Abstract
The function of Notch signaling was previously studied in two cnidarians, Hydra and Nematostella, representing the lineages Hydrozoa and Anthozoa, respectively. Using pharmacological inhibition in Hydra and a combination of pharmacological and genetic approaches in Nematostella, it was shown in both animals that Notch is required for tentacle morphogenesis and for late stages of stinging cell maturation. Surprisingly, a role for Notch in neural development, which is well documented in bilaterians, was evident in embryonic Nematostella but not in adult Hydra. Adult neurogenesis in the latter seemed to be unaffected by DAPT, a drug that inhibits Notch signaling. To address this apparent discrepancy we studied the role of Notch in Hydractinia echinata, an additional hydrozoan, in all life stages. Using CRISPR-Cas9 mediated mutagenesis, transgenesis, and pharmacological interference we show that Notch is dispensable for Hydractinia normal neurogenesis in all life stages but is required for the maturation of stinging cells and for tentacle morphogenesis. Our results are consistent with a conserved role for Notch in morphogenesis and nematogenesis across Cnidaria, and a lineage specific loss of Notch dependence in neurogenesis in hydrozoans.
Keywords: Neurogenesis, Cnidaria, morphogenesis, nematocyte
1. Introduction
Cnidarians (such as corals, sea anemones, hydroids and jellyfish) constitute the sister group to all bilaterian animals (Hejnol et al., 2009; Ryan et al., 2013; Technau and Steele, 2011) and are among the earliest diverging phyla possessing neurons (Galliot and Quiquand, 2011). The cnidarian nervous system consists of three main cell types: sensory neurons, ganglionic neurons, and a phylum-specific sensory/effector cell called a cnidocyte or nematocyte (also known as stinging cells); all of these are thought to develop from a common proliferative precursor population (Miljkovic-Licina et al., 2007; Richards and Rentzsch, 2014). Cnidarian and bilaterian nervous systems are considered homologous, having evolved from their common ancestors some 700 million years ago (Kelava et al., 2015).
Cnidarians employ all major cellular signaling systems known from bilaterians, but the roles many of these pathways play in cnidarian development are not well understood. Notch signaling has been studied in two cnidarians in several contexts but a clear consensus on its function is lacking. Research carried out on the sea anemone Nematostella, a member of the cnidarian clade Anthozoa, revealed that embryonic neural progenitors in these animals are epithelial and their numbers are controlled by Notch signaling (Layden and Martindale, 2014; Marlow et al., 2012; Richards and Rentzsch, 2014; Richards and Rentzsch, 2015), similar to their bilaterian counterparts. In hydrozoan cnidarians, however, neurons develop from non-epithelial, migratory stem cells, called i-cells, which are segregated during gastrulation (Gahan et al., 2016; Hager and David, 1997; Leclere et al., 2012; Miljkovic-Licina et al., 2007). Pharmacological inhibition of Notch signaling in adult Hydra, a hydrozoan cnidarian, revealed no effect on the numbers of adult neurons (Käsbauer et al., 2007; Khalturin et al., 2007), but this treatment did affect nematocyte differentiation and tentacle development (Käsbauer et al., 2007; Khalturin et al., 2007; Münder et al., 2013), defects that were also observed in Nematostella following pharmacological Notch inhibition (DuBuc et al., 2014; Fritz et al., 2013; Marlow et al., 2012). Hence, the role of Notch in tentacle morphogenesis and nematogenesis seems to be conserved in cnidarians, but its function in neural development requires further research across the phylum. In particular, a role for Notch in de novo adult and embryonic neurogenesis in hydrozoans has yet to be assessed (Rentzsch et al., 2016). Furthermore, genetic approaches have not been utilized in hydrozoan Notch studies.
To extend the knowledge on Notch signaling in cnidarians we focused on Hydractinia echinata, a hydrozoan that allows experimental access to all life stages (Flici et al., 2017; Gahan et al., 2016) (Fig. S1). To manipulate Notch signaling, we used genetic gain and loss of function, and pharmacological interference. We analyzed embryos, metamorphosing larvae, and regenerating adults. Our collective data show that Notch signaling is not required for the initial development of at least a subset of neural cells in embryos and adults. However, we confirm the role of Notch signaling in nematocyte differentiation and tentacle morphogenesis.
2. Materials and methods
2.1 Animals
Hydractinia echinata colonies were sampled in Galway Bay, Ireland, and in Roscoff, France. They were cultured in artificial seawater at 18–20°C under a 10/14 dark/light regime. Animals were fed three times a week with brine shrimp nauplii and twice with ground oyster. Daily spawned gametes from males and females were mixed to enable fertilization and embryos were allowed to develop to larva stages in plastic Petri dishes. Metamorphosis was induced using a pulse treatment with CsCl (Müller and Buchal, 1973). Metamorphosing animals were positioned on glass coverslips in seawater and allowed to metamorphose.
2.2 Pharmacological inhibition of Notch signaling
N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT; Abcam, ab120633) was diluted to a stock concentration of 10 mM or 0.1 mM in DMSO and then diluted 1:1000 in artificial seawater to give working solutions of 10 µM and 0.1 µM, respectively. 0.1% DMSO was used as a control in all experiments. Embryos were treated from the 1–2 cell stage until fixation. For metamorphosis experiments animals were first induced with CsCl and then placed in DAPT. Regenerating animals were placed in DAPT immediately post decapitation. In all cases the solution was changed every 24 hours.
