Abstract
Background
The determinants of parasite persistence or elimination after treatment and clinical resolution of cutaneous leishmaniasis (CL) are unknown. We investigated clinical and parasitological parameters associated with the presence and viability of Leishmania after treatment and resolution of CL caused by L. Viannia.
Methods
Seventy patients who were treated with meglumine antimoniate (n = 38) or miltefosine (n = 32) and cured, were included in this study. Leishmania persistence and viability were determined by detection of kDNA and 7SLRNA transcripts, respectively, before, at the end of treatment (EoT), and 13 weeks after initiation of treatment in lesions and swabs of nasal and tonsillar mucosa.
Results
Sixty percent of patients (42/70) had evidence of Leishmania persistence at EoT and 30% (9/30) 13 weeks after treatment initiation. A previous episode of CL was found to be a protective factor for detectable Leishmania persistence (OR: 0.16, 95%CI: 0.03–0.92). kDNA genotyping could not discern differences between parasite populations that persisted and those isolated at diagnosis.
Conclusions
Leishmania persist in skin and mucosal tissues in a high proportion of patients who achieved therapeutic cure of CL. This finding prompts assessment of the contribution of persistent infection in transmission and endemicity of CL, and in disease reactivation and protective immunity.
Author summary
Control of cutaneous leishmaniasis (CL) in the Americas is dependent upon active case detection and treatment. The efficacy and effectiveness of therapeutic interventions is based on clinical resolution of disease, not on parasitological clearance. The detection of dermotropic Leishmania in tissues such as nasal and conjunctival mucosa, blood and healthy skin in the absence of signs and symptoms of disease, suggests that despite clinical resolution, parasites persist subclinically. We examined clinical and parasitological factors associated with Leishmania persistence after standard-of-care treatment of CL caused by L. Viannia. We found that a high proportion of CL patients with therapeutically achieved clinical resolution of CL harbor viable Leishmania. A previous episode of CL was found to be a protective factor for parasite persistence. Treated, clinically cured CL patients constitute an important proportion of a persistently infected human population whose clinical and epidemiological significance remains to be determined.
Introduction
Over 95% of clinical manifestations of human infections caused by Leishmania species of the Viannia subgenus consist of cutaneous lesions. Although L. Viannia species are typically considered dermotropic, infection is systemic [1, 2]. The presence and viability of parasites in lesion scars and in otherwise healthy tissues, including blood, skin, nasal and conjunctival mucosa, have been documented in patients with active cutaneous disease [3–6] and in individuals with asymptomatic infection residing in endemic areas of L. Viannia transmission [7]. The ability of L. Viannia parasites to colonize host tissues without causing signs or symptoms of disease reflects a host-pathogen relationship that is permissive for microbial persistence.
Loss of susceptibility for meglumine antimoniate and miltefosine has been reported after a single treatment course [8, 9]. Thus, subclinical persistence of Leishmania after chemotherapeutic interventions could favor the development of acquired drug resistance and selection of non-susceptible parasite populations, risking the usefulness of available and potentially new drugs.
The systemic detection of subclinical Leishmania infection after clinical resolution of disease whether therapeutically achieved or after self-resolution, together with persistent asymptomatic infection, could reveal a previously unrecognized magnitude of the human population harboring viable parasites. The clinical and epidemiological impact of subclinical infection, and the factors that underlie parasite persistence or elimination, are unknown. In this study we explored clinical and parasitological factors associated with Leishmania persistence after standard-of-care treatment of cutaneous leishmaniasis (CL) caused by L. Viannia.
Methods
Ethics statement
This study was approved and monitored by the Institutional Review Board for Ethical Conduct of Research Involving Human Subjects of the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) with approval code CIEIH 1221, in accordance with national and international guidelines. All individuals voluntarily participated in the study. Written informed consent was obtained from each participant.
Study design
This study was designed to explore the associations of clinical and parasitological factors with the persistence of Leishmania after supervised treatment and follow-up, and documented clinical resolution of CL. We included a cohort of CL patients who consulted CIDEIM outpatient clinics in Cali (Valle, Colombia) and Tumaco (Nariño, Colombia) between years 2011 and 2014, who participated in clinical studies that included prospective treatment follow-up. Demographic and clinical information from study participants was obtained at diagnosis and follow up visits.
The primary outcome of analysis was post-treatment parasite persistence. We qualitatively determined this by molecular detection of Leishmania nucleic acids (kDNA) from cutaneous lesions and nasal and tonsillar mucosa samples obtained from patients before and after treatment (end of treatment and at 13 weeks after initiation of treatment). Parasite viability was assessed by amplification and quantification of Leishmania 7SLRNA gene transcripts in all available lesion samples and for kDNA-positive mucosal samples [10]. Independent variables included sex, age, self-reported ethnicity, weight, self-reported time of lesion evolution, number of lesions, previous episode of leishmaniasis, and parasite loads in lesion aspirates, as well as drug-related variables including prescribed drug and adherence to treatment.
Participants and samples
Clinical histories and samples from 70 adult patients with parasitological diagnosis of CL were included in this study. All participants were ≥18 years of age, received supervised standard-of-care treatment with meglumine antimoniate or miltefosine according to the national guidelines [11], and had clinical follow-up at the end of treatment and 13 weeks after initiation of treatment, the time at which therapeutic outcome was determined. Cure was defined as the re-epithelialization of all cutaneous lesions without inflammatory signs.
