lipid parameter values as indicated in Table 12.
As a comparison system, it has been suggested to use the decision-making values, represented by the desirable values defined in the European guidelines and presented in Table 13. These values should be accompanied by the explanatory comment indicated in the table. It should be clearly indicated in the report that the reference is constituted by decision-making values rather than reference values.
We suggest that TC and C-LDL values indicative of FH as well as TG values associated with a risk of acute pancreatitis are highlighted in the report, accompanied by an explanatory note and that the clinician is promptly informed. Examples of accompanying notes:
TC ≥310 mg/dL (≥8.00 mmol/L): value requiring clinical evaluation for FH.
C-LDL ≥190 mg/dL (≥4.90 mmol/L): value requiring clinical evaluation for FH.
TG ≥880 mg/dL (≥10.0 mmol/L): value requiring clinical assessment due to a possible risk of acute pancreatitis.
The therapeutic target for C-LDL depends on the individual cardiovascular risk profile, information that can be obtained from the patient’s physician care giver.
Unmet clinical needs in the management of hypercholesterolaemia
Statin intolerance
In clinical practice, we refer to SI in those cases in which the onset of clinically significant side effects and/or relevant adverse reactions require treatment discontinuation. However, to date, there is no univocal and universally accepted definition of this phenomenon in Italy. This issue is particularly important, given its clinical and regulatory implications, especially in view of the introduction of new, non-statin lipid-lowering agents (PCSK9 inhibitors) that could be useful in the presence of SI. The occurrence of adverse events during statin treatment may require posology changes (dose and/or a reduction in the frequency of administration) or treatment discontinuation either temporarily or permanently.
SI could be defined as a condition in which, during therapy with statins, the patient experiences unacceptable symptoms and/or lab parameter alterations, suggesting the possibility of a significant clinical risk. Both symptoms and lab test alterations must be reversible and indisputably associated with statin therapy. The occurrence of these adverse events may require treatment interruption.128 In the vast majority of cases, the SI condition is characterized by the patient’s perception of the impossibility of continuing therapy due to the presence of disabling symptoms, whereas cases in which SI is associated with asymptomatic alterations in lab parameters are less common. In a non-negligible percentage of cases, the clinician discontinues the drug due to an abnormal perception of the clinical risk associated with treatment, even in the absence of significant clinical issues.129 The correct identification of a true condition of SI is particularly important in order to avoid inappropriate interruptions in treatment. Indeed, statin discontinuation can expose patients to the risk of adverse cardiovascular events.129–131
International definitions
In recent years, a number of different scientific and professional associations have attempted to define the characteristics of the clinical condition known as SI. Several possible definitions for this complex phenomenon have been proposed.132,133 More specifically, in 2013, the Canadian Working Group Consensus Conference on Diagnosis, Prevention and Management of Statin Adverse Effects and Intolerance published an article on the SI issue.132 The Canadian group suggested defining SI as a clinical syndrome characterized by:
the inability to use statins to reduce C-LDL and cardiovascular risk due to the presence of symptoms and/or lab test alterations that can be temporally associated with the start of statin treatment or the increase in the dose. The relationship between statins and disorders should be confirmed by the interruption and subsequent reintroduction of the treatment (rechallenge intervention);
SI can be either complete (intolerance to any statin and at any dose) or partial (intolerance to certain statins at certain doses); and
SI is not associated with modifiable clinical conditions (hypothyroidism, drug-drug interactions and intercurrent conditions).
In 2014, the US National Lipid Association (NLA) published a document about SI.133 The NLA suggests identifying SI as a set of symptoms, signs, and lab test alterations that the patient and/or doctor attribute to statin treatment. The patient finds these disorders invalidating as they interfere in an unacceptable way with normal daily activities and require a treatment interruption or dose reduction. In some cases, the decision to interrupt or reduce the dose of the medication may be made by the doctor due to the onset of asymptomatic blood test alterations [elevation in creatine kinase (CK) or transaminase values], suggesting the presence of a significant risk of adverse events. The NLA highlights the need for an in-depth assessment of each individual case that should take into account all the aspects of communication between the doctor and patient (with a ‘patient-centred approach’), thereby avoiding inappropriate interruptions for symptoms that are not actually related to statin toxicity. The NLA also suggests an operative definition of SI:
Inability to tolerate at least 2 statins: one at the lowest starting daily dose AND another at any daily dose, due to either objectionable symptoms or abnormal lab determinations which are temporally related to statin treatment and reversible when the statin is discontinued, but reproducible by re- challenge. Any modifiable possible cause of SI should be excluded (hypothyroidism, drug-drug interactions, intercurrent conditions, intense physical exercise, underlying muscle disease). More specifically, the lowest starting doses for statins are: rosuvastatin 5 mg per day, simvastatin 10 mg per day, atorvastatin 10 mg per day, lovastatin 20 mg per day, pravastatin 40 mg per day and fluvastatin 40 mg per day.
Lastly, despite avoiding terms such as ‘intolerance’, the EAS recently published an in-depth review containing a classification of the adverse events affecting the skeletal muscles that could be associated with statin therapy.134 The EAS experts focus their attention on the potential muscle disorders caused by statin therapy, making a detailed distinction between the various possible forms (Table 14).
Table 14.
Definition of the muscle symptoms associated with statin therapy proposed by the Consensus Panel of the European Atherosclerosis Society134
| Symptoms | Biomarker | Comment |
|---|---|---|
| Muscle pain, muscle weakness, cramps | Normal CK values | Commonly defined as ‘myalgia’; causal relationship with statin therapy not always certain and obvious. Clinical investigation necessary. |
| Muscle pain, muscle weakness, cramps | Increase in CK < 4× ULN | The appearance of symptoms associated with a modest elevation in CK values can usually be attributed to exercise. Clinical investigation is necessary (i.e. thyroid function tests) with redefinition of the cardiovascular risk profile. Possible interruption of statin therapy. |
| Muscle pain, muscle weakness, cramps | Increase in CK 4–10× ULN | Condition of greater clinical importance, associated with greater risk of significant muscle problems. Interruption of statin therapy appropriate. |
| Muscle pain, muscle weakness, cramps | Increase in CK > 10× ULN | Referred to as ‘myopathy’ or ‘myositis’ by international regulatory authorities. Incidence of 1 in every 10,000 treated patients/year. Intense proximal muscle pain with loss of strength. Significant pre-existing muscle condition often present. Statin therapy must be discontinued. |
| Muscle pain, muscle weakness, cramps | Increase in CK > 40× ULN | Known as ‘rhabdomyolysis’ if accompanied by a reduction in renal function and/or myoglobinuria. |
| None | Increase in CK < 4× ULN | Incidental finding of CK elevation in patient on statin therapy. The assessment of thyroid function and of the relationship with exercise are appropriate. |
| None | Increase in CK > 4× ULN | Situation of uncertain clinical significance, requiring repeated tests and in-depth clinical assessment. |
CK, creatine kinase; ULN, upper limit of normal range.
Operative synopsis for clinical practice
Considering the challenges, in many cases, of achieving a diagnosis of a true SI, a simplified approach aimed at the identification and characterization of this condition can be of great help in clinical practice. The Panel believes that the suspicion of SI should be based on the characteristics of muscle symptoms, the presence of an increase in CK and/or transaminases, and temporal association with the use of statins, their interruption and treatment reintroduction (rechallenge).
More specifically, in the SI document proposed by ANMCO (see ‘The proposals in ANMCO consensus documents’ section subsequently), the following definitions are suggested:
‘statin-associated muscle symptoms’: the patient presents clinically relevant muscle symptoms that may or may not be associated with a significant increase in CK and that regress after statin discontinuation but recur after a ‘rechallenge’;
‘statin-related liver damage’: the patient presents a significant increase in transaminases (>3 times the upper limit of normal range) that regress after statin therapy discontinuation and recur after ‘rechallenge’;
‘complete intolerance’ signifies that the adverse reactions occur with all statins at any dose; and
‘incomplete intolerance’ indicates that the patient is able to tolerate low doses of any statin.
Failure to meet therapeutic goals: the lower, the better; the sooner, the better
The large-scale epidemiological studies conducted since the 1960s (Seven Countries Study, Framingham Heart Study, MRFIT) reported the existence of a direct relationship between plasma cholesterol levels and the incidence of clinical presentations of atherosclerotic cardiovascular disease. Overall, the clinical and epidemiological evidence indicates the presence of a linear relationship between cardiovascular morbidity and cholesterolaemia. The number of adverse cardiovascular events grows progressively with an increase in plasma cholesterol values. In the MRFIT study, e.g. for every 20 mg increase in the total cholesterolaemia values, there is an increase of approximately two deaths for ischaemic cardiomyopathy per 1000 inhabitants over a 6-year observational period.118 The most recent large-scale studies on pharmacological intervention with statins completed over the past 25 years confirmed the observational evidence previously reported.25 The introduction of these pharmacological agents into clinical practice significantly revolutionized the approach to patients with high cardiovascular risk in both primary and secondary prevention. Indeed, in all clinical studies, the reduction in cholesterolaemia values obtained with statins is associated with a significant reduction in cardiovascular morbidity.25 More specifically, an approximately 40 mg/dL reduction in C-LDL corresponds to an approximately 25% reduction in the relative risk of ischaemic cardiovascular events (Figure 7).
Figure 7.
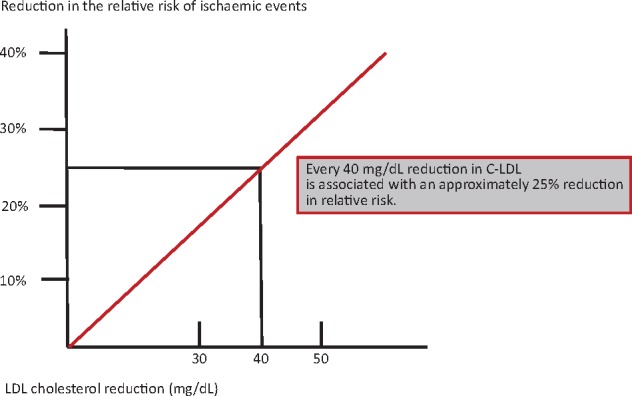
Relationship between reduction in LDL cholesterol (C-LDL) levels and the reduction in the relative risk of ischaemic cardiovascular events. Modified from Baigent et al.25
Large-scale studies also made it possible to define the optimal C-LDL levels to achieve in each patient in order to reduce cardiovascular adverse events in a clinically relevant manner. Indeed, in patients with very high cardiovascular risk, such as in cases of a prior myocardial infarction, C-LDL values must be reduced to below 70 mg/dL. This therapeutic target is recommended both in the cardiovascular prevention guidelines issued by the ESC8 and in the most recent AIFA note (Table 15).
Table 15.
Risk categories in AIFA note number 13 and in ESC guidelines of various clinical types of patients and related treatment targets
| Clinical type | AIFA circular number 13 |
ESC guidelines |
||
|---|---|---|---|---|
| Risk level | Treatment target | Risk level | Treatment target | |
| C-LDL | C-LDL | |||
| Diabetes mellitus | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% |
| Type 1 and type 2 with other risk factors or organ damage | ||||
| Diabetes mellitus | High | <100 mg/dL | High | <100 mg/dL |
| Type 1 and type 2 without other risk factors or organ damage | ||||
| Ischaemic cardiomyopathy | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% |
| Prior infarction, prior coronary artery bypass grafting, prior PCI | ||||
| Prior stroke, prior TIA | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% |
| Peripheral occlusive arterial disease, aortic aneurysm | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% |
| Severe chronic kidney disease (eGFR<30 mL/min) | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% | Very high | <70 mg/dL and/or reduction in C-LDL of ≥ 50% |
| Moderate chronic kidney disease (eGFR<60 mL/min) | High | <100 mg/dL | High | <100 mg/dL |
AIFA, Agenzia Italiana del Farmaco (Italian Medicines Agency); C-LDL, LDL cholesterol; eGFR, estimated glomerular filtration rate; ESC, European Society of Cardiology; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
Even the last version of AIFA note number 13, published in July 2014, recommends reaching and maintaining C-LDL values <70 mg/dL in subjects with very high cardiovascular risk and at least <100 mg/dL in high-risk subjects.93 In addition, AIFA note number 13 also indicates that in order to maintain therapeutic appropriateness without unnecessarily using limited NHS resources, lipid-lowering pharmacological interventions must achieve and maintain the lipid targets established. Only in this way will it be possible to effectively reduce cardiovascular events in the populations at greatest risk.
In light of the points summarized above, it is clear that C-LDL reduction is a fundamental priority in the management of patients with high or very high cardiovascular risk. The lipid targets indicated in the guidelines and in AIFA note number 13 should be reached and maintained over time. However, statins alone are not always adequate. Indeed, these drugs do not make it possible to achieve the therapeutic targets in all patients, especially when it is necessary to reduce C-LDL under 70 mg/dL. More specifically, patients with initial C-LDL values >150 mg/dL will have difficulties in achieving the recommended target, even if the highest efficacy statins are used. Indeed, only the highest doses of atorvastatin (80 mg) and rosuvastatin (20–40 mg) make it possible to achieve a 50% reduction in C-LDL values.8 In addition, the use of high doses of statins can cause the onset of significant side effects and adverse reactions. More specifically, available data135,136 indicate that just 60–70% of patients with a high cardiovascular risk achieve the lipid targets set by the guidelines, despite being correctly treated with highly efficacious statins and complying with their treatment prescriptions.
In recent years, ezetimibe, a new non-statin molecule that is effective in reducing plasma levels of C-LDL, has become available.137 This drug is a selective inhibitor of the intestinal absorption of cholesterol. When used alone, ezetimibe reduces C-LDL by 5–22%. However, when combined with a statin, it favours a further 15–20% reduction in C-LDL levels.138 In addition, the recent IMPROVE-IT trial27 has shown that combining ezetimibe with a statin is associated not only with a greater reduction in C-LDL values but also with a significant improvement in clinical prognosis. Precisely because of these results, a recent consensus document issued by the American College of Cardiology (ACC) proposes ezetimibe as first-choice therapy in patients who are unable to achieve a reduction in C-LDL values adequate to their level of cardiovascular risk, despite statin therapy.139
Non-compliance with treatment prescriptions
Over the past 20 years, several clinical studies have shown a widespread underutilization of the pharmacological treatments recommended by international guidelines for the treatment of cardiovascular diseases.140–142 This obvious lack of intervention results in a failure to achieve the therapeutic targets recommended by international guidelines and is caused by a combination of factors associated with the complex operation of health care systems, as well as the behaviour of individual patients.140–142 In current clinical practice, the clinical management of the main cardiovascular risk factors appear to be inadequate because of the inertia of physicians. This inertia can be characterized by one or more of the following143:
The failure to prescribe treatments recommended for the control of risk factors (antihypertensive and lipid-lowering therapy).
The prescription of lower and potentially inadequate doses of the various pharmacological agents.
The absence of adequate intervention and effective therapeutic modifications in cases of failure to achieve therapeutic targets recommended by guidelines.
The lack of adherence to therapeutic prescriptions has now become a proper ‘additional occult risk factor’.144 Indeed, preventive interventions reach their favourable effects over a period of time that is significantly longer than acute-phase therapies, and it is therefore necessary for patients to follow medical prescriptions in a continuous manner and for drugs to be taken at the doses shown to be efficacious in clinical studies. Patients are considered to be ‘treatment compliant’ when they take more than 80% of the medication prescribed, ‘partially compliant’ if they take between 20% and 70%, and ‘non-compliant’ if they take less than 20%.
Extent of the phenomenon
Non-compliance is a widespread phenomenon among patients with cardiovascular risk factors or cardiovascular disease. This phenomenon is estimated to involve 50–60% of patients in primary cardiovascular prevention and 30–40% of those in secondary prevention.145–147
Studies conducted in the US and Canada suggest that lipid-lowering treatment with statins is interrupted in 30–40% of cases within 6 months of the first prescription.147 In the Dutch PHARMO database, of the approximately 60 000 patients who had been prescribed a statin over a 13-year period, more than 50% interrupted the drug intake within 2 years from the start of treatment.148 Similar data have also been recorded in Italy129,149.
In clinical studies, the interruption of treatment or intermittent use are caused by several factors, which can be divided into five categories (Table 16).
Table 16.
Factors associated with non-adherence to therapeutic prescriptions
| Patient related | Related to the clinical condition | Therapy related | Health Service related | Related to socio- economic system |
|---|---|---|---|---|
|
|
|
|
|
Clinical assessment
In clinical practice, treatment adherence is usually assessed by direct patient interviews, they are asked what drugs they are taking and how. This assessment is highly subjective and significantly conditioned by the quality of the doctor–patient relationship, with a possible 20–30% overestimation of the drug actual use.144 A direct question cannot provide accurate assessments, especially if a closed answer is expected (‘do you always take your medication as it was prescribed?’). On the contrary, however, problems of non-compliance can be better identified using directly administered questionnaires, such as the Morisky scale (Table 17).150
Table 17.
Morisky Medication Adherence Scale
| Morisky scale |
|---|
|
|
Interventions to improve therapeutic adherence
Different types of interventions have been proposed in order to improve patient adherence (Table 18). Interventions to improve compliance can be classified into four types:
Table 18.
Intervention intended to improve prescription adherence
| Changes to therapeutic prescriptions | Training initiatives | Behavioural intervention | ‘Complex’ intervention |
|---|---|---|---|
|
|
|
|
interventions regarding prescription, with simplification and modification of drug posology;
programmes aimed at informing and educating the patient;
initiatives aimed at changing patients’ individual behaviour; and
‘complex and combined’ interventions split into different levels and implemented through combined multidisciplinary approaches.
Overall, the quality of communication between health care staff (doctors and nurses) and patients represents the most important element in conditioning effective treatment adherence. Only clinical meetings of an appropriate duration and frequent follow-up appear successful in improving adherence.144
The proposals in ANMCO consensus documents
Diagnostic-therapeutic pathways in patients with statin-induced myalgia
In the event that a patient complains of muscle symptoms during statin treatment, it is necessary to first of all check the CK levels (Figure 8). In general, it is always advisable to assess the CK values before starting treatment with any statin.
Figure 8.
Diagnostic and therapeutic pathways in patients with statin-induced myopathy. ULN, upper limit of normal.
If high CK levels are found (CK values in the rhabdomyolysis range), it is necessary to withhold drug intake, carefully monitor renal function, and, if needed, to arrange hospitalization. When CK values are >5 times higher than the normal upper limit, it is recommended to stop the drug and evaluate the presence of factors that increase myopathy/myalgia risk. More specifically, it is necessary to exclude the presence of hypothyroidism, rheumatic polymyalgia, osteo-articular disease, or recent intense physical activity. If there are not secondary causes, a rechallenge, using the same statin previously used or a different one based on the pharmacokinetic characteristics, is encouraged. In the event that muscle symptoms recur (regardless of whether or not they are associated with CK increase), the presence of an SI can be considered confirmed.
If CK levels are <5 times normal values, but the patient considers the myalgia symptoms to be intolerable, the statin may be discontinued. Once the symptoms have disappeared, a ‘rechallenge’ must be performed by re-prescribing a statin (either one that has already been used, or a different one, depending on pharmacokinetic characteristics). If the muscle symptoms (with or without CK elevation) reappear, the presence of intolerance can be considered confirmed.
