Abstract
Enantioselective syntheses of (−)-alloyohimbane and (−)-yohimbane was accomplished in a convergent manner. The key step involved a modified mild protocol for the enantioselective enzymatic desymmetrization of meso-diacetate. The protocol provided convenient access to an optically active monoacetate in multi-gram scale in high enantiomeric purity. This monoacetate was converted to (−)-alloyohimbane. Reductive amination of the derived aldehyde causes the isomerization leading to the trans-product and allows the synthesis of (−)-yohimbane.
Keywords: alkaloids, Bischler-Napieralski, enzymatic resolution, indole, polycyclic, synthesis, yohimbine
Introduction
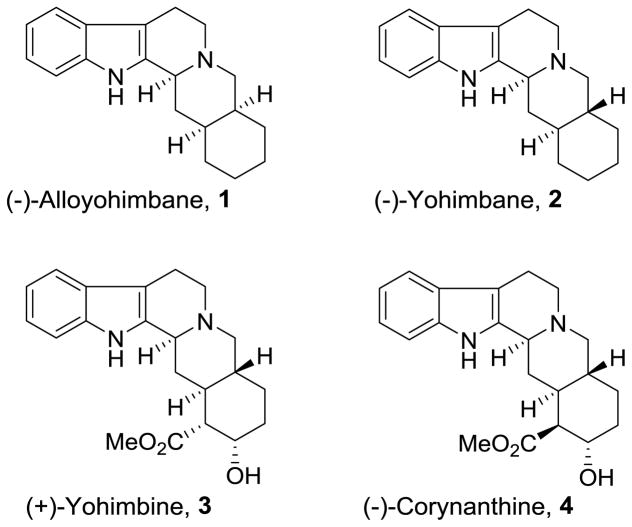
The yohimbine alkaloids, featuring a characteristic pentacyclic indole ring framework, display a wide range of pharmacological properties.1,2 These alkaloids belong to the Rubiaceae family and they are isolated from the bark of the Pausinystalia Yohimbe tree in central Africa. Over the years, this family of alkaloids has received much attention due to its antihypertensive and antipsychotic activity.3–6 Yohimbine (Figure 1) and related derivatives have been used as traditional medicines, specifically as adrenergic blocking agents for Angina pectoris and arteriosclerosis. They are also used as herbal medicine for the treatment of impotency. Furthermore, yohimbines have been shown to lower body fat and relieve symptoms of dry mouth.7,8 The biological mechanism of action of the yohimbine family may be due to its potent, competitive, and selective alpha-2-adrenergic receptor antagonist properties.9,10 They have been shown to block dopamine pathways in schizophrenic patients. Also, they weakly interact with alpha-1-adrenoreceptors. Not surprisingly, the chemistry and biology of yohimbines attracted immense interest in their synthesis and biological studies. To date, several racemic syntheses,11–13 as well as enantioselective syntheses14–16 of yohimbine and related alkaloids are reported. Racemic17–20 and enantioselective16,21–26 syntheses of alloyohimbane and yohimbane, the pentacyclic skeleton of yohimbine alkaloids have also been repoted in the literature. As part of our interest to explore the use of polycyclic indoles in medicinal chemistry, we have devised practical and enantioselective syntheses of (−)-yohimbane and (−)-alloyohimbane. Herein we report convergent syntheses of these molecules using enzymatic desymmetrization as the key step. Of particular interest, we have developed a modified protocol which provides convenient access to (1R, 6S)-6(hydroxymethylcyclohexenyl) methyl acetate in high optical purity on multigram scale.
Figure 1.
Structures of yohimbine and related compounds
Results and Discussion
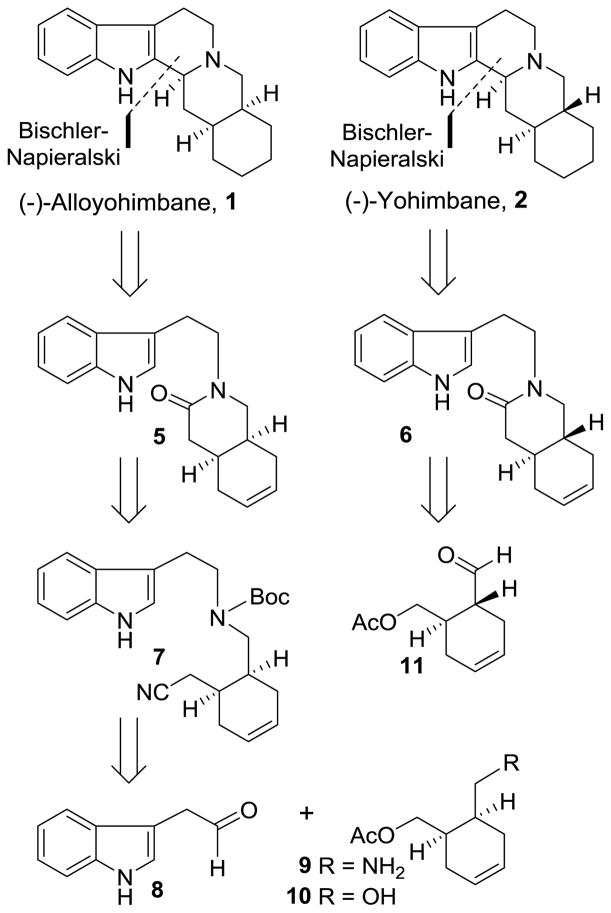
Our retrosynthetic analysis of (−)-alloyohimbane and (−)-yohimbane is shown in Figure 2. For the synthesis of (−)-alloyohimbane, we planned a Bischler-Napierlaski reaction to construct the pentacyclic indole ring framework from the corresponding lactams 5 and 6. A similar saturated lactam intermediate has been previously converted to yohimbane derivative.19,22 Lactam 5 would be obtained from nitrile derivative 7 through its conversion to the corresponding acid followed by removal of the Boc group and amidation of the resulting amino acid. Nitrile derivative 7 can be obtained by reductive amination of commercially available 3-indolyl acetaldehyde acid 8 and (1R, 6S)-6-(aminomethylcyclohexenyl)methyl acetate 9 and its conversion to nitrile by standard synthetic steps. The requisite optically active amine would be derived from the corresponding (1R, 6S)-6-(hydroxymethylcyclohexenyl)methyl acetate 10. Such optically active alcohol 10 can be obtained by enzymatic desymmetrization of the corresponding meso-diacetate. For the synthesis of (−)-yohimbane, we planned to carry out reductive amination of (1R, 6R)-6-(formylcyclohexenyl)methyl acetate 11, which could be obtained by oxidation of alcohol 10 and in situ epimerization of the resulting aldehyde via an enamine intermediate. Therefore, optically active alcohol 10 can be manipulated for the synthesis of alloyohimbane and yohimbane and their derivatives.
Figure 2.
Retrosynthetic analysis for yohimbanes.
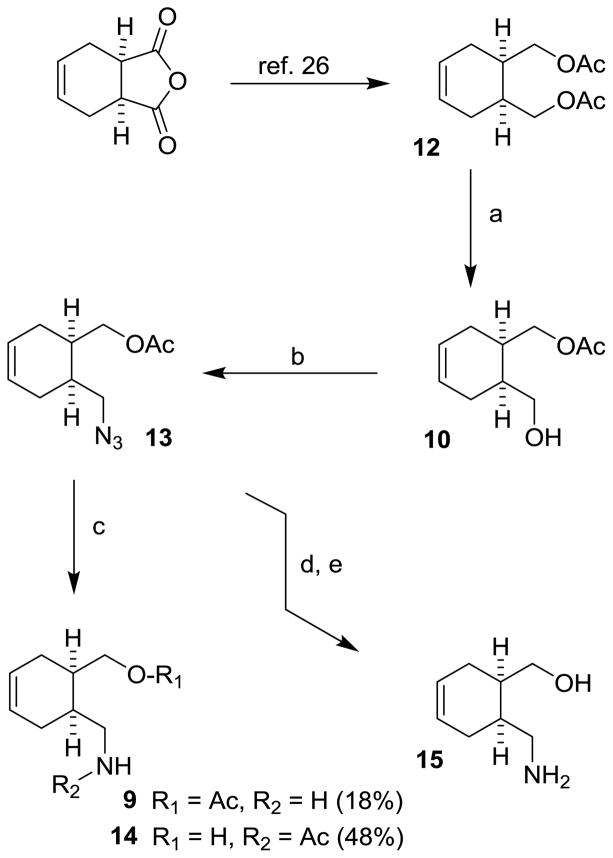
Our synthesis of optically active alcohol 10 via desymmetrization is shown in Scheme 1. Commercially available meso-1, 2, 3, 6-tetrahydrophthalic anhydride was converted to meso-diacetate 12 by LAH reduction followed by acetylation of the resulting diol as reported in the literature.27 For optical resolution, we planned to utilize commercially available and inexpensive porcine pancreatic lipase (PPL), an enzyme which hydrolyzes dietary triglycerides to monoglycerides and free fatty acids.28 This enzyme has been extensively utilized in the selective hydrolysis of esters in aqueous solution. As reported, exposure of meso-diacetate 12 with porcine pancreatic lipase in 0.1 M phosphate buffer (pH 7) provided monoacetate 10 with high optical purity.27,29 The reaction protocol required continuous addition of 1N NaOH solution to neutralize the acetic acid released in the reaction.
Scheme 1.
Reagents and conditions: (a) PPL (5% w/w), pH 7 buffer, 23 °C, 1 N NaHCO3 (84%); (b), Ph3P, DEAD, (PhO)2P(O)N3, 0 °C, THF (92%); (c) PPh3, H2O, THF, 60 °C; (d) K2CO3, MeOH, 0 °C; (e) PPh3, THF, 60 °C, then, H2O, 60 °C (78% from 13).
