Abstract
Setting: Ten hospitals managing drug-resistant tuberculosis (TB) in Pakistan.
Objective: To assess the implementation of TB infection control (IC) practices and reasons for non-adherence to guidelines.
Design: This was a descriptive study conducted between April and October 2016 with three components: 1) non-participant observation of service delivery areas (SDAs) (n = 82) in hospitals (n = 10) using structured checklists; 2) exit interviews with 100 patients (10 per hospital); and 3) interviews with 100 health-care workers (HCWs, 10/hospital).
Results: Of the 82 SDAs, posters were displayed in 34 (41%), mechanical ventilation was implemented in 79% and functional ultraviolet germicidal irradiation (UVGI) was available in only 26%. Patient interviews showed 50–65% adherence to triage and use of personal protective measures. Key reasons for non-adherence were lack of adequate supplies, discomfort using N-95 masks, a lack of knowledge or training, perceived non-cooperation by patients, poor maintenance of mechanical ventilators and UVGI due to unstable electricity supply, a lack of clarity in roles (no-one designated in charge) and staff shortages and subsequent workloads. Adherence to natural ventilation usage was poor for reasons related to climate and privacy.
Conclusion: Implementation of TBIC measures in hospitals was suboptimal. Urgent measures need to be put in place, including retraining of HCWs, addressing weaknesses in mask and poster supplies and constant supervision and monitoring.
Keywords: infection control, health-care workers, Pakistan, adherence, monitoring
Abstract
Contexte : Dix hôpitaux prenant en charge la tuberculose (TB) pharmacorésistante au Pakistan.
Objectif : Evaluer la mise en œuvre des pratiques de lutte contre l'infection TB (CITB) et les raisons de la non-adhésion aux directives.
Schéma : Étude descriptive réalisée entre avril et octobre 2016 avec trois composants : 1) observation non participative des zones de prestations de service (SDA) (n = 82) dans des hôpitaux (n = 10) grâce à des checklists structurées ; 2) entretiens de sortie avec 100 patients (10 par hôpital) ; 3) entretiens avec 100 prestataires de soins de santé (HCW, 10 par hôpital).
Résultats : Parmi 82 SDA, des affiches ont été déployées dans 34 (41%) d'entre elles, une ventilation mécanique a été mise en œuvre dans 79% et un système fonctionnel d'irradiation par ultraviolets germicides (UVGI) a été disponible dans seulement 26%. Les entretiens avec les patients ont mis en évidence 50–65% d'adhérence au triage et à l'utilisation de mesures de protection personnelles. Les raisons majeures de la non-adhésion ont été le manque de fournitures appropriées, l'inconfort d'utilisation des masques N-95, le manque de connaissance ou de formation, la perception d'une non-coopération par les patients, la maintenance médiocre des ventilateurs mécaniques et de l'UVGI à cause de l'instabilité de l'alimentation électrique et le manque de clarification des responsabilités (aucune personne désignée responsable), et la pénurie de personnel avec surcharge de travail du personnel présent. L'adhésion à la ventilation naturelle a été médiocre en raison du climat et pour des problèmes de confidentialité.
Conclusion : La mise en œuvre de mesures de CITB dans les hôpitaux a été sous-optimale. Des mesures urgentes sont requises, notamment la formation continue des HCW, la lutte contre les problèmes de fourniture de masques et d'affiches et une supervision et un suivi constants.
Abstract
Marco de referencia: Diez hospitales que se ocupan del tratamiento de la tuberculosis (TB) multirresistente en el Pakistán.
Objetivo: Evaluar la aplicación de las prácticas de control de la infección TB (CITB) y determinar las razones del incumplimiento de las normas.
Método: De abril a octubre del 2016 se realizó un estudio descriptivo que comportó los siguientes elementos: 1) personas no vinculadas evaluaron las zonas de prestación de servicios (n = 82) en los hospitales (n = 10), con listas de verificación estructuradas; 2) se realizaron entrevistas de salida a 100 pacientes (10 por cada hospital) y 3) entrevistas a 100 profesionales de salud (HCW; 10 por cada hospital).
