Abstract
Setting: India has one of the highest global rates of multidrug-resistant tuberculosis (MDR-TB), which is associated with poor treatment outcomes. A better understanding of the risk factors for unfavourable outcomes is needed.
Objectives: To describe 1) the demographic and clinical characteristics of MDR-TB patients registered in three states of India during 2009–2011, 2) treatment outcomes, and 3) factors associated with unfavourable outcomes.
Design: A retrospective cohort study involving a record review of registered MDR-TB patients.
Results: Of 788 patients, 68% were male, 70% were aged 15–44 years, 90% had failed previous anti-tuberculosis treatment or were retreatment smear-positive, 60% had a body mass index < 18.5 kg/m2 and 72% had additional resistance to streptomycin and/or ethambutol. The median time from sputum collection to the start of MDR-TB treatment was 128 days (IQR 103–173). Unfavourable outcomes occurred in 40% of the patients, mostly from death or loss to follow-up. Factors significantly associated with unfavourable outcomes included male sex, age ⩾ 45 years, being underweight and infection with the human immunodeficiency virus. Adverse drug reactions were reported in 24% of patients, with gastrointestinal disturbance, psychiatric morbidity and ototoxicity the most common.
Conclusion: Long delays from sputum collection to treatment initiation using conventional methods, along with poor treatment outcomes, suggest the need to scale up rapid diagnostic tests and shorter regimens for MDR-TB.
Keywords: MDR-TB, treatment outcomes, India, operational research
Abstract
Contexte : L'Inde a l'un des taux les plus élevés au monde de tuberculose multirésistante (TB-MDR), qui est associée à des résultats médiocres du traitement. Une meilleure compréhension des facteurs de risque de résultats défavorables est requise.
Objectifs : Décrire : 1) les caractéristiques démographiques et cliniques des patients TB-MDR enregistrés dans trois états d'Inde de 2009 à 2011, 2) les résultats du traitement, et 3) les facteurs associés à des résultats défavorables.
Schéma : Une étude de cohorte rétrospective impliquant une revue des dossiers des patients TB-MDR enregistrés.
Résultats : Il y a eu 788 patients, dont 68% d'hommes, 70% âgés de 15–44 ans, 90% ayant eu un échec de leur traitement anti-tuberculose précédent ou ayant un frottis positif en retraitement, 60% ayant un index de masse corporelle < 18,5 kg/m2 et 72% ayant en plus une résistance à la streptomycine et/ou à l'éthambutol. Le délai médian entre le recueil de crachats et la mise en route du traitement de la TB-MDR a été de 128 jours (IQR 103–173). Les résultats ont été défavorables pour 40% des patients, en majorité des décès ou des pertes de vue. Les facteurs significativement associés à un résultat défavorable ont inclus le sexe masculin, l'âge ⩾ 45 ans, la maigreur et le fait d'être positif pour le virus de l'immunodéficience humaine. Des effets secondaires des médicaments ont été notés dans 24% des cas, avec des troubles gastro-intestinaux, des problèmes psychiatriques et une ototoxicité comme symptômes les plus fréquents.
Conclusion : De longs délais entre le recueil de crachats et la mise en route du traitement basé sur des méthodes conventionnelles et des résultats médiocres du traitement signalent la nécessité d'intensifier la mise en œuvre des tests de diagnostic rapide et des protocoles de traitement court de la TB-MDR.
Abstract
Marco de referencia: La tasa de tuberculosis multirresistente (TB-MDR) en la India es una de las tasas más altas en el mundo y se asocia con desenlaces terapéuticos desfavorables. Es preciso lograr un mejor conocimiento de los factores de riesgo que determinan la ineficacia del tratamiento.
Objetivos: 1) Describir las características demográficas y clínicas de los pacientes con TB-MDR registrados en tres estados de la India del 2009 al 2011; 2) analizar los desenlaces terapéuticos; y 3) describir los factores asociados con los resultados desfavorables del tratamiento.
Método: Un estudio retrospectivo de cohortes a partir del análisis de las historias clínicas de los pacientes registrados con diagnóstico de TB-MDR.