While this protocol provided optically active alcohol 10, the observed enantiomeric excess and reaction yield of the product alcohol varied, particularly during large scale preparation of alcohol 10. We presume that the erosion of ee could be due to competing non-enzymatic hydrolysis of the meso-diacetate 12. Also, the reason for variability of yields may be due to non-enzymatic hydrolysis of the monoacetate 10. In an effort to minimize this competing non-enzymatic reaction, we investigated a number of other organic and inorganic bases that can effectively neutralize liberated acetic acid without non-enzymatic side reaction. We found that enzymatic desymmetrization of meso-diacetate27 in the presence of 1N NaHCO3 aqueous solution provided mono-acetate 10 in high optical purity (>95% ee) with reproducible yields (>80% yield). In a typical experiment, we carried out enzymatic desymmetrization of meso-diacetate with 5% (w/w) PPL (Sigma, type II, crude) in pH 7 phosphate buffer at 23 °C in the presence of 1N aqueous NaHCO3 solution over 24 h. We have scaled this enzymatic desymmetrization to 51 grams and obtained monoacetate 10 in 84% yield with >95% ee as determined by HPLC analysis. Please see supporting information for further details. We utilized this optically active alcohol in a unified synthesis of (−)-alloyohimbane and (−)-yohimbane.
For synthesis of (−)-alloyohimbane, alcohol 10 was subjected to Mitsunobu azidation,30,31 using diphenylphosphoryl azide and triphenylphosphine in THF at 0 °C for 40 min to provide azide 13 in 92% yield. Staudinger reduction,32 of 13 with triphenylphosphine in aqueous THF at 60 °C, provided desired amine 9 in only 18% yield. Acetamide 14, resulting from intramolecular acetate transfer, was obtained as a major product in 48% yield. Acetate 13 was therefore converted to aminoalcohol 15 by exposure to K2CO3 in MeOH at 0 °C for 1 h followed by Staudinger reduction to provide 15 in 78% yield over two steps.
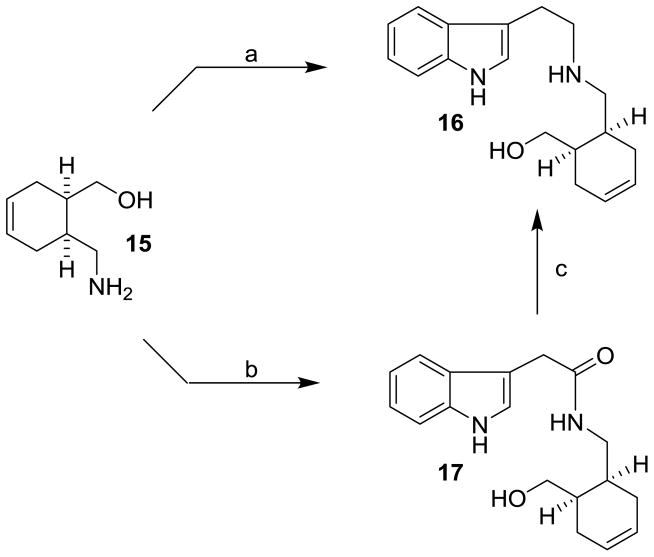
Our initial coupling of amine 15 with 3-indolyl acetaldehyde33 did not provide satisfactory results. As shown in Scheme 2, reductive amination of 15 with 3-indolyl acetaldehyde was carried out with sodium cyanoborohydride in MeOH in the presence of acetic acid. However, this reductive amination provided amine 16 in poor yields under a variety of reaction conditions, presumably due to the instability of 3-indolyl acetaldehyde. In an alternative route, amine 15 was coupled with 3-indolylacetic acid using EDC and HOBt in THF at 23 °C for 12 h to provide amide 17 in 75% yield. Reduction of this amide with lithium aluminum hydride in THF at 80 °C for 12 h afforded amine 16 in 74% yield.
Scheme 2.
Reagents and conditions: (a) 3-indolyl acetaldehyde 8, AcOH, MeOH, Na(CN)BH3, 23 °C (24%); (b) 3-indolylacetic acid, EDC, HOBt, iPr2NEt, THF, 23 °C (75%); (c) LiAlH4, THF, 80 °C (74%).
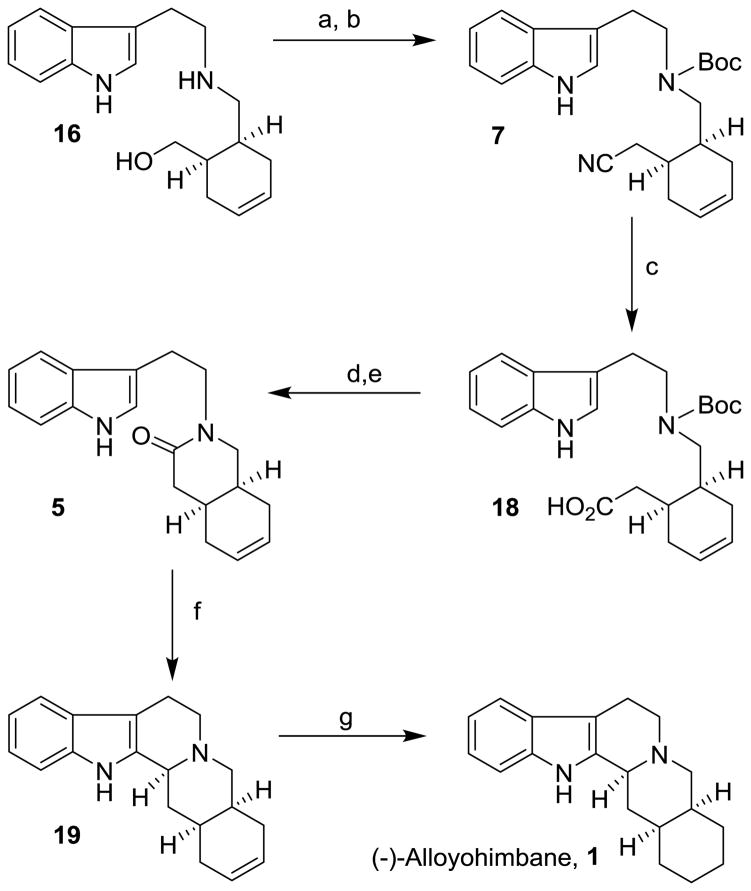
The synthesis of (−)-alloyohimbane from amine 16 is shown in Scheme 3. Amine 16 was protected as the Boc derivative by treatment with 0.95 equivalent of Boc2O in CH2Cl2 at 23 °C for 5 h. This condition mainly provided the corresponding N-Boc derivative and only trace amount of the corresponding tert-butylcarbonate. The free alcohol was converted to cyanide 7 using a Mitsunobu reaction with acetone cyanohydrin34 in the presence of diisopropyl azodicarboxylate (DIAD) and triphenylphosphine in THF at 23 °C for 6 h. Cyanide derivative 7 was obtained in 83% yield over two steps. This was treated with 20% aqueous NaOH in ethanol at reflux for 12 h to provide acid 18 in 76% yield. The Boc group was deprotected by exposure to trifluoroacetic acid in CH2Cl2 at 23 °C for 1 h. Reaction of the resulting amino acid with EDC and HOBt in the presence of diisopropylethylamine at 23 °C for 18 h afforded lactam 5 in 45% yield over two steps. The lactam was converted to (−)-dehydroalloyohimbane 19 by a Bischler-Napieralski reaction22 using phosphorus oxychloride in CH2Cl2 (1:1) at 50 °C for 3 h followed by sodium borohydride reduction of the resulting iminium ion to provide 19 as a single isomer in 74% yield. The stereochemical outcome was dictated by the existing ring stereochemistry and preferred hydride attack from the sterically less hindered α-face. Catalytic hydrogenation of olefin 19 over 10% Pd/C in ethyl acetate under a hydrogen-filled balloon at 23 °C for 1 h furnished (−)-alloyohimbane (−)-1 in 91% yield. The spectroscopic data of our synthetic (−)-alloyohimbane and observed specific rotation {[α]20D = −78.8 (c, 0.3 EtOH)} are in complete agreement with the synthetic (−)-alloyohimbane reported in the literature.22
Scheme 3.
Reagents and conditions: (a) Boc2O, CH2Cl2, 23 °C; (b) Ph3P, DIAD, THF, Me2C(OH)CN, 23 °C (83% for 2-steps); (c) 20% aq NaOH, EtOH (1:1), reflux (76%); (d) CF3CO2H, CH2Cl2, 23 °C; (e) EDCI, HOBt, iPr2NEt, CH2Cl2, 23 °C (45% for 2-steps); (f) POCl3, CH2Cl2, 50 °C, then, NaBH4, MeOH/H2O, 0 °C (74%); (g) H2, 10% Pd-C, EtOAc, 23 °C (91%).
