Abstract
There continues to be a major effort in the United States to develop mitigators for the treatment of mass casualties that received high-intensity acute ionizing radiation exposures from the detonation of an improvised nuclear device during a radiological terrorist attack. The ideal countermeasure should be effective when administered after exposure, and over a wide range of absorbed doses. We have previously shown that the administration of a subcutaneous incision of a defined length, if administered within minutes after irradiation, protected young adult female C57BL/6 mice against radiation-induced lethality, and increased survival after total-body exposure to an LD50/30 X-ray dose from 50% to over 90%. We refer to this approach as “protective wounding”. In this article, we report on our efforts to further optimize, characterize and demonstrate the validity of the protective wounding response by comparing the response of female and male mice, varying the radiation dose, the size of the wound, and the timing of wounding with respect to administration of the radiation dose. Both male and female mice that received a subcutaneous incision after irradiation were significantly protected from radiation lethality. We observed that the extent of protection against lethality after an LD50/30 X-ray dose was independent of the size of the subcutaneous cut, and that a 3 mm subcutaneous incision is effective at enhancing the survival of mice exposed to a broad range of radiation doses (LD15–LD100). Over the range of 6.2–6.7 Gy, the increase in survival observed in mice that received an incision was associated with an enhanced recovery of hematopoiesis. The enhanced rate of recovery of hematopoiesis was preceded by an increase in the production of a select group of cytokines. Thus, a thorough knowledge of the timing of the cytokine cascade after wounding could aid in the development of novel pharmacological radiation countermeasures that can be administered several days after the actual radiation exposure.
INTRODUCTION
Over the past 15 years, research efforts to develop mitigators for use against high-intensity acute ionizing radiation exposures have been intensified due to the increased threat of a radiological terrorist strike and the mass casualties that would result from such an incident. Doses of 1–15 Gy of X rays or γ rays would result in the development of the acute radiation syndromes (ARS) (1), with the lethal dose to 50% of humans exposed (LD50) to total-body irradiation often being cited to be ~3.25 Gy without medical intervention (2). Doses of 1–3 Gy might simply be temporarily debilitating, while doses in excess ≥10 Gy would almost certainly result in death from the gastrointestinal (GI) syndrome within 3–10 days postirradiation, due to depopulation of the epithelial lining of the GI tract and infection (1, 3). Doses ranging from ~3–9 Gy induce the hematopoietic syndrome, with death occurring within 60 days of the exposure without medical intervention (1, 3). In the case of the hematopoietic syndrome, death would be attributed to the normal attrition of the terminally differentiated functional blood cells (or rapid apoptosis of radiosensitive lymphocytes), and failure to replace them due to depletion of the more radiosensitive stem and precursor cells. Many victims of an improvised nuclear device (IND) could be rescued by conventional supportive therapies such as antibiotics and blood transfusions; such treatments would likely double the LD50 (4–6). Recently, the FDA approved Neupogen (filgrastim; G-CSF, granulocyte colony stimulating factor) and Neulasta (pegfilrastim), and these are the only agents that can be provided by the government for mitigation of the radiation-induced hematopoietic syndrome via stimulation of recovery of peripheral blood cells during neutropenia resulting from a radiation incident without an Emergency Use Authorization (7–9). While Neupogen and Neulasta are maintained in the Strategic National Stockpile for treatment of mass casualties (9, 10), the drugs are perishable, require several doses after treatment to be effective (e.g., must be given daily or weekly), and are only useful over a limited radiation dose range. Thus, the search has continued for other compounds or strategies that could be employed as mitigators after irradiation.
We previously demonstrated that the creation of a small subcutaneous (SC) wound, if administered shortly after irradiation, was extremely effective in preventing the death of female C57BL/6 mice that received an LD50/30 dose of X rays (total-body exposure), and that the mitigation of lethality may be attributed, at least in part, to an enhanced recovery of hematopoietic elements (red blood cells, neutrophils, platelets) after irradiation (11). We now show that our protective wounding strategy is effective at enhancing the survival and stimulating hematopoeisis of mice exposed to a broad range of radiation doses. In our quest to determine the optimal wounding parameters, we now show that protection against radiation lethality is independent of wound size. Finally, to demonstrate the validity of the protective wounding strategy in both sexes, we show that the creation of a small subcutaneous wound mitigates radiation lethality in male as well as female mice.
MATERIALS AND METHODS
Female and male C57BL/6 mice (in our hands, LD50/30 = ~6.9 Gy and 6.7 Gy for females and males, respectively) were obtained from Jackson Laboratories (Bar Harbor, ME) at 9–11 weeks of age. Mice were housed in micro-isolator cages on ventilated racks (5 mice per cage) and were given food and acidified water ad libitum. All animal procedures were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee. At 12 weeks of age, after acclimating for at least one week, mice were randomly assigned to control and irradiated groups that either would or would not receive a subcutaneous incision or “cut”. Mice were anesthetized with a ketamine cocktail (~100 mg/ml ketamine, ~0.45 mg/ml atropine and ~2.2 mg/ml acepromazine) and either sham-irradiated (0 Gy) or irradiated in a rectangular Lucite box (inner dimensions were 16 × 17.5 cm), which was placed within a flat-beam field; 2–10 mice were positioned in the box during irradiations. Mice were placed in the box such that all heads were pointed toward the mid-line of the box, and we ensured that mice and their tails didn’t overlap one another. Mice were irradiated with a single uniform total-body exposure of 250 kVp X rays (various doses; dose rate = ~65 cGy/min) using a Precision X-ray machine (North Branford, CT) with a 2-mm aluminum filter. Mice were irradiated in the morning, and all irradiations were performed within the same 3 h window. Dosimetry was performed using a Farmer-type ionization chamber (PTW Model N30013, Freiburg, Germany) in conjunction with a Keithley electrometer (Model K602, Cleveland, OH). Survival experiments were repeated 2–5 times with irradiated treatment groups consisting of 10–21 mice per experiment; these same mice were used for analysis of blood cell elements. For cytokine studies, groups consisted of 10–41 mice.