The management of patients with ‘confirmed SI’ should contemplate:
a further attempt at prescribing another statin, different from that/those initially used (hydrophilic vs. lipophilic molecules) and/or with different metabolism (CYP3A4 or CYP2C9), starting with a minimum dosage then increasing the dose until the optimal dose is achieved;
prescription of a low statin dose combined with ezetimibe (intestinal cholesterol absorption inhibitor);
prescription of statins with longer half-life (atorvastatin and rosuvastatin) administered on alternate days or every 2 days at low/minimum dosages; and
ezetimibe prescription as monotherapy or combined with nutraceuticals, based on the target of C-LDL reduction.
The sequence of these interventions must take into account the relative efficacy of single options. Indeed, using ezetimibe or nutraceutical allows only a modest C-LDL reduction when compared with statin treatment. In conclusion, an additional possibility is offered by the new non-statin lipid-lowering agents, particularly the PCSK9 inhibitors.
Diagnostic–therapeutic pathways in patients with statin-induced liver injury
It should be take into account that an increase of transaminases values <3 times the normal upper limit is not a contraindication to statin therapy (Figure 9). Several patients with diabetes, metabolic syndrome, or obesity have transaminases values fluctuating around 1–3 times the normal upper limit values151 because of a non-alcoholic steatohepatitis. In the case that an increase >3 times the normal value occurs during statin treatment, it is recommended to discontinue the drug. There is not a consensus on when the best time is to recheck transaminases values. In some clinical trials with statins, hepatic function tests were rechecked after 2–3 weeks, and in 70% of these cases the values had normalized. Some authors suggest repeating the tests after 6 weeks.152
Figure 9.
Diagnostic and therapeutic pathways in patients with statin-induced liver injury. ULN, upper limit of normal.
After verifying the absence of other factors that could be responsible for transaminase increases, a rechallenge should be considered in which the new intake of a statin is prescribed (either the same previously used or a different one based on pharmacokinetic characteristics). If a transaminase increase reoccurs, different options should be contemplated. Since a greater incidence of hepatic abnormalities occurs when higher statin doses are used, it may be more appropriate to prescribe low doses or non-daily dosing regimens. Transaminase levels should be checked monthly during the first 3–4 months and then every 3 months thereafter. In addition, the use of statins not metabolized in the liver (rosuvastatin or pravastatin) or non-statin compounds may also be considered.
New drugs for the treatment of dyslipidaemia
Introduction
Aimed at optimizing the treatment of patients at a high risk of cardiovascular disease, several new lipid-lowering drugs are in ongoing development and in various clinical trial phases. The need for new therapeutic strategies is motivated by the fact that the therapies currently available for the treatment of dyslipidaemia, including statins, which represent the milestone in hypercholesterolaemia treatment and in the primary and secondary prevention of atherosclerotic disease, do not make it possible to achieve the target lipid level in all patients. Indeed, just 20% of patients with FH treated with statins reach target C-LDL levels. Furthermore, there is a subgroup of patients who are intolerant to high-dose statins due to the occurrence of adverse effects, especially myotoxicity and hepatotoxicity.153,154
In light of the limits of current dyslipidaemia treatment, the introduction of new lipid-lowering therapies, such as TG microsomal transfer protein (MTP) inhibitors, antisense oligonucleotides directed against ApoB, ApoA1 mimetic peptides, cholesterol ester transfer protein (CETP) inhibitors and PCSK9 inhibitors, may represent an important complement or alternative to statin therapy in reducing C-LDL.154,155
Drugs for the treatment of severe genetic dyslipidaemia
MTP, which is expressed primarily by hepatocytes and enterocytes, plays a key role in the synthesis of the lipoproteins containing ApoB. MTP is responsible for the transfer of TG, phospholipids, and cholesterol esters to ApoB in the endoplasmic reticulum. MTP inhibition causes a reduced synthesis and secretion of VLDL in the liver and a reduction in overall plasma TG levels. Lomitapide, an oral MTP inhibitor, was approved by the Food and Drug Administration (FDA) in December 2012 for the treatment of patients with HoFH. Figure 10 shows the mechanism of action of lomitapide.154 In preclinical studies, lomitapide caused a dose-dependent decrease in VLDL and C-LDL values in the range of 19–89% and a decrease in TG in the range of 8–49%.156,157 The effects of lomitapide in humans were evaluated in a Phase 3 clinical study that enrolled 29 HoFH patients with mean baseline values of C-LDL equal to 336 mg/dL. The drug caused a 50% reduction in C-LDL after 26 weeks of treatment and a reduction of 38% after 52 weeks.158 Regarding the tolerability profile, lomitapide seems to be associated mainly with gastrointestinal adverse events (as diarrhoea, nausea, and abdominal pain).154
Figure 10.
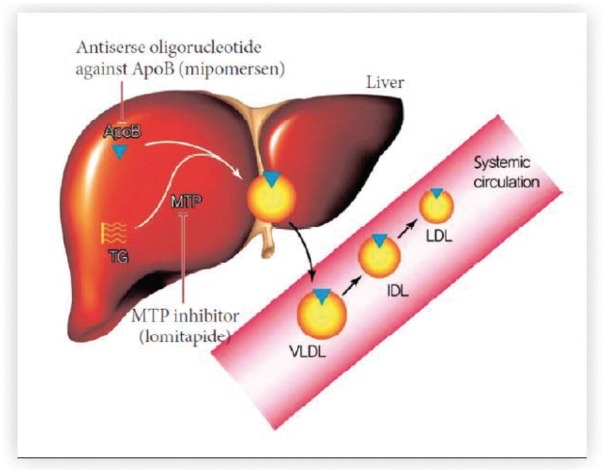
Mechanism of action of lomitapide and mipomersen. Lomitapide blocks the synthesis of lipoproteins containing apolipoprotein B (ApoB) by inhibiting the triglyceride microsomal transfer protein (MTP), the protein responsible for the transfer of triglycerides (TGs), phospholipids, and cholesterol esters to the ApoB in the endoplasmic reticulum; mipomersen is an antisense oligonucleotide that reduces the hepatic secretion of VLDL by binding messenger RNA encoding ApoB and preventing its transcription. Reproduced from Ahn and Choi.154
The antisense oligonucleotides directed against ApoB are another emerging group of lipid-lowering agents. The ApoB is the most important structural protein of the atherogenic lipid particles and plays a key role in the assembly and secretion of VLDL by the liver.159 Mipomersen is a second-generation antisense oligonucleotide constituting of 20–22 bases that targets messenger RNA (mRNA) encoding ApoB, thereby preventing mRNA transcription. Figure 10 shows the mechanism of action of mipomersen.154 This medicinal product, administered in weekly subcutaneous doses of 200 mg, was approved by the FDA in January 2013 as an additional lipid-lowering treatment in patients already on treatment for HoFH. Many preclinical studies have evaluated the effects of mipomersen in several animal species, showing that this drug reduces, in a dose-dependent manner, hepatic mRNA ApoB-100, plasma concentrations of ApoB, C-LDL, and TC levels.160 In patients with mild dyslipidaemia, the administration of mipomersen for 12 weeks at a dose of 50–400 mg every 3 weeks caused a dose-dependent reduction of ApoB and C-LDL of 50% and 35%, respectively.161 Other Phase 3 clinical studies have also confirmed the beneficial effects of mipomersen.162 With regard to mipomersen tolerability, this drug is often associated with the occurrence of injection site reactions, ‘flu-like’ reactions and hepatic enzyme elevation. Since the therapeutic indication of lomitapide and mipomersen is the HoFH, a rare hereditary form that affects approximately 1/1 000 000 patients in the USA, these medicinal products have been authorized as orphan drugs.
Another novel class of lipid-lowering drugs are the ApoA1 mimetic peptides. ApoA1 is one of the main structural apolipoproteins present in mature HDL. ApoA1 picks up cholesterol from the macrophages of atherosclerotic lesions through the adenosine triphosphate-binding cassette transporter A1 (ABCA1), a membrane cholesterol transporter. ApoA1 mimetic peptides imitate the effects of ApoA1 and C-HDL and favour the regression of atherosclerosis.163,164 ApoA1 mimetic peptides include ApoA1 Milano, known as ETC-216, which is obtained by combining mutant HDL and phospholipids. Clinical data on the efficacy of ETC-216 in reducing the volume of the atheroma remain controversial.165,166
A further therapeutic alternative in lipid-lowering treatment is eprotirom, a thyroid hormone analogue with minimal extrahepatic uptake that has been shown to reduce C-LDL plasma concentration in Phase 1 and 2 clinical studies. The long-term efficacy and safety of eprotirom, administered at doses of 50–100 μg/day, have recently been evaluated in a Phase 3 clinical study. The Efficacy and Safety Study of Eprotirome in HeFH Patients Who Are on Optimal Standard of Care (AKKA) study included 236 patents with HeFH who did not reach target C-LDL values after 8 weeks’ statin treatment with or without ezetimibe. This study showed that the mean plasma concentrations of C-LDL increased by 9% in the group of patients treated with placebo, decreased by 12% in the group of patients receiving eprotirom 50 μg, and decreased by 22% in the group of patients treated with eprotirom 100 μg. However, although it is efficacious in reducing C-LDL, this drug may cause liver damage,167 as its employment was associated with a significant increase in alanine transaminase, aspartate transaminase and gamma-glutamyl transferase. Because Phases 2 and 3 clinical studies showed an unacceptable risk/benefit ratio, the development of this drug has been discontinued.
Lastly, other therapeutic strategies for reducing the risk of cardiovascular events include CETP inhibitors, such as torcetrapib, dalcetrapib, anacetrapib, and evacetrapib. CETP is a plasma protein that promotes the transfer of the cholesteryl ester from C-HDL to VLDL or LDL.168 CETP inhibition is thought to increase the concentration of C-HDL, thereby reducing the risk of CHD.169 However, the data obtained from clinical studies do not confirm the efficacy of these products in reducing cardiovascular risk, with the exception of anacetrapib, which is currently being examined in the Phase 3 Randomized EValuation of the Effects of Anacetrapib Through Lipid-modification (REVEAL) clinical study.170,171
PCSK9 inhibitors
The discovery of mutations in the gene encoding for PCSK9 associated with a ‘gain of function’, identified as the genetic causes of FH, dates back to 2003. Since the discovery of PCSK9, over 50 mutations/variations in the gene encoding for this protein have been identified as the genetic causes of FH. The subsequent characterization of PCSK9 ‘loss-of-function’ mutations, which translate into markedly reduced plasma levels of C-LDL, paved the way for the development of a new class of lipid-lowering drug, the monoclonal antibodies against PCSK9.172
In physiological conditions, LDLR has a domain for ApoB that allows binding with C-LDL and the internalization of the LDL/LDLR complex in a clathrin-coated vesicle. Inside the endosome, the LDL/LDLR complex is dissociated due to the presence of an acidic environment. This dissociation leaves the LDLR free to return to the cell surface by means of a recirculation mechanism, whereas the LDL particles are transported to the lysosomes, where they are degraded. PCSK9 is a key protein in the metabolism of cholesterol and is expressed primarily in the liver and intestine. Circulating PCSK9 binds with LDLR on the cell surface. When LDL binds LDLR in the presence of PCSK9, the recirculation of LDLR on the cell surface is inhibited, the amount of LDLR on the cell surface drops, and circulation clearance of LDL is decreased. Therefore, the density of LDLR on the surface of the hepatocytes is inversely proportionate to PCSK9 levels, whereas there is a directly proportionate relationship between the levels of C-LDL and those of PCSK9 (Figure 11A).173,174
Figure 11.
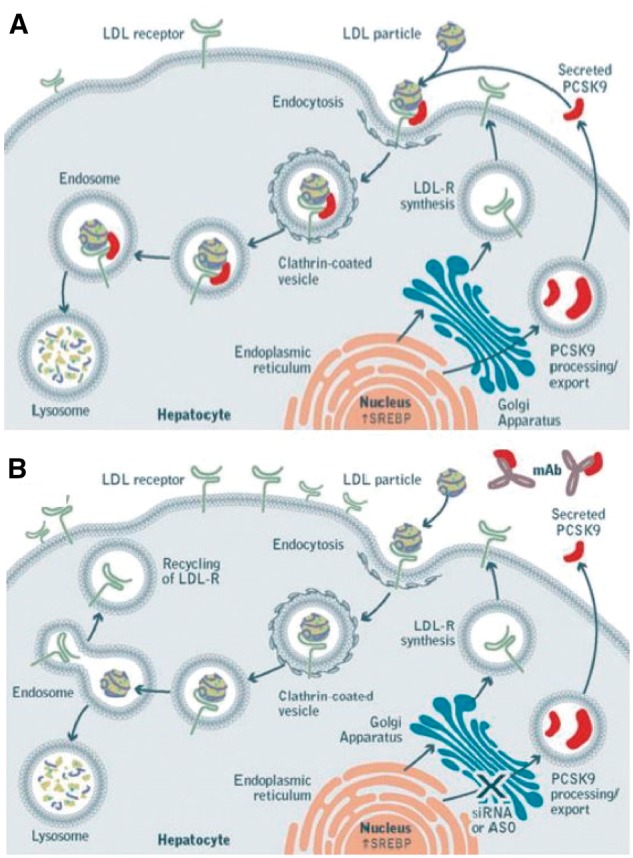
(A) PCSK9 function. (B) effects mediated by PCSK9 inhibitor therapy. Reproduced from Do et al.173
The monoclonal antibodies that inhibit PCSK9 by binding circulating PCSK9 neutralize this protein, inhibit the degradation of LDLR, and increase its expression on the surface of hepatocytes (Figure 11B).172 Over the past 5 years, many monoclonal antibody PCSK9 inhibitors have been developed including evolocumab, alirocumab, and bococizumab.175
Alirocumab: the pharmacodynamic and pharmacokinetic aspects
Alirocumab is a human monoclonal immunoglobulin G1 (IgG1) antibody that binds to PCSK9 with high affinity and specificity. By inhibiting PCSK9 binding with LDLR, alirocumab increases the number of LDLR available to eliminate LDL and therefore lowers the levels of C-LDL.176 As the LDLR also binds to the residues of VLDL and intermediate-density lipoprotein, alirocumab can also cause a reduction in these residues, which is demonstrated by decreases in ApoB, non-HDL cholesterol, and TG. Alirocumab also causes a reduction in Lp(a), a form of LDL bound to ApoA. The pharmacokinetic characteristics of alirocumab are described in Table 19.
Table 19.
Pharmacokinetic characteristics of alirocumab and evolocumab
| Monoclonal antibody | Tmax (days) | Bioavailability (%) | Distribution value (L) | Half-life (days) | Metabolism |
|---|---|---|---|---|---|
| Alirocumab | 3–7 | 85 | 2.8–3.5 | 17–20 | It is eliminated through the immunoglobulin clearance pathways and broken down into small peptides and single amino acids. |
| Evolocumab | 3–4 | 72 | 3.3 | 11–17 | It is eliminated through the immunoglobulin clearance pathways and broken down into small peptides and single amino acids. |
This drug received a favourable review from the Committee for Medicinal Products for Human Use (CHMP) in July 2015; it was approved by the FDA in July 2015 and by the European Medicines Agency (EMA) in September 2015. Alirocumab is approved for subcutaneous administration at a starting dose of 75 mg every 2 weeks for the treatment of HeFH, of non-familial hypercholesterolaemia and of mixed dyslipidaemia. In patients requiring greater reductions in C-LDL levels, therapy with alirocumab may be started at a dose of 150 mg every 2 weeks.176
Clinical studies on alirocumab
The ODYSSEY clinical trial programme for the development of alirocumab included 17 Phase III clinical studies, of which 12 have been completed (7 placebo-controlled, 3 controlled vs. ezetimibe, and 2 controlled vs. ezetimibe and statins) and 5 are currently ongoing (2 double-blind placebo-controlled studies and 3 open-label extension studies). The results of 10 of the 12 completed studies have been published and included in the Assessment report for Praluent® (Table 20). These studies included a total of 5172 patients with HeFH, non-familial hypercholesterolaemia, and mixed dyslipidaemia. Three of the 10 studies enrolled only patients with HeFH; the OPTIONS I and II studies enrolled both patients with HeFH and non-familial hypercholesterolaemia at high cardiovascular risk. In all the 10 studies, patients were treated with a starting dose of 75 mg every 2 weeks and titrated at Week 12 to 150 mg, with the exception of the LONG TERM and HIGH FH studies, which used a 150 mg dose from the outset. All studies showed stable reductions in C-LDL up until their end.176 The remaining two completed studies (CHOICE I and EFC13672) evaluated the efficacy and safety of alirocumab in monotherapy or in combination with statins or other lipid-lowering therapy. The CHOICE I study compared alirocumab vs. placebo in patients with hypercholesterolaemia and moderate-to-high cardiovascular risk, whereas study EFC13672 compared alirocumab with placebo in Japanese patients with HeFH or at high cardiovascular risk with hypercholesterolaemia not adequately controlled by lipid-lowering therapy. The Phase III clinical studies of the ODYSSEY programme currently ongoing are the CHOICE II study that will end in June 2017; the OUTCOMES study that will end in February 2018; and the OLE, ALTERNATIVE, and CHOICE open-label extension studies.
Table 20.
Pre-authorization clinical studies evaluating the efficacy of alirocumab
| Forms of hypercholesterolaemia | Clinical studies in the ODYSSEY programme |
||
|---|---|---|---|
| Clinical trial | Population/comparison | Results at week 24 | |
| Heterozygous familial | FH I: 78 week, multi-centred, double-blind, controlled trial | 485 patients alirocumab (75–150mg/EOW) + lipid-lowering therapy vs. placebo + lipid-lowering therapy | 58% reduction in C-LDL |
| FH II: 78-week, multi-centred, double-blind, controlled trial | 247 patients alirocumab (75–150mg/EOW) + lipid-lowering therapy vs. placebo + lipid-lowering therapy | 51% reduction in C-LDL | |
| HIGH FH: 78 week, multi-centred, double-blind, controlled trial | 106 patients alirocumab (150 mg/EOW) + lipid-lowering therapy vs. placebo + lipid-lowering therapy | 39% reduction in C-LDL | |
| Statin-intolerant patients | ALTERNATIVE: 24 week, multicentre, double-blind, controlled trial | 248 patients alirocumab (75–150 mg/EOW) vs ezetimibe | 30% reduction in C-LDL |
| Patients at moderate cardiovascular risk | MONO: 24 week, multicentre, double-blind, controlled trial | 103 patients alirocumab (75–150 mg/EOW) vs. ezetimibe | 32% reduction in C-LDL |
| Patients at high cardiovascular risk | LONG TERM: 78 week, multicentre, double-blind, controlled trial | 2310 patients alirocumab (150 mg/EOW) + lipid-lowering therapy vs. placebo + lipid-lowering therapy | 62% reduction in C-LDL |
| COMBO I: 52 week, multicentre, double-blind, controlled trial | 311 patients alirocumab (75–150 mg/EOW) + lipid-lowering therapy vs. placebo + lipid-lowering therapy | 46% reduction in C-LDL | |
| COMBO II: 104 week, multicentre, double-blind, controlled trial | 707 patients alirocumab (75–150 mg/EOW) + statins vs. ezetimibe + statins | 30% reduction in C-LDL | |
| OPTIONS I: 24 week, randomized, double-blind, controlled trial | 355 patients Alirocumab (75–150 mg/EOW) + atorvastatin (20–40 mg) vs. ezetimibe + atorvastatin vs. high-dose atorvastatin vs. rosuvastatin | Alirocumab + atorvastatin (20 mg) reduced C-LDL by 24% vs. ezetimibe and by 39% vs. statins; Alirocumab + atorvastatin (40 mg) reduced C-LDL by 31% vs. ezetimibe, by 49% vs. statins and by 33% vs. rosuvastatin | |
| OPTIONS II: 24 week, randomized, double-blind, controlled trial | 300 patients alirocumab (75–150 mg/EOW) + rosuvastatin vs. ezetimibe + rosuvastatin vs. high-dose rosuvastatin | Alirocumab + atorvastatin (10 mg) reduced C-LDL by 36% vs. ezetimibe, by 34% vs. statins | |
| Alirocumab + rosuvastatin (20 mg) reduced C-LDL by 25% vs. ezetimibe and by 20% vs. statins | |||
C-LDL, LDL cholesterol.
