Abstract
Objective
Gynecologic Oncology Group (GOG) 177 demonstrated that addition of paclitaxel to a backbone of adriamycin/cisplatin improves overall survival (OS) and progression-free survival (PFS) for patients with advanced or recurrent endometrial cancer. Using patient specimens from GOG-177, our objective was to identify potential mechanisms underlying the improved clinical response to taxanes. Stathmin (STMN1) is a recognized poor prognostic marker in endometrial cancer that functions as a microtubule depolymerizing protein, allowing cells to transit rapidly through mitosis. Therefore, we hypothesized that one possible mechanism underlying the beneficial effects of paclitaxel could be to counter the impact of stathmin.
Methods
We analyzed the expression of stathmin by immunohistochemistry (IHC) in 69 specimens from patients enrolled on GOG-177. We also determined the correlation between stathmin mRNA expression and clinical outcomes in The Cancer Genome Atlas (TCGA) dataset for endometrial cancer.
Results
We first established that stathmin expression was significantly associated with shorter PFS and OS for all analyzed cases in both GOG-177 and TCGA. However, subgroup analysis from GOG-177 revealed that high stathmin correlated with poor PFS and OS particularly in patients who received adriamycin/cisplatin only. In contrast, there was no statistically significant association between stathmin expression and OS or PFS in patients treated with paclitaxel/adriamycin/cisplatin.
Conclusions
Our findings demonstrate that high stathmin expression is a poor prognostic marker in endometrial cancer. Paclitaxel may help to negate the impact of stathmin overexpression when treating high risk endometrial cancer cases.
Keywords: endometrial cancer, paclitaxel, stathmin
Introduction
Endometrial cancer is the most common gynecologic malignancy in women [1]. Early stage disease can be treated surgically, with some cases receiving radiation treatment to decrease local and regional recurrence. However, for recurrent disease or cases diagnosed at an advanced stage, chemotherapy plays a crucial role in management.
GOG-177 was a phase III randomized controlled trial that enrolled patients with advanced (stage III or IV) or recurrent endometrial cancer response between two treatment arms: adriamycin (doxorubicin)/cisplatin (AP) vs. adriamycin/cisplatin/paclitaxel with G-CSF (TAP). Results from this trial showed that patients treated with the three-drug regimen TAP had a better overall response rate (57% vs 34%), median progression-free survival (PFS) (8.3 vs. 5.3 months), and median overall survival (OS) (15.3% vs 12.3%) [1]. The TAP regimen, however, was associated with a 27% and 12% incidence of grade 2 and 3 peripheral neuropathy, respectively [2]. Following this trial, the GOG performed a phase III non-inferiority trial (GOG-209), which evaluated the differences in survival and toxicity between the triple regimen (TAP) vs. paclitaxel/carboplatin (TC). The interim analysis showed that the PFS and OS achieved on TC are not inferior to TAP, with TC showing a better toxicity profile [3]. The outcomes of GOG-177 and GOG-209 suggest that while TAP and TC are both active regimens for advanced or recurrent disease, TC is now preferred due to the lower toxicity. Therefore, GOG-177 and GOG-209 have defined the modern therapeutic treatment for advanced and recurrent endometrial cancer.
Using patient specimens from GOG-177, our objective in this study was to identify biomarkers of clinical response, and to determine biological differences between tumors that respond to AP and those that respond better with the addition of paclitaxel (TAP). One molecule of particular interest as a biomarker of poor outcome is stathmin. Stathmin expression has been widely analyzed in endometrial tumors and is known to be part of the machinery required for rapid cell transit through mitosis [4]. In addition, stathmin expression has been shown to negatively correlate with response to chemotherapeutic regimens that contain paclitaxel, an association that has been observed in multiple cancer types [5-11]. As paclitaxel is a microtubule stabilizing agent that prevents successful completion of mitosis [12], i.e., a potential molecular antagonist to stathmin, we hypothesized that it could counteract the impact of high stathmin expression in high risk cases.
Materials And Methods
Human Subjects
Formalin-fixed, paraffin- embedded (FFPE) tissue slides were received from patients who were enrolled on the GOG-177 protocol. Out of the 263 eligible patients in the protocol, specimens were available for 86 patients. A total of 69 patients had adequate quantity of available slides to evaluate for IHC. Among the 4 patients alive at last contact, the minimum follow-up was 5 years; the others were followed between 8 and 9 years. All studies were approved by the University of Iowa Institutional Review Board (Approval #200907769: GOG Core Laboratory).