Resultados: De las 82 zonas de prestación de servicios evaluadas, en 34 había afiches expuestos (41%), el 79% contaba con sistemas de ventilación mecánica y solo en el 26% existía un dispositivo funcional de radiación ultravioleta germicida (UVGI). Las entrevistas a los pacientes revelaron un cumplimiento de 50% a 65% con la selección de los pacientes y las medidas de protección personal. Las principales explicaciones del incumplimiento fueron la insuficiencia de suministros, la incomodidad de utilización de las mascarillas N-95, la carencia de conocimientos o capacitación adecuada, la percepción de una falta de cooperación por parte de los pacientes, un mantenimiento deficiente de los ventiladores mecánicos y los dispositivos de UVGI debido a la inestabilidad del suministro eléctrico y la poca claridad con respecto a las funciones (falta de designación de una persona encargada), la escasez de personal y la consecuente sobrecarga de trabajo. El cumplimiento de las normas de ventilación natural era deficiente por causa de las condiciones climáticas y aspectos relacionados con el respeto de la intimidad.
Conclusión: La aplicación de las medidas de CITB en los hospitales es deficiente. Se precisa de manera urgente instaurar medidas como la actualización de la formación de los HCW, la corrección de las deficiencias en el abastecimiento de mascarillas y afiches y la práctica constante de la supervisión y la vigilancia.
An estimated 10.4 million people worldwide developed tuberculosis (TB) in 2015 and 1.4 million people died from it.1 Pakistan, a geographically diverse country with a population of 185 million, currently ranks fifth worldwide in terms of estimated TB incidence with an annual incidence of 510 000, and it ranks sixth in estimated incidence of multidrug-resistant TB (MDR-TB).1 Urgent interventions are needed to reduce TB transmission.
TB transmission is well documented in health-care settings, with alarmingly high levels of TB among health-care workers (HCWs).2–4 TB infection control (TBIC) programmes are therefore necessary in each health facility. To reduce the risk of TB transmission in health-care facilities, in 2009 the World Health Organization (WHO) issued a TBIC policy including administrative, environmental and personal protection measures.5 These measures have been adopted in Pakistan's national TBIC guidelines.6 The implementation of TBIC measures takes on greater significance in sites managing MDR-TB patients, as such sites have increased following the scale-up of the programmatic management of drug-resistant TB (PMDT).
As part of the PMDT scale-up, Pakistan's National TB Programme (NTP) started upgrading selected health-care facilities to make them compliant with the global recommended TBIC policy.5 The key intervention involved providing all PMDT sites with a TBIC package, which includes infrastructure upgrades, the provision of TBIC supplies (surgical masks, N-95 masks and materials on information, communication and education) and training for HCWs on TBIC.
Implementation of these measures has not been systematically evaluated. TBIC, particularly airborne TBIC, has until recently been neglected globally as an approach to disease prevention.7 There is little information worldwide regarding TBIC implementation and the attitudes and practices of health-care providers in health-care settings, and no published evidence on this issue from Pakistan.
We aimed to assess the extent of the implementation of TBIC measures at selected PMDT sites in Pakistan and reasons for non-adherence to TBIC practices from the provider's perspective.
METHODS
Study design
This was a descriptive study involving observation of health facilities and interviews with patients and HCWs.
Setting
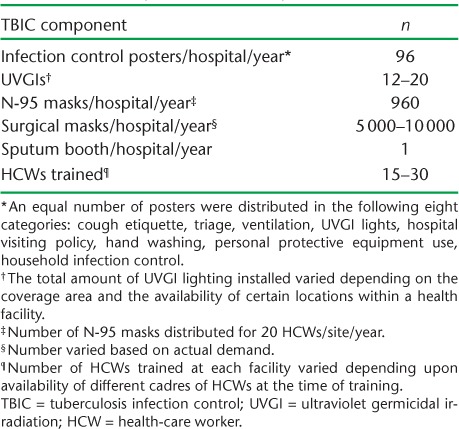
In 2010, Pakistan's NTP decided to upgrade 30 MDR-TB treatment sites and 22 laboratories by introducing a TBIC package consisting of infrastructure upgrades and the training of all cadres of hospital staff, including doctors, nurses, paramedics and laboratory, radiography, pharmacy and cleaning staff. Training was conducted on site for a duration of 1 day. The objective of the infrastructure upgrade was to put in place safeguards for airborne infection transmission. Following risk assessments, site renovation in the form of construction work (mainly new windows and the installation of ventilators to aid indoor cross-ventilation) and electrical work (the indoor installation of fans, exhaust fans and ultraviolet germicidal irradiation [UVGI]) was undertaken. A site-specific TBIC committee was then established at each facility. A written document with assigned roles and responsibilities for each TBIC practice (e.g., triage, working UVGIs, provision of masks to patients, etc.) was developed with the approval of the head of the institution. A detailed description of the components of the TBIC package is shown in Table 1. By the end of March 2016, complete TBIC packages had been successfully delivered to 14 PMDT sites; the remaining sites are due to be covered by the end of 2017.
