Abstract
Objectives
A CD1d-binding invariant natural killer T (iNKT)-cell stimulatory glycolipid, namely 7DW8–5, is shown to enhance the efficacy of radiation-attenuated sporozoites (RAS)-based malaria vaccine in mice. In the current study, we aim to determine whether 7DW8-5 can display a potent adjuvant effect in human immune system (HIS) mice.
Methods
HIS-A2/hCD1d mice, which possess both functional human iNKT cells and CD8+ T cells, were generated by the transduction of NSG mice with adeno-associated virus serotype 9 expressing genes that encode human CD1d molecules and HLA-A*0201, followed by the engraftment of human hematopoietic stem cells. The magnitudes of human iNKT-cell response against 7DW8-5 and HLA-A*0201-restricted human CD8+ T-cell response against a human malaria antigen in HIS-A2/hCD1d mice were determined by using human CD1d tetramer and human HLA-A*0201 tetramer, respectively.
Results
We found that 7DW8-5 stimulates human iNKT cells in HIS-A2/hCD1d mice, as well as those derived from HIS-A2/hCD1d mice in vitro. We also found that 7DW8-5 significantly increases the level of a human malarial antigen-specific HLA-A*0201-restricted human CD8+ T-cell response in HIS-A2/hCD1d mice.
Conclusions
Our study indicates that 7DW8-5 can display a potent adjuvant effect on RAS vaccine-induced anti-malarial immunity by augmenting malaria-specific human CD8+ T-cell response.
Keywords: malaria, sporozoites, humanized mice, adjuvant, glycolipid, CD1d, NKT cell
1. Introduction
Malaria is a mosquito-borne infectious disease affecting humans and other animals caused by the parasitic protozoan of the genus Plasmodium. Although medications and mosquito elimination are widely used as treatment and control methods, respectively, malaria is still a pandemic disease, with 198 million cases of malaria resulting in 584,000 deaths globally in 2013 [1]. Plasmodium falciparum, P. vivax, P. ovale, and P. malariae together account for almost all human infections with Plasmodium species, with P. falciparum accounting for the most of the mortality. Therefore, a vaccine that can induce protective anti-malarial immunity is essential to eradicate malaria. Most vaccine efforts are directed against the pre-erythrocytic stages [sporozoites (Spz) and liver stages] and the blood stages in the life cycle of the malaria parasite [2]. Vaccination with radiation-attenuated sporozoites (IrSpz) has been found to induce complete protection (i.e., sterile immunity) against malarial infection not only in experimental animals but also in humans [3–7], demonstrating the feasibility of effective vaccination against this disease. Beyond the mouse model, intravenous (IV) immunization of IrSpz of P. falciparum, named the PfSPZ vaccine, has been shown to effectively induce a high frequency of malarial-specific CD8+ T cells in the livers of nonhuman primates [8]. More recently, IV immunization with multiple doses of the PfSPZ vaccine induced high levels of PfSPZ-specific T-cell responses, including that of CD8+ T cells, and conferred protection in six out of six (100%) human vaccine recipients against malaria challenge [9]. Another recent study showed that administration of live P. falciparum Spz through bites from infected mosquitoes, followed by chloroquine treatment, induced significant malaria-specific pluripotent effector memory T-cell responses in vaccinated volunteers and protected all of them (10 out of 10) upon malaria challenge [10].
We and others have shown that α-galactosylceramide (α-GalCer), which binds CD1d molecules and stimulates invariant natural killer T (iNKT) cells, displays an adjuvant effect that enhances the efficacy of various vaccines (e.g., adenovirus, DNA, live-attenuated pathogen, recombinant protein, peptide, and irradiated parasite vaccines) [11–17]. We found that a synthetic analog of α-GalCer, 7DW8-5, displays stronger biological activity and ultimately a more potent adjuvant effect than that of α-GalCer when co-administered with a malaria vaccine in mice [18, 19]. 7DW8-5 was also shown to exert a significant adjuvant effect on the cellular immunogenicity, particularly with respect to CD8+ T cells, of an adenovirus-based malaria vaccine in non-human primates [20]; this led us to choose 7DW8-5 as a lead candidate for clinical evaluation with a malaria vaccine [21].
We previously established a human immune system (HIS) mouse model by transducing genes encoding human HLA-A*0201 and human cytokines using a recombinant adeno-associated virus serotype 9 (AAV9) vector, followed by the engraftment of HLA-A*0201-matched, human hematopoietic stem cells (HSCs) [22]. Several weeks after HSC engraftment, the HIS-A2 mice mounted HIV− and malaria-specific HLA-A*0201-restricted human CD8+ T-cell responses following immunization with HIV− and malaria-derived antigens; thereby demonstrating that these mice could mount functional human CD8+ T cells. In view of a previous study showing that human CD4 molecules can weakly interact with murine class II molecules [23], it is possible that some of human CD4+ T cells in HIS-A2 mice may be functional. Nevertheless, we have most recently shown that in vivo depletion of human CD8+ T cells almost completely abolished the protective anti-malaria immunity induced in HIS-A2 mice immunized with a recombinant adenovirus expressing the Plasmodium falciparum circumsporozoite protein (AdPfCSP) [24].
