Abstract
Setting: National Institute for Research in Tuberculosis, Madurai, India.
Objective: To determine the efficacy of physician's advice on quitting smoking compared with standard counselling in patients with tuberculosis (TB) and patients with human immunodeficiency virus (HIV) infection.
Design/Methods: This was a clinical trial conducted in Madurai, south India, among 160 male patients (80 with TB and 80 with HIV), randomised and stratified by nicotine dependence (low/high according to the Fagerström scale), who received physician's advice with standard counselling or standard counselling alone for smoking cessation. Abstinence at 1 month was assessed by self-report and carbon monoxide breath analysis.
Results: The patients' mean age was 39.4 years (SD 8.5). Overall, 35% of the patients had high nicotine dependence. Most patients (41%) smoked both cigarettes and bidis. In a combined analysis including both the HIV and the TB groups, quit rates were 41% of the 68 patients in the physician group and 35% of the 68 patients in the standard counselling arm.
Conclusions: Physician's advice to quit smoking delivered to patients with TB or HIV is feasible and acceptable. Smoking cessation could easily be initiated in TB patients in programme settings. Future studies should assess long-term abstinence rates with a larger sample size to demonstrate the efficacy of physician's advice.
Keywords: tuberculosis, human immunodeficiency virus, smoking cessation, physician's advice
Abstract
Contexte : National Institute for Research in Tuberculosis (NIRT), Madurai, Inde.
Objectif : Déterminer l'efficacité des conseils d'un médecin comparés aux conseils standard d'arrêt du tabac chez des patients atteints de tuberculose (TB) et chez des patients infectés par le virus de l'immunodéficience humaine (VIH).
Schéma/Méthodes : Cet essai clinique a été réalisé à Madurai, dans le sud de l'Inde. Cent soixante patients masculins (80 TB et 80 VIH) ont été randomisés, stratifiés en fonction de leur dépendance à la nicotine (faible/élevée selon l'échelle de Fagerström) pour bénéficier soit des conseils d'un médecin avec des conseils standard ou des conseils standard seuls pour l'arrêt du tabac. L'abstinence à un mois a été évaluée par déclaration des patients et analyse du monoxyde de carbone dans l'air expiré.
Résultats : L'âge moyen (DS) a été de 39,4 (±8,5) ans. Dans l'ensemble, 35% des patients avaient une dépendance élevée à la nicotine. La majorité des patients (41%) fumait à la fois des cigarettes et des bidis (cigarettes indiennes). En analyse combinée (à la fois le groupe VIH et TB), les taux de cessation ont été de 41% sur 68 patients dans le groupe « médecin » et de 35% de 68 patients dans le bras « conseil standard » (non significatif).
Conclusions: Les conseils d'un médecin en matière d'arrêt de tabac délivrés aux patients atteints de TB ou de VIH sont faisables et acceptables. L'arrêt du tabac pourrait bien être mis en œuvre dans le contexte des programmes chez les patients TB. De futures études devraient évaluer le taux d'abstinence à long terme avec des échantillons de plus grande taille afin de démontrer l'efficacité des conseils d'un médecin.
Abstract
Marco de referencia: El Instituto Nacional de Investigación en Tuberculosis de Madurai, en la India.
Objetivo: Comparar la eficacia de los consejos que da el médico con el asesoramiento corriente sobre el abandono del tabaco, en pacientes aquejados de tuberculosis (TB) o infección por el virus de la inmunodeficiencia humana (VIH).
Métodos: El ensayo clínico inicial se llevó a cabo en Madurai, en el sur de la India. Se escogieron de manera aleatoria 160 pacientes de sexo masculino (80 con diagnóstico de TB y 80 de infección por el VIH), se estratificaron en función de la dependencia de la nicotina (baja y alta, según la escala de Fagerström) y se asignaron a un grupo que recibiría consejos del médico además del asesoramiento corriente sobre el abandono del tabaco o un grupo que solo recibiría el asesoramiento corriente. Al cabo de un mes, se evaluó la abstinencia a partir de la información proporcionada por los pacientes y la medición de monóxido de carbono en el aire espirado.