2.3 Notch intracellular domain overexpression
In order to generate the Notch intracellular domain (NICD) overexpression construct, the NICD was amplified from cDNA using primers NICDfwd (5'ATGAAAAGGACCTATGGTGCCC3') and NICDrev (5'GCATTGAAATTATATAGTTCGTGCTACAAC3') and cloned into the β-tubulin::GFP plasmid using Gibson assembly to generate an NICD-GFP fusion separated by a linker region as used previously (Flici et al., 2017). Injections were carried out as previously described (Millane et al., 2011).
2.4 Staining of nematocysts
Nematocyst staining was carried out as outlined (Szczepanek et al., 2002). Animals were anesthetized, if necessary, for 30 minutes in 4% MgCl2 in 50% ASW/ 50% H20. Fixation was carried out for 30 minutes at room temperatures in 4% PFA in PBS supplemented with Triton X-100 to a final concentration of 0.3% and EDTA to a final concentration of 10 mM (PBSTE). Following fixation animals were washed three times in PBSTE and then stained overnight in 1:200 DAPI (10 mg/ml) in deionised water. After staining, animals were washed three times in PBSTE and mounted in Fluoromount (Sigma F4680). To combine with antibody staining the immunofluorescence (IF) protocol was carried out as below except EDTA was added to all solutions to a final concentration of 10 mM and DAPI staining was carried out after the secondary antibody.
2.5 mRNA in situ hybridization
Whole mount RNA in-situ hybridizations were carried out as previously published (Gajewski et al., 1996) with minor changes (see supplemental materials and methods).
2.6 Immunofluorescence
Prior to fixation adult stages were anesthetized in 4% MgCl2 in 50% ASW/ 50% H2O. Animals were fixed in 4% PFA in PBS for 20–60 minutes at room temperature or overnight at 4°C. This was followed by three washes for 10 minutes in PBSTx. For larva an additional wash overnight at 4°C was carried out. Animals were then blocked in 3% BSA in PBSTx for one hour at room temperature before primary antibody (anti acetylated tubulin, Sigma Aldrich T7451; Anti-FRamide (Seipp et al., 2010); Anti-GLWamide (Schmich et al., 1998)) incubation in 3% BSA in PBSTx overnight at 4°C. Animals were then washed four times in PBSTx for at least 10 minutes each. An additional overnight wash in PBSTx was carried out on larva. Following this, blocking was carried out in 5% goat serum, 3% BSA in PBSTx and then secondary antibody incubation (goat anti-mouse 594, Abcam 150116; goat anti-rabbit 488, Invitrogen A11008) was carried out at room temperature for two hours at a concentration of 1:500 in 5% goat serum, 3% BSA in PBSTx. Animals were then washed once for 20 minutes in 10 ng/µl Hoechst 33258 (25 mg/ml, Sigma B2883) in PBSTx followed by three washes with PBSTx. Animals were mounted in Fluoromount (Sigma F4680).
2.7 Microscopy and cell counting
Fluorescence microscopy was carried out using the Olympus BX51 compound microscope. Confocal microscopy was carried out on either an Olympus FV1000 with an inverted IX71 microscope or Andor revolution spinning disc confocal microscope (Yokagawa CSU22). Image analysis was carried out in all cases using ImageJ (Version 1.6.0_20).
Cell counting was carried out using ImageJ cell counting feature. All counting experiments were carried out in triplicate and at least 8 animals were counted per treatment. For GLWamide+ neurons, confocal z-stacks were counted manually, without projecting. Only the aboral domain of the larva was counted, as this is the site where the majority of the GLWamide+ neurons reside at this stage. For DAPI stained nematocytes images were taken at the surface of the larva. These were then manually counted. Statistical significance was assessed using two-tailed Student t-test if the data were normally distributed (tested by the Shapiro-Wilk test), and the Mann-Whitney U test was carried out otherwise. All data is shown +/− standard deviation and represent either individual biological replicates or pooled data from 3 replicates as indicated in the figure legends.
CRISPR-Cas9 mutagenesis
Four sgRNAs were generated targeting exons of the Notch locus found in both isoforms (Fig. 1C). These sgRNAs were injected into zygotes at a concentration of 125ng/ul each along with 1ug/ul Cas9 protein (PNAbio, CP02) and 1× NEB buffer 3 (NEB). Before injection the mixture was incubated for 10 minutes at 37°C.
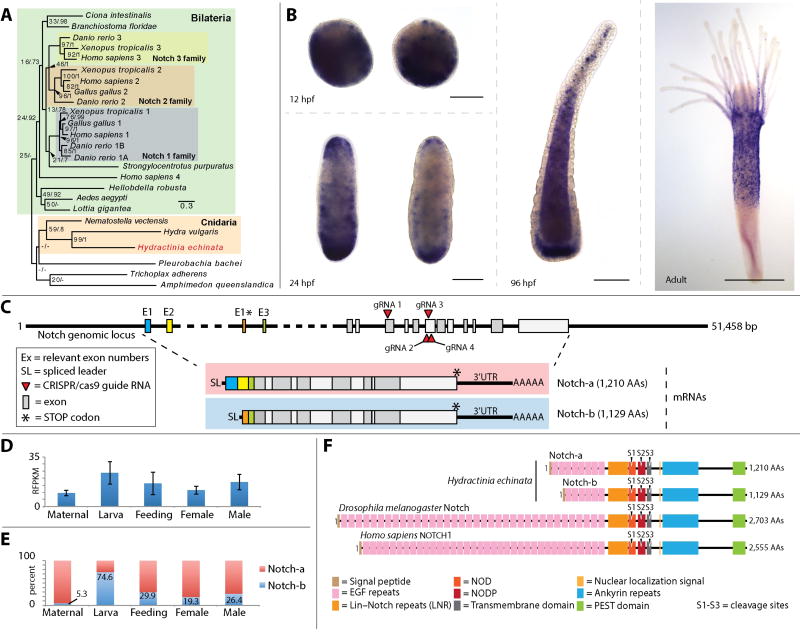
Figure 1. Notch phylogeny, expression, genomic architecture and alternative splicing.