Tolerability of alirocumab
Overall, the drug is well tolerated. The adverse events observed during the studies are shown in Table 21.
Table 21.
Adverse events associated with alirocuman and evolocumab therapy
| System and organ classification according to MedDRA | Alirocumab | Evolocumab |
|---|---|---|
| Infections and infestations | — | Nasopharyngitis, upper airway infections, influenza |
| Immune system disorders | Hypersensitivity reactions, hypersensitivity vasculitis | Rash, urticaria |
| Gastrointestinal disorders | — | Nausea |
| Musculoskeletal and connective tissue disorders | — | Back ache, joint pain |
| General disorders and administration site conditions | Injection site reactions, erythema/redness, swelling, pain | Injection site reactions, such as redness, pain and bruising |
| Respiratory, thoracic and mediastinal disorders | Oropharyngeal pain, rhinorrhoea, and sneezing | — |
| Skin and subcutaneous tissue disorders | Pruritus, urticaria, nummular eczema | — |
Data provided on the printed materials for Praluent® (alirocumab) and Repatha® (evolocumab).
Evolocumab: pharmacodynamic and pharmacokinetic aspects
Evolocumab is a human monoclonal antibody belonging to the IgG2 class and has been shown to reduce circulating PCSK9, C-LDL, TC, ApoB, non-HDL cholesterol, TC/C-HDL, ApoB/ApoA1, VLDL cholesterol, TG, and Lp(a) and to induce an increase in C-HDL and ApoA1 in patients with primary hypercholesterolaemia and mixed dyslipidaemia.177 The kinetic characteristics of alirocumab are described in Table 19. Evolocumab received a favourable review from the CHMP in May 2015 and was approved by the EMA in June 2015 and by the FDA in August 2015. This drug is approved for subcutaneous administration at a dose of 140 mg every 2 weeks or 420 mg once a month for primary hypercholesterolaemia and mixed dyslipidaemia or at a dose of 420 mg once a month for FH. In the absence of a clinically relevant response in HF, this latter dose can be increased to 420 mg every 2 weeks after 12 weeks of treatment.177
Clinical studies on evolocumab
The Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK9 In Different Populations (PROFICIO) clinical trial programme for evolocumab development included 16 Phase 3 clinical studies. Of these studies, 8 have been completed and introduced as registration studies, enrolling a total of 4409 patients with HeFH, HoFH, primary hypercholesterolaemia, or mixed dyslipidaemia (Table 22). Of these studies, one included only patients with HeFH and one only patients with HoFH. In all the studies, patients were treated with evolocumab at a dose of 140 mg every other week or 420 mg once a month. All the studies showed stable reductions in C-LDL up until their end. In the FLOREY study, evolocumab as a monotherapy or in combination with atorvastatin was compared with a placebo in patients with primary hyperlipidaemia or mixed dyslipidaemia; in the YUKAWA-2 study evolocumab was compared with a placebo in Japanese patients with hyperlipidaemia or mixed dyslipidaemia and at high cardiovascular risk. Six studies are still ongoing and include the GLAGOV Phase III clinical study, which is due to end in July 2016; the FOURIER and EBBINGHAUS studies, which will end in February 2018; the OSLER-2, which will end in August 2018; the GAUSS-3, which will end in November 2017; and the TAUSSIG, which will end in March 2020.177,178
Table 22.
Pre-authorization clinical studies evaluating the efficacy of evolocumab
| Forms of hypercholesterolaemia | Clinical studies in the PROFICIO programme |
||
|---|---|---|---|
| Clinical trial | Population/comparison | Results | |
| Heterozygous familial | TESLA: 12-week, multicentre, randomised, double-blind, controlled trial | 49 patients evolocumab (420 mg/month) + lipid-lowering therapy vs. placebo + lipid-lowering therapy | At week 12 there was a 32% reduction in C-LDL |
| Heterozygous familial | RUTHERFORD-2: 12-week, multicentre, randomised, double-blind, controlled trial | 329 patients evolocumab (140 mg/EOW and 420 mg/month) + lipid-lowering therapy vs. placebo + lipid-lowering therapy | At weeks 10 and 12, evolocumab 140 mg/EOW and 420 mg/month had reduced C-LDL by an average of 61% and 66%, respectively. |
| Primary hypercholesterolaemia and mixed dyslipidaemia | LAPLACE-2: 12-week, multicentre, randomised, double-blind, controlled trial | 1896 patients evolocumab (140 mg/EOW and 420 mg/month) + statins vs placebo or ezetimibe | At weeks 10 and 12, evolocumab 140 mg/EOW and 420 mg/month had reduced C-LDL by an average of 72% and 69%, respectively vs. placebo and by 43% and 46% vs. ezetimibe |
| MENDEL-2: 12 week, multicentre, randomised, double-blind, controlled trial | 614 patients evolocumab (140 mg/EOW and 420 mg/month) vs. placebo or ezetimibe | At weeks 10 and 12, evolocumab 140 mg/EOW and 420 mg/month had reduced C-LDL by an average of 40% and 41%, respectively vs. ezetimibe and at week 12 by 57% and 55% vs. placebo | |
| DESCARTES: 52 week, multicentre, randomised, double-blind, controlled trial | 901 patients evolocumab 420 mg/month + diet or statins or ezetimibe vs. placebo + diet or lipid-lowering therapy | At week 52 there was a 59% reduction in C-LDL | |
| THOMAS: 14 week, multicentre, randomised, open-label trial | 149 patients evolocumab (140 mg/EOW) pre-filled syringe + lipid-lowering therapy vs. evolocumab (140 mg/EOW) pre-filled pen + lipid-lowering therapy | At week 6 evolocumab pre-filled syringe and evolocumab pre-filled pen reduced C-LDL by 61% and 64%, respectively | |
| THOMAS: 28 week, multicentre, randomised, open-label trial | 164 patients evolocumab (420 mg/month) automatic microinfusion pump + lipid-lowering therapy vs. evolocumab (420 mg/month) pre-filled pen + lipid-lowering therapy | At weeks 10 and 12, the evolocumab automatic microinfusion pump and evolocumab pre-filled pen had reduced C-LDL by an average of 69% and 67%, respectively | |
| Statin-intolerant patients | GAUSS-2: 12-week, multicentre, randomised, double-blind, controlled trial | 307 patients evolocumab (140 mg/EOW and 420 mg/month) vs. ezetimibe | At weeks 10 and 12, evolocumab 140 mg/EOW and 420 mg/month had reduced C-LDL by an average of 38% and 39%, respectively |
C-LDL, LDL cholesterol.
Tolerability of evolocumab
Overall, evolocumab is well tolerated. The adverse events observed during the studies are shown in Table 21.
Other PCSK9 inhibitors
The PCSK9 inhibitor class includes bococizumab. This is a humanized monoclonal IgG2 antibody, which is currently being evaluated in a Phase III trial. This drug, administered subcutaneously at a dose of 150 mg every 2 weeks, is currently being evaluated against a placebo in the SPIRE-1 and SPIRE-2 clinical studies. The first study will enrol 12 000 high-risk patients who are on lipid-lowering therapy and have laboratory C-LDL values between 70 and 100 mg/dL or non-HDL cholesterol values between 100 and 130 mg/dL.179 The study aim is to evaluate the reduction in major cardiovascular events, including cardiovascular death, myocardial infarction, stroke, and unstable angina. The SPIRE-2 study will evaluate the same outcomes in 6300 patients on treatment with maximum statin doses or with other lipid-lowering drugs, and with C-LDL and non-HDL cholesterol levels higher than 100 or 130 mg/dL, respectively.180
The other medicinal products belonging to the monoclonal antibody class that are inhibitors of PCSK9, such as RG7652, LGT-209, ALN-PCS02, LY3015014 and BMS-962476, are currently undergoing Phase 2 and 3 trial.154
Conclusions
As shown by the clinical studies conducted up to now, the new therapeutic strategies for the treatment of dyslipidaemia have favourable effects on the reduction of C-LDL when administered either as a monotherapy or in combination with statins. Although lomitapide and mipomersenhave shown their efficacy in patients with HoFH, some doubts remain regarding their safety profiles. Indeed, because of the mechanism of action of these medicinal products, namely their inhibition of hepatic VLDL secretion, they cause an accumulation of TG in the liver and therefore require long-term monitoring of their safety. Further studies on ApoA1 mimetics and CETP inhibitors are required in order to confirm their efficacy and safety. Lastly, to date, PCSK9 inhibitors appear to be one of the most important pharmacological innovations for reducing hypercholesterolaemia, both in combination with statins and as an alternative to them in intolerant patients, as these drugs have shown a good safety profile and, above all, good efficacy in achieving C-LDL values <70 mg/dL. To conclude, the new pharmacological classes for the treatment of dyslipidaemia could represent a valid option for improving anti-atherosclerotic therapy.
Eligibility for treatment with PCSK9 inhibitors
How to identify patients to be treated
Until further data are available from clinical studies showing that PCSK9 inhibitors have an impact on clinical outcomes, it would be appropriate to identify the categories of patients in which these new drugs could have the greatest clinical benefits. On the basis of the scientific evidence available, international regulatory authorities—the FDA in the USA and the EMA in Europe—have posed some general indications for the use of PCSK9 inhibitors in clinical practice. Despite acknowledging the absence of data supporting the efficacy of PCSK9 inhibitors in reducing the incidence of adverse cardiovascular events, the EMA considers the use of these drugs indicated in patients with primary hypercholesterolaemia (heterozygous familial or non-familial) and mixed dyslipidaemia who are unable to meet the recommended C-LDL target despite using the maximum tolerated dose of statins.181,182 The EMA also states that PCSK9 inhibitors can be used in patients with hypercholesterolaemia who do not tolerate or who have specific contraindications for the use of statins.181,182 The FDA, on the other hand, allows PCSK9 inhibitor use in addition to the maximum tolerated dose of statins in patients with HeFH and in patients with atherosclerotic cardiovascular disease requiring a further reduction in C-LDL.183,184 Lastly, both regulatory authorities believe that evolocumab can be used in patients with HoFH.181,183
Overall, the approach adopted by the FDA appears to be far stricter than that adopted by the European Agency. The North American regulations do not consider statin intolerance and the use of PCSK9 inhibitors is limited to the genetic forms of hypercholesterolaemia and the secondary prevention of atherosclerotic cardiovascular disease. Primary prevention in high- or very high-risk subjects is neglected.
These decisions of the FDA appear to be affected by the widespread debate in progress in the USA concerning the cost-effectiveness of these new drugs. The publication of a preliminary cost-effectiveness analysis for PCSK9 inhibitors by the Institute for Clinical and Economic Review (ICER), an independent, non-profit organization, triggered a large, heated debate on the high costs of this new pharmacological therapy.185,186 ICER analysed all the evidence available (8 Phase II studies, 16 Phase III studies, 1 long-term follow-up study, and a meta-analysis) regarding the predicted costs of the new therapy (approximately $14 000/year/patient). The conclusion was that PCSK9 inhibitors would be cost-effective only if there was an 80% reduction in the predicted drug price.187 This evaluation could change if the ongoing clinical studies show that PCSK9 inhibitors have a particularly favourable effect on the incidence of atherosclerotic cardiovascular disease.
The considerable costs of these new therapies (between €5000 and 7000/year/patient in Europe) pose serious doubts as to the cost-effectiveness of their use also in the complex setting of the Italian NHS.
Possible criteria for National Health Service reimbursement of PCSK9 inhibitors
Prescription restricted to specialists (cardiologists, internal medicine specialists, and lipidologists).
Establishment of an easy to use web-based register for the assessment of prescriptive appropriateness and the efficacy/safety of treatment in the first 6 months of use (the register should have a limited duration of no more than 2 years or until data on clinical events becomes available).
- Target population:
- FH patients (using validated score for the definition of the probability of the disease);
- patients with very high cardiovascular risk (recent atherothrombotic event within 1 year) not meeting C-LDL targets on optimum C-LDL-lowering therapy; and
- very high-risk patients, after the first reoccurrence of a cardiovascular event;
Pharmaceutical dispensing billed to the NHS.
Assessment of co-morbidities: the internal medicine patient
Introduction
Most internal medicine patients are elderly or very elderly, they usually have multiple co-morbidities, including acute conditions, but more commonly chronic ones, that restrict their degree of autonomy.188,189 In these patients, it is important to look for co-morbidities, however, this is often not enough to explain the overall patient complexity and frailty.190 In clinical practice, there is no co-morbidity indicator or parameter to define patient complexity, which relies solely on the specific skills that internal medicine specialists possess191,192 on account of their training.
Co-morbidity and polymorbidity
The presence of multiple conditions in the same individual constitutes a challenge for all clinicians treating elderly people.193–196 A distinction should be made between co-morbidity and polymorbidity and the complications that arise during hospitalization,197 which make defining co-morbidity in a patient a matter that is far from simple.198–200 For example, in the REgistro POliterapie SIMI (REPOSI) study, the term ‘cluster’ of illnesses is used to describe the presence of two or more specific chronic illnesses.201 In actual fact, we need to have a fully comprehensive definition of polymorbidity, especially in long-term care settings, which would also be of use to general practitioners.
Functional dependence
In individuals aged over ≥75 years, polymorbidity is more often associated with disability,202 to the extent that in one large Swedish study, the presence of a functional disability was observed in almost 20% of participants, with a prevalence that rose with the number of chronic conditions. The prevalence of disability varies significantly for the different clusters of illnesses: from 6.7% in those with hypertension and atrial fibrillation to 82.4% in those with dementia and hip fractures.203 The World Health Organization’s International Classification of Impairments, Disabilities, and Handicaps (ICIDH)204 defined the taxonomy of the consequences of an illness with three main conditions: impairments, disability, and handicap. Another scheme describes four fundamental situations: active illness, impairment, functional limitation, and disability.205 Functional dependence refers to people who are not self-sufficient in at least one activity of daily living (ADL, e.g. washing, getting dressed, eating, getting into or out of bed, getting on to a chair, getting around, using the toilet, and sphincter continence) or in an instrumental ADL (preparing meals, doing the shopping, managing money, using the telephone, doing housework, and going out).
Frailty
Complexity and frailty can be present in the same subject; however, these terms are often used incorrectly and should be considered separately.206,207 It is not easy to define frailty according to a univocal concept, to the extent that certain authors208 define it as a vulnerable state of health, deriving from the complex interaction of medical and social issues, with a reduced ability to respond to stress, associated with a functional reduction in performance. Generally speaking, frail elderly subjects are debilitated individuals who are either old or very old, with disabilities of varying severity and associated geriatric syndromes. From a strictly clinical standpoint, frailty is characterized by a high susceptibility to developing illnesses often with an atypical clinical course, limitation of motor capacities, propensity to immobility, rapid fluctuations in health, events with a domino effect, risk of adverse events and complications, need for constant medical observation and increased risk of death.209 In clinical practice, doctors use the Clinical Frailty Scale to evaluate the diagnosed illnesses, patient motivation, symptom control, functional status, and the degree of dependence.210
The definition of complexity
What makes patients complex and how can we measure their complexity? These questions are yet to receive an adequate answer,211 to the extent that in hospitalized patients, the complexity of the case mix is an expression used by clinicians and administrators to describe a series of multiple attributes that include, more than co-morbidity itself, the severity of the disease, the risk of death, prognosis, treatment difficulty, health care needs, and the entity of the health care resources involved. For clinicians, it refers to the patient, his or her overall needs and the methods used to guarantee the necessary health care, taking into account different aspects such as the severity of disease, increased risk of mortality, therapy and patient management difficulties, worse prognosis, and overall greater health care needs. Administrators and providers tend to emphasize in the complexity of the case mix the greater consumption of resources, with an increase in the cost of care. The concept of complexity is not univocal212 and extends from clinical to psychosocial and economic to organizational spheres. The term complexity refers to uncertainty, dynamicity, unpredictability, and risk. When dealing with complex patients, we do not need centric approaches that would be excessively simple and reductive. On the contrary, we need eccentric approaches, able to decentralize know-how regarding the many facets of the patient.213 In reality, the concept of complexity lacks a precise definition and often includes interrelations between each component of a complex system, in relation to their number, interfaces, contingent conditions, and possible decision-making options.214 A complex system is characterized by a vast quantity of interacting elements with great variability and is burdened by considerable risks.215 Complexity is the real world, and it requires an alternative outlook in health care, based on a fair judgement of the dynamics of the possible interactions between the different parts considered. Complexity means the impossibility to reduce to linear terms not so much the situation being studied, but rather the different ways in which the situation is studied. A physician treating complex patient should aim for multidimensional and multidisciplinary knowledge but is aware of the impossibility of full knowledge. Complexity makes medicine a probabilistic science, with a high risk of error, due to the uncertainty that permeates medical decisions, which must in any case be made within a limited time frame and in a context of knowledge that is not always defined.216 The definition of a complex patient, adopted by the Agency for Healthcare Research and Quality (AHRQ), refers to a person with two or more chronic illnesses, in whom each of the conditions is able to influence the outcome of the treatment of the other co-morbidities, in various ways: limitation of life expectancy, increased intercurrent morbidity, drug–drug interactions, and the impossibility of making full use of adequate treatment due to contraindications.217 In the most complete concept of complexity, the biological, socio-economical, cultural, behavioural, and environmental components all become important determinants of health,218 although—paradoxically—these elements are considered in the inclusion/exclusion criteria of clinical studies as potentially confounding factors for an objective evaluation of the results. In the internal medicine setting, the management of a complex patient means, depending on his/her specific characteristics, possessing special skills for tackling the challenges posed by the single case in the specific clinical context. Complex patients often represent a grey area and require individualized treatments, based on suitable clinical judgement and adequate decisions at the highest levels of knowledge of the context. Complexity of care can also require that more time be dedicated to patients in order to evaluate and deal with the health care needed,219,220 with a varied case mix and a vast range of possible decisions to be made.