Resultados: Se incluyeron en el estudio 788 pacientes; el 68% era de sexo masculino, en el 70% la edad estaba comprendida entre 15 años y 44 años, el 90% tenía antecedente de fracaso de un tratamiento antituberculoso o estaba en retratamiento con baciloscopia positiva, el índice de masa corporal era inferior a 18,5 en el 60% de los casos y el 72% presentaba resistencia adicional a estreptomicina, etambutol o ambos. La mediana del lapso entre la recogida de la muestra de esputo y el comienzo del tratamiento de la TB-MDR fue 128 días (intervalo intercuartil 103–173). Se observaron desenlaces desfavorables en 40% de los pacientes y consistieron en su mayoría en defunciones o pérdidas durante el seguimiento. Los factores que se asociaron de manera significativa con estos desenlaces fueron el sexo masculino, la edad ⩾ 45 años, el bajo peso y la serología positiva frente del virus de la inmunodeficiencia humana. Se notificaron reacciones adversas a los medicamentos en el 24% de los casos, de las cuales las más frecuentes fueron los trastornos gastrointestinales, las afecciones psiquiátricas y la ototoxicidad.
Conclusión: La observación de plazos prolongados entre la recogida de las muestras de esputo y la iniciación del tratamiento cuando se utilizan los medios diagnósticos corrientes y de desenlaces terapéuticos desfavorables destaca la necesidad de ampliar la escala de aplicación de las pruebas rápidas de diagnóstico y la administración de pautas más cortas de tratamiento de la TB-MDR.
Global efforts to control tuberculosis (TB) are being hampered by the emergence of drug-resistant disease, which is a major concern for TB control programmes worldwide. Globally, in 2014 an estimated 3.3% of new cases and 20% of previously treated cases were multidrug-resistant TB (MDR-TB, defined as TB resistant to at least isoniazid [INH] and rifampicin [RMP]), resulting in an estimated total of 480 000 new cases of MDR-TB associated with 190 000 deaths worldwide for the year.1
The standard treatment for MDR-TB is a 24-month regimen largely comprising second-line drugs that are less effective, more costly and associated with a high number of adverse events.2 Not surprisingly, treatment outcomes in MDR-TB are significantly worse than for standard first-line therapy. Globally, the proportion of MDR-TB patients in the 2012 cohort who successfully completed treatment (i.e., were cured or completed treatment) was 50%, due largely to high rates of mortality and loss to follow-up (LTFU).1 Under study conditions, treatment outcomes are marginally better. A systematic review of 36 studies reported that 62% of patients with MDR-TB achieved successful outcomes, with 11% deaths, 8% failures, 13% LTFU and 2% transferred out. Data were not available for the remaining 4%.3 In another review of 29 studies of individualised treatment regimens for MDR-TB, the reported treatment success rate was 64%.4
India has one of the highest burdens of MDR-TB, with 71 000 estimated MDR-TB cases among 300 000 TB cases notified in 2014.1,5 India's Revised National Tuberculosis Control Programme (RNTCP) introduced the programmatic management of drug-resistant TB (PMDT) services in 2007 to address the needs of this growing patient population, and services have been rapidly scaled up across the country to achieve universal access.6 Cumulative outcomes have been reported in 31 365 MDR-TB patients; of these, 14 632 (47%) were successfully treated, 6811 (22%) died and 6229 (20%) were lost to follow-up.5 Although a few studies in India have reported on treatment outcomes, there is insufficient knowledge about the sociode-mographic and clinical factors associated with unfavourable treatment outcomes. A better understanding of these risk factors is necessary to design effective interventions that might help reduce morbidity and mortality and thereby improve treatment success.
The objectives of this study were therefore 1) to describe the demographic and clinical characteristics of MDR-TB patients enrolled in three states of India during the period 2009–2011, 2) to describe programme-defined treatment outcomes, and 3) to describe factors associated with unfavourable treatment outcomes.
METHODS
Study design
This was a retrospective cohort study involving a review of the records of MDR-TB patients registered under India's RNTCP.
General setting
India, the second-most populous country in the world, has the highest number of annual incident TB cases. Of the estimated global annual incidence of 9.6 million TB cases in 2015, 2.2 million occurred in India, and were associated with 0.22 million TB deaths, also the highest for any country.5
Programmatic management of MDR-TB
The RNTCP in India provides free diagnostic and treatment services, including for MDR-TB, throughout the country according to national and international guidelines.7,8 All patients with presumptive MDR-TB, currently defined as contacts of index MDR-TB cases, human immunodeficiency virus (HIV) infected TB patients, those who have failed treatment, retreatment smear-positive or smear-negative cases and patients identified as smear-positive during TB treatment follow-up, are identified at peripheral health centres; sputum samples are collected and sent to designated intermediate reference laboratories (IRLs) for detection of MDR-TB.
