Abstract
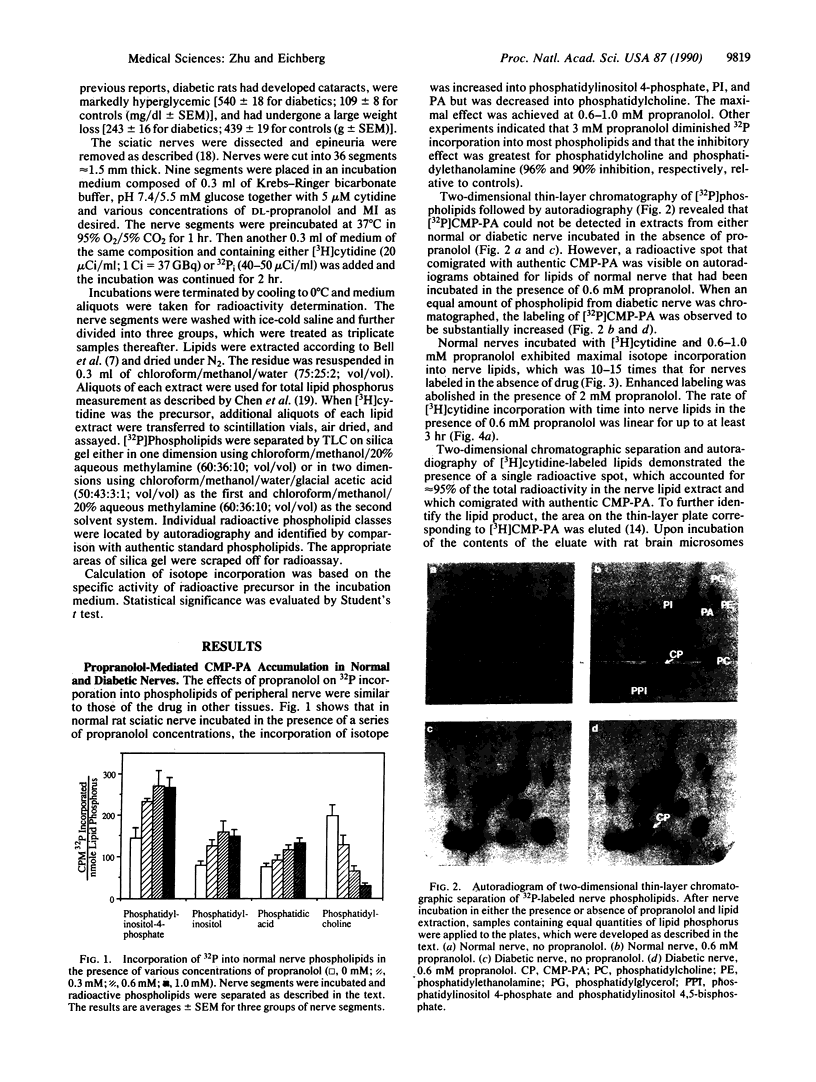
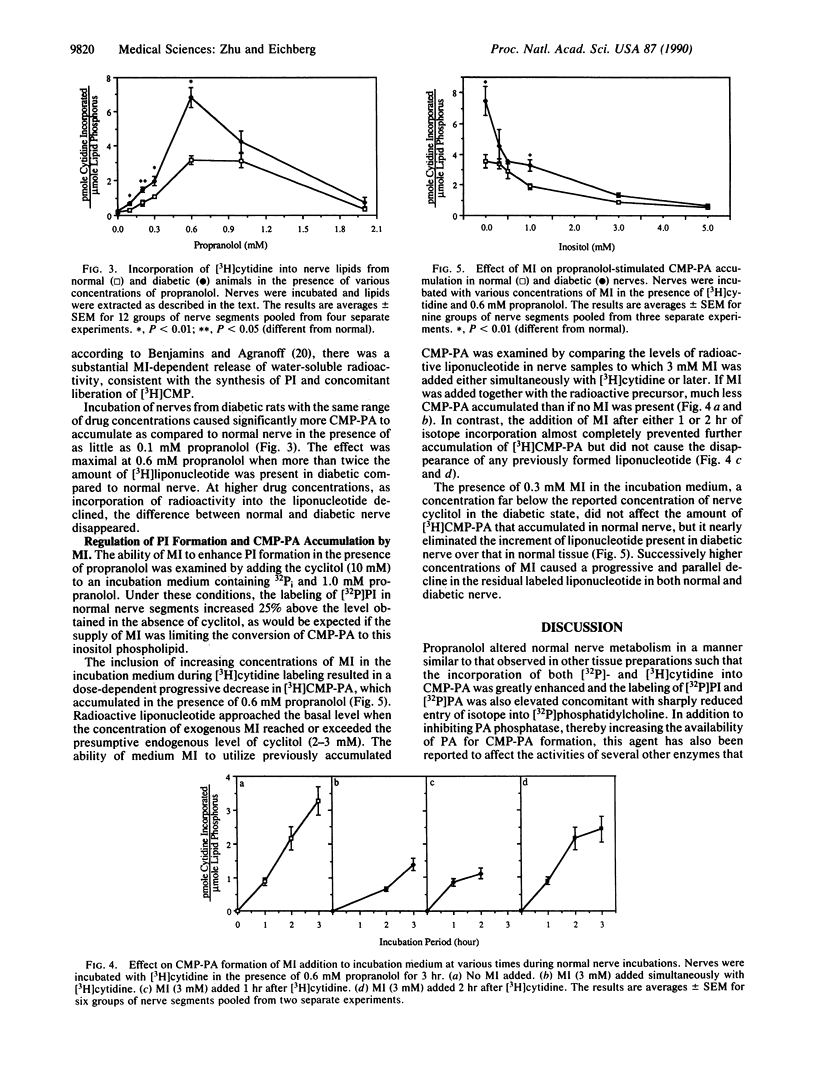
Peripheral nerve from experimentally diabetic rats exhibits lowered levels of myo-inositol (MI) and decreased incorporation of [3H]MI into phosphatidylinositol (PI). There are indications that diminished PI turnover may be causally related to reduced Na+,K(+)-ATPase activity in diabetic nerve. We have investigated whether a metabolic compartment of MI that is essential for PI synthesis is decreased in this tissue. Sciatic nerve segments from streptozotocin-induced diabetic and age-matched normal rats were incubated in vitro with either 32Pi or [3H]cytidine in the presence of propranolol. This cationic amphiphilic agent redirected nerve phospholipid metabolism to produce enhanced 32P incorporation into PI and decreased labeling of phosphatidylcholine and phosphatidyl-ethanolamine. The accumulation of phosphatidyl CMP (CMP-PA) was also demonstrated by chromatographic and enzymatic means. The incorporation of [3H]cytidine into CMP-PA in normal nerve increased up to 15-fold when 0.6 mM propranolol was present. In diabetic nerve, the liponucleotide incorporated 2- to 3-fold more isotope and was more readily labeled at lower drug concentrations as compared to normal nerve. The buildup of [3H]CMP-PA was reduced in a dose-dependent manner in the presence of MI in the incubation medium at concentrations up to 3 mM. However, if MI was added after liponucleotide accumulation, preformed CMP-PA could not be utilized for PI synthesis. The difference in liponucleotide labeling between normal and diabetic nerve was nearly abolished at 0.3 mM medium MI, a concentration much less than the level of cyclitol in the tissue. These results strongly suggest the presence in nerve of a pool of MI that is not in equilibrium with the bulk of nerve MI and that is preferentially used for PI synthesis. This metabolic compartment is depleted in diabetic nerve but can be readily replenished by exogenous MI and may correspond to the MI pool that has been proposed to be required for the turnover of a portion of tissue PI involved in maintenance of normal Na+,K(+)-ATPase activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A., Smith J. P., Akhtar R. A. Studies on the mechanism of alteration by propranolol and mepacrine of the metabolism of phosphoinositides and other glycerolipids in the rabbit iris muscle. Biochem Pharmacol. 1983 Dec 15;32(24):3815–3821. doi: 10.1016/0006-2952(83)90154-5. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif A. A., Smith J. P. Effects of DL-propranolol on the synthesis of glycerolipids by rabbit iris muscle. Biochem Pharmacol. 1976 Aug 1;25(15):1697–1704. doi: 10.1016/0006-2952(76)90401-9. [DOI] [PubMed] [Google Scholar]