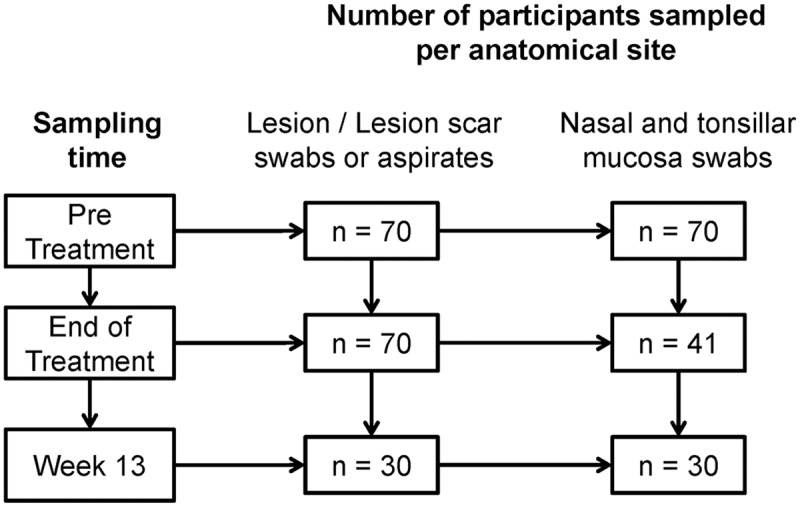
Samples of active lesions and lesion scars were taken by needle aspirate of the border of the lesion or at the periphery of the scar. Swab samples of cutaneous lesions, nasal mucosa, and palatine tonsil mucosa were obtained by gently rubbing sterile swabs (BuccalAmp kit; Epicenter Biotechnologies) over the mucosal surface [10]. Lesion and mucosal samples were collected from all patients before treatment, as were lesion samples at the end of treatment (Fig 1). Mucosal swabs were obtained from 41 participants at the end of treatment. Samples were also obtained from 30 patients at 13 weeks of follow-up. All samples were preserved in TRIzol Reagent (Life Technologies) and stored at -80°C until processed.
Fig 1. Schematic representation of study participants and samples obtained at different time points during treatment and clinical follow-up.
Detection of Leishmania
DNA and RNA were extracted using the AllPrep DNA/RNA Minikit (Qiagen) according to manufacturer´s recommendations. Leishmania was detected by PCR amplification and southern blot of minicircle kDNA as previously described [4], which has a limit of detection of 0.3 fg of parasite DNA (~10−2 parasites per reaction) [12]. A positive control of L. V. panamensis (MHOM/CO/86/1166) DNA and a negative water control were included in each PCR run. PCR amplification of the human GAPDH gene was used as quality control of the extracted DNA [7]. DNAse treated RNA was used for quantitative reverse transcriptase PCR (qRT-PCR) of the Leishmania 7SLRNA transcript to evaluate parasite viability and quantify parasite burden. The limit of detection of this method is 102 parasites per reaction [10]. Parasite loads were calculated by extrapolation to a standard curve and normalized to the number of human nucleated cells using TATA box binding protein amplification [7]. Detection of amplification products was performed using SYBR Green Master Mix (Applied Biosystems) on a BioRad CFX-96 platform. For those kDNA positive samples that were below the limit of detection of the 7SLRNA qRT-PCR, a maximum likelihood estimate of 0.0001 parasites per reaction was calculated as previously described [7].
kDNA genotyping
A nested PCR reaction to amplify and sequence the conserved region of Leishmania minicircle kDNA was performed using external primers LVp1-Fw and LVp1-Rv and internal primers LVp1-Fw and LVp5-Rv [7]. Sequences were analyzed using BioEdit v7.2.5. Genetic distances and trees were calculated and constructed using MEGA 7.0. Population structure was explored with the STRUCTURE 2.3.4 software [13]. Runs were performed under the following parameters: burn-in period of 20,000 iterations, 200,000 Markov Chain Monte Carlo iterations and admixture model. A series of three runs was performed for each K value between 1 and 10. STRUCTURE outputs were visualized using STRUCTURE HARVESTER and used for selection of the number of genetic groups that best fitted the data [14]. Estimated fixation index (FST) values were retrieved from STRUCTURE.
Multilocus microsatellite typing (MLMT)
Fourteen microsatellite loci distributed in 13 Leishmania chromosomes were amplified by PCR from log-phase promastigote DNA [15]. The size of the microsatellites was determined by mobility of the PCR products in 4.5% agarose gels. Genetic distances were estimated using MSA4.05 and Populations-2.1 software. UPGMA trees were constructed using MEGA7.0 and compared to those generated by kDNA genotyping.
Statistical analyses
Descriptive statistics were used to summarize the demographic, clinical and parasitological characteristics of the sample. Differences in frequencies were explored using McNemar’s test for paired nominal data, and X2 and Fisher's exact tests for unpaired data. Logistic regression analysis was used to identify independent predictors of post-treatment parasite persistence at the lesion site [16]. All variables associated with parasite persistence at the p<0.2 level in unadjusted analyses were entered into a multivariable model. The strength of association was determined by calculating the odds ratio (OR) and 95% confidence interval (CI) using the Wolf method [16, 17]. For the multivariable model, model fit using the goodness-of-fit test and model discrimination using the c-statistic, were analyzed [17]. All statistical analyses were performed using STATA 12 (StataCorp, College Station, TX, USA).
Results
Patient characteristics
Seventy patients with active CL, who received standard-of-care treatment with parenteral meglumine antimoniate (n = 38) or oral miltefosine (n = 32), were included in this study. Characteristics of the sample are summarized in Table 1. Patients were mostly young adult males of Afrocolombian or mestizo ethnicity. For the majority of participants (86%), the diagnosis was the first episode of CL. Clinical manifestations were predominantly characterized by single lesions with a median evolution time of 2.5 months. L.V. panamensis was the most frequently isolated species (in 93% of patients). Adherence to greater than 90% of the treatment regimen was achieved in 91% of study participants. None of the patients had symptoms or clinical signs of mucosal involvement.