Resultados: El promedio de edad de los participantes fue 39,4 años (desviación estándar 8,5 años). En general, el 35% de los participantes exhibía una alta dependencia de la nicotina. La mayoría fumaba cigarrillos y también bidis (41%). En el análisis conjunto (ambos grupos: TB y VIH), las tasas de abandono fueron 41% en los 68 pacientes del grupo que recibió consejo médico y 35% en los 68 pacientes del grupo que obtuvo asesoramiento corriente (diferencia no significativa).
Conclusión: El consejo sobre el abandono del tabaco ofrecido por el médico a los pacientes con diagnóstico de TB o infección por el VIH es factible y bien aceptado. Es posible iniciar intervenciones de abandono del tabaco con los pacientes TB en el marco programático. Futuros estudios podrían evaluar las tasas de abstinencia a largo plazo con muestras más grandes, a fin de demostrar la eficacia del consejo dado por los médicos.
Tobacco consumption is currently the single leading preventable cause of death globally.1 Of the estimated 1.1 billion smokers worldwide, approximately 182 million (16.6%) are in India; it is predicted that tobacco will account for 13% of all deaths in India by 2020.2 The National Family Health Survey (NFHS) is a large-scale multi-round survey conducted in a representative sample of households throughout India. According to NFHS-3,3 which collected data between 2005 and 2006 directly on tobacco use by asking respondents to report on their own tobacco use, the percentage of women and men aged 15–49 years who smoked cigarettes or bidis (thin, often flavoured cigarettes made of tobacco wrapped in a tendu leaf) was respectively 1.4% and 32.7%. In NFHS-2,4 conducted in 1998–1999, smoking prevalence among females and males was respectively 2.3% and 29.3%. A comparison of NFHS-2 and NFHS-3 shows that the prevalence of smoking among males (both rural and urban) has increased. Furthermore, the rates of smoking among illiterate, rural and lower socio-economic group populations were consistently higher in both NFHS-2 and NFHS-3.
The growing body of literature describing a causal association between active or passive exposure to tobacco smoke and various outcomes of tuberculosis (TB) should be reason enough to join forces in tackling both the TB and the tobacco epidemics.5 Both passive and active exposure to tobacco smoke have been shown to be associated with tuberculous infection6 and increase the risk of developing TB disease.7,8 In a nested case-control study at the National Institute for Research in Tuberculosis (NIRT), Chennai, India, a positive association between tobacco smoking and pulmonary TB (PTB) was observed (crude odds ratio [OR] 2.48, 95% confidence interval [CI] 1.42–4.37, P < 0.001), with a strong dose response relationship.7 In a cross-sectional observational study, TB patients who smoked were more likely to develop pulmonary disease (adjusted OR [aOR] 1.5, 95%CI 1.3–1.6) and cavitary lesions (aOR 1.9, 95%CI 1.6–2.3) and to require hospitalisation than those who did not smoke (aOR 1.8, 95%CI 1.5–2.2). Smoking also led to faster and more severe progression of TB, and the cost of TB-related treatment was comparatively higher.9 Smoking has also been found to be an independent predictor of relapse of PTB (aOR 3.1, 95%CI 1.6–6.0),10 while a retrospective study of 43 000 adult male deaths and 35 000 controls performed in both rural and urban areas in Tamil Nadu, India, mortality from TB was four times greater among smokers than among non-smokers.11
Smokers who are also infected with the human immunodeficiency virus (HIV) face additional risks besides the general health consequences of smoking. It has been shown that among women on highly active antiretroviral therapy (HAART), smokers had poorer viral and immunological response, greater risk of virological rebound and more frequent immunological failure than non-smokers.12 In India, which has a large burden of people living with HIV and with almost half of its population infected with TB, more people are likely to develop co-infection. As TB is one of the most common opportunistic infections among HIV-infected patients, the additive risks of smoking are likely to be detrimental and contribute to the observed high mortality and morbidity.
Smoking cessation messages delivered by a physician, even when brief, are known to be effective as a strategy to promote smoking cessation.13,14 Smokers cite physician's advice to quit as an important motivator for attempting to stop smoking, and there is growing evidence that smokers who receive clinician advice and assistance with quitting report greater satisfaction with their health care than those who do not.15 With this background information, we conducted a study aimed to determine the efficacy of physicians' advice in addition to standard counselling compared with standard counselling alone in quitting smoking (defined as abstinence at 1 month), in patients with TB and patients with HIV. Our hypothesis was that the efficacy of the physicians' advice given in addition to standard counselling for quitting smoking would be at least 30%.