Within 20 min postirradiation, a 3-, 6- or 12-mm SC incision was administered as described by Garrett et al. (11). Briefly, the entire mid-section was rinsed with 70% isopropyl alcohol and then swabbed with Betadine (Purdue Pharma, Stamford, CT) before the hair was separated to expose a clean line of skin; aseptic techniques were used for all subsequent procedures to create the wound. After separating the hair to expose a clean line of skin, a SC cut of various lengths (but ~1 mm in depth) was made midway down the dorsal surface, parallel to the plane of each mouse. An incision was made with a pair of surgical scissors, and a pair of hemostats was inserted 1.5 cm underneath the skin and then opened to approximately 6 or 12 mm to separate the fascia, producing a final incision length of 6 or 12 mm, respectively. To produce a 3-mm incision, after the initial incision, one side of the hemostat was inserted 1.5 cm underneath the skin; this itself resulted in the formation of a 3-mm final incision length. Wounds were closed with a 9-mm MikRon Autoclip. Wound clips either fell out without provokation, or were removed by day 14 postirradiation. A group of “sham-cut” mice were included in every experiment, and were treated the same as mice that received the wound, except that no incision was ever made nor wound clip applied (thus, observers could not be blinded to treatment). Mice did not receive supportive care after irradiation. The Mantel-Haenszel test was used to determine whether there was a difference between the 30-day all-cause mortality of the different treatment groups. The test was run using the total number of surviving mice at day 30 and total number of mice irradiated initially from all experiments for that particular dose (2–5 experiments per dose).
To determine the effects of wounding on survival as a function of time after irradiation, it was necessary to anesthetize mice twice (once prior to irradiation and once prior to making a 6-mm incision at various times after irradiation). To reduce the risk of mortality that can occur from multiple dosings of anesthesia within a short time period, the decision was made to reduce the anesthetic dosage used prior to irradiation. For example, for mice that received an incision approximately 24 h postirradiation, mice were anesthetized with 2/3 of the normal dose of anesthesia for irradiations, and approximately 24.3 h later, mice were given a full dose of anesthesia; the cut was administered approximately 24 h postirradiation.
Mice were observed for signs of illness twice daily, and were euthanized if they could no longer respond to stimulation, right themselves, or if a combination of hunching, decreased activity and eye squint, reached a level of 7 on a 9-point grading scale (scale of 0–3 for each of the latter three criteria) (12).
Complete blood counts (CBCs) were performed as described previously (11), using a HemaVet cell counter (Drew Scientific, Dallas, TX). Mice from each of the aforementioned treatment groups were bled at least 24 h prior to irradiation (for baseline measurements) and on days 2, 6, 9, 13, 16, 20, 23, 27 and 30 postirradiation. Briefly, for acquisition of blood samples, mice were restrained and ~30–44 µl of blood drawn from clipped tails. Bleeding was stopped, and mice were released into their cages. For each experiment, at least two mice per group were bled as controls prior to irradiation. Blood analysis was performed on 2–6 animals per experiment, per group, per time point. Since each experiment was repeated at least 1 time (experiments with male mice only) or up to 3 times, each time point represents the mean of 4–15 mice. As in our previous study (11), mice were rotated in the bleeding schedule such that no mouse was bled twice within the 30-day period of observation unless all other mice in the group had been bled at least once, and at least 10 days were allowed to elapse between bleeds to allow for recovery from the initial bleed. Mean blood cell counts and hemoglobin levels at each time point for each dose and treatment group were analyzed using a 2-tailed, uneven variance t test to determine if significant differences existed between the treatment groups at various days during the recovery period (days 13–23). Additionally, two-way ANOVA was done on the datum points from the recovery period to determine overall, whether treatment significantly affected the means.
Cytokine concentrations in the sera of cut and sham-cut mice (irradiated or sham irradiated) were assayed using Millipore kits (Billerica, MA) designed to separately quantify the mouse proteins RANTES, Interleukin (IL)-1a, IL-3, IL-6, IL-9, IL-12(p40), IL-12(p70), IL-13, IL-17, Eotaxin, G-CSF, keratinocyte chemoattractant (KC), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1a and 1b (MIP-1a and MIP-1b, respectively), IL-1b, IL-2, IL-4, IL-5, IP-10, granulocyte-macrophage colony-stimulating factor (GMCSF), interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α). Assays were performed according to the manufacturer’s protocol. Serum matrix was used as sample matrix for protein detection. Serum samples from 4–8 mice from 2–3 experiments were run undiluted, unless there was not enough serum, in which case the sample was diluted minimally (1.5 fold for 1 sample) with assay buffer according to the manufacturer’s recommendations. After preparation, samples were processed (50 beads per bead set in 25-µl sample size) on a Bio-Plex 200 System with High Throughput Fluidics (HTF) Multiplex Array System (Bio-Rad Laboratories, Hercules, CA).
RESULTS
We previously showed that 30-day all-cause mortality was greatly reduced (by ~40%) in female C57BL/6 mice that received a single subcutaneous 6-mm incision (cut) within 20 min after total-body irradiation with a single LD50/30 dose of 250 kVp X rays compared to mice that were sham irradiated (11). In the current study, we sought to optimize the parameters of our protective wounding approach, further demonstrate its validity, and gain further insight into the mechanisms by which the approach results in enhanced survival after total-body irradiation.
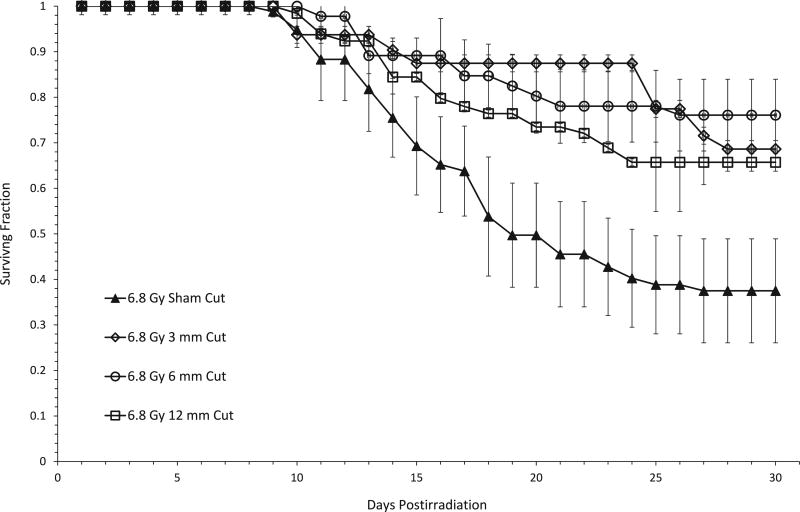
To determine whether the extent of protection was dependent upon the size of the incision, female mice were X irradiated with 6.8 Gy and incisions of 3, 6 or 12 mm were administered within 20 min thereafter. This dose represents the LD50/30 dose for sham-cut female mice. As shown in Fig. 1, 30-day all-cause mortality was significantly decreased in mice that received our standard 6-mm cut. Administration of a 3- or 12-mm cut also provided dramatic protection against radiation lethality, with no significant differences in survival noted between groups that received incisions of various lengths. That is, a 3-mm cut conferred as much protection against acute radiation lethality as our standard 6-mm incision or a 12-mm incision. Thus, the enhancement of survival when measured 30 days after total-body irradiation was independent of cut size.