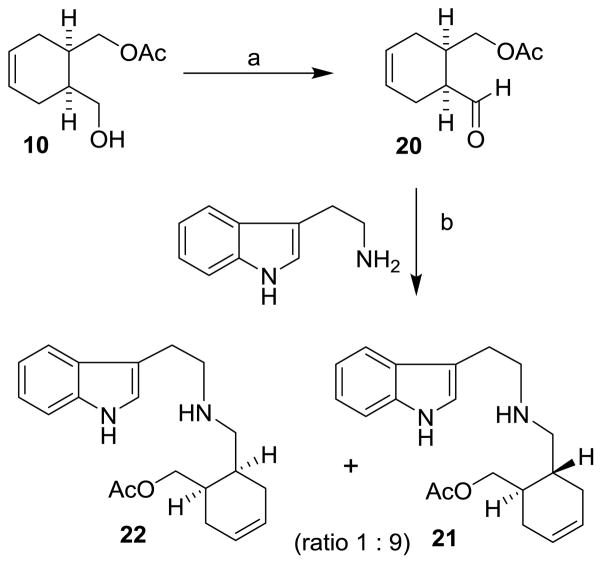
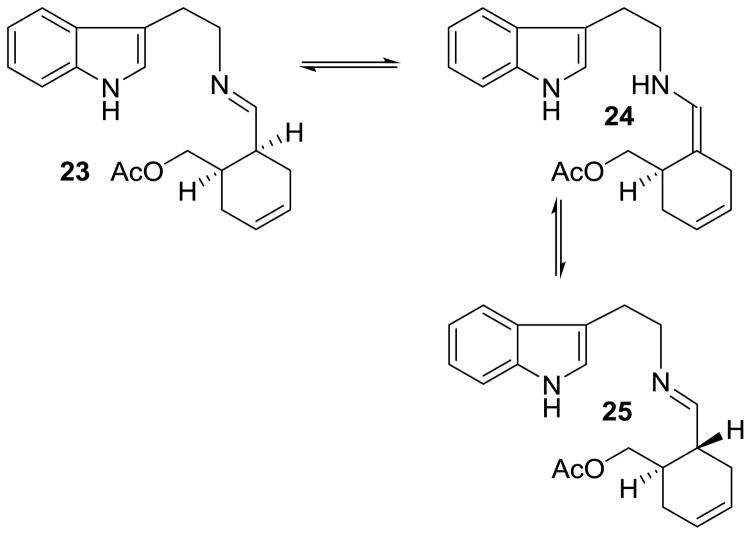
The synthesis of (−)-yohimbane, 2 from (1R, 6S)-6-(hydroxymethylcyclohexenyl)methyl acetate 10 is shown in Scheme 4. Swern oxidation of alcohol 10 provided aldehyde 20, which was immediately subjected to reductive amination with tryptamine in the presence of acetic acid and sodium cyanoborohydride at 23 °C for 10 h. To our delight, the reductive amination conditions led to the formation of the epimerized product 21 along with the unepimerized product 22 in a 9:1 diastereomeric ratio by 1H-NMR analysis. Swern oxidation conditions mainly provided cis-aldehyde 20 and only trace amount of epimerized trans-aldehyde 11 by 1H-NMR analysis. The reductive amination provided epimerized product 21 as a major product presumably due to epimerization via an imine-enamine pathway. As shown in Figure 3, initial imine 23 isomerized to the more stable trans-imine 25 via enamine 24 under the reaction conditions. Interestingly, hydrogenation of cis-aldehyde with tryptamine using 10% Pd-C in methanol at 23 °C for 24 h resulted in significantly less epimerization and the corresponding saturated cis-isomer of 22 was formed as the major product (7:3 diastereomeric ratio). The acetate group was hydrolyzed under this reaction condition.
Scheme 4.
Reagents and conditions: (a) (COCl)2, DMSO, Et3N, −78 °C, CH2Cl2 (93%); (b) AcOH, MeOH, Na(CN)BH3, 23 °C (74%).
Figure 3.
Plausible epimerization pathway
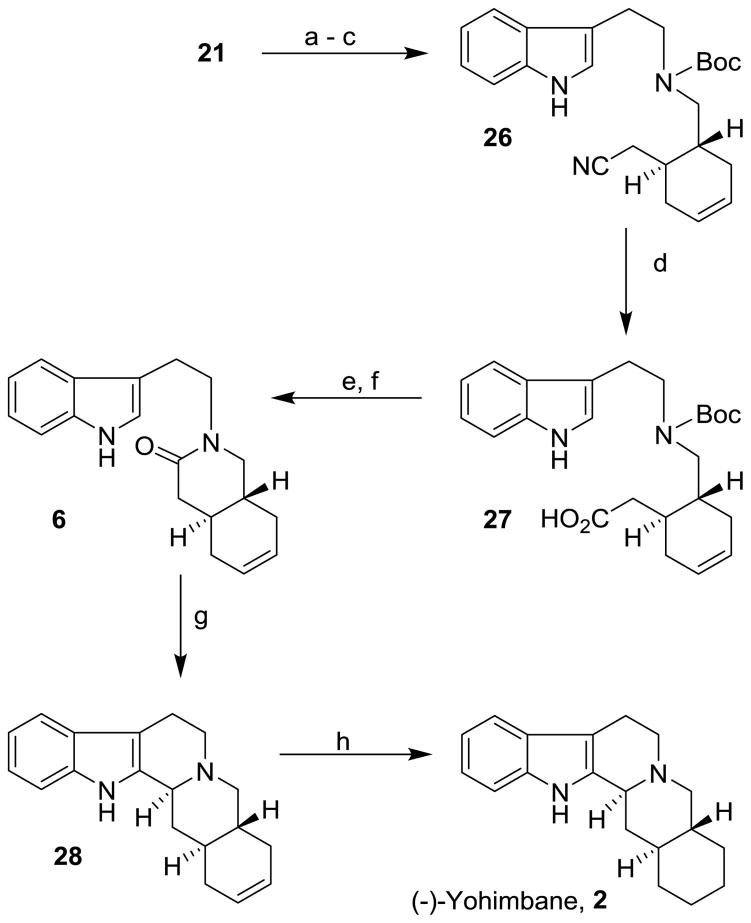
Amine 21 was converted to (−)-yohimbane as shown in Scheme 5. Treatment of amine 21 with Boc2O provided the corresponding Boc-amine in 92 % yield. Saponification of acetate afforded the corresponding alcohol in 96% yield. The resulting alcohol was converted to cyanide 26 in 94% yield. The cyanide was hydrolyzed to carboxylic acid 27 as described above. Carboxylic acid 27 was subjected to trifluoroacetic acid and the resulting aminoacid was converted to lactam 6 in 42% yield over two steps. The lactam 6 was reacted with phosphorus oxychloride in CH2Cl2 at 50 °C for 3 h followed by treatment with NaBH4 to afford the pentacyclic indole derivative 28 in 72% yield. Catalytic hydrogenation of 28 using 10% Pd-C under a hydrogen filled balloon furnished (−)-yohimbane 2 in 92% yield. The spectroscopic data of our synthetic (−)-yohimbane {[α]20D = −77.6 (c 0.22, EtOH)} is in complete agreement with the synthetic (−)-yohimbane {[α]22 D= −81 (c 0.5, EtOH)} reported in the literature.23
Scheme 5.
Reagents and conditions: (a) Boc2O, Et3N, CH2Cl2, 23 °C; (b) K2CO3, MeOH, 0 °C; (c) Ph3P, DIAD, Me2C(OH)CN, THF, 23 °C (83% for 3-steps); (d) 20% aq NaOH, EtOH (1:1), reflux (81%); (e) CF3CO2H, CH2Cl2, 23 °C; (f) EDCI, HOBt, iPr2NEt, CH2Cl2, 23 °C (42% for 2-steps); (g) POCl3, CH2Cl2, 50 °C, then, NaBH4, MeOH/H2O, 0 °C (72%); (h) H2, 10% Pd-C, EtOAc, 23 °C (92%).
Conclusions
In summary, we have developed stereoselective syntheses of (−)-yohimbane and (−)-alloyohimbane which feature enantioselective enzymatic desymmetrization using sodium bicarbonate to neutralize the acetic acid formed. To the best of our knowledge, this procedure has not been reported in literature and should serve as an excellent technique for large-scale synthesis of optically active starting material due to the mild basicity of sodium bicarbonate. Epimerization during reductive amination was taken to our advantage and utilized for the synthesis of (−)-yohimbane. Different stereochemical consequences attained by reductive amination and amide coupling reactions led to efficient paths towards synthesis of the pentacyclic cores of yohimbine alkaloids. The strategically positioned unsaturated site in the pentacyclic indole derivatives 19 and 28 can be used conveniently to synthesize yohimbol, yohimbine, alloyohimbine, corynanthine and related alkaloids. The efficacy and brevity of this synthesis underlines the value of enantioenriched products easily accessible by enzymatic desymmetrization.
Experimental Section
All reactions were carried out under an atmosphere of argon in oven-dried (120 °C) glassware with magnetic stirring unless otherwise noted. Solvents, reagents and chemicals were purchased from commercial suppliers. Solvents were distilled as follows: dichloromethane from calcium hydride, tetrahydrofuran from sodium/benzophenone, and methanol from activated magnesium. Purification of reaction products was carried out by flash chromatography using Silicycle silica gel 230–400 mesh, 60 Å pore diameter. Analytical thin layer chromatography was performed on glass-backed thin-layer silica gel chromatography plates (0.25 mm thickness, 60 Å, F-254 indicator). Visualization was realized with UV light and ethanolic phosphomolybdic acid solution or ethanolic acidic p-anisaldehyde solution followed by heating. Optical rotations were measured on a Perkin Elmer 341 polarimeter with a sodium lamp and are reported as follows: [α]DT °C (c = g/100 mL, solvent). Infrared spectra were recorded on a Varian 2000 Infrared spectrophotometer and are reported as cm−1. 1H NMR spectra were recorded at room temperature on a Bruker ARX-400 spectrometer and are reported in ppm using solvent (CDCl3 at 7.26 ppm, CD3OD at 3.31 ppm) as an internal standard. Data are reported as (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublets, ddd = doublet of doublet of doublets, td = triplet of doublets, qd = quartet of doublets, dt = doublet of triplets, dq = doublet of quartets, brs = broad singlet; coupling constant(s) in Hz; integration). Proton-decoupled 13C NMR spectra were recorded on a Bruker ARX-400 spectrometer and are reported in ppm using solvent as the internal standard (CDCl3 at 77.16 ppm, CD3OD at 49.00 ppm). Low and High resolution mass spectra were obtained at the Purdue University Department of Chemistry Mass Spectrometry Center.