STUDY POPULATION, DESIGN AND METHODS
The study was conducted at the NIRT, Madurai, India. Subject recruitment and follow-up to review the smoking cessation information after 1 month took place from March 2010 to May 2012.
Approval was obtained from the Scientific Advisory Board of Brown University Fogarty, Providence, RI, USA, the Scientific Advisory Committee and Institutional Ethics Committee of the NIRT, Madurai, India, and from the Brown University Institutional Review Board. The trial was registered in the Clinical Registry of India (CTRI/2009/091/000962, 14.12.2009). Written informed consent was obtained from all participants.
Eligibility, recruitment and randomisation
Patients aged >18 years, with either TB or HIV and with a history of current smoking (at least one cigarette in the past 1 week), referred to the NIRT clinic, Madurai, were enrolled. Patients with HIV and TB co-infection were included in the HIV group.
A total of 160 patients, 80 with HIV and 80 with TB, were enrolled. Each subject's clinical and demographic profile and a detailed smoking history were collected by the registered nurses at our centres and the social workers involved in the study. The nicotine dependence of each participant was assessed using the Fagerström test.16 To assess the impact of physician's advice in highly nicotine-dependent patients, the patients were stratified according to the Fagerström scale: a score of ⩽5 was considered low and >5 was high. Stratified block randomisation was used separately for the TB and HIV groups for allocation; sealed envelopes were used to ensure allocation concealment, and outcome assessment was not blinded.
The physicians who participated in the study intervention followed the 5As approach recommended by the US Department of Health and Human Services for primary care clinicians/health care personnel: Ask the patient if he/she uses tobacco, Advise him/her to quit, Assess willingness to make a quit attempt, Assist him/her in making a quit attempt, and Arrange for follow-up contact to prevent relapse.14 The principles used in these guidelines were formulated after several clinical trials.17–22
The intervention group received physician's advice using a modified version of the 5As strategy14 for smoking cessation plus a brochure containing smoking cessation information, and counselling from a counsellor. The comparator group received a brochure containing smoking cessation information plus counselling from a counsellor. For the intervention group, a standard format for delivering the physician's advice to quit smoking was developed and used in the study. The modified 5As strategy was followed systematically.
We termed our approach ‘modified 5As’, as some additional approaches were added. The physician delivered brief structured advice both to the subject and to family members, which is a change from the standard ‘Assist’ step of the 5As approach. The subjects were educated on setting a quit date and on the procedures to follow during the process of quitting. Patients were educated on the harms of smoking and TB and smoking and HIV.
The physicians who performed the study intervention were provided with a checklist for TB and HIV patients separately detailing the points to be discussed; they documented their notes in the respective study forms. Subjects were asked to attend on the next working day for review. Subjects were also encouraged to bring close family members to attend on the next day for counselling by the physician.
In addition to the physician's advice, brochures were provided with pictorial presentations of the complications of smoking, how to quit and withdrawal symptoms. Standard counselling by a counsellor was also provided.
For the comparator group, the brochure containing smoking cessation information was also given to the patient, as well as standard counselling by a counsellor. Standard counselling was structured based on the recommendations of the National Tobacco Control Programme of India, and lasted for approximately 15–20 min. The counsellors were provided with a checklist of what needed to be discussed with the subjects and initially elicited their knowledge about tobacco smoking and what were the triggering factors, and then counselled the subjects by explaining the harms of smoking, the benefits of quitting smoking, how to set a quit date and anticipated challenges.
Outcome
Patients in both arms were asked to set a quit date no later than 3 weeks from the date of the first visit. At the first monthly visit, smoking abstinence was determined by self-reported abstinence and confirmed by carbon monoxide monitor reading (<10 parts per million [ppm]). Wherever there were discrepancies, the final outcome would be still smoking when any of the methods said still smoking. Smoking cessation was defined as not currently smoking and a carbon monoxide concentration of <10 ppm at the first monthly visit.
Sample size and analyses
The sample size was calculated based on two proportions. It was assumed that standard smoking cessation counselling by counsellors with brochures would have an efficacy of about 10%. By adding physician's advice, it was expected that efficacy would increase to 30%. To detect the difference with 95%CI and 90% power, the minimum number of patients required was approximately 70 per arm. Allowing for 10% upward adjustment for loss to follow-up, the minimum sample size required was calculated at about 80 per arm.