Immunohistochemistry
IHC was performed on slides from GOG-177. FFPE tumor tissue sections from GOG-177 were evaluated for expression of stathmin (#3352, Cell Signaling, Danvers, MA), via IHC per the manufacturer's recommendations and as shown by other investigators [5,13]. A western blot analysis using 8 different cell lines and IHC utilizing positive and negative control slides were performed to confirm specificity of the antibody. Staining was independently quantified by three investigators blinded to sample identity using a modified H-scoring system that is calculated by multiplying the percentage of tissue with positive staining (0-100%) by the intensity of staining (0-4) as previously described [14].
The Cancer Genome Atlas (TCGA)
Using 333 identified endometrial cancer patients from the TCGA database (271 endometrioid, and 62 serous), we examined the relationship of STMN1 (the gene that codes for the protein stathmin) gene expression with OS and PFS. Of the 333 patients, 102 received chemotherapy (N=55 paclitaxel-containing chemotherapy; N=52 no paclitaxel). The remaining 229 patients did not receive chemotherapy. The mean follow-up time was 33.8 months. Dataset extraction of clinicopathologic parameters (age, weight, tumor grade, invasion, histology, stage, residual tumor, lymph node status, total pelvic, and total aortic lymph nodes), risk group stratification, and STMN1 gene expression were performed.
“High risk” patients were defined as those at risk of having extrauterine disease and most likely needing adjuvant treatment after surgery. Specifically, all patients presenting with stage II, III and IV as defined by 2009 FIGO classification (and sanctioned in 2014) [15], and patients with initial stage I and high-intermediate risk features by GOG 99 criteria [16] were classified as high risk. High-intermediate features of stage I included three risks factors: 2 or 3 tumor grade, presence of lymphovascular invasion, and outer-third myometrial invasion, with the following criteria: 1) at least 70 years of age with only one of the risk factors, 2) at least 50 years of age with any two of the other risk factors, or 3) any age with all three of the other risk factors. “Low risk” patients were the remaining stage I patients, either with no myometrial invasion or low-intermediate risk features by GOG 99 criteria [16]. STMN1 gene expression was divided into quartiles and data analyzed as ≤50% vs. >50% or ≤75% vs. >75%.
Statistical Analysis
Protein expression of stathmin was evaluated by IHC in 69 patients from GOG-177 and dichotomized using modified H-score results of >50, >75, or >100 as the cut-off. Mean age was compared via a two-sided Wilcoxon rank sum test. Tumor grade, tumor stage (as defined by 1988 FIGO classification [17]), and tumor type were compared via a Chi-squared test. Serous vs. non-serous and endometrioid vs. non-endometrioid types were compared via two-sided Fisher's tests. For the TCGA data, multi- and univariate analysis were performed via Cox's proportional hazards ratio. STMN1 gene expression was dichotomized (High or Low) using either the 50th percentile or 75th percentile of the expression as the cut-off. The associations of STMN1 expression with PFS and OS were evaluated in patients with endometrial cancer, adjusted for all clinicopathologic parameters of interest, using the Cox proportional Hazard Ratio, and Kaplan-Meier survival curves were plotted when significant. PFS and OS analysis was also further stratified according to histologic type (serous or endometrioid). Significance was assessed using log rank tests. For all statistical tests, a level of <0.05 was considered statistically significant.
Results
Stathmin overexpression predicts for poorer overall survival and progression-free survival
We first performed a retrospective analysis of FFPE tumors from 69 patients enrolled on GOG-177. Patient characteristics are presented in Table 1. Representative images of stathmin expression by IHC are provided in Figure 1. Stathmin expression was significantly associated with PFS and OS (Figure 2A, B). However, stathmin was significantly associated with PFS and OS in arm 1 AP (Figure 2C, D) but not in arm 2 TAP (Figure 2E, F).
Table 1. Demographics and clinicopathological features of patients on GOG177 and association with stathmin expression.
Stathmin was dichotomized into low (≤50 H-score) or high (>50 H-score) by IHC (N=69). Mean age is compared via a two-sided Wilcoxon rank sum test. Tumor grade, tumor stage, and tumor type are compared via a Chi-squared test, except for serous vs. non-serous and endometrioid vs. non-endometrioid that were compared via two-sided Fisher's tests. Overall survival and progression free survival were compared with a log rank test.