((1R,6S)-6-(hydroxymethyl)cyclohex-3-en-1-yl)methyl acetate (10)
To a suspension of diacetate 12 (51 g, 0.23 mol) in 0.1 M Phosphate buffer (650 mL, pH 7) was added Porcine Pancreatic Lipase (2.55 g, Sigma type II, crude). 1 N Sodium bicarbonate solution (690 mL) was added and the heterogeneous mixture was stirred for 24 hours. The mixture was then filtered through Celite®. The filtrate was extracted with dichloromethane (×3). The organic layer was washed with brine, dried over sodium sulfate, filtered and evaporated. The residue was purified by chromatography on silica gel (25% to 30% ethyl acetate/hexanes) to obtain 10 (34.7 g, 0.19 mol, 84% yield) as a colorless oil. Rf 0.6 (50% ethyl acetate/hexanes); [α]D20= −20.1 (c 2.0, CHCl3); lit.1, [α]D23 = −17.0 (c 0.42, CHCl3); 1H NMR (400 MHz, CDCl3) δ 5.75 – 5.54 (m, 2H), 4.19 (dd, J = 11.0, 6.0 Hz, 1H), 3.95 (dd, J = 11.0, 8.0 Hz, 1H), 3.68 (dd, J = 10.8, 7.1 Hz, 1H), 3.59 (dd, J = 10.7, 6.9 Hz, 1H), 2.29 – 2.08 (m, 3H), 2.06 (s, 3H), 2.01 – 1.68 (m, 3H), 1.80 (brs, 1H, OH); 13C NMR (101 MHz, CDCl3) δ 171.5, 125.8, 125.3, 77.4, 65.1, 63.9, 37.4, 33.4, 27.2, 26.1, 21.2; LRMS-ESI (m/z): 207.2 [M + Na]+.
((1R,6S)-6-(azidomethyl)cyclohex-3-en-1-yl)methyl acetate (13)
To a solution of alcohol 10 (1.25 g, 6.8 mmol) and PPh3 (3.6 g, 13.6 mmol) in tetrahydrofuran (65 mL) at 0 °C was added diethyl azodicarboxylate (5.3 mL, 13.6 mmol) and the solution was stirred for 5 minutes. Diphenyl phosphoryl azide (2.9 mL, 13.6 mmol) was then added at room temperature and the solution was stirred for 20 minutes. The solvent was evaporated and the crude mixture was purified over silica gel using 5% ethyl acetate/hexanes to yield 13 (1.3 g, 6.2 mmol, 92% yield) as a yellow liquid. Rf 0.25 (5% ethyl acetate/hexanes); [α]D25 −1.7 (c 0.35, CHCl3); IR: 3028, 2900, 2845, 2094, 1976, 1737, 1653, 1439, 1396, 1387, 1336, 1035, 922, 909 cm−1; 1H-NMR (400 MHz, CDCl3) δ 5.57 (s, 2H), 4.02 (dd, J = 11.0, 7.0 Hz, 1H), 3.93 (dd, J = 11.1, 7.3 Hz, 1H), 3.31 (dd, J = 12.1, 6.3 Hz, 1H), 3.18 (dd, J = 12.0, 8.3 Hz, 1H), 2.22 – 2.02 (m, 4H), 1.99 (s, 3H), 1.94 – 1.79 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 170.9, 125.0, 124.9, 64.6, 52.4, 34.4, 33.8, 27.0, 26.5, 20.8; HRMS (ESI) m/z calcd for C10H15N3O2Na [M+Na]+: 232.1062, found: 232.1058.
N-(((1S,6R)-6-(hydroxymethyl)cyclohex-3-en-1-yl)methyl)acetamide (14)
To a solution of azide 13 (60 mg, 0.29 mmol) in tetrahydrofuran (4 mL) was added triphenylphosphine (151 mg, 0.58 mmol), followed by few drops of water. The solution was heated at 60 °C for 2 hours. The solvent was evaporated, and the residue was purified over silica gel with 5–10% (5% ammonia/methanol)/dichloromethane) to afford acetamide 14 as a brown oil (25 mg, 0.14 mmol, 48% yield) along with amino-acetate 9 (vide article) (10 mg, 0.05 mmol, 18% yield). Analytical data for 9 could not be collected because it gradually converted to 14 under purification conditions and upon standing. Analytical data for 14: Rf 0.1 (5% (5% ammonia / methanol) / dichloromethane); [α]D25 +27.1 (c 1.9, CHCl3); 1H NMR (400 MHz, CD3OD) δ 5.73 – 5.53 (m, 2H), 3.62 (dd, J = 10.8, 6.1 Hz, 1H), 3.48 (dd, J = 10.8, 7.5 Hz, 1H), 3.24 (dd, J = 13.3, 5.5 Hz, 1H), 3.14 – 3.03 (m, 1H), 2.20 – 1.96 (m, 5H), 1.94 (s, 3H), 1.89 – 1.78 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 170.9, 125.7, 125.3, 63.0, 40.1, 36.9, 34.1, 27.8, 26.6, 23.0; LRMS-ESI (m/z): 183.2; HRMS (ESI) m/z calcd for C10H17NO2Na [M+Na]+: 206.1157, found: 206.1155.
((1R,6S)-6-(aminomethyl)cyclohex-3-en-1-yl)methanol (15)
To a solution of azido-acetate 14 (1.02 g, 4.9 mmol) in methanol (30 mL) was added potassium carbonate (672 mg, 4.9 mmol), and stirred for 2 hours. Methanol was removed under reduced pressure, dichloromethane was added and the mixture was washed with water. The organic layer was washed with brine, dried over sodium sulfate and the solvent was evaporated to produce 774 mg (4.7 mmol, 96% yield) of the corresponding alcohol as a pale yellow viscous liquid. Rf 0.75 (40% ethyl acetate/hexanes); 1H NMR (400 MHz, CDCl3) δ 5.72 – 5.52 (m, 2H), 3.63 (dd, J = 10.8, 6.9 Hz, 1H), 3.55 (dd, J = 10.8, 6.7 Hz, 1H), 3.40 (dd, J = 12.2, 6.3 Hz, 1H), 3.23 (dd, J = 12.1, 8.1 Hz, 1H), 2.22 – 2.01 (m, 4H), 2.00 – 1.81 (m, 2H).
The azido-alcohol (750 mg, 4.5 mmol) obtained above was dissolved in tetrahydrofuran (25 mL) and triphenylphosphine (2.35 g, 9 mmol) was added. The mixture was heated at 60 °C for 1 hour. A few drops of water were added, and further heated at 60 °C for 1 hour. The solvent and water were removed under vacuum, and the residue was purified by column chromatography using 5–20% (5% ammonia/methanol)/dichloromethane to obtain amino-alcohol 15 (513 mg, 3.63 mmol, 81% yield) as a yellow oil in 78% yield over 2 steps. Rf 0.3 [20% (5% ammonia/methanol)/dichloromethane]; [α]D20 −7.95 (c 2, Methanol); 1H NMR (400 MHz, CD3OD) δ 5.62 (s, 2H), 3.57 (dt, J = 10.0, 5.0 Hz, 1H), 3.45 (dd, J = 10.9, 6.2 Hz, 1H), 2.70 (dd, J = 12.5, 6.0 Hz, 1H), 2.57 (dd, J = 12.6, 7.1 Hz, 1H), 2.17 – 1.89 (m, 6H); 13C NMR (101 MHz, CD3OD) δ 126.9, 126.6, 63.3, 43.1, 39.0, 28.8, 27.8; LRMS (TWA-CI) m/z: 142.25 [M+H]+; HRMS (ESI) m/z calcd for C8H16NO [M+H]+: 142.1232, found: 142.1226.
N-(((1S,6R)-6-(hydroxymethyl)cyclohex-3-en-1-yl)methyl)-2-(1H-indol-3-yl)acetamide (17)
A solution of indole-3-acetic acid (666 mg, 3.82 mmol) and HOBt hydrate (703 mg, 5.2 mmol) in dry tetrahydrofuran (15 mL) was cooled to 0 °C. EDCI.HCl (732 mg, 3.82 mmol) was added and the mixture was stirred for 1 h at 0 °C. A solution of amino-alcohol 15 (490 mg, 3.47 mmol) and DIPEA (1.81 mL, 10.4 mmol) in tetrahydrofuran (10 mL) was then added at rt, and the reaction mixture was stirred for 12 hours. The solvent was evaporated and the residue was dissolved in ethyl acetate. The solution was washed with saturated aqueous sodium bicarbonate, brine and dried over Na2SO4. Ethyl acetate was evaporated and the residue was purified over silica gel using 75% ethyl acetate/hexanes to ethyl acetate to furnish light yellow solid 17 (850 mg, 2.85 mmol, 75% yield). Rf 0.19 (ethyl acetate); [α]D20 +32.7 (c 2, CHCl3); 1H NMR (400 MHz, CDCl3) δ 9.26 (s, 1H), 7.51 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.19 (t, J = 7.3 Hz, 1H), 7.10 (t, J = 7.4 Hz, 1H), 7.05 (s, 1H), 6.39 (s, 1H), 5.50 (q, J = 9.9 Hz, 2H), 3.70 (s, 2H), 3.58 – 3.48 (m, 1H), 3.39 (ddd, J = 25.9, 11.7, 5.7 Hz, 3H), 2.87 (dt, J = 13.1, 6.3 Hz, 1H), 2.00 – 1.80 (m, 4H), 1.79 – 1.60 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 172.8, 127.0, 125.7, 125.2, 124.3, 122.4, 119.8, 118.5, 111.7, 108.3, 63.4, 40.1, 36.9, 34.0, 33.4, 27.8, 26.3; LRMS (ESI) m/z: 321.3 [M+Na]+; HRMS (ESI) m/z calcd for C18H22N2O2Na [M+Na]+: 321.1279, found: 321.1569.