In the present study, we further studied a humanized mice model (HIS-A2/hCD1d) reconstituted with functional HLA-A*0201-restricted human CD8+ T cells, as well as human CD1d restricted human iNKT cells. We investigated whether co-administration of 7DW8-5 with IrSpz of PfCSP/Py, a transgenic P. yoelii parasite that expresses PfCSP, enhances the immunogenicity and protective efficacy of the IrSpz-based malaria vaccine in HIS mice.
2. Materials and methods
Ethics statement
All animal experiments were conducted in strict accordance with the Policy on Humane Care and Use of Laboratory Animals of the United States Public Health Service. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at The Rockefeller University (Assurance # A3081-01). CO2 was used for euthanasia, and all efforts were made to minimize suffering. Human fetal liver samples were obtained via a non-profit partner (Advanced Bioscience Resources, Alameda, CA); the samples were devoid of any information that would identify the subjects from whom they were derived. Therefore, IRB approval was not required for their use, as previously described [22, 24].
Mice
NOD.Cg-B2mtm1Unc Prkdcscid Il2rgtm1Wjl/SzJ (NSG-B2m) triple mutant mice, which combined the features of severe combined immune deficiency mutation (SCID) with IL-2 receptor γ chain and β2-microglobulin (β2m) deficiencies, were purchased from The Jackson Laboratories. Mice were maintained under specific pathogen-free conditions in the animal facilities at the Comparative Bioscience Center of The Rockefeller University.
Generation of AAV9 vectors and transduction of selected human genes by AAV9 vectors
Recombinant AAV9 vectors, which encode human IL-3, IL-15, GM-CSF (AAV9-hucytokines) and HLA-A*0201 (AAV9-A2), were constructed as previously described [22]. A plasmid consisting of a human CD1d gene covalently linked to a human β2m (hβ2m), hCD1d-hβ2m, was constructed as described previously [25] and then sub-cloned into a pAAV CMV plasmid (Stratagene La Jolla, CA). AAV9 vector encoding hCD1d-hβ2m, AAV9-hCD1d, was made at Penn Vector Core, Gene Therapy Program, Perelman School of Medicine, University of Pennsylvania. Four-week-old NSG-B2m mice were transduced with AAV9-A2 and/or AAV9-hCD1d by intrathoracic (IT) injection, together with AAV9 encoding human IL-3, IL-15, and GM-CSF, and/or AAV9-A2 and AAV9-CD1d and by IV injection, as previously described [22].
Purification of human HSCs and xenogeneic transplantation
Lymphocytes were isolated from fetal liver samples as described previously [26]. CD34+ human HSCs were isolated from lymphocytes using the Human CD34 Positive Selection Kit (STEMCELL Technologies Inc., Vancouver, BC, Canada), according to the manufacturer’s instructions. HSC purity was evaluated using flow cytometric analysis, and the percentage of CD34+ cells was confirmed to be higher than 90%. One to two weeks after the transduction of selected human genes by AAV9 vectors, mice were exposed to 150-Gy whole-body sub-lethal irradiation for myeloablation. A few hours later, each transduced, irradiated mouse was engrafted IV with 1 × 105 HLA-A*0201+ matched, CD34+ human HSCs.
Phenotypic analyses of human CD45+ cells in the blood of HIS-A2/hCD1d mice
Fourteen weeks after the engraftment of human HSCs, the reconstitution status of human CD45+ cells in the blood of HIS-A2/hCD1d mice was monitored by determining the percentage of human CD45+ cells and subsets of human lymphocytes in the peripheral blood using flow cytometric analysis, as previously described [22]. Briefly, upon lysing red blood cells, peripheral blood mononuclear cells (PBMCs) were purified from HIS-A2/hCD1d mice peripheral blood samples. After washing the cells twice, PBMCs were blocked for 5 min on ice using normal mouse sera supplemented with anti-CD16/CD32 (clone 93, BioLegend). Cells were washed once and stained for 40 min on ice in the dark with the following antibodies: Pacific Blue anti-human CD45 (clone HI30 - BioLegend), Pacific Orange anti-mouse CD45 (clone 30-F11, Life Technologies), phycoerythrin (PE)-TexasRed anti-human CD3 (clone UCHT1, Life Technologies), allophycocyanin (APC)-Cy7 anti-human CD4 (clone RPA-T4, BioLegend), fluorescein isothiocyanate (FITC) anti-human CD8 (clone HIT8a - BioLegend), peridinin chlorophyll protein complex (PerCp)-Cy5.5 anti-human TCR Vα24 (clone C15, Biolegend), Alexa Fluor 647 anti-human CD161 (clone HP-3G10, BioLegend), PE-Cy7 anti-human CD19 (clone HIB19, BioLegend), Alexa Fluor 700 anti-HLA-DR (clone L243-Biolegend), PE anti-CD11c (clone 3.9, Biolegend), PerCp-Cy5.5 anti-human CD14 (clone M5E2, Biolegend) and APC anti-human CD11b (clone ICRF44, BioLegend). After staining, cells were washed twice with phosphate-buffered saline (PBS) containing 2% fetal bovine serum (FBS), fixed with 1% paraformaldehyde, and analyzed using a BD LSR II Flow Cytometer (BD Biosciences).