TABLE 1.
Details of the TBIC packages provided to each of 10 selected hospitals, Pakistan, April–October 2016
Study population
To assess the extent of the implementation, we selected 10 of the 14 PMDT sites in Punjab, Sindh, Khyber Pakhtunkhwa and Baluchistan provinces in Pakistan. Four sites were excluded because they could not be visited due to the security situation, geographical remoteness of the locations and time constraints.
To assess the reasons for non-adherence to TBIC practices, patients and HCWs in the same institution were selected for interview. Ten patients per health facility were consecutively selected after seeing a physician and 10 HCWs at each PMDT site were interviewed to assess reasons for non-adherence. The HCWs were purposively selected, and included those identified as non-adherent to TBIC practices during health-facility observation and/or patient interviews. All cadres of HCWs were selected to obtain different perspectives: doctors, nurses, counsellors, DOTS staff, paramedic staff, registration clerks, pharmacists, radiology staff and cleaning staff.
Data collection, data variables and sources
The head of each PMDT site provided administrative approval to conduct the assessment. Two investigators who were fully trained in TBIC (YW and MAK) collected the data; one was the main trainer and the nodal person responsible for the IC component at the NTP. For the assessment of IC activities, non-participant observation of the health facility was performed by the investigators using a structured checklist. The checklist included variables on the availability and functional status of TBIC posters, natural and mechanical ventilation and UVGI lights. The facility observation of the TBIC protocols was held at different service delivery areas (SDA) (chest out-patient departments, chest wards, PMDT areas, waiting areas, sputum booths, radiography and procedure rooms) over 2 days to ensure a more accurate representation of the situation.
To interview patients exiting the chest and MDR-TB out-patient departments, a structured questionnaire was used that included variables on screening, segregation and fast-tracking of coughing patients, cough etiquette, designated areas for sputum collection, surgical masks and N-95 masks.
Finally, the HCWs were interviewed using a structured questionnaire that included variables on the causes that prevented HCWs from performing triage, i.e., screening, segregating and fast-tracking coughers; instructing coughers on cough hygiene; asking presumptive and confirmed TB patients to wear surgical masks; wearing N-95 masks at all times around TB patients; collecting sputum samples in designated areas; displaying posters; keeping doors and windows open; and keeping the fans/extraction fans/UVGI lights operational.
All data collection tools were pre-tested and validated before use. Data collection was conducted between April and October 2016. At the conclusion of the assessment at each site, the investigators gave feedback and made recommendations to the head of the PMDT site.
Data entry and analysis
Data were double-entered and analysed using EpiData v. 3.1 for entry and v. 2.2.2.182 for analysis (EpiData Association, Odense, Denmark). The analysis was descriptive and included cross-tabulation. Each service delivery site observed in the hospital constituted a unit of analysis. The proportion of SDAs complying with a particular TBIC measure was used as a measure of adherence to that specific TBIC measure. From the patient interviews, we assessed the number and proportion of patients who reported being screened, segregated and fast-tracked, receiving education on cough etiquette, using a sputum booth for sputum expectoration, being provided with a surgical mask and observing the use of N-95 masks by HCWs. This information was used to assess the extent of adherence to the recommended TBIC practices. The questionnaire used for identifying reasons for non-adherence to TBIC practices by the HCWs had numerous responses. Similar and relevant responses were aggregated and categorised for the sake of simplicity and ease of communication.
Ethics approval
Permission for the study was provided by the NTP (Islamabad, Pakistan). Ethics approval was obtained from the Ethics Advisory Group of the International Union Against Tuberculosis and Lung Disease (Paris, France) and the National Bioethics Committee (Islamabad, Pakistan). Written informed consent was obtained from the patients and HCWs interviewed. For purposes of confidentiality, the names of the hospitals assessed have been concealed.