Table 1. Clinical and demographic characteristics in patients with and without evidence of Leishmania persistence at the lesion site after clinical cure.
| Variable | Total n = 70 |
kDNA / 7SLRNAa | pb | |
|---|---|---|---|---|
| Positive n = 32 |
Undetectable n = 33 |
|||
| Sex, No. (%) | ||||
| Male | 47 (67) | 24 (56) | 19 (44) | 0.138* |
| Female | 23 (33) | 8 (36) | 14 (64) | |
| Age, median (range), years | 31 (18–72) | 30(18–72) | 31(20–65) | 0.762** |
| Ethnicity, No. (%) | ||||
| Afrocolombian | 50 (71) | 23 (49) | 24 (51) | 0.939* |
| Other | 20 (29) | 9 (50) | 9 (50) | |
| Municipality of infection No.(%) | ||||
| Nariño | 46 (66) | 18 (42) | 25 (58) | 0.097* |
| Other | 24 (34) | 14 (64) | 8 (36) | |
| Weight, median (range), Kg | 70 (11) | 66 (48–102) | 69 (50–94) | 0.328** |
| Time of oldest lesion evolution, median (range), months | 2.5 (1–40) | 2(1–24) | 2(1–40) | 0.481** |
| Lesions per patient, median (range) | 1 (1–8) | 1 (1–8) | 1 (1–6) | 0.217** |
| Previous episode of leishmaniasis, No. (%) | ||||
| No | 60 (86) | 30 (55) | 25 (45) | 0.082¶ |
| Yes | 10 (14) | 2 (20) | 8 (80) | |
| Treatment, No. (%) | ||||
| Meglumine antimoniate | 38 (46) | 20 (59) | 14 (41) | 0.105* |
| Miltefosine | 32 (54) | 12 (39) | 19 (61) | |
| Adherence to meglumine antimoniate (% ampules received/prescribed), median (range) | 100 (50–100) | 100 (50–100) | 100 (70–100) | 0.616** |
| Adherence to miltefosine (% capsules received/prescribed), median (range) | 100 (76–100) | 100 (76–100) | 100 (88–100) | 0.626** |
a Patients from whom end of treatment lesion samples were analyzed for Leishmania persistence. Samples from five patients were unavailable for molecular analyses.
b Contrasts between samples from patients with and without molecular evidence of parasite persistence;
* X2 test;
** Mann-Whitney U test;
¶ 2-sided Fisher's exact test.
Leishmania persistence after clinical cure of CL
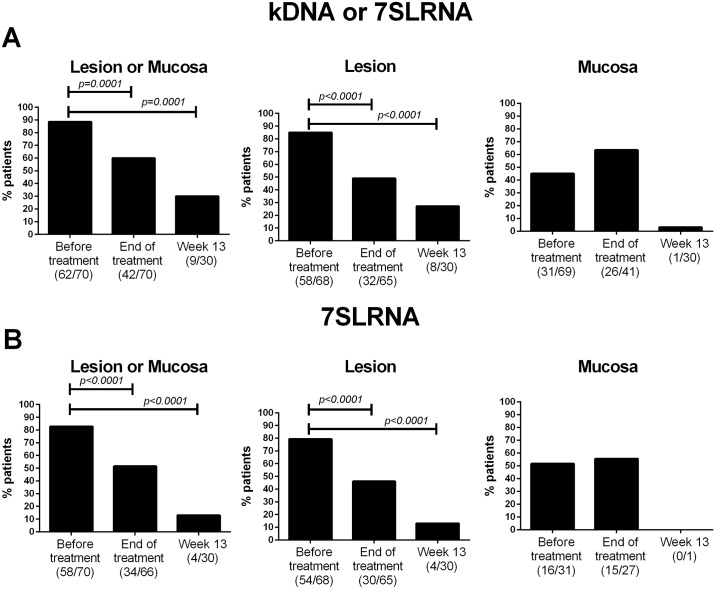
Evidence of Leishmania persistence (defined by amplification of minicircle kDNA or 7SLRNA transcripts in at least one lesion or mucosal sample) was found in 60% of patients (42/70) at the end of treatment and in 30% (9/30) at 13 weeks follow-up. Both of these proportions were significantly lower than the baseline parasite detection rate of 88% (62/70) before treatment (Fig 2A, left panel). Molecular detection of Leishmania in lesions was achieved in 49% of patients at the end of treatment and in 27% at 13 weeks, compared with 85% before treatment (Fig 2A, center panel). Leishmania was found in swab samples from mucosa in 45% of patients before treatment (Fig 2A, right panel). In contrast to the decline in the proportion of detection of parasites at the lesion site, no significant decrease in the frequency of patients with Leishmania-positive mucosal samples was found at the end of treatment compared with pre-treatment samples. However, only one of 30 patients had a positive mucosal sample at 13 weeks follow-up.
Fig 2. Frequency of detection of Leishmania in samples from CL patients before and after treatment.
Presence of Leishmania was determined by detection of Leishmania kDNA or 7SLRNA in lesion and mucosal samples (A) and parasite viability (B) was determined by detection of 7SLRNA transcripts. Graphs represent frequency of positivity in at least one sample (left panels), or independently in lesions (center panels) and in any mucosal tissue (right panels). Data are shown as relative frequencies based on the total number of patients at each sampling time. Differences were analyzed by the McNemar’s test.
Parasite viability was evidenced by detection of 7SLRNA transcripts in 83% of patients (58/70) before treatment, indicating underestimation of the proportion of patients in which viable parasites were detected. This proportion decreased to 51% at the end of treatment and to 13% at 13 week of follow-up (Fig 2B, left panel). At the end of treatment, viable Leishmania were detected in a similar proportion of patients’ lesion and mucosal samples (46% and 55%, respectively; Fig 2B, center and right panels). At week 13, 7SLRNA transcripts were found in samples from lesions of only four patients. Parasite loads at the lesion site were overall lower at the end of treatment compared with parasite loads quantified before treatment (median parasite loads 14.5 and 40 parasites/1000 mammalian cells, respectively).
Factors related with Leishmania persistence at the lesion site after clinical cure
Among the clinical and parasitological factors analyzed (Table 1), individuals with a prior history of CL were less likely to have detectable Leishmania after treatment and clinical cure than those without a previous episode of CL (20% vs. 55%, respectively). Adjusting for sex, the received drug and the municipality of infection (which were significant at p<0.2), a previous symptomatic episode of leishmaniasis was significantly associated with decreased risk of parasite persistence, with an adjusted odds ratio of 0.16 (95% CI: 0.03–0.92). The model fit well and discrimination was good (Table 2, c-statistic = 0.73).