Glycolipid, human CD1d tetramer, human HLA-A*0201 tetramer and parasites
α-galactosylceramide (α-GalCer) was purchased from Avanti Polar Lipids. APC-labeled human CD1d tetramer loaded with or without PBS-57 [27], and HLA-A*0201 tetramer loaded with PfCSP-derived peptide, YLNKIQNSL [28], were all kindly supplied by the NIH Tetramer Core Facility. A transgenic P. yoelii parasite line expressing full-length PfCSP, the PfCSP/Py parasite was recently constructed and its infectivity determined, as described previously [28]. The PfCSP/Py parasites were maintained at the Insectary Core Facility of the Department of Microbiology, New York University School of Medicine.
Determine HLA-A*0201-restricted CD8+ T-cell response by tetramer staining
PfCSP/Py sporozoites (Spz) were obtained from dissected salivary glands of infected Anopheles stephensi mosquitoes 2 weeks after infective blood meal. For immunization, PfCSP/Py Spz were radiation-attenuated by exposing them to 12,000 rad. Next, HIS-A2/hCD1d mice were immunized intramuscularly (IM) with 2 × 105 radiation-attenuated PfCSP/Py Spz. Ten days later, the spleens were harvested from immunized or naïve HIS-A2/hCD2d mice. Splenocytes were isolated and incubated with YLNKIQNSL-loaded HLA-A*0201 tetramer and the following antibodies: Pacific Blue anti-human CD45, Pacific Orange anti-mouse CD45, PE-Texas Red anti-human CD3, APC-Cy7 anti-human CD4, FITC anti-human CD8 and PE-Cy7 anti-human CD19. Finally, the percentage of PfCSP-specific human CD8+ T cells was analyzed using a BD LSR II Flow Cytometer.
Sporozoite challenge and assessment of parasite burden in the liver
The sporozoite challenge experiment was conducted as described previously [24]. Briefly, a group of HIS-A2/hCD1d humanized mice were immunized with 2 × 105 radiation-attenuated PfCSP/Py Spz, and 10 days later, groups of immunized as well as naïve HIS-A2/hCD2d mice were challenged with 2 × 104 live PfCSP/Py Spz by IV injection. Forty-two hours later, livers were collected from both groups of challenged HIS-A2/hCD1d mice, and the amount of parasite-specific ribosomal RNA was determined by using a 7300 Real-Time PCR System (Applied Biosystems), as described previously [24]. Parasite burden was calculated as a ratio of the absolute copy number of parasite ribosomal RNA to that of mouse GAPDH mRNA.
Statistical analysis
All statistical analyses were performed using GraphPad Prism (ver. 7) (GraphPad Software, Inc.). Bars in each figure represent means. For most studies, statistical analysis of experimental and control data were evaluated with one-way ANOVA and Student t test. For protection experiments, the values were log-transformed and then one-way ANOVA followed by Dunnett’s test were employed to determine the differences between the groups. For correlation analysis, linear regression analysis was applied to determine the percentage of PfCSP-specific CD8+ T cells among the total human CD8+ T-cell population and the percentage of relative parasite burden in the liver, 100% being the parasite burden in naïve and challenged mice.
3. Results
Establishment of HIS-A2/hCD1d mice
In this study, HLA-A*0201 and human CD1d molecules were transduced by AAV9-mediated gene transfer into NOD scid gamma (NSG) mice that lack mouse β2m, called NSG-B2m mice. Because the mouse β2m gene had been deleted, NSG-B2m mice were unable to form functional complexes of both mouse MHC I and mouse CD1d, which might cross-educate human CD8+ T cells and iNKT cells, respectively. For the purpose of educating human CD8+ T cells in vivo, the HLA-A*0201 gene was transduced into NSG-B2m mice using the AAV9 vector, as we have previously shown [22]. To facilitate the development and differentiation of human iNKT cells, we first constructed a plasmid that consisted of a human CD1d gene fused to a human β2m gene (hCD1d-hβ2m), and then generated AAV9 that expressed the human CD1d gene AAV9-hCD1d. After in vitro transfection with AAV9-hCD1d, HT1080 cells not only expressed hCD1d-hβ2m molecules (Fig. 1A), but the hCD1d- hβ2m molecules expressed by HT1080 cells were actually able to present glycolipids, 7DW8-5, and αGalCer to human iNKT hybridoma cells, thereby stimulating them to secrete human IFN-γ in a dose-dependent fashion (Fig 1B).
Fig 1. Establishment of HIS-A2/hCD1d humanized mice.