For statistical analyses and data handling, SPSS software version 20.0 was used (IBM SPSS Systems, Armonk, NY, USA). The results are presented in percentages, means and standard deviation (SD). To compare the differences between the groups, Pearson's χ2 test was used for categorical variables. Univariate and multivariate logistic regression analysis was also performed to evaluate the association between baseline characteristics with quitting smoking. P < 0.05 was considered statistically significant.
RESULTS
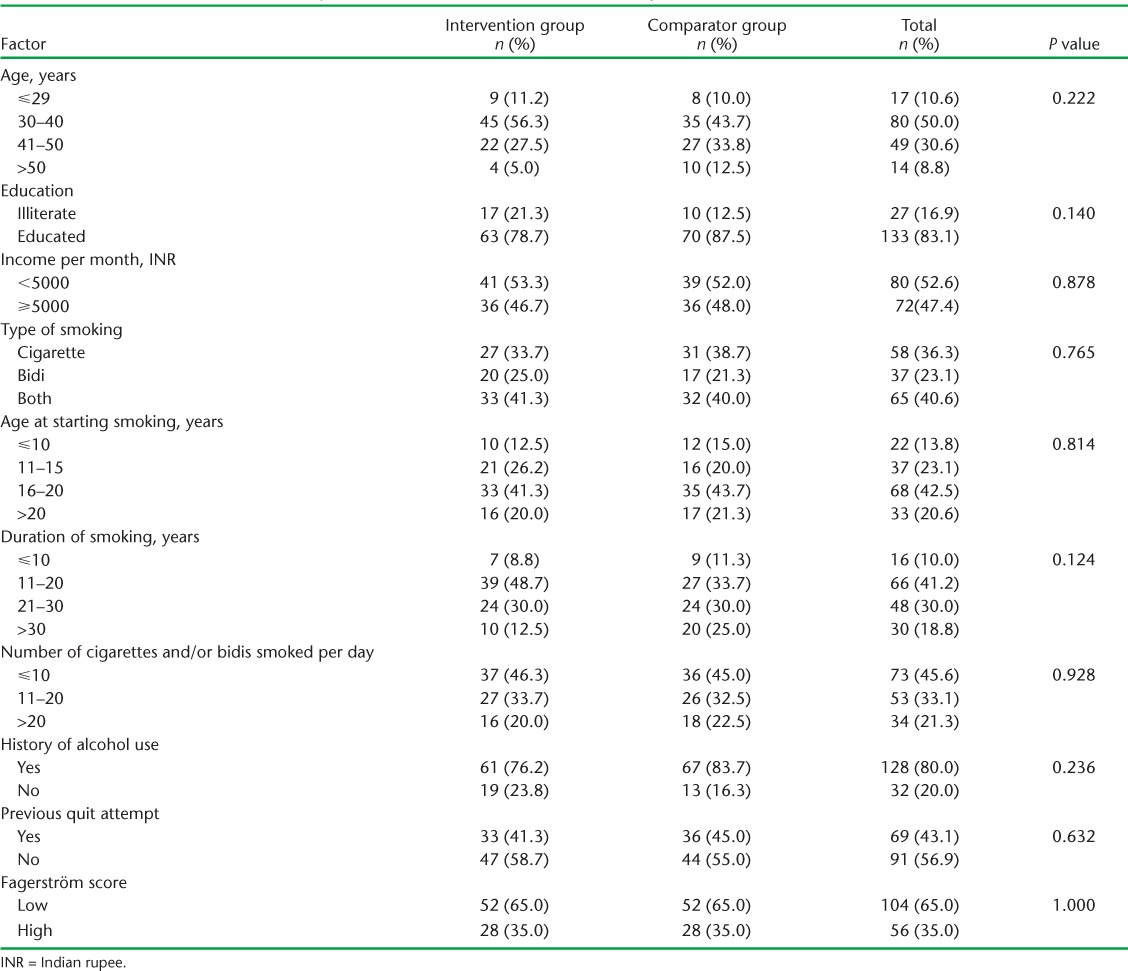
Of 179 subjects screened, 160 (80 TB and 80 HIV) were enrolled in the study. The Figure shows the enrolment and follow-up of the study subjects. All subjects were male. Table 1 describes the baseline characteristics of the subjects enrolled in the two arms: intervention and comparator. The mean age was 39.4 (SD ± 8.5) years. Nearly half (45%) were educated to primary level. The mean age at starting smoking was 17.4 (±5.5) years and the mean duration of smoking was 22 (±9.8) years. The mean number of cigarettes and/or bidis per day was 14.3 (±10.8). Overall, 35% (56/160) of the patients had high dependence on nicotine as assessed by the Fagerström scale. Many of the patients (41%) had a habit of smoking both cigarettes and bidis. Smoking habits differed between TB patients and HIV patients, in that more people in the HIV group exclusively smoked cigarettes (P = 0.025).