FIG. 1.
Enhancement of 30-day survival of female C57BL/6 mice after irradiation is independent of the size of the subcutaneous incision. The groups designated as “cut” received either a 3-, 6- or 12-mm cut (a sterile incision, as described in Materials and Methods) approximately 20 min postirradiation with 6.8 Gy of X rays. Data shown are from 2–5 independent experiments, with 14–18 mice per experimental group. Error bars represent the standard error of the mean (SEM) from mice across all experiments. Day 30 survival between the cut groups was compared using the Mantel-Haenszel method and was found to be not significant (P = 0.39). Comparing each cut length group vs. the sham-cut group separately was significantly different each time (3-mm cut, P < 0.001; 6-mm cut, P = 0.001; 12-mm cut, P = 0.002). There was no difference in 30-day survival when different cut lengths were compared at other doses as well (6.2 Gy, P = 0.57; 6.7 Gy, P = 0.15; 7.1 Gy, P = 0.39, data not shown).
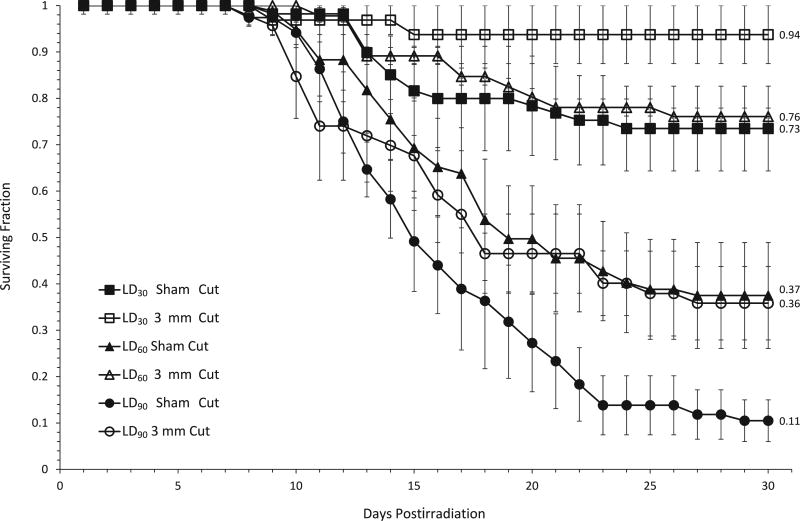
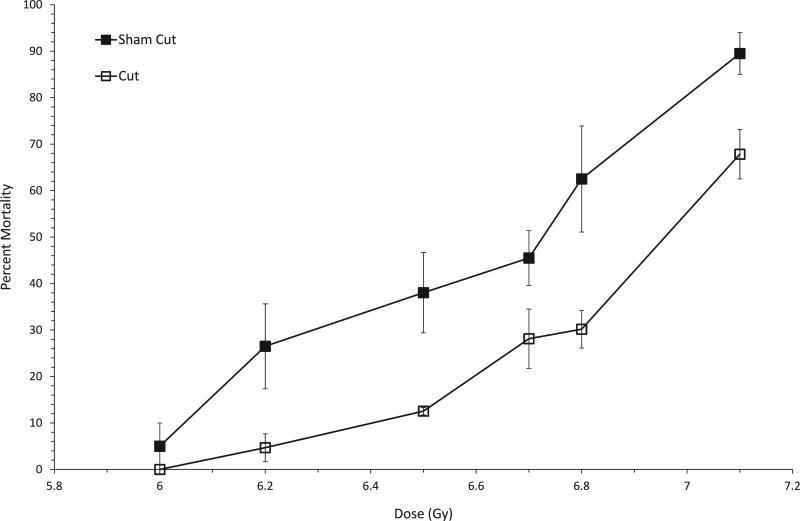
We next assessed the effectiveness of the protective wounding approach over a wide range of doses. Mice received total-body exposures of 6–7.1 Gy and all-cause mortality was measured through day 30 postirradiation, along with the overall timing of deaths. Survival after exposure to three representative doses approximating the LD30, LD60 and LD90 is shown in Fig. 2. A comparison of the curves for cut vs. sham-cut mice reveals a significant enhancement of survival at each dose for mice receiving a 3-mm cut. Although in general the temporal pattern of onset of deaths did not appear to be different (Fig. 2), with the first deaths in all groups beginning on approximately day 10 and the final deaths occuring by day 24 postirradiation, wounding was found to confer a significant survival benefit compared to sham-cut mice, at all dose levels greater than 6 Gy (Fig. 3).
FIG. 2.
Subcutaneous wounding enhances 30-day survival of female C57BL/6 mice over a wide range of doses. Female C57Bl/6 mice received a subcutaneous cut approximately 20 min postirradiation with X rays. Data shown are from 2–5 independent experiments in which mice received a 3-mm incision, with 10–17 mice irradiated per experimental group. Error bars represent the standard error of the mean (SEM) from mice across all experiments. Day 30 survival between the sham-cut and cut groups within each dose was compared using the Mantel-Haenszel method [since data sets consisted of dichotomous data (e.g., mice would either die or survive)] and was found to be significant at all 3 doses; LD30 P < 0.0001, LD60 P = 0.0001, LD90 P = 0.0010. Numbers to the right of the 30-day survival data points indicate the fraction of mice surviving 30 days postirradiation for that particular treatment group.
FIG. 3.
