Abstract
Background
Abnormal childhood growth may affect future health. Maternal tenofovir (TFV) use was associated with lower body length and head circumference at one year of age in HIV-exposed uninfected (HEU) US children.
Methods
We studied 509 HEU children in the US-based Surveillance Monitoring of ART Toxicities cohort whose HIV-infected mothers were not using antiretrovirals at the last menstrual period and began combination antiretroviral therapy (cART) in pregnancy (cART-initiators). We examined adjusted associations between antiretrovirals and CDC 2000 growth z-scores at 2 years of age within trimester of cART initiation: weight (WTZ), length (LNZ), weight-for-length (WFLZ), triceps skinfold z-score (TSFZ), and head circumference (HCZ).
Results
Mothers mean age was 28.6 years; 57% were black non-Hispanic and 19% delivered at <37 weeks gestation. At 2 years, mean WTZ, LNZ, WFLZ, and HCZ, were above average (P<0.05), while TSFZ (P=0.57) did not differ from average. WFLZ was >1.64 SD (>95th percentile) in 13%. Among children of first trimester cART-initiators, TFV+emtricitabine -exposed children had slightly higher mean WFLZ (0.45 SD, 95%CI −0.10, 1.00) and lower TSFZ (−0.55 SD, 95%CI −1.07, −0.02) compared to zidovudine+lamivudine-exposed. TSFZ was lower in those exposed to boosted protease inhibitors. In contrast, growth in children of second trimester cART-initiators did not differ by antiretroviral exposures.
Conclusion
Growth was above average in HEU; 13% were obese. Maternal TFV use was not associated with lower length or head circumference at age two, as hypothesized, but may be related to greater weight among those exposed to cART early in pregnancy.
Keywords: Maternal antiretroviral therapy, HIV-exposed uninfected children, early childhood growth, tenofovir, obesity, trimester of pregnancy
INTRODUCTION
Most HIV-infected (HIV+) women in the United States (US) are on antiretroviral (ARVs) medications at conception or begin early in pregnancy to prevent mother-to-child HIV transmission (PMTCT).1 Prenatal exposures to infections, including HIV, may alter fetal and postnatal growth,2 through decreases in placental size or morphologic changes 3,4 or mitochondrial abnormalities. 5Little is known about antenatal ARV exposure and growth. Factors that affect fetal programming may affect subsequent growth 6. Since both fetal growth restriction and subsequent infant obesity are linked to diabetes and cardiovascular disease in adulthood,7 characterizing growth in general and the effects of specific antenatal ARV exposure on postnatal growth in HIV-exposed uninfected (HEU) children is necessary to optimize therapy and maximize safety. Exposures that occur during the first trimester (organogenesis) compared to later in gestation when there is rapid growth may differentially affect outcomes. In Europe, HEU children to age ten, on average, had normal growth in the periods before and during widespread use of ARVs in pregnancy.8 In Botswana, HEU children exposed antenatally to cART after the first trimester had significantly lower length but only small differences in weight (lower) and weight-for-length (higher) z-scores at six months than those exposed to short course zidovudine (ZDV).9 Effects of specific antenatal ARVs on growth in HEU children have not been adequately evaluated in the era of combination antiretroviral therapy (cART),10 and some results are discrepant. Tenofovir-exposed HEU children had lower weight11, length and head circumference12 in two studies, but no difference in growth in other cohorts.13,14
We examined average growth and effects of antenatal ARV exposures on growth outcomes in HEU children at 2 years of age. We studied HEU children in the US-based Surveillance Monitoring of ART Toxicities (SMARTT) cohort of the Pediatric HIV/AIDS Cohort Study within trimester of cART initiation, comparing common therapies such as tenofovir (TFV) versus zidovudine (ZDV)-containing regimens and any protease inhibitor (PI) regimen boosted with ritonavir (bPI) versus any PI regimen not boosted with ritonavir.
METHODS
Population and data collection
SMARTT prospectively enrolled two cohorts of HEU children at 22 clinical sites across the US and Puerto Rico to evaluate the safety of ARVs used in pregnancy on childhood outcomes. Between 2007 and 2009, the Static cohort enrolled HEU children <12 years of age and their mother or caregiver. Since 2007, the Dynamic cohort has enrolled HIV+ mothers and their infants from 22 weeks gestation until one week postpartum with continued enrollment to monitor safety of newly prescribed ARVs in pregnancy. At entry, the mother or caregiver was interviewed to obtain socio-demographic characteristics and history of substance use during this pregnancy. Pregnancy data abstracted from medical charts included obstetric complications, ARV use, CD4 count and percent (CD4%), and HIV viral load. At annual visits, children’s growth measurements and health histories were obtained. The SMARTT protocol was approved by the Institutional Review Boards at Harvard and each site. Informed consent was obtained from all mothers or caregivers for their own and their children’s participation.
Inclusion criteria
Estimates of the exposure-outcome relationship may be biased when prevalent and incident exposures are included together, as it is difficult to control for confounding by indication.15,16 Thus, we studied incident cART users and excluded those who were exposed to ARV at conception. We included HEU children whose mothers were not on any ARV at their last menstrual period (LMP) and for whom the first ARV regimen during this pregnancy was cART (cART-initiators). cART was defined as at least 3 ARVs from at least 2 different classes. Any mothers who had received ARVs before the current pregnancy (during a prior pregnancy or for their own health) were only included in this analysis if they had stopped ARVs before their LMP. Others may never have used ARVs. We further limited this analysis to HEU children whose mothers had complete ARV information by trimester and who had weight and length measurements at 2 years ± 4 months of age as of April 1, 2013.
Growth outcomes
Birth weight and diagnosis of intrauterine growth restriction were abstracted from the obstetrical chart. Anthropometric measurements were performed in triplicate at entry and annually thereafter by trained personnel.17 In the Dynamic cohort, neonatal measures were generally obtained within 72 hours of birth (72%), but accepted within 14 days of birth. When weights (kg) were measured, infants could wear undergarments, but not diapers. Length (cm) was measured in the recumbent position on a board with legs fully extended until age three. If the child’s length exceeded that of the board, standing height was measured without shoes using a wall-mounted stadiometer, based on CDC recommendations when our protocol was written. Triceps skinfold thickness (TSF, mm) and head circumference (HC, cm) were measured by standard techniques18 at age 2.
CDC 2000 z-scores for age and sex19 were calculated for weight (WTZ), length/height (LNZ), weight-for-length (WFLZ), TSF (TSFZ), and HC (HCZ). Body mass index z-score (BMIZ) was calculated, rather than WFLZ, when standing height was measured. For preterm children (<37 weeks gestation), z-scores for birth weight and neonatal weight and length (within 2 weeks of birth) were calculated for gestational age.20 WTZ, LNZ, and WFLZ values < −5 and > 5 standard deviations (SD) were excluded because they were highly influential in the analysis. At age 2, we compared the mean of each growth z-score to a mean of zero using a Student’s t test. WTZ, LNZ, WFLZ/BMIZ, and TSFZ were each categorized into low, normal, and high: < −1.64, −1.64 to 1.64, and >1.64. These correspond to < 5th, 5th-95th, > 95th percentiles, respectively. HCZ was categorized as <-2 SD, −2 to 2 SD, and >2 SD, per the protocol. Children with WFLZ > 1.64 were considered obese. Small for gestational age (SGA) was defined as birth weight <10th percentile corrected for gestational age.
Statistical methods
We fit unadjusted and adjusted general linear regression models to evaluate the association of each ARV exposure at maternal cART initiation on each growth outcome at age 2 years, separately for first trimester (LMP through 14 weeks gestation) and second trimester (15 through 28 weeks gestation) of cART initiation. We compared common regimens including TFV + emtricitabine (FTC) versus ZDV + lamivudine (3TC) and bPI versus unboosted PI based on results of previous studies of fetal growth or early infant growth. 11,12,21 This is a comparative safety strategy.15,16,22 We also compared exposed versus unexposed to each PI and NRTI (nucleoside reverse transcriptase inhibitors) used by at least 10% of women during pregnancy. Changes in regimen were not considered as this analysis was the observational study equivalent of an intention-to-treat analysis.22 SMARTT is a drug safety study so our priority was to limit Type II statistical errors (falsely missing a safety signal) rather than Type I errors (falsely rejecting the null hypothesis). Thus, we made no adjustments for multiple testing and pointed out results with P <0.10.23,24 Adjusted models included site geographic region and maternal covariates that were associated with the outcome with P<0.20 in univariable analyses and deemed to be confounders if they changed the estimate of exposure >10% or improved estimate precision for that outcome in the adjusted analysis. We performed sensitivity analyses limited to children whose mothers had CD4% and viral load before cART initiation in pregnancy. In addition, we evaluated the effect of tenofovir on growth among children whose mothers were on a PI-based regimen.
Based on a priori knowledge, directed acyclic graphs (DAGs) were drawn using DAGitty v2.025 to identify potential confounders of the relationship between the main exposure (ARV type) and growth outcomes at age 2 years. Potential confounders measured at or before cART initiation included mothers’ age, born on mainland US, race/ethnicity, English only spoken at home, household income, first trimester use of illicit drugs, alcohol, or tobacco during the pregnancy, CD4%, and HIV RNA > 10,000 copies/mL before cART initiation in pregnancy, pre-pregnancy BMI, ever used ARV prior to this pregnancy, birth year of child, and geographic region of the clinic. We used CD4% rather than CD4 count because this is more stable in pregnancy. We also tested random effect models to account for clustering of children within site or between siblings and performed sensitivity analyses omitting siblings and twins.