FIGURE.
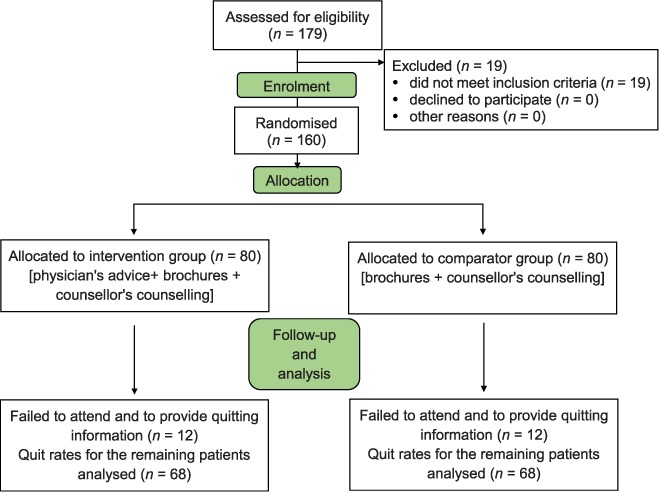
Consort diagram showing enrolment and follow-up of the study subjects.
TABLE 1.
Baseline characteristics of subjects enrolled in the intervention and comparator arms
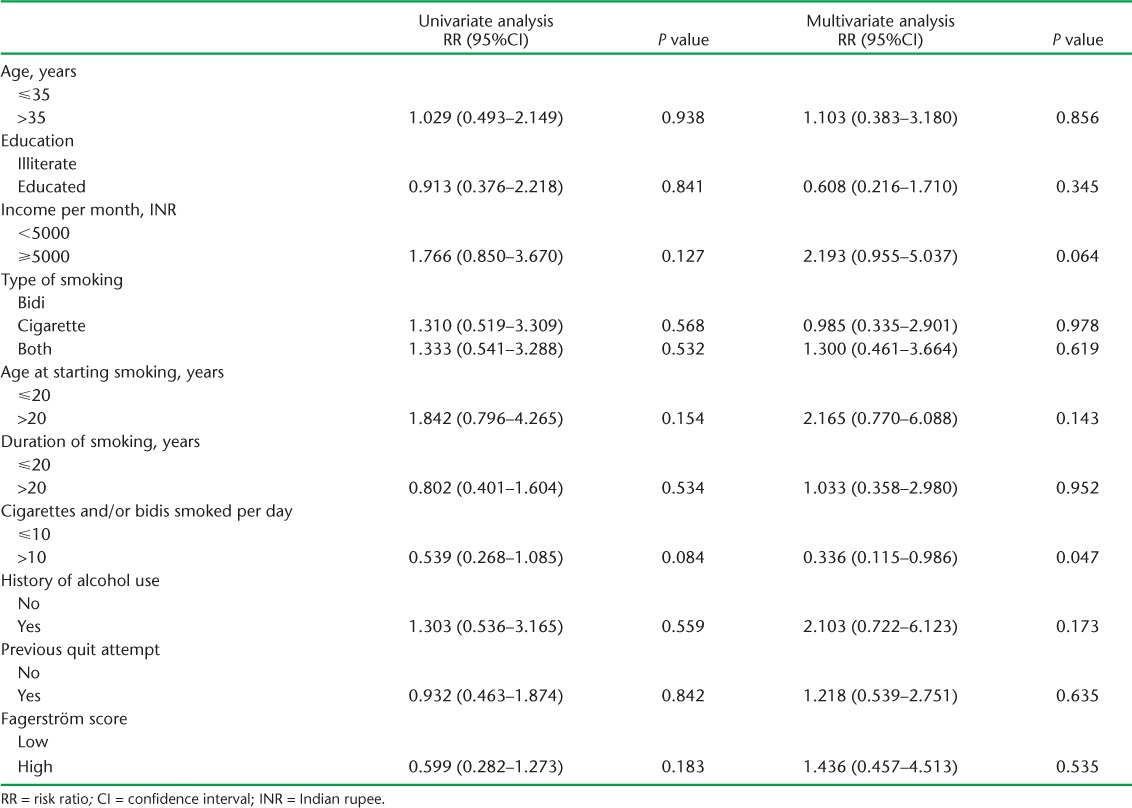
Twenty-four patients (12 patients in each intervention group) did not return after the first smoking cessation visit. Information on quitting smoking was available for the remaining 136 patients (Table 2), of whom 52 (38%) quit smoking at 1 month. Among the patients receiving physician's advice, 41% (28/68) quit smoking, while 35% (24/68) quit smoking with counselling from the counsellors (relative risk [RR] 1.167, 95%CI 0.759–1.792, P = 0.480). Intention-to-treat analysis was also performed, as this is a preferred approach, and showed that the quit rate among the intervention group vs. the comparator group was 35% vs. 30% (P = 0.500). Among the highly nicotine-dependent smokers, 30% (in the intervention or the comparator group) quit smoking. No significant association was found between the quit rate and the type of tobacco smoked (P = 0.506), past history of quitting (P = 0.842), number of cigarettes/bidis smoked (P = 0.175), duration of smoking (P = 0.864), age at starting smoking (P = 0.538) or educational status (P = 0.349). Univariate and multivariate regression analysis could not ascertain any association between these factors and quitting smoking (Table 3).
TABLE 2.
Quit rates among the intervention * and comparator † arms among TB and HIV subgroups
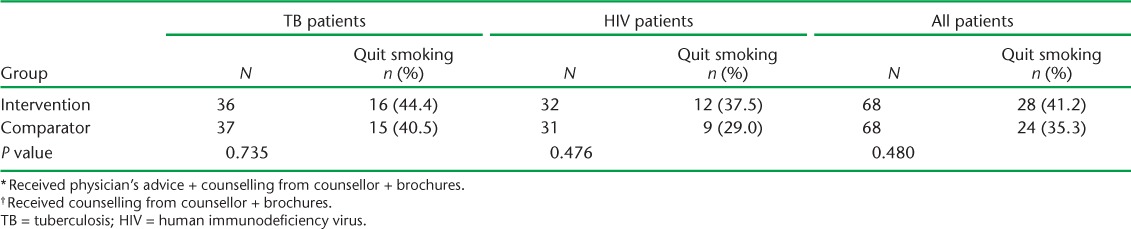
TABLE 3.
Univariate and multivariate logistic regression analysis to evaluate the association between baseline characteristics and quitting smoking
We collected information on attempting to quit from patients in both arms, to assess how many subjects tried to follow our instructions to aid in smoking cessation. Among those who still smoked (n = 84), 80% attempted to quit, i.e., fixed a quit date and abstained from smoking <1 month, but could not refrain from smoking after that period.