(A) Unrooted maximum likelihood/Baysian phylogeny of Hydractinia echinata Notch; scale bar = nucleotide changes per site. (B) In-situ hybridization showing Notch mRNA expression patterns at 12, 24 and 96 hours post fertilization (hpf), and adult polyp; scale bar = 100 µm in embryos and larva, and 300 µm in the polyp. (C) Notch genomic locus showing CRISPR-Cas9 guide RNA sites, alternative exons and the complete architecture of the mRNA for the two notch isoforms. (D) Notch expression levels in Hydractinia eggs (maternal transcripts), larvae, feeding, and male and female polyps. (E) Ratio of expression of Notch isoforms in percent across the same life stages shown in (D). (F) Domain structure of Notch isoform 1 and isoform 2 alongside Drosophila Notch and Human Notch1.
Phylogenetic analysis
Phylogenetic analysis was performed using maximum likelihood and Bayesian inference as detailed in the supplemental materials and methods.
3. Results
3.1 Notch signaling components are encoded in the Hydractinia genome
We first investigated the presence of Notch pathway components in Hydractinia. We found that the Hydractinia genome encodes a single Notch gene (Fig. 1), several DSL (Delta/Serrate/LAG-2)-type Notch ligands, and a single member of the CSL (CBF1/Suppressor of Hairless/Lag-1) family of transcription factors. Notch displayed a dynamic expression pattern throughout the life cycle, as assessed by mRNA in situ hybridization and transcriptomic data (Fig. 1B, D, E). During embryogenesis, Notch appeared to be expressed in both the ectoderm and endoderm with higher expression towards the aboral pole, but was somewhat variable between individuals. In the adult polyp, Notch was expressed in the body column, excluding the most aboral section, and at the base of the tentacles (Fig 1B). This expression pattern is similar to HyNotch expression in Hydra (Münder et al., 2013; Wenger et al., 2016). We also found that Notch is alternatively spliced to generate two main isoforms that share all major Notch domains and differ only in the first two exons, which encode EGF repeats (Fig. 1C, F).
3.2 Notch signaling is not required for the normal development of the embryonic RFamide+ and GLWamide+ nervous systems
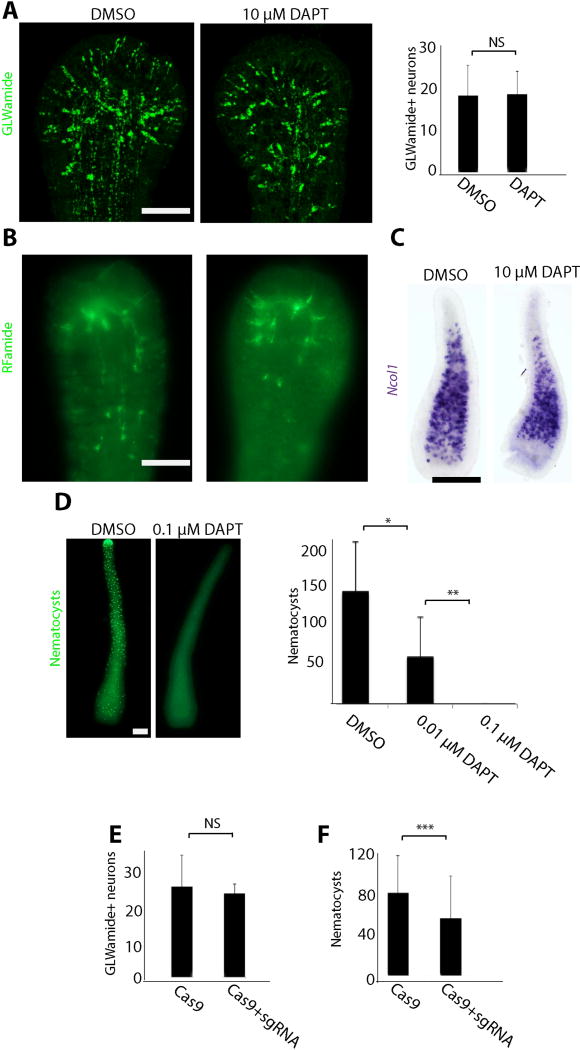
We then studied the function of Notch signaling, starting with embryonic neurogenesis. For this, we performed CRISPR-Cas9 mediated mutagenesis in Hydractinia to target its single Notch gene. We developed a strategy whereby we co-inject four sgRNAs with Cas9 protein. To test the efficiency of this approach, genomic DNA was extracted from two independent replicates of pooled, injected larvae. PCR was then carried out, covering the region targeted by all four sgRNAs using primers flanking the four predicted cut sites. Gel electrophoresis revealed extra bands at lower sizes than expected (Fig. S2A). Sanger sequencing has shown either a large deletion resulting from simultaneous cutting by two or more sgRNAs (band 2 in Fig. S2A) or a large resection after cutting with a single sgRNA (band 1 in Fig. S2A). The upper band, i.e. the band at the expected wild type size, was excised, extracted, cloned, and sequenced, revealing the presence of mutations within that region (Fig. S2B). Analysis of multiple clones was used to evaluate the percentage of mutated alleles. Overall, the normal sized alleles were mutated at one or more sites in about 70% of the cases (Fig. S2C) and all scored mutations resulted in frameshifts. Hence, combined with the lower bands, which represented alleles with larger deletions, the rate of mutated alleles was well over 70% in the injected F0 animals using this strategy. We also used a pharmacological approach, by treating developing embryos with the γ-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT); this drug prevents release of the Notch intracellular domain (NICD), an essential step in activation of the pathway (Geling et al., 2002; Käsbauer et al., 2007; Micchelli et al., 2003). We then monitored the development of the nervous system in these embryos using markers of early and late neurogenesis. We