((1R,6S)-6-(((2-(1H-indol-3-yl)ethyl)amino)methyl)cyclohex-3-en-1-yl)methanol (16)
To a solution of amide 17 (800 mg, 2.68 mmol) in tetrahydrofuran (40 mL) was added lithium aluminum hydride (407 mg, 10.7 mmol). The suspension was stirred at 80° C for 12 hours. The mixture was cooled to 0° C and ethyl acetate was added until bubbling ceased. 5N sodium hydroxide solution (9 mL) was added, followed by solid sodium sulfate. The mixture was filtered through Celite® and the filtrate was evaporated. The residue was taken in ethyl acetate, washed with water and brine. The solvent was evaporated, and the residue was purified over silica gel with 5% (5% ammonia/methanol)/dichloromethane to afford amino-alcohol 16 (561 mg, 1.97 mmol, 74% yield) as a yellow oil. Rf 0.5 [10% (5% ammonia/methanol)/dichloromethane]; [α]D20 −20.7 (c 1.46, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.92 (s, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.36 (d, J = 7.9 Hz, 1H), 7.19 (t, J = 7.2 Hz, 1H), 7.12 (d, J = 7.2 Hz, 1H), 6.98 (s, 1H), 5.75 – 5.51 (m, 2H), 4.40 (s, 1H), 3.75 – 3.53 (m, 2H), 3.07 – 2.80 (m, 4H), 2.74 (t, J = 10.7 Hz, 1H), 2.42 (d, J = 12.2 Hz, 1H), 2.29 – 2.15 (m, 2H), 2.08 – 1.78 (m, 5H); 13C NMR (101 MHz, CDCl3) δ 136.4, 127.2, 126.6, 124.6, 122.2, 122.0, 119.2, 118.7, 113.1, 111.2, 64.6, 50.3, 49.3, 39.1, 37.1, 30.8, 25.3, 25.1; LRMS (ESI) m/z: 285.3 [M+H]+; HRMS (ESI) m/z calcd for C18H25N2O [M+H]+: 285.1967, found: 285.1961.
tert-butyl (2-(1H-indol-3-yl)ethyl)(((1S,6S)-6-(cyanomethyl)-cyclohex-3-en-1-yl)methyl)carbamate (7)
To a solution of amino-alcohol 16 (450 mg, 1.58 mmol) in dichloromethane (10 mL) was added di-tert-butyl dicarbonate (Boc anhydride, 328 mg, 1.5 mmol). The solution was stirred for 5 hours at room temperature. The solvent was evaporated under reduced pressure and the residue was purified over silica gel using 15% ethyl acetate/hexanes to yield the N-boc derivative (535 mg, 1.39 mmol, 88% yield) along with traces of the corresponding tert-butylcarbonate (O-Boc derivative). Rf 0.25 (40% ethyl acetate/hexanes); [α]D20 +7.8 (c 0.5, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 7.62 (d, J = 7.5 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.19 (d, J = 7.4 Hz, 1H), 7.12 (t, J = 7.4 Hz, 1H), 6.99 (s, 1H), 5.81 – 5.46 (m, 2H), 3.86 – 3.40 (m, 5H), 3.36 – 3.20 (m, 1H), 3.07 – 2.89 (m, 2H), 2.18 (s, 1H), 2.00 (d, J = 15.3 Hz, 3H), 1.77 (d, J = 14.2 Hz, 1H), 1.61 (d, J = 19.2 Hz, 2H); 13C NMR (101 MHz, CDCl3) δ 156.4, 136.3, 127.3, 126.0, 125.6, 121.9, 119.2, 118.6, 113.1, 111.2, 79.8, 63.4, 49.3, 47.2, 37.1, 33.1, 28.3, 27.9, 26.6, 24.5.
To a solution of the above Boc-protected amino-alcohol (500 mg, 1.3 mmol) in dry tetrahydrofuran (8 mL) was added triphenylphosphine (1.02 g, 3.9 mmol), followed by dropwise addition of diisopropyl azodicarboxylate (0.77 mL, 3.9 mmol) at 0 °C. The solution turned cloudy after 5 minutes. After 5 more minutes, acetone cyanohydrin (0.6 mL, 6.5 mmol) was added and the reaction mixture turned clear yellow. The solution was warmed to room temperature and stirred for 6 hours. The mixture was then poured into water and extracted with ethyl acetate (×3). The organic layer was washed with brine, dried over Na2SO4, filtered and evaporated to dryness. The residue was purified by silica gel chromatography (5% to 30% ethyl acetate/hexanes) to yield pale yellow amorphous solid 7 (480 mg, 1.22 mmol, 94% yield. Rf 0.6 (40% ethyl acetate/hexanes); [α]D20 −4.0 (c 0.42, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.29 (s, 1H), 7.62 (d, J = 6.5 Hz, 1H), 7.36 (d, J = 7.5 Hz, 1H), 7.24 – 7.16 (m, 1H), 7.16 – 7.05 (m, 1H), 6.97 (s, 1H), 5.62 (s, 2H), 3.58 (dt, J = 14.0, 7.2 Hz, 1H), 3.50 – 3.19 (m, 2H), 3.14 – 2.91 (m, 3H), 2.14 (dd, J = 36.6, 21.1 Hz, 6H), 1.99 (d, J = 19.5 Hz, 1H), 1.86 – 1.63 (m, 1H), 1.52 (s, 3H), 1.46 (s, 6H); 13C NMR (101 MHz, CDCl3) δ 136.4, 125.6, 124.6, 122.3, 122.0, 119.6, 118.8, 111.4, 71.7, 48.0, 35.0, 31.4, 29.5, 28.6, 26.6, 26.3, 22.1; LRMS-ESI (m/z): 416.3 [M+Na]+; HRMS (ESI) m/z calcd for C24H31N3O2Na [M+Na]+ : 416.2314, found: 416.2308.
2-((1S,6S)-6-(((2-(1H-indol-3-yl)ethyl)(tert-butoxycarbonyl)-amino)methyl)cyclohex-3-en-1-yl)acetic acid (18)
To 7 (430 mg, 1.09 mmol) was added 1:1 20% aqueous sodium hydroxide/ethanol (6 mL). The mixture was stirred at room temperature for 1 hour, followed by addition of water (1 mL). The reaction was then refluxed for 18 hours. The mixture was cooled to 0 °C, acidified to pH 3 by careful addition of saturated aqueous citric acid, and then extracted with ethyl acetate (×3). The organic layer was washed with brine, dried over Na2SO4, filtered and the solvent was evaporated. The residue was purified by silica gel chromatography with 60% ethyl acetate/hexanes to obtain carboxylic acid 18 (341 mg, 0.83 mmol, 76% yield) as a yellow amorphous solid. Rf 0.2 (60% ethyl acetate/hexanes); [α]D20 +28.9 (c 1.9, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 7.60 (d, J = 8.3 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.23 – 7.16 (m, 1H), 7.12 (t, J = 7.1 Hz, 1H), 6.98 (s, 1H), 5.61 (s, 2H), 3.60 (dq, J = 15.4, 8.1, 7.5 Hz, 1H), 3.46 (dd, J = 14.2, 8.9 Hz, 1H), 3.35 (dt, J = 13.5, 6.8 Hz, 1H), 3.12 – 2.95 (m, 2H), 2.90 (dd, J = 14.8, 5.6 Hz, 1H), 2.55 (d, J = 10.3 Hz, 1H), 2.37 – 2.06 (m, 4H), 2.04 – 1.76 (m, 3H), 1.44 (m, 9H). 13C NMR (101 MHz, CDCl3) δ 177.2, 136.4, 127.5, 125.6, 125.4, 122.2, 122.1, 119.5, 118.7, 113.1, 111.4, 80.6, 48.4, 35.8, 33.7, 30.9, 29.8, 28.6, 26.3, 24.5; LRMS (ESI) m/z: 435.3 [M+Na]+; HRMS (ESI) m/z calcd for C24H32N2O4 [M+Na]+ : 435.2260, found: 435.2256.
(4aS,8aS)-2-(2-(1H-indol-3-yl)ethyl)-1,4,4a,5,8,8a-hexahydroiso-quinolin-3(2H)-one (5)
To a solution of carboxylic acid 18 (165 mg, 0.4 mmol) in dichloromethane (2 mL) at 0 °C was added trifluoroacetic acid (0.67 mL). The solution turned from yellow to orange as it was gradually warmed to room temperature. After 1 hour, the reaction mixture was concentrated in vacuo. The residue was taken in dichloromethane (4 mL), and diisopropylethylamine (0.35 mL, 2 mmol) was added at 0 °C. HOBt hydrate (73 mg, 0.48 mmol) was then added, followed by EDCI.HCl (84 mg, 0.44 mmol). Diisopropylethylamine (0.35 mL, 2 mmol) was added again and the solution was allowed to warm to room temperature. After 18 hours, water was added to the reaction mixture, and it was extracted with ethyl acetate (×3). The combined organic layers washed with brine, dried over Na2SO4, filtered and the solvent was evaporated. The residue was subjected to silica gel chromatography with ethyl acetate to afford lactam 5 (74 mg, 0.25 mmol, 45% yield) as a white solid. Rf 0.1 (ethyl acetate); [α]D20 −18.1 (c 0.16, CHCl3); 1H-NMR (400 MHz, CDCl3) δ 8.31 (s, 1H), 7.67 (d, J = 7.9 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.23 – 7.09 (m, 2H), 7.03 (s, 1H), 5.58 (s, 2H), 3.72 (dt, J = 14.7, 7.7 Hz, 1H), 3.61 (dt, J = 13.6, 7.6 Hz, 1H), 3.17 (qd, J = 12.0, 5.5 Hz, 2H), 3.04 (t, J = 6.2 Hz, 2H), 2.38 (qd, J = 17.8, 5.8 Hz, 2H), 2.21 – 2.06 (m, 4H), 1.90 – 1.80 (m, 2H). 13C NMR (101 MHz, CDCl3): δ 169.1, 136.2, 127.4, 124.7, 124.4, 122.0, 121.8, 119.2, 118.7, 113.0. 111.1, 50.9, 48.0, 35.7, 30.0, 29.3, 28.0, 26.1, 22.8; LRMS-ESI (m/z): 317.3 [M+Na]+; HRMS (ESI) m/z calcd for C19H22N2ONa [M+Na]+: 317.1630, found: 317.1628.
