Abstract
Parvovirus B19 infections are typically mild in healthy individuals, but can be life threatening in individuals with sickle cell disease (SCD). A Saccharomyces cerevisiae-derived B19 VLP vaccine, now in pre-clinical development, is immunogenic in wild type mice when administered with the adjuvant MF59®. Because SCD alters the immune response, we evaluated the efficacy of this vaccine in a mouse model for SCD. Vaccinated mice with SCD demonstrated similar binding and neutralizing antibody responses to those of heterozygous littermate controls following a prime-boost-boost regimen. Due to the lack of a mouse parvovirus B19 challenge model, we employed a natural mouse pathogen, Sendai virus, to evaluate SCD respiratory tract responses to infection. Normal mucosal and systemic antibody responses were observed in these mice. Results demonstrate that mice with SCD can respond to a VLP vaccine and to a respiratory virus challenge, encouraging rapid development of the B19 vaccine for patients with SCD.
Keywords: Parvovirus B19 vaccine, yeast-derived, intramuscular, sickle cell disease, systemic antibody, mucosal antibody response
INTRODUCTION
In parvovirus B19 infection, erythroid progenitor cells of the bone marrow are targeted, leading to their destruction. Brief interruption of red blood cell production does not produce significant anemia in normal persons due to the long survival of erythrocytes in circulation. However, in patients with sickle cell disease (SCD), parvovirus B19 infection causes a precipitous drop in hemoglobin concentration due to the short supply and abbreviated longevity of red blood cells (15–20 days versus the normal 3–4 months). The consequent disease outcome, transient aplastic crisis, can be life-threatening and frequently requires hospitalization and blood transfusions. Potential sequelae include stroke with permanent neurologic deficits, glomerulonephritis, and cardiac dysfunction [1–3].
Decades of research have been expended in the development of a vaccine candidate to protect children with SCD from parvovirus B19-induced transient aplastic crisis. Virus-like particle (VLP) vaccine candidates have been produced in baculovirus-infected insect cells, but significant local adverse reactions observed in clinical trials prevented product advancement [4, 5]. More recently, a yeast-based VLP was shown to be immunogenic in wild type mice when co-administered with the adjuvant MF59 [6]. This vaccine was produced by expression of viral proteins (VP) 1 and 2 from a single construct. The dual expression strategy improved control of the VP1/VP2 ratio in the VLP. The vaccine also included a point mutation in VP1 that eliminated its phospholipase A2 activity, a potential cause of the adverse reactions observed in earlier clinical trials.
In the study described here, rather than testing wild type mice, we employed a transgenic mouse model of SCD (Berkeley; BERK) to test the usefulness of the yeast-derived VLP vaccine candidate in a clinically relevant model. These animals express human α, βS, and γ globins, and have homozygous deletions of murine α and β globin genes [7, 8]. Control littermates express the same human genes, but retain a mouse β globin gene that rescues the wild type phenotype. Mice with SCD exhibit many of the major genetic, hematologic and histopathologic features of humans with SCD in that: 1) red blood cells are irreversibly sickled; 2) mice suffer from anemia and multi-organ pathology; 3) mice suffer from exaggerated inflammatory responses; and 4) mice are at a risk of invasive disease due to encapsulated bacterial infections [7–10].
Here we show that mice with SCD respond well to vaccination when parvovirus B19 VLPs are co-administered with MF59, and respond well to a viral challenge by the respiratory route, the typical point of entry for B19.
METHODS
Mice
All animal experiments were performed in accordance with the Institutional Animal Care and Use Committee protocols at St. Jude. Heterozygous BERK mice were obtained from a colony maintained at St. Jude and bred to produce homozygous mice with SCD. At 3 weeks of age, mice were bled, and SCD status was determined by both hemoglobin gel electrophoresis and complete blood count analysis. Heterozygous littermates were used as controls. Both female and male mice were used in the study.