also stained poly-γ-glutamate+ nematocyst capsules as markers for mature nematocytes (Szczepanek et al., 2002). Overall, genetic or pharmacological inhibition of Notch signaling had no effect on RFamide+ and GLWamide+ neurons but did reduce the numbers of mature nematocytes (Fig. 2), a phenomenon also known from other cnidarians (Käsbauer et al., 2007; Khalturin et al., 2007; Marlow et al., 2012; Richards and Rentzsch, 2015). A detailed analysis of DAPT treated embryos revealed normal numbers and distribution of GLWamide+ and RFamide+ neurons, as well as Ncol1+ nematoblasts (i.e. committed, immature nematocytes: Fig. 2A–C) in planula larvae at all concentrations tested, but mature nematocyte numbers were drastically reduced in a dose dependent manner (Fig. 2D). Importantly, genetic inhibition of Notch signaling did not compromise the animals' development to metamorphosis competence - a stage that, in Hydractinia, requires GLWamide+ neurons (Schmich et al., 1998). Together, these experiments show that Notch signaling is not involved in Hydractinia embryonic neurogenesis, at least with respect to RFamide+ and GLWamide+ neurons. We also conclude that Notch signaling plays a role in late nematogenesis, which has also been shown in other cnidarians (Käsbauer et al., 2007; Marlow et al., 2012); in Hydractinia this role is limited to later differentiation stages but does not include the control of their early commitment. The absence of a neurogenic effect (i.e. excessive neurons) following Notch inhibition in Hydractinia embryos, is markedly different from earlier Notch inhibition experiments on bilaterian and anthozoan cnidarian embryos that result in an increase in the numbers of neural cells (Chitnis and Kintner, 1996; Hartenstein and Stollewerk, 2015; Henrique et al., 1997; Louvi and Artavanis-Tsakonas, 2006; Richards and Rentzsch, 2015).
Figure 2. Notch signaling is not involved in embryonic neural commitment.
(A) Pharmacological inhibition of γ-secretase by DAPT during embryogenesis does not affect the pattern and numbers of GLWamide+ neurons in larvae. (B,C) DAPT treatment during embryogenesis does not affect the pattern of RFamide+ neurons (B) or the expression pattern of Ncol1 (C) in larvae. (D) γ-secretase inhibition leads to a dose dependent loss of mature poly-γ-glutamate positive nematocysts in larvae. (E, F) CRISPR-Cas9 mediated mutagenesis of Notch does not affect the number of GLWamide+ neurons (E) but causes a reduction in the numbers of poly-γ-glutamate+ nematocysts (F). Scale bars: 10 µm in (A, B and D) and 40 µm in (C). Data represents combined counting from 3 independent biological replicates. * Student t-test with p<0.05. ** Student t-test with p<0.0001. *** Mann-Whitney U-test with p<0.05.
3.3 Notch signaling is not involved in development or regeneration of the adult nervous system
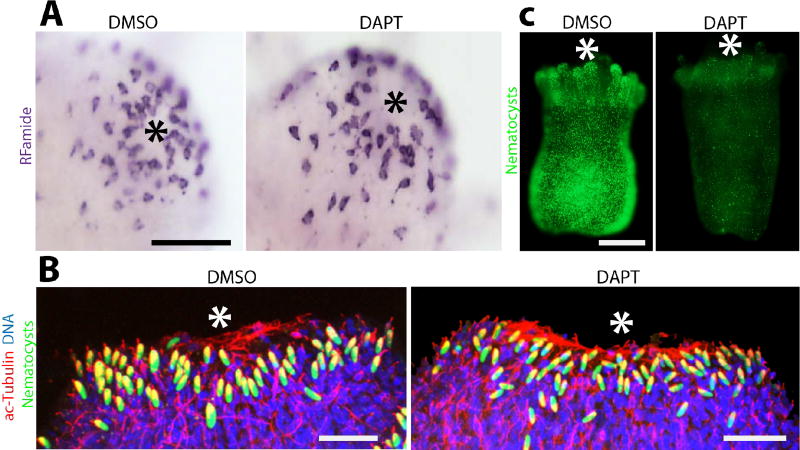
We then investigated the effect of Notch signaling inhibition on the formation of the adult nervous system during metamorphosis, a process that transforms a planula larva into a polyp (Fig. S1). Pharmacological inhibition of Notch signaling during the course of metamorphosis resulted in polyps with normal Rfamide+ oral nervous systems (Fig. 3A). We next studied the effect of inhibition of Notch signaling on adult neurogenesis during regeneration. Hydractinia can regenerate a fully functional head, including its nervous system, 2–3 days post-amputation (Bradshaw et al., 2015). Hence, we treated decapitated animals with DAPT, allowed them to regenerate and stained with anti-acetylated tubulin antibodies to mark the nervous system. We found no gross difference in the regenerated, acetylated tubulin positive nervous system between DAPT treated and control animals (Fig. 3B). Finally, we analyzed the effect of DAPT treatment on adult nematogenesis by treating adult polyps with DAPT while feeding them to deplete existing mature nematocytes. This led to a reduction in the number of mature nematocytes (Fig. 3C). Therefore, Hydractinia neurogenesis in embryos and adults is similar with regard to Notch function.