(−)-Dehydroalloyohimbane (19)
To a solution of the lactam 5 (45 mg, 0.15 mmol) in dichloromethane (1 mL) was added phosphorus oxychloride (1 mL). The mixture was heated for 4 hours at 50 °C. The reaction was then cooled to room temperature and phosphorus oxychloride was removed in vacuo. The residue was dissolved in methanol/water (9:1) (1 mL) and cooled to 0 °C. Sodium borohydride was added until the pH > 7. Then 1 mL of saturated aqueous ammonium chloride solution and ice were added to the reaction mixture. The mixture was then extracted with dichloromethane (×3). The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated under reduced pressure. The crude residue was purified by silica gel chromatography with 30% ethyl acetate/hexanes to obtain 19 (31 mg, 0.11 mmol, 73% yield). Rf 0.3 (30% ethyl acetate/hexanes); [α]D20 −59.2 (c 1.00, CHCl3); 1H-NMR (400 MHz, CDCl3) δ 7.73 (s, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.27 (d, J = 6.8 Hz, 1H), 7.10 (dt, J = 14.8, 7.0 Hz, 2H), 5.80 – 5.41 (m, 2H), 3.24 (d, J = 10.7 Hz, 1H), 3.04 – 2.90 (m, 2H), 2.83 (d, J = 11.2 Hz, 1H), 2.73 – 2.51 (m, 4H), 2.43 (d, J = 17.9 Hz, 1H), 2.08 – 1.95 (m, 3H), 1.90 – 1.69 (m, 3H). 13C NMR (101 MHz, CDCl3): δ 136.1, 135.6, 126.2, 123.4, 121.3, 119.5, 118.2, 110.8, 108.3, 60.7, 60.4, 53.3, 33.3, 32.2, 32.0, 31.4, 25.7, 22.0; LRMS (ESI) m/z: 279.4 [M+H]+; HRMS (ESI) m/z calcd for C19H23N2 [M + H]+: 279.1861, found: 279.1857.
(−)-Alloyohimbane (1)
A 2-neck round-bottomed flask was evacuated and filled with argon. 10% Pd/C (5 mg) was added under argon. 0.5 mL of ethyl acetate was added down the sides of the flask to wash down any Pd/C in the walls. A solution of 19 (8 mg, 0.029 mmol) in 0.5 mL ethyl acetate was then added. The mixture was stirred. The flask was evacuated and re-filled with argon 3 times. A hydrogen balloon was then attached. The flask was evacuated and filled with hydrogen 3 times. After 2 hours, the balloon was removed and the flask was filled with Argon. The mixture was filtered over Celite® and the solvent was concentrated in vacuo. The residue was purified by chromatography over silica gel (30% ethyl acetate/hexanes) to yield (−)-Alloyohimbane 1 (7.4 mg, 0.026 mmol, 92% yield). Rf 0.3 (20% ethyl acetate/hexanes); [α]D20 −78.8 (c 0.3, Ethanol); 1H-NMR (400 MHz, CDCl3) δ 7.75 (s, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.30 (d, J = 7.7 Hz, 1H), 7.10 (dt, J = 17.9, 7.1 Hz, 2H), 3.24 – 3.18 (m, 1H), 3.00-2.93 (m, 2H), 2.79 (d, J = 11.3 Hz, 1H), 2.68 (d, J = 14.9 Hz, 1H), 2.52 (dq, J = 14.3, 8.7, 6.1 Hz, 2H), 2.01 – 1.86 (m, 3H), 1.75 – 1.57 (m, 5H), 1.42 (ddd, J = 16.8, 9.8, 5.7 Hz, 2H), 1.38-1.26 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 135.9, 135.4, 127.4, 121.1, 119.3, 118.0, 110.6, 108.1, 61.7, 60.3, 53.3, 36.5, 34.7, 31.8, 30.4, 26.4, 21.6, 20.8.; LRMS (ESI) m/z: 281.4 [M+H]; HRMS (ESI) m/z calcd for C19H25N2 [M+H]+: 281.2018, found: 281.2009.
((1R,6S)-6-formylcyclohex-3-en-1-yl)methyl acetate (20)
To a solution of oxalyl chloride (0.76 mL, 8.69 mmol) in dichloromethane (10 mL) at −78 °C was added dropwise a solution of dry dimethyl sulfoxide (1.23 mL, 17.4 mmol) in dichloromethane (10 mL). The mixture was stirred at −78 °C for 5 minutes. A solution of the alcohol 10 (800 mg, 4.35 mmol) in dichloromethane (10 mL) was then added dropwise to the mixture. After 30 minutes, triethylamine (2.43 mL, 17.4 mmol) was added and the mixture was stirred at −78 °C for 10 minutes. The reaction was then allowed to warm to room temperature. The mixture was washed successively with water, 2N HCl, water, brine and dried over Na2SO4. The solvent was evaporated to obtain aldehyde 20 (736 mg, 4.05 mmol, 93% yield) as a colorless oil. Rf 0.8 (50% ethyl acetate/hexanes); 1H-NMR (400 MHz, CDCl3) δ 9.69 (s, 1H), 5.74 – 5.54 (m, 2H), 4.23 – 3.85 (m, 2H), 2.59 (q, J = 7.8, 7.1 Hz, 2H), 2.31 – 2.17 (m, 3H), 2.05 – 1.98 (m, 1H), 1.96 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 202.9, 170.2, 125.2, 124.5, 64.1, 46.9, 32.5, 26.5, 22.4, 20.3.
((1R,6R)-6-(((2-(1H-indol-3 yl)ethyl)amino)methyl)cyclohex-3-en-1-yl)methyl acetate (21)
To a solution of aldehyde 20 (700 mg, 3.85 mmol) and tryptamine (801 mg, 5 mmol) in methanol (25 mL) was added glacial acetic acid (0.22 mL, 3.85 mmol) and 4 Å molecular sieves (1.5 g, powdered, activated). The orange solution was stirred for 1 hour. Sodium cyanoborohydride was then added and the reaction was stirred for 10 hours. The mixture was then filtered and the methanol was evaporated. The residue was purified by silica gel chromatography with ethyl acetate to obtain the secondary amine 21 (917 mg, 2.81 mmol, 73% yield) as an orange semi-solid. Rf 0.2 [5%(5% ammonia/methanol)/dichloromethane]; [α]D20 −30.7 (c 7.0, Methanol); 1H NMR (400 MHz, CD3OD) δ 7.60 (d, J = 7.8 Hz, 1H), 7.39 (d, J = 8.1 Hz, 1H), 7.16 – 7.00 (m, 3H), 5.56 (s, 2H), 3.93 (h, J = 5.6 Hz, 2H), 3.20 – 3.07 (m, 4H), 2.97 (dd, J = 12.5, 5.0 Hz, 1H), 2.78 (dd, J = 12.6, 9.0 Hz, 1H), 2.15 (d, J = 18.1 Hz, 1H), 2.05 – 1.98 (m, 5H), 1.90 – 1.76 (m, 3H). 13C NMR (101 MHz, CD3OD) δ 127.8, 125.9, 124.6, 124.0, 122.6, 120.0, 118.9, 112.4, 110.1, 66.5, 51.7, 49.7, 34.8, 32.5, 26.6, 25.9, 22.9, 20.9; LRMS (ESI) m/z: 327 [M+H]+; HRMS (ESI) m/z calcd for C20H27N2O2 [M+H]+: 327.2073, found: 327.2062.
Reductive amination under hydrogenation condition
To a solution of aldehyde 20 (63 mg, 0.35 mmol) in dry methanol (2 mL) under argon was added tryptamine (55 mg, 0.35 mmol). Molecular sieves (150 mg, 4 Å, powdered, oven-dried) were then added, followed by 10% Pd-C (19 mg). The argon balloon was replaced with a hydrogen balloon, and the reaction was stirred for 24 hours at room temperature. The mixture was then filtered through Celite®, the methanol was removed under reduced pressure, and the residue was purified with 5%(5% ammonia/methanol)/dichloromethane to afford saturated cis-amine (31 mg, 0.11 mmol, 31% yield) and saturated trans-amine (13 mg, 0.045 mmol, 13% yield). 1H NMR for 26 (400 MHz, CDCl3) δ 9.00 (s, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.38 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 7.7 Hz, 1H), 7.13 (d, J = 5.0 Hz, 1H), 7.07 (d, J = 7.7 Hz, 1H), 3.48 (dd, J = 11.1, 2.3 Hz, 1H), 3.43 – 3.27 (m, 1H), 3.24-3.18 (m, 2H), 3.15 – 3.10 (m, 1H), 3.07-3.00 (m, 1H), 2.76 – 2.73 (m, 1H), 1.70 – 1.53 (m, 3H), 1.45-1.37 (m, 2H), 1.26 – 1.18 (m, 2H), 1.16 – 0.99 (m, 3H), 0.78-0.68 (m, 1H); LRMS (APCI) m/z: 287 [M+H]+.
1H NMR (400 MHz, CDCl3) δ 8.41 (s, 1H), 7.61 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.20 (t, J = 7.3 Hz, 1H), 7.12 (t, J = 7.4 Hz, 1H), 7.01 (d, J = 1.9 Hz, 1H), 3.67-3.55 (m, 1H), 3.55 – 3.48 (m, 1H), 2.99 – 2.93 (m, 2H), 2.93 – 2.86 (m, 1H), 2.86 – 2.75 (m, 1H), 2.52 – 2.40 (m, 1H), 1.79 (s, 2H), 1.62 – 1.46 (m, 3H), 1.46 – 0.99 (m, 6H); LRMS (ESI) m/z: 287.4 [M+H]+.
tert-butyl (2-(1H-indol-3-yl)ethyl)(((1S,6S)-6-(cyanomethyl)-cyclohex-3-en-1-yl)methyl)carbamate (26)
To a solution of amine 21 (800 mg, 2.45 mmol) in dichloromethane (10 mL) was added triethylamine (0.38 mL, 2.7 mmol). The mixture was cooled to 0 °C. A solution of di-tert-butyl dicarbonate (Boc anhydride, 589 mg, 2.7 mmol) in dichloromethane (5 mL) was then added. The reaction was stirred for 90 minutes. The solvent was evaporated and the residue was purified by silica gel chromatography with 15% ethyl acetate/hexanes to obtain the –Boc protected amino-acetate (961 mg, 2.25 mmol, 92% yield). Rf 0.6 (40% ethyl acetate/hexanes); [α]D20 −20.9 (c 2, CHCl3); 1H-NMR (400 MHz, CDCl3) δ 8.13 (s, 1H), 7.63 (d, J = 7.4 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.19 (t, J = 7.3 Hz), 7.12 (t, J = 7.5 Hz), 6.99 (s, 1H), 5.61 (d, J = 11.6 Hz, 2H), 4.01 (dd, J = 13.2, 5.1 Hz, 2H), 3.48 (d, J = 5.3 Hz, 2H), 3.21 (s, 2H), 3.00 (s, 2H), 2.15 – 2.05 (m, 3H), 2.03 (s, 3H), 1.89 (d, J = 9.9 Hz, 1H), 1.84 – 1.77 (m, 2H), 1.51 – 1.41 (m, 9H). 13C NMR (101 MHz, CDCl3) δ 171.2, 155.9, 136.2, 127.4, 125.7, 124.9, 122.1, 121.8, 119.1, 118.6, 112.9, 111.1, 79.3, 66.5, 64.7, 48.4, 33.9, 32.5, 28.4, 26.0, 25.4, 22.6, 23.7, 20.9.