Vaccination
Parvovirus B19 VLPs were generated from a yeast cell line that expresses VP1 and VP2 in a fixed ratio [6]. At 8–12 weeks of age, mice were vaccinated intramuscularly (i.m.) with phosphate-buffered saline [PBS] (negative control), 5 μg VLP + PBS, 0.5 μg VLP + MF59, or 5 μg VLP + MF59®. The mice were boosted twice, at 4 week intervals (for a total of 3 doses). Sera were collected by retro-orbital bleeding from unvaccinated and vaccinated mice at 2-week intervals following the first vaccination. One month following the final boost (3 months after the first vaccination), animals were sacrificed, and sera were collected as previously described [11].
Immune response to an intranasal virus challenge
Unfortunately, a parvovirus B19 challenge is not available in the mouse model. To examine the capacity of mice with SCD to respond to a pathogen in the respiratory tract (a common route for B19 infections), we utilized Sendai virus (SeV, strain Enders), a natural respiratory pathogen of mice. Specifically, mice were infected intranasally (i.n.) with 250 plaque forming units (pfu) SeV and sacrificed at either 1 or 4 months post-infection. Lungs, nasal washes (NW), bronchoalveolar lavages (BAL), and sera were collected and prepared for immune assays as previously described [12].
AFC and antibody measurements
B cells were isolated from the lungs of SeV-infected mice at 1 and 4 months post-infection [12]. SeV-specific IgG and IgA-producing antibody forming cells (AFCs) were detected by ELISPOT, and VLP-specific or SeV-specific binding antibodies were measured in sera, NW, and BAL by ELISA [13]. For both ELISPOTS and ELISAs, plates were coated with 0.1 μg/mL of VLP or 10 μg/mL disrupted SeV in PBS. ELISA titers were defined as the inverse serum dilution with an O.D. reading of 0.1 at 405 nm. O.D. readings for samples from naïve mice were generally below 0.1 at 405 nm, and hence titers were not detectable.
Neutralizing antibody assays against parvovirus were performed as previously described [4]. Briefly, serially diluted (1:10) sera were pre-incubated for 1 hour at room temperature with 2×106 genome equivalents of parvovirus B19 (strain V2), and then the mixture was added to 2×104 erythroid progenitor cells, propagated as previously described [14], for 2 hours in a 96-well plate. Cells were cultured for 3 days, after which RNA was extracted from the culture using a TurboCapture ®96 mRNA kit (Qiagen) and converted to cDNA using a MMLV-RT kit (ThermoFisher Scientific). A real time PCR assay was performed to detect parvovirus B19 NS and β-actin transcripts in a multiplex quantitative PCR using the PerfeCTa Multiplex qPCR Super Mix (Quanta Biosciences Inc.). Probes were custom manufactured by Integrated DNA Technologies. Probe and primer sequences used were: NS-1354 F (5′-GGGCAGCATGTGTTAAAGTGGA-3′), NS-1453 R (5′-TGGCCATTGCCAAGTTTGT-3′), NS-1413 probe (5TYE665-TTATGGGCCGCCAAGTACAGGAAA-31AbRQsp), β-actin F (5′-GGCACCCAGCACAATGAAG-3′), β-actin R (5′-GCCGATCCACACGGAGTACT-3′) and β-Actin probe (5MAX550-TCAAGATCATTGCTCCTCCTGAGCGC-3IABlk_FQ). The genome copies were determined by extrapolating from a standard curve generated from known quantities of target. To validate the number of infectious particles in the stock serum, 10-fold serial dilutions of virus stock V2 were used to infect cells, as described above, using a seronegative control sample for the pre-incubation. Titers were defined as the inverse of the highest serum dilution that reduced virus growth by at least 75%. Samples failing to neutralize virus growth 75% at the lowest dilution evaluated were assigned a titer of zero.