Table 2. Multivariable logistic regression analysis of factors related to Leishmania parasite persistence at the lesion site at the end of the treatment.
| Variable | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|
| Male sex | 2.21 (0.77–6.36) | 2.02 (0.61–6.71) |
| Previous episode of leishmaniasis | 0.21 (0.04–1.07) | 0.16 (0.03–0.92) |
| Treatment with meglumine antimoniate | 2.26 (0.84–6.11) | 2.18 (0.70–6.85) |
| Municipality of infection (Nariño vs. Other) | 0.41 (0.14–1.19) | 0.37 (0.12–1.16) |
Note: The final model included 65 patients; Goodness-of-Fit Test p = 0.62; c-statistic = 0.73.
Genotyping of persistent parasite populations after treatment and clinical resolution of CL
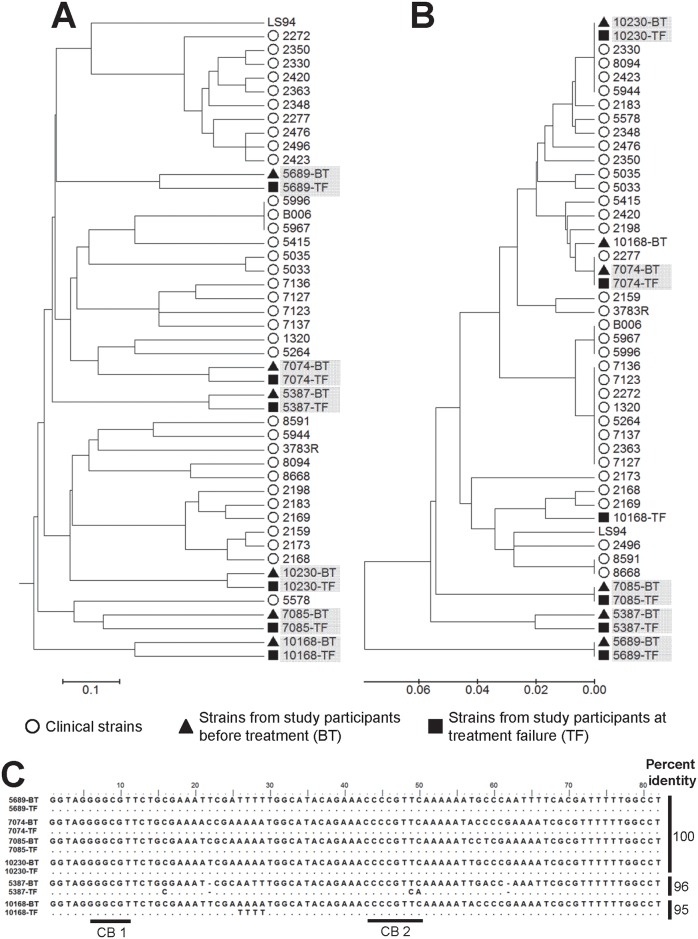
We have reported the use of genotyping of the conserved region of minicircle kDNA to explore the genetic diversity of Leishmania strains causing subclinical and asymptomatic infections [7]. To examine the relatedness of Leishmania populations during active disease and those that persist after treatment, we conducted an initial comparative analysis of multilocus microsatellite typing (MLMT) and kDNA genotyping of strains isolated before treatment and at treatment failure (S1 Table) from six CL patients. MLMT showed that parasites isolated before treatment grouped within the same branch as those isolated at treatment failure for each individual patient (Fig 3A, S2 Table). For five out of the six analyzed pairs of strains, groups defined by kDNA genotyping (Fig 3B, S3 Table) were concordant with those obtained by MLMT. Strains that were concordantly grouped by kDNA genotyping and MLMT shared ≥96% sequence homology in the sequenced region of the conserved block of minicircle kDNA (Fig 3C).
Fig 3. Comparative analysis of pre-treatment and post-treatment clinical strains by kDNA genotyping and multilocus microsatellite typing.
UPGMA trees of distances calculated from MLMT data (A) and conserved block minicircle kDNA genotyping (B) from three pairs of L. V. panamensis and three pairs of L. V. braziliensis strains isolated before treatment (BT; black triangles) and at treatment failure (TF; black squares), alongside 34 L. V. panamensis clinical strains (open circles) and the reference L. V. panamensis strain LS94. Shadowed codes denote pairs of strains isolated from the same patient. (C) Pairwise sequence alignment of pre and post-treatment strains indicating % base-pair identity.