| Characteristic | Low Stathmin (n=62) | High Stathmin (n=7) | Association with stathmin (P-value) |
|---|---|---|---|
|
| |||
| Age at Study Entry (Mean, SD) | 62.45,10.25 | 60.83,6.63 | P=0.5183 |
|
| |||
| Tumor Grade (Counts) | P=0.2230 | ||
| 1 | 14 | 0 | |
| 2 | 21 | 5 | |
| 3 | 25 | 2 | |
| N/A | 2 | 0 | |
|
| |||
| Tumor Stage (Counts) | P=0.7861 | ||
| 3 | 6 | 0 | |
| 3C | 3 | 0 | |
| 4 | 14 | 1 | |
| 4B | 6 | 1 | |
|
| |||
| Recurrent | 33 | 5 | |
| Tumor Type (Counts) | P=0.9274 | ||
| Adenocarcinoma | 1 | 0 | |
| Clear Cell | 3 | 0 | |
| Endometrioid | 46 | 6 | |
| Mixed Epithelial | 5 | 0 | |
| Undifferentiated | 1 | 0 | |
| Serous | 6 | 1 | |
|
| |||
| Tumor Type (Counts) | P=0.5442 | ||
| Non-Serous | 56 | 6 | |
| Serous | 6 | 1 | |
|
| |||
| Tumor Type (Counts) | P=0.6742 | ||
| Endometrioid | 46 | 6 | |
| Non-Endometrioid | 16 | 1 | |
|
| |||
| Median PFS (Months) | 8.07 | 2.79 | P=0.0470 |
| Median OS (Months) | 15.10 | 10.28 | P=0.0096 |
Figure 1. Representative images of stathmin staining by IHC.
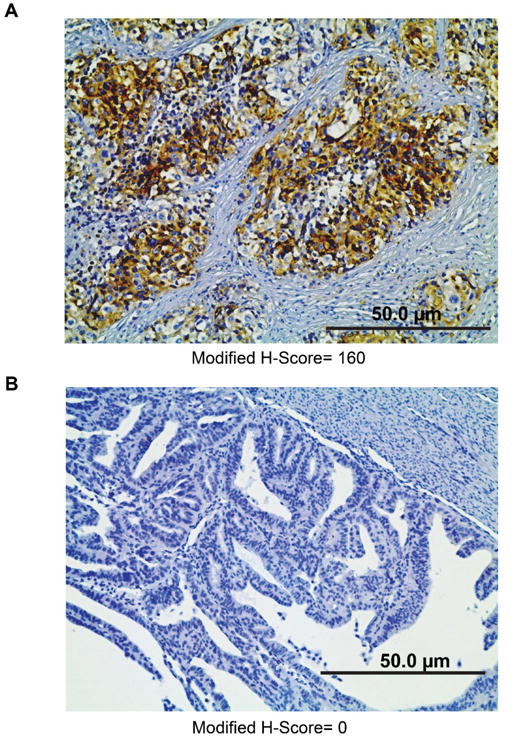
High modified H-score (A) and low modified H-score (B). Images were acquired at 200X magnification.
Figure 2. Stathmin expression is associated with progression-free survival and overall survival in GOG177; addition of paclitaxel blunts the impact of stathmin overexpression.
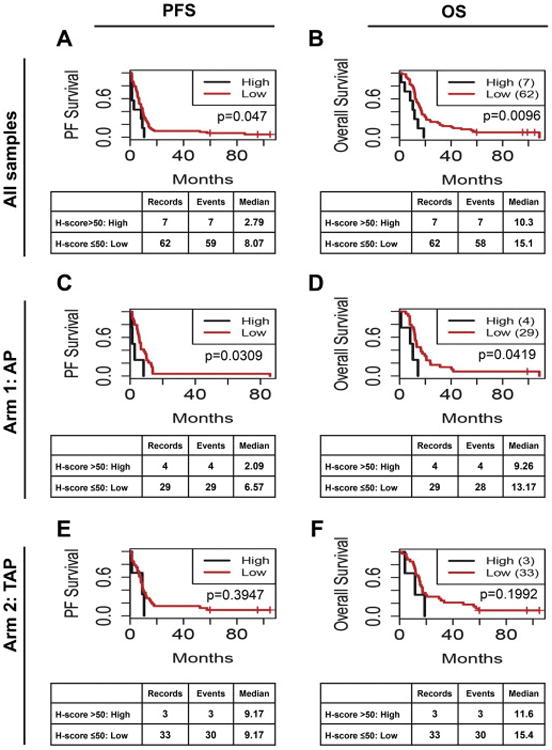
Kaplan-Meier analysis of the association of stathmin with PFS (A, C, E) and OS (B, D, F) for all samples regardless of treatment (A, B), or for only Arm 1 cisplatin/adriamycin (AP, C, D) or only Arm 2 cisplatin/adriamycin/paclitaxel (TAP, E, F).
Relationship between stathmin expression and histologic subtype
We next examined whether there was a relationship between stathmin expression and histologic type in GOG-177 samples. However, there was no significant association (P=0.5442) observed when tumors were dichotomized into serous and non-serous types nor when tumors were dichotomized into endometrioid and non-endometrioid types (P=0.6724, Table 1). Similarly, stathmin expression was not associated with age at study entry, tumor grade, or stage. Nevertheless, we extended this analysis to a larger dataset from TCGA.