RESULTS
Maternal and HIV-exposed infant characteristics
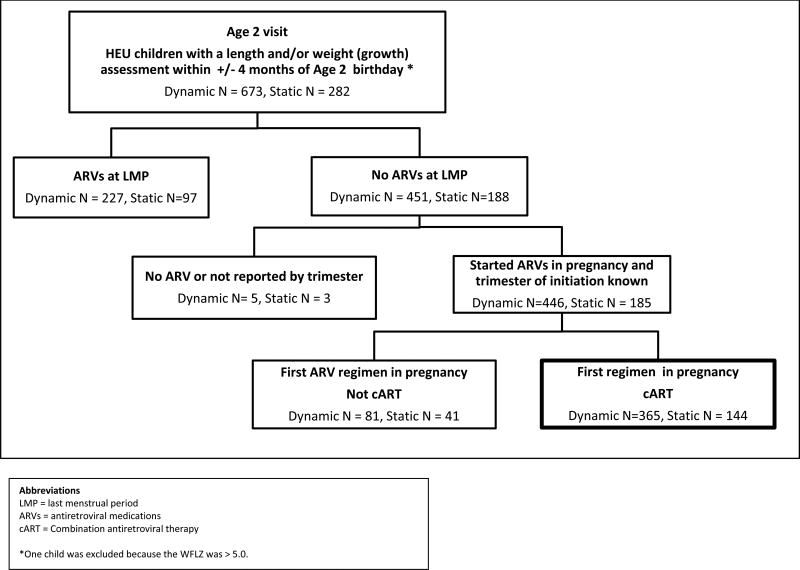
We included 509 children (Figure 1), 72% (365/509) of whom were from the Dynamic and 28% (144 of 509) from the Static cohort. HIV+ mothers had a mean age of 28.6 years and 57% were black non-Hispanic (Table 1). First-trimester use of illicit drugs, alcohol, and tobacco was reported by 7%, 8%, and 19%, respectively. Of the 83% with CD4% and viral load information available prior to cART initiation in pregnancy, 45% had CD4% <25 and 41% had viral load >10,000 copies/mL (Table 2). Ninety-seven (19%) deliveries were preterm; nine of these infants were SGA. Of the 28 sibling pairs, seven pairs were twins, and one twin in each of 2 pairs was SGA. Mean WTZ and LNZ at birth, adjusted for gestational age, were −0.44 and −0.34, respectively. ARV use prior to this pregnancy was reported by 148 (29%), not used by 132 (26%), and unknown in 229 (45%) women. Of the 509 women, 27.1% initiated cART in the first trimester, 64% in the second trimester and 8.5% in the third trimester. First trimester cART-initiators were significantly older. Trimester of initiation varied by geographic site.
Figure 1.
Flowchart for selection of HEU children with anthropometric measures at 2 years of age (+/−4 months) whose HIV-infected mothers initiated cART during this pregnancy as of April 1, 2013
Table 1.
Maternal and HIV-exposed but uninfected infant characteristics at delivery by trimester first exposed to cART
| Trimester in pregnancy started cART | ||||||
|---|---|---|---|---|---|---|
| Characteristic1 | 1 (N=138) |
2 (N=328) |
3 (N=43) |
Total (N=509) |
P Value |
|
| Maternal age (years) | Mean (SD) | 30.2 (6.3) | 28.1 (5.9) | 27.7 (7.2) | 28.6 (6.2) | 0.003 |
| Maternal birthplace | U.S. (mainland) | 85 (62%) | 214 (66%) | 31 (72%) | 330 (65%) | 0.48 |
| Puerto Rico | 12 (9%) | 42 (13%) | 3 (7%) | 57 (11%) | ||
| Other | 17 (12%) | 30 (9%) | 1 (2%) | 48 (9%) | ||
| Africa | 10 (7%) | 14 (4%) | 4 (9%) | 28 (6%) | ||
| Mexico | 8 (6%) | 11 (3%) | 2 (5%) | 21 (4%) | ||
| Dominican Republic | 2 (1%) | 10 (3%) | 1 (2%) | 13 (3%) | ||
| Haiti | 3 (2%) | 3 (1%) | 1 (2%) | 7 (1%) | ||
| Cuba | 1 (1%) | 1 (0%) | 0 (0%) | 2 (0%) | ||
| Race/ethnicity Mom/caregiver | Black Non-Hispanic | 79 (58%) | 180 (56%) | 26 (60%) | 285 (57%) | 0.80 |
| Hispanic | 46 (34%) | 106 (33%) | 11 (26%) | 163 (33%) | ||
| White Non-Hispanic | 11 (8%) | 25 (8%) | 5 (12%) | 41 (8%) | ||
| Other unreported | 1 (1%) | 8 (3%) | 1 (2%) | 10 (2%) | ||
| High school graduate | 87 (63%) | 215 (66%) | 21 (51%) | 323 (64%) | 0.15 | |
| Annual Income | <$10K | 62 (45%) | 141 (43%) | 22 (51%) | 225 (44%) | 0.88 |
| ≥$10K | 59 (43%) | 148 (45%) | 16 (37%) | 223 (44%) | ||
| Unknown | 17 (12%) | 39 (12%) | 5 (12%) | 61 (12%) | ||
| English only spoken at home | 86 (62%) | 212 (65%) | 27 (63%) | 325 (64%) | 0.84 | |
| Single (not living with partner/spouse) | 78 (57%) | 177 (54%) | 21 (49%) | 276 (54%) | 0.67 | |
| Illicit drugs - used in first trimester | 12 (9%) | 20 (6%) | 3 (8%) | 35 (7%) | 0.67 | |
| Alcohol - used in first trimester | 15 (11%) | 21 (7%) | 3 (8%) | 39 (8%) | 0.30 | |
| Tobacco - used in first trimester | 31 (23%) | 54 (17%) | 7 (18%) | 92 (19%) | 0.38 | |
| Clinical site by region | NY-NJ | 38 (28%) | 79 (24%) | 12 (28%) | 129 (25%) | 0.013 |
| South | 20 (14%) | 84 (26%) | 10 (23%) | 114 (22%) | ||
| West | 30 (22%) | 39 (12%) | 4 (9%) | 73 (14%) | ||
| Puerto Rico | 12 (9%) | 52 (16%) | 4 (9%) | 68 (13%) | ||
| Florida | 19 (14%) | 34 (10%) | 9 (21%) | 62 (12%) | ||
| Chicago | 19 (14%) | 36 (11%) | 3 (7%) | 58 (11%) | ||
| Mid-Atlantic | 0 (0%) | 4 (1%) | 1 (2%) | 5 (1%) | ||
| Pre-pregnancy BMI (kg/m2) 2 | Mean (SD) | 29.4 (8.3) | 30.2 (8.3) | 27.6 (6.7) | 29.8 (8.2) | 0.39 |
| Toxemia/pre-eclampsia2 | 9 (7%) | 19 (6%) | 0 (0%) | 28 (6%) | -- | |
| Intrauterine growth retardation2 | 2 (1%) | 3 (1%) | 0 (0%) | 5 (1%) | -- | |
| Pre-gestational diabetes2 | 5 (4%) | 4 (1%) | 0 (0%) | 9 (2%) | -- | |
| Gestational diabetes2 | 10 (7%) | 13 (4%) | 0 (0%) | 23 (5%) | -- | |
| Male gender of child | 68 (49%) | 168 (51%) | 23 (53%) | 259 (51%) | 0.87 | |
| Preterm (< 37 weeks)2 | 33 (24%) | 59 (18%) | 5 (12%) | 97 (19%) | 0.14 | |
| Birth weight z-score2,3 | Mean (SD) | −0.55 (0.88) | −0.61 (0.77) | −0.75 (0.64) | −0.61 (0.80) | 0.36 |
| Length z-score < 14 days old3 | Mean (SD) | −0.15 (1.01) | −0.12 (0.93) | −0.16 (1.01) | −0.13 (0.96) | 0.87 |
| Weight-for-length < 10th percentile at birth2,3 | 10 (10%) | 27 (13%) | 6 (18%) | 43 (13%) | 0.51 | |
| Small for gestational age (WT < 10th percentile)2 | 28 (20%) | 60 (19%) | 9 (21%) | 97 (19%) | 0.87 | |
| Received antiretroviral therapy before this pregnancy1 | Yes | 56 (41%) | 82 (25%) | 10 (23%) | 148 (29%) | 0.001 |
| No | 26 (19%) | 88 (27%) | 18 (42%) | 132 (26%) | ||
| Unknown | 56 (41%) | 158 (48%) | 15 (35%) | 229 (45%) | ||
| Days after LMP started cART (days) | Mean (SD) | 68.4 (26.3) | 133.2 (25.5) | 225.0 (19.0) | 123.4 (48.9) | <0.001 |
| Days after LMP started prenatal care1 | Mean (SD) | 64.9 (27.7) | 92.9 (38.3) | 161.3 (74.0) | 92.0 (48.8) | <0.001 |
cART=combination antiretroviral therapy, NY-NJ=New York/New Jersey, SD=standard deviation, BMI=body mass index, WT=weight, LMP=last menstrual period, SGA=small-for-gestational age.