Protective wounding results in decreased 30-day mortality of female C57BL/6 mice over a range of radiation doses. Mortality Curves are shown for sham-cut mice or mice that received a subcutaneous incision (3, 6 or 12 mm in length) within 20 min postirradiation with various doses of X rays. Groups consisted of 10–21 mice per experimental group and experiments were repeated 2–5 times. Using the Mantel-Haenzel method on 30-day survival data, a significant difference between sham cut and cut groups was found at all radiation doses except 6.0 Gy, P = 0.15. For 6.2 Gy, P < 0.0001; 6.5 Gy, P = 0.0219; 6.7 Gy, P = 0.0199; 6.8 Gy, P < 0.0001, 7.1 Gy, P = 0.0017.
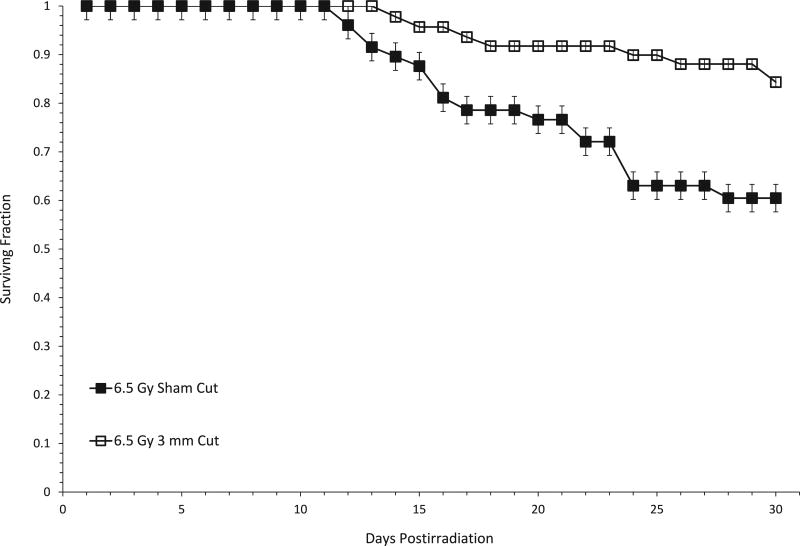
In various rodent models, we and others have found that the radiation-induced response of normal tissues (timing and severity of the normal tissue response) may be different depending on the sex of the animal [see ref. (13) and references therein]. Therefore, we investigated whether male mice might also be protected from radiation lethality after administration of a subcutaneous incision. Survival at day 30 postirradiation was about 25% greater in male mice that received a 3-mm incision after irradiation compared to male mice that received a sham cut (Fig. 4).
FIG. 4.
Subcutaneous wounding enhances 30-day survival of male C57BL/6 mice. Mice either received a sham cut, or a 3-mm SC incision approximately 20 min postirradiation with 6.5 Gy of X rays. Analyzing the 30-day surviving fraction using the Mantel-Hanenzel method shows a significant difference between cut and sham-cut mice; P = 0.0134. Experimental groups consisted of 13–18 mice in 2 independent experiments. Error bars represent SEM from mice across both experiments.
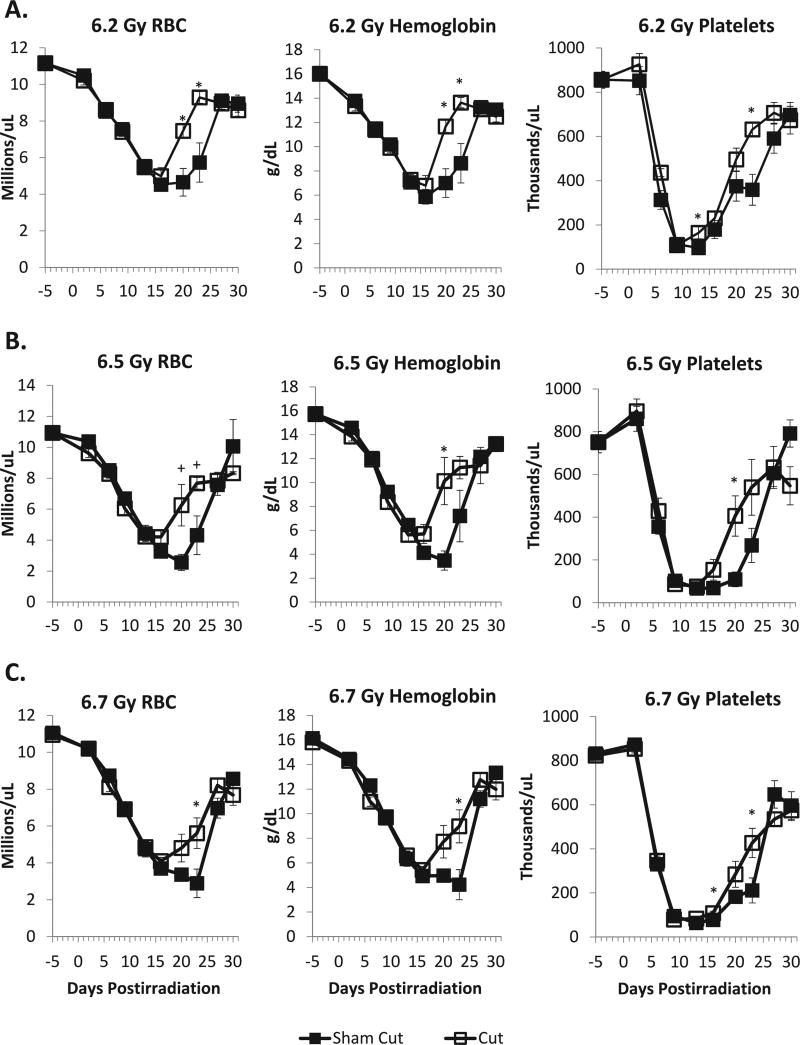
By performing serial CBCs and differential analysis of peripheral blood samples, we sought to determine whether there was a relationship between enhanced survival and either initial hematopoietic damage or recovery of blood-forming elements. In our previous studies, which were confined to one dose (LD50) in mice that either received a standard 6-mm SC wound after irradiation or were sham cut, we found that there were no significant differences in the time to reach the nadir of the RBC, neutrophil or platelet counts, or in the RBC, neutrophil or platelet counts themselves at the nadir, when mice that either received a cut or a sham cut after irradiation were compared (11). However, the recovery of these blood cell elements was significantly faster in the mice that received a cut after irradiation compared to irradiated sham-cut mice. In the current study, we obtained similar results for RBC and platelets after mice were exposed to a range of doses (6.2, 6.5 and 6.7 Gy) of X rays (Fig. 5A – C). Both cut and sham-cut mice showed similar nadirs in RBC and platelet counts, as well as hemoglobin levels, by approximately 9–15 days postirradiation; enhanced recovery of RBC counts and hemoglobin levels was observed between days 16 and 20 postirradiation over the entire dose range, while significantly enhanced recovery of platelets was observed over the entire dose range by day 23. By day 27 postirradiation, at all doses, levels of RBCs, platelets and hemoglobin were similar in both sham-cut mice and mice that were given a SC incision after irradiation; while recovery had reached a plateau, the levels never fully recovered to baseline levels. There were no significant differences in white blood cell and neutrophil counts between mice that received cuts or mice which were sham cut (e.g., no significant difference in recovery rates; data not shown).