Elevated expression of stathmin portends poor outcomes in TCGA dataset
Using RNA-seq data from all 333 endometrial cancer patients in TCGA dataset for endometrial cancer [18], we confirmed that high stathmin mRNA levels are associated with shorter PFS in both multi- and univariate analysis (Figure 3A), even after adjusting for tumor grade, myometrial invasion, FIGO stage, lymph node status, and residual tumor after surgery. Specifically, stathmin mRNA expression at >50th percentile significantly correlated with poorer 5-year PFS of 62.5% (P=0.0485) as compared to cases with low expression (5-year PFS of 82.5%). Stathmin expression at the >75th percentile also was associated with poorer OS of 75% vs. 89% for patients with stathmin mRNA levels <75th percentile by univariate analysis (Figure 3B). In the multivariate analysis, only FIGO stage and myometrial invasion, but not stathmin expression, were independently associated with OS (P<0.01).
Figure 3. High stathmin mRNA levels predicts for poor PFS and OS in TCGA dataset for endometrial cancer.
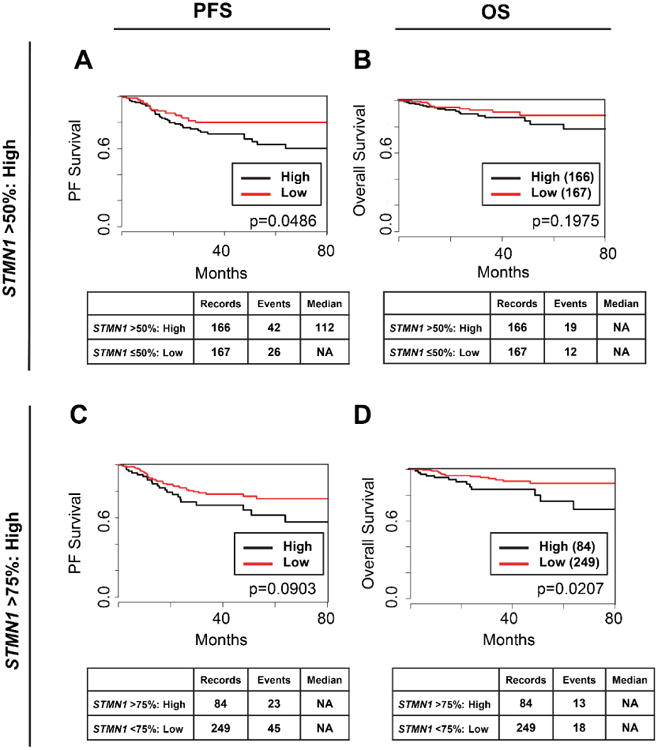
Kaplan-Meier analysis of the association of stathmin mRNA levels (STMN1) with PFS (A, C) or OS (B, D). STMN1 was dichotomized into low and high categories using a cut-off of either >50% (A, B) or >75% (C, D). NA= not achieved, the number of observed events (deaths) did not reach threshold to calculate median survival; Median= median survival, the amount of time (months) wherein 50% of the patients are alive.
We next stratified patients in TCGA dataset by histologic type and found that high stathmin mRNA levels were associated with worse PFS in serous tumors by multivariate analysis (Figure 4A). Specifically, stathmin expression >75th percentile in the serous group showed significantly worse 5-year PFS of 22% compared to those with low stathmin expression (5-year PFS of 67%). Stathmin expression was not significantly associated with PFS in the endometrioid group (P=0.0811), yet we did detect a similar trend for stathmin expression >50th percentile linked to worse PFS, with a 5-year PFS of 70% for those with high expression and 85% for those with low expression (Figure 4B).
Figure 4. High stathmin mRNA expression is associated significantly decreased PFS for endometrial patients with the serous histologic subtype.