Prior to 2012, all laboratories used conventional culture and drug susceptibility testing (CDST) with Löwenstein-Jensen medium and the proportion method.9 Since 2012 there has been widespread scale-up of rapid molecular diagnostics tests, such as Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) and the GenoType® MTBDRplus (Hain LifeScience, Nehren, Germany) line probe assay (LPA).
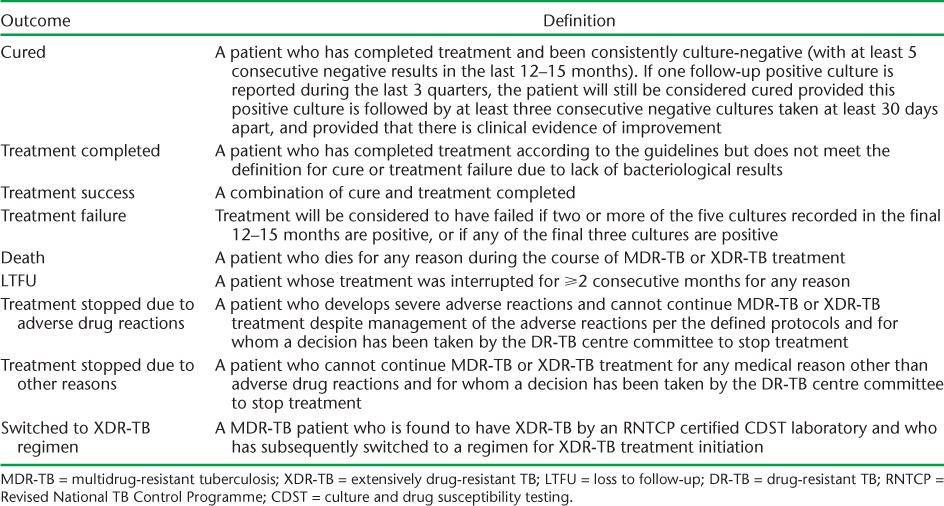
Whichever laboratory method is used, results are communicated back to the referring district TB centre from where patients are traced and referred to DOTS-Plus sites for evaluation and initiation of treatment. After a period of 7–10 days for initial hospitalisation, patients are referred to their respective district TB control/DOTS centres for continuation of therapy. Treatment is given for an average of 24 months, according to PMDT guidelines,6 and patients are followed up and assessed according to standardised programmatic treatment outcome definitions (Table 1).6
TABLE 1.
Definitions of final treatment outcomes for patients with MDR-TB
Study setting and study population
For this study, three states in India, Kerala, Delhi and West Bengal, were purposively selected, as MDR-TB diagnostic and treatment services were initiated in 2008 in all three states. The study included all patients enrolled for MDR-TB treatment under PMDT in the three states from 1 January 2009 to 31 December 2011.
Sources of data, data variables and data collection
The sources of data were treatment cards and DOTS-Plus treatment registers at all the drug-resistant TB centres in the three states. Information on patient characteristics (sociodemographic, clinical and treatment related) and treatment outcomes were collected (see Table 2 for variables). MDR-TB treatment outcomes were further grouped as favourable (cured or treatment completed) or unfavourable (failed, died, LTFU, stopped treatment due to adverse drug reactions or other reasons, switched to extensively drug-resistant [XDR] TB treatment or transferred out).6
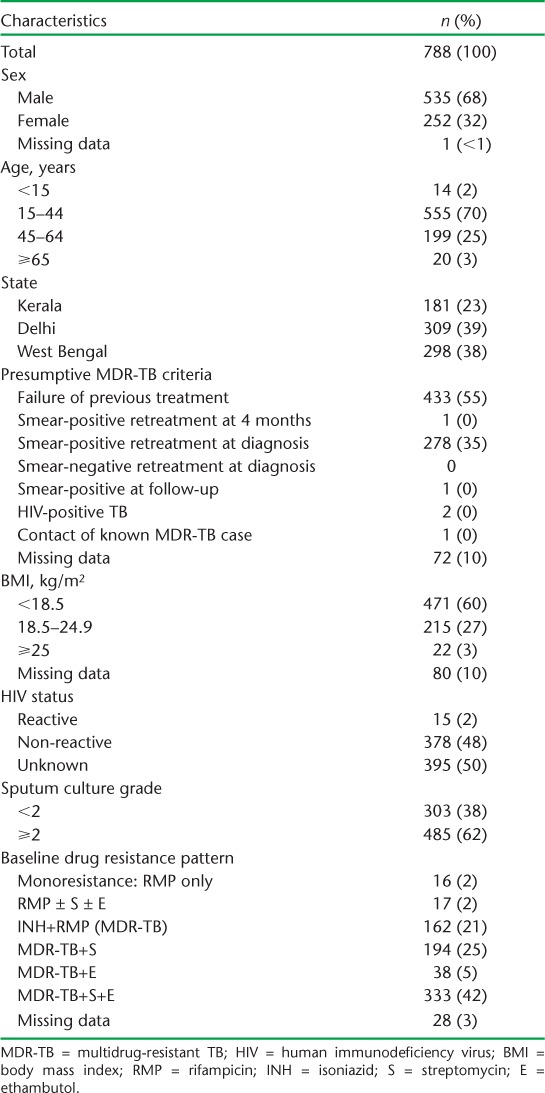
TABLE 2.