Figure 3. Notch signaling is does not play a role in adult neural commitment.
(A) Pharmacological inhibition of γ-secretase by DAPT during metamorphosis does not affect the pattern of RFamide+ neurons in the oral nerve net. (B) γ-secretase inhibition following decapitation does not affect the regeneration of the oral nervous system (C) DAPT treatment over 5 days with daily feeding causes a reduction in the numbers of poly-γ-glutamate+ nematocysts. Asterisk marks the oral pole in all images. Scale bars: 50 µm in (A), 10 µm in (B), 250 µm in (C).
3.4 Notch signaling is required for tentacle morphogenesis
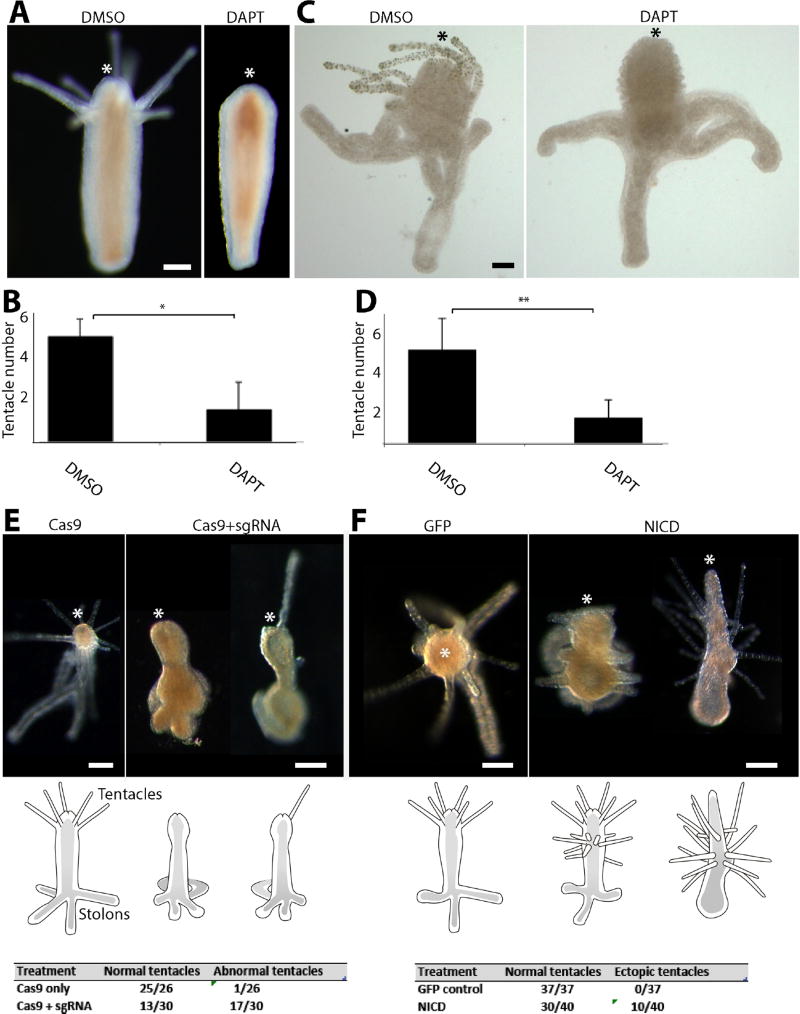
Next, we studied the role of Notch signaling in morphogenesis. Notch signaling is frequently used in metazoan development to generate sharp boundaries between domains (Bray, 2006). Indeed, in Hydra it was shown to have a role in boundary formation during budding (Münder et al., 2010). DAPT treatment during regeneration led to a block in tentacle formation (Fig. 4A and B), a phenotype also known from other cnidarians (DuBuc et al., 2014; Fritz et al., 2013; Marlow et al., 2012; Münder et al., 2013). However, as shown above, the acetylated tubulin positive oral nervous system in regenerating, Notch inhibited polyps was normal (Fig 3B). Pharmacological or genetic inhibition of Notch signaling during metamorphosis led to a striking reduction in the number of formed tentacles without affecting stolon outgrowth or hypostome development (i.e. the region between the tentacles and the mouth; Fig 4C–E). This is consistent with results obtained in other cnidarians.
Figure 4. Notch signaling is required for tentacle formation.
(A, B) DAPT treatment following decapitation inhibits tentacle but not hypostome regeneration. (C, D) DAPT treatment during metamorphosis blocks tentacle development but has no effect on hypostome, body column or stolon formation. (E) CRISPR-Cas9 mediated mutagenesis results in defective tentacle patterning during metamorphosis. (F) Ectopic expression of NICD leads to the development of ectopic tentacles. Scale bars: 250 µm in (A), 40 µm in (C) 100 µm in (E) and (F). Asterisk marks the oral pole in all images. * Student t-test with p<0.0001. ** Student t-test with p<0.000001.
Finally, we studied the effect of ectopic Notch activation on Hydractinia development. For this, we ectopically expressed NICD in Hydractinia embryos using random integration transgenesis, which produced transgenic mosaic animals (Fig. S3). These animals developed normally to metamorphosis-competent planula larvae and were then induced to metamorphose. Consistent with the Notch inhibition experiments described above, following metamorphosis induction they developed multiple ectopic tentacles (Fig. 4F), phenotypes never seen in control transgenic animals that expressed a GFP transgene driven by the same promoter (Fig. 4F).