RESULTS
All 10 hospitals studied had a TBIC committee, although there was no documented evidence of regular committee meetings. None of the hospitals displayed the TBIC plan of the hospital.
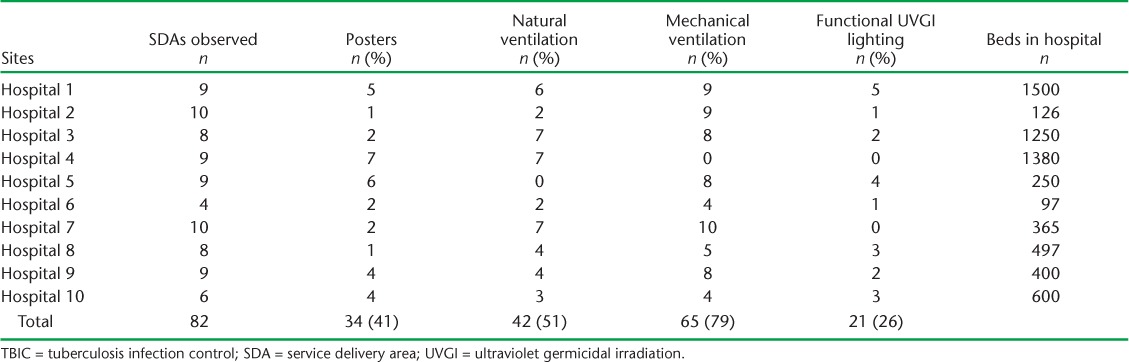
A total of 82 SDAs were observed in the 10 hospitals, with 4–10 SDAs per hospital. TBIC posters were displayed in only 34 of these (41%). While mechanical ventilation was implemented in most SDAs (79%), natural ventilation was observed in 51%; only 26% had functional UVGIs (Table 2).
TABLE 2.
Adherence to TBIC activities observed at 10 selected hospitals, Pakistan, April–October 2016
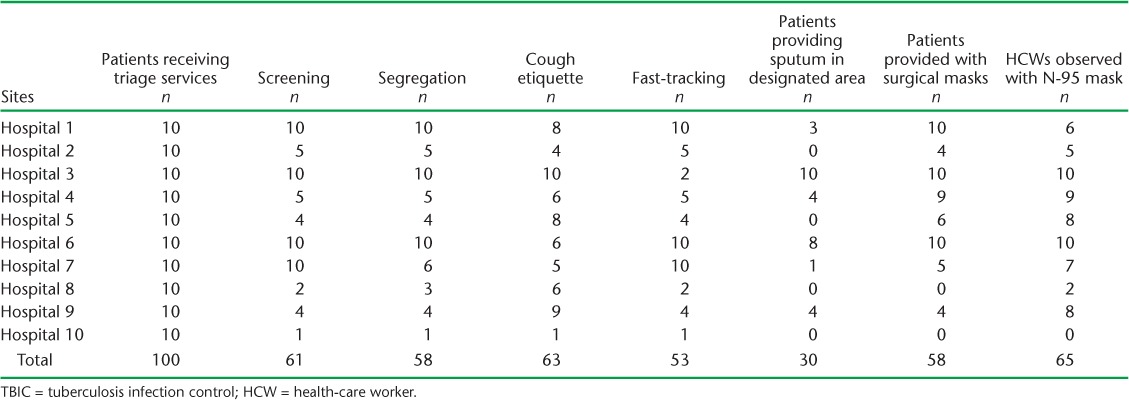
Among the patients interviewed, approximately 50–60% reported having been screened for chest symptoms, segregated, educated about cough etiquette, fast-tracked for availing services and provided with surgical masks. Only 30% of the patients mentioned expectorating sputum in the designated sputum booth. Approximately two thirds of patients reported seeing the HCWs wearing N-95 masks (Table 3).
TABLE 3.