To perform neutralization assays against SeV, 10–100 TCID50 of virus per well was incubated in a 96-well plate with 1:2 serial serum dilutions (starting at a serum dilution of 1:40) for 1 hour at 37°C in humid air with 5% CO2. The mixtures were then inoculated into wells of 96-well plates in which confluent monolayers of LLC-MK2 cells had been grown. Wells with medium alone or virus without antibodies served as control wells. Following an overnight incubation at 37°C in humid air with 5% CO2, the inoculum was removed and replaced with medium (DMEM, 0.2% BSA, glutamine, and gentamicin) and acetylated trypsin (2 μg/ml) for further incubation. After 4 days, virus-infected cells were scored by ELISA. Briefly, supernatants were removed from wells and cells were fixed using 100 μl Acetone/DPBS (80%/20%). Cells were washed with DPBS and a mixture of mouse monoclonal anti-SeV antibodies was added for a 1 hour incubation at room temperature. Plates were washed with DPBS. Then, horse radish peroxidase-conjugated goat anti-mouse IgG was added for 1 hour. The plates were washed with DPBS and developed with TMB peroxidase. Reactions were stopped by adding 100 μl 4N H3PO4, and plates were read (OD 450 nm) on a microplate reader. The neutralization titer for a sample was the highest dilution that resulted in ≥50% test wells scoring at a level <50% compared to the average score for wells with virus and no antibodies.
Statistics were performed using GraphPad Prism. Specifically, differences amongst heterozygous littermate controls and mice with SCD, as well as differences between treatment groups, were evaluated by two-way Anova followed by Bonferroni’s correction for multiple tests.Fisher’s exact tests were used to evaluate differences in production of parvovirus-specific neutralizing antibodies amongst treatment groups. All experiments were repeated to ensure reproducibility, except for preliminary tests of SeV-specific NW and BAL antibodies, and SeV-specific serum neutralization titers.
RESULTS AND DISCUSSION
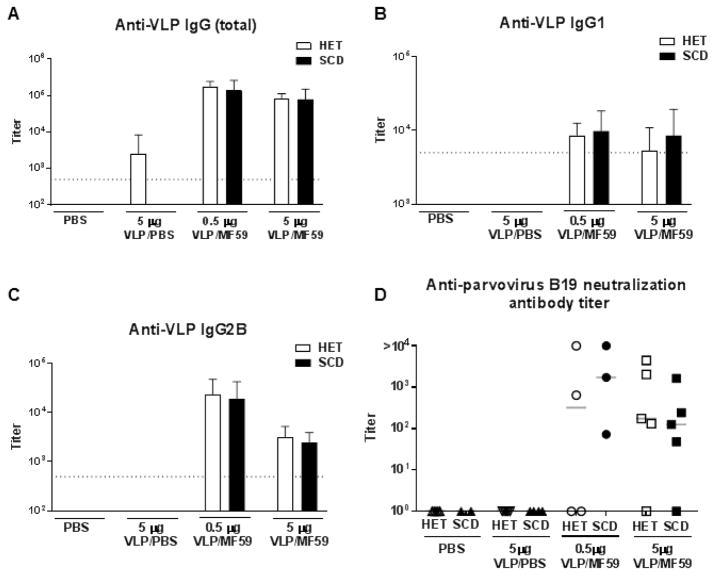
The BERK mouse model was employed to evaluate the immune response to vaccination with the parvovirus B19 VLP in the context of SCD. SCD and control mice were vaccinated intramuscularly (i.m.) with either PBS, 5 μg VLP in PBS, 0.5 μg VLP plus MF59, or 5 μg VLP plus MF59. Each animal received 3 doses (prime-boost-boost) of the appropriate preparation at 1 month intervals, after which animals were tested for the presence of VLP-specific antibodies in the blood. Figure 1 shows the results from mice examined at 3 months from the start of the experiment (1 month after the final boost). Notably, IgG serum responses were strongest following immunization with 0.5 or 5 μg VLP with MF59 (p<0.001 and p=0.02, respectively compared to 5 μg VLP in PBS), and were nearly indistinguishable between vaccinated SCD and control animals (p>0.5 for all isotypes examined). This was the case when we examined total parvovirus B19-specific IgG (Figure 1A) or when we examined IgG1 and IgG2B isotypes separately (Figures 1B and 1C, respectively). Parvovirus B19 neutralizing assays were also conducted with samples from animals that had received the full prime-boost-boost regimen. With this assay, functional antibodies were detectable in most animals that received VLP with MF59 (Figure 1D; p<0.005 and p<0.001 for 0.5 μg and 5 μg dose groups, respectively, compared to 5 μg VLP in PBS), similar to the previous experience with wild type mice [6]. No significant difference in production of neutralization antibodies was observed between vaccinated control and SCD groups (p>0.4). Consistent with previously published data [6], in the absence of adjuvant no neutralizing antibodies were detected in either control mice or mice with SCD.