Baseline demographic and clinical characteristics of MDR-TB patients in three states, India, 2009–2011
Patients whose initial sputum cultures showed resistance to INH and RMP (MDR-TB) or monoresistance to RMP, with or without resistance to other drugs, plus those who had their treatment outcomes documented in cards and registers, were included in the analysis. Treatment delays were calculated from the time of receipt of sputum specimens in the laboratory to initiation of treatment. Data collection was carried out between October 2015 and April 2016 using a paper-based structured form.
Data analysis
Data were entered using EpiData software v. 3.1 and analysed using EpiData v. 2.2.2.182 (EpiData Association, Odense, Denmark). The data were double-entered and validated. Frequencies and proportions were used to summarise categorical variables and medians and interquartile ranges (IQR) to summarise continuous variables. The χ2 test was used to compare categorical variables, with risk ratios quantifying the strength of association between potential risk factors and treatment outcomes. Backward conditional multivariate logistic regression was performed to explore the predictors of unfavourable treatment outcomes after controlling for confounders. Levels of significance were set at 5% (P < 0.05). Variables with P < 0.2 on bivariate analysis were included in the multivariate logistic regression model.
Ethics approval
Permission for the study was obtained from the Central TB Division and the State TB cells of Kerala, Delhi and West Bengal. Ethics approval for the study was obtained from the Institutional Ethics Committee at the National Institute for Research in Tuberculosis, Chennai, India, and the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease, Paris, France.
RESULTS
Patient characteristics
From January 2009 to December 2011, 836 patients were initiated on MDR-TB treatment in all three states. Of the 48 patients excluded from the analysis, the reasons included non-availability of records (n = 12), negative or absent pre-treatment sputum cultures (n = 28), DST profile not available (n = 6), and cultures resistant only to streptomycin and ethambutol (n = 2).
The baseline characteristics of the remaining 788 patients are shown in Table 2. Most patients were male and aged 15–44 years. Over 50% had failed previous anti-tuberculosis treatment, and 35% were retreatment smear-positive pulmonary TB cases. Nearly two thirds were underweight, with a body mass index (BMI) < 18.5 kg/m2. Half of the patients had no record of HIV status, but where testing had been performed, about 4% were HIV-positive. Nearly two thirds of the patients had a sputum culture grade ⩾ 2. Monoresistance to RMP was uncommon, at <5%. Just over 20% of patients had resistance to only RMP and INH, with the remainder having additional resistance to streptomycin, ethambutol, or both.
Time to treatment initiation
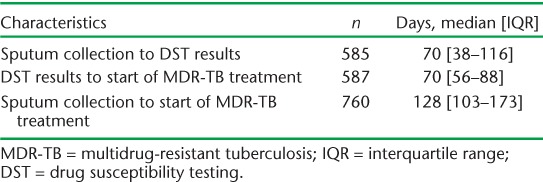
The median time from sputum collection to start of MDR-TB treatment was 128 days (IQR 103–173). The time from sputum collection to DST results and from DST results to start of MDR-TB treatment is shown in Table 3.
TABLE 3.