There were missing values for the following variables: birthplace (N=3), race/ethnicity (N=10), high school grad (N=6), language spoken at home (N= 2), living arrangement (N=2), alcohol (N=24), tobacco (N=24) or illicit drugs (N=26), pre-pregnancy BMI (N=115), pregnancy complications: toxemia/pre-eclampsia (N= 6), intrauterine growth retardation (N= 6), pre-gestational diabetes (N=7), gestational diabetes (N=6), SGA (N=5), birth weight (N=5), neonatal length (N=22 among Dynamic cohort). The questions regarding ARV use before pregnancy and date of prenatal visit were first collected in 2010 and thus, were unavailable for 218 participants. Of those administered the questionnaire, ARV use before pregnancy was unknown for N=11 and prenatal date was unknown for 220 (13 additional had uninterpretable prenatal care dates). P values for pregnancy complications were not computed because there were different frequencies of missing by trimester.
Birth weight, maternal weight and height, and pregnancy complications were abstracted from the clinical charts.
Length was measured in SMARTT within 14 days after birth. For children born < 37 weeks gestation, both weight and length were adjusted for gestational age using Fenton et al, norms.20 For children born ≥ 37 weeks Centers for Disease Control (CDC) norms were used. Length within 2 weeks of delivery was missing on N= 166. This was primarily because children in the Static cohort did not have measures of length at or near delivery (N=155), so weight-for-length could also not be calculated.
Table 2.
Maternal CD4 count and HIV viral load before cART initiation and ARV medication use at time of cART initiation, by trimester
| Trimester in pregnancy started cART | ||||||
|---|---|---|---|---|---|---|
| Characteristic1 | 1 (N=138) |
2 (N=328) |
3 (N=43) |
Total (N=509) |
P Value |
|
| HIV RNA > 10K copies/mL2 | 43 (43%) | 116 (40%) | 16 (44%) | 175 (41%) | 0.81 | |
| CD4 percent < 25%2 | 38 (39%) | 135 (48%) | 17 (46%) | 190 (45%) | 0.32 | |
| Combinations of NRTIs | 3TC+ZDV | 83 (60%) | 229 (70%) | 29 (67%) | 341 (67%) | 0.74 |
| TFV+FTC | 39 (28%) | 68 (21%) | 13 (30%) | 120 (24%) | ||
| 3TC+ZDV+ABC | 7 (5%) | 16 (5%) | 1 (2%) | 24 (5%) | ||
| 3TC+ABC | 6 (4%) | 6 (2%) | 0 (0%) | 12 (2%) | ||
| TFV+3TC+ZDV | 2 (1%) | 3 (1%) | 0 (0%) | 5 (1%) | ||
| 3TC+D4T | 0 (0%) | 2 (1%) | 0 (0%) | 2 (0%) | ||
| 3TC+DDI | 0 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | ||
| TFV+3TC+ABC | 0 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | ||
| TFV+DDI | 0 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | ||
| TFV+FTC+DDI | 0 (0%) | 1 (0%) | 0 (0%) | 1 (0%) | ||
| TFV+ZDV | 1 (1%) | 0 (0%) | 0 (0%) | 1 (0%) | ||
| Boosted PI | PI with RTV | 97 (75%) | 239 (76%) | 38 (93%) | 374 (77%) | 0.04 |
| PI without RTV | 33 (25%) | 74 (24%) | 3 (7%) | 110 (23%) | ||
| Atazanavir | 28 (20%) | 42 (13%) | 7 (16%) | 77 (15%) | 0.12 | |
| Nelfinavir | 29 (21%) | 69 (21%) | 2 (5%) | 100 (20%) | 0.03 | |
| Lopinavir/ritonavir | 58 (42%) | 167 (51%) | 29 (67%) | 254 (50%) | 0.01 | |
| Any NNRTI | 7 (5%) | 15 (5%) | 2 (5%) | 24 (5%) | 0.97 | |
ABC= abacavir, DDI=didanosine, FTC=emtricitabine, TFV=tenofovir, ZDV=zidovudine, 3TC=lamivudine, NRTI=non-nucleoside reverse transcriptase inhibitors, PI=protease inhibitor, RTV=ritonavir, NNRTI-non-nucleoside reverse transcriptase inhibitors, cART= combination antiretroviral therapy,
Values for CD4% and HIV viral before cART initiation were unavailable on 90 and 87 women, respectively.
ARV exposure by trimester of cART initiation
At cART initiation, maternal NRTI use included 3TC plus ZDV (67%), TFV plus FTC (24%), 3TC plus ZDV plus abacavir (ABC) (5%), and 3TC plus ABC (2%) (Table 2). Any TFV was used by 25%. Boosted PI-based cART was received by 74%, non-boosted PI 22% and no PI by 4%; 5% received NNRTI. Other ARV agents were used by <10% of women. First trimester cART-initiators used a boosted PI less frequently and were more likely to receive nelfinavir. Appendix 1 shows the relationship of socio-demographic and clinical factors with TFV+FTC and boosted PI use by trimester of cART initiation.
HEU growth outcomes at 2 years of age
At 2 years of age, mean WTZ, LNZ, WFLZ, and HCZ were all greater than zero (all P < 0.05), while TSFZ did not differ from zero (P=0.57) (Table 3). The percentage with high z-scores (see methods) was 13% for WFLZ, 11% for WTZ, 10% for TSFZ, 7% for HCZ, and 6% for LNZ. In contrast, the percentage with low z-scores was small for each of these outcomes: 5% WFLZ, 7% for WTZ, 6% for TSFZ, 1% for HCZ, and 5% for LNZ. Standing height rather than length was measured in 2 of 138 children aged 21–24 months and 16 of 371 aged 24–27 months.
Table 3.
CDC growth outcomes at age 2 years (+/− 4 months) in HIV-exposed uninfected children by trimester of maternal cART initiation
| Trimester in pregnancy started cART | ||||||
|---|---|---|---|---|---|---|
| Characteristic1 | 1 (N=138) |
2 (N=328) |
3 (N=43) |
Total (N=509) |
P Value2 | |
| Age at anthropometry (months) | Mean (SD) | 24.6 (1.6) | 24.5 (1.4) | 24.9 (1.4) | 24.6 (1.4) | 0.13 |
| Weight z-score | Mean (SD) | 0.02 (1.33) | 0.18 (1.18) | 0.37 (1.26) | 0.15 (1.23) | 0.45 |
| Weight z-score category | Low (<-1.64) | 14 (10%) | 22 (7%) | 2 (5%) | 38 (7%) | 0.46 |
| Mid (−1.64–1.64) | 111 (80%) | 272 (83%) | 34 (79%) | 417 (82%) | ||
| High (>1.64) | 13 (9%) | 34 (10%) | 7 (16%) | 54 (11%) | ||
| Length z-score | Mean (SD) | 0.03 (1.12) | 0.15 (1.02) | 0.18 (1.00) | 0.12 (1.05) | 0.70 |
| Length z-score category | Low (<-1.64) | 11 (8%) | 12 (4%) | 2 (5%) | 25 (5%) | 0.39 |
| Mid (−1.64–1.64) | 118 (86%) | 296 (90%) | 39 (91%) | 453 (89%) | ||
| High (>1.64) | 9 (7%) | 20 (6%) | 2 (5%) | 31 (6%) | ||
| Weight-for-length z-score | Mean (SD) | 0.20 (1.26) | 0.31 (1.22) | 0.54 (1.26) | 0.30 (1.24) | 0.32 |
| Weight-for-length z-score category | Low (<-1.64) | 8 (6%) | 14 (4%) | 1 (2%) | 23 (5%) | 0.55 |
| Mid (−1.64–1.64) | 115 (84%) | 266 (82%) | 34 (79%) | 415 (82%) | ||
| High (>1.64) | 14 (10%) | 46 (14%) | 8 (19%) | 68 (13%) | ||
| Triceps skinfold z-score | Mean (SD) | 0.12 (1.20) | −0.12 (1.16) | 0.13 (1.30) | −0.03 (1.18) | 0.14 |
| Triceps skinfold z-score category | Low (<-1.64) | 7 (5%) | 22 (7%) | 2 (5%) | 31 (6%) | 0.57 |
| Mid (−1.64–1.64) | 107 (82%) | 264 (85%) | 36 (86%) | 407 (84%) | ||
| High (>1.64) | 17 (13%) | 25 (8%) | 4 (10%) | 46 (10%) | ||
| Head circumference z | Mean (SD) | 0.26 (1.14) | 0.37 (1.14) | 0.07 (1.08) | 0.31 (1.14) | 0.27 |
| Head circumference z category | Low (< −2.0) | 4 (3%) | 2 (1%) | 0 (0%) | 6 (1%) | 0.38 |
| Mid (−2.0 – 2.0) | 123 (90%) | 292 (92%) | 39 (95%) | 454(92%) | ||
| High (>2.0) | 10 (7%) | 24 (8%) | 2 (5%) | 36 (7%) | ||
Abbreviations: cART = combination antiretroviral therapy.
There were missing values for triceps skinfold (n=25) and head circumference (n=13).
The average z-score for all children at 2 years of age for each growth outcome was compared to a z-score of zero using a Student’s t-test. The p values for each were weight z (P=0.005), length z (P=0.013), weight-for-length z (P< 0.001), head circumference z (< 0.0001), and triceps skinfold z (P=0.57).