FIG. 5.
Recovery of red blood cells (RBC), hemoglobin and platelets was enhanced in C57BL/6 female mice receiving a subcutaneous cut within 20 min after exposure to various doses of X rays (panel A = 6.2 Gy; panel B = 6.5 Gy; panel C = 6.7 Gy). Experiments were performed 2–4 times, with 2–3 mice bled per time point postirradiation, per experiment. Thus, from all experiments involved, each datum point represents 4–16 sham-cut or cut mice; baseline CBCs were obtained from both the sham-cut and cut mice, leading to n = 16, instead of n = 8. The 6.2 and 6.7 Gy cut data represent pooled data from mice that received 3 and 6 mm, or 3-, 6- and 12-mm incisions, respectively; we elected to pool these data since we had previously determined that length of the cut did not significantly alter 30-day all-cause mortality. Blood cell counts obtained during the recovery period (days 13–23), were analyzed by two-tail t tests and two-way ANOVA. Asteriks (*) indicate where P < 0.05 in the two-tail t test between the sham-cut and cut treatment group datum points. Plus signs (+) indicate where P < 0.07. Two-way ANOVA indicated P < 0.05 for treatment (sham cut vs. cut) for all three types of blood measurements, for all 3 radiation doses. Additionally, the interaction between time and treatment was significant (P < 0.05) for 6.2 and 6.5 Gy in RBC and hemoglobin, and for 6.7 Gy for all 3 types of blood measurements.
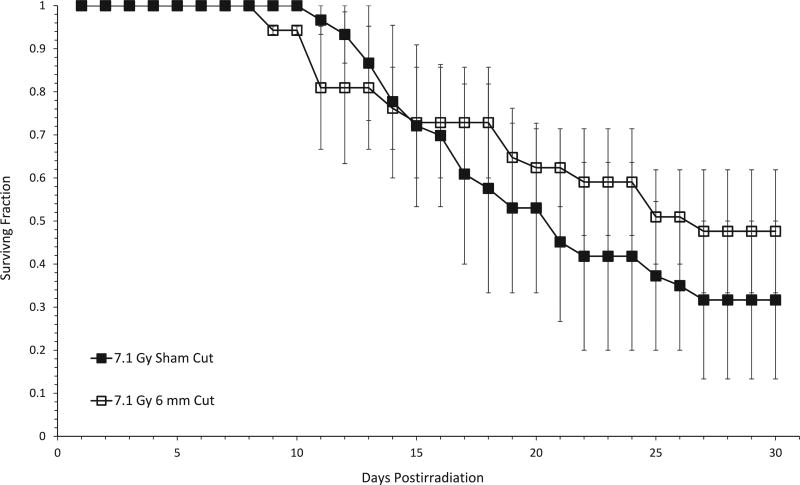
Since a SC cut afforded significant protection from radiation lethality across a wide dose range if administered immediately after irradiation, and this protection may have been due, at least in part, to enhanced recovery of hematopoeisis that occurred over two weeks after the time of irradiation, but several days before the well-established crisis period for the hematopoietic syndrome (2) we hypothesized that administration of an SC cut hours to several days after irradiation would still be protective. However, when a 6-mm incision was made 12–24 h postirradiation with doses approximating the LD50 (not shown) or LD70, no protection against lethality was noted compared to sham-cut controls [Fig. 6; similar results were obtained for mice that received a 3-mm cut (data not shown)].
FIG. 6.
A subcutaneous incision cannot mitigate the lethal effects of radiation when administered 24 h postirradiation. Mice were irradiated with a dose approximating the LD70/30 (7.1 Gy) for that series of experiments (in which mice were anesthetized twice) and were returned to their cages for 24 h, after which a 6-mm subcutaneous incision was made. Using the Mantel-Haenzel method on 30-day surviving fractions data, no significant difference between sham-cut and 6-mm cut groups after irradiation was found (P = 0.17).