Patients receiving triage and other TBIC services at 10 selected hospitals, Pakistan, April–October 2016
A total of 100 HCWs were interviewed: 24 doctors, 32 nurses, 23 paramedical staff, including pharmacists and radiography technicians, 8 cleaning staff, 2 from administration and 11 with missing data on cadre. The distribution was similar across the hospitals. Of the 100 HCWs, only 44 (44%) reported being trained in TBIC. The reasons for HCWs' non-adherence to the TBIC package are shown in Tables 4–6. The main reasons for not advising patients on cough etiquette were workload, language barriers and the perception that patients would not comply even if advised. The most common reported reason for not implementing triage services was a lack of knowledge or training. The most common reason for not collecting sputum in a designated area was site unavailability and distant location. Posters were not displayed because they were either not available or were not permitted by the hospital to be displayed on the walls. Reasons for the non-use of surgical masks and N-95 masks were non-availability and discomfort.
TABLE 4.
Reasons for non-adherence to TBIC practices (administrative control measures) by HCWs (n = 100) at 10 selected hospitals, Pakistan, April–October, 2016

TABLE 5.
Reasons for non-adherence to TBIC practices (personal protective measures) by HCWs (n = 100) at 10 selected hospitals, Pakistan, April–October 2016

TABLE 6.
Reasons for non-adherence to TBIC practices (environmental control measures) by HCWs (n = 100) at 10 selected hospitals, Pakistan April–October 2016
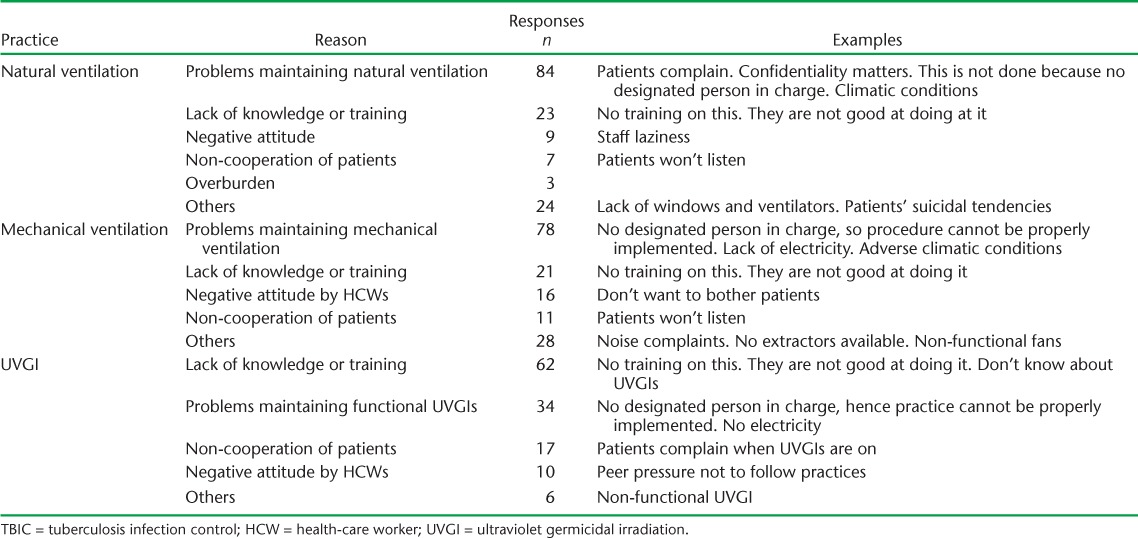
In responses to questions about non-adherence to environmental control measures, many HCWs reported that natural cross-ventilation was not possible due to climate or privacy concerns. Poor maintenance of mechanical ventilators was reported to be due to a lack of clarity about roles (not clear of who would be the nodal person to oversee its implementation) and unstable power supplies. Not using UVGI was reported to be due to a lack of knowledge and a lack of clarity about roles.
DISCUSSION
This is the first study from Pakistan on TBIC in hospitals, and it will provide a baseline for future comparisons. We found that approximately half of the measures recommended by the NTP were implemented in the hospitals. Implementation levels were fairly similar across the 10 hospitals. There was poor adherence to administrative measures such as triage and sputum collection in designated areas, with ‘lack of knowledge’ cited as the main reason. Low rates of use of N-95 and surgical masks were explained by a lack of supplies, confirmed by the NTP. Adherence to environmental measures such as functional UVGIs and natural ventilation was poor.