Figure 1. Robust anti-parvovirus B19 antibody forming cells (AFCs) and antibody responses follow VLP with MF59 immunization of mice with SCD.
Mice were vaccinated 3 times at 1 month intervals with either PBS (negative control), 5 μg VLP in PBS, 0.5 μg VLP in MF59, or 5 μg VLP in MF59 by the i.m. route. Three months after the first immunization, animals were sacrificed, and sera were tested for parvovirus B19 VLP-specific (A) IgG (all subtypes), (B) IgG1, and (C) IgG2B (N=4–5 animals per group). Clear and black bars represent heterozygous littermate controls (HET) and animals with SCD, respectively. Titers shown represent the dilution of sera that would produce an OD 405 reading of 0.1 within each assay. (D) Residual sera, when available, were also tested for neutralizing antibodies. Individual heterozygous and SCD are shown as open and solid symbols, respectively. The numbers of mice tested for neutralization in each group were as follows: 5 HETs and 2 SCD for PBS only; 5 HETs and 4 SCD for VLP in PBS; 4 HETs and 3 SCD for 0.5 μg VLP in MF59; 5 HETs and 5 SCD for 5 μg VLP in MF59. Samples failing to neutralize virus growth by 75% at the lowest dilution evaluated were assigned a titer of 100. Dotted lines indicate the lowest dilution of sera evaluated.
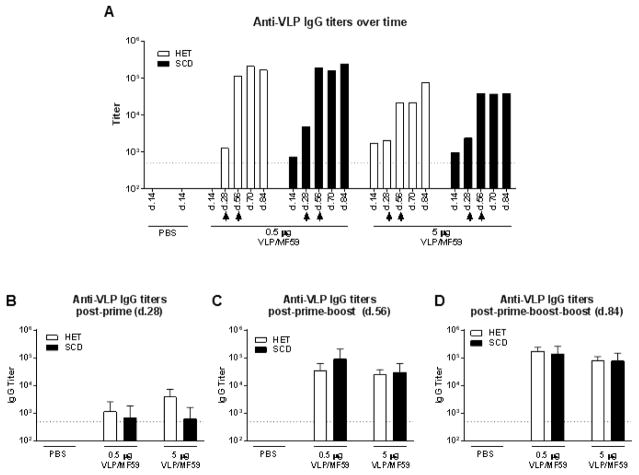
A kinetic analysis of parvovirus B19 VLP-specific serum antibodies was conducted throughout the course of repeated immunizations. Figure 2A shows results from a representative mouse for both heterozygous control and SCD groups receiving either 0.5 μg or 5 μg VLP with MF59 over the entire course of the experiment. Additionally, average serum antibody titers for each of these groups are shown at 1 month intervals following each immunization (Figures 2B–D). Parvovirus B19 VLP-specific responses were detectable as early as 2 weeks following the first immunization and increased most substantially after the first booster immunization. The 0.5 and 5 μg doses of VLP administered with MF59 were most immunogenic and produced similar antibody response profiles to one another as observed in Figure 1. Elevated anti-parvovirus B19 titers in the immunized mice persisted for at least 84 days after the first immunization (28 days after the final boost), the last time point tested (Figure 2D). There were no clear differences between SCD and control mice, either in the titer (p>0.5) or the persistence of the anti-VLP antibody immune response.
Figure 2. Durable anti-parvovirus B19 antibody responses in animals with SCD.