DISCUSSION
Our study has shown that delivering physicians' advice on smoking cessation is feasible and acceptable in two groups of the population: TB and HIV patients. The physicians involved had ample time to deliver and document the advice given, and the patients were very receptive. This makes it clear that physician advice for smoking cessation is feasible in TB programmes, supported by a recent study conducted in India.23 We found that, in addition to the TB patients, patients with HIV were keen to obtain advice on smoking cessation from the physicians, showing the level of acceptability for this approach. The evidence for this conclusion, however, may be graded as low,24 as we analysed compiled qualitative information recounted by the patients passively, as documented by the physicians in the study forms.
It has been shown that brief advice delivered by a physician as part of a minimal intervention vs. no advice, or usual care, results in an increase in quit rates;14 almost half of the patients who were offered physician's advice in our study quit smoking. It is therefore justified to promote physician's advice on quitting smoking among smokers with TB as an important and essential component of TB management in TB programmes, and as a simple strategy in which a doctor in a TB clinic in a programme delivers brief advice to aid in TB control.
As the study was time bound, we could only assess a 1-month abstinence period. We feel, however, that smoking cessation in TB and HIV patients is very important and that every instance of a patient quitting smoking in these populations makes a difference. A smaller sample size would have failed to demonstrate the statistical difference between the two arms.