The implementation of TBIC activities, especially early diagnosis, segregation and HCW training, has proven effective, although very challenging to implement, and our study demonstrates findings similar to others.9–11 We also found that although initial TBIC training and the provision of all necessary IC items were largely in place, there remains a gap in adherence, especially on the part of the HCWs. A recent survey in low- and middle-income countries reported a ‘lack of knowledge/training’, although training had previously been conducted; this may be explained by the high turnover of staff in hospital settings.12
The assessment of key administrative measures relating to the overall provision of triage services remained consistently low, around 50%, at all sites. There was no evidence of active involvement of the hospital IC committees. Provision of education about cough etiquette was found to be inconsistent. Although not directly comparable, this finding has been documented in a study from Nigeria, which showed that only 20% of TB clinics were observed to be providing consistent delivery of health education on cough etiquette to patients.13
The main reasons for non-adherence to IC measures in our study were lack of training, non-availability of a segregation area, perceived non-cooperation of patients and overburdened staff. More than half of the HCWs interviewed reported that they had not received any training in the hospital. In contrast to the recommendations in the TBIC guidelines, the cascade of continuous onsite training has not been replicated by the responsible focal persons of the facilities studied due to the practice of relocation of intra-hospital staff. Our study also showed very low adherence to the practice of collecting sputum in a designated area (30%), which is similar to other settings,13 due to the distant location of collection areas and patient non-cooperation.
Regarding environmental measures, the use of UVGI showed poor adherence, at 26%, similar to the audits of TBIC in facilities by Claassens et al.14 The main underlying reasons identified in our study were a lack of knowledge and training and an unstable electricity supply. Furthermore, in contrast to the study by Tenna et al., our study indicated greater adherence to mechanical ventilation, as natural ventilation was used less frequently due to issues of privacy, patient-related complaints and climatic conditions.15
Adherence to the use of personal protective measures was suboptimal, with the main reasons for non-adherence being the non-availability of the requisite items and discomfort in wearing personal protective equipment, a factor for both patients and HCWs. Other studies have shown the same barriers, i.e., ‘not enough N95’ and ‘N95 are uncomfortable’, preventing the implementation of personal protective measures.16,17
One of the strengths of the study was the systematic assessment of TBIC implementation from three angles: the direct observations of the researchers, exit interviews with patients, and interviews with health staff as to why measures were not being implemented. Key limitations include the selected sampling of hospital sites with TBIC packages due to constraints of security, time and logistics. Another limitation is that the personnel involved in conducting training onsite were also the investigators of this study, which might have affected the responses of the HCWs. This was partly mitigated by the non-participant observation and the patient interview components of the study. Finally, we did not have information from hospitals where the TBIC package had not been delivered. Future assessments should include such hospitals, including an analysis of IC practices before and after the provision of the TBIC package.
As the NTP in Pakistan plans to scale up the delivery of TBIC packages to other PMDT sites, the lessons learnt from this study should be used to optimise implementation. These lessons include the following: 1) to improve the participation of the senior administration of the hospital in the implementation of TBIC measures, it is important for a communiqué to be sent from the highest level in the Ministry of Health to the hospital; 2) the initial training by a central team should be extended over a 2–3 day period and cover all health-care providers, instead of relying on master trainers in each facility to pass on the knowledge; this should be supplemented by onsite refresher training during routine supervisory visits; 3) frequent supervisory visits by the central NTP team will be necessary, especially in the initial period of implementation, to identify implementation bottlenecks, including weaknesses in supplies of TBIC consumables, and to take the appropriate corrective measures.
CONCLUSION
To limit the nosocomial spread of the disease, effective TBIC in hospitals is fundamental. In view of the suboptimal implementation of the TBIC measures observed in this study, staff retraining is needed, coupled with uninterrupted supplies, regular supervision and implementation monitoring.
Acknowledgments
This research was conducted through the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Program for Research and Training in Tropical Diseases at the World Health Organization (WHO-TDR, Geneva, Switzerland). The training model is based on a course developed jointly by the International Union Against Tuberculosis and Lung Disease (The Union, Paris, France) and Médecins Sans Frontières (MSF, Geneva, Switzerland). The specific SORT IT programme that resulted in this publication was implemented by the National Tuberculosis Programme of Pakistan (Islamabad) through support from the Global Fund to Fight AIDS, Tuberculosis and Malaria (Geneva, Switzerland), WHO-TDR, the University of Bergen (Bergen, Norway), The Union and The Union South-East Asia Office (New Delhi, India).