Discussion
Taken together, these results show that Notch signaling in Hydractinia is required for tentacle morphogenesis and for late stage nematocyte differentiation, but is dispensable for the development and normal distribution of Rfamide+ and GLWamide+ neurons. Given that Notch signaling is generally considered to be a negative regulator of neurogenesis (Hartenstein and Stollewerk, 2015), the lack of a neurogenic effect, i.e. increase in neural cells following Notch inhibition, is striking. Hence, our data not only confirm previous results on adult neurogenesis in Hydra, but also extend the understanding of Notch signaling in hydrozoans to adult regeneration, metamorphosis, and embryonic development.
The evolution of Notch signaling as a negative regulator of neurogenesis is debatable, being either a primitive or a derived trait. According to current literature, this function of Notch is present in nearly all studied bilaterians (Hartenstein and Stollewerk, 2015) and in anthozoan cnidarians (Layden and Martindale, 2014; Marlow et al., 2012; Richards and Rentzsch, 2015). Our data, and those of previous authors working on Hydra (Käsbauer et al., 2007; Khalturin et al., 2007), suggest that it is absent in hydrozoans. Given the phylogenetic position of the Anthozoa as a basal cnidarian clade (Bridge et al., 1992; Zapata et al., 2015), with Hydrozoa considered derived, a scenario of a primitive Notch function in neurogenesis inhibition in eumetazoans is parsimonious (Fig. 5). Hence, the absence of this trait in Hydra and Hydractinia reflects a specific loss in the hydrozoan lineage rather than multiple acquisitions in other lineages. Of note, early neurogenesis in the annelid Platynereis is Notch independent (Gazave et al., 2017), like in hydrozoans, and could represent an additional, independent loss. Analysis of the role of Notch signaling in other cnidarian clades and in the basal bilaterian phylum, Xenacoelomorpha, would help resolve this issue.
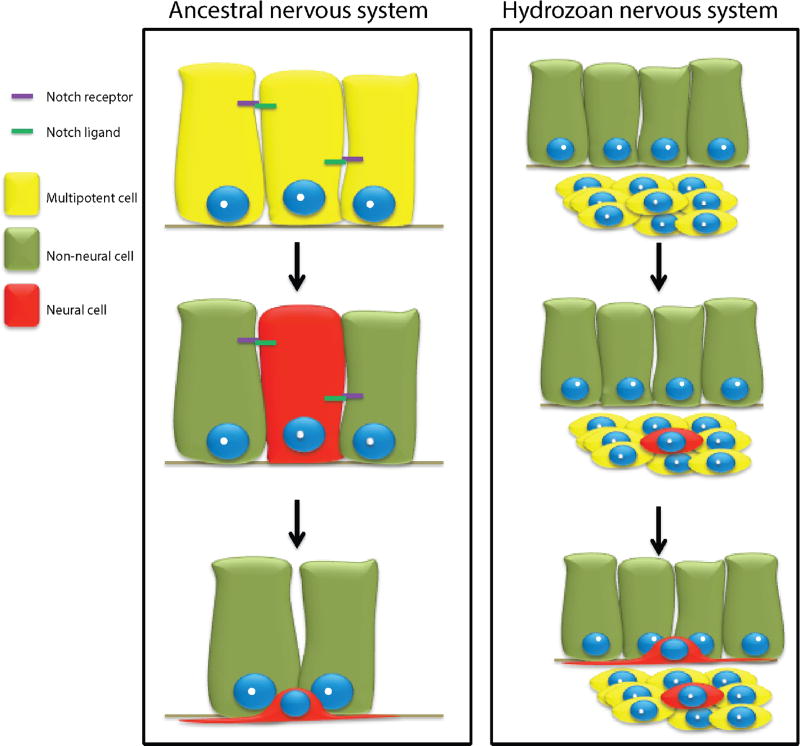
Figure 5. A hypothetical scenario for the evolution of neurogenesis in hydrozoans.
Ancestral neural progenitors were segregated from epithelial cells via Notch mediated lateral inhibition. In the hydrozoans ancestor, neural progenitors became Notch independent through a yet unknown, mechanism.
Other than most studied animals, anthozoan cnidarians included, whose neuronal progenitors are epithelial (Hartenstein and Stollewerk, 2015; Richards and Rentzsch, 2014), hydrozoan neural cells develop from migratory, non-epithelial stem cells, called i-cells that also give rise to non-neural cell types (Gahan et al., 2016). Furthermore, neurogenesis occurs in both the ectoderm and endoderm in Nematostella. Hydrozoan i-cells are first found in the endoderm, and embryonic neurogenesis occurs within this tissue (Flici et al., 2017; Martin, 1990), also pointing to a derived mode of neurogenesis in hydrozoans. Co-occurrence of the i-cell lineage, exclusively endodermal embryonic neurogenesis, and the loss of Notch mediated neurogenesis inhibition in hydrozoans are consistent with a link between these phenomena.
Supplementary Material
Acknowledgments
We thank members of our lab for comments and discussions. We are indebted to Werner A Müller for drawings in Fig. 3. Thanks are also due to the staff of the Station Biologique Roscoff, France for providing Hydractinia echinata colonies. We also thank Thomas Leitz for providing GLWamide antibodies. This research was funded by Science Foundation Ireland (SFI) through a Principal Investigator Award to UF (grant No. 11/PI/1020). It was also supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health (NIH). JMG was a recipient of a Beckman Fund Scholarship. KT was funded by the Irish Higher Education Authority, Programme for Research in Third Level Institutions. SGG was funded by a Starting Investigator Research Grant (SIRG) from SFI and by a Marie Curie Incoming International Fellow award. TQD was funded by an EMBO Long Term Fellowship.