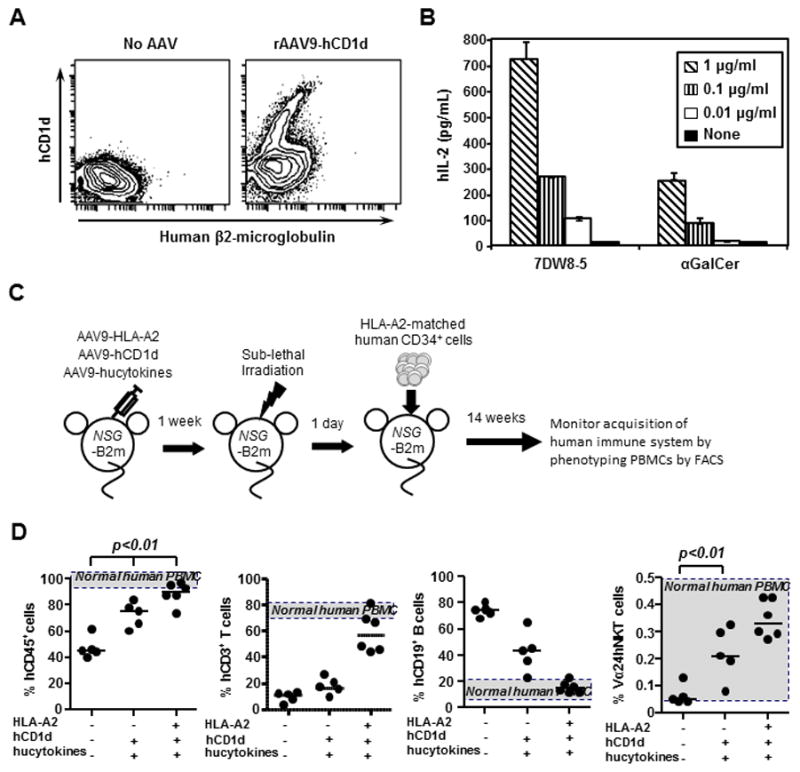
(A). HT1080 cells were transduced with rAAV9-hCD1d at 1010 GC/ml for 3 days, and then the expression of human β2-microglobulin-CD1d was determined by flow cytometry analysis. (B). Three days after transduction, 5×104 rAAV9-hCD1d transduced HT1080 cells were co-cultured with 5 × 104 58ab hybridoma cells expressing human iNKT Vα24/β11 TCR at the presence of escalating doses of 7DW8-5 or α-GalCer, respectively. After 24 hours, mouse IL-2 levels in the culture supernatants were determined by ELISA. (C) Schematic representation of the strategic methodology for engrafting human CD34+ cells in AAV9-transduced NSG-B2m mice. NSG-B2m mice were inoculated with AAV9-hCD1d, AAV9-hucytokines or/and AAV9-A2, and 2 weeks later, mice were irradiated to myeloablate mouse immune cells at 150 rad. The next day, mice were engrafted IV with 1 × 105 HLA-A2+ human CD34+ cells previously isolated from human fetal liver. A group of B2m-NSG mice that did not receive rAAV9 injection (No AAV) were also engrafted with HSCs. (D) Fourteen weeks after engraftment, human immune system reconstitution in blood was evaluated by flow cytometric analyses by detecting human CD45+PBMC, CD3+ T cells, CD4+ T cells, CD8+ T cells, CD3-CD161+ NK cells, CD19+ B cells, CD3+Vα24+ iNKT cells, CD14+CD11b+ macrophages, and CD11c+HLA-DR+ DCs.
Next, NSG-B2m mice were administered with AAV9-hCD1d and/or AAV9-A2 by IV and IT routes in the presence or absence of IV injection of AAV9 vectors that encode human IL-3, IL-15 and GM-CSF (Fig. 1C). The transduced NSG-B2m mice then received sub-lethal γ-irradiation immediately followed by the engraftment of HLA-A*0201+ CD34+ human HSCs (Fig 1C). Fourteen weeks later, peripheral blood mononuclear cells (PBMCs) were isolated from the blood of human gene-transduced, HSCs-engrafted NSG-B2m mice, and the state of the development of the human immune system, including total human CD45+ leukocytes, human T and B cells, and human iNKT cells, was monitored by flow cytometric analysis. NSG-B2m mice transduced with all of the human genes, including HLA-A*0201, human CD1d, and human cytokines, mounted a significantly higher percentage of human CD45+ cells upon HSC engraftment, compared to NSG-B2m mice that had not received AAV9-mediated human gene transfer (No AAV), or even NSG-B2m mice transduced with human CD1d and human cytokines (HIS-hCD1d) (Fig. 1D). The percentages of human T and B cells observed in HIS-A2/hCD1d mice were very similar to those found in humans. Of importance, both HIS-CD1d mice and HIS-A2/hCD1d mice mount a significantly higher percentage of human iNKT cells than that seen in HIS mice without AAV9-mediated human gene transfer (Fig. 1D). Collectively, these results indicated that HIS-A2/hCD1d mice were able to reconstitute a high percentage of not only human CD45+ cells, but also human T cells, as well as human iNKT cells.