To a solution of the above amino-acetate (800 mg, 1.88 mmol) in methanol (10 mL) was added potassium carbonate (260 mg, 1.88 mmol). The mixture was stirred for 2 hours at room temperature. The methanol was evaporated, water was added and extracted with dichloromethane (×3). The combined organic layers were washed with water, brine, dried over sodium sulfate and the solvent was evaporated. The residue was purified by silica gel chromatography (30% to 40% ethyl acetate/hexanes) to obtain the amino-alcohol (694 mg, 1.8 mmol, 96% yield). Rf 0.3 (40% ethyl acetate/hexanes); [α]D20 −7.7 (c 1.7, CHCl3); 1H-NMR (400 MHz, CDCl3) δ 8.07 (s, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.36 (d, J = 8.1 Hz, 1H), 7.20 (t, J = 7.4 Hz, 1H), 7.12 (t, J = 7.3 Hz, 1H), 6.99 (s, 1H), 5.66 – 5.57 (m, 2H), 3.58-3.47 (m, 5H), 3.02-2.98 (m, 3H), 2.19 – 2.02 (m, 2H), 1.98 (m, 2H), 1.78 (d, J = 15.9 Hz, 1H), 1.67 – 1.56 (m, 2H), 1.44 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 155.9, 136.2, 127.4, 125.5, 125.0, 121.9, 121.8, 119.2, 118.7, 113.4, 111.1, 79.4, 63.4, 50.3, 48.7, 47.1, 37.4, 33.1, 28.3, 26.5, 24.5; LRMS (ESI) m/z: 407.3 [M+Na]+; HRMS (ESI) m/z calcd for C23H32N2O3Na [M+Na]+: 407.2311, found: 407.2303.
To a solution of the above alcohol (615 mg, 1.6 mmol) in dry tetrahydrofuran (10 mL) was added triphenylphosphine (1.26 g, 4.8 mmol), followed by dropwise addition of diisopropyl azodicarboxylate (0.94 mL, 4.8 mmol) at 0 °C. The solution turned cloudy after 5 minutes. After 5 more minutes, acetone cyanohydrin (0.73 mL, 8 mmol) was added. The solution was warmed to room temperature and stirred for 6 hours. The mixture was then poured into water and extracted with ethyl acetate (×3). The organic layer was washed with brine, dried over sodium sulfate, filtered and evaporated to dryness. The residue was purified by silica gel chromatography (5% to 30% ethyl acetate/hexanes) to yield yellow liquid 26 (592 mg, 1.5 mmol, 83% yield over 3 steps). Rf 0.7 (40% ethyl acetate/hexanes); [α]D25 −7.1 (c 3.9, CHCl3); 1H-NMR (400 MHz, CDCl3) δ 8.49 (s, 1H), 7.61 (t, J = 7.1 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 7.17 (t, J = 7.3 Hz, 1H), 7.11 (t, J = 7.0 Hz, 1H), 6.97 (s, 1H), 5.63-5.55 (m, 2H), 3.61-3.50 (m, 1H), 3.31 (s, 1H), 3.16 – 2.89 (m, 3H), 2.34 – 1.91 (m, 6H), 1.83 (m, 1H), 1.68 (s, 2H), 1.48 (m, 9H); 13C NMR (101 MHz, CDCl3) δ 156.1, 136.4, 127.4, 125.5, 125.0, 124.4, 122.2, 121.9, 119.2, 118.6, 112.8, 111.4, 79.8, 71.4, 47.8, 34.8, 34.4, 28.4, 26.3, 26.1, 22.0, 21.2; LRMS (ESI) m/z: 416.3 [M+Na]+; HRMS (ESI) m/z calcd for C24H31N3O2Na [M+Na]+ : 416.2314, found: 413.2304.
2-((1S,6S)-6-(((2-(1H-indol-3-yl)ethyl)(tert-butoxycarbonyl)-amino)methyl)cyclohex-3-en-1-yl)acetic acid (27)
To compound 26 (500 mg, 1.27 mmol) was added 20% aqueous sodium hydroxide (4 mL) and ethanol (4 mL, to make the solution homogenous). The mixture was stirred at room temperature for 1 hour, followed by addition water (1 mL). The reaction was then refluxed for 12 hours. The mixture was cooled to 0 °C, acidified to pH 3 by careful addition of saturated aqueous citric acid, and then extracted with ethyl acetate (×3). The combined organic layers were dried over sodium sulfate, filtered and the solvent was evaporated. The residue was purified by silica gel chromatography with 60% ethyl acetate/hexanes to obtain carboxylic acid 27 (424 mg, 1.03 mmol, 81% yield) as a colorless oil. Rf 0.2 (60% ethyl acetate/hexanes); [α]D20 +28.9 (c 1.9, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.31 (s, 1H), 7.62 (d, J = 7.2 Hz, 2H), 7.34 (d, J = 7.0 Hz, 1H), 7.22 – 7.15 (m, 1H), 7.15 – 7.04 (m, 1H), 6.96 (s, 1H), 5.68 – 5.50 (m, 2H), 3.66 – 3.38 (m, 2H), 3.37 – 3.08 (m, 2H), 2.99 (s, 2H), 2.61 – 2.06 (m, 5H), 1.86 (dd, J = 42.4, 9.0 Hz, 3H), 1.54 – 1.37 (m, 9H); 13C NMR (101 MHz, CDCl3) δ 178.7, 136.4, 127.6, 125.0, 122.0, 119.3, 118.8, 113.3, 111.3, 79.7, 48.5, 38.2, 35.0, 31.3, 28.6, 28.0, 24.4; LRMS (ESI) m/z: 411.3 [M-H]; HRMS (ESI) m/z calcd for C23H32N2O3Na [M+Na]+: 435.2260, found: 435.2249.
(4aS,8aS)-2-(2-(1H-indol-3-yl)ethyl)-1,4,4a,5,8,8a-hexahydroiso-quinolin-3(2H)-one (6)
To a solution of the carboxylic acid 27 (300 mg, 0.73 mmol) in dichloromethane (3 mL) at 0 °C was added trifluoroacetic acid (1 mL). The solution turned yellow to orange to purple as it was gradually warmed to room temperature. After 1 hour, the reaction mixture was concentrated in vacuo. The residue was taken in dichloromethane (4 mL), and diisopropylethylamine (0.64 mL, 3.65 mmol) was added at 0 °C. HOBt hydrate (134 mg, 0.88 mmol) was then added, followed by EDCI.HCl (154 mg, 0.8 mmol). Diisopropylethylamine (0.64 mL, 3.65 mmol) were added and the solution was allowed to warm to room temperature. After 48 hours, water was added to the reaction mixture, and it was extracted with ethyl acetate (×3). The combined organic layers washed with brine, dried over sodium sulfate, filtered and the solvent was evaporated. The residue was subjected to silica gel chromatography (50% to 75% ethyl acetate/hexanes) to afford lactam 6 (90 mg, 0.31 mmol, 42% yield) as an amorphous solid. Rf 0.4 (5%(5% ammonia/methanol)/dichloromethane); [α]D20 −13.2 (c 1, CHCl3); 1H NMR (400 MHz, CDCl3) δ 8.06 (s, 1H), 7.67 (dq, J = 7.8, 0.9 Hz, 1H), 7.36 (d, J = 8.0 Hz, 1H), 7.22 – 7.16 (m, 1H), 7.13 (td, J = 7.5, 7.1, 1.0 Hz, 1H), 7.06 (d, J = 2.2 Hz, 1H), 5.65 (t, J = 4.7 Hz, 2H), 3.77 – 3.57 (m, 2H), 3.23 (dd, J = 12.2, 4.2 Hz, 1H), 3.13 – 2.96 (m, 2H), 2.99 – 2.88 (m, 1H), 2.64 (dd, J = 17.6, 4.8 Hz, 1H), 2.21 (dt, J = 14.6, 3.6 Hz, 1H), 2.10 – 2.02 (m, 2H), 1.81 – 1.71 (m, 2H), 1.67 – 1.63 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 169.5, 132.3, 132.2, 128.7, 128.6, 125.8, 125.1, 122.1, 119.4, 118.8, 111.3, 54.5, 48.2, 39.4, 33.8, 32.8, 32.0, 28.8, 23.1; LRMS-ESI (m/z): 293.5 [M-H]; HRMS (ESI) m/z: calcd for C19H22N2ONa [M+Na]+: 317.1630, found: 317.1626.