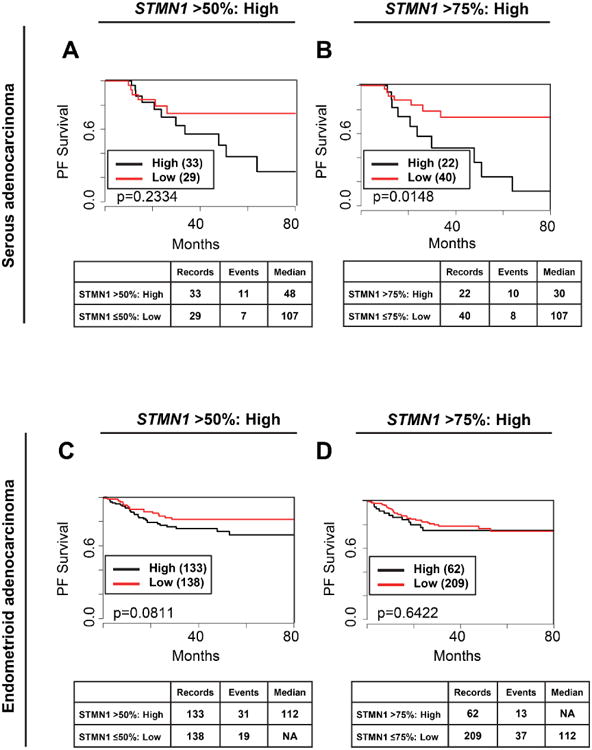
Kaplan-Meier analysis of TCGA dataset for endometrial cancer for the association of STMN1 with PFS for serous adenocarcinoma (A, B) or endometrioid adenocarcinoma (C, D). STMN1 was dichotomized into low and high categories using a cut-off of either >50% (A, C) or >75% (B, D). NA= not achieved, the number of observed events (deaths) did not reach threshold to calculate median survival; Median= median survival, the amount of time (months) wherein 50% of the patients are alive.
Discussion
Stathmin expression at the mRNA and protein levels has been attributed to poor prognosis in a variety of solid tumors. In cancer cells, the overall function of elevated stathmin is to speed cell progression into and out of mitosis [4]. High stathmin has been reported to underlie resistance to paclitaxel by directly antagonizing paclitaxel's stabilizing effect on the microtubule. Using tumor specimens from patients treated on GOG-177, we confirmed that high expression of stathmin correlated with worse OS and PFS. However, the impact of high stathmin was most significant in patients on arm 1, TA. The addition of paclitaxel in arm 2 (TAP) resulted in a significant overall improvement in PFS and OS, highlighting the important role of paclitaxel in the treatment of patients with advanced endometrial cancer. Importantly, even high stathmin expression did not negate the improved outcomes with the addition of paclitaxel.
Stathmin was first identified based on its overexpression in leukemia cell lines. It is now widely recognized to be overexpressed in nearly all solid tumors examined to date, including hepatocellular, gastric, breast, prostate, cervical, pancreatic, and ovarian cancers. Moreover, stathmin expression is generally considered to be a marker for an aggressive tumor based on the correlation between stathmin levels and tumor size, metastatic potential, histopathologic grade and overall survival in multiple cancer types [19-29].
Elevated expression of stathmin has also been documented in endometrial tumors [5, 16, 30-34]. For example, an integrated analysis of genome-wide expression data for primary endometrial carcinoma found that STMN1 is upregulated at the mRNA level [30]. In addition, qRT-PCR demonstrated 2.5-fold higher STMN1 expression in tumor vs. normal endometrium [31]. Our analysis of TCGA dataset using a STMN1 cut-off of 50% suggests that approximately half of all endometrial tumors have elevated stathmin transcripts. In our IHC study of stathmin protein expression (GOG-177 samples), we found that 75% of tumors (53 out of 69) had no detectable stathmin expression by IHC, and only 7 tumors (10%) had an H-score >50. Similarly, a study of metastatic endometrial cancer in Norway demonstrated that only 18% of primary tumors had high stathmin levels [5]. By contrast, using a cut-off of >10% cells staining positive for stathmin, others have reported that 60.7% of endometrial tumors express stathmin protein, whereas 36.7% of normal endometrium samples have positive staining [31]. Nevertheless, stathmin expression, while relatively uncommon overall in GOG-177, appears to identify some of the highest risk cases from this study based upon the poor prognosis associated with its presence. The high proportion of tumors with no detectable stathmin protein in the GOG specimens cannot be explained by the difference in scoring systems, since such a large number of tumors were negative by IHC in our study.
Studies in lung cancer suggest that stathmin expression is significantly increased in poorly differentiated lung adenocarcinoma as compared to moderately or well differentiated tumors [35]. Similarly, stathmin expression has been associated with more aggressive phenotypes, including higher expression in late vs. early stage endometrial cancer and in lymph node metastases and increased invasion into the myometrium and lymphovascular space [30,31,34,36]. However, we did not observe a significant correlation between stathmin expression and age, pathological type, grade in GOG-177 subjects or in TCGA. There was a pronounced association between stathmin expression and serous subtype of endometrial cancer in TCGA dataset, with a large difference in PFS associated with low stathmin in this cohort of serous cases. This observation of higher stathmin in serous cases was not replicated in the GOG-177 dataset, possibly because of the relatively small number of serous and stathmin positive cases.