Unadjusted and adjusted models of ARVs on growth outcomes in HEU at age 2 years
Table 4 shows unadjusted and adjusted models of the association of each ARV with each growth outcome by trimester of cART initiation.
Table 4.
Difference in CDC growth z-scores in HEU at 2 years of age (+/− 4 months) for each ARV medication by trimester of maternal cART initiation
| 1st trimester cART initiation1,2 N= 138 | 2nd trimester cART initiation1,2 N= 328 | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| ARV at cART initiation |
Unadj Estimate (95%CI) |
Unadj P value |
Adj Estimate (95%CI) |
Adj P value |
Unadj Estimate (95%CI) |
Unadj P value |
Adj Estimate (95%CI) |
Adj P value |
| Weight z-score | Weight z-score | |||||||
| TFV+FTC vs. ZDV+3TC | 0.35 (−0.16,0.87) | 0.18 | 0.44 (−0.15,1.03) | 0.14 | −0.04 (−0.36,0.27) | 0.79 | −0.13 (−0.48,0.22) | 0.47 |
| TFV vs. noTFV | 0.30 (−0.18,0.79) | 0.22 | 0.50 (−0.05,1.05) | 0.08 | −0.09 (−0.39,0.22) | 0.58 | −0.20 (−0.54,0.13) | 0.24 |
| bPI vs. unboosted PI | −0.26 (−0.79,0.27) | 0.33 | −0.13 (−0.70,0.44) | 0.65 | 0.06 (−0.25,0.37) | 0.69 | 0.02 (−0.32,0.37) | 0.90 |
| ATV vs. noATV | 0.04 (−0.52,0.60) | 0.88 | 0.06 (−0.52,0.64) | 0.84 | 0.07 (−0.31,0.46) | 0.71 | 0.01 (−0.39,0.41) | 0.95 |
| NFV vs. noNFV | 0.16 (−0.40,0.71) | 0.57 | 0.12 (−0.48,0.72) | 0.69 | −0.05 (−0.37,0.26) | 0.75 | 0.03 (−0.32,0.38) | 0.87 |
| Length z-score | Length z-score | |||||||
| TFV+FTC vs. ZDV+3TC | 0.26 (−0.18,0.70) | 0.24 | 0.25 (−0.26,0.76) | 0.33 | −0.11 (−0.38,0.16) | 0.41 | −0.15 (−0.45,0.15) | 0.31 |
| TFV vs. noTFV | 0.22 (−0.19,0.63) | 0.29 | 0.28 (−0.19,0.76) | 0.23 | −0.14 (−0.41,0.12) | 0.30 | −0.20 (−0.50,0.09) | 0.17 |
| bPI vs. unboosted PI | −0.16 (−0.61,0.28) | 0.47 | −0.22 (−0.72,0.27) | 0.37 | 0.22 (−0.05,0.48) | 0.11 | 0.23 (−0.06,0.53) | 0.12 |
| ATV vs. noATV | 0.10 (−0.37,0.57) | 0.67 | 0.03 (−0.47,0.53) | 0.91 | 0.05 (−0.29,0.38) | 0.78 | 0.05 (−0.29,0.40) | 0.77 |
| NFV vs. noNFV | 0.01 (−0.46,0.47) | 0.98 | 0.07 (−0.45,0.58) | 0.80 | −0.17 (−0.44,0.10) | 0.22 | −0.16 (−0.47,0.14) | 0.29 |
| Weight-for-length z-score | Weight-for-length z-score | |||||||
| TFV+FTC vs. ZDV+3TC | 0.24 (−0.25,0.72) | 0.33 | 0.45 (−0.10,1.00) | 0.11 | 0.05 (−0.28,0.38) | 0.76 | −0.03 (−0.40,0.34) | 0.89 |
| TFV vs. noTFV | 0.21 (−0.25,0.67) | 0.37 | 0.50 (−0.02,1.02) | 0.06 | −0.01 (−0.33,0.31) | 0.94 | −0.11 (−0.47,0.25) | 0.55 |
| bPI vs. unboosted PI | −0.17 (−0.67,0.33) | 0.50 | 0.07 (−0.44,0.57) | 0.79 | −0.12 (−0.44,0.20) | 0.47 | −0.16 (−0.52,0.20) | 0.37 |
| ATV vs. noATV | −0.10 (−0.63,0.43) | 0.70 | 0.01 (−0.53,0.55) | 0.97 | 0.05 (−0.35,0.45) | 0.81 | −0.04 (−0.46,0.38) | 0.85 |
| NFV vs. noNFV | 0.16 (−0.36,0.68) | 0.55 | 0.01 (−0.53,0.55) | 0.96 | 0.10 (−0.22,0.43) | 0.53 | 0.17 (−0.20,0.54) | 0.37 |
| Triceps skinfold z-score | Triceps skinfold z-score | |||||||
| TFV+FTC vs. ZDV+3TC | −0.05 (−0.51,0.42) | 0.84 | −0.55 (−1.07,-0.02) | 0.04 | 0.02 (−0.30,0.33) | 0.91 | −0.04 (−0.38,0.31) | 0.84 |
| TFV vs. noTFV | −0.13 (−0.57,0.32) | 0.58 | −0.47 (−0.97,0.02) | 0.06 | −0.05 (−0.35,0.26) | 0.76 | −0.11 (−0.45,0.23) | 0.52 |
| bPI vs. unboosted PI | −0.17 (−0.67,0.33) | 0.51 | −0.61 (−1.26,0.04) | 0.065 | −0.02 (−0.32,0.29) | 0.91 | 0.15 (−0.18,0.48) | 0.38 |
| ATV vs. noATV | 0.24 (−0.27,0.75) | 0.36 | 0.14 (−0.40,0.67) | 0.61 | 0.43 (0.06,0.81) | 0.024 | 0.29 (−0.09,0.67) | 0.15 |
| NFV vs. noNFV | −0.03 (−0.55,0.48) | 0.89 | 0.41 (−0.28,1.09) | 0.24 | 0.0 (−0.32,0.32) | 0.99 | −0.18 (−0.52,0.17) | 0.31 |
| Head circumference z-score | Head circumference z-score | |||||||
| TFV+FTC vs. ZDV+3TC | 0.31 (−0.13,0.75) | 0.17 | 0.18 (−0.34,0.70) | 0.49 | 0.05 (−0.26,0.36) | 0.74 | −0.06 (−0.40,0.28) | 0.72 |
| TFV vs. noTFV | 0.27 (−0.15,0.69) | 0.20 | 0.18 (−0.30,0.66) | 0.45 | 0.01 (−0.29,0.31) | 0.97 | −0.15 (−0.48,0.18) | 0.38 |
| bPI vs. unboosted PI | 0.37 (−0.09,0.84) | 0.11 | 0.15 (−0.45,0.76) | 0.62 | −0.07 (−0.37,0.24) | 0.67 | −0.18 (−0.52,0.15) | 0.28 |
| ATV vs. noATV | 0.22 (−0.26,0.69) | 0.38 | 0.23 (−0.28,0.74) | 0.37 | −0.03 (−0.41,0.35) | 0.87 | −0.09 (−0.48,0.30) | 0.64 |
| NFV vs. noNFV | −0.35 (−0.83,0.13) | 0.15 | −0.07 (−0.71,0.57) | 0.83 | 0.02 (−0.29,0.33) | 0.91 | 0.15 (−0.19,0.49) | 0.39 |
cART = combination antiretroviral therapy, Unadj = unadjusted, TFV=tenofovir, FTC=emtricitabine, ZDV=zidovudine, 3TC=lamivudine, ATV=atazanavir, NFV=nelfinavir, bPI = boosted protease inhibitor. WTZ = weight z, LNZ = length z, WFL = weight-for-length z, TSFZ = triceps skinfold z, HCZ = head circumference z, 95% CI = 95% confidence interval.
| First trimester: | TFV+FTC vs. ZDV+ FTC: WTZ, LNZ, and WFLZ (N=127), TSFZ (N=120), HCZ (N=126). |
| TFV, ATV, and NFV: WTZ, LNZ and WFLZ (N= 135), TSFZ (N=128), HCZ (N=134). | |
| bPI vs. unboosted PI: WTZ, LNZ, and WFLZ (N=128), TSFZ (N=121), HCZ (N=127). | |
| Second trimester | TFV+FTC vs. ZDV+ FTC: WTZ (N=297), LNZ (N= 297), WFLZ (N= 297), TSFZ (N=280), HCZ (N289) |
| TFV, ATV, and NFV: WTZ (N=310), LNZ and WFLZ (N= 310), TSFZ (N=293), HCZ (N=300). | |
| bPI vs. unboosted PI: WTZ, LNZ, and WFLZ (N=295), TSFZ (N=280), HCZ (N=285). |
Separate unadjusted and adjusted linear regression models fit for each ARV and each z-score outcome at trimester of cART initiation in pregnancy. Average difference is in those exposed compared to those unexposed for each ARV.
Alcohol and tobacco use are during the first trimester.