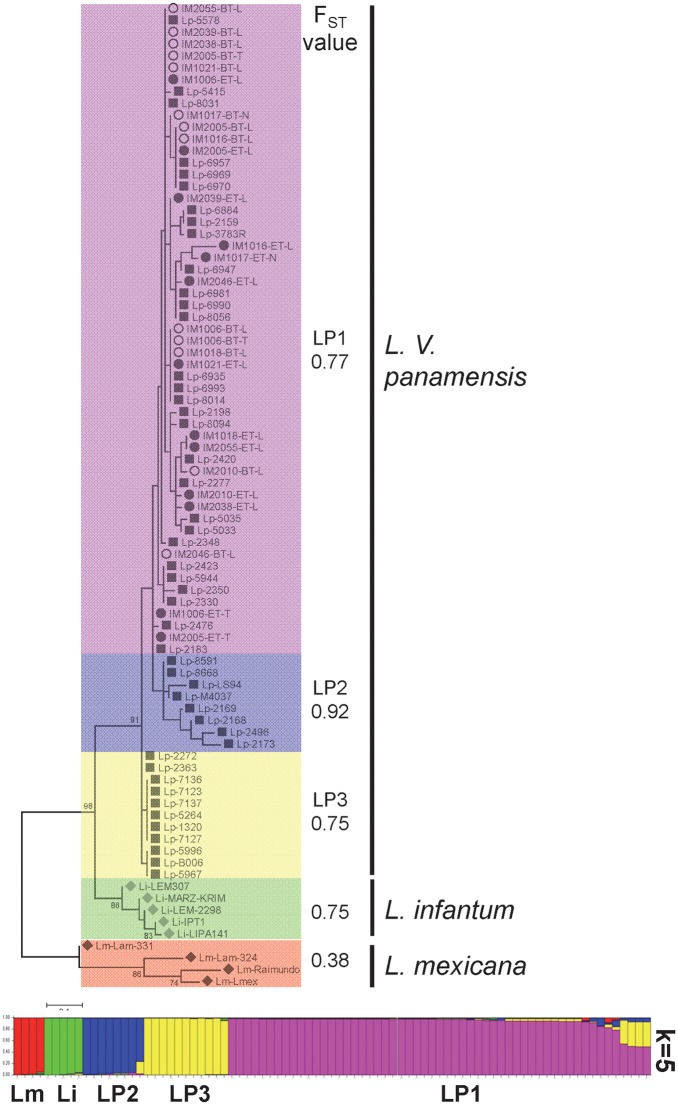
We performed kDNA genotyping on paired samples obtained pre-treatment and at the end of treatment from mucosal tissues (nasal and tonsillar) and lesions of 21 patients. Good quality sequences were obtained from 11 of the 21 patients: 10 sample pairs from lesion aspirates, two from tonsillar swabs, and one from nasal swabs (S3 Table). Genetic distances were calculated and population structure analyses were conducted alongside a panel of 46 L.V. panamensis clinical isolates obtained from CL patients across Colombia (Nariño [n = 31], Valle del Cauca [n = 5], Risaralda [n = 3], Choco [n = 6] and Putumayo [n = 1]), one L.V. panamensis reference strain, and one L.V. panamensis, 5 L. infantum and 4 L. mexicana kDNA sequences retrieved from NCBI (S1 Table). The selection of L.V. panamensis strains included in this panel and their proportions were reflective of the geographic distribution (municipality of infection) of study participants. Within the L.V. panamensis cluster, three subpopulations could be discerned responding primarily to geographic distribution: LP1 and LP2 corresponding to strains predominantly isolated from Nariño and LP3 to strains from Valle del Cauca and Risaralda. All minicircle kDNA sequences obtained from study participants pre- and post-treatment grouped within the LP1 cluster and fixation indices >0.3 supported the identified clusters (Fig 4). These data indicate that persistent Leishmania subpopulations within this group of CL patients were not genetically distinct from those isolated at diagnosis.
Fig 4. Analysis of Leishmania genetic diversity in clinical samples obtained from patients before and at the end of treatment.
Maximum Likelihood tree of distances calculated from sequences of the conserved block of minicircle kDNA from lesion (-L), tonsillar (-T) and nasal (-N) mucosa samples obtained before treatment (BT) and at the end of treatment (ET), alongside a panel of L. V. panamensis (Lp; n = 48), L. infantum (Li; n = 5), and L. mexicana complex (Lm; n = 4) sequences. Color blocks denote subpopulations defined by population structure analysis and include the estimated fixation index (FST). LP1-3: L. V. panamensis subpopulations. The bottom panel is the visual representation of the STRUCTURE analysis. Each strain/sequence (n = 83) is represented by a single vertical line divided into K colors, where K is the number of populations assumed (K = 5). Details of strains and kDNA sequences retrieved from NCBI are shown in S1 Table.
Discussion
The outcome of antileishmanial chemotherapy is determined by clinical parameters of healing and non-healing responses. Although the parasitological response in spleen or bone marrow aspirates is a secondary indicator of therapeutic responsiveness during treatment of visceral leishmaniasis, microbiological clearance is not considered a reliable measure of healing of dermal disease [18, 19]. Our results revealed a high frequency of Leishmania persistence in mucosal tissues and at the lesion site in patients who were systematically followed 13 weeks after end of treatment, had a median overall treatment adherence of 100% and achieved therapeutic clinical cure of CL. Detection of kDNA molecules does not demonstrate parasite viability despite being rapidly degraded after parasite death [20]. However, due to the short half-life and lability of RNA molecules, detection of Leishmania 7SLRNA gene transcripts substantiates the persistence of viable parasites after clinical cure [10]. Although our current and previous findings [21] and that of others [22] show that clinical resolution of CL is accompanied by a reduction in parasite burden at the lesion site, Leishmania persistence is the norm rather than the exception, suggesting that other factors beyond parasite elimination contribute to the efficacy of antileishmanial therapy. In contrast to the significant reduction in the frequency of Leishmania-positive lesion samples, no decrease in the frequency of parasite detection at mucosal sites (either tonsillar or nasal mucosal samples) was found at the end of treatment. This observation suggests that mucosal sites could be a privileged niche for parasite persistence after drug treatment, either by pharmacokinetic differences in drug distribution and accumulation and/or immunological divergence between mucosal vs. skin tissues. Indeed, the detection of Leishmania in mucosal tissues in asymptomatic individuals and in CL patients without signs or symptoms of mucosal involvement [2, 7], and the development of mucosal disease years after an episode of CL, support the silent persistence of Leishmania in these anatomical sites.
Whether antileishmanial therapy should be aimed at complete parasite elimination (sterile cure) is a matter of debate. Persistent infection without signs of disease in animal models has provided evidence that such infection may promote immunity to subsequent infections [23–25]. In contrast, well documented clinical cases of disease reactivation in the context of immune suppression [26–28] or local trauma [29, 30] and mucosal involvement years after an episode of CL [31–33] support parasite persistence as a risk factor for reactivation of disease. Notably, the presence of a scar typical of CL and/or a positive Montenegro skin test reaction, both indicative of prior infection, have been shown to significantly increase the risk of re-activation of infection and development of CL in a prospective investigation of incidence of infection and disease in endemically exposed communities [33]. We have previously shown that amastigotes were less frequently observable in biopsies of active lesions of patients having scars suggestive of prior leishmaniasis [34]. Concordantly, our present results demonstrate a significant association between history of a previous episode of CL and a lower frequency of detectable Leishmania persistence at the end of treatment in CL patients who received supervised standard-of-care treatment with meglumine antimoniate or miltefosine. Interestingly, a negative skin test at diagnosis has been identified as a risk factor for relapse after treatment with pentavalent antimonials [35]. Therefore, it is plausible that acquired protective immune responses could contribute to enhanced parasite control during therapeutic intervention, thereby reducing the parasite burden, detectable parasite persistence and treatment failure.