The significant associations between stathmin mRNA levels and PFS/OS are consistent with several studies in multiple cancer types documenting a link between high stathmin expression and poor response to treatment [5,20-22]. Consistent with a previous report [34], we found a significant inverse relationship between stathmin overexpression and OS as well as PFS in both the GOG-177 specimens as well as TCGA dataset for endometrial cancer. One of the most interesting findings of our study is that the addition of paclitaxel negated the impact of stathmin overexpression on PFS and OS, consistent with a study of ovarian clear cell adenocarcinoma patients that received taxane-containing or taxane-free therapy [37]. However, the precise role of stathmin as a marker for paclitaxel sensitivity or resistance is not clear. For example, a Norwegian study showed of 38 patients with metastatic endometrial cancer treated with paclitaxel-containing chemotherapy reported a significantly lower disease-specific survival in patients with high stathmin [5]. Similarly, stathmin has been associated with resistance to antimicrotubule drugs including paclitaxel in breast cancer [3,6,7] non-small cell lung cancer [8,9], epithelial ovarian cancer [10], and bladder cancer [11].
At the molecular level, stathmin destabilizes microtubules to promote cell division. Conversely, paclitaxel promotes microtubule polymerization, leading to mitotic arrest and ultimately cell death [12]. We propose that the transition through mitosis in the setting of paclitaxel therapy for cancer is a balance between the microtubule depolymerizing activity of stathmin and the microtubule stabilizing activity of paclitaxel. Our translational analyses herein suggest that paclitaxel negates the deleterious effects of stathmin overexpression.
Research Highlights.
This study sought to define markers of response on GOG Study 177
Addition of paclitaxel to adriamycin/cisplatin regimen improves clinical outcomes
High stathmin is associated with poor outcomes on the adriamycin/cisplatin arm
Paclitaxel may negate the effects of stathmin overexpression
Acknowledgments
This work was supported by NIH R01CA99908 and R01CA184101 (KKL), K12-HD063117 (HDR and KKL), and the Department of Obstetrics and Gynecology Research Development Fund (KKL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This study was supported by National Cancer Institute grants to NRG Oncology (1U10 CA180822) and NRG Operations (U10CA180868). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Community Clinical Oncology Program, Abington Memorial Hospital, University of Colorado Cancer Center – Anschutz Cancer Pavilion, Tufts-New England Medical Center, University of California Medical Center at Irvine – Orange Campus, Ohio State University Comprehensive Cancer Center, University of Mississippi Medical Center, Penn State Milton S. Hershey Medical Center, Women's Cancer Center of Nevada, Tacoma General Hospital, University of Alabama at Birmingham, University of Minnesota Medical Center – Fairview, University of Cincinnati, University of North Carolina at Chapel Hill, Rush University Medical Center, Stony Brook University Medical Center, Cooper Hospital University Medical Center, Fox Chase Cancer Center, Duke University Medical Center, Fred Hutchinson Cancer Research Center, University of Iowa Hospitals and Clinics, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Washington University School of Medicine, University of Oklahoma Health Sciences Center, University of Virginia, University of Chicago and Moffitt Cancer Center and Research Institute.
Dr. Jeffrey Miecznikowski receives grant funding from NRG Oncology Statistical and Data Management Center. Dr. Kristina Thiel is a co-founder of Immortagen and holds an equity stake in the company. Dr. Krishnansu Tewari receives payment for lectures, including service on speakers bureaus, from Roche, Astra Zeneca and Merck. He also receives travel/accommodations/meeting expenses unrelated to activities listed from Roche. Dr. Floor Backes serves as consultant for Advasix on clinical trial steering committee. She also has grants/grants pending from Eisai. Dr. Nilsa Ramirez receives grant funding from GOG Tissue Bank/NRG Oncology Biospecimen Bank-Columbus. Dr. Ramirez also has grants/grants pending through the GOG/NRG Oncology Biospecimen Bank support via NCI awarded U24 and U10 grants. Dr. Kimberly Leslie receives grant funding through the NIH – R01CA99908.
Footnotes
Authors' contributions: H.D.R. and K.K.L. conceptualized the hypotheses and designed the research strategy; P.H., S.G., K.S.T., F.B., N.R., G.F.F., V.F., and M.J.B. accrued patients for the study; H.D.R, E.J.D., Y.Z.,, J.-M.S., and M.M. performed the experiments; J. M., and J. G.-B. performed the statistical analysis; H.D.R., Y.Z., and M.S. performed blinded histologic analyses, H.D.R. J. M., K.W.T., and K.K.L. analyzed data; and H.D.R., K.W.T., E.J.D., and K.K.L. wrote the paper. All authors read and approved the final manuscript.