Adjusted models include the following covariates for each outcome by trimester of cART initiation: WTZ 1st (site region, alcohol, tobacco, language at home); WTZ 2nd (site region, language at home); LNZ 1st (site region, alcohol, tobacco); LNZ 2nd (site region, tobacco, language at home, living arrangement); WFLZ 1st (site region, alcohol, language at home); WFLZ 2nd (site region, tobacco, language at home); TSFZ 1st (site region, alcohol, tobacco, birth year); TSFZ 2nd (site region, living arrangement); HCZ 1st (region, alcohol, tobacco); HCZ 2nd (site region, tobacco, income, language at home). Marital status, race/ethnicity, birth outside the mainland US, and prior ARV use did not cause confounding and were not included.
First-trimester cART initiators
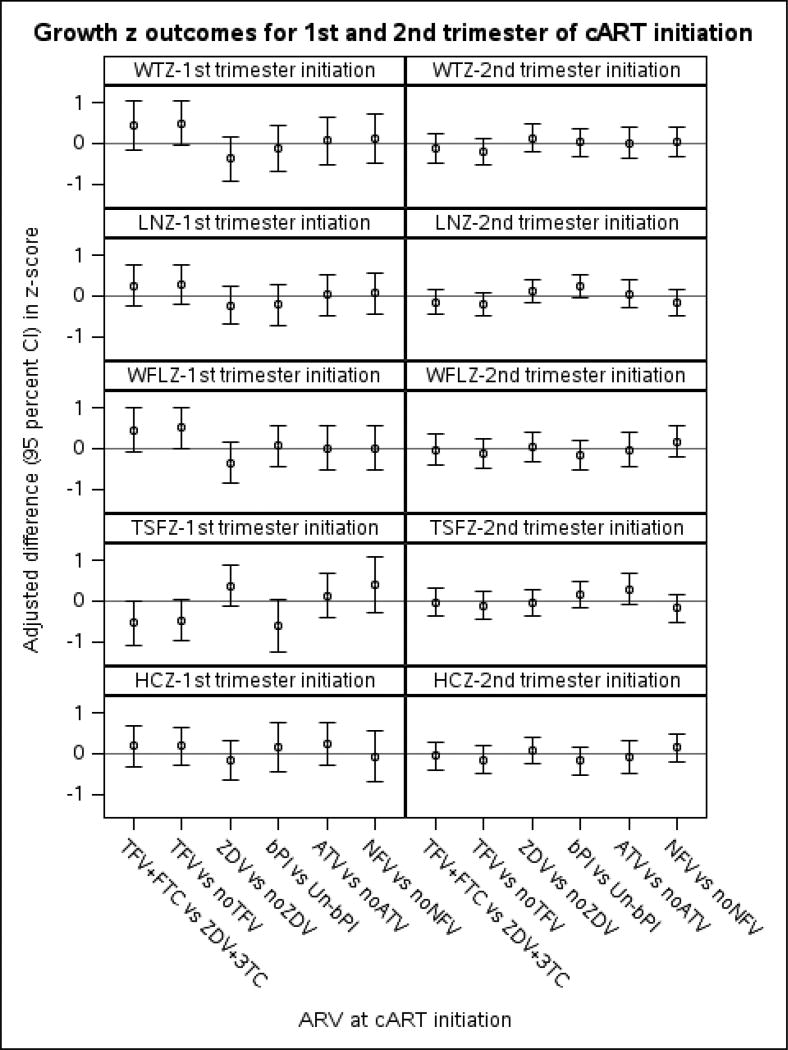
Among children of first-trimester cART-initiators (Table 4 and Figure 2), WTZ at 2 years of age was, on average, 0.44 SD higher in HEU children exposed to TFV+FTC compared to ZDV+3TC at maternal cART initiation after adjustment (Table 4). When TFV exposure was compared to no TFV exposure, ignoring other NRTIs used, WTZ was 0.50 higher. No other ARVs were associated with WTZ. For all ARVs studied, differences in mean LNZ between those exposed versus unexposed were small and 95% confidence intervals (CI’s) were wide (Table 4 and Figure 2). WFLZ was, on average, 0.45 SD greater in TFV+FTC compared to ZDV+3TC and 0.50 higher for TFV-exposed versus TFV-unexposed regardless of other NRTI used. In sensitivity analyses among PI-exposed, WTZ and WFLZ were each higher in the TFV+FTC-exposed vs. ZDV+3TC-exposed and remained when adjusted for bPI. TSFZ was 0.55 lower in those whose mother received TFV+FTC compared to ZDV+3TC and 0.61 lower in bPI versus non-bPI. The association of TFV+FTC on TSFZ remained after adjustment for bPI (−0.60, 95%CI −1.10, −0.095), while that for bPI was attenuated (−0.49, 95%CI −1.10, 0.10) when both were in the model. No associations were found for HCZ.
Figure 2.
This figure shows the adjusted differences in growth z-scores at 2 years of age (+/− 4 months) in HEU children for each ARV within each trimester of maternal cART initiation.1
1 ARV= antiretroviral type of regimen; cART = combination antiretroviral therapy; TFV = tenofovir; FTC = emtricitabine; ZDV = zidovudine; 3TC = lamivudine; bPI =Boosted protease inhibitor; Un-bPI = Unboosted PI; ATV = atazanavir; NFV = nelfinavir. WTZ =weight z; LNZ = length z; WFLZ = weight-for-length z; TSFZ = triceps skinfold z; HCZ = head circumference z. Adjusted models include the following covariates for each outcome by trimester of cART initiation: WTZ 1st (site region, alcohol, tobacco, language at home); WTZ 2nd (site region, language at home); LNZ 1st (site region, alcohol, tobacco); LNZ 2nd (site region, tobacco, language at home, living arrangement); WFLZ 1st (site region, alcohol, language at home); WFLZ 2nd (site region, tobacco, language at home); TSFZ 1st (site region, alcohol, tobacco, birth year); TSFZ 2nd (site region, living arrangement); HCZ 1st (region, alcohol, tobacco); HCZ 2nd (site region, tobacco, income, language at home). Marital status, race/ethnicity, birth outside the mainland US, and prior ARV use did not cause confounding and were not included.
In sensitivity analyses restricted to children of mothers with CD4% and viral load measures available before cART initiation, results were similar to those reported in the complete dataset above (not shown). In addition, adjustment for CD4% and viral load did not affect estimated ARV associations, suggesting no confounding by disease severity. The results did not change when a random effect was included for site or siblings or when twins were excluded.
Second-trimester cART initiators
In contrast to children of first-trimester cART-initiators, there were no differences at P<0.10 in WTZ, LNZ, WFLZ, TSFZ, or HCZ, by exposure to tenofovir or other ARVs among children of second-trimester cART-initiators. (Table 4, Figure 2). However, LNZ was 0.23 (95%CI −0.06, 0.53, p= 0.12) higher in those exposed to bPI versus non-boosted PI. All of these results were consistent in the subset with available measures of maternal CD4 and viral load data before cART initiation (not shown). Again, the results did not change when a random effect was included for site or siblings or when twins were excluded.
DISCUSSION
With the success of PMTCT, the HEU population is increasing worldwide. It is essential to monitor this population for possible adverse outcomes related to antenatal ARV exposure. This group of HEU children in the US whose mothers initiated cART during pregnancy had lower than average birth weight and length, but 13% were obese at 2 years of age. The prevalence of low growth was not greater than expected. Among children of first-trimester cART-initiators, WTZ and WFLZ were greater and TSFZ lower for those exposed to TFV+FTC versus ZDV+3TC and persisted when comparing TFV-exposed to TFV-unexposed children. Boosted PI was associated with lower TSFZ. However, we observed no effects of ARV exposure on growth among second-trimester cART initiators.
Exposures during critical fetal development periods can induce persistent changes in body structure or function, and have long-term effects on health.26 Thirteen percent of our cohort was obese at 2 years of age as compared to a national sample of similar age where the prevalence was 8.1% (95%CI 5.8–11.7) for all races and 8.4% (95%CI 4.6–14.9) in non-Hispanic blacks.27 In addition, in unpublished data from our cohort, among 3-year old children, HEU had a significantly higher rate of obesity compared to HIV-unexposed children (13.6% vs. 3.2%) from the same clinical sites, even after adjustment for race/ethnicity. However, these HIV-unexposed children may differ from the HEU on other factors, including other socioeconomic status, birth weight and length on which we do not have data, which can influence later growth. Low birth weight and more rapid catch-up growth in early childhood can increase the risk of obesity, insulin resistance, and metabolic syndrome in childhood28, and early coronary heart disease in adulthood. 7 Childhood obesity is now epidemic in most racial, ethnic, and economic categories in the US, but it is especially high in black, Hispanic, and poor communities, which comprise the majority of our HEU. Formula feeding, poor diet, and lack of physical activity also impact obesity. These factors, combined with potential additional effects of in utero ARV exposures, may elevate HEU children’s risk of cardiovascular disease in adulthood.
We expected that length and head circumference at age 2 years would be lower in HEU children exposed to tenofovir in utero, based on previous results at age one in SMARTT.12 However, we found no significant differences in these outcomes at 2 years, regardless of trimester of cART initiation. It is possible that TFV-exposed children caught up in length by age 2, as shown by Gibb et al.13 Alternatively, our results may differ due to study design as we limited analyses to women who initiated cART during the current pregnancy to decrease bias from combining prevalent and incident users.16 We also had limited power to detect small differences by TFV or other ARVs.