Although not statistically significant, Leishmania persistence was more frequently detected in men, during treatment with meglumine antimoniate and among individuals from municipalities other than Nariño. In the case of parasitic infectious diseases, sexually mature men are often more susceptible to infection due to hormonal factors [36–38]. Higher susceptibility of male hamsters to L.V. panamensis infection has been associated with a more permissive immune environment for parasite survival [39]. However, the extent and causality of the relationship between sex and persistent infection after drug exposure in humans remains unknown. Pharmacokinetic and pharmacodynamic differences between drugs could also influence the post treatment persistence and burden of infection. Meglumine antimoniate has a short elimination half-life (~20h, [40]), in contrast to miltefosine which has a first elimination half-life of 7.05 days and a terminal elimination half-life of 30.9 days [41]. The short half-life of antimonials could result in reduced time of drug exposure of intracellular parasites promoting the higher frequency of parasite persistence. In addition, based on a retrospective cohort study of 230 CL patients, we have recently found that treatment with meglumine antimoniate (vs. miltefosine) is a risk factor for therapeutic failure [42]. The potential contribution of other demographic variables to Leishmania persistence such as genetic background or race remains to be determined. However, that individuals from municipalities other than Nariño presented higher frequency of parasite persistence could be indicative of parasite subpopulations or host determinants related to geographic origin, specifically contributing to Leishmania persistence.
Selection of parasite subpopulations within the human host is considered to contribute to emergence of drug resistance. Characterization of parasites causing subclinical infections is restricted by the inability to isolate, culture, and propagate the parasite. Based on kDNA sequence homology and population structure analyses, strains and patient samples could be clustered based on geographical origin. However, we found no evidence of selection of parasite subpopulation after treatment and clinical cure. Phenotypic analyses such as drug susceptibility testing, and sequence analyses of kDNA and ITS rDNA have revealed differences in parasites isolated at diagnosis and at treatment failure in individuals with cutaneous and visceral leishmaniasis [8, 9, 43, 44]. It is plausible that implementation of sequencing methods at the genome level could contribute to discriminate and characterize subclinically persistent Leishmania subpopulations.
Association analyses of clinical, parasitological and drug-related factors with parasite persistence at later time points (eg. week 13 and 26 after end of treatment) were precluded in this study due to sample size limitations. However, our results and those of others demonstrate that currently available treatments do not eliminate Leishmania. This contributes to the proportion of the persistently infected human population, which includes patients with therapeutically achieved cure, those with self-resolution of active disease and individuals with asymptomatic infection. Despite compelling circumstantial evidence, anthroponotic transmission of Leishmania (Viannia) remains uncertain. Nevertheless, the high proportion of individuals harboring infection in endemic areas, coupled with the rising importance of domestic transmission of CL, and the demonstrated risk of disease re-activation [45–47], highlight the importance of this subclinically infected human population in control strategies for CL. Prospective studies designed to interrogate the dynamics of parasite clearance and reactivation of disease are needed to establish the clinical and epidemiological impact of Leishmania persistence.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We gratefully acknowledge the support of the CIDEIM Clinical Units in Tumaco and Cali and the collaboration of CIDEIM BioBank personnel. We specially thank all the patients who participated in this study. Thanks to Juan David Escobar and Diego Echeverry for fruitful discussions and support on genetic analyses.
Data Availability
All molecular data (sequences, microsatelite profiles, parasite loads, etc.) are available as supporting information files. Due to patient privacy concerns, patient demographics and related metadata will be available on request to the institutional review board of CIDEIM, contacting Ms. Jackeline Bravo at jbravo@cideim.org.co.
Funding Statement
This work was supported, in part, by COLCIENCIAS Grant 250-2010 Code 2229-519-28930 (http://www.colciencias.gov.co/), US National Institutes of Health (NIH) Grants R01AI104823 and R01AI093775 (https://www.niaid.nih.gov/), and US NIH International Fogarty Center Global Infectious Disease Research Training Program, Award Number D43 TW006589 (https://www.fic.nih.gov/). AJM and CFD were supported by the COLCIENCIAS Young Investigators and Innovators Program Contract Number 0714-2013. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.de Oliveira Camera P, Junger J, do Espirito Santo Silva Pires F, Mattos M, Oliveira-Neto MP, Fernandes O, et al. Haematogenous dissemination of Leishmania (Viannia) braziliensis in human American tegumentary leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100(12):1112–7. doi: 10.1016/j.trstmh.2006.02.014 . [DOI] [PubMed] [Google Scholar]
- 2.Figueroa RA, Lozano LE, Romero IC, Cardona MT, Prager M, Pacheco R, et al. Detection of leishmania in unaffected mucosal tissues of patients with cutaneous leishmaniasis caused by leishmania (viannia) species. The Journal of infectious diseases. 2009;200(4):638–46. Epub 2009/07/03. doi: 10.1086/600109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mendonca MG, de Brito ME, Rodrigues EH, Bandeira V, Jardim ML, Abath FG. Persistence of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure? The Journal of infectious diseases. 2004;189(6):1018–23. doi: 10.1086/382135 . [DOI] [PubMed] [Google Scholar]