Among the 73 TB patients for whom information on quitting was available, 31 (43%) had quit smoking at 1 month using either of the two interventions. We feel that TB patients can be targeted for smoking cessation because they are in regular contact with health workers and may be more motivated than healthy persons to quit smoking. A system has already been established in TB programmes and a system for recording events (diagnosis, treatment, treatment outcome) is already in place. The introduction of a few additional interventions would be relatively easy—for example, directly observed treatment (DOT) providers could be trained to advise, encourage and monitor smoking cessation attempts. Furthermore, with ample evidence that smoking in TB causes more relapse, morbidity and mortality, TB patients should be targeted for smoking cessation interventions.
Among those who did not quit (n = 84), 80% attempted to quit, i.e., they set a quit date and followed the instructions and remained abstinent for a period of <1 month. Any amount of motivation, therefore, when delivered towards a positive motive, may aid in successful quitting.
The results showed that the intervention delivered by the counsellors alone, without the physician's advice, also had fairly good quit rates. This justifies the need to include social workers or counsellors as personnel in the TB management team of the TB programme who could aid in the process, given the busy schedules of the medical officers in TB management in the programme.
Finally, although high-dose preparations of nicotine gum, patches and lozenges are recommended in guidelines15 for highly dependent smokers, our study results show that even among patients with high nicotine dependence, 30% quit smoking without recourse to pharmacological methods. Thus a simple counselling intervention even for patients with high nicotine dependence can be effective to assist in quitting smoking.
One of the limitations of this study was that smoking cessation rates were measured at 1 month follow-up only. As this was a time-limited project, we could not follow the patients and assess their long-term quit rates. Furthermore, the power of the study to determine differences between the arms was limited due to the relatively small sample size. Another limitation was that all the subjects were males, with no females enrolled. This is because the history of smoking is rarely revealed by females in south India. Also, although the patients in the comparator group received counselling from the counsellors and it was ensured that they were not to be reviewed by the physicians involved to avoid spill-over, seven patients did report to the physicians; such situations could not be avoided due to ethical reasons.
To conclude, physician's advice on quitting smoking delivered to patients with TB or HIV is feasible and acceptable. Smoking cessation can easily be initiated in TB patients in programme settings. Future studies should assess the long-term abstinence rates with larger sample sizes to demonstrate the efficacy of physician's advice.
Acknowledgments
SRK was the recipient of a Fogarty AIDS International Training and Research Programme (D43-TW000237) grant funded by the US National Institutes of Health at Miriam Hospital/Brown University, Providence, RI, USA, in 2006. The authors thank the funding agency for sponsoring the study findings presented at the 43rd Union Against Tuberculosis and Lung Disease World Conference on Lung Health held in Kuala Lumpur, Malaysia, in November 2012. The authors also thank E Caffrey, Brown University, for administrative and secretarial assistance, all the staff members of the National Institute for Research in Tuberculosis, Madurai Unit, involved in conducting the study, and medical social workers M Rajasakthivel and E Thirivalluvan for providing technical support.
Footnotes
Conflicts of interest: none declared.