Time from sputum collection to start of treatment in MDR-TB patients in three states, India, 2009–2011
Treatment outcomes and adverse drug reactions
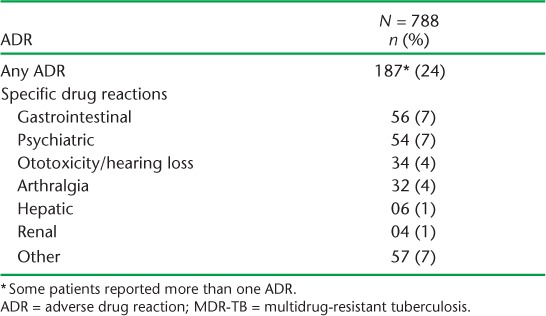
Treatment outcomes of MDR-TB patients in the three states and altogether are shown in Table 4. Overall treatment success was 60%, with no programmatically significant differences between the three states. Of the 40% of patients with unfavourable treatment outcomes, most (85%) were due to death or LTFU, with no significant differences between the three states. Factors associated with unfavourable outcomes are shown in Table 5. In the univariate and multivariate analyses, significant factors included male sex, age ⩾ 45 years, being underweight with a BMI of <18.5 kg/m2 and being HIV-positive. Nearly a quarter of the patients reported adverse drug reactions, with gastrointestinal disturbances, psychiatric morbidity and ototoxicity/hearing loss being the most common (Table 6).
TABLE 4.
Treatment outcomes for patients started on MDR-TB treatment in three states in India, 2009–2011
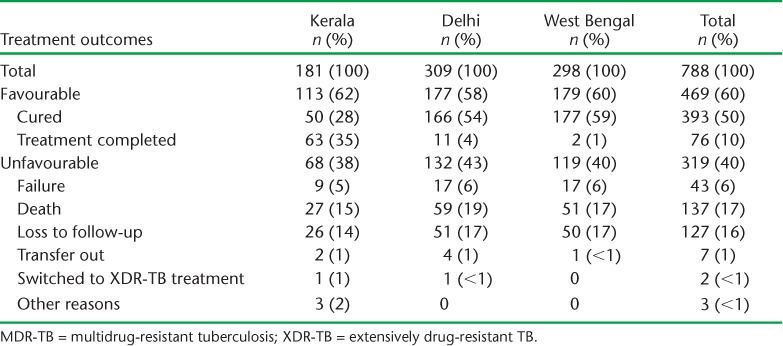
TABLE 5.
Characteristics of MDR-TB patients associated with unfavourable treatment outcomes in three states of India, 2009–2011
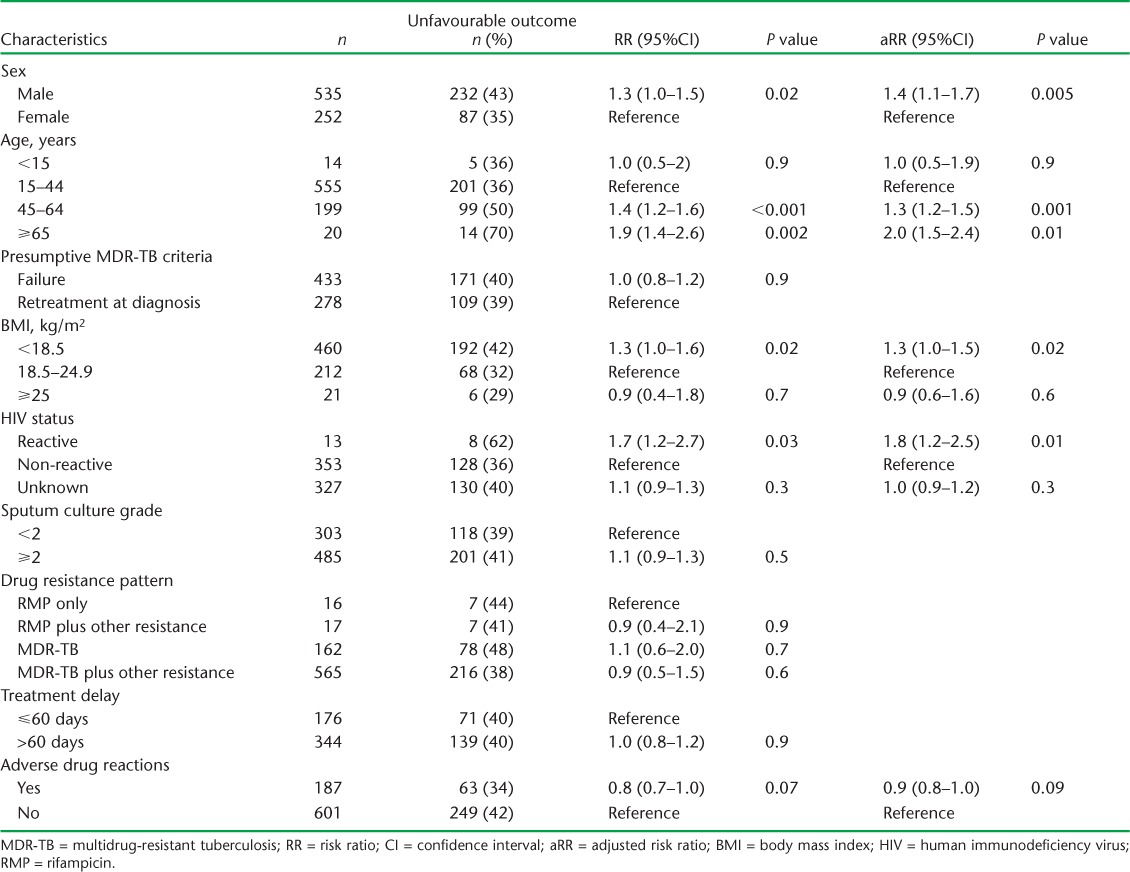
TABLE 6.