Footnotes
Author contributions
JMG and UF conceived the study; JMG performed the experiments; CES, and ADB provided genomics data; TQD performed Notch in situ hybridization; LBD, SGG, and SB performed transcriptome analysis; JK provided gene expression data, PS provided transcriptomic data on Notch isoforms; KT performed spinning disk confocal microscopy; JMG and UF wrote the paper.
References
- Bradshaw B, Thompson K, Frank U. Distinct mechanisms underlie oral vs aboral regeneration in the cnidarian Hydractinia echinata. eLife. 2015:4. doi: 10.7554/eLife.05506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- Bridge D, Cunningham CW, Schierwater B, DeSalle R, Buss LW. Class-level relationships in the phylum Cnidaria: Evidence from mitochondrial genome structure. PNAS. 1992;89:8750–8753. doi: 10.1073/pnas.89.18.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis A, Kintner C. Sensitivity of proneural genes to lateral inhibition affects the pattern of primary neurons in Xenopus embryos. Development. 1996;122:2295–2301. doi: 10.1242/dev.122.7.2295. [DOI] [PubMed] [Google Scholar]
- DuBuc TQ, Traylor-Knowles N, Martindale MQ. Initiating a regenerative response; cellular and molecular features of wound healing in the cnidarian Nematostella vectensis. BMC biology. 2014;12:24. doi: 10.1186/1741-7007-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flici H, Schnitker N, Millane RC, Govinden G, Houlihan A, Boomkamp SD, Shen S, Baxevanis AD, Frank U. An evolutionarily conserved SoxB-Hdac2 crosstalk regulates neurogenesis in a cnidarian. Cell reports. 2017;18:1395–1409. doi: 10.1016/j.celrep.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz AE, Ikmi A, Seidel C, Paulson A, Gibson MC. Mechanisms of tentacle morphogenesis in the sea anemone Nematostella vectensis. Development. 2013;140:2212–2223. doi: 10.1242/dev.088260. [DOI] [PubMed] [Google Scholar]
- Gahan JM, Bradshaw B, Flici H, Frank U. The interstitial stem cells in Hydractinia and their role in regeneration. Curr Opin Genet Dev. 2016;40:65–73. doi: 10.1016/j.gde.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Gajewski M, Leitz T, Schloherr JR, Plickert GN. LWamides from Cnidaria constitute a novel family of neuropeptides with morphogenetic activity. Roux’s Arch Dev Biol. 1996:205. doi: 10.1007/BF00365801. [DOI] [PubMed] [Google Scholar]
- Galliot B, Quiquand M. A two-step process in the emergence of neurogenesis. Eur J Neurosci. 2011;34:847–862. doi: 10.1111/j.1460-9568.2011.07829.x. [DOI] [PubMed] [Google Scholar]
- Gazave E, Lemaitre QI, Balavoine G. The Notch pathway in the annelid Platynereis: insights into chaetogenesis and neurogenesis processes. Open Biol. 2017:7. doi: 10.1098/rsob.160242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A γ-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G, David CN. Pattern of differentiated nerve cells in hydra is determined by precursor migration. Development. 1997;124:569–576. doi: 10.1242/dev.124.2.569. [DOI] [PubMed] [Google Scholar]
- Hartenstein V, Stollewerk A. The Evolution of Early Neurogenesis. Dev Cell. 2015;32:390–407. doi: 10.1016/j.devcel.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejnol A, Obst M, Stamatakis A, Ott M, Rouse GW, Edgecombe GD, Martinez P, Baguna J, Bailly X, Jondelius U, Wiens M, Muller WE, Seaver E, Wheeler WC, Martindale MQ, Giribet G, Dunn CW. Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc Biol Sci. 2009;276:4261–4270. doi: 10.1098/rspb.2009.0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrique D, Hirsinger E, Adam J, Le Roux I, Pourquie O, Ish-Horowicz D, Lewis J. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol. 1997;7:661–670. doi: 10.1016/s0960-9822(06)00293-4. [DOI] [PubMed] [Google Scholar]
- Käsbauer T, Towb P, Alexandrova O, David CN, Dall'Armi E, Staudigl A, Stiening B, Böttger A. The Notch signaling pathway in the cnidarian Hydra. Developmental Biology. 2007;303:376–390. doi: 10.1016/j.ydbio.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Kelava I, Rentzsch F, Technau U. Evolution of eumetazoan nervous systems: insights from cnidarians. Philos Trans R Soc Lond B Biol Sci. 2015:370. doi: 10.1098/rstb.2015.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalturin K, Anton-Erxleben F, Milde S, Plotz C, Wittlieb J, Hemmrich G, Bosch TCG. Transgenic stem cells in Hydra reveal an early evolutionary origin for key elements controlling self-renewal and differentiation. Dev Biol. 2007;309:32–44. doi: 10.1016/j.ydbio.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Layden MJ, Martindale MQ. Non-canonical Notch signaling represents an ancestral mechanism to regulate neural differentiation. Evodevo. 