Mice were vaccinated 3 times at 1 month intervals with either 0.5 μg or 5 μg parvovirus VLP with MF59 by the i.m. route. Serum anti-parvovirus B19 VLP antibodies were examined throughout the course of the vaccination scheme for individual SCD (clear bars) and control (black bars) mice. (A) A single representative mouse is shown for each dose group. Results from day 14 sera from SCD and control mice given PBS only are shown to demonstrate background levels of the assay. Arrow heads indicate when mice received second and third vaccinations. Average serum anti-parvovirus B19 VLP antibody levels are shown for each group (N=4–5 mice per group) at 1 month following the first (B), second (C) and third (D) immunizations. A dotted line indicates the lowest dilution of sera evaluated.
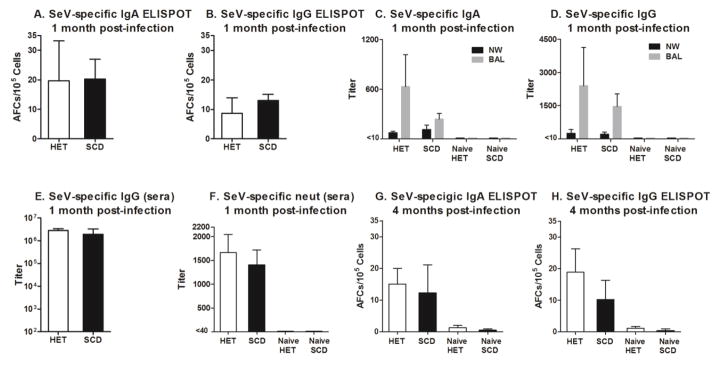
An appropriate parvovirus challenge comparable to the human B19 virus is not available for use in mouse models, leaving open the question of whether SCD and wild type mice have comparable antibody responses to a virus introduced in the respiratory tract, a common point of entry for parvovirus B19. This is a concern as sickled blood cells can reduce blood flow to respiratory tract tissues and patients with SCD often suffer from severe disease following a respiratory tract infection [15, 16]. To test immune responses of the respiratory tract, we administered Sendai virus (SeV), a natural mouse respiratory pathogen, i.n. to mice with SCD and control littermates. In healthy mice, antibody responses to SeV infection typically peak at one month after infection. Antibodies may then wane over many months but are sustained for the lifetime of the animals [13]. As demonstrated in Figure 3, we found that SCD and heterozygous control animals both responded well to SeV infection. One month after the i.n. infection, SeV-specific IgA and IgG AFCs were present in the lung (Figures 3A and 3B). SeV-specific IgA and IgG antibodies were also present in respiratory secretions (preliminary findings, Figures 3C and 3D show IgA and IgG levels in NW and BAL). Anti-SeV IgG antibodies were present in the sera at comparable levels in SCD and control mice (Figure 3B). Preliminary studies also showed that SeV-specific serum antibodies were neutralizing, demonstrating the protective potential of the response (Figure 3F). Responses were durable in both SCD and control mice, as SeV-specific IgA and IgG AFCs were still present in the respiratory tract four months after infection in the majority of animals (Figures 3G and 3H).
Figure 3. Acute and durable responses following an intranasal (i.n.) virus exposure in mice with SCD.
Mice were infected i.n. with Sendai virus (SeV), a natural mouse respiratory pathogen. Virus-specific IgA (A) and IgG (B) antibody forming cells (AFCs) from the lungs are shown for infected heterozygous littermate control mice (HET) and mice with SCD (N=4 per group) tested 1 month after SeV infection. Panels C and D: Nasal wash (NW) and bronchoalveolar lavages (BAL) from HET mice and mice with SCD (N=5 per group) were evaluated for SeV-specific antibody titers in a preliminary experiment. (E) Serum IgG antibody responses are shown for the same animals as in panel A. (F) Serum neutralization titers were tested in a preliminary experiment with samples from HET mice and mice with SCD (N=5 per test group; N=2–5 per naïve group). (G) IgA and (H) IgG AFCs from the lung were measured in HET and SCD mice (N=5 per group) 4 months after SeV infection. Results from naïve animals that received no vaccine (N=2 per group) are also shown.