Overdiagnosis and the increase in co-morbidities
Overdiagnosis occurs when an illness that will not cause any symptoms, morbidity, or premature mortality is identified.221,222 Overdiagnosis is often the result of an exasperated quest for a diagnosis at all costs, where the identification of a hypothetical disease is sought and constantly evaluated improperly. In other cases, the thresholds for the assessment of the illness or risk, such as blood pressure or cholesterol, are shifted to the point where people who are healthy or have very mild problems or who are at low risk of illness are considered sick.223 Overdiagnosis inevitably entails exposure to potential damage caused by treatment, without receiving any benefit.224 Moreover, broadening the frontiers of illnesses that can be potentially cured using medication causes an expansion in profit-making markets,225 thereby generating a further waste of public health resources. In this scenario, it is therefore necessary to identify those subjects at greatest cardiovascular risk on the basis of set and universally accepted parameters, an approach that becomes all the more evident in light of the Ministerial Decree on prescriptive appropriateness that recently came into force.
Complexity constitutes a daily challenge for physicians and in order to obtain efficacious results they should systematically evaluate patient needs and prognoses, going beyond the individual treatments to consider the health care process as a whole,226 by filtering useful specialist opinions with monitoring and systematic feedback of the interactions existing between the elements and the potential associated risks.227,228 The illusion of simplicity requires a review of the methods with which clinicians today deal with complex patients188; however, simplifying complexity does not mean standardizing it or dealing with problems in a simplistic manner. For this reason, an overall patient-oriented assessment of problems, integration, cooperation and coordination, and communication are some of the simple rules that can prove useful to obtaining tangible results in a complex system.229,230 These concepts, together with a specific attempt to investigate, in a multidimensional manner, the topic of complexity and co-morbidity, must become tools to be used by the internal medicine specialist in order to reconsolidate his/her natural vocation as a ‘physician of the person’.
Co-morbidity assessment: common clinical cases
The European Commission has approved the use of the first PCSK9 inhibitor in adult patients with primary hypercholesterolaemia or mixed dyslipidaemia, in addition to diet and in combination with a statin and/or other lipid-lowering therapies in patients who are unable to meet the C-LDL targets with the maximum tolerated dose of a statin or in combination with other lipid-lowering agents in subjects with statin intolerance or for whom statins are contraindicated. The assessment of these co-morbidities in this type of patient has a number of implications.
Firstly, the identification and characterization of co-morbidities are the first element for the estimation of cardiovascular risk and therefore for the establishment of the target C-LDL value. The presence of myocardial ischaemia, hypertension, or cardiac insufficiency are important elements for the identification of subjects at the highest cardiovascular risk.231 On the basis of the data provided by the 2001–2006 NHANES survey,232 the most common cardiovascular co-morbidities among dyslipidaemic subjects ≥65 years of age are CHD (27.0%), diabetes mellitus (26.5%), prior stroke (10.4%), and congestive HF (9.9%), with 51.2% of these subjects having at least one of these co-morbidities.
Among the patients enrolled in the ODYSSEY LONG TERM study,84 68.9% had a history of coronary heart disease and the average age was 60 years. The inclusion criteria were based not only on cholesterol levels (>70 mg/dL despite treatment with statins) but on a high cardiovascular risk associated with the presence of a documented coronary disease or a coronary artery disease equivalent (defined as peripheral arterial disease, prior ischaemic stroke, moderate chronic kidney disease with GFR 30–60 mL/min/1.73 m2 body surface area), or diabetes mellitus plus two or more additional risk factors (hypertension; ankle–brachial index ≤0.90; microalbuminuria, macroalbuminuria, or urine dipstick >2+ proteins; retinopathy; or a family history of premature coronary disease).84 In the USA, 47.5% of high-risk adult dyslipidaemics are currently not on statins and just 25% of high-risk patients receive high-intensity statins.233 This rate is even lower in Europe, where a high-intensity statin is prescribed in less than 10% of all high-risk patients.234 Dedicating greater attention to the assessment of co-morbidities may modify an incorrect perception of the degree of cardiovascular risk.
Furthermore, the identification of co-morbidities has specific relevance. As PCSK9 inhibitors allow a reduction in C-LDL levels that is greater than that obtained with statin therapy, it is important to pay attention to certain co-morbidities that could be associated with drug safety aspects. In the studies performed to date, the number of adverse events observed was no higher than for conventional therapy or in those with a C-LDL level <25 mg/dL (0.6 mmol/L). However, the follow-up periods were somewhat short, and hence, specific neurocognitive function assessment is required.235
Atherogenic dyslipidaemia
Of the various lipid metabolism disorders, the profile characterized by the presence of small, dense LDL particles, low HDL levels (<1.0 mmol/L in men and <1.3 mmol/L in women) and high levels of TG (>1.7 mmol/L), and VLDL has been defined the ‘atherogenic lipid profile’, or ‘atherogenic dyslipidaemia’ and is associated with a three- to six-fold increase in the risk of cardiovascular disease.236
The lipid components that define atherogenic dyslipidaemia, in particular HDL and TG and their remnants, have a complex pathogenic role in relation to the progression of the atherosclerotic process, which is still in part unclear. Observational studies have shown that a 1 mg/dL increase in HDL is associated with a 2–3% reduction in cardiovascular risk and that high levels of HDL, even in the presence of C-LDL levels >160 mg/dL, protect against cardiovascular events, as shown by the Framingham study237 and the Prospective Cardiovascular Münster (PROCAM) study.238 Furthermore, a series of observations from clinical studies and meta-analyses suggest that high TG levels are an independent cardiovascular risk factor.238 Prospective studies have shown that a 1 mmol/L increase in TG is associated with a 14% increase in the risk of CHD in men and a 37% increase in women, regardless of the C-HDL levels.239 Moreover, an increase in the concentration of lipoproteins rich in TG, such as VLDL, kilomicrons and catabolism products are associated with a greater risk of atherosclerotic plaque and CHD progression.236
Given the marked increase in cardiovascular risk in subjects with an atherogenic lipid profile, especially those with type 2 diabetes, a therapeutic approach that acts on the non-LDL components of the lipid profile is required.
It has been proved that an efficacious reduction in abdominal fat reduces the prevalence of small dense LDL particles and increases the levels of HDL; thus, lifestyle changes combining diet and regular exercise represent the first aspect of therapy for atherogenic dyslipidaemia.240 Current ESC/EAS guidelines on dyslipidaemia8 recommend regular daily exercise (at least 30 min) in order to improve atherogenic dyslipidaemia, insulin resistance, and C-HDL levels.
From a pharmacological standpoint, the approach to the treatment of atherogenic dyslipidaemia in patients on statin therapy can involve the addition of fenofibrate, the only fibrate approved by the EMA that is compatible, from a safety point of view, with statin co-administration. Although there are not ad hoc interventional studies on the treatment of atherogenic dyslipidaemia, a series of observations from previous clinical studies, especially in diabetic subjects, clearly indicate that fibrates have a favourable effect on the reduction of cardiovascular events in patients with atherogenic dyslipidaemia and diabetes mellitus. In the FIELD study, which recruited diabetic patients with or without ischaemic cardiomyopathy, although no significant reduction was observed in coronary events, myocardial infarction, the Study’s primary endpoint, decreased by 24% in patients treated with fibrate.71 Moreover, in a posthoc analysis of the FIELD study, over 5 years of fenofibrate therapy reduced cardiovascular events by 27% in the subgroup of subjects with atherogenic dyslipidaemia (hypertriglyceridaemia and low HDL levels).241 These results were confirmed by the ACCORD Lipid study,72 which enrolled patients with high cardiovascular risk, type 2 diabetes mellitus, and high levels of C-LDL, who were on simvastatin therapy. At the end of the study, the primary outcome of non-fatal myocardial infarction, non-fatal ischaemic stroke, and cardiovascular death occurred in 12.4% of patients treated with fenofibrate against 17.3% (31% reduction in relative risk) of patients treated with a placebo. On the other hand, no significant differences were observed in patients without atherogenic dyslipidaemia: in this case, adding fenofibrate to statin therapy did not cause a reduction in cardiovascular events compared with those subjects treated with simvastatin monotherapy. Lastly, in a meta-analysis on patients with atherogenic dyslipidaemia enrolled in five large-scale interventional studies with fenofibrate, the use of fibrates was associated with a 35% reduction in coronary events. The use of fenofibrate is currently approved by the EMA in patients with severe hypertriglyceridaemia, in those with mixed dyslipidaemia who are intolerant to statins, and in those with atherogenic dyslipidaemia and currently on statin therapy, if at high cardiovascular risk.
The hypercholesterolaemic patient with neurological co-morbidities
The relationship between lipid-lowering therapies and neurological disorders has always been the object of heated debate. As far as acute cerebrovascular events are concerned, the benefit of cholesterol-lowering therapies would appear to be irrefutable. Indeed, statins have been shown to reduce the progression of atherosclerotic carotid lesions, induce (when used at high doses) lesion regression, and modify the composition of atherosclerotic plaques, favouring their stabilization. Similar results are also expected to be observed with PCSK9 inhibitors, as during the long-term safety studies (ODYSSEY LONG TERM and OSLER II) a reduction in cardio- and cerebrovascular events was observed. If this trend is confirmed by the ongoing outcome studies, the protective role of these drugs will be definitively confirmed in patients with both symptomatic [prior transient ischaemic attack (TIA)/stroke] and asymptomatic hypercholesterolaemia and atherosclerotic carotid artery disease.
However, the relationship between cholesterol-lowering therapy and cognitive deterioration is a far more controversial matter. Indeed, cholesterol is the main component of myelin, which is known to play a fundamental role in the transmission of neuronal signals and in the integrity of the blood–brain barrier. The decrease in serum cholesterol caused by PCSK9 inhibitors, as was suspected in the past for statins, could, therefore, potentially cause an alteration in the myelin sheath and therefore in signal transmission. Currently, this hypothesis is not supported by dedicated studies, as no observational studies on subjects with genetic PCSK9 defects have shown any reduction in cognitive functions.242 However, concerns regarding a possible unfavourable impact of PCSK9 inhibitors on neurological function were rekindled by a recent meta-analysis of all the RCTs comparing PCSK9 inhibitors with a placebo. This meta-analysis reconfirmed the extreme efficacy of these medicinal products in reducing cardio- and cerebrovascular outcomes [odds ratio (OR) 0.43; 95% CI 0.22–0.82; P = 0.01) but also showed that therapy with PCSK9 inhibitors is associated with adverse neurocognitive events when compared with a placebo (OR 2.34; 95% CI 1.11–4.93; P = 0.02).243
On the other hand, therapy with PCSK9 inhibitors may also be associated with neurological protective effects in the very long term. Indeed, PCSK9 binds with LDLR causing its deterioration. This receptor, which is also present in the central nervous system, would also seem to be responsible for the degradation of ApoE, which is known to increase the accumulation of amyloid B (the peptide responsible for Alzheimer’s disease). PCSK9 inhibition could, therefore, theoretically cause an increase in LDLR and, consequently, a reduction in the accumulation of amyloid substance. This mechanism was also observed for statins, which also increase LDLR expression, and which, in a number of studies, have been associated with a reduction in the development of Alzheimer’s disease.
Future randomized, double-blind studies with a long duration and having cognitive deterioration as a primary endpoint will be needed to definitively clarify this aspect.
The hypercholesterolaemic patient with thrombophilic status
Lp(a) is an independent cardiovascular risk factor. EAS/ESC guidelines on the treatment of dyslipidaemia recommend testing Lp(a) in high-risk patients and in those with a family history of premature cardiovascular events.
Lp(a), a particle similar to LDL, which contains a single molecule of ApoB-100 bound to a specific glycoprotein, Apo(a), seems to be involved in the atherosclerotic process due to its richness in cholesterol and to Apo(a)’s similarities to plasminogen. These two processes could explain why a number of studies have shown a relationship between Lp(a) levels and cardiovascular risk.
Unfortunately, unlike the other modifiable cardiovascular risk factors, the treatment options for Lp(a) are currently limited. Indeed, unlike the potent effect that statins have on C-LDL levels, these drugs have very little effect on Lp(a) levels, reducing them just a few percentage points. Among the drugs currently available for dyslipidaemia, only nicotinic acid causes a substantial decrease in lipid levels; however, this medicinal product has been almost shelved in clinical practice on account of its significant side effects.
In light of these observations, a great deal of interest has been generated by the evidence that in several Phase II and III studies the new lipid-lowering agents, PCSK9 inhibitors (alirocumab, evolocumab, and bococizumab) have been shown to cause a statistically significant reduction in the levels of Lp(a).244 More specifically, these drugs have shown a significant reduction in Lp(a) compared with a placebo (reduction of approximately 25–30%), with a similar percentage reduction regardless of Lp(a) baseline values, but with a higher absolute decrease in patients with higher baseline values, especially in high-risk patients with baseline Lp(a) values >125 nmol/L.245
Furthermore, significant and dose-dependent reductions in Lp(a) compared with the controls have been observed, regardless of background statin therapy. The reduction in Lp(a) was also seen to be reversible when therapy was discontinued, whereas it persisted over time during the treatment. The reduction was also seen to be regardless of sex, age, and baseline levels of C-LDL.
The mechanism by means of which Lp(a) is synthesized, metabolized, and removed from circulation is still unclear. One of the hypotheses is that LDLR upregulation, combined with the low levels of circulating LDL obtained with PCSK9 inhibitors, allows greater Lp(a) uptake by the LDLR, for which, under normal conditions, Lp(a) has a low affinity.
To conclude, the effect of PCSK9 inhibitors on the concentration of Lp(a), in addition to that on C-LDL, could have a beneficial effect on the prevention of cardiovascular events in patients with thrombophilic status; however, it is yet to be clarified to what extent the reduction in Lp(a) influences these events regardless of the reduction in LDL cholesterol.
The role of statin therapy in elderly patients
The gradual ageing of the population and better survival following ACS increasingly require a solution to the issue of cardiovascular risk in the elderly population, both in primary and in secondary prevention settings. Indeed, more than 80% of individuals who die from ischaemic cardiomyopathy are aged over 65 years.246 The high absolute cardiovascular risk associated with age, the frequent presence of co-morbidities, and/or the presence of a prior ischaemic cardiovascular event make the elderly population that in which the benefits of statin therapy are the most obvious. However, the elderly population, which is often on polydrug regimens, is also that which is most exposed to the side effects of drugs, including statins and in which lower doses should be used.
Because of the shortage of data concerning the use of statins for primary prevention in elderly patients, their employment in these patients without a prior ischaemic cardiovascular event (primary prevention) has a Class II recommendation with a B level of evidence in the ESC guidelines.8 Indeed, just one study, the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) study,247 prospectively assessed the use of pravastatin 40 mg vs. placebo in patients with no prior cardiovascular events and aged between 70 and 82 years, showing a reduction in coronary death, non-fatal myocardial infarction, and stroke risk at 3 years, though without a reduction in all-cause mortality. However, one recent meta-analysis,248 which included 24 674 elderly individuals (>65 years) in primary prevention and at high cardiovascular risk enrolled in 8 large trials on statins, showed that there was a 39% reduction in the risk of myocardial infarction and a 24% reduction in the risk of stroke in patients randomized to statins when compared with the control group, though there was no effect on all-cause mortality, cardiovascular death, and on the risk of new malignancies. Failure to reduce mortality in the elderly population undergoing primary prevention intervention is, of course, expected, given the high prevalence of other potentially fatal age-related medical conditions that outweigh the advantage of statin therapy. However, in order to guarantee the quality and not merely the quantity of life in the elderly population, reducing potentially disabling events such as stroke and myocardial infarction, which are also related to significant health care costs, represents an aspect that should guide cardiovascular prevention in the elderly population. Moreover, this evidence should influence the recommendation of future guidelines regarding primary prevention with statin therapy in the elderly and is of particular importance considering the current increase of the elderly population.
Data regarding the use of statins in the elderly population with prior ischaemic cardiovascular events are more numerous. The 4S study (Scandinavian Simvastatin Survival Study)249 showed a reduction in 5-year mortality in patients randomized to simvastatin vs. placebo, aged over 60 years and undergoing secondary prevention. In the Cholesterol and Recurrent Events (CARE),250 pravastatin reduced the risk of major coronary events, death by coronary disease, and stroke in elderly patients with a reported history of myocardial infarction. This evidence was confirmed by a meta-analysis that included subjects over 65 years of age (19 569 patients) recruited in nine RCTs and reported a 22% reduction in the risk of all-cause death at 5 years, a 30% reduction in death by coronary disease, a 26% reduction in non-fatal myocardial infarction, a 30% reduction in revascularization procedures and a 25% reduction in stroke with statin treatment.251 Based on these data, current guidelines suggest statin treatment in elderly patients in secondary prevention settings as a Class I recommendation with a level B of evidence, such as for the secondary prevention in the population <65 years.8
The diabetic patient
Diabetic patients, especially those with type 2 diabetes, are characterized by specific lipid profile alterations that constitute a substantial part of the disease: hypertriglyceridaemia, reductions in C-HDL, increases in LDL, smaller and denser LDL and HDL, and a postprandial increases in TG-rich lipoproteins. The combination of these alterations constitute what is known as ‘atherogenic dyslipidaemia in diabetes’, which contributes to the increased cardiovascular risk of diabetic patients.
Although C-LDL elevation is not strictly dependent on the presence of diabetes, it represents the main lipid factor of cardiovascular risk in diabetic patients.
Statin therapy is the treatment of choice for diabetic patients with C-LDL values that are off-target with non-pharmacological intervention alone. If full-dose statin therapy is unable to achieve optimal C-LDL values, combination with ezetimibe should be considered. Recently, the IMPROVE-IT study,27 which included approximately 5000 patients with diabetes, confirmed the efficacy of ezetimibe in the diabetic sub-population (RR 0.86 vs. 0.98). The IMPROVE-IT study results support the hypothesis that achieving a more ambitious therapeutic target in terms of C-LDL determines further cardiovascular benefit.
With regard to the effect of PCSK9 inhibitors in patients with type 2 diabetes, there are currently no data from clinical studies that have been conducted exclusively on these subjects. In the studies published with alirocumab (ODYSSEY LONG TERM)84 or evolocumab (OSLER),85 the percentage of diabetic subjects was 35% and 13%, respectively. The sub-analyses conducted on diabetic subjects showed that the effect on C-LDL, non-HDL cholesterol, Lp(a), and TG is similar to that of subjects without diabetes and irrespective of gender, type of statin used, insulin treatment, presence of cardiovascular disease, reduced GFR, and degree of glycaemic compensation. The most common side effects reported with these drugs were neurocognitive (regardless of the levels of C-LDL achieved), injection site reactions (6 vs. 4%), myalgia (6 vs. 3%), and ophthalmological events (3 vs. 2%), without a difference in incidence rates between the diabetic and the non-diabetic groups.
More recently, other safety and efficacy studies conducted in subjects with high cardiovascular risk were published in which the percentage of patients with diabetes was approximately 50% (ODYSSEY OPTIONS I and OPTIONS II with alirocumab and YUKAWA-2 with evolocumab). In these studies, PCSK9 inhibitors were more effective in reducing C-LDL values than statin dose increases or their combination with ezetimibe, even in diabetic subjects.