- 4.Vergel C, Palacios R, Cadena H, Posso CJ, Valderrama L, Perez M, et al. Evidence for leishmania (viannia) parasites in the skin and blood of patients before and after treatment. The Journal of infectious diseases. 2006;194(4):503–11. doi: 10.1086/505583 . [DOI] [PubMed] [Google Scholar]
- 5.Schubach A, Marzochi MC, Cuzzi-Maya T, Oliveira AV, Araujo ML, Oliveira AL, et al. Cutaneous scars in American tegumentary leishmaniasis patients: a site of Leishmania (Viannia) braziliensis persistence and viability eleven years after antimonial therapy and clinical cure. The American journal of tropical medicine and hygiene. 1998;58(6):824–7. . [DOI] [PubMed] [Google Scholar]
- 6.Schubach A, Haddad F, Oliveira-Neto MP, Degrave W, Pirmez C, Grimaldi G Jr., et al. Detection of Leishmania DNA by polymerase chain reaction in scars of treated human patients. The Journal of infectious diseases. 1998;178(3):911–4. . [DOI] [PubMed] [Google Scholar]
- 7.Rosales-Chilama M, Gongora RE, Valderrama L, Jojoa J, Alexander N, Rubiano LC, et al. Parasitological Confirmation and Analysis of Leishmania Diversity in Asymptomatic and Subclinical Infection following Resolution of Cutaneous Leishmaniasis. PLoS neglected tropical diseases. 2015;9(12):e0004273 doi: 10.1371/journal.pntd.0004273 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojas R, Valderrama L, Valderrama M, Varona MX, Ouellette M, Saravia NG. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. The Journal of infectious diseases. 2006;193(10):1375–83. doi: 10.1086/503371 . [DOI] [PubMed] [Google Scholar]
- 9.Obonaga R, Fernandez OL, Valderrama L, Rubiano LC, Castro Mdel M, Barrera MC, et al. Treatment failure and miltefosine susceptibility in dermal leishmaniasis caused by Leishmania subgenus Viannia species. Antimicrobial agents and chemotherapy. 2014;58(1):144–52. doi: 10.1128/AAC.01023-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero I, Tellez J, Suarez Y, Cardona M, Figueroa R, Zelazny A, et al. Viability and burden of Leishmania in extralesional sites during human dermal leishmaniasis. PLoS neglected tropical diseases. 2010;4(9). doi: 10.1371/journal.pntd.0000819 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministerio de la Protección Social INdS. Guía de Atención Clínica Integral del Paciente con Leishmaniasis. In: OPS/OMS OPdlS, editor. 2010.
- 12.Vergel C, Walker J, Saravia NG. Amplification of human DNA by primers targeted to Leishmania kinetoplast DNA and post-genome considerations in the detection of parasites by a polymerase chain reaction. The American journal of tropical medicine and hygiene. 2005;72(4):423–9. Epub 2005/04/14. . [PubMed] [Google Scholar]
- 13.Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular ecology. 2005;14(8):2611–20. doi: 10.1111/j.1365-294X.2005.02553.x . [DOI] [PubMed] [Google Scholar]
- 14.Earl DA, vonHoldt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources. 2012;4(2):359–61. doi: 10.1007/s12686-011-9548-7 [Google Scholar]
- 15.Oddone R, Schweynoch C, Schonian G, de Sousa Cdos S, Cupolillo E, Espinosa D, et al. Development of a multilocus microsatellite typing approach for discriminating strains of Leishmania (Viannia) species. Journal of clinical microbiology. 2009;47(9):2818–25. Epub 2009/07/10. doi: 10.1128/JCM.00645-09 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz MH. Multivariable analysis: a primer for readers of medical research. Ann Intern Med. 138 United States 2003. p. 644–50. [DOI] [PubMed] [Google Scholar]
- 17.Hosmer DW L S. Applied Logistic Regression. New York: Wiley; 1989. p. 187–215. [Google Scholar]
- 18.Olliaro P, Vaillant M, Arana B, Grogl M, Modabber F, Magill A, et al. Methodology of clinical trials aimed at assessing interventions for cutaneous leishmaniasis. PLoS Negl Trop Dis. 2013;7(3):e2130 Epub 2013/04/05. doi: 10.1371/journal.pntd.0002130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez U, Pinart M, Rengifo-Pardo M, Macaya A, Alvar J, Tweed JA. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane database of systematic reviews (Online). 2009;(2):CD004834 doi: 10.1002/14651858.CD004834.pub2 . [DOI] [PubMed] [Google Scholar]
- 20.Prina E, Roux E, Mattei D, Milon G. Leishmania DNA is rapidly degraded following parasite death: an analysis by microscopy and real-time PCR. Microbes and infection / Institut Pasteur. 2007;9(11):1307–15. doi: 10.1016/j.micinf.2007.06.005 . [DOI] [PubMed] [Google Scholar]
- 21.Castro MD, Gomez MA, Kip AE, Cossio A, Ortiz E, Navas A, et al. Pharmacokinetics of miltefosine in children and adults with cutaneous leishmaniasis. Antimicrobial agents and chemotherapy. 2016. doi: 10.1128/AAC.02198-16 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Meide WF, Peekel I, van Thiel PP, Schallig HD, de Vries HJ, Zeegelaar JE, et al. Treatment assessment by monitoring parasite load in skin biopsies from patients with cutaneous leishmaniasis, using quantitative nucleic acid sequence-based amplification. Clin Exp Dermatol. 33 England 2008. p. 394–9. doi: 10.1111/j.1365-2230.2007.02680.x [DOI] [PubMed] [Google Scholar]
- 23.Belkaid Y, Mendez S, Lira R, Kadambi N, Milon G, Sacks D. A natural model of Leishmania major infection reveals a prolonged "silent" phase of parasite amplification in the skin before the onset of lesion formation and immunity. J Immunol. 2000;165(2):969–77. Epub 2000/07/06. . [DOI] [PubMed] [Google Scholar]
- 24.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194(10):1497–506. Epub 2001/11/21. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420(6915):502–7. Epub 2002/12/06. doi: 10.1038/nature01152 . [DOI] [PubMed] [Google Scholar]
- 26.Bogdan C. Leishmaniasis in rheumatology, haematology and oncology: epidemiological, immunological and clinical aspects and caveats. Ann Rheum Dis. 2012;71 Suppl 2:i60–6. Epub 2012/04/04. doi: 10.1136/annrheumdis-2011-200596 . [DOI] [PubMed] [Google Scholar]