Incidence of ADRs among patients undergoing MDR-TB treatment in three states of India registered during 2009–2011
DISCUSSION
This study, carried out in three states of India, highlights the large burden of MDR-TB, occurring largely as result of patients failing previous anti-tuberculosis treatment or becoming smear-positive again after successful completion of previous treatment. There was a long median delay between sputum collection and start of MDR-TB treatment, with the reasons being equally divided between the time to get CDST results and the time taken to feed these results back to the referring centres and initiate treatment. While the treatment success was 60%, which is better than the global average,1 a large proportion of patients had unfavourable treatment outcomes, due largely to death and LTFU. An examination of baseline characteristics identified male sex, older age, being underweight and HIV-positive status as significantly associated with unfavourable treatment outcomes.
The factors in our study associated with unfavourable treatment outcomes are similar to what has been reported elsewhere. A systematic review in 2009 of 31 treatment programmes from 21 countries found that male sex was associated with worse treatment outcomes in MDR-TB.3 A more recent study in India amongst MDR-TB patients reported the same findings.10 The reasons are unclear, but males have higher rates of smoking and alcohol consumption, both of which in themselves are associated with poor outcomes, and males seem to be less vigilant and less adherent to drug treatment than females.
The poorer outcomes in our older patients are in agreement with a recent report from India showing poor treatment outcomes in older drug-susceptible TB patients, especially those aged ⩾70 years.11 In countries in Asia, Eastern Europe and Latin America, patients aged ⩾40 years with MDR-TB have also been reported to have worse outcomes than younger patients.12–14 The higher rate of unfavourable outcomes among older adults observed in our study and elsewhere could be due in part to co-morbidities such as diabetes mellitus, hypertension and associated cardiovascular diseases, which are becoming increasingly prevalent in Asia and India.15 In particular, diabetes mellitus in TB patients is known to be associated with an increased risk of failure and death during anti-tuberculosis treatment.16
Being underweight, with a BMI < 18.5 kg/m2, in patients with MDR-TB has been found to be a risk factor for unfavourable outcomes, particularly mortality.3,12,17,18 Being underweight is a manifestation of severe disease and possibly poor socio-economic circumstances, and as it also impairs host immunity against mycobacteria, it is not surprising that there are higher rates of mortality in such patients. HIV infection, which may be associated with malnutrition, has also been reported as a risk factor for death in patients with MDR-TB,19,20 although observational cohort studies have shown that timely antiretroviral therapy can improve survival.21 In our study, there were few patients with HIV infection; these patients had worse outcomes than those who were HIV-negative. Unfortunately, there is no information from the records about whether or not they were undergoing antiretroviral therapy.
Adverse drug reactions were documented in nearly a quarter of the study patients, with gastrointestinal, psychiatric and ototoxic reactions being the most common. This is consistent with other recent reports.22–24 High frequencies of adverse drug reactions lead to poor adherence and treatment interruptions, and this in turn can contribute to poor treatment outcomes and high LTFU.25
The strengths of this study are its being conducted within the routine programmatic setting in three large states of India and the large sample size. The study also adhered to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines for conducting and reporting on observational studies.26 The main limitations are the retrospective nature of the study and the missing data for some of the variables, especially with respect to HIV status and BMI. Although the patients were referred for HIV testing, the results were unavailable in many records. Patient height was missing in the majority of the records, and BMI could thus not be calculated. Considerable improvement is needed in routine data recording and monitoring in registers and treatment cards so that the programme can be better informed and more accurate, and so that reliable data-driven operational research can be conducted in the future on MDR-TB outcomes.