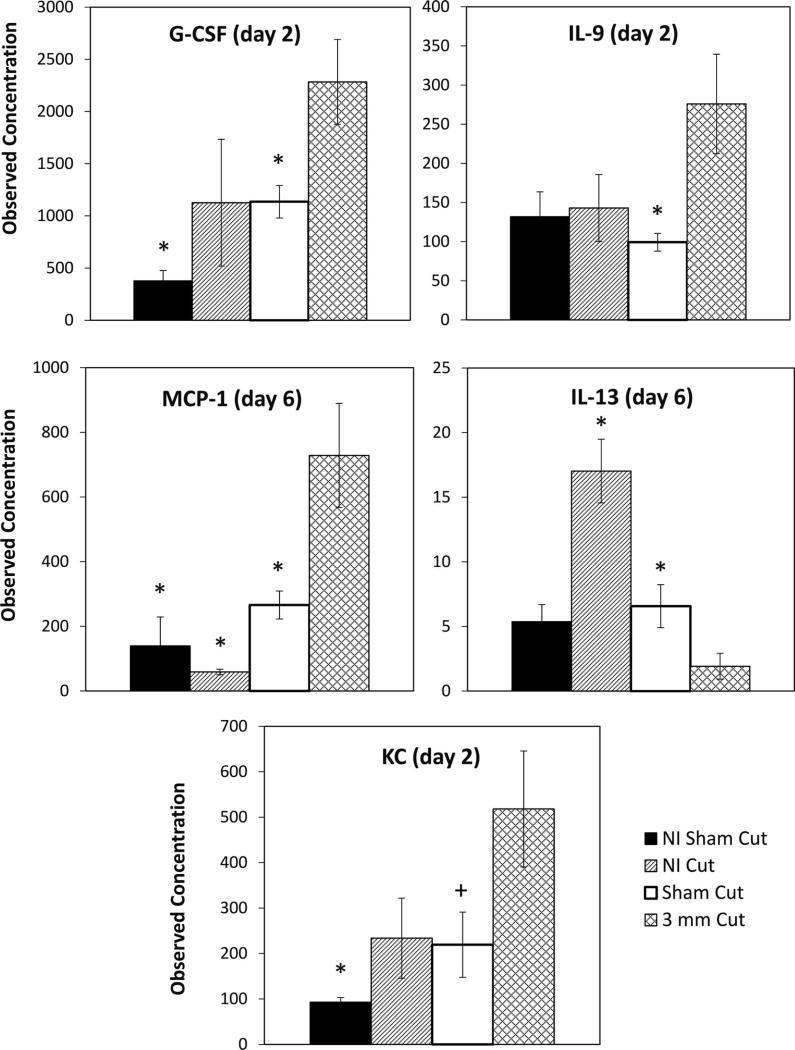
Since the only compounds currently approved by the FDA to treat hematopoietic ARS are granulocyte colony-stimulating factor (G-CSF) [filgrastim; brand name = Neupogen) and PEGylated G-CSF (pegfilgrastim; brand name = Neulasta), and these growth factors stimulate bone marrow granulopoiesis, the enhancement of which may explain the enhanced survival in mice receiving cuts after irradiation, we sought to determine whether specific plasma cytokines or growth factors were “overexpressed” or “under-expressed” in wounded vs. sham-cut mice within a 6-day window postirradiation. Several cytokines were assayed (see Materials and Methods) but only a minority showed a significant difference between sham-cut and cut mice. We found that G-CSF and IL-9 levels were significantly increased on day 2, and MCP-1 levels were increased on day 6 postirradiation, in female mice receiving a 3-mm cut after irradiation compared to those that were sham cut. Levels of KC in mice that received a 3-mm cut after irradiation were significantly higher than in the nonirradiated sham-cut mice (P = 0.0125), and showed a trend towards significance compared to the sham-cut mice (P = 0.0660). However, IL-13 levels on day 6 were significantly reduced in irradiated mice that received a cut, compared to irradiated sham-cut and nonirradiated cut control mice (P = 0.0489 and P = 0.000904, respectively; see Fig. 7).
FIG. 7.
Effects of protective wounding on radiation-induced plasma cytokine production in female mice. Mice were exposed to either 0 or 6.7 Gy and either received a 3-mm subcutaneous cut 15–20 min postirradiation or received a sham-cut. On days –1, 1, 2 and 6 postirradiation, peripheral blood was obtained and analyzed for plasma cytokines. On day 2 postirradiation, the observed concentration of G-CSF and IL-9 was significantly higher in the irradiated 3-mm cut mice than in the irradiated sham-cut mice; P = 0.0271 and 0.0274, respectively. Additionally, at day 2, the observed concentration of G-CSF was significantly higher in the irradiated 3-mm cut mice than in the nonirradiated sham-cut mice (P = 0.002). On day 6 postirradiation, significant differences between irradiated 3-mm cut mice and other groups were seen for observed concentrations of MCP-1 and IL-13. Irradiated mice that received a 3-mm cut showed higher MCP-1 levels than the nonirradiated sham-cut mice (P = 0.0006), the nonirradiated cut mice (P = 0.0005), and the irradiated sham-cut mice (P = 0.0241). Irradiated mice that received a 3-mm cut had a lower observed concentration of IL-13 than nonirradiated (NI) cut mice and irradiated sham-cut mice; P = 0.0009 and P = 0.0489, respectively. The observed concentration of keritinocyte chemoattractant (KC) in mice receiving a 3-mm cut after irradiation was significantly higher than in the nonirradiated sham-cut mice (P = 0.0125) and showed a trend towards significance compared to the irradiated sham-cut mice (P = 0.0660). All P values were determined using two-way t tests with unequal variance. Data from serum samples from 3 experiments were averaged (4–8 mice per group). Asteriks (*) indicate where P < 0.05 in the two-tail t test between that particular group and the cut group. Plus signs (+) indicate where P < 0.07 in the two-tail t test between that particular group and the cut group.
DISCUSSION
Building upon our earlier studies in which we demonstrated that mortality was significantly decreased in female C57BL/6 mice if given a small SC incision within 20 min after receiving an LD50/30 total-body dose of X rays (11), we endeavored to determine optimal wounding parameters (size, timing of wounding relative to the irradiation to obtain the greatest decrease in 30-day all-cause mortality) and further demonstrate the validity of the protective wounding approach by testing its effectiveness over a large dose range and in male mice. We also attempted to gain greater insight into the mechanisms behind the enhancement of survival of mice that received an incision after irradiation.
Since the detonation of an improvised nuclear device (IND) during a terrorist attack in a metropolitan area would result in mass casualties suffering from acute high-dose radiation exposure as well as burns and other wounds, many investigators in recent years have focused their efforts to develop mitigators that will reduce the increased mortality that would be predicted [in part from animal studies dating back to the 1960s (14, 15)] to result from such combined injuries. Interestingly, Ledney et al. (16) found that mortality was decreased if a very large wound was created in mice (corresponding to ~4% of the total-skin surface area) within 10 min after irradiation with 9 Gy and the wound was left open; this reduction of mortality greatly diminished or disappeared if the wound was created 1–2 days postirradiation or following larger exposures. In our studies, we found that mortality after exposure to a single acute dose or X rays is independent of incision size made after irradiation, and that a very small (3 mm) closed wound was sufficient to significantly protect female mice against radiation lethality over a wide range of doses (Figs. 1–3). Similar to the results of Ledney et al. (16), we found that the protective effect of the SC incision disappeared if administered at longer intervals (12–24 h) after irradiation (see below).
An ideal mitigator of the acute radiation syndromes and radiation lethality must not only be effective over a wide range of doses (since absorbed dose would be difficult to assess in situations involving mass casualties), but it must also be effective in both female and male subjects. Since males may be more sensitive to acute and late effects of ionizing radiation [see ref. (13) and references therein], and since there are differences in normal tissue response between the sexes, it would not have been unreasonable to hypothesize that males would respond differently to our protective wounding approach compared to females. While the decrease in mortality was not as great as we observed in female mice, we found that administration of a 3-mm incision after irradiation also significantly reduced mortality in male mice exposed to an LD50/30 dose of X rays compared to mice that received a sham cut (Fig. 4).