Altogether, our results demonstrate that, despite the presence of SCD, mice responded both to a candidate parvovirus B19 VLP vaccine and to an intranasal Sendai virus challenge. The VLP-based vaccine was most effective in eliciting binding and neutralizing antibodies when co-delivered with the adjuvant MF59 in a prime-boost-boost regimen in both SCD and control animals. Comparable responses were observed when either 0.5 or 5 μg VLP were administered with MF59, indicating that both antigen doses had likely achieved the maximum dose-response for the VLP-adjuvant formulation. A 0.5 μg antigen dose with MF59 elicited a much higher antibody titer than a 5 μg antigen dose without MF59, indicating a dose-sparing effect of the adjuvant.
Our results in mice are consistent with the clinical data described by Long et al. [17], who showed that children with SCD respond well to an influenza virus vaccine. The animal model results are also consistent with our own observations that children with SCD respond well immunologically to parvovirus B19 antigens after a natural infection, and that parvovirus sero-prevalence is similar between children with SCD and their unaffected counterparts [18]. Importantly, we noted that IgG responses against parvovirus B19 persisted at least 13 years (the longest period of time evaluated in the study) in children with SCD; responses were not dampened by hydroxyurea, a common therapy for SCD that is known to cause myleosuppression [19]; and no child who was previously exposed to parvovirus B19 experienced a repeat occurrence of parvovirus-induced disease. All of these factors suggest that parvovirus B19 vaccination has the potential to confer durable protection against infection in patients with SCD [18]. In total, results encourage the rapid development and testing of a VLP candidate vaccine in individuals with SCD to protect against the morbidity and mortality caused by parvovirus B19 infections in this patient population.
HIGHLIGHTS.
Parvovirus B19 infection can be life threatening in patients with SCD
A B19 parvovirus VLP vaccine candidate is in pre-clinical development
Vaccinated mice with SCD responded with binding and neutralizing antibodies
Mice with SCD responded well to a respiratory virus challenge
Rapid clinical development of the B19 VLP vaccine for people with SCD is encouraged
Acknowledgments
We thank Dr. Jeffrey Ulmer (Head Preclinical R&D USA, GlaxoSmithKline) for manuscript review. Research was funded in part by the Children’s Infection Defense Center at St. Jude, NIH NCI P30 CA21765, the Intramural Research Program of the NIH, NHLBI, and the American Lebanese Syrian Associated Charities (ALSAC).
Abbreviations
- i.n
intranasal
- i.m
intramuscular
- VLP
virus-like particle
- SeV
Sendai virus
- NW
nasal wash
- BAL
bronchoalveolar lavage
- SCD
sickle cell disease
- HET mice-control
heterozygous mice
- DPBS
Dulbecco’s phosphate-buffered saline
Footnotes
Author Contributions: RRP, SLS, RES, PRD, ECS, SW, and JLH contributed to the initial study design. RRP, NSY, SLS, RES, JR, PRD, ECS, SC, SW, JSH, and JLH contributed to the acquisition of data, interpretation of data, drafting and revision of the article, and final approval of the submitted paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelleher JF, Jr, Luban NL, Cohen BJ, Mortimer PP. Human serum parvovirus as the cause of aplastic crisis in sickle cell disease. Am J Dis Child. 1984;138:401–3. doi: 10.1001/archpedi.1984.02140420067020. [DOI] [PubMed] [Google Scholar]
- 2.Rao SP, Miller ST, Cohen BJ. Transient aplastic crisis in patients with sickle cell disease. B19 parvovirus studies during a 7-year period. Am J Dis Child. 1992;146:1328–30. doi: 10.1001/archpedi.1992.02160230086025. [DOI] [PubMed] [Google Scholar]