Function of human iNKT cells present in HIS-A2/hCD1d mice
To determine the function of human iNKT cells in HIS-A2/hCD1d mice, we first sought to determine whether human iNKT cells in HIS-A2/hCD1d mice recognized glycolipid antigen in the context of human CD1d molecules. For this purpose, we isolated splenocytes from HIS-A2/hCD1d mice and incubated them with APC-labeled human CD1d tetramer loaded with PBS57, which is known to detect human iNKT cells [27]. Unloaded human CD1d tetramer was used as a negative control. We found that PBS57-loaded human CD1d tetramer, but not unloaded tetramer, was able to detect splenocytes (Fig. 2A) as well as peripheral blood and liver-resident mononuclear cells collected from HIS-A2/hCD1d mice (Fig. 2B). Almost no human iNKT cells were found in HIS mice that did not receive AAV, as evidenced by the fact that PBS57-loaded human CD1d tetramer failed to stain the cells.
Fig 2. Function of human iNKT cells in HIS-A2/hCD1d mice.
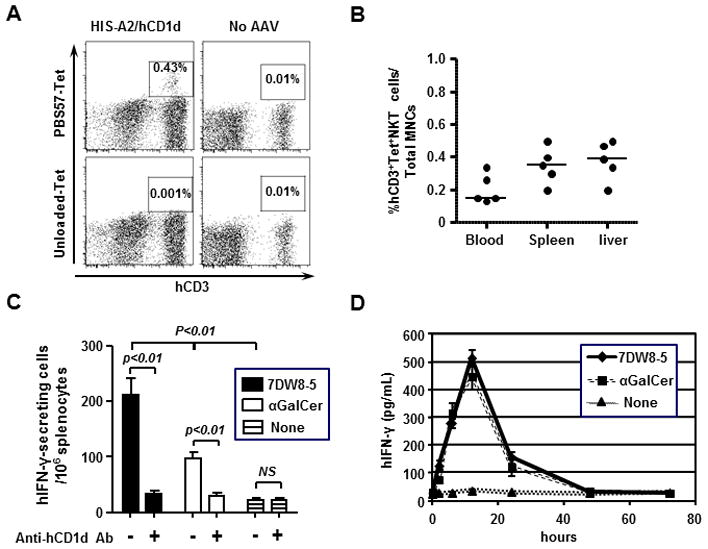
(A) Spleen mononuclear cells (MNC) from groups (n=5) of HIS-A2/hCD1d mice and No AAV mice were stained with PBS57-loaded/unloaded human CD1d tetramer and the following antibodies: anti-human CD45, anti-human CD3, and anti-mouse CD45 antibodies. The result came from one representative mouse in each group. (B) Peripheral blood, spleen and liver MNC from groups (n=5) of HIS-A2/hCD1d mice and No AAV mice were stained with PBS57-loaded human CD1d tetramer and antibodies as in (A). (C) To produce immature DCs, bone marrow MNCs from HIS-A2/hCD1d mice were stimulated with 40 ng/ml human GM-CSF and IL-4 for 7 days. Then, 5 × 104 DCs and 1 × 105 autologous spleen MNCs were co-cultured in an ELISPOT plate for 24 hours with 1 μg 7DW8-5 or α-GalCer, respectively, in the presence of 50 μg/ml anti-human CD1d antibody (clone 51.1) or mouse IgG2b isotype antibody. (D). Groups of HIS-A2/hCD1d mice (n=4) were IV. administered 1 μg 7DW8-5 or α-GalCer, respectively. Blood samples were collected at 2, 6, 12, 24, 48, and 72 hours after injection, and the concentration of human IFN-γ in the sera was determined by ELISA.
Next, to determine whether human iNKT cells in HIS-A2/hCD1d mice can display biological activity in vitro, we isolated immature dendritic cells (DCs) from splenocytes collected from HIS-A2/hCD1d mice and co-cultured them with splenocytes in the presence or absence of CD1d-binding iNKT-cell ligands, 7DW8-5, and α-GalCer (17). We found that 7DW8-5 was able to stimulate splenocytes more potently than α-GalCer, resulting in higher secretion of human IFN-γ (Fig. 2C) and that the responses were almost completely inhibited when anti-human CD1d antibody was added into the culture (Fig. 2C). To determine the in vivo biological activity of human iNKT cells present in HIS-A2/hCD1d mice, we administered 7DW8-5 and α-GalCer to groups of HIS-A2/hCD1d mice. After collecting the sera from treated HIS-A2/hCD1d mice, we found that both glycolipids were actually able to induce robust human IFN-γ production in vivo, as determined by ELISA (Fig. 2D). Collectively, these results indicated that HIS-A2/hCD1d mice can possess human iNKT cells that are functionally able to respond to human CD1d-binding and iNKT-cell stimulatory glycolipids both in vitro and in vivo.