Clinical and laboratory criteria for prescriptive appropriateness pending outcome data
Taking into account the evidence currently available, the use of PCSK9 inhibitors could be reasonably considered in the following conditions.
Heterozygous familial hypercholesterolaemia
PCSK9 inhibitors could be used in patients with HeFH who are unable to achieve the C-LDL target recommended by the guidelines,8 despite a substantiated use of statins at the maximum tolerated dose combined with ezetimibe. More specifically, the lipid objectives should be as follows:
C-LDL < 100 mg/dL in patients in primary prevention, who do not present clinical or instrumental signs of atherosclerotic cardiovascular disease and
C-LDL < 70 mg/dL in patients is secondary prevention, who present clinical or instrumental signs of atherosclerotic cardiovascular disease.
For the clinical diagnosis of FH (prevalence of 1:200–1:500 subjects in the general population), the Italian Regulatory Agency, AIFA, has issued the following criteria93:
baseline C-LDL > 190 mg/dL and
vertical disease transmission, confirmed by the presence of hypercholesterolaemia (with C-LDL > 190 mg/dL) among patient’s first-degree relatives.
If data regarding the lipid profile of the patient’s family are not available, FH can be diagnosed in the presence of C-LDL > 190 mg/dL in combination with at least one of the following conditions:
patient presenting tendon xanthoma;
evidence of premature ischaemic cardiomyopathy in at least one first-degree relative (earlier than 55 years of age in men and 60 years of age in women); and
severe hypercholesterolaemia in prepubescent children among first-degree relatives.
The AIFA diagnostic criteria indicated above are preferable to others, such as the DLCN score,104 due to their simplicity and their regulatory values.
Patients with atherosclerotic cardiovascular disease and diabetes mellitus
PCSK9 inhibitors could be used in patients with atherosclerotic cardiomyopathy (those with ischaemic cardiomyopathy, ischaemic cerebrovascular disease (CVD), and peripheral occlusive arterial disease), and/or diabetes mellitus with evidence of organ damage (e.g. microalbuminuria) who do not reach the C-LDL target <70 mg/dL, as recommended by the guidelines,8 despite the use of the maximum tolerated dose of statin in combination with ezetimibe.
Closing comments
At present, statins remain the main therapeutic strategy for the treatment of hypercholesterolaemia.8 The diagnostic and therapeutic pathway of each patient starting treatment with PCSK9 inhibitors should be carefully recorded. More specifically, the ‘maximum tolerated dose of statin’ should be adequately verified and each therapeutic change recorded. In this way, it will be possible to guarantee the best use of NHS resources. For the diagnosis of statin intolerance, which may restrict or prevent statin use, the ANMCO position paper on this specific subject should be consulted.252 This document defines the pathway for the diagnosis of a condition that actually limits the clinical use of statins.
‘Real-world’ data regarding the use of cholesterol-lowering agents in patients with recent cardiovascular events in Italy
Introduction
Atherothrombotic cardiovascular diseases have now come to be the most common cause of death in both men and women and in all parts of the world, not merely in industrialized countries. Indeed, we are currently witnessing a globalization, not just in economic and cultural terms but also in terms of clinical epidemiology.253,254 The spread of cardiovascular disease is expected to increase further as the population ages: in Europe, the number of subjects aged >65 years is expected to rise from 85 million in 2008 to 151 million in 2060.255 Another determinant of this increase is the improvement in the quality and efficiency of care for acute cardiovascular diseases, which are able to increase the survival of affected patients, but at the same time, increase the size of the population of subjects requiring secondary prevention intervention.256
Over the past three decades, several clinical studies have highlighted the efficacy of various pharmacological treatments able to reduce the incidence of new atherothrombotic episodes in subjects surviving a first episode; more specifically, antiplatelet drugs, renin–angiotensin system blockers, beta–blockers, and cholesterol-lowering drugs have shown significant efficacy. In particular, statins have been shown to reduce cardiovascular mortality and non-fatal atherothrombotic events in heterogeneous patient populations in primary and secondary prevention. These treatments are recommended according to both US and European guidelines and should be prescribed to all patients who have already had a cardiovascular event and do not have any specific contraindication.5,16
Clinical studies reporting this evidence were conducted in patients populations selected according to the eligibility criteria of each trial, whereas the positive results of these studies are transferred to real-world patients who often have different characteristics than those of clinical research. In addition, the levels of physician adherence to guideline recommendations may vary not only with patient characteristics but also with different sociocultural and economic situations.
For these reasons, it is interesting to evaluate the CORE report that used an administrative database to describe: (i) the characteristics of real-world patients with a recent atherothrombotic event; (ii) statin treatments at discharge, an essential decision-making time point for secondary prevention strategies; (iii) their doses and prescriptive continuity; (iv) the rate and causes of rehospitalization; and (v) the overall costs for the Italian NHS for patients with these conditions in the year following the acute event.
Analysis methodology
The CORE report analyses were conducted using data from the ARNO (CINECA) database, a clinical Data Warehouse that combines, for each patient, data from various administrative databases (prescriptions for the medication dispensed by the NHS to each citizen, hospital discharge extracts, specialist ambulatory services, etc.), demographical data, and other information (socio-demographic data). The ARNO database is currently composed of a network of 32 local health authorities from all over Italy and reports data for approximately 11 million inhabitants.
The CORE report provides a very extensive and detailed analysis concerning resource utilization and the costs for patients with cardiovascular events. Reported data are extracted from two abstracts presented at the 18th International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Annual European Congress held in 2015.257,258
The cohort analysed
Patients with cardiovascular events were defined as those hospitalized with a main diagnosis of ACS or CVD (including stroke and TIA) or peripheral occlusive arterial disease (POAD) or who had had a coronary artery bypass grafting or coronary angioplasty procedure.
From a population of 2 989 512 potential patients from seven Italian local health unities, a cohort was extracted consisting of patients hospitalized from 1 January 2011 to 31 December 2011 (on an ordinary admission or day hospital basis) with ACS, CVD, or POAD as a main diagnosis.
All selected patients were observed for a follow-up period of 12 months following the index hospitalization, with the aim of quantifying the resource utilization and health care costs.
Statin use and cost assessment
The analyses regarding the quantitative use of resources (statin consumption and treatment adherence and cases of hospitalization) refer to the follow-up period alone (and therefore exclude the index hospitalization) and consequently only refer to patients who were discharged alive. More specifically:
regarding statin use, the analysis focuses on the first month of follow-up, as the most important decisions concerning secondary prevention strategies are made at discharge (additionally, for certain treatments, prescriptions at discharge are different from those issued subsequently, e.g. higher doses of statins, dual antiplatelet therapy, etc.);
compliance with statin therapy was attributed to those patients who in the 12-month follow-up period received a total of posology units compatible with the daily treatment indicated in the summary of product characteristics (SPC) for the statin in question (considering a 20% tolerance over 365 days). Therefore, in this case, the assessment was carried out on a whole year’s follow-up; and
the new cases of hospitalization were analysed from the day following the index hospitalization for the whole year’s follow-up.
The overall costs refer to the initial cohort (not merely those discharged alive) at the 1 year follow-up and include the cost of the index hospitalization and co-morbidity. The index hospitalization was taken into account because to attribute an average cost to patients with cardiovascular events, it is important to also consider the cost of the first hospitalization, regardless of its outcome.
Results
Overall, the cohort recruited for the analysis (17 213 patients) represented almost 0.6% of the total potential population (2 989 512 patients) (Table 23). The average age was 73 years and males accounted for a bit more than half the population (56%).
Table 23.
General characteristics of the sample cohort (potential patient population n = 2 989 512)
| Characteristics | ACS | CVD | POAD | Sum/mean |
|---|---|---|---|---|
| Enrolled patients (n) | 6226 | 9939 | 1048 | 17 213 |
| (% of the population) | 0.21 | 0.33 | 0.04 | 0.58 |
| Mean age, years | 71 | 75 | 73 | 73 |
| Mean age M | 68 | 73 | 71 | |
| Mean age F | 77 | 77 | 76 | |
| Gender (M %) | 65% | 49% | 68% | 56% |
| Patients with diabetes | 31% | 26% | 35% | 28% |
| Patients who died during index hospitalization (n) | 289 | 688 | 10 | 987 |
| Intrahospital mortality for all causes (%) | 4.6% | 6.9% | 1.0% | 5.7% |
| Patients at follow-up (n) | 5937 | 9251 | 1038 | 16 226 |
F, females; M, males; CVD, cerebrovascular disease; POAD, peripheral occlusive arterial disease; ACS, acute coronary syndrome.
The subgroup of patients with CVD is the largest (9939 patients), with characteristics that were significantly different (P < 0.001) from those of the other two subgroups. They had a higher average age (75 years), lower male presence (less than half, whereas in the other subgroups it was approximately two-thirds), and higher baseline in-hospital mortality (6.9%). Among patients with CVD, diabetes is present with a lower frequency (26%), whereas its frequency is highest among patients with POAD (35%).
Statin use
Statin use in the first month’s follow-up is expressed in terms of patients treated. It is therefore analysed in terms of prescription rates on two levels: by active substance and active substance/dose.
Among patients admitted for a cardiovascular event who were discharged alive (16 226), almost half (7220 or 44%) were treated with statins (Table 24). The highest prescription rate was observed among patients with ACS (more than two-thirds: 70%), whereas rates were approximately one-third in patients with CVD (29%) and POAD (37%).
Table 24.
Use of statins in patients with cardiovascular events by active substance (in the first month follow-up)
| ACS | CVD | POAD | Sum/mean | |
|---|---|---|---|---|
| Patients at follow-up (n) | 5937 | 9251 | 1038 | 16 226 |
| Of whom were treated with statins (n) | 4148 | 2684 | 388 | 7220 |
| Percentage | 70% | 29% | 37% | 44% |
| Of whom were treated with: | ||||
| Simvastatin | 695 | 1088 | 117 | 1900 |
| (% of those treated) | 16.8% | 40.5% | 30.2% | 26.3% |
| Lovastatin | 4 | 17 | 0 | 21 |
| (% of those treated) | 0.1% | 0.6% | 0.0% | 0.3% |
| Pravastatin | 37 | 114 | 14 | 165 |
| (% of those treated) | 0.9% | 4.2% | 3.6% | 2.3% |
| Fluvastatin | 8 | 14 | 3 | 25 |
| (% of those treated) | 0.2% | 0.5% | 0.8% | 0.3% |
| Atorvastatin | 2524 | 1048 | 138 | 3710 |
| (% of those treated) | 60.8% | 39.0% | 35.6% | 51.4% |
| Rosuvastatin | 885 | 405 | 96 | 1386 |
| (% of those treated) | 21.3% | 15.1% | 24.7% | 19.2% |
| Simvastatin and ezetimibe | 93 | 63 | 24 | 180 |
| (% of those treated) | 2.2% | 2.3% | 6.2% | 2.5% |
In each subgroup, adding together the number of patients treated according to the single active substances identified would provide a total higher than the corresponding value in the second row (which, e.g. for patients with ACS is equal to 4148). This can be explained by the fact that this total would count patients treated with more than one active substance more than once because of a switch during the month considered; whereas the total indicated in the second row is net of duplications.
CVD, cerebrovascular disease; POAD, peripheral occlusive arterial disease; ACS, acute coronary syndrome.
More than half (51.4%) of treated patients received atorvastatin. Almost all patients were treated with atorvastatin, simvastatin, or rosuvastatin, which combined account for 97% of the market in question. Treatment with atorvastatin was preferred among patients with ACS (60.8%) and POAD (35.6%), whereas simvastatin was preferred among those with CVD (42.7%, if we also consider the combination with ezetimibe).
Compliance to treatment with statins
The evaluation of compliance is based on the whole year’s follow-up. On average, treatment compliance is maintained by two patients of the every three (64.5%). Peak compliance is achieved among patients with ACS (71.7%).
There are more compliant patients than non-compliant ones only among patients treated with atorvastatin and rosuvastatin (at least considering the whole cohort level and the ACS subgroup).
Among diabetics, the distribution of the frequency of compliant patients among the three subgroups is very similar to that in the overall population: 68.7 vs. 71.7% in patients with ACS; 58.7 vs. 57.4% in those with CVD; and 54.7 vs. 55.8% in those with POAD.
New admissions
During the year of follow-up, more than half of all patients (55.7%) were readmitted (at least once, for various reasons). Of these, 63.3% of patients were from the ACS subgroup (the subgroup with the highest rehospitalization rate), 49.1% were from the CVD subgroup, and 57.6% were from the POAD subgroup. Each patient had an average of two hospitalizations (Table 25).
Table 25.
Admissions during follow-up for cardiovascular and other events in the three groups of patients
| ACS | CVD | POAD | Sum/mean | |||||
|---|---|---|---|---|---|---|---|---|
| Patients at follow-up (n) | 5937 | 9251 | 1038 | 16 226 | ||||
| Hospitalised patients | ||||||||
| Admission diagnosis | Pt hosp | Hosp/pt | Pt hosp | Hosp/pt | Pt hosp | Hosp/pt | Pt hosp | Hosp/pt |
| ACS | 1347 | 1.3 | 147 | 1.2 | 36 | 1.4 | 1530 | 1.3 |
| (% of patients at follow-up) | 22.7% | 1.6% | 3.5% | 20.2% | ||||
| CVD | 165 | 1.2 | 1512 | 1.2 | 35 | 1.3 | 1712 | 1.2 |
| (% of patients at follow-up) | 2.8 | 16.3% | 3.4% | 14.8% | ||||
| POAD | 1 | 2 | 1 | 1 | 2 | 1.5 | ||
| (% of patients at follow-up) | 0% | 0.1% | 0.1% | |||||
| Other CV causesa | 2653 | 1.4 | 1614 | 1.3 | 452 | 1.6 | 4719 | 1.4 |
| (% of patients at follow-up) | 44.7% | 17.4% | 43.5% | 35.3% | ||||
| Total CV causes | 2994 | 1.9 | 2781 | 1.5 | 457 | 1.8 | 6232 | 1.7 |
| (% of patients at follow-up) | 50.4% | 30.1% | 44% | 40.9% | ||||
| Total non-CV causes | 1703 | 1.6 | 2714 | 1.6 | 300 | 1.7 | 4717 | 1.6 |
| (% of patients at follow-up) | 28.7% | 29.3% | 28.9% | 29.1% | ||||
| Total for all causes | 3761 | 2.2 | 4541 | 1.9 | 598 | 2.2 | 8900 | 2 |
| (% of patients at follow-up) | 63.3% | 49.1% | 57.6% | 55.7% | ||||
A patient admitted more than once with different diagnoses (causes) will count in the incidence rate of each diagnosis. Total CV causes is net of duplications when the causes are of a CV nature (and similarly for the total non-CV causes). If the patient was admitted for CV causes and non-CV causes, he/she is counted once in the first total and another in the second. The total for all causes is also net of this duplication.
CV, cardiovascular; CVD, cerebrovascular disease; POAD, peripheral occlusive arterial disease; Pt hosp, patients hospitalised; Hosp/pt, average number of hospitalizations per hospitalized patient; ACS, acute coronary syndrome.
Coronary artery bypass grafting/coronary angioplasty + decompensated heart failure + other CV causes.
It is important to stress that the proportion of hospitalizations for causes other than cardiovascular events is not negligible: 3 patients of the 10 (29.1%) were hospitalized for non-cardiovascular causes during the follow-up, compared with 4 of the 10 (40.9%) for cardiovascular causes. The proportion of admissions for non-cardiovascular causes is similar in all three subgroups, whereas there are differences between the three groups in the proportion of hospital admissions for cardiovascular causes: 50.4% in patients with ACS, 30.1% in patients with CVD, and 44% in patients with POAD.
The probability of being readmitted with the same diagnosis as assigned at the index hospitalization is higher among patients with ACS (22.7%), lower among patients with CVD (16.3%), and minimal among patients with POAD (0.1%).
Rehospitalization rates among diabetic patients for all causes are higher than the corresponding ones in the general population: 69.3 vs. 63.3% (ACS subgroup); 52.3 vs. 49.1% (CVD subgroup); and 65.3 vs. 57.6% (POAD subgroup).
Cost analysis
The mean annual costs incurred by the NHS for diabetic patients with ACS was €16 897: €14 199 for hospital admissions, €1691 for medication, and €1008 for diagnostic procedures and outpatient clinic visits. Figure 12 shows how the costs are allocated between the two cohorts. It is important to remember that the costs refer to all enrolled patients (not just those discharged alive), during the 1 year follow-up, and include the cost of the index hospitalization and co-morbidities. A patient with cardiovascular events costs the NHS €11 617 a year on average, of which 86.6% is due to hospital admissions—the dominant costdriver (Table 26).
Figure 12.
Mean annual cost of diabetic patients with acute coronary syndrome (ACS) incurred by the National Health Service. CV, cardiovascular.
Table 26.
Components of health care expenditure for patients with cardiovascular events
| ACS | CVD | POAD | Sum/mean | |||||
|---|---|---|---|---|---|---|---|---|
| Enrolled patients (n) | 6226 | 9939 | 1048 | 17 213 | ||||
| Mean annual spending/patient | € | % | € | % | € | % | € | % |
| Expenditure item | ||||||||
| Pharmaceuticals (CV drugs)a | 821 | 5.5 | 353 | 3.7 | 571 | 4.8 | 536 | 4.6 |
| Pharmaceuticals (other medication) | 494 | 3.3 | 442 | 4.6 | 635 | 5.3 | 473 | 4.1 |
| Hospitalisations | 12 836 | 86.3 | 8338 | 87.4 | 9911 | 82.6 | 10 061 | 86.6 |
| Specialist services | 720 | 4.8 | 404 | 4.2 | 887 | 7.4 | 548 | 4.7 |
| Total | 14 871 | 100 | 9537 | 100 | 12 004 | 100 | 11 617 | 100 |
CV, cardiovascular; CVD, cerebrovascular disease; POAD, peripheral occlusive arterial disease; ACS, acute coronary syndrome.
Drugs with ATC code = ‘C’ (cardiovascular system) or = ‘B01’ (antithrombotic agents).
Patients with ACS have a higher overall mean cost (€14 871) when compared with the €9537 cost for patients with CVD and the €12 004 cost for patients with a POAD.
Patients with CVD cost least. The highest cost for non-cardiovascular drugs (i.e. those relating to the co-morbidities) and for specialist services, on the other hand, is accrued by patients with POAD (€635 and €887, respectively).
In the overall expenditure for pharmaceuticals, the cost for cardiovascular drugs prevails over that for non-cardiovascular drugs in patients with ACS (€821 vs. €494). Conversely, in the other two subgroups, the expenditure for other drugs is prevalent.
In diabetic patients the mean expenditure is higher—both for the individual items and the totals—when compared with the overall patient population. Indeed, in diabetic patients, the overall mean expenditure (€13 045) is 12.3% higher than the mean expenditure in the total patient population (€11 617) as indicated in Table 26.
Discussion and conclusions
Patients in the real world are known to differ from clinical study populations because the latter have a more careful follow-up. This gives rise to the need to conduct further research in order to obtain realistic information about the actual conditions and costs to manage in daily clinical practice.