- 27.van Griensven J, Carrillo E, Lopez-Velez R, Lynen L, Moreno J. Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect. 2014;20(4):286–99. Epub 2014/01/24. doi: 10.1111/1469-0691.12556 . [DOI] [PubMed] [Google Scholar]
- 28.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, et al. The relationship between leishmaniasis and AIDS: the second 10 years. Clinical microbiology reviews. 2008;21(2):334–59. Epub 2008/04/11. doi: 10.1128/CMR.00061-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wortmann GW, Aronson NE, Miller RS, Blazes D, Oster CN. Cutaneous leishmaniasis following local trauma: a clinical pearl. Clin Infect Dis. 2000;31(1):199–201. Epub 2000/07/29. doi: 10.1086/313924 . [DOI] [PubMed] [Google Scholar]
- 30.Mendes MST GD, Ribeiro JBP, Alfani AOS, Lima BD, et al. Cutaneous Leishmaniasis Following Local Trauma: A Case Report. Clinical Research in Dermatology: Open Access Open Access: J Clin Exp Dermatol Res; 2014. p. 250 [Google Scholar]
- 31.Saravia NG, Weigle K, Segura I, Giannini SH, Pacheco R, Labrada LA, et al. Recurrent lesions in human Leishmania braziliensis infection—reactivation or reinfection? Lancet. 1990;336(8712):398–402. . [DOI] [PubMed] [Google Scholar]
- 32.Netto EM, Marsden PD, Llanos-Cuentas EA, Costa JM, Cuba CC, Barreto AC, et al. Long-term follow-up of patients with Leishmania (Viannia) braziliensis infection and treated with Glucantime. Trans R Soc Trop Med Hyg. 1990;84(3):367–70. Epub 1990/05/01. . [DOI] [PubMed] [Google Scholar]
- 33.Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: a longitudinal study of the natural history, prevalence, and incidence of infection and clinical manifestations. The Journal of infectious diseases. 1993;168(3):699–708. Epub 1993/09/01. . [DOI] [PubMed] [Google Scholar]
- 34.Gutierrez Y, Salinas GH, Palma G, Valderrama LB, Santrich CV, Saravia NG. Correlation between histopathology, immune response, clinical presentation, and evolution in Leishmania braziliensis infection. The American journal of tropical medicine and hygiene. 1991;45(3):281–9. . [DOI] [PubMed] [Google Scholar]
- 35.Passos VM, Barreto SM, Romanha AJ, Krettli AU, Volpini AC, Lima e Costa MF. American cutaneous leishmaniasis: use of a skin test as a predictor of relapse after treatment. Bull World Health Organ. 2000;78(8):968–74. Epub 2000/09/20. . [PMC free article] [PubMed] [Google Scholar]
- 36.Zuk M, McKean KA. Sex differences in parasite infections: patterns and processes. Int J Parasitol. 26 England 1996. p. 1009–23. [PubMed] [Google Scholar]
- 37.Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 26 England 2004. p. 247–64. doi: 10.1111/j.0141-9838.2004.00710.x [DOI] [PubMed] [Google Scholar]
- 38.Nava-Castro K, Hernandez-Bello R, Muniz-Hernandez S, Camacho-Arroyo I, Morales-Montor J. Sex steroids, immune system, and parasitic infections: facts and hypotheses. Ann N Y Acad Sci. 2012;1262:16–26. Epub 2012/07/25. doi: 10.1111/j.1749-6632.2012.06632.x . [DOI] [PubMed] [Google Scholar]
- 39.Travi BL, Osorio Y, Melby PC, Chandrasekar B, Arteaga L, Saravia NG. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmania spp. Infection and immunity. 2002;70(5):2288–96. doi: 10.1128/IAI.70.5.2288-2296.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz A, Rainey PM, Herwaldt BL, Stagni G, Palacios R, Trujillo R, et al. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. The Journal of infectious diseases. 2007;195(4):602–8. doi: 10.1086/510860 . [DOI] [PubMed] [Google Scholar]
- 41.Dorlo TP, van Thiel PP, Huitema AD, Keizer RJ, de Vries HJ, Beijnen JH, et al. Pharmacokinetics of miltefosine in Old World cutaneous leishmaniasis patients. Antimicrobial agents and chemotherapy. 2008;52(8):2855–60. doi: 10.1128/AAC.00014-08 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro MDM, Cossio A, Velasco C, Osorio L. Risk factors for therapeutic failure to meglumine antimoniate and miltefosine in adults and children with cutaneous leishmaniasis in Colombia: A cohort study. PLoS neglected tropical diseases. 2017;11(4):e0005515 doi: 10.1371/journal.pntd.0005515 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhandari V, Kulshrestha A, Deep DK, Stark O, Prajapati VK, Ramesh V, et al. Drug susceptibility in Leishmania isolates following miltefosine treatment in cases of visceral leishmaniasis and post kala-azar dermal leishmaniasis. PLoS neglected tropical diseases. 2012;6(5):e1657 doi: 10.1371/journal.pntd.0001657 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodrigues EH, Soares FC, Werkhauser RP, de Brito ME, Fernandes O, Abath FG, et al. The compositional landscape of minicircle sequences isolated from active lesions and scars of American cutaneous leishmaniasis. Parasit Vectors. 2013;6:228 doi: 10.1186/1756-3305-6-228 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell-Lendrum D, Dujardin JP, Martinez E, Feliciangeli MD, Perez JE, Silans LN, et al. Domestic and peridomestic transmission of American cutaneous leishmaniasis: changing epidemiological patterns present new control opportunities. Memorias do Instituto Oswaldo Cruz. 2001;96(2):159–62. . [DOI] [PubMed] [Google Scholar]
- 46.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2001;95(3):239–43. . [DOI] [PubMed] [Google Scholar]
- 47.Yadon ZE, Rodrigues LC, Davies CR, Quigley MA. Indoor and peridomestic transmission of American cutaneous leishmaniasis in northwestern Argentina: a retrospective case-control study. The American journal of tropical medicine and hygiene. 2003;68(5):519–26. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All molecular data (sequences, microsatelite profiles, parasite loads, etc.) are available as supporting information files. Due to patient privacy concerns, patient demographics and related metadata will be available on request to the institutional review board of CIDEIM, contacting Ms. Jackeline Bravo at jbravo@cideim.org.co.