Glycolipid adjuvant enhanced the efficacy of the irradiated sporozoite vaccine in HIS-A2/hCD1d mice
Our previous study showed that 7DW8-5 displays greater potent adjuvant activity than does α-GalCer, by enhancing the T-cell immunogenicity of malarial and HIV vaccines and the protective efficacy of various forms of a malarial vaccine in mice [18–21]. Therefore, using the HIS-A2/hCD1d mouse model that we have established, we sought to determine whether these glycolipid adjuvants could enhance human immune responses, particularly those mediating protective anti-malarial immunity, induced by a malarial vaccine. For this purpose, we immunized HIS-A2/hCD1d mice with radiation-attenuated PfCSP/Py Spz conjointly administered i.m. with either 7DW8-5 or α-GalCer. Ten days after immunization, we determined the human malaria antigen-specific human CD8+ T-cell response by assessing the percentage of human CD8+ T cells that were positive for YLNKIQNSL-loaded HLA-A*0201 tetramer by a flow cytometric analysis. We found that both 7DW8-5 and α-GalCer were able to enhance the level of the PfCSP-specific, HLA-A*0201-restricted human CD8+ T-cell response: 7DW8-5 resulted in an almost 4-fold higher PfCSP-specific CD8+ T-cell response induced by irradiated PfCSP/Py Spz, whereas the enhancement by α-GalCer was less than 3-fold (Fig. 3A). The immunized groups, with or without glycolipid-treatment, as well as a naïve group of HIS-A2/hCD1d mice were then challenged with live PfCSP/Py Spz, and the parasite burden in the liver measured to assess the level of protective anti-malarial immunity. We found that both glycolipids displayed significant adjuvant activity by enhancing protective anti-malarial immunity, with 7DW8-5 showing a more potent adjuvant effect than that of α-GalCer (Fig. 3B). When we compared the level of the PfCSP-specific human CD8+ T-cell response and the level of protective anti-malarial immunity induced by the PfCSP/Py Spz vaccine, these two parameters showed a significant correlation (Fig. 3C).
Fig 3. Enhancement of PfCSP-specific, HLA-A*0201-restricted CD8+ T-cell response and protective anti-malarial immunity by CD1d-binding glycolipids in HIS-A2/hCD1d mice.
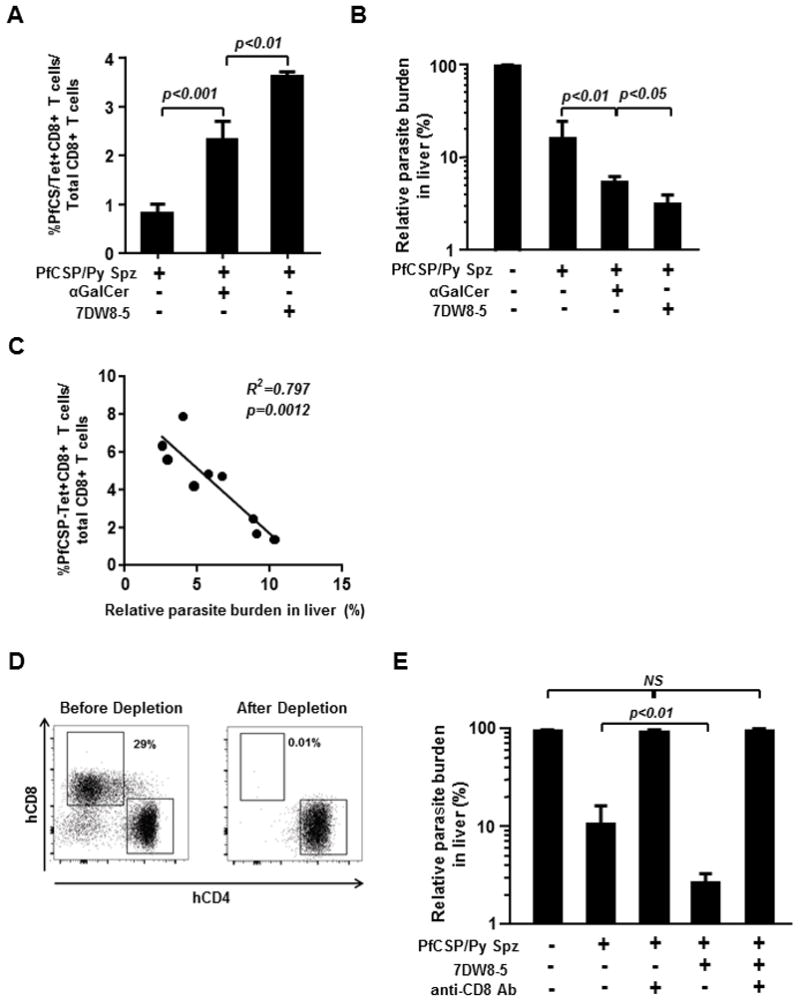
A group of twelve HIS-A2/hCD1d mice were immunized with 1×105 irradiated PfCSP/Py Spz by IM injection. Of those, four HIS-A2/hCD1d mice received conjoint administration of α-GalCer, whereas another group of four HIS-A2/hCD1d mice received 7DW8-5 co-administration. Ten days after immunization, PfCSP/Py Spz-immunized mice, PfCSP/Py Spz-immunized and glycolipid-treated mice, as well as four naïve HIS-CD8 mice, were challenged intravenously with 2×104 live PfCSP/Py Spz. (A) Forty-two hours after the challenge, all groups of HIS-A2/hCD1d mice were sacrificed, and the percentage of HLA-A2–restricted, PfCSP-specific CD8+ T cells among the total CD8+ T cells in the spleen was determined using HLA-A2 tetramer staining and flow cytometric analysis. (B) The parasite load in the liver was determined by qRT-PCR analysis. (C) The correlation between the percentage of PfCSP-specific CD8+ T cells and the relative parasite load in the liver was assessed using Prism GraphPad 5.0 software. (D) A group of six HIS-A2/hCD1d mice were immunized with irradiated PfCSP/Py Spz. Another group of six HIS-A2/hCD1d mice received joint administration of α-GalCer with PfCSP/Py Spz immunization. Among the PfCSP/Py Spz-immunized HIS-A2/hCD1d mice and the PfCSP/Py Spz-immunized, 7DW8-5-administered HIS-A2/hCD1d mice, a group of three each received two 0.1 mg/mouse doses of anti-CD8 mAb, MT807R1, by IP injection on days 5 and 8 post-immunization. Ten days after immunization, blood was collected from all four groups of mice, and the percentages of human CD8+ and