The temporal pattern of onset of deaths did not appear to be different when mortality was measured in sham-cut female mice compared to mice that received a 3-, 6- or 12-mm incision immediately after exposure to an LD50/30 dose or in mice that received a 3-mm incision after exposure to a range of radiation doses (Fig. 2). This finding was in contrast to what we observed in previous studies (10) in which female mice were irradiated with an LD50/30 dose and received a 6-mm incision immediately thereafter. It is difficult to speculate why we observed this difference. However, it should be noted that in the previous study, mice were purchased from a different vendor that bred and maintained the mice under very different husbandry techniques, which likely resulted in variations in microflora.
The enhanced survival we observed in animals receiving an incision immediately after irradiation was likely not due to a reduction in damage to, or enhanced repopulation of the stem cells of the GI tract. While total-body doses of less than 7 Gy might be sufficient to trigger mucosal barrier breakdown in a subset of mice, thereby allowing bacteria to translocate into the circulation, such doses lie below the threshold for the classical GI syndrome; thus, it was not surprising that we found no differences in the number of crypts or regeneration of the intestinal epithelium between cut or sham-cut mice irradiated with doses approximating the LD50/30 (not shown). Rather, as we proposed previously, the reduced mortality of mice wounded after irradiation may be attributed, at least in part, either to the enhanced recovery of hematopoietic elements compared to sham-cut mice, or an increased activity of blood element precursors. While administration of a postirradiation incision did not significantly alter the timing or magnitude of the nadirs of the RBC, platelet and hemoglobin levels, recovery to near-normal levels was statistically significantly faster if mice received a 3-mm subcutaneous incision after exposure to various doses of X rays, compared to irradiated sham-cut mice (Fig. 5). The increase in platelet recovery in wounded mice suggests enhanced activity of colony forming unit-megakaryocytes (CFU-Meg).
Our finding that there was no reduction in mortality in mice after administration of an incision 12 or 24 h postirradiation (Fig. 6) was somewhat disappointing; although the Department of Defense and/or first responder populations may find it useful to employ the protective wounding strategy shortly after exposure, an ideal counter-measure for use with civilian populations should afford protection to exposed individuals even if given hours to days after irradiation. However, such a finding was not totally unexpected due to the complicated nature of the experiments and the fact that an experiment to test efficacy of wound administration several hours to days after irradiation cannot be performed under exactly the same conditions as an experiment to test wound administration immediately after irradiation. This is because of the need for mice to be anesthetized both during the irradiation and also subsequently, at the time of wounding. Mice that receive a cut immediately after irradiation would have already been anesthetized minutes earlier and would still be under anesthesia at the time the wound would need to be made, but mice that would require a wound to be made several hours after the irradiation would have to be anesthetized a second time. In our experience, mice are hypersensitive to a second dose of anesthesia unless spatially separated by several days. Thus, a direct comparison of 30-day all-cause mortality in mice that were given an incision immediately after irradiation (as per our standard protocol) with those that were given an incision up to 24 h later cannot be made directly.
It is worth speculating, though, that the reason for our failure to observe protection from lethality when the length of time between the irradiation and administration of the wound was increased to 12–24 h may have involved the lack of a synergistic interaction between the responses to the radiation injury and physical injury that might occur when the two injuries are not significantly separated temporally. This interaction could directly or indirectly lead to a unique cytokine cascade.
We posited that wounding after irradiation could result in the modulation of key cytokines normally involved in hematopoiesis. The design of our cytokine study was predicated on 1. the numerous findings that several cytokines, including the newly FDA-approved mitigator G-CSF (Neupogen), are known to stimulate or regulate hematopoiesis (17–21), and 2. that while the up- or down-regulation of cytokines could occur within days or weeks after irradiation and/or wounding, the most critical period of observation, for purposes of the potential development of phamacologic cytokine-based mitigator strategies, would occur within 1–6 days postirradiation.
Three cytokines were found to be significantly up-regulated and one was significantly down-regulated in wounded mice within this time period (Fig. 7). G-CSF, IL-9 and MCP-1 all increased at least twofold either 2 days (MCP-1 and IL-9) or 6 days (MCP-1) postirradiation in mice that had been given an SC cut; IL-13, showed at least a twofold decrease at day 6 postirradiation. IL-9 is often referred to as a factor that regulates hematopoiesis, as it synergizes with IL-3 to enhance erythroid burst forming unit (BFU-E), CFU-E and multi-lineage colony formation in combination with other factors from bone marrow precursors, and acts as an activator of megakaryocyte progenitor cells (22). MCP-1 regulates migration and infiltration of monocytes/macrophages (23) while G-CSF promotes neutrophil production and mobilizes hematopoietic stem cells from the bone marrow into the bloodstream (24). Finally, IL-13 down-regulates macrophage activity, thus inhibiting the production of pro-inflammatory cytokines and chemokines (25). Further studies are necessary to link the modulation of these cytokines with the enhanced recovery of hematopoiesis in mice that receive an incision after irradiation.
While we could not demonstrate efficacy of our protective wounding approach when wounds were administered 12–24 h postirradiation, we speculate that the lack of effectivness may be due to lack of effect of the cytokine cascade when it is induced (e.g., by a SC cut) outside of a critical time interval. For example, a specific cytokine may work in concert with other cytokines if it is triggered to be up-regulated day 1 postirradiation and wounding, but may be ineffective if induced day 2 postirradiation if the cut itself is delayed 1 day postirradiation. Further studies are necessary to determine whether the administration of combinations of cytokines at tightly-timed intervals after irradiation (following a pattern similar to that which is induced when wounding occurs immediately after irradiation) can provide a mitigating effect similar to that observed by wounding immediately after irradiation.
Since combinations of cytokines that are strong stimulators of hematopoiesis have been demonstrated to improve reconstitution of the hematopoietic system and decrease 30-day mortality (17–21), and recent data indicate that postirradiation administration of specific early-acting cytokines may lead to enhanced short- and long-term survival (26, 27), further insight into the mechanisms by which protection is afforded through subcutaneous wounding could lead to the development of novel pharmacological radiation countermeasures.