2014;5:30. doi: 10.1186/2041-9139-5-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclere L, Jager M, Barreau C, Chang P, Le Guyader H, Manuel M, Houliston E. Maternally localized germ plasm mRNAs and germ cell/stem cell formation in the cnidarian Clytia. Dev Biol. 2012;364:236–248. doi: 10.1016/j.ydbio.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Marlow H, Roettinger E, Boekhout M, Martindale MQ. Functional roles of Notch signaling in the cnidarian Nematostella vectensis. Developmental Biology. 2012;362:295–308. doi: 10.1016/j.ydbio.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin VJ. Development of Nerve Cells in Hydrozoan Planulae: III. Some Interstitial Cells Traverse the Ganglionic Pathway in the Endoderm. Biol Bull. 1990;178:10–20. doi: 10.2307/1541532. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Esler WP, Kimberly WT, Jack C, Berezovska O, Kornilova A, Hyman BT, Perrimon N, Wolfe MS. Gamma-secretase/presenilin inhibitors for Alzheimer's disease phenocopy Notch mutations in Drosophila. Faseb j. 2003;17:79–81. doi: 10.1096/fj.02-0394fje. [DOI] [PubMed] [Google Scholar]
- Miljkovic-Licina M, Chera S, Ghila L, Galliot B. Head regeneration in wild-type hydra requires de novo neurogenesis. Development. 2007;134:1191–1201. doi: 10.1242/dev.02804. [DOI] [PubMed] [Google Scholar]
- Millane RC, Kanska J, Duffy DJ, Seoighe C, Cunningham S, Plickert G, Frank U. Induced stem cell neoplasia in a cnidarian by ectopic expression of a POU domain transcription factor. Development. 2011;138:2429–2439. doi: 10.1242/dev.064931. [DOI] [PubMed] [Google Scholar]
- Müller WA, Buchal G. Metamorphoseinduktion bei Planulalarven. II. Induktion durch monovalente Kationen: Die Bedeutung des Gibbs-Donnan Verhältnisses und der Ka+Na+-ATPase. Wilhelm Roux Arch. 1973;173:122–135. doi: 10.1007/BF00575138. [DOI] [PubMed] [Google Scholar]
- Münder S, Käsbauer T, Prexl A, Aufschnaiter R, Zhang X, Towb P, Böttger A. Notch signalling defines critical boundary during budding in Hydra. Developmental Biology. 2010;344:331–345. doi: 10.1016/j.ydbio.2010.05.517. [DOI] [PubMed] [Google Scholar]
- Münder S, Tischer S, Grundhuber M, Buchels N, Bruckmeier N, Eckert S, Seefeldt CA, Prexl A, Kasbauer T, Bottger A. Notch-signalling is required for head regeneration and tentacle patterning in Hydra. Dev Biol. 2013;383:146–157. doi: 10.1016/j.ydbio.2013.08.022. [DOI] [PubMed] [Google Scholar]
- Rentzsch F, Layden M, Manuel M. The cellular and molecular basis of cnidarian neurogenesis. Wiley Interdisciplinary Reviews: Developmental Biology. 2016 doi: 10.1002/wdev.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards GS, Rentzsch F. Transgenic analysis of a SoxB gene reveals neural progenitor cells in the cnidarian Nematostella vectensis. Development. 2014 doi: 10.1242/dev.112029. [DOI] [PubMed] [Google Scholar]
- Richards GS, Rentzsch F. Regulation of Nematostella neural progenitors by SoxB, Notch and bHLH genes. Development. 2015;142:3332–3342. doi: 10.1242/dev.123745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JF, Pang K, Schnitzler CE, Nguyen AD, Moreland RT, Simmons DK, Koch BJ, Francis WR, Havlak P, Smith SA, Putnam NH, Haddock SHD, Dunn CW, Wolfsberg TG, Mullikin JC, Martindale MQ, Baxevanis AD. The Genome of the Ctenophore Mnemiopsis leidyi and Its Implications for Cell Type Evolution. Science. 2013;342:1242592–1242592. doi: 10.1126/science.1242592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmich J, Trepel S, Leitz T. The role of GLWamides in metamorphosis of Hydractinia echinata. Dev Genes Evol. 1998;208:267–273. doi: 10.1007/s004270050181. [DOI] [PubMed] [Google Scholar]
- Seipp S, Schmich J, Will B, Schetter E, Plickert G, Leitz T. Neuronal cell death during metamorphosis of Hydractina echinata (Cnidaria, Hydrozoa) Invert Neurosci. 2010;23:23. doi: 10.1007/s10158-010-0109-7. [DOI] [PubMed] [Google Scholar]
- Szczepanek S, Cikala M, David CN. Poly-{gamma}-glutamate synthesis during formation of nematocyst capsules in Hydra. J Cell Sci. 2002;115:745–751. doi: 10.1242/jcs.115.4.745. [DOI] [PubMed] [Google Scholar]
- Technau U, Steele RE. Evolutionary crossroads in developmental biology: Cnidaria. Development. 2011;138:1447–1458. doi: 10.1242/dev.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger Y, Buzgariu W, Galliot B. Loss of neurogenesis in Hydra leads to compensatory regulation of neurogenic and neurotransmission genes in epithelial cells. Philos Trans R Soc Lond B Biol Sci. 2016:371. doi: 10.1098/rstb.2015.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata F, Goetz FE, Smith SA, Howison M, Siebert S, Church SH, Sanders SM, Ames CL, McFadden CS, France SC, Daly M, Collins AG, Haddock SHD, Dunn CW, Cartwright P. Phylogenomic Analyses Support Traditional Relationships within Cnidaria. Plos One. 2015;10:e0139068. doi: 10.1371/journal.pone.0139068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.