CD4+ T cells among the total human T-cell population in PBMCs were determined by flow cytometric analysis. One of the representative figures is shown. (E) Groups of PfCSP/Py Spz-immunized HIS-A2/hCD1d mice and PfCSP/Py Spz-immunized, 7DW8-5-treated HIS-A2/hCD1d mice, and groups of these HIS-A2/hCD1d mice depleted of human CD8+ T cells, as well as a group of naïve HIS-A2/hCD1d mice were challenged by IV injection with 2 × 104 live PfCSP/Py Spz. Forty-two hours later, all groups of HIS-A2/hCD1d mice were sacrificed, livers were collected, and the parasite load in the liver was determined by qRT-PCR.
Finally, we were interested to see if the protective anti-malarial immunity enhanced by 7DW8-5 in HIS-A2/hCD1d mice was mediated by human CD8+ T cells. To address this issue, we administered anti-human CD8+ T-cell antibody, which we most recently used to deplete human CD8+ T cells from our HIS-A2 mice [24], to PfCSP/Py-immunized HIS-A2/hCD1d mice with or without 7DW8-5-co-administration, as well as to naïve HIS-A2/hCD1d mice. The administration of anti-human CD8+ T-cell antibody not only abolished the protective anti-malarial immunity induced by PfCSP/Py Spz vaccine, but also the immunity induced by PfCSP/Py vaccinated HIS-A2/hCD1d mice conjointly injected with 7DW8-5 (Fig. 3E). These results strongly indicate that human CD8+ T cells mediate the PfCSP/Py Spz-induced protective anti-malarial immunity enhanced by 7DW8-5.
4. Discussion
The finding that vaccination with radiation-attenuated sporozoites (RAS) can induce complete protection (i.e., sterile immunity) against malarial infection not only in experimental animals but also in humans [3–7], demonstrated the feasibility of effective vaccination against this disease. In fact, live RAS or live non-attenuated sporozoites with chloroquine chemoprophylaxis are the only two immunogens that have ever induced high-level (80–90%) and long-lasting (10–25 months) protection against malaria in humans [8–10]. A radiation-attenuated PfSPZ vaccine, isolated from the salivary glands of “aseptically raised” infected Anopheles stephensi mosquitoes, purified, vialed, and cryopreserved under GMP, has been shown to be well tolerated, safe, immunogenic and highly protective in multiple phase I and II clinical trials [30, 31].
We have previously shown that co-administration of α-GalCer and 7DW8-5 enhanced both the immunogenicity and efficacy of various malarial vaccines, including irradiated P. yoelii Spz [17–21]. More recently, we have found that 7DW8-5 exerts a more potent adjuvant effect than that of α-GalCer by co-localizing with irradiated P. yoelii Spz in lymph node-resident dendritic cells upon conjoint i.m. administration [19].
In the current study, we established humanized mice, HIS-A2/hCD1d mice, which possess functional human CD8+ T cells and human iNKT cells, to investigate whether 7DW8-5 and α-GalCer can display the adjuvant effect, thus enhancing the human CD8+ T-cell response induced by a malarial vaccine. Both 7DW8-5 and α-GalCer stimulated human iNKT cells derived from HIS-A2/hCD1d mice in the context of human CD1d molecules in vitro as well as human iNKT cells in HIS-A2/hCD1d mice. When these glycolipids were co-administered to HIS-A2/hCD1d mice with PfCSP/Py Spz, a higher level of PfCSP-specific, HLA-A*0201-restricted human CD8+ T-cell response was induced, resulting in more robust protective immunity against malaria. It appears that 7DW8-5 could exert a more potent adjuvant effect than α-GalCer. Lastly, protective anti-malarial immunity enhanced by 7DW8-5 conjointly administered with PfCSP/Py Spz was found to be mediated by human CD8+ T cells, as evidenced by the fact that the in vivo depletion of human CD8+ T cells by anti-human CD8 antibody abolished the protection.