- Bell M. E., Eichberg J. Decreased incorporation of [3H]inositol and [3H]glycerol into glycerolipids of sciatic nerve from the streptozotocin diabetic rat. J Neurochem. 1985 Aug;45(2):465–469. doi: 10.1111/j.1471-4159.1985.tb04011.x. [DOI] [PubMed] [Google Scholar]
- Bell M. E., Peterson R. G., Eichberg J. Metabolism of phospholipids in peripheral nerve from rats with chronic streptozotocin-induced diabetes: increased turnover of phosphatidylinositol-4,5-bisphosphate. J Neurochem. 1982 Jul;39(1):192–200. doi: 10.1111/j.1471-4159.1982.tb04718.x. [DOI] [PubMed] [Google Scholar]
- Benjamins J. A., Agranoff B. W. Distribution and properties of CDP-diglyceride:inositol transferase from brain. J Neurochem. 1969 Apr;16(4):513–527. doi: 10.1111/j.1471-4159.1969.tb06850.x. [DOI] [PubMed] [Google Scholar]
- Berti-Mattera L. N., Lowery J., Day S. F., Peterson R. G., Eichberg J. Alteration of phosphoinositide metabolism, protein phosphorylation, and carbohydrate levels in sciatic nerve from Wistar fatty diabetic rats. Diabetes. 1989 Mar;38(3):373–378. doi: 10.2337/diab.38.3.373. [DOI] [PubMed] [Google Scholar]
- Clements R. S., Jr, Stockard C. R. Abnormal sciatic nerve myo-inositol metabolism in the streptozotocin-diabetic rat: effect of insulin treatment. Diabetes. 1980 Mar;29(3):227–235. doi: 10.2337/diab.29.3.227. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Stone M. A. Lithium-induced reduction in intracellular inositol supply in cholinergically stimulated parotid gland. Biochem J. 1986 Feb 15;234(1):199–204. doi: 10.1042/bj2340199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck P. J., Zimmerman B. R., Vilen T. H., Minnerath S. R., Karnes J. L., Yao J. K., Poduslo J. F. Nerve glucose, fructose, sorbitol, myo-inositol, and fiber degeneration and regeneration in diabetic neuropathy. N Engl J Med. 1988 Sep 1;319(9):542–548. doi: 10.1056/NEJM198809013190904. [DOI] [PubMed] [Google Scholar]
- Eichberg J., Gates J., Hauser G. The mechanism of modification by propranolol of the metabolism of phosphatidyl-CMP (CDP-diacylglycerol) and other lipids in the rat pineal gland. Biochim Biophys Acta. 1979 Apr 27;573(1):90–106. doi: 10.1016/0005-2760(79)90176-0. [DOI] [PubMed] [Google Scholar]
- Freinkel N., El Younsi C., Dawson M. C. Inter-relations between the phospholipids of rat pancreatic islets during glucose stimulation, and their response to medium inositol and tetracaine. Eur J Biochem. 1975 Nov 1;59(1):245–252. doi: 10.1111/j.1432-1033.1975.tb02448.x. [DOI] [PubMed] [Google Scholar]
- Ghalayini A., Eichberg J. Purification of phosphatidylinositol synthetase from rat brain by CDP-diacylglycerol affinity chromatography and properties of the purified enzyme. J Neurochem. 1985 Jan;44(1):175–182. doi: 10.1111/j.1471-4159.1985.tb07128.x. [DOI] [PubMed] [Google Scholar]