A drug utilization study has been conducted that not only evaluated diagnostic and therapeutic pathways but also the costs of health care (from an NHS prospective) for patients with cardiovascular conditions. Here, we recap some of the critical issues that the study brought to light, starting with those regarding treatment with statins.
Less than half (44%) of the patients requiring therapy are treated (Table 24). More specifically, only a few patients with CVD (29%) and with POAD (37%) are treated; in these groups, the low-dose intensity treatment prevails.
With regard to statin therapy adherence, two patientsof every three with cardiovascular disease (64.5%) can be considered compliant, with a slightly higher compliance in the ACS subgroup (71.7%). Adherence is, therefore, unsatisfactory overall, especially among patients with CVD (57.4%) and POAD (55.8%).
CORE report results do not provide information concerning the achievement of C-LDL targets in Italy.
Diabetic patients with atherothrombotic events have a higher cost (+12.3%), in terms of both pharmaceutical expenditure (for cardiovascular medication: +19.8%; for non-cardiovascular medication: +53.4%) and expenditure for hospitalization (+8.4%).
Spending for readmission for non-cardiovascular causes is not negligible (18% of the total), due to patient age (average age of 73 years for the general population) and co-morbidities (such as in patients with POAD).
The annual payment of the NHS for a cardiovascular patient is high (€11 617) and expenditures for hospitalization (86.6% of the total) are the main costdriver (for a comparison in the general population, the mean expenditure on health care per capita in 2013 was estimated at €1816).259
As shown by the critical aspects discussed above, there is still a gap between evidence-based recommendations and routine clinical practice—a gap that generates a high social and economic burden for Italy’s health care facilities.
From efficacy and safety to clinical effectiveness. The challenge of monoclonal antibodies in sustainable cardiovascular prevention: the reuse of savings in rehospitalizations
The cost-effectiveness of statin therapy has been extensively documented,25 and there is an ongoing debate about the possible favourable cost-effectiveness of low-potency statin therapy as a primary prevention strategy in subjects with a low to moderate risk of events in the next 10 years.260 Indeed, because of the wide use of low-cost statins, these drugs no longer represent a cost issue. For PCSK9-inhibiting monoclonal antibodies, on the other hand, the cost-effectiveness debate has just begun.
In the USA, the New England Comparative Effectiveness Public Advisory Council (CEPAC) report on behalf of the ICER entitled ‘PCSK9 Inhibitors for Treatment of High Cholesterol: Effectiveness, Value, and Value-Based Price Benchmarks’ was published on 24 November 2015.261 These two institutions are independent non-profit research organizations working in the medical and economic field and the published report concludes an in-depth analysis involving researchers, physicians, and the pharmaceutical industry.
The analysis started from the ‘unmet clinical needs’ (such as the adequate treatment in patients who, despite maximum dose statin therapy, are unable to meet the therapeutic target) and considered the data included in a meta-analysis conducted by Navarese et al.,262 which predicted costs for the new therapies of between $14 100 and $14 600/year/patient in the USA. The conclusions of this analysis generate certain concerns, especially considering the wide range of potential treatment users. Indeed, although these agents make it possible to obtain reductions in LDL levels of between 52.6% and 63.5% (vs. no PCSK9 inhibitors) and between 31.7% and 39.3% (vs. ezetimibe) and a short-term reduction in OR for total mortality and myocardial infarction of approximately 50% (but with a modest number of events and a very large 95% CI). PCSK9 inhibitors would have a cost of $135 000–$290 000/QALY (the former figure for statins + ezetimibe and the latter for statins + PCSK9 inhibitors) in patients with FH, and of $145 000–$274 000/QALY in patients undergoing secondary prevention intervention who are statin intolerant and of $135 000–$302 000 for patients undergoing secondary prevention intervention who do not meet their therapeutic goal with statins. PCSK9 inhibitors would only be cost-effective if their predicted cost is reduced 80%. Indeed, only with a cost of $2100–$2600/year/patient would it be possible to meet the cost-effectiveness threshold of $50 000/QALY. A cost of approximately $5200/year/patient would meet a more generous threshold of $150 000/QALY. It should be noted that these latter costs are closer to the current cost of PCSK9 inhibitors proposed in some European countries ($6800 in the UK, $8200 in Austria, and $8800 in Finland).
In Italy, we are still lacking an essential element for this kind of analysis because the amount of reimbursement for these drugs from the NHS is unknown. It is therefore still very difficult to estimate the total economic impact of PCSK9 inhibitors, and it is impossible to verify whether it is possible to associate a reduction in events (currently unknown in the medium and long term) with an acceptable cost-effectiveness of therapy in the various potential treatment clinical settings: in patients with HeFH, and in patients undergoing primary (high-risk) and secondary prevention interventions who despite maximum dose statin therapy did not reach their therapeutic targets, and in statin-intolerant patients.
It is, however, worth highlighting the fact that the CORE report257 revealed that:
not even half (44%) of the patients requiring therapy are treated;
compliance to statin therapy is unsatisfactory;
spending for readmissions is not negligible; and
the annual cost to the NHS for a cardiovascular patient is high and spending for hospitalization is the main costdriver.
We also know that the aim of reducing LDL by ≥50% is rarely possible in both primary preventions in patients at a high cardiovascular risk [the Statins Target Assessment in Real practice (STAR) study revealed that this goal is met in <30% of naive patients263] and in secondary preventions where the target of C-LDL <70 mg/dL is met and maintained with difficulty.
There is therefore an urgent need to more effectively treat patients with the high-dose, high-efficacy compounds currently available, while implementing all potential strategies for reducing SI and improving compliance over time. It is also necessary that clinical and organizational tools proved to be useful in the therapeutic optimization of patients undergoing secondary prevention intervention after coronary events are implemented and spread out as far as possible over the whole national territory. A structured secondary prevention pathway such as that proposed in cardiac rehabilitation has shown that high statin doses and the therapeutic target can be maintained at 1 year in almost 70% of patients.264
Conflict of Interest: none declared.
References
- 1. Nichols M, Townsend N, Scarborough P, et al. ; European Society of Cardiology; European Heart Network; British Heart Foundation Health Promotion Research Group. European Cardiovascular Disease Statistics, 2012 edition. http://www.ehnheart. org/component /downloads/ downloads/1436 (19 April 2017).
- 2. Neaton JD, Blackburn H, Jacobs D, Kuller L, Lee DJ, Sherwin R, Shih J, Stamler J, Wentworth D.. Serum cholesterol level mortality findings for men screened in the Multiple Risk Factor Intervention Trial. Multiple Risk Factor Intervention Trial Research Group. Arch Intern Med 1992;152:1490–1500. [PubMed] [Google Scholar]
- 3. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 4. Prospective Studies Collaboration. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007;370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 5. Perk J, De Backer G, Gohlke H, et al. ; European Association for Cardiovascular Prevention and Rehabilitation (EACPR) ESC Committee for Practice Guidelines (CPG). European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 6. Giampaoli S, Vanuzzo D.. La salute cardiovascolare degli italiani. Terzo Atlante Italiano delle Malattie Cardiovascolari. Edizione 2014. G It Cardiol 2014;15(4 Suppl 1):1S–31S. [Google Scholar]
- 7. Giampaoli S, Palmieri L, Donfrancesco C, Lo Noce C, Pilotto L, Vanuzzo D.. Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey Research Group Cardiovascular health in Italy. Ten-year surveillance of cardiovascular diseases and risk factors: Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey 1998-2012. Eur J Prev Cardiol 2015;22(2 Suppl):9–37. [DOI] [PubMed] [Google Scholar]
- 8. Reiner Z, Catapano AL, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011;32:1769–1818. [DOI] [PubMed] [Google Scholar]
- 9. Stamler J. Low risk—and the "No more than 50%" myth/dogma. Arch Intern Med 2007;167:537–539. [DOI] [PubMed] [Google Scholar]
- 10. Daviglus ML, Liu K, Pirzada A, et al. Favorable cardiovascular risk profile in middle age and health-related quality of life in older age. Arch Intern Med 2003;163:2460–2468. [DOI] [PubMed] [Google Scholar]
- 11. Daviglus ML, Liu K, Pirzada A, et al. Cardiovascular risk profile earlier in life and Medicare costs in the last year of life. Arch Intern Med 2005;165:1028–1034. [DOI] [PubMed] [Google Scholar]
- 12. Liu K, Daviglus M, Loria C, et al. Healthy lifestyle through young adulthood and the presence of low cardiovascular diseases risk profile in middle age: the Coronary Artery Risk Development in (Young) Adults (CARDIA) study. Circulation 2012;125:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lloyd-Jones DM, Hong Y, Labarthe D, et al. ; American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- 14. Giampaoli S, Palmieri L, Panico S, et al. Favorable cardiovascular risk profile (low risk) and 10-year stroke incidence in women and men: findings on twelve Italian population samples. Am J Epidemiol 2006;163:893–902. [DOI] [PubMed] [Google Scholar]
- 15. Palmieri L, Donfrancesco C, Giampaoli S, et al. Favorable cardiovascular risk profile and 10-year coronary heart disease incidence in women and men: results from the Progetto CUORE. Eur J Cardiovasc Prev Rehabil 2006;13:562–570. [DOI] [PubMed] [Google Scholar]
- 16. Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force. On Practice Guidelines. Circulation 2014;129(25 Suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 17. Giampaoli S, Rizzo C, Vanuzzo D.. L’applicazione italiana delle linee guida europee sulla prevenzione delle malattie cardiovascolari. G Ital Cardiol 2008;9:60–67. [PubMed] [Google Scholar]
- 18. Donfrancesco C, Palmieri L, Coneey MT, et al. Italian cardiovascular mortality charts of the CUORE Project: are they comparable with the score charts?. Eur J Cardiovasc Prev Rehabil 2010;17:403–409. [DOI] [PubMed] [Google Scholar]
- 19. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med 1996;335:1001–1009. [DOI] [PubMed] [Google Scholar]
- 20. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo controlled trial. Lancet 2002;360:7–22.12114036 [Google Scholar]
- 21. LaRosa JC, Grundy SM, Waters DD, et al. ; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 22. Pedersen TR, Faergeman O, Kastelein JJP, et al. ; Incremental Decrease in End Points Through Aggressive Lipid Lowering (IDEAL) Study Group . High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: The IDEAL study: a randomized controlled trial. JAMA 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 23. Kulik A, Brookhart MA, Levin R, Ruel M, Solomon DH, Choudhry NK.. Impact of statin use on outcomes after coronary artery bypass graft surgery. Circulation 2008;118:1785–1792. [DOI] [PubMed] [Google Scholar]
- 24. Baigent C, Keech A, Kearney PM, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 25. Baigent C, Blackwell L, Emberson J; Cholesterol Treatment Trialists’ (CTT) Collaboration, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Catapano AL, Averna M, Faggiano P, Novo S; Federazione Italiana di Cardiologia; Società Italiana per lo Studio dell’Arteriosclerosi, con l’endorsement della European Society of Atherosclerosis e della European Association of Cardiovascular Prevention and Rehabilitation . Nuove linee guida americane 2013 ACC/AHA sul trattamento del colesterolo plasmatico per ridurre il rischio cardiovascolare aterosclerotico: confronto con le raccomandazioni ESC/EAS per la gestione delle dislipidemie. G Ital Cardiol 2014;15:19–20. [DOI] [PubMed] [Google Scholar]
- 27. Cannon CP, Blazing MA, Giugliano RP, IMPROVE-IT Investigators, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 28. The Final Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III): Executive Summary. Bethesda, MD: US National Heart, Lung, and Blood Institute, National Institutes of Health; 2001. NIH publication No. 01-3670. [Google Scholar]
- 29. Palmieri L, Bennett K, Giampaoli S, Capewell S.. Explaining the decrease in coronary heart disease mortality in Italy between 1980 and 2000. Am J Public Health 2010;100:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S76–S99. [DOI] [PubMed] [Google Scholar]
- 31. Mente A, de Koning L, Shannon HS, Anand SS.. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med 2009;169:659–669. [DOI] [PubMed] [Google Scholar]
- 32. Mensink RP, Zock PL, Kester ADM, Katan MB.. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 2003;77:1146–1155. [DOI] [PubMed] [Google Scholar]
- 33. Mozaffarian D, Aro A, Willett WC.. Health effects of trans-fatty acids: experimental and observational evidence. Eur J Clin Nutr 2009;63(Suppl 2):S5–21. [DOI] [PubMed] [Google Scholar]
- 34. GISSI-Prevenzione Investigators (Gruppo Italiano per la Sopravvivenza nell’Infarto Miocardico). Dietary supplementation with n-3 polyunsatured fatty acids and vitamine E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 1999;354:447–455. [PubMed] [Google Scholar]
- 35. Brown L, Rosner B, Willet W, Sacks SM.. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69:30–42. [DOI] [PubMed] [Google Scholar]
- 36. Nordmann AJ, Nordmann A, Briel M, et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med 2006;166:285–293. [DOI] [PubMed] [Google Scholar]
- 37. Keys A. Serum cholesterol response to dietary cholesterol. Am J Clin Nutr 1984;40:351–359. [DOI] [PubMed] [Google Scholar]
- 38. Estruch R, Ros E, Salas-Salvadó J, et al. ; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–1290.23432189 [Google Scholar]
- 39. de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N.. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999;99:779–785. [DOI] [PubMed] [Google Scholar]
- 40. Keys AB, Keys M.. How to Eat Well and Stay Well: The Mediterranean Way. New York: Doubleday; 1975. [Google Scholar]
- 41. Fidanza F, Alberti A, Fruttini D, The Nicotera diet: the reference Italian Mediterranean diet In: Simopoulos AP, ed. Nutrition and Fitness: Mental Health, Aging, and the Implementation of a Healthy Diet and Physical Activity Lifestyle. Basel: Karger; 2005. p115–21. [DOI] [PubMed] [Google Scholar]
- 42. Appel L, Brands M, Daniels S, Karanja N, Elmer P, Sacks F.. Dietary approaches to prevent and treat hypertension. A Scientific Statement from the American Heart Association. Hypertension 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 43. Giampaoli S, Krogh V, Grioni S, et al. ; Gruppo di Ricerca dell’Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey. Comportamenti alimentari degli italiani: risultati dell’Osservatorio Epidemiologico Cardiovascolare/Health Examination Survey. Epidemiol Prev 2015;39:373–379. [PubMed] [Google Scholar]
- 44. Stamler J. Toward a modern Mediterranean diet for the 21st century. Nutr Metab Cardiovasc Dis 2013;23:1159–1162. [DOI] [PubMed] [Google Scholar]
- 45. Huffman KM, Hawk VH, Henes ST, et al. Exercise effects on lipids in persons with varying dietary patterns-does diet matter if they exercise? Responses in Studies of a Targeted Risk Reduction Intervention through Defined Exercise I. Am Heart J 2012;164:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Piepoli MF, Corrà U, Benzer W, et al. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur J Cardiovasc Prev Rehabil 2010;17:1–17. [DOI] [PubMed] [Google Scholar]
- 47. Shaw K, Gennat H, O’Rourke P, Del Mar C.. Exercise for overweight or obesity. Cochrane Database Syst Rev 2006;4:CD003817.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dattilo AM, Kris-Etherton PM.. Effects of weight reduction on blood lipids and lipoproteins: a meta- analysis. Am J Clin Nutr 1992;5:320–328. [DOI] [PubMed] [Google Scholar]
- 49. NHS Foundation Trust . Guidelines on Statin Prescribing in the Prevention of Cardiovascular Disease. London: University College London Hospitals; 2006. [Google Scholar]
- 50. Rapporto ARNO. 2015. http://www.siditalia.it/images/Documenti/NEWS/Rapporto_Arno_Diabete_2015.pdf (19 April 2017).
- 51. Brun E, Nelson RG, Bennett PH, et al. Verona Diabetes Study. Diabetes duration and cause- specific mortality in the Verona Diabetes Study. Diabetes Care 2000;23:1119–1123. [DOI] [PubMed] [Google Scholar]
- 52. Vaccaro O, Eberly LE, Neaton JD, et al. ; Multiple Risk Factor Intervention Trial Research Group. Impact of diabetes and previous myocardial infarction on long-term survival: 25-year mortality follow-up of primary screenees of the Multiple Risk Factor Intervention Trial. Arch Intern Med 2004;164:1438–1443. [DOI] [PubMed] [Google Scholar]
- 53. Rivellese AA, Riccardi G, Vaccaro O.. Cardiovascular risk in women with diabetes. Nutr Metab Cardiovasc Dis 2010;20:474–480. [DOI] [PubMed] [Google Scholar]
- 54. Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 55. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia 2015;58:886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Andrikoula M, McDowell IF.. The contribution of ApoB and ApoA1 measurements to cardiovascular risk assessment. Diabetes Obes Metab 2008;10:271–278. [DOI] [PubMed] [Google Scholar]
- 57. Kearney PM, Blackwell L, et al. Cholesterol Treatment Trialists’ (CTT) Collaborator.s Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125. [DOI] [PubMed] [Google Scholar]
- 58. Standards SID/AMD. 2014. http://www.standarditaliani.it/skin/www.standarditaliani.it/pdf/STANDARD_2014_May28.pdf (19 April 2017).