(4aS,13bS,14aS)-1,4,4a,5,7,8,13,13b,14,14a-decahydroindolo-[2′,3′:3,4]pyrido[1,2-b]isoquinoline (28)
To a solution of lactam 6 (67 mg, 0.23 mmol) in dichloromethane (1 mL) was added freshly distilled phosphorus oxychloride (1 mL). The mixture was heated for 4 hours at 50 °C. The reaction was then cooled to room temperature and the phosphorus oxychloride was evaporated in vacuo. The residue was dissolved in methanol/water (9:1, 1 mL) and cooled to 0 °C. Sodium borohydride was added until the pH > 7. Then 1 mL of saturated ammonium chloride and ice were added to the reaction mixture. The mixture was then extracted with dichloromethane (×3). The combined organic layers were washed with brine, dried over sodium sulfate, filtered and concentrated in vacuo. The crude residue was purified by silica gel chromatography with 90% ethyl acetate/toluene to obtain the 28 (46 mg, 0.16 mmol, 72% yield). Rf 0.4 (90% ethyl acetate/toluene); [α]D20 −55.3 (c 0.49, CHCl3); 1H-NMR (400 MHz, CDCl3) δ 7.74 (brs, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.30 (d, J = 7.9 Hz, 1H), 7.13-7.08 (m, 2H), 5.69 (d, J = 2.5 Hz, 2H), 3.38-3.34 (m. 1H), 3.13-2.96 (m, 3H), 2.74 (dd, J = 3.5 Hz, 7.8 Hz, 1H), 2.79 – 2.69 (m, 1H), 2.63 (td, J = 11.1, 4.4 Hz, 1H), 2.53 – 2.27 (m, 1H), 2.21 – 2.08 (m, 3H), 1.92 – 1.69 (m, 3H), 1.61 – 1.49 (m, 1H), 1.47 – 1.35 (m, 1H). 13C NMR (101 MHz, CDCl3): δ 136.2, 134.9, 127.6, 126.5, 126.2, 121.5, 119.6, 118.3, 110.8, 108.4, 62.0, 60.2, 53.3, 37.0, 36.9, 32.2, 30.7, 29.6, 21.8; LRMS-ESI (m/z): 279.4 [M+H]+; HRMS (ESI) m/z calcd for C19H23N2 [M+H]+: 279.1861, found: 279.1857.
(4aS,13bS,14aS)-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydro-indolo[2′,3′:3,4]pyrido[1,2-b]isoquinoline (2)
A 2-neck round-bottomed flask was evacuated and filled with Argon. 10% Pd/C (8 mg) was added under Argon. 0.5 mL of ethyl acetate was added down the sides of the flask to wash down any Pd/C in the walls. A solution of the 28 (21 mg, 0.075 mmol) in 0.5 mL ethyl acetate was then added. The mixture was stirred. The flask was evacuated and filled with Argon 3 times. A H2 balloon was then attached. The flask was evacuated and re-filled with H2 3 times. After 2 hours, the balloon was removed and the flask was filled with Argon. The mixture was filtered over Celite® and the solvent was removed in vacuo. The residue was purified by chromatography over silica gel (90% Ethyl acetate/Toluene) to yield (−)-Yohimbane 2 (19 mg, 0.069 mmol, 82% yield). Rf 0.3 (40% ethyl acetate/hexanes); [α]D20 −77.6 (c 0.22, ethanol); 1H-NMR (400 MHz, CDCl3) δ 7.73 (s, 1H), 7.47 (d, J = 7.3 Hz, 1H), 7.30 (d, J = 7.7 Hz, 1H), 7.10 (dt, J = 14.6, 6.7 Hz, 2H), 3.29 (d, J = 10.7 Hz, 1H), 3.09 (dt, J = 13.2, 6.3 Hz, 1H), 3.04 – 2.95 (m, 1H), 2.95 – 2.80 (m, 1H), 2.72 (d, J = 15.3 Hz, 1H), 2.62 (dt, J = 11.0, 5.8 Hz, 1H), 2.13 (t, J = 10.9 Hz, 1H), 2.00 (d, J = 12.1 Hz, 1H), 1.80 – 1.60 (m, 4H), 1.50 – 1.41 (m, 1H), 1.31 (m, 4H), 1.16 – 0.99 (m, 2H); 13C NMR (101 MHz, CDCl3): δ 135.9, 135.0, 127.4, 121.1, 119.2, 118.0, 110.6, 108.0, 62.0, 60.2, 53.1, 41.9, 36.9, 32.8, 30.3, 26.3, 25.9, 21.7; LRMS (ESI) m/z: 281.3 [M+H]+; HRMS (ESI) m/z calcd for C19H25N2 [M+H]+: 281.2018, found: 281.2005.
Supplementary Material
Acknowledgments
Financial support of this work was provided by the National Institutes of Health and Purdue University.
References
- 1.Baxter EW, Marino PS. In: Alkaloids: Chemical and Biological Perspectives. Pelletier SW, editor. Vol. 8. Springer-Verlag; New York: 1992. pp. 197–31. [Google Scholar]
- 2.Yang VW, Hong BC, Kao HK, Tu TH, Shen JY, Chen CL, Lee GH, Chou PT. Org Lett. 2015;17:5816–5819. doi: 10.1021/acs.orglett.5b02949. [DOI] [PubMed] [Google Scholar]
- 3.Szantay C, Blasko G, Honty K, Domye G. The Alkaloids. Vol. 27. Academic; New York: 1986. pp. 131–268. [Google Scholar]
- 4.Chatterjee A. Pure Appl Chem. 1986;58:685–692. [Google Scholar]
- 5.Lounasmaa M, Jokela R. Tetrahedron. 1990;46:615–622. [Google Scholar]
- 6.Scott JA, Crews FT. J Pharmacol Exp Ther. 1983;224:640–646. [PubMed] [Google Scholar]
- 7.Abebe W. J Dent Hyg. 2003;77:37–46. [PubMed] [Google Scholar]
- 8.Bagheri H, Schmitt L, Berlan M, Montastruc JL. Eur J Clin Pharmacol. 1997;52:339–342. doi: 10.1007/s002280050298. [DOI] [PubMed] [Google Scholar]
- 9.Reid K, Surridge DH, Morales A. Lancet. 1987;2:421–423. doi: 10.1016/s0140-6736(87)90958-5. [DOI] [PubMed] [Google Scholar]
- 10.Morales A. Int J Impot Res. 2000;12:S70–S74. [PubMed] [Google Scholar]
- 11.Naito T, Hirata Y, Miyata O, Ninomiya I. Chem Soc Perkin Trans 1. 1988;2219–2225 [Google Scholar]
- 12.Stork G, Guthikonda RN. J Am Chem Soc. 1972;94:5109–5110. doi: 10.1021/ja00769a068. [DOI] [PubMed] [Google Scholar]
- 13.Van Tamelen EE, Shamma M, Burgstahler AW, Wolinsky J, Tamm R, Aldrich PE. J Am Chem Soc. 1969;91:7315–7333. doi: 10.1021/ja01054a021. [DOI] [PubMed] [Google Scholar]
- 14.Herlé B, Wanner MJ, van Maarseveen JH, Hiemstra H. J Org Chem. 2011;76:8907–8912. doi: 10.1021/jo201657n. [DOI] [PubMed] [Google Scholar]
- 15.Mergott DJ, Zuend SJ, Jacobsen EN. Org Lett. 2008;10:745–748. doi: 10.1021/ol702781q. [DOI] [PubMed] [Google Scholar]
- 16.Aube J, Ghosh S, Tanol M. J Am Chem Soc. 1994;116:9009–9018. [Google Scholar]
- 17.Chen HW, Hsu RT, Chang MY, Chang NC. Org Lett. 2006;8:3033–3035. doi: 10.1021/ol060958k. [DOI] [PubMed] [Google Scholar]
- 18.Kaoudi T, Miranda LD, Zard SZ. Org Lett. 2001;3:3125–3127. doi: 10.1021/ol016424v. [DOI] [PubMed] [Google Scholar]
- 19.Wender PA, Smith TE. J Org Chem. 1996;61:824–825. and references cited therein. [Google Scholar]
- 20.van Tamelen EE, Shamma M, Aldrich P. J Am Chem Soc. 1956;78:4628–4632. doi: 10.1021/ja01054a021. [DOI] [PubMed] [Google Scholar]
- 21.Chiou WH, Wang YW, Kao CL, Chen PC, Wu CC. Organometallics. 2014;33:4240–4244. [Google Scholar]
- 22.Miyafujia A, Ito K, Katsuki T. Heterocycles. 2000;52:261–272. and references cited therein. [Google Scholar]
- 23.Bergmeier SC, Seth PP. J Org Chem. 1999;64:3237–3243. doi: 10.1021/jo9825097. [DOI] [PubMed] [Google Scholar]
- 24.Hu DH, Bharathi SN, Panangadan JAK, Tsujimoto A. J Org Chem. 1991;56:6998–7007. [Google Scholar]
- 25.Meyers AI, Highsmith TK, Buonora PT. J Org Chem. 1991;56:2960. [Google Scholar]
- 26.Riva R, Banfi L, Danieli B, Guanti G, Lesma G, Palmisano G. J Chem Soc, Chem Commun. 1987:299–300. [Google Scholar]
- 27.Langen DJV, Tolman RL. Tetrahedron: Asymmetry. 1997;8:677–681. [Google Scholar]
- 28.Chapus C, Rovery M, Sarda L, Verger R. Biochimie. 1988;70:1223–1234. doi: 10.1016/0300-9084(88)90188-5. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt JP, Beltrán-Rodil S, Cox RJ, McAllister GD, Reid M, Taylor RJK. Org Lett. 2007;9:4041–4044. doi: 10.1021/ol701772d. [DOI] [PubMed] [Google Scholar]
- 30.Mitsunobu O. Synthesis. 1981:1–28. [Google Scholar]
- 31.Lal B, Pramanik BN, Manha MS, Bose AK. Tetrahedron Lett. 1977;23:1977–1980. [Google Scholar]
- 32.Gololobov YG, Zhmurova IN, Kasukhin LF. Tetrahedron. 1981;37:437–472. [Google Scholar]
- 33.Sugawara S, Hishiyama S, Jikumaru Y, Hanada A, Nishimura T, Koshiba T, Zhao Y, Kamiya Y, Kasahara H. Proc Natl Acad Sci. 2009;106:5430–5435. doi: 10.1073/pnas.0811226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilk BK. Synth comm. 1993;23:2481–2484. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.