There are two important programmatic implications from this study. First, the long delays between sputum collection and CDST results are unacceptable. Rapid, molecular diagnostic tests such as LPA and Xpert have revolutionised the diagnosis of TB, and particularly drug-resistant TB. India has taken action since 2012 to procure these new diagnostic tests to replace the slow and cumbersome CDST; this should result in more rapid diagnosis and initiation to treatment and potentially better outcomes. The World Health Organization (WHO) now recommends Xpert as the initial diagnostic test for all people with presumptive TB, regardless of the presence of risk factors for MDR-TB.27
Second, the poor overall treatment outcomes, with high rates of death and LTFU, need to be addressed. Many of the risk factors identified in this study, such as male sex and older age, cannot be modified, so a shorter and easier to follow MDR-TB treatment regimen is probably the answer. Observational cohort studies in the last few years have shown that a 9-month regimen is effective and well tolerated, and the conditional recommendation by the WHO in May 2016 that countries could use this regimen under certain conditions might improve treatment outcomes and reduce LTFU rates.28 The RNTCP in India is already considering its adoption and roll-out. It is tempting to think that nutritional support, especially for malnourished patients with MDR-TB, might improve treatment success. The evidence to date to support such an intervention is limited,29 so there is scope here to assess this through clinical trials and programmatic implementation.
In conclusion, this study in three states in India showed a long median delay between sputum collection and start of MDR-TB treatment and a high proportion of patients with unfavourable treatment outcomes, due largely to death and LTFU. Moving from conventional CDST methodology to rapid molecular diagnostic tests, and giving due consideration to implementing a shorter, better tolerated MDR-TB treatment regimen, might help to improve the management and outcomes of this serious disease.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR), Geneva, Switzerland. The model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Brussels, Belgium). The specific SORT IT programme that resulted in this publication was jointly developed and implemented by The Union South-East Asia Office, New Delhi, India; the Centre for Operational Research, The Union, Paris, France; the Operational Research Unit (LUXOR), MSF Brussels Operational Centre, Luxembourg; the Department of Preventive and Social Medicine, Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India; the Department of Community Medicine, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India; the Department of Preventive and Social Medicine, Medical College Baroda, Vadodara, India; and the National Institute for Research in Tuberculosis, Chennai, India.
The training programme and open access publication costs were funded by the Department for International Development (London, UK), The Union, MSF and La Fondation Veuve Emile Metz-Tesch (Luxembourg). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
In accordance with the WHO's open-access publication policy for all work funded by the WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO licence (http://creativecommons.org/licenses/by/3.0/igo/legalcode) which permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . Global tuberculosis report, 2015. WHO/HTM/TB/2015.22 Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 2. Lange C, Abubakar I, Alffenaar J W, . et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: A TBNET Consensus Statement. Eur Respir J 2014; 44: 23– 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johnston J C, Shahidi N C, Sadatsafavi M, Fitzgerald J M.. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLOS ONE 2009; 4: e6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orenstein E W, Basu S, Shah N S, . et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis 2009; 9: 153– 161. [DOI] [PubMed] [Google Scholar]
- 5. Central TB Division. . TB India 2016. Revised National TB Control Programme: annual status report. Unite to end TB. New Delhi, India: Ministry of Health and Family Welfare, 2016. http://www.tbcindia.nic.in/showfile.php?lid=3180 Accessed December 2016. [Google Scholar]
- 6. Central TB Division. . Guidelines on Programmatic Management of Drug-resistant TB (PMDT) in India. New Delhi, India: Ministry of Health and Family Welfare, 2012. [Google Scholar]