- 59. Collins R, Armitage J, Parish S, et al. ; Heart Protection Study Collaborative Group . MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–2016. [DOI] [PubMed] [Google Scholar]
- 60. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo controlled trial. Lancet 2004;364:685–696. [DOI] [PubMed] [Google Scholar]
- 61. Armitage J. The safety of statins in clinical practice. Lancet 2007;370:1781–1790. [DOI] [PubMed] [Google Scholar]
- 62. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 63. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 64. Bozzetto L, Annuzzi G, Corte GD, et al. Ezetimibe beneficially influences fasting and postprandial triglyceride-rich lipoproteins in type 2 diabetes. Atherosclerosis 2011;217:142–148. [DOI] [PubMed] [Google Scholar]
- 65. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351–364. DOI: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 66. Gudzune KA, Monroe AK, Sharma R, et al. Effectiveness of combination therapy with statin and another lipid-modifying agent compared with intensified statin monotherapy. A systematic review. Ann Intern Med 2014;160:468–476. [DOI] [PubMed] [Google Scholar]
- 67. Hansen M, Sonne DP, Knop FK.. Bile acid sequestrants: glucose-lowering mechanisms and efficacy in type 2 diabetes. Curr Diab Rep 2014;14:482.. [DOI] [PubMed] [Google Scholar]
- 68. Frick MH, Elo O, Haapa K, et al. Helsinki Heart Study: Primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia: Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med 1987;317:1237–1245. [DOI] [PubMed] [Google Scholar]
- 69. Rubins HB, Robins SJ, Collins D, et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med 1999;341:410–418. [DOI] [PubMed] [Google Scholar]
- 70. Haim M, Benderly M, Brunner D, et al. Elevated serum triglyceride levels and long-term mortality in patients with coronary heart disease: The Bezafibrate Infarction Prevention (BIP) Registry. Circulation 1999;100:475–482. [DOI] [PubMed] [Google Scholar]
- 71. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849–1861. [DOI] [PubMed] [Google Scholar]
- 72. Ginsberg HN, Elam MB, Lovato LC, et al. ; ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010;375:1875–1884. [DOI] [PubMed] [Google Scholar]
- 74. Sacks FM, Carey VJ, Fruchart JC.. Combination lipid therapy in type 2 diabetes. N Engl J Med 2010;363:692–694. [DOI] [PubMed] [Google Scholar]
- 75. Hegele RA, Ginsberg HN, Chapman MJ, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol 2014;2:655–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Morgan CL, Owens DR, Aubonnet P, et al. Primary prevention of diabetic retinopathy with fibrates: a retrospective, matched cohort study. BMJ Open 2013;3:e004025.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ding Y, Li Y, Wen A.. Effect of niacin on lipids and glucose in patients with type 2 diabetes: a meta-analysis of randomized, controlled clinical trials. Clin Nutr 2015;34:838–844. [DOI] [PubMed] [Google Scholar]
- 78. Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA 1975;231:360–381. [PubMed] [Google Scholar]
- 79. Boden WE, Probstfield JL, Anderson T, et al. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 80. Landray MJ, Haynes R, Hopewell JC, et al. ; HPS2-THRIVE Collaborative Group . Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 81. McKenney J, Bays H, Gleim G, et al. Safety and tolerability of extended-release niacin laropiprant: pooled analyses for 11,310 patients in 12 controlled clinical trials. J Clin Lipidol 2015;9:313–325. [DOI] [PubMed] [Google Scholar]
- 82. Ginsberg HN, Reyes-Soffer G.. Niacin: a long history, but a questionable future. Curr Opin Lipidol 2013;24:475–479. [DOI] [PubMed] [Google Scholar]
- 83. Sattar N, Preiss D, Robinson JG, et al. Lipid-lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta-analysis of individual patient data. Lancet Diabetes Endocrinol 2016;4:403–410. [DOI] [PubMed] [Google Scholar]
- 84. Robinson JG, Farnier M, Krempf M, et al. ; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 85. Sabatine MS, Giugliano RP, Wiviott SD, et al. ; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 86. Masana L, Pedro-Botet J, Civeira F.. IMPROVE-IT clinical implications: Should the “high-intensity cholesterol-lowering therapy” strategy replace the “high-intensity statin therapy”. Atherosclerosis 2015;240:161–162. [DOI] [PubMed] [Google Scholar]
- 87. Santos RD. PCSK9 inhibition in type 2 diabetes: so far so good, but not there yet. Lancet Diabetes Endocrinol 2016;4:377–379. [DOI] [PubMed] [Google Scholar]
- 88. DeFilippis AP, Sperling LS.. Understanding omega-3’s. Am Heart J 2006;151:564–570. [DOI] [PubMed] [Google Scholar]
- 89. Kris-Etherton PM, Harris WS, Appel LJ; Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arterioscler Thromb Vasc Biol 2003;23:e20–e30. [DOI] [PubMed] [Google Scholar]
- 90. Balk EM, Lichtenstein AH, Chung M, et al. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 2006;189:19–30. [DOI] [PubMed] [Google Scholar]
- 91. Ballantyne C, Abate N, Zhong Y, et al. Dose comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia. the Vytorin Versus Atorvastatin (VYVA Study). Am Heart J 2005;149:464–473. [DOI] [PubMed] [Google Scholar]
- 92. Catapano A, Brady WE, King TR, et al. Lipid altering-efficacy of ezetimibe co-administered with simvastatin compared with rosuvastatin: a meta-analysis of pooled data from 14 clinical trials. Curr Med Res Opin 2005;21:1123–1130. [DOI] [PubMed] [Google Scholar]
- 93. Determinazione AIFA 19.6.2014, n. 617. Gazzetta Ufficiale n. 156 dell’8.7.2014: Modifica alla nota 13 di cui alla Determina del 26.3.2013. http://www.agenziafarmaco.gov. it/sites/default /files/Nota13 _GU156_08072014.pdf (19 April 2017).
- 94. Donfrancesco C, Palmieri L, Vanuzzo D.. Omogeneità delle carte del rischio del Progetto CUORE per la valutazione della mortalità cardiovascolare e le carte del Progetto SCORE. G Ital Cardiol 2010;11:148–153. [PubMed] [Google Scholar]
- 95. Downs JR, O’Malley PG.. Management of Dyslipidemia for Cardiovascular Disease Risk Reduction: Synopsis of the 2014 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guideline. Ann Intern Med 2015;163:291–297. [DOI] [PubMed] [Google Scholar]
- 96. Filippi A, Medea G, Catapano A. La diagnosi delle principali dislipidemie familiari in Medicina Generale. Sintesi per la Medicina Generale italiana—Aggiornamento 2010. Pisa: Rivista della Società Italiana di Medicina Generale; 2010. p41–8.
- 97. Il Progetto CUORE. Calcolo del punteggio individuale. http://www.cuore.iss.it/ sopra/calc- rischio.asp (19 April 2017).
- 98. Law MR, Wald NJ, Rudnicka AR.. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1423–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heart Score. The Interactive Tool for Predicting and Managing the Risk of Heart Attack and Stroke. http://www.heartscore. org/Pages/ welcome.aspx (19 April 2017).
- 100. Stone NJ, Blum CB.. Primary risk prevention in the elderly: use clinician-patient discussions, not automatic statin prescribing. Am J Med 2015;128:804–806. [DOI] [PubMed] [Google Scholar]
- 101.Nota 13: tra ombre e luci … poche. http://www.informazionisuifarmaci.it/nota-13-tra-ombre-e-lucipoche (19 April 2016).
- 102. Bianchi A, Casula M, Linee guida e nota 13 AIFA. http://www.sisa.it/index.php?class= Comp&className=Content&op= Show& param= cid,393,preview,0 (19 April 2017).
- 103.La nuova nota 13 AIFA. https://www.simg.it/documenti/aree_ cliniche/Cardiovascolare /supporti/nota13/commento _nota_13_marzo_2013.pdf (19 April 2017).
- 104. Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Consensus Statement of the European Atherosclerosis Society. Eur Heart J 2013;34:3478–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Goldstein JK, Hobbs HH, Brown MS, Familial hypercholesterolemia In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The Metabolic and Molecular Bases of Inherited Disease. 8th ed.New York, NY: McGraw-Hill; 2001. p2863–913. [Google Scholar]
- 106. Austin MA, Hutter CM, Zimmern RL, Humphries SE.. Genetic causes of monogenic heterozygous familial hypercholesterolemia: a HuGE prevalence review. Am J Epidemiol 2004;160:407–420. [DOI] [PubMed] [Google Scholar]
- 107. Versmissen J, Oosterveer DM, Yazdanpanah M, et al. Efficacy of statins in familial hypercholesterolaemia: a long term cohort study. BMJ 2008;337:a2423.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Marks D, Thorogood M, Neil HA, Humphries SE.. A review on the diagnosis, natural history, and treatment of familial hypercholesterolaemia. Atherosclerosis 2003;168:1–14. [DOI] [PubMed] [Google Scholar]
- 109. World Health Organization. World Health Statistics2012. http://www.who.int/gho/publications /world_health_ statistics/2012 /en/ (19 April 2017).
- 110. Nordestgaard BG, Chapman MJ, Humphries SE, et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease. Consensus statement of the European Atherosclerosis Society. Eur Heart J 2013;34:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Sjouke B, Kusters DM, Kindt I, et al. Homozygous autosomal dominant hypercholesterolemia in the Netherlands: prevalence, genotype-phenotype relationship and clinical outcome. Eur Heart J 2015;36:560–565. [DOI] [PubMed] [Google Scholar]
- 112. Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the consensus panel on familial hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.British Heart Foundation. UCL LDLR Database http://www.ucl.ac.uk/ldlr/LOVDv. 1.1.0/index.php?select _db=LDLR (19 April 2017).
- 114. Talmud PJ, Shah S, Whittall R, et al. Use of low-density lipoprotein cholesterol gene score to distinguish patients with polygenic and monogenic familial hypercholesterolemia: a case-control study. Lancet 2013;381:1293–1301. [DOI] [PubMed] [Google Scholar]
- 115. Goldberg AC, Hopkins PN, Toth PP, et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: clinical guidance from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J Clin Lipidol 2011;5:133–140. [DOI] [PubMed] [Google Scholar]
- 116. Olesen H. Properties and units in the clinical laboratory sciences. I. Syntax and semantic rules (recommendation 1995). International Union of Pure and Applied Chemistry (IUPAC) and International Federation of Clinical Chemistry (IFCC). Eur J Clin Chem Clin Biochem 1995;33:627–636. [PubMed] [Google Scholar]
- 117. Spandrio L, Pagani F, Panteghini M.. Criteri interpretativi per l’utilizzo ottimale degli esami di laboratorio In Panteghini M, ed. Interpretazione degli esami di laboratorio. Padova: Piccin Nuova Libraria; 2008. p25–46. [Google Scholar]
- 118. Clinical and Laboratory Standards Institute. Defining, Establishing and Verifying Reference Intervals in the Clinical Laboratory. Approved Guideline—Third Edition CLSI Document EP28-A3c. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. [Google Scholar]
- 119. Stamler J, Wentworth D, Neaton JD.. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT). JAMA 1986;256:2823–2828. [PubMed] [Google Scholar]
- 120. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the Third Report on the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 121. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guidelines on the assessment of cardiovascular risk: a report of the American College of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nordestgaard BG, Langsted A, Mora S, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points - a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J 2016;37:1944–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. National Heart, Lung, and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. https://www.nhlbi.nih.gov/files /docs/peds_ guidelines_sum.pdf (19 April 2017). [DOI] [PMC free article] [PubMed]
- 124. Westgard QC. Quality Requirements. Desirable Biological Variation Database Specifications. https://www.westgard.com/ biodatabase1.htm (19 April 2017).
- 125. Solétormos G, Duffy MJ, Hayes DF, et al. Design of tumor biomarker-monitoring trials: a proposal by the European Group on Tumor Markers. Clin Chem 2013;59:52–59. [DOI] [PubMed] [Google Scholar]
- 126. Lippi G, Caputo M, Banfi G, Buttarello M, et al. Raccomandazioni per l’identificazione e la gestione dei valori critici nei laboratori clinici. Biochim Clin 2008;32:209–216. [Google Scholar]
- 127. Averna M, Brignoli O, Bucci M, et al. ; per ANMCO, SIMG e SISA. Linee guida cliniche per la prevenzione della cardiopatia ischemica nella ipercolesterolemia familiare. G Ital Arterioscler 2013;4(Suppl 1):35–63. [Google Scholar]
- 128. Hovingh GK, Gandra SR, McKendrick J, et al. Identification and management of patients with statin-associated symptoms in clinical practice: a clinician survey. Atherosclerosis 2016;245:111–117. [DOI] [PubMed] [Google Scholar]
- 129. Colivicchi F, Bassi A, Santini M, Caltagirone C.. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke 2007;38:2652–2657. [DOI] [PubMed] [Google Scholar]
- 130. Daskalopoulou SS, Delaney JA, Filion KB, Brophy JM, Mayo NE, Suissa S.. Discontinuation of statin therapy following an acute myocardial infarction: a population-based study. Eur Heart J 2008;29:2083–2091. [DOI] [PubMed] [Google Scholar]
- 131. Kim MC, Cho JY, Jeong HC, et al. Impact of postdischarge statin withdrawal on long-term outcomes in patients with acute myocardial infarction. Am J Cardiol 2015;115:1–7. [DOI] [PubMed] [Google Scholar]
- 132. Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can. J Cardiol 2013;29:1553–1568. [DOI] [PubMed] [Google Scholar]
- 133. Guyton JR, Bays HE, Grundy SM, Jacobson TA.. The National Lipid Association Statin Intolerance Panel An assessment by the Statin Intolerance Panel: 2014 update. J Clin Lipidol 2014;8(3 Suppl):S72–S81. [DOI] [PubMed] [Google Scholar]
- 134. Stroes ES, Thompson PD, Corsini A, et al. ; European Atherosclerosis Society Consensus Panel . Statin-associated muscle symptoms: impact on statin therapy—European Atherosclerosis Society Consensus Panel Statement on Assessment, aetiology and management. Eur Heart J 2015;36:1012–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Santos RD, Waters DD, Tarasenko L, et al. A comparison of non-HDL and LDL cholesterol goal attainment in a large, multinational patient population: the Lipid Treatment Assessment Project 2. Atherosclerosis 2012;224:150–153. [DOI] [PubMed] [Google Scholar]
- 136. Urbinati S, Olivari Z, Gonzini L, et al. ; BLITZ-4 Investigators. Secondary prevention after acute myocardial infarction: drug adherence, treatment goals, and predictors of health lifestyle habits. The BLITZ-4 Registry. Eur J Prev Cardiol 2015;22:1548–1556. [DOI] [PubMed] [Google Scholar]
- 137. Daskalopoulou SS, Mikhailidis DP.. Reaching goal in hypercholesterolaemia: dual inhibition of cholesterol synthesis and absorption with simvastatin plus ezetimibe. Curr Med Res Opin 2006;22:511–528. [DOI] [PubMed] [Google Scholar]
- 138. Ballantyne CM, Houri J, Notarbartolo A, et al. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation 2003;107:2409–2415. [DOI] [PubMed] [Google Scholar]
- 139. Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol 2016;68:92–125. [DOI] [PubMed] [Google Scholar]
- 140. Kotseva K, Jennings CS, Turner EL, et al. ; ASPIRE-2-PREVENT Study Group . ASPIRE-2-PREVENT: a survey of lifestyle, risk factor management and cardioprotective medication in patients with coronary heart disease and people at high risk of developing cardiovascular disease in the UK. Heart 2012;98:865–871. [DOI] [PubMed] [Google Scholar]
- 141. Vedin O, Hagstrom E, Stewart R, et al. Secondary prevention and risk factor target achievement in a global, high-risk population with established coronary heart disease: baseline results from the STABILITY study. Eur J Prev Cardiol 2013;20:678–685. [DOI] [PubMed] [Google Scholar]
- 142. EUROASPIRE Investigators. Patients with coronary artery disease and diabetes need improved management: a report from the EUROASPIRE IV survey: a registry from the EuroObservational Research Programme of the European Society of Cardiology. Cardiovasc Diabetol 2015;14:133.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med 2001;135:825–834. [DOI] [PubMed] [Google Scholar]
- 144. Colivicchi F, Abrignani MG, Santini M.. Aderenza terapeutica: il fattore di rischio occulto. G Ital Cardiol 2010;11(5 Suppl 3):124S–127S. [PubMed] [Google Scholar]
- 145. Patel MX, David AS.. Medication adherence: predictive factors and enhancement strategies. Psychiatry 2004;10:41–45. [Google Scholar]
- 146. Ho PM, Bryson CL, Rumsfeld JS.. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028–3035. [DOI] [PubMed] [Google Scholar]
- 147. De Vera MA, Bhole V, Burns LC, Lacaille D.. Impact of statin adherence on cardiovascular disease and mortality outcomes: a systematic review. Br J Clin Pharmacol 2014;78:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Penning-van Best FJ, Termorshuizen F, Goettsch WG, Klungel OH, Kastelein JJP, Herings RM.. Adherence to evidence-based statin guidelines reduces the risk of hospitalizations for acute myocardial infarction by 40%: a cohort study. Eur Heart J 2007;28:154–159. [DOI] [PubMed] [Google Scholar]
- 149. Colivicchi F, Uguccioni M, Ragonese M, et al. Cardiovascular risk factor control among diabetic patients attending community-based diabetics care clinics in Italy. Diabetes Res Clin Prac 2007;75:176–183. [DOI] [PubMed] [Google Scholar]
- 150. Morisky DE, Green LW, Levine DM.. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 151. Argo CK, Loria P, Caldwell SH, Lonardo A.. Statins in liver disease: a molehill, an iceberg, or neither? Hepatology 2008;48:662–669. [DOI] [PubMed] [Google Scholar]
- 152. Anfossi G, Massucco P, Bonomo K, Trovati M.. Prescription of statins to dyslipidemic patients affected by liver diseases: a subtle balance between risks and benefits. Nutr Metab Cardiovasc Dis 2004;14:215–224. [DOI] [PubMed] [Google Scholar]
- 153. Hovingh GK, Davidson MH, Kastelein JJ, O’Connor AM. . Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J 2013;34:962–971. [DOI] [PubMed] [Google Scholar]
- 154. Ahn CH, Choi SH.. New drugs for treating dyslipidemia: beyond statins. Diabetes Metab J 2015;39:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rodriguez F, Knowles JW.. PCSK9 inhibition: current concepts and lessons from human genetics. Curr Atheroscler Rep 2015;17:487.. [DOI] [PubMed] [Google Scholar]
- 156. Wetterau JR, Gregg RE, Harrity TW, et al. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science 1998;282:751–754. [DOI] [PubMed] [Google Scholar]
- 157. Dhote V, Joharapurkar A, Kshirsagar S, et al. Inhibition of microsomal triglyceride transfer protein improves insulin sensitivity and reduces atherogenic risk in Zucker fatty rats. Clin Exp Pharmacol Physiol 2011;38:338–344. [DOI] [PubMed] [Google Scholar]
- 158. Cuchel M, Meagher EA, Du Toit Theron H, et al. ; Phase 3 HoFH Lomitapide Study Investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet 2013;381:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Sahebkar A, Watts GF.. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovasc Drugs Ther 2013;27:559–567. [DOI] [PubMed] [Google Scholar]
- 160. Rader DJ, Kastelein JJ.. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 2014;129:1022–1032. [DOI] [PubMed] [Google Scholar]
- 161. Kastelein JJ, Wedel MK, Baker BF, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation 2006;114:1729–1735. [DOI] [PubMed] [Google Scholar]
- 162. Akdim F, Visser ME, Tribble DL, et al. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol 2010;105:1413–1419. [DOI] [PubMed] [Google Scholar]
- 163. Sherman CB, Peterson SJ, Frishman WH.. Apolipoprotein A-I mimetic peptides: a potential new therapy for the prevention of atherosclerosis. Cardiol Rev 2010;18:141–147. [DOI] [PubMed] [Google Scholar]
- 164. Smith JD. Apolipoprotein A-I and its mimetics for the treatment of atherosclerosis. Curr Opin Investig Drugs 2010;11:989–996. [PMC free article] [PubMed] [Google Scholar]
- 165. Nissen SE, Tsunoda T, Tuzcu EM, et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 2003;290:2292–2300. [DOI] [PubMed] [Google Scholar]
- 166. Tardif JC, Gregoire J, L’Allier PL, et al. Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 2007;297:1675–1682. [DOI] [PubMed] [Google Scholar]
- 167. Sjouke B, Langslet G, Ceska R, et al. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): a randomised, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol 2014;2:455–463. [DOI] [PubMed] [Google Scholar]
- 168. Lagrost L, Gambert P, Dangremont V, et al. Role of cholesteryl ester transfer protein (CETP) in the HDL conversion process as evidenced by using anti-CETP monoclonal antibodies. J Lipid Res 1990;31:1569–1575. [PubMed] [Google Scholar]
- 169. Okamoto H, Yonemori F, Wakitani K, et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature 2000;406:203–207. [DOI] [PubMed] [Google Scholar]
- 170. Mohammadpour AH, Akhlaghi F.. Future of cholesteryl ester transfer protein (CETP) inhibitors: a pharmacological perspective. Clin Pharmacokinet 2013;52:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Randomized EValuation of the Effects of Anacetrapib Through Lipid-modification (REVEAL). https://clinicaltrials. gov/ct2/show/ record/ NCT01252953 (16 May 2016).