- Gillon K. R., Hawthorne J. N. Transport of myo-inositol into endoneurial preparations of sciatic nerve from normal and streptozotocin-diabetic rats. Biochem J. 1983 Mar 15;210(3):775–781. doi: 10.1042/bj2100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey P. P. Potentiation by lithium of CMP-phosphatidate formation in carbachol-stimulated rat cerebral-cortical slices and its reversal by myo-inositol. Biochem J. 1989 Mar 1;258(2):621–624. doi: 10.1042/bj2580621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983 Sep;72(3):1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Hauser G., Eichberg J. Identification of cytidine diphosphate-diglyceride in the pineal gland of the rat and its accumulation in the presence of DL-propranolol. J Biol Chem. 1975 Jan 10;250(1):105–112. [PubMed] [Google Scholar]
- Hothersall J. S., McLean P. Effect of experimental diabetes and insulin onphosphatidyl-inositol synthesis in rat sciatic nerve. Biochem Biophys Res Commun. 1979 May 28;88(2):477–484. doi: 10.1016/0006-291x(79)92073-4. [DOI] [PubMed] [Google Scholar]
- Imai A., Gershengorn M. C. Independent phosphatidylinositol synthesis in pituitary plasma membrane and endoplasmic reticulum. Nature. 1987 Feb 19;325(6106):726–728. doi: 10.1038/325726a0. [DOI] [PubMed] [Google Scholar]
- Kennedy E. D., Challiss R. A., Ragan C. I., Nahorski S. R. Reduced inositol polyphosphate accumulation and inositol supply induced by lithium in stimulated cerebral cortex slices. Biochem J. 1990 May 1;267(3):781–786. doi: 10.1042/bj2670781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumara-Siri M. H., Gould R. M. Enzymes of phospholipid synthesis: axonal versus Schwann cell distribution. Brain Res. 1980 Mar 31;186(2):315–330. doi: 10.1016/0006-8993(80)90978-6. [DOI] [PubMed] [Google Scholar]
- Lattimer S. A., Sima A. A., Greene D. A. In vitro correction of impaired Na+-K+-ATPase in diabetic nerve by protein kinase C agonists. Am J Physiol. 1989 Feb;256(2 Pt 1):E264–E269. doi: 10.1152/ajpendo.1989.256.2.E264. [DOI] [PubMed] [Google Scholar]
- Lowery J. M., Berti-Mattera L. N., Zhu X., Peterson R. G., Eichberg J. Relationship of ATP turnover, polyphosphoinositide metabolism, and protein phosphorylation in sciatic nerve and derived peripheral myelin subfractions from normal and streptozotocin diabetic rats. J Neurochem. 1989 Mar;52(3):921–932. doi: 10.1111/j.1471-4159.1989.tb02543.x. [DOI] [PubMed] [Google Scholar]
- Mayer J. H., Tomlinson D. R. Prevention of defects of axonal transport and nerve conduction velocity by oral administration of myo-inositol or an aldose reductase inhibitor in streptozotocin-diabetic rats. Diabetologia. 1983 Nov;25(5):433–438. doi: 10.1007/BF00282524. [DOI] [PubMed] [Google Scholar]