- 7. Central TB Division. . Revised National TB Control Programme technical and operational guidelines for tuberculosis control in India. New Delhi, India: Ministry of Health and Family Welfare, 2016. http://tbcindia.gov.in/show-file.php?lid=3197 Accessed December 2016. [Google Scholar]
- 8. World Health Organization. . Treatment of tuberculosis guidelines, 2010. WHO/HTM/TB/2009.420 Geneva, Switzerland: WHO, 2009. [PubMed] [Google Scholar]
- 9. Canetti G, Fox W, Khomenko A, . et al. Advances in techniques of testing mycobacterial drug sensitivity and the use of sensitivity trusts in tuberculosis control programmes. Bull World Health Organ 1969; 41: 21– 43. [PMC free article] [PubMed] [Google Scholar]
- 10. Jain K, Desai M, Solanki R, Dikshit R K.. Treatment outcome of standardized regimen in patients with multidrug-resistant tuberculosis. J Pharmacol Pharmacother 2014; 5: 145– 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ananthakrishnan R, Kumar K, Ganesh M, . et al. The profile and treatment outcomes of the older (aged 60 years and above) tuberculosis patients in Tamilnadu, South India. PLOS ONE 2013; 8: e67288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kurbatova E V, Taylor A, Gammino V M, . et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis (Edinb) 2012; 92: 397– 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmed N, Javaid A, Basit A, . et al. Management and treatment outcomes of MDR-TB: results from a setting with high rates of drug resistance. Int J Tuberc Lung Dis 2015; 19: 1109– 1114. [DOI] [PubMed] [Google Scholar]
- 14. Khan M A, Mehreen S, Basit A, . et al. Characteristics and treatment outcomes of patients with multidrug-resistant tuberculosis at a tertiary hospital in Peshawar, Pakistan. Saudi Med J 2015; 36: 1463– 1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramachandran A, Ma R C W, Snehalatha C.. Diabetes in Asia. Lancet 2010; 375: 408– 418. [DOI] [PubMed] [Google Scholar]
- 16. Baker M A, Harries A D, Jeon C Y, . et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Podewils L J, Holtz T, Riekstina V, . et al. Impact of malnutrition on clinical presentation, clinical course, and mortality in MDR-TB patients. Epidemiol Infect 2011; 139: 113– 120. [DOI] [PubMed] [Google Scholar]
- 18. Tang S, Tan S, Yao L, . et al. Risk factors for poor treatment outcomes in patients with MDR-TB and XDR-TB in China: retrospective multi-centre investigation. PLOS ONE 2013; 8: e82943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wells C D, Cegielski J P, Nelson L J, . et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis 2007; 196 ( Suppl 1): S86– S107. [DOI] [PubMed] [Google Scholar]
- 20. Gandhi N R, Shah N S, Andrews J R, . et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med 2010; 181: 80– 86. [DOI] [PubMed] [Google Scholar]
- 21. Palacios E, Franke M, Munoz M, . et al. HIV-positive patients treated for multidrug-resistant tuberculosis: clinical outcomes in the HAART era. Int J Tuberc Lung Dis 2012; 16: 348– 354. [DOI] [PubMed] [Google Scholar]
- 22. Törün T, Güngör G, Ozmen I, . et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005; 9: 1373– 1377. [PubMed] [Google Scholar]
- 23. Bloss E, Kuksa L, Holtz T H, . et al. Adverse events related to multidrug-resistant tuberculosis treatment, Latvia, 2000–2004. Int J Tuberc Lung Dis 2010; 14: 275– 281. [PubMed] [Google Scholar]
- 24. Hoa N B, Nhung N V, Khanh P H, Hai N V, Quyen B T.. Adverse events in the treatment of MDR-TB patients within and outside the NTP in Pham Ngoc Thach hospital, Ho Chi Minh City, Vietnam. BMC Res Notes 2015; 8: 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deshmukh R D, Dhande D J, Sachdeva K S, . et al. Patient and provider reported reasons for lost to follow up in MDRTB treatment: a qualitative study from a drug resistant TB centre in India. PLOS ONE 2015; 10: e0135802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Elm E, Altman D G, Egger M, . et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453– 1457. [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization. . Xpert MTB/RIF assay for diagnosis of pulmonary and extra-pulmonary TB in adults and children. Policy update. WHO/HTM/TB/2013.16 Geneva, Switzerland: WHO, 2013. [Google Scholar]
- 28. World Health Organization. . The shorter MDR-TB regimen. Geneva, Switzerland: WHO, 2016. http://www.who.int/tb/Short_MDR_regimen_factsheet.pdf Accessed December 2016. [Google Scholar]
- 29. Van Lettow M, Fawzi W W, Semba R D.. Triple trouble: the role of malnutrition in tuberculosis and human immunodeficiency virus co-infection. Nutr Rev 2003; 61: 81– 90. [DOI] [PubMed] [Google Scholar]