Among children of first-trimester cART-initiators, we found greater WTZ and WFLZ at age 2 years in those exposed to TFV+FTC vs. ZDV+3TC. Mean WTZ and WFLZ were greater than zero in both groups, but we cannot determine if TFV+FTC had a biological effect on growth or if ZDV+3TC might have mitigated expected weight gain.29 However, we observed no association of TFV+FTC with WTZ or WFLZ among children of second-trimester cART-initiators. The first trimester may be a vulnerable time for programming of later growth in HEU children, despite similarities in fetal growth. Fetal transfer of drug can be influenced by specific drug transporters 30 and the levels of drug transporters change during gestation. In addition, TFV and ZDV are transported on different receptors. It is possible that these factors may explain why we only saw differences in growth between TFV and ZDV only among those exposed during the first trimester. The placenta regulates nutrient transfer to the fetus. However, some nutrient transporters, such as equilibrative nucleoside transporter 1 (ENT1/SLC29A1), also transport ARVs such as ZDV. Thus, ZDV may competitively inhibit nutrient transfer to the fetus, potentially affecting growth outcomes.31,32
Among children of first trimester cART-initiators, TSFZ was lower in TFV+FTC vs. ZDV+3TC and in bPI vs. non-boosted PI exposed. Limb fat atrophy due to PIs has been described in children and adults.33 In contrast, switching from ZDV to TFV improved limb fat in adults.34 It is not known if or how ARVs may affect fat accumulation of HEU in-utero or during early childhood. The timing of these NRTI exposures may explain their differential effects by trimester.35,36
We do not know why women were prescribed their current or previous ARVs. Among women with available data, pre-cART CD4 and viral load did not differ by trimester of initiation, but first-trimester initiators were more likely to have been on ARV prior to this pregnancy. We do not know why mothers had a lapse in treatment and restarted after the pregnancy began. While we did not observe confounding by clinical markers or prior ARV use, unmeasured confounding could explain differences across trimester. Also, infants of women treated with ARV in the first trimester had longer exposure to ARV and were less likely to experience exposure to prolonged maternal viremia, but there was no difference in the percent with suppressed viral load in the third trimester. However, women may have experienced greater cytokine production in the first trimester as a result of ART initiation.37 However, it is not known whether increased inflammation during this critical time period could impact growth.
We evaluated the total effect of ARVs on growth outcomes, not distinguishing indirect and direct effects (e.g., through low birth weight and/or prematurity). Factors associated with prematurity were previously reported.38 There could also be survival bias due to early fetal loss related to specific ARVs. We did not collect dietary data and could not determine if certain dietary habits explained the higher than expected rate of obesity in HEU children. Similarly, we did not have a comparison group of HIV-unexposed two year olds of similar sociodemographic status to compare rates of obesity. We only had a group of HIV-unexposed three year olds as mentioned above. Additionally, our results may only apply to the US population of HEU.
The rate of childhood obesity has climbed dramatically in the US over the last 30 years with a concomitant and worrisome rise in risk factors for cardiovascular disease and diabetes. The differential associations of ARV exposures with growth that we found underscore the importance of determining if and how specific antenatal ARV exposures contribute to the risk of obesity and its long-term consequences so that specific ARVs perhaps can be avoided during critical periods in pregnancy.
Acknowledgments
Funding source: The Pediatric HIV/AIDS Cohort Study (PHACS) was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1).
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson).
Abbreviations
- 3TC
lamivudine
- 95%CI
95% confidence interval
- ARV
antiretroviral medication
- ATV
atazanavir
- bPI
boosted protease inhibitor
- BWTZ
birth weight z-score
- cART
combination antiretroviral therapy
- FTC
emtricitabine
- HEU
HIV-exposed uninfected
- HCZ
head circumference z-score
- LMP
last menstrual period
- LNZ
length z-score
- NFV
nelfinavir
- NNRTI
non-nucleoside reverse transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
protease inhibitor
- PMTCT
prevention of mother to child HIV transmission
- SD
standard deviation
- SMARTT
Surveillance Monitoring of ART Toxicities cohort
- TFV
tenofovir
- TSFZ
triceps skinfold z-score
- WFLZ
weight-for-length z-score
- WTZ
weight z-score
- ZDV
zidovudine
Appendix 1
Table 5.
Characteristics of mothers who started cART in the first trimester with TFV+FTC versus ZDV+3TC
| Characteristic | ZDV and 3TC (N=90) |
TFV and FTC (N=39) |
P Value |
|
|---|---|---|---|---|
| Maternal age (yr) | Median | 28.7 | 30.5 | 0.51 |
| (Q1, Q3) | (25.0, 33.9) | (24.4, 35.7) | ||
| Mother born on US mainland | 54 (60%) | 28 (72%) | 0.20 | |
| High school graduate | 57 (63%) | 25 (64%) | 0.93 | |
| Annual Income < 10K | 44 (49%) | 13 (33%) | 0.11 | |
| Pre-pregnancy BMI (kg/m2) | Median | 27.0 | 28.3 | 0.26 |
| (Q1, Q3) | (22.5, 34.6) | (26.0, 34.8) | ||
| Single (not living with partner/spouse) | 51 (57%) | 21 (54%) | 0.77 | |
| Race/ethnicity Mom/caregiver | Black Non-Hispanic | 49 (54%) | 25 (64%) | 0.60 |
| Hispanic | 33 (37%) | 10 (26%) | ||
| White Non-Hispanic | 7 (8%) | 4 (10%) | ||
| Multiracial/other/unknown | 1 (1%) | 0 (0%) | ||
| English only spoken at home | 53 (59%) | 30 (77%) | 0.05 | |
| Not currently working | 66 (73%) | 29 (74%) | 0.90 | |
| Illicit drugs - used in first trimester | 6 (7%) | 5 (13%) | 0.24 | |
| Alcohol - used in first trimester | 8 (9%) | 6 (16%) | 0.26 | |
| Tobacco - used in first trimester | 21 (24%) | 7 (18%) | 0.52 | |
| Clinical site by region | NY-NJ | 17 (19%) | 18 (46%) | <0.001 |
| West | 24 (27%) | 3 (8%) | ||
| South | 17 (19%) | 2 (5%) | ||
| Chicago | 14 (16%) | 4 (10%) | ||
| Florida | 6 (7%) | 12 (31%) | ||
| Puerto Rico | 12 (13%) | 0 (0%) | ||
| CD4 percent (%) before cART | Median | 27 | 23 | 0.15 |
| (Q1, Q3) | (22, 33) | (13., 33) | ||
| Log10 HIV RNA before cART | Median | 3.9 | 3.8 | 0.81 |
| (Q1, Q3) | (3.0, 4.2) | (3.5, 4.4) | ||
| CD4 percent (%) 3rd trimester | Median | 32 | 23 | 0.002 |
| (Q1, Q3) | (24, 38) | (14, 31) | ||
| Log10 RNA 3rd trimester | Median | 1.7 | 1.8 | 0.24 |
| (Q1, Q3) | (1.7, 1.9) | (1.7, 2.1) | ||
| Year of birth | 2005 | 4 (4%) | 0 (0%) | 0.02 |
| 2006 | 12 (13%) | 0 (0%) | ||
| 2007 | 18 (20%) | 4 (10%) | ||
| 2008 | 20 (22%) | 11 (28%) | ||
| 2009 | 19 (21%) | 7 (18%) | ||
| 2010 | 16 (18%) | 16 (41%) | ||
| 2011 | 1 (1%) | 1 (3%) | ||
| ARV use before this pregnancy | Yes | 29 (32%) | 24 (62%) | 0.008 |
| No | 19 (21%) | 5 (13%) | ||
| Unknown | 42 (47%) | 10 (26%) |
Abbreviations: ARV=antiretroviral therapy, BMI=body mass index, FTC=emtricitabine, TFV=tenofovir, ZDV=zidovudine, 3TC=lamivudine, cART= combination antiretroviral therapy, Q1=25th percentile, Q3=75th percentile
Table 6.