Conflicts Of Interest: All other co-authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Atlanta, GA: American Cancer Society; 2015. Cancer Facts and Figures 2015. Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 2.Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 3.Miller D, Fillaci V, Fleming GF, Mannel R, Cohn D, Matsumoto T, et al. Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771. abst. [Google Scholar]
- 4.Gupta KK, Li C, Duan A, Alberico EO, Kim OV, Alber MS, et al. Mechanism for the catastrophe-promoting activity of the microtubule destabilizer Op18/stathmin. Proc Natl Acad Sci USA. 2013;110:20449–20454. doi: 10.1073/pnas.1309958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Werner HM, Trovik J, Halle MK, Wik E, Akslen LA, Birkeland E, et al. Stathmin protein level, a potential predictive marker for taxane treatment response in endometrial cancer. PloS One. 2014;9:e90141. doi: 10.1371/journal.pone.0090141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–6869. [PubMed] [Google Scholar]
- 7.Meng XL, Su D, Wang L, Gao Y, Hu YJ, Yang HJ, et al. Low expression of stathmin in tumor predicts high response to neoadjuvant chemotherapy with docetaxel-containing regimens in locally advanced breast cancer. Genet Test Mol Biomarkers. 2012;16:689–694. doi: 10.1089/gtmb.2011.0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosell R, Scagliotti G, Danenberg KD, Lord RV, Bepler G, Novello S, et al. Transcripts in pretreatment biopsies from a three-arm randomized trial in metastatic non-small-cell lung cancer. Oncogene. 2003;22:3548–3553. doi: 10.1038/sj.onc.1206419. [DOI] [PubMed] [Google Scholar]
- 9.Sun R, Liu Z, Wang L, Lv W, Liu J, Ding C, et al. Overexpression of stathmin is resistant to paclitaxel treatment in patients with non-small cell lung cancer. Tumour Biol. 2015;36:7195–7204. doi: 10.1007/s13277-015-3361-y. [DOI] [PubMed] [Google Scholar]
- 10.Su D, Smith SM, Preti M, Schwartz P, Rutherford TJ, Menato G, et al. Stathmin and tubulin expression and survival of ovarian cancer patients receiving platinum treatment with and without paclitaxel. Cancer. 2009;115:2453–2463. doi: 10.1002/cncr.24282. [DOI] [PubMed] [Google Scholar]
- 11.Wosnitzer MS, Domingo-Domenech J, Castillo-Martin M, Ritch C, Mansukhani M, Petrylack DP, et al. Predictive value of microtubule associated proteins tau and stathmin in patients with nonmuscle invasive bladder cancer receiving adjuvant intravesical taxane therapy. J Urol. 2011;186:2094–2100. doi: 10.1016/j.juro.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 12.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 13.Berg A, Hoivik EA, Mios S, Holst F, Werner HM, Tangen IL, et al. Molecular profiling of endometrial carcinoma precursor, primary and metastatic lesions suggests different targets for treatment in obese compared to non-obese patients. Oncotarget. 2015;6:1327–1339. doi: 10.18632/oncotarget.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleming GF, Filiaci VL, Marzullo B, Zaino RJ, Davidson SA, Pearl M, et al. Temsirolimus with or without megestrol acetate and tamoxifen for endometrial cancer: a gynecologic oncology group study. Gynecol Oncol. 2014;132:585–592. doi: 10.1016/j.ygyno.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.FIGO Committee on Gynecologic Oncology. FIGO staging for carcinoma of the vulva, cervix, and corpus uteri. Int J Gynaecol Obstet. 2014;125:97–8. doi: 10.1016/j.ijgo.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–51. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 17.FIGO Announcements, Stages - 1988 Revision. Gynecologic Oncology. 1989;35:125–7. [Google Scholar]
- 18.Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akhtar J, Wang Z, Yu C, Li CS, Shi YL, Liu HJ. STMN-1 is a potential marker of lymph node metastasis in distal esophageal adenocarcinomas and silencing its expression can reverse malignant phenotype of tumor cells. BMC Cancer. 2014;14:28. doi: 10.1186/1471-2407-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Karst AM, Levanon K, Duraisamy S, Liu JF, Hiarsch MS, Hecht JL, et al. Stathmin 1, a marker of PI3K pathway activation and regulator of microtubule dynamics, is expressed in early pelvic serous carcinomas. Gynecol Oncol. 2011;123:5–12. doi: 10.1016/j.ygyno.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Liu C, Cheng H, Xu Y, Jiang J, Xu J, Long J, Liu L, Yu X. Stathmin, interacting with Nf-kappaB, promotes tumor growth and predicts poor prognosis of pancreatic cancer. Curr Mol Med. 2014;14:328–339. doi: 10.2174/1566524014666140228120913. [DOI] [PubMed] [Google Scholar]