Acknowledgments
This work was supported by a grant from the National Institute of Environmental Health Sciences (ES021631). We thank Hui Lin Chua, Hailin Feng and the Multiplex Analysis Core at the Indiana University Simon Cancer Center for providing expert support in analyzing cytokine samples and interpretation of data. We also thank Helen Chin-Sinex and Chris Roberts for providing technical support, and George Eckert (Indiana University School of Medicine Department of Biostatistics Core) for assistance with biostatistics. This paper is dedicated to the memory of Sigmund Dynlacht, father of JRD; he survived the Holocaust and came to the United States with nothing except “street smarts” and a good sense of humor, but succeeded in giving his three sons the tools to live better lives than their parents had.
References
- 1.Lushbaugh C. Reflections on some recent progress in human radiobiology. In: Augenstein L, Mason R, Zelle M, editors. Advances in Radiation Biology. New York: Academic Press; 1969. pp. 277–314. [Google Scholar]
- 2.Hall E, Giaccia A. Radiobiology for the Radiologist. Seventh. Philadelphia: Lippincott, Williams, and Wilkins Company; 2012. [Google Scholar]
- 3.Thomas GE, Jr, Wald N. The diagnosis and management of accidental radiation injury. J Occup Med. 1959;1:421–47. [Google Scholar]
- 4.Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, et al. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat Res. 2003;159:812–34. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 5.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, et al. Models for evaluating agents intended for the prophylaxis and mitigation, treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162:711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 6.Pellmar TC, Rockwell S. Priority list of research areas for radiological nuclear threat countermeasures. Radiat Res. 2005;163:115–23. doi: 10.1667/rr3283. [DOI] [PubMed] [Google Scholar]
- 7.Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–51. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 8.Farese AM, MacVittie TJ. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today. 2015;51:537–48. doi: 10.1358/dot.2015.51.9.2386730. [DOI] [PubMed] [Google Scholar]
- 9.News Releases, Collected by the U.S. Department of Health and Human Services. http://bit.ly/2ngYC0t.
- 10.CDC Strategic National Stockpile. https://www.cdc.gov/phpr/stockpile/
- 11.Garrett J, Orschell CM, Mendonca MS, Bigsby RM, Dynlacht JR. Subcutaneous wounding post-irradiation reduces radiation lethality in mice. Radiat Res. 2014;181:578–83. doi: 10.1667/RR13267.1. [DOI] [PubMed] [Google Scholar]
- 12.Plett PA, Sampson CH, Chua HL, Joshi M, Booth C, Gough A, et al. Establishing a murine model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys. 2012;103:343–55. doi: 10.1097/HP.0b013e3182667309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dynlacht JR. The role of age, sex and steroid sex hormones in radiation cataractogenesis. Radiat Res. 2013;180:559–566. doi: 10.1667/RR13549.1. [DOI] [PubMed] [Google Scholar]
- 14.Koslowski L, Messerschmidt O. Intermedes Proceedings: Combined Injuries and Shock. Vol. 80. Research Institute of National Defense; Stockholm: 1968. The role of the time factor in the combined injury syndrome; p. 21. [Google Scholar]
- 15.Stromberg LW, Woodward KT, Mahin DT, Donati RM. Combined surgical radiation injury The effect of timing of wounding and whole body gamma irradiation on 30 day mortality and rate of wound contracture in the rodent. Ann Surg. 1968;167:18–22. doi: 10.1097/00000658-196801000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ledney GD, Exum ED, Jackson WE., 3rd Wound-induced alterations in survival of 60Co irradiated mice: importance of wound timing. Experientia. 1985;41:614–6. doi: 10.1007/BF02007684. [DOI] [PubMed] [Google Scholar]
- 17.Van der Meeren A, Mouthon MA, Gaugler MH, Vandamme M, Gourmelon P. Administration of recombinant human IL11 after supralethal radiation exposure promotes survival in mice: interactive effect with thrombopoietin. Radiat Res. 2002;157:642–9. doi: 10.1667/0033-7587(2002)157[0642:aorhia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.DiCarlo AL, Poncz M, Cassatt DR, Shah JR, Czarniecki CW, Maidment BW. Development and licensure of medical counter-measures for platelet regeneration after radiation exposure. Radiat Res. 2011;176:134–7. doi: 10.1667/rr2610.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Meeren A, Mouthon MA, Vandamme M, Squiban C, Aigueperse J. Combinations of cytokines promote survival of mice and limit acute radiation damage in concert with amelioration of vascular damage. Radiat Res. 2004;161:549–59. doi: 10.1667/rr3164. [DOI] [PubMed] [Google Scholar]
- 20.Waddick KG, Song CW, Souza L, Uckun FM. Comparative analysis of the in vivo radioprotective effects of recombinant granulocyte colony-stimulating factor (G-CSF), recombinant granulocyte-macrophage CSF, and their combination. Blood. 1991;77:2364–71. [PubMed] [Google Scholar]
- 21.Neta R, Abrams JS, Pelrlstein RS, Rokita H, Sipe JD. Interactions of the cytokines IL-1, and IL-6 in in vivo host responses. In: Revel M, editor. IL-6: Physiopathology and Clinical Potentials. New York: Raven Press; 1992. [Google Scholar]
- 22.Goswami R, Kaplan MH. A Brief History of IL-9. J Immunol. 2011;186:3283–88. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satish L, Kremlev S, Amini S, Sawaya BE. Monocyte chemo-attractant protein-1 (MCP-1): An overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendall LJ, Bradstock KF. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25:355–367. doi: 10.1016/j.cytogfr.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 25.IL13 interleukin 13 [Homo sapiens (human)] https://www.ncbi.nlm.nih.gov/gene/3596.
- 26.Herodin F, Bourin P, Mayol JF, Lataillade JJ, Drouet M. Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma-irradiation promotes survival. Blood. 2003;101:2609–16. doi: 10.1182/blood-2002-06-1634. [DOI] [PubMed] [Google Scholar]
- 27.Drouet M, Grenier N, Herodin F. Revisiting emergency anti-apoptotic cytokinotherapy: erythropoietin synergizes with stem cell factor, FLT-3 ligand, trombopoietin and interleukin-3 to rescue lethally-irradiated mice. Eur Cytokine Netw. 2012;23:56–63. doi: 10.1684/ecn.2012.0306. [DOI] [PubMed] [Google Scholar]