Characteristics of mothers who started cART in the second trimester with TFV+FTC versus ZDV+3TC
| Characteristic | ZDV and 3TC (N=245) |
TFV and FTC (N=69) |
P Value |
|
|---|---|---|---|---|
| Maternal age (yr) | Median (Q1, Q3) | 27.3 (23.1, 32.9) | 26.9 (23.8, 30.6) | 0.69 |
| Mother born on US mainland | 149 (62%) | 58 (84%) | < 0.001 | |
| High school graduate | 158 (66%) | 46 (67%) | 0.86 | |
| Annual Income < 10K | 104 (42%) | 32 (45%) | 0.17 | |
| Pre-pregnancy BMI (kg/m2) | Median (Q1, Q3) | 27.3 (23.8, 36.6) | 27.0 (23.9, 36.9) | 0.50 |
| Single (not living with partner/spouse) | 129 (53%) | 42 (61%) | 0.25 | |
| Race/ethnicity Mom/caregiver | Black Non-Hispanic | 125 (52%) | 48 (73%) | 0.002 |
| Hispanic | 91 (38%) | 9 (14%) | ||
| White Non-Hispanic | 17 (7%) | 8 (12%) | ||
| Multiracial/other/unknown | 7 (3%) | 1 (2%) | ||
| English only spoken at home | 149 (61%) | 57 (83%) | <0.001 | |
| Not currently working | 168 (69%) | 46 (67%) | 0.70 | |
| Illicit drugs - used in first trimester | 16 (7%) | 3 (5%) | 0.50 | |
| Alcohol - used in first trimester | 20 (9%) | 1 (2%) | 0.05 | |
| Tobacco - used in first trimester | 41 (18%) | 10 (15%) | 0.62 | |
| Clinical site by region | South | 47 (19%) | 37 (54%) | <0.001 |
| NY-NJ | 55 (22%) | 17 (25%) | ||
| Puerto Rico | 52 (21%) | 0 (0%) | ||
| Chicago | 32 (13%) | 4 (6%) | ||
| West | 34 (14%) | 2 (3%) | ||
| Florida | 23 (9%) | 8 (12%) | ||
| Mid-Atlantic | 2 (1%) | 1 (1%) | ||
| CD4 percent (%) before cART | Median (Q1, Q3) | 25 (18, 32) | 24.50 (20, 37) | 0.35 |
| Log10 HIV RNA before cART | Median (Q1, Q3) | 3.8 (3.2, 4.4) | 4.1 (3.1, 4.7) | 0.40 |
| CD4 percent (%) 3rd trimester | Q1, Q3 | 31 (23, 37) | 30 (23, 38) | 0.93 |
| Log RNA 3rd trimester | Median (Q1, Q3) | 1.9 (1.7, 2.4) | 1.9 (1.7, 2.3) | 0.98 |
| Year of birth | 2005 | 16 (7%) | 1 (1%) | 0.004 |
| 2006 | 33 (13%) | 3 (4%) | ||
| 2007 | 47 (19%) | 8 (12%) | ||
| 2008 | 51 (21%) | 19 (28%) | ||
| 2009 | 51 (21%) | 11 (16%) | ||
| 2010 | 41 (17%) | 23 (33%) | ||
| 2011 | 6 (2%) | 4 (6%) | ||
| Use of ARV before this pregnancy | Yes | 58 (24%) | 21 (30%) | 0.26 |
| No | 64 (26%) | 21 (30%) | ||
| Unknown | 123 (50%) | 27 (39%) |
Abbreviations: ARV=antiretroviral therapy, BMI=body mass index, FTC=emtricitabine, TFV=tenofovir, ZDV=zidovudine, 3TC=lamivudine, cART= combination antiretroviral therapy, Q1=25th percentile, Q3=75th percentile
Table 7.
Characteristics of mothers who started cART in the first trimester with boosted PI versus non-boosted PI
| Characteristic | PI not boosted (N=33) |
Boosted PI (N=97) |
P Value |
|
|---|---|---|---|---|
| Maternal age (yr) | Median (Q1, Q3) | 30.3 (24.0, 33.9) | 29.6 (25.7, 33.6) | 0.72 |
| Mother born on US mainland | 19 (58%) | 60 (62%) | 0.66 | |
| High school graduate | 18 (55%) | 66 (68%) | 0.16 | |
| Annual Income < 10K | 21 (64%) | 39 (40%) | 0.03 | |
| Pre-pregnancy BMI (kg/m2) | Median (Q1, Q3) | 27.5 (24.4, 36.2) | 26.6 (24.2, 32.9) | 0.53 |
| Single (not living with partner/spouse) | 20 (61%) | 55 (57%) | 0.69 | |
| Race/ethnicity Mom/caregiver | Black Non-Hispanic | 14 (42%) | 59 (61%) | 0.20 |
| Hispanic | 16 (48%) | 28 (29%) | ||
| White Non-Hispanic | 3 (9%) | 8 (8%) | ||
| Multiracial/other/unknown | 0 (0%) | 1 (1%) | ||
| English only spoken at home | English | |||
| Not currently working | 22 (67%) | 74 (76%) | 0.28 | |
| Illicit drugs - used in first trimester | 2 (6%) | 10 (10%) | 0.48 | |
| Alcohol drugs - used in first trimester | 1 (3%) | 14 (15%) | 0.08 | |
| Tobacco drugs - used in first trimester | 11 (34%) | 19 (20%) | 0.09 | |
| Clinical site by region | NY-NJ | 7 (21%) | 28 (29%) | 0.03 |
| West | 8 (24%) | 20 (21%) | ||
| Florida | 1 (3%) | 18 (19%) | ||
| South | 6 (18%) | 13 (13%) | ||
| Chicago | 4 (12%) | 13 (13%) | ||
| Puerto Rico | 7 (21%) | 5 (5%) | ||
| CD4 percent (%) before cART | Median (Q1, Q3) | 27 (24, 33) | 25 (16, 32) | 0.28 |
| Log10 HIV RNA before cART | Median (Q1, Q3) | 3.8 (3.3, 4.2) | 3.9 (3.2, 4.4) | 0.64 |
| CD4 percent (%) 3rd trimester | Median (Q1, Q3) | 32 (27, 36) | 26 (20, 38) | 0.15 |
| Log RNA 3rd trimester | Median (Q1, Q3) | 1.9 (1.7, 2.2) | 1.7 (1.7, 2.0) | 0.11 |
| Year of birth | 2005 | 3 (9%) | 1 (1%) | <0.001 |
| 2006 | 11 (33%) | 2 (2%) | ||
| 2007 | 9 (27%) | 13 (13%) | ||
| 2008 | 4 (12%) | 28 (29%) | ||
| 2009 | 2 (6%) | 24 (25%) | ||
| 2010 | 4 (12%) | 26 (27%) | ||
| 2011 | 0 (0%) | 3 (3%) | ||
| Use of ARV before this pregnancy | Yes | 9 (27%) | 43 (44%) | 0.07 |
| No | 5 (15%) | 20 (21%) | ||
| Unknown | 19 (58%) | 34 (35%) |
Abbreviations: ARV=antiretroviral therapy, BMI=body mass index, FTC=emtricitabine, TFV=tenofovir, ZDV=zidovudine, 3TC=lamivudine, cART= combination antiretroviral therapy, Q1=25th percentile, Q3=75th percentile
Table 8.
Characteristics of mothers who started cART in the second trimester with boosted PI versus non-boosted PI
| Characteristic | PI not boosted (N=74) |
Boosted PI (N=239) |
P Value |
|
|---|---|---|---|---|
| Maternal age (yr) | Median (Q1, Q3) | 27.9 (23.4, 33.4) | 27.1 (23.1, 32.1) | 0.61 |
| Mother born on US mainland | 33 (45%) | 167 (71%) | <0.001 | |
| High school graduate | 45 (61%) | 161 (69%) | 0.22 | |
| Annual Income <10K | <10K | 37 (50%) | 97 (41%) | 0.17 |
| Pre-pregnancy BMI (kg/m2) | Median (Q1, Q3) | 30.6 (25.4, 35.8) | 26.8 (23.3, 36.7) | 0.27 |
| Single (not living with partner/spouse) | 31 (42%) | 138 (58%) | 0.014 | |
| Race/ethnicity Mom/caregiver | Black Non-Hispanic | 27 (38%) | 142 (61%) | <0.001 |
| Hispanic | 42 (58%) | 63 (27%) | ||
| White Non-Hispanic | 3 (4%) | 20 (9%) | ||
| Multirace/other/unknown | 0 (0%) | 8 (3%) | ||
| English only spoken at home | 43 (58%) | 70 (30%) | <0.001 | |
| Not currently working | 47 (64%) | 167 (70%) | 0.26 | |
| Illicit drugs - used in first trimester | 7 (10%) | 11 (5%) | 0.11 | |
| Alcohol - used in first trimester | 5 (7%) | 15 (7%) | 0.86 | |
| Tobacco - used in first trimester | 13 (19%) | 38 (17%) | 0.70 | |
| Clinical site by region | NY-NJ | 18 (24%) | 60 (25%) | <0.001 |
| South | 8 (11%) | 69 (29%) | ||
| Puerto Rico | 28 (38%) | 24 (10%) | ||
| Chicago | 6 (8%) | 30 (13%) | ||
| West | 9 (12%) | 27 (11%) | ||
| Florida | 4 (5%) | 27 (11%) | ||
| Mid-Atlantic | 1 (1%) | 2 (1%) | ||
| CD4 percent before cART | Q1, Q3 | 29 (22, 35) | 24 (18, 33) | 0.03 |
| Log10 RNA before cART | Median (Q1, Q3) | 3.6 (3.1, 4.2) | 3.8 (3.2, 4.4) | 0.34 |
| CD4 3rd trimester | Median (Q1, Q3) | 33 (28, 38) | 29 (23, 38) | 0.07 |
| Log10 HIV RNA 3rd trimester | Median (Q1, Q3) | 1.9 (1.7, 2.6) | 1.9 (1.7, 2.3) | 0.03 |
| Year of birth | 2005 | 11 (15%) | 7 (3%) | <0.001 |
| 2006 | 18 (24%) | 19 (8%) | ||
| 2007 | 21 (28%) | 35 (15%) | ||
| 2008 | 7 (9%) | 62 (26%) | ||
| 2009 | 8 (11%) | 53 (22%) | ||
| 2010 | 6 (8%) | 56 (23%) | ||
| 2011 | 3 (4%) | 7 (3%) | ||
| Use of ARV before this pregnancy | Yes | 12 (16%) | 67 (28%) | 0.12 |
| No | 22 (30%) | 61 (26%) | ||
| Unknown | 40 (54%) | 111 (46%) |
Abbreviations: ARV=antiretroviral therapy, BMI=body mass index, FTC=emtricitabine, TFV=tenofovir, ZDV=zidovudine, 3TC=lamivudine, cART= combination antiretroviral therapy, PI= protease inhibitor, Q1=25th percentile, Q3=75th percentile
Footnotes
The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
Conflicts of Interest and Source of Funding: The authors have no conflicts of interest to disclose. This work was supported by the National Institutes of Health
Contributor’s Statement:
Denise L. Jacobson: Dr. Jacobson conceptualized and designed the study, analyzed and interpreted the data, drafted the article and approved the final manuscript as submitted.