- 22.Tan HT, Wu W, Ng YZ, Zhang X, Yan B, Ong CW, et al. Proteomic analysis of colorectal cancer metastasis: stathmin-1 revealed as a player in cancer cell migration and prognostic marker. J Proteome Res. 2012;11:1433–1445. doi: 10.1021/pr2010956. [DOI] [PubMed] [Google Scholar]
- 23.Belletti B, Nicoloso MS, Schiappacassi M, Gerton S, Lovat F, Wolf K, et al. Stathmin activity influences sarcoma cell shape, motility, and metastatic potential. Mol Biolo Cell. 2008;19:2003–2013. doi: 10.1091/mbc.E07-09-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh SY, Huang SF, Yu MC, Yeh TS, Chen TC, Lin YJ, et al. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol Carcinog. 2010;49:476–487. doi: 10.1002/mc.20627. [DOI] [PubMed] [Google Scholar]
- 25.Cheng AL, Huang WG, Chen ZC, Peng F, Zhang PF, Li MY, et al. Identification of novel nasopharyngeal carcinoma biomarkers by laser capture microdissection and proteomic analysis. Clin Cancer Res. 2008;14:435–445. doi: 10.1158/1078-0432.CCR-07-1215. [DOI] [PubMed] [Google Scholar]
- 26.Jeon TY, Han ME, Lee YW, Lee YS, Kim GH, Song GA, et al. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010;102:710–718. doi: 10.1038/sj.bjc.6605537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brattsand G. Correlation of oncoprotein 18/stathmin expression in human breast cancer with established prognostic factors. Br J Cancer. 2000;83:311–318. doi: 10.1054/bjoc.2000.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi M, Yang XJ, Lavery TT, Furge KA, Williams BO, Tretiakova M, et al. Gene expression profiling of favorable histology Wilms tumors and its correlation with clinical features. Cancer research. 2002;62:6598–6605. [PubMed] [Google Scholar]
- 29.Watanabe A, Suzuki H, Yokobori T, Tsukagoshi M, Altan B, Kubo N, et al. Stathmin1 regulates p27 expression, proliferation and drug resistance, resulting in poor clinical prognosis in cholangiocarcinoma. Cancer Sci. 2014;105:690–696. doi: 10.1111/cas.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salvesen HB, Carter SL, Mannelgvist M, Duff A, Getz G, Stefansson IM, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci USA. 2009;106:4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao X, He Y, Lu W, Xu G, Tong H, Ke J, et al. Elevated STMN1 promotes tumor growth and invasion in endometrial carcinoma. Tumour Biol. 2016 doi: 10.1007/s13277-016-4869-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 32.Vandenput I, Trovik J, Leunen K, Wik E, Stefansson I, Akslen L, et al. Evolution in endometrial cancer: evidence from an immunohistochemical study. Int J Gynecol Cancer. 2011;21:316–322. doi: 10.1097/IGC.0b013e31820575f5. [DOI] [PubMed] [Google Scholar]
- 33.Wik E, Birkeland E, Trovik J, Werner HM, Hoivik EA, Mios S, et al. High phospho-Stathmin(Serine38) expression identifies aggressive endometrial cancer and suggests an association with PI3K inhibition. Clin Cancer Res. 2013;19:2331–2341. doi: 10.1158/1078-0432.CCR-12-3413. [DOI] [PubMed] [Google Scholar]
- 34.Trovik J, Wik E, Stefansson IM, Marcickiewicz J, Tingulstad S, Staff AC, et al. Stathmin overexpression identifies high-risk patients and lymph node metastasis in endometrial cancer. Clin Cancer Res. 2011;17:3368–3377. doi: 10.1158/1078-0432.CCR-10-2412. [DOI] [PubMed] [Google Scholar]
- 35.Chen G, Wang H, Gharib TG, Huang CC, Thomas DG, Shedden KA, et al. Overexpression of oncoprotein 18 correlates with poor differentiation in lung adenocarcinomas. Mol Cell Proteomics. 2003;2:107–116. doi: 10.1074/mcp.M200055-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Ring KL, Connor EV, Atkins KA, Ricketts W, Kashlan B, Modesitt SC. Women 50 years or younger with endometrial cancer: the argument for universal mismatch repair screening and potential for targeted therapeutics. Int J Gynecol Cancer. 2013;23:853–860. doi: 10.1097/IGC.0b013e31828eed9c. [DOI] [PubMed] [Google Scholar]
- 37.Aoki D, Oda Y, Hattori S, Taguchi K, Ohishi Y, Basaki Y, et al. Overexpression of class III beta-tubulin predicts good response to taxane-based chemotherapy in ovarian clear cell adenocarcinoma. Clin Cancer Res. 2009;15:1473–1480. doi: 10.1158/1078-0432.CCR-08-1274. [DOI] [PubMed] [Google Scholar]