- 3.Smith-Whitley K, Zhao H, Hodinka RL, Kwiatkowski J, Cecil R, Cecil T, et al. Epidemiology of human parvovirus B19 in children with sickle cell disease. Blood. 2004;103:422–7. doi: 10.1182/blood-2003-01-0069. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein DI, El Sahly HM, Keitel WA, Wolff M, Simone G, Segawa C, et al. Safety and immunogenicity of a candidate parvovirus B19 vaccine. Vaccine. 2011;29:7357–63. doi: 10.1016/j.vaccine.2011.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballou WR, Reed JL, Noble W, Young NS, Koenig S. Safety and immunogenicity of a recombinant parvovirus B19 vaccine formulated with MF59C.1. J InfectDis. 2003;187:675–8. doi: 10.1086/368382. [DOI] [PubMed] [Google Scholar]
- 6.Chandramouli S, Medina-Selby A, Coit D, Schaefer M, Spencer T, Brito LA, et al. Generation of a parvovirus B19 vaccine candidate. Vaccine. 2013;31:3872–8. doi: 10.1016/j.vaccine.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 7.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, et al. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278:876–8. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 8.Szczepanek SM, McNamara JT, Secor ER, Jr, Natarajan P, Guernsey LA, Miller LA, et al. Splenic morphological changes are accompanied by altered baseline immunity in a mouse model of sickle-cell disease. AmJ Pathol. 2012;181:1725–34. doi: 10.1016/j.ajpath.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andemariam B, Adami AJ, Singh A, McNamara JT, Secor ER, Guernsey LA, et al. The sickle cell mouse lung: proinflammatory and primed for allergic inflammation. Transl Res. 2015;166:254–68. doi: 10.1016/j.trsl.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosch JW, Boyd AR, Hinojosa E, Pestina T, Hu Y, Persons DA, et al. Statins protect against fulminant pneumococcal infection and cytolysin toxicity in a mouse model of sickle cell disease. J Clin Invest. 2010;120:627–35. doi: 10.1172/JCI39843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sealy R, Surman S, Hurwitz JL, Coleclough C. Antibody response to influenza infection of mice: different patterns for glycoprotein and nucleocapsid antigens. Immunology. 2003;108:431–9. doi: 10.1046/j.1365-2567.2003.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surman SL, Jones BG, Rudraraju R, Sealy RE, Hurwitz JL. Intranasal administration of retinyl palmitate with a respiratory virus vaccine corrects impaired mucosal IgA response in the vitamin A-deficient host. Clin Vaccine Immunol. 2014;21:598–601. doi: 10.1128/CVI.00757-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudraraju R, Surman S, Jones B, Sealy R, Woodland DL, Hurwitz JL. Phenotypes and functions of persistent Sendai virus-induced antibody forming cells and CD8+ T cells in diffuse nasal-associated lymphoid tissue typify lymphocyte responses of the gut. Virology. 2011;410:429–36. doi: 10.1016/j.virol.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong S, Zhi N, Filippone C, Keyvanfar K, Kajigaya S, Brown KE, et al. Ex vivo-generated CD36+ erythroid progenitors are highly permissive to human parvovirus B19 replication. J Virol. 2008;82:2470–6. doi: 10.1128/JVI.02247-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manci EA, Culberson DE, Yang YM, Gardner TM, Powell R, Haynes J, Jr, et al. Causes of death in sickle cell disease: an autopsy study. British journal of haematology. 2003;123:359–65. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- 16.Overturf GD. Infections and immunizations of children with sickle cell disease. Advances in pediatric infectious diseases. 1999;14:191–218. [PubMed] [Google Scholar]
- 17.Long CB, Ramos I, Rastogi D, Manwani D, Janow G, Del Rio M, et al. Humoral and cell-mediated immune responses to monovalent 2009 influenza A/H1N1 and seasonal trivalent influenza vaccines in high-risk children. J Pediatr. 2012;160:74–81. doi: 10.1016/j.jpeds.2011.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hankins JS, Penkert RR, Lavoie P, Tang L, Sun Y, Hurwitz JL. Original Research: Parvovirus B19 infection in children with sickle cell disease in the hydroxyurea era. Exp Biol Med (Maywood) 2016;241:749–54. doi: 10.1177/1535370216636723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lederman HM, Connolly MA, Kalpatthi R, Ware RE, Wang WC, Luchtman-Jones L, et al. Immunologic effects of hydroxyurea in sickle cell anemia. Pediatrics. 2014;134:686–95. doi: 10.1542/peds.2014-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]