Kunjal Patel: Dr. Patel conceptualized and designed the study, contributed to analysis and interpretation of the data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Paige L. Williams: Dr. Williams provided statistical support for analysis and interpretation of the data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Mitchell E. Geffner: Dr. Geffner contributed to the conception of the study, interpretation of the data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
George K. Siberry: Dr. Siberry contributed to the interpretation of the data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Linda A. DiMeglio: Dr. DiMeglio provided input on the design of the study, interpreted data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Marilyn Crain: Dr. Crain supervised collection of the data at one clinical site, provided input on the design of the study, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Ayesha Mirza: Dr. Mirza contributed to interpretation of data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Janet S. Chen: Dr. Chen contributed to interpretation of data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Elizabeth McFarland: Dr. McFarland supervised collection of the data at one site, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Deborah Kacanek: Dr. Kacanek contributed to study design, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Margarita Silio: Dr. Silio contributed to interpretation of the data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Kenneth Rich: Dr. Rich supervised collection of the data at one site, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
William Borkowsky: Dr. Borkowsky supervised collection of the data at one site, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
Tracie L. Miller: Dr. Miller conceptualized and designed the study, interpreted the data, reviewed and critically revised the manuscript, and approved the final manuscript as submitted.
The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2012, in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Anna Cintron; Children’s Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Doyle Patton, Deyana Leon; Ann & Robert H. Lurie Children’s Hospital of Chicago: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Helen Rozelman; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Hermann Mendez, Ava Dennie, Susan Bewley; Tulane University Health Sciences Center: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Newana Beatty, Julie Huldtquist; University of California, San Diego: Stephen Spector, Jean Manning, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Florida/Jacksonville: Mobeen Rathore, Kristi Stowers, Ann Usitalo; University of Illinois, Chicago: Kenneth Rich, Lourdes Richardson, Delmyra Turpin, Renee Smith; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Claudia Florez, Elizabeth Willen; University of Southern California: Toinette Frederick, Mariam Davtyan, Maribel Mejia; University of Puerto Rico Medical Center: Zoe Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
References
- 1.WHO. World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: toward universal access (2010 version) [PubMed] [Google Scholar]
- 2.Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146(5):R151–62. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jauniaux E, Nessmann C, Imbert MC, Meuris S, Puissant F, Hustin J. Morphological aspects of the placenta in HIV pregnancies. Placenta. 1988;9(6):633–42. doi: 10.1016/0143-4004(88)90007-0. [DOI] [PubMed] [Google Scholar]
- 4.Vermaak A, Theron GB, Schubert PT, et al. Morphologic changes in the placentas of HIV-positive women and their association with degree of immune suppression. Int J of Gynaecol Obs. 2012;119(3):239–43. doi: 10.1016/j.ijgo.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Ross AC, Leong T, Avery A, et al. Effects of in utero antiretroviral exposure on mitochondrial DNA levels, mitochondrial function and oxidative stress. HIV Med. 2012;13(2):98–106. doi: 10.1111/j.1468-1293.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker DJ, Fall CH. Fetal and infant origins of cardiovascular disease. Arch Dis Child. 1993;68(6):797–9. doi: 10.1136/adc.68.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ. 1999;318(7181):427–31. doi: 10.1136/bmj.318.7181.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newell ML, Borja MC, Peckham C, European Collaborative S. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111(1):e52–60. doi: 10.1542/peds.111.1.e52. [DOI] [PubMed] [Google Scholar]
- 9.Powis KM, Smeaton L, Ogwu A, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr. 2011;56(2):131–8. doi: 10.1097/QAI.0b013e3181ffa4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidari S, Mofenson L, Cotton MF, Marlink R, Cahn P, Katabira E. Antiretroviral drugs for preventing mother-to-child transmission of HIV: a review of potential effects on HIV-exposed but uninfected children. J Acquir Immune Defic Syndr. 2011;57(4):290–6. doi: 10.1097/QAI.0b013e318221c56a. [DOI] [PubMed] [Google Scholar]
- 11.Ransom CE, Huo Y, Patel K, et al. Infant growth outcomes after maternal tenofovir disoproxil fumarate use during pregnancy. J Acquir Immune Defic Syndr. 2013;64(4):374–81. doi: 10.1097/QAI.0b013e3182a7adb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siberry GK, Williams PL, Mendez H, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS. 2012;26(9):1151–9. doi: 10.1097/QAD.0b013e328352d135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibb DM, Kizito H, Russell EC, et al. Pregnancy and infant outcomes among HIV-infected women taking long-term ART with and without tenofovir in the DART trial. PLoS Med. 2012;9(5):e1001217. doi: 10.1371/journal.pmed.1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vigano A, Mora S, Giacomet V, et al. In utero exposure to tenofovir disoproxil fumarate does not impair growth and bone health in HIV-uninfected children born to HIV-infected mothers. Antivir Ther. 2011;16(8):1259–66. doi: 10.3851/IMP1909. [DOI] [PubMed] [Google Scholar]
- 15.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–20. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 16.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 2012;175(4):250–62. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jacobson DL, Patel K, Siberry GK, et al. Body fat distribution in perinatally HIV-infected and HIV-exposed but uninfected children in the era of highly active antiretroviral therapy: outcomes from the Pediatric HIV/AIDS Cohort Study. Am J Clin Nutr. 2011;94(6):1485–95. doi: 10.3945/ajcn.111.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Health and Nutrition Examination Survey: Anthropometry Procedures Manual. 2002 [Google Scholar]
- 19.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Advance data from vital and health statistics. 2000;314:1–27. [PubMed] [Google Scholar]
- 20.Fenton TR, Sauve RS. Using the LMS method to calculate z-scores for the Fenton preterm infant growth chart. Eur J Clin Nutr. 2007;61(12):1380–5. doi: 10.1038/sj.ejcn.1602667. [DOI] [PubMed] [Google Scholar]
- 21.Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346(24):1863–70. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 22.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–79. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams PL, Seage GR, 3rd, Van Dyke RB, et al. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. Am J Epidemiol. 2012;175(9):950–61. doi: 10.1093/aje/kwr401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 25.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. doi: 10.1097/EDE.0b013e318225c2be. [DOI] [PubMed] [Google Scholar]
- 26.Barker DJ. The origins of the developmental origins theory. J Int Med. 2007;261(5):412–7. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 27.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ong KK, Dunger DB. Birth weight, infant growth and insulin resistance. European J Endocrinol / European Federation of Endocrine Societies. 2004;151(Suppl 3):U131–9. doi: 10.1530/eje.0.151u131. [DOI] [PubMed] [Google Scholar]
- 29.Miller TL, Easley KA, Zhang W, et al. Maternal and infant factors associated with failure to thrive in children with vertically transmitted human immunodeficiency virus-1 infection: The prospective, P2C2 human immunodeficiency virus multicenter study. Pediatrics. 2001;108(6):1287–96. doi: 10.1542/peds.108.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahakangas K, Myllynen P. Drug transporters in the human blood-placental barrier. Brit J Pharmacol. 2009;158(3):665–78. doi: 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Govindarajan R, Bakken AH, Hudkins KL, et al. In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta. Am J Physiol Reg Integrative Comparative Physiol. 2007;293(5):R1809–22. doi: 10.1152/ajpregu.00293.2007. [DOI] [PubMed] [Google Scholar]
- 32.MacFarland A, Abramovich DR, Ewen SW, Pearson CK. Stage-specific distribution of P-glycoprotein in first-trimester and full-term human placenta. Histochem J. 1994;26(5):417–23. doi: 10.1007/BF00160054. [DOI] [PubMed] [Google Scholar]
- 33.Anuurad E, Bremer A, Berglund L. HIV protease inhibitors and obesity. Curr Opin Endocrinol Diabet Obes. 2010;17(5):478–85. doi: 10.1097/MED.0b013e32833dde87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher M, Moyle GJ, Shahmanesh M, et al. A randomized comparative trial of continued zidovudine/lamivudine or replacement with tenofovir disoproxil fumarate/emtricitabine in efavirenz-treated HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2009;51(5):562–8. doi: 10.1097/QAI.0b013e3181ae2eb9. [DOI] [PubMed] [Google Scholar]
- 35.Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2006;27(6–7):602–9. doi: 10.1016/j.placenta.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Meyer zu Schwabedissen HE, Jedlitschky G, Gratz M, et al. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metab Disposition. 2005;33(7):896–904. doi: 10.1124/dmd.104.003335. [DOI] [PubMed] [Google Scholar]
- 37.Fiore S, Newell ML, Trabattoni D, et al. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. J Reprod Immunol. 2006;70(1–2):143–50. doi: 10.1016/j.jri.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Watts DH, Williams PL, Kacanek D, et al. Combination antiretroviral use and preterm birth. J Infect Dis. 2013;207(4):612–21. doi: 10.1093/infdis/jis728. [DOI] [PMC free article] [PubMed] [Google Scholar]