- Palmano K. P., Whiting P. H., Hawthorne J. N. Free and lipid myo-inositol in tissues from rats with acute and less severe streptozotocin-induced diabetes. Biochem J. 1977 Oct 1;167(1):229–235. doi: 10.1042/bj1670229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu A. S., Hauser G. Changes in brain polyphosphoinositide metabolism induced by cationic amphiphilic drugs in vitro. Biochem Pharmacol. 1981 Dec 1;30(23):3243–3246. doi: 10.1016/0006-2952(81)90525-6. [DOI] [PubMed] [Google Scholar]
- Pappu A. S., Hauser G. Propranolol-induced inhibition of rat brain cytoplasmic phosphatidate phosphohydrolase. Neurochem Res. 1983 Dec;8(12):1565–1575. doi: 10.1007/BF00964158. [DOI] [PubMed] [Google Scholar]
- Sima A. A., Lattimer S. A., Yagihashi S., Greene D. A. Axo-glial dysjunction. A novel structural lesion that accounts for poorly reversible slowing of nerve conduction in the spontaneously diabetic bio-breeding rat. J Clin Invest. 1986 Feb;77(2):474–484. doi: 10.1172/JCI112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A., Kern E. F., Winegrad A. I., Martin D. B. Basal phosphatidylinositol turnover controls aortic Na+/K+ ATPase activity. J Clin Invest. 1986 Feb;77(2):503–513. doi: 10.1172/JCI112330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. A., Winegrad A. I., Martin D. B. Significance of tissue myo-inositol concentrations in metabolic regulation in nerve. Science. 1982 Aug 27;217(4562):848–851. doi: 10.1126/science.6285474. [DOI] [PubMed] [Google Scholar]
- Takenawa T., Egawa K. CDP-diglyceride:inositol transferase from rat liver. Purification and properties. J Biol Chem. 1977 Aug 10;252(15):5419–5423. [PubMed] [Google Scholar]
- Whiting P. H., Palmano K. P., Hawthorne J. N. Enzymes of myo-inositol and inositol lipid metabolism in rats with streptozotocin-induced diabetes. Biochem J. 1979 Jun 1;179(3):549–553. doi: 10.1042/bj1790549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winegrad A. I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes. 1987 Mar;36(3):396–406. doi: 10.2337/diab.36.3.396. [DOI] [PubMed] [Google Scholar]
- Yorek M. A., Dunlap J. A., Ginsberg B. H. Effect of increased glucose levels on Na+/K+-pump activity in cultured neuroblastoma cells. J Neurochem. 1988 Aug;51(2):605–610. doi: 10.1111/j.1471-4159.1988.tb01081.x. [DOI] [PubMed] [Google Scholar]
- Zhu X., Eichberg J. 1,2-diacylglycerol content and its arachidonyl-containing molecular species are reduced in sciatic nerve from streptozotocin-induced diabetic rats. J Neurochem. 1990 Sep;55(3):1087–1090. doi: 10.1111/j.1471-4159.1990.tb04604.x. [DOI] [PubMed] [Google Scholar]