- 172. Langslet G, Emery M, Wasserman SM.. Evolocumab (AMG 145) for primary hypercholesterolemia. Expert Rev Cardiovasc Ther 2015;13:477–488. [DOI] [PubMed] [Google Scholar]
- 173. Do RQ, Vogel RA, Schwartz GG.. PCSK9 inhibitors: potential in cardiovascular therapeutics. Curr Cardiol Rep 2013;15:345.. [DOI] [PubMed] [Google Scholar]
- 174. Perrone FP, Paolillo S, Trimarco B.. Controllo lipidico in pazienti ad elevato rischio cardiovascolare: focus sull’inibizione di PCSK9. G Ital Cardiol 2015;16:44–51. [DOI] [PubMed] [Google Scholar]
- 175. Cainzos-Achirica M, Martin SS, Cornell JE, Mulrow CD, Guallar E.. PCSK9 inhibitors: a new era in lipid-lowering treatment? Ann Intern Med 2015;163:64–65. [DOI] [PubMed] [Google Scholar]
- 176. European Medicines Agency. Assessment report Praluent International non-proprietary name: alirocumab Procedure No. EMEA/H/C/003882/0000. 23 July 2015. EMA/CHMP/392430/2015 Committee for Medicinal Products for Human Use (CHMP). http://www.ema.europa. eu/docs/en_GB/ document_library/EPAR_- _Public_assessment_report/ human/003882 /WC500194524.pdf (19 April 2017).
- 177. European Medicines Agency. Assessment report Repatha International non-proprietary name: evolocumab. Procedure No. EMEA/H/C/003766/0000. 21 May 2015. EMA/CHMP/222019/2015. Committee for Medicinal Products for Human Use (CHMP). http://www.ema.europa.eu/docs/en_ GB/document_library/EPAR_ -_Public_ assessment_report/ human/003766/ WC500191400.pdf (19 April 2017).
- 178. European Medicines Agency. Product Information Repatha International non-proprietary name: evolocumab. ANNEX I Summary of product characteristics. EMEA/H/C/003766 03. 2015. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003766/WC500191398.pdf (19 April 2017).
- 179. The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1). https://clinicaltrials.gov/ct2/show/record/NCT01975376 (19 April 2017).
- 180. The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). https://clinical trials.gov/ct2/show /NCT01975389 (19 April 2017).
- 181.Repatha: EPAR-product information. http://www.ema.europa.eu/ema/ index.jsp?curl=pages /medicines/human/ medicines/003766/human_med_ 001890.jsp&mid =WC0b01ac058001d124 (19 April 2017).
- 182.Praluent: EPAR-product information. http://www.ema.europa.eu/ema/index.jsp?curl=pages/ medicines/human/medicines/ 003882/human_med_001915. jsp&mid= WC0b01ac 058001d124 (19 April 2017).
- 183.Repatha prescibing information. http://www.accessdata.fda.gov/ drugsatfda_docs/label /2015/1255 22s000lbl.pdf (19 April 2017).
- 184.Praluent prescribing information. http://www.accessdata.fda. gov/drugsatfda_docs/ label/2015/125559 Orig1s000 lbledt.pdf (19 April 2017).
- 185. Institute for Clinical and Economic Review. PCSK9 Inhibitors for Treatment of High Cholesterol: Effectiveness, Value, and Value-Based Price Benchmarks Draft Report Boston: Institute for Clinical and Economic Review; 2015.
- 186. O’Riordan M. PCSK9 Inhibitors Not Cost-Effective at Current Price: ICER Review Medscape; 2015. http://www.medscape.com/ viewarticle/850715 (19 April 2017).
- 187. Joszt L. PCSK9 Inhibitors Should Cost 85% Less, ICER Reports 2015. http://www.ajmc.com /newsroom/ pcsk9-inhibitors- should-cost-85- less-icer-reports (19 April 2017).
- 188. Nardi R, Scanelli G, Borioni D, et al. The assessment of complexity in internal medicine patients. The FADOI Medicomplex Study. Eur J Intern Med 2007;18:283–287. [DOI] [PubMed] [Google Scholar]
- 189. Nardi R, Scanelli G.. Multimorbidity and complexity: a current research priority not only in the uK and in primary care, but all over the world and in every care setting. bmj 2007;334:1016.17510108 [Google Scholar]
- 190. Nardi R, Scanelli G, Corrao S, et al. Co-morbidity does not reflect complexity in internal medicine patients. Eur J Intern Med 2007;18:359–368. [DOI] [PubMed] [Google Scholar]
- 191. Nardi R, Corbetta L, Muratori M, et al. Metodologia clinica, strumenti di valutazione e gestione dei pazienti anziani affetti da BPCO e comorbilità croniche. Ital J Med 2011;5:S171–S178. [Google Scholar]
- 192. Leipzig RM, Hall WJ, Fried LP.. Treating our societal scotoma: the case for investing in geriatrics, our nation’s future, and our patients. Ann Intern Med 2012;156:657–658. [DOI] [PubMed] [Google Scholar]
- 193. Stange KC. Assessing and acting on complexity. Ann Fam Med 2012;10:98–99. [Google Scholar]
- 194. Akker M, van den Buntinx F, Knottnerus JA.. Comorbidity or multimorbidity: what’s in a name? A review of literature. Eur J Gen Pract 1996;2:65–70. [Google Scholar]
- 195. Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci 2004;59:255–263. [DOI] [PubMed] [Google Scholar]
- 196. Guralnik JM. Assessing the impact of comorbidity in the older population. Ann Epidemiol 1996;6:376–380. [DOI] [PubMed] [Google Scholar]
- 197. Canadian Institute for Health Information Partnership for Health Information Standards. WHIC Chronic Disease Management Infostructure. Toronto: CDM data standards; Fall; 2005. [Google Scholar]
- 198. Huntley AL, Johnson R, Purdy S, et al. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med 2012;10:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Fortin M, Stewart M, Poitras M, et al. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med 2012;10:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bayliss EA, Ellis JL, Shoup JA, et al. Association of patient-centered outcomes with patient-reported and ICD-9-based morbidity measures. Ann Fam Med 2012;10:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Nobili A, Licata G, Salerno F, et al. Polypharmacy, length of hospital stay, and in-hospital mortality among elderly patients in internal medicine wards. The REPOSI study. Eur J Clin Pharmacol 2011;67:507–519. [DOI] [PubMed] [Google Scholar]
- 202. Newman AB, Comorbidity and multimorbidity In: Newman AB, Cauley JA, eds. The Epidemiology of Aging. Dordrecht: Springer-Verlag; 2012. p119–33. [Google Scholar]
- 203. Marengoni A, Angleman S, Fratiglioni L.. Prevalence of disability according to multimorbidity and disease clustering: a population-based study. J Comorbidity 2011;1:11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. World Health Organization. The International Classification of Impairments, Disabilities, and Handicaps (ICIDH) Defined a Taxonomy of Disease Impacts by the World Health Organization. Geneva: World Health Organization; 1980. [Google Scholar]
- 205. Nagi SZ, Disability concepts revised: implications for prevention In: Pope AM, Tarlov AR, eds. Disability in America: Toward a National Agenda for Prevention. Washington, DC: National Academy Press; 1991. p309–27. [Google Scholar]
- 206. Cerimele JM, Peccoralo LA.. Defining patient complexity. Ann Intern Med 2012;156:606–607. [DOI] [PubMed] [Google Scholar]
- 207. Olde Rikker MG, Schers HJ, Melis RJ.. Defining patient complexity. Ann Intern Med 2012;156:607.. [DOI] [PubMed] [Google Scholar]
- 208. Rockwood K, Stadnyk K, Carver D, et al. A clinimetric evaluation of specialized geriatric care for rural dwelling, frail older people. J Am Geriatr Soc 2000;48:1080–1085. [DOI] [PubMed] [Google Scholar]
- 209. Nardi R, L’anziano fragile In: Mongardi M, ed. L’assistenza all’anziano—ospedale, territorio, domicilio. Cap. 21. Milano: McGraw-Hill; 2010. p351–67. [Google Scholar]
- 210. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Turner BJ, Cuttler L.. The complexity of measuring clinical complexity. Ann Intern Med 2011;155:851–852. [DOI] [PubMed] [Google Scholar]
- 212. Grant RW, Ashburner JM, Hong CS, et al. Defining patient complexity from the primary care physician’s perspective: a cohort study. Ann Intern Med 2011;155:797–804. [DOI] [PubMed] [Google Scholar]
- 213. Cavicchi I. Medicina interna, paziente complesso: verso una clinica relazionale e ragionevole. Ital J Med 2012;6:259–264. [Google Scholar]
- 214. Evans T, Michael W, Marciniak J.. Software Quality Assurance and Management. New York, NY: John Wiley & Sons Inc; 1987. [Google Scholar]
- 215. Morin E. Introduzione al pensiero complesso. Milano: Sperling & Kupfer, 1993. [Google Scholar]
- 216. Wilson T, Holt T, Greenlagh T.. Complexity and clinical care. BMJ 2001;323:685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Plsek PE, Wilson T.. Complexity, leadership, and management in healthcare organisations. BMJ 2001;323:746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Agency for Healthcare Research and Quality (AHRQ). Definition: Complex Patient, Funding Opportunity Announcement (FOA)—Technical Assistance Teleconference Call. https://archive.ahrq.gov/fund/tac101507txt.htm (19 April 2017).
- 219. Safford MM, Allison JJ, Kiefe CI.. Complexity: more than comorbidity. The vector model of complexity. J Gen Intern Med 2007;22:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Whittle J, Bosworth H.. Studying complexity is complex. J Gen Intern Med 2007;22:379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Reich J. A critical appraisal of overdiagnosis: estimates of its magnitude and implications for lung cancer screening. Thorax 2008;63:377–383. [DOI] [PubMed] [Google Scholar]
- 222. Welch G, Schwartz L, Woloshin S.. Overdiagnosed: Making People Sick in Pursuit of Health. Boston, MA: Beacon Press; 2011. [Google Scholar]
- 223. Moynihan R, Cassels A.. Selling Sickness: How the World’s Biggest Pharmaceutical Companies Are Turning us All into Patients. New York, NY: Nation Books; 2005. [Google Scholar]
- 224. Hoffman JR, Cooper RJ.. Overdiagnosis of disease: a modern epidemic. Arch Intern Med 2012;172:1123–1124. [DOI] [PubMed] [Google Scholar]
- 225. Moynihan R, Heath I, Henry D.. Selling sickness: the pharmaceutical industry and disease mongering. BMJ 2002;324:886–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Upshur REG. Chronicity and complexity, Is what’s good for the diseases always good for the patients? Can Fam Physician 2008;54:1655–1658. [PMC free article] [PubMed] [Google Scholar]
- 227. Sturmberg JP, Martin CM.. Complexity and health-yesterday’s traditions, tomorrow’s future. J Eval Clin Pract 2009;15:543–548. [DOI] [PubMed] [Google Scholar]
- 228. Singh H, Petersen LH, Thomas EJ.. Understanding diagnostic errors in medicine: a lesson from aviation. Qual Health Saf Care 2006;15:159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Gensini GF, Fabbri LM, Fini M, Nozzoli C.. La Medicina della complessità: BPCO e comorbidità. Firenze: Firenze University Press; 2010. [Google Scholar]
- 230. Carmel M, Martin CM, Sturmberg JP.. General practice—chaos, complexity and innovation. MJA 2005;183:106–109. [DOI] [PubMed] [Google Scholar]
- 231. Simoons ML, Deckers JW, Intensive LDL.. lowering therapy for prevention of recurrent cardiovascular events: a word of caution. Eur Heart J 2016;37:520–523. [DOI] [PubMed] [Google Scholar]
- 232. Candrilli SD, Kuznik A, Mendys PM, Wilson DJ.. Prevalence and coexistence of cardiovascular comorbidities among the US dyslipidemic population aged ≥ 65 years by lipid-lowering medication use status. Postgrad Med 2010;122:142–149. [DOI] [PubMed] [Google Scholar]
- 233. Rodriguez F, Olufade T, Heithoff K, Friedman HS, Navaratnam P, Foody JM.. Frequency of high-risk patients not receiving high-potency statin (from a large managed care database). Am J Cardiol 2015;115:190–195. [DOI] [PubMed] [Google Scholar]
- 234. Halcox JP, Tubach F, Lopez-Garcia E, et al. Low rates of both lipid-lowering therapy use and achievement of low-density lipoprotein cholesterol targets in individuals at high-risk for cardiovascular disease across Europe. PLoS One 2015;10:e0115270.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Stone NJ, Lloyd-Jones DM, Lowering LDL.. cholesterol is good, but how and in whom? N Engl J Med 2015;372:1564–1565. [DOI] [PubMed] [Google Scholar]
- 236. Aguiar C, Alegria E, Bonadonna RC, et al. A review of the evidence on reducing macrovascular risk in patients with atherogenic dyslipidaemia: a report from an expert consensus meeting on the role of fenofibrate-statin combination therapy. Atheroscler Suppl 2015;19:1–12. [DOI] [PubMed] [Google Scholar]
- 237. Wilson PW, Abbott RD, Castelli WP.. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 1988;8:737–741. [DOI] [PubMed] [Google Scholar]
- 238. Assmann G, Schulte H, von Eckardstein A, Huang Y.. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 1996;124(Suppl):S11–S20. [DOI] [PubMed] [Google Scholar]
- 239. Hokanson JE, Austin MA.. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996;3:213–219. [PubMed] [Google Scholar]
- 240. Grundy SM, Hansen B, Smith SC Jr, Cleeman JI, Kahn RA; American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 2004;109:551–556. [DOI] [PubMed] [Google Scholar]
- 241. Scott R, O’Brien R, Fulcher G, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 2009;32:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Swiger KJ, Martin SS.. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1 trials. Drug Saf 2015;38:519–526. [DOI] [PubMed] [Google Scholar]
- 243. Lipinski MJ, Benedetto U, Escarcega RO, et al. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 244. Gaudet D, Kereiakes DJ, McKenney JM, et al. Effect of alirocumab, a monoclonal proprotein convertase subtilisin/kexin 9 antibody, on lipoprotein(a) concentrations (a pooled analysis of 150 mg every two weeks dosing from phase 2 trials). Am J Cardiol 2014;114:711–715. [DOI] [PubMed] [Google Scholar]
- 245. Raal FJ, Giugliano RP, Sabatine MS, et al. Reduction in lipoprotein(a) with PCSK9 monoclonal antibody evolocumab (AMG 145): a pooled analysis of more than 1,300 patients in 4 phase II trials. J Am Coll Cardiol 2014;63:1278–1288. [DOI] [PubMed] [Google Scholar]
- 246. Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2012 update: a report from the American Heart Association. Circulation 2012;125:e2–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Shepherd J, Blauw GJ, Murphy MB, et al. ; PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 248. Savarese G, Gotto AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62:2090–2099. [DOI] [PubMed] [Google Scholar]
- 249. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–1389. [PubMed] [Google Scholar]
- 250. Lewis SJ, Moye LA, Sacks FM, et al. Effect of pravastatin on cardiovascular events in older patients with myocardial infarction and cholesterol levels in the average range. Results of the Cholesterol and Recurrent Events (CARE) trial. Ann Intern Med 1998;129:681–689. [DOI] [PubMed] [Google Scholar]
- 251. Afilalo J, Duque G, Steele R, et al. Statins for secondary prevention in elderly patients: a hierarchical Bayesian meta-analysis. J Am Coll Cardiol 2008;51:37–45. [DOI] [PubMed] [Google Scholar]
- 252. Gulizia MM, Colivicchi F, Arca M, et al. Position paper ANMCO: Percorso diagnostico-terapeutico nel paziente con ipercolesterolemia e intolleranza alla terapia con statine. G Ital Cardiol 2016;17:447–455. [DOI] [PubMed] [Google Scholar]
- 253. Anderson GF, Chu E.. Expanding priorities - confronting chronic disease in countries with low income. N Engl J Med 2007;356:209–211. [DOI] [PubMed] [Google Scholar]
- 254. Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 255. Giannakouris K. Ageing characterises the demographic perspectives of the European societies. Eurostat Statistics in Focus 72; 2008http://www.apapr.ro/images/BIBLIOTECA/demografie/eurostat%20focus%202008.pdf (19 April 2017). [Google Scholar]
- 256. Kotseva K, Wood D, De Backer G, et al. ; EUROASPIRE Study Group . EUROASPIRE III: a survey on the lifestyle, risk factors and use of cardioprotective drug therapies in coronary patients from twenty-two European countries. Eur J Cardiovasc Prev Rehabil 2009;16:121–137. [DOI] [PubMed] [Google Scholar]
- 257. Maggioni AP, Cinconze E, Rossi E, et al. Outcomes, health costs and use of statins in 6226 patients admitted in 2011 for an acute coronary syndrome (ACS) occurring in a large community setting of 2,989,512 subjects. Presented at ISPOR 18th Annual European Congress, Milan, Italy, 7–11 November 2015.
- 258. Maggioni AP, Cinconze E, Rossi E, et al. Costs and outcomes of patients admitted for a cardiovascular ischemic disease in a large community setting of 2,989,512 subjects of the Italian National Health Service (NHS). Presented at ISPOR 18th Annual European Congress, Milan, Italy, 7–11 November 2015.
- 259. Il Sole-24 Ore. Info Data Blog, 8/4/2015. Sanità, la spesa pubblica pro capite scende a 1816 euro. E nelle regioni? http://www.infodata.ilsole24ore. com/2015/04/08/sanita- spesa-pubblica-pro- capite-scende-a-1816- euro-e-nelle-regioni/ (16 May 2016).
- 260. Lazar LD, Pletcher MJ, Coxon PG, et al. Effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation 2011;124:146–153. [DOI] [PubMed] [Google Scholar]
- 261. Comparative effectiveness Public Advisory Council (CEPAC) and Institute for Clinical and Economic Review (ICER) Review. PCSK9 inhibitors for treatment of high cholesterol: effectiveness, value, and value-based price benchmarks draft report. Final Report. November 24, 2015.
- 262. Navarese EP, Kolodzejczac M, Shulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia. A systematic review and meta-analysis. Ann Intern Med 2015;163:40–51. [DOI] [PubMed] [Google Scholar]
- 263. Degli EL, Sangiorgi D, Arca M.. Il raggiungimento dei livelli di colesterole LDL nella pratica clinica. Risultati dello studio STAR (Statins Target Assessment in Real Practice). Monaldi Arch Chest 2011;76:160–167. [DOI] [PubMed] [Google Scholar]
- 264. Griffo R, Ambrosetti M, Tramarin R, et al. Effective secondary prevention through cardiac rehabilitation and predictors of poor adherence to lifestyle modification and medication. Results of the ICAROS survey. Int J Cardiol 2013;167:1390–1395. [DOI] [PubMed] [Google Scholar]