The programme was funded by the WHO and The Global Fund in Pakistan. The publication costs were covered by the WHO/TDR. In accordance with the WHO's open-access publication policy for all work funded by WHO or authored/co-authored by WHO staff members, the WHO retains the copyright of this publication through a Creative Commons Attribution IGO license (http://creativecommons.org/licenses/by/3.0/igo/legalcode) that permits unrestricted use, distribution and reproduction in any medium provided the original work is properly cited.
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 2. Baussano I, Nunn P, Williams B, Pivetta E, Bugiani M, Scano F.. Tuberculosis among health care workers. Emerg Infect Dis 2011; 17: 488– 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klimuk D, Hurevich H, Harries A D, . et al. Tuberculosis in health care workers in Belarus. Public Health Action 2014; 4 ( Suppl 2): S29– S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farley J E, Tudor C, Mphahlele M, . et al. A national infection control evaluation of drug-resistant tuberculosis hospitals in South Africa. Int J Tuberc Lung Dis 2012; 16: 82– 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. . WHO policy on TB infection control in health-care facilities, congregate settings and households. WHO/HTM/TB/2009.419 Geneva, Switzerland: WHO, 2009. [PubMed] [Google Scholar]
- 6. National Tuberculosis Control Programme. . National guidelines for TB infection control. Islamabad, Pakistan: Ministry of National Health Services, 2016. http://www.ntp.gov.pk/resource.php Accessed February 2017. [Google Scholar]
- 7. Institute of Medicine and Institute of Microbiology, Chinese Academy of Sciences. . The global crisis of drug-resistant tuberculosis and leadership of China and the BRICS. Washington, DC, USA: National Academies Press, 2014. [PubMed] [Google Scholar]
- 8. World Bank. . Land area. Washington, DC, USA: World Bank, 2016. http://data.worldbank.org/indicator/AG.LND.TOTL.K2 Accessed February 2017. [Google Scholar]
- 9. Joshi R, Reingold A L, Menzies D, Pai M.. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLOS Med 2006; 3: e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brouwer M, Coelho E, das Dores Mosse C, van Leth F.. Implementation of tuberculosis infection prevention and control in Mozambican health care facilities. Int J Tuberc Lung Dis 2015; 19: 44– 49. [DOI] [PubMed] [Google Scholar]
- 11. Turusbekova N, Ljungqvist I, Davidavičiene E, Mikaityte J, van der Werf M J.. Tuberculosis infection control in health facilities in Lithuania: lessons learnt from a capacity support project. Public Health Action 2016; 6: 22– 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Godfrey C, Tauscher G, Hunsberger S, . et al. A survey of tuberculosis infection control practices at the NIH/NIAID/DAIDS-supported clinical trial sites in low and middle income countries. BMC Infect Dis 2016; 16: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuyinu Y A, Mohammed A S, Adeyeye O O, Odugbemi B A, Goodman O O, Odusanya O O.. Tuberculosis infection control measures in health care facilities offering TB services in Ikeja local government area, Lagos, South West, Nigeria. BMC Infect Dis 2016; 16: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Claassens M M, van Schalkwyk C, du Toit E, . et al. Tuberculosis in healthcare workers and infection control measures at primary healthcare facilities in South Africa. PLOS ONE 2013; 8: e76272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tenna A, Stenehjem E A, Margoles L, Kacha E, Blumberg H M, Kempker R R.. Infection control knowledge, attitudes, and practices among healthcare workers in Addis Ababa, Ethiopia. Infect Control Hosp Epidemiol 2013; 34: 1289– 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanjee Z, Catterick K, Moll A P, Amico K R, Friedland G H.. Tuberculosis infection control in rural South Africa: survey of knowledge, attitude and practice in hospital staff. J Hosp Infect 2011; 79: 333– 338. [DOI] [PubMed] [Google Scholar]
- 17. Tamir K, Wasie B, Azage M.. Tuberculosis infection control practices and associated factors among health care workers in health centers of West Gojjam zone, Northwest Ethiopia: a cross-sectional study. BMC Health Serv Res 2016; 16: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]