References
- 1. World Health Organization. . WHO Framework Convention on Tobacco Control: why is it important? Geneva, Switzerland: WHO, 2014. http://www.who.int/topics/tobacco/qa/en/ Accessed December 2016. [Google Scholar]
- 2. Shimkhada R, Peabody J W.. Tobacco control in India. Bull World Health Organ 2003; 81: 48– 52. [PMC free article] [PubMed] [Google Scholar]
- 3. International Institute for Population Sciences. . National Family Health Survey–3. Mumbai, India: IIPS, 2007. http://rchiips.org/nfhs/NFHS-3%20Data/VOL-1/India_volume_I_corrected_17oct08.pdf Accessed December 2016. [Google Scholar]
- 4. International Institute for Population Sciences. . National Family Health Survey–2. Mumbai, India: IIPS, 2000. http://www.dhsprogram.com/pubs/pdf/FRIND2/FRIND2.pdf Accessed December 2016. [Google Scholar]
- 5. World Health Organization. . A WHO/The UNION monograph on TB and tobacco control, 2007. WHO/HTM/TB/2007.390 Geneva, Switzerland: WHO, 2007. http://www.who.int/tobacco/resources/publications/tb_tobac_monograph.pdf Accessed December 2016. [Google Scholar]
- 6. Chiang C Y, Slama K, Enarson D A.. Associations between tobacco and tuberculosis. Int J Tuberc Lung Dis 2007; 11: 258– 262. [PubMed] [Google Scholar]
- 7. Kolappan C, Gopi P G.. Tobacco smoking and pulmonary tuberculosis. Thorax 2002; 57: 964– 966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Podhipak A, Akarasewi P, Tornee S, Smithtikarn S, Thongprathum P.. Cigarette smoking and its relation to pulmonary tuberculosis in adults. Southeast Asian J Trop Med Public Health 2004; 35: 219– 227. [PubMed] [Google Scholar]
- 9. Altet-Gomez M N, Alcaide J, Godoy P, Romero M A, Hernandez del Rey I.. Clinical and epidemiological aspects of smoking and tuberculosis: a study of 13 038 cases. Int J Tuberc Lung Dis 2005; 9: 430– 436. [PubMed] [Google Scholar]
- 10. Thomas A, Gopi P G, Santha T, . et al. Predictors of relapse among pulmonary tuberculosis patients treated in a DOTS programme in South India. Int J Tuberc Lung Dis 2005; 9: 556– 561. [PubMed] [Google Scholar]
- 11. Gajalakshmi V, Peto R, Kanaka T S, Jha P.. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43 000 adult male deaths and 35 000 controls. Lancet 2003; 362: 507– 515. [DOI] [PubMed] [Google Scholar]
- 12. Feldman J G, Minkoff H, Schneider M F, . et al. The association of cigarette smoking with HIV prognosis among women in the HAART era—a report from the Women's Interagency HIV Study. Am J Public Health 2006; 96: 1060– 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demers R Y, Neale A V, Adams R, Trembath C, Herman S C.. The impact of physicians' brief smoking cessation counseling: a MIRNET study. J Fam Pract 1990; 31: 625– 629. [PubMed] [Google Scholar]
- 14. Stead L F, Buitrago D, Preciado N, Sanchez G, Hartmann-Boyce J, Lancaster T.. Physician advice for smoking cessation. Cochrane Database Syst Rev 2013; 5: CD000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agency for Healthcare Research and Quality. . Treating tobacco use and dependence: 2008 update. Rockville, MD, USA: AHRQ, 2008. [Google Scholar]
- 16. Heatherton T F, Kozlowski L T, Frecker R C, Fagerström K O.. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991; 86: 1119– 1127. [DOI] [PubMed] [Google Scholar]
- 17. Glynn T J, Manley M W, Pechacek T F.. Physician-initiated smoking cessation program: the National Cancer Institute trials. Prog Clin Biol Res 1990; 339: 11– 25. [PubMed] [Google Scholar]
- 18. Glynn T J, Manley M W.. How to help your patients stop smoking: a National Cancer Institute manual for physicians. Bethesda, MD, USA: National Institutes of Health, 1989. [Google Scholar]
- 19. American Medical Association. . American Medical Association guidelines for the diagnosis and treatment of nicotine dependence: how to help patients stop smoking. Washington, DC, USA: AMA, 1994. [Google Scholar]
- 20. American Psychiatric Association. . Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996; 153 ( Suppl): S1– S31. [DOI] [PubMed] [Google Scholar]
- 21. Kottke T E, Solberg L I, Brekke M L.. Beyond efficacy testing: introducing preventive cardiology into primary care. Am J Prev Med 1990; 6 ( Suppl): 77– 83. [PubMed] [Google Scholar]
- 22. Mecklenburg R E, Christen A G, Gerbert B, Gift M C.. How to help your patients stop using tobacco: a National Cancer Institute manual for the oral health team. Bethesda, MD, USA: National Institutes of Health, National Cancer Institute, 1991. [Google Scholar]
- 23. Kaur J, Sachdeva K, Modi B, . et al. Promoting tobacco cessation by integrating ‘brief advice’ in tuberculosis control programme. WHO South-East Asia J Public Health 2013; 2: 28– 33. [DOI] [PubMed] [Google Scholar]
- 24. Atkins D, Best D, Briss P A, . et al. Grading quality of evidence and strength of recommendations. BMJ 328: 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]