5. Conclusion
Using HIS-A2/hCD1d mice that we generated, we are able to show that our glycolipid adjuvant, 7DW8-5, can enhance human anti-malaria immunity induced by RAS-based vaccine. Thus, HIS-A2/hCD1d mice are proven to be a useful animal model not only to study the biological activity of CD1d-binding glycolipids against human iNKT cells in vivo, but also to assess the adjuvant effect of CD1d-binding glycolipids in a human immune system setting. We hope that the HIS-A2/hCD1d mouse model may facilitate the pre-clinical development of CD1d-binding, iNKT-cell-stimulatory glycolipids in the future.
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health (R01-AI070258) and the Mark S. Bertuch AIDS Research Fund (both to M Tsuji) and from KAKENHI (26293091, 26305009, and 25253027) to M Yuda).
We thank Dr. Vincent Sahi for assistance with flow cytometric analyses.
Non-standard abbreviations used
- CSP
circumsporozoite protein
- HIS
human immune system
- HIS
hematopoietic stem cell, HSC
- NKT
natural killer T
- PfCSP
Plasmodium falciparum CSP
Footnotes
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- 1.World Health Organization (WHO) World Malaria Report. 2014 http://www.who.int/malaria/publications/world_malaria_report_2014/en/
- 2.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120:4168–78. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nussenzweig RS, Vanderberg J, Most H, et al. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 4.Gwadz RW, Cochrane AH, Nussenzweig V, et al. Preliminary studies on vaccination of rhesus monkeys with irradiated sporozoites of Plasmodium knowlesi and characterization of surface antigens of these parasites. Bull World Health Organ. 1979;57(Suppl 1):165–73. [PMC free article] [PubMed] [Google Scholar]
- 5.Clyde DF, McCarthy VC, Miller RM, et al. Specificity of protection of man immunized against sporozoite-induced falciparum malaria. Am J Med Sci. 1973;266:398–403. doi: 10.1097/00000441-197312000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Herrington D, Davis J, Nardin E, et al. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–47. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science. 2011;334:475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 9.Seder RA, Chang L, Enama ME, et al. Protection against malaria by intravenous immunization with a non-replicating sporozoite vaccine. Science. 2013;341:1359–65. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 10.Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–77. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 11.Bartkowiak T, Singh S, Yang G, et al. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc Natl Acad Sci U S A. 2015;112:E5290–9. doi: 10.1073/pnas.1514418112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkataswamy MM, Baena A, Goldberg MF, et al. Incorporation of NKT cell-activating glycolipids enhances immunogenicity and vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guerin. J Immunol. 2009;183:1644–56. doi: 10.4049/jimmunol.0900858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y, Chen A, Li X, et al. Enhancement of HIV DNA vaccine immunogenicity by the NKT cell ligand, alpha-galactosylceramide. Vaccine. 2008;26:1807–16. doi: 10.1016/j.vaccine.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Ko SY, Ko HJ, Chang WS, et al. alpha-Galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–17. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 15.Hermans IF, Silk JD, Gileadi U, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 16.Fujii S, Shimizu K, Smith C, et al. Activation of natural killer T cells by alpha-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a coadministered protein. J Exp Med. 2003;198:267–9. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Aseguinolaza G, Van Kaer L, Bergmann CC, et al. Natural killer T cell ligand alpha-galactosylceramide enhances protective immunity induced by malaria vaccines. J Exp Med. 2002;195:617–24. doi: 10.1084/jem.20011889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Fujio M, Imamura M, et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci USA. 2010;10:13010–5. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Kawamura A, Andrews CD, et al. Colocalization of a CD1d-Binding Glycolipid with a Radiation-Attenuated Sporozoite Vaccine in Lymph Node-Resident Dendritic Cells for a Robust Adjuvant Effect. J Immunol. 2015;195:2710–21. doi: 10.4049/jimmunol.1403017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padte NN, Boente-Carrera M, Andrews CD, et al. A glycolipid adjuvant, 7DW8-5, enhances CD8+ T cell responses induced by an adenovirus-vectored malaria vaccine in non-human primates. PLoS One. 2013;8:e78407. doi: 10.1371/journal.pone.0078407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padte NN, Li X, Tsuji M, Vasan S. Clinical development of a novel CD1d-binding NKT cell ligand as a vaccine adjuvant. Clin Immunol. 2011;140:142–51. doi: 10.1016/j.clim.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Li X, Coelho-dos-Reis JG, et al. An AAV vector-mediated gene delivery approach facilitates reconstitution of functional human CD8+ T cells in mice. PLoS One. 2014;9:e88205. doi: 10.1371/journal.pone.0088205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barzaga-Gilbert E, Grass D, Lawrance SK, et al. Species specificity and augmentation of responses to class II major histocompatibility complex molecules in human CD4 transgenic mice. J Exp Med. 1992;175:1707–15. doi: 10.1084/jem.175.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Huang J, Zhang M, et al. Human CD8+ T cells mediate protective immunity induced by a human malaria vaccine in human immune system mice. Vaccine. 2016;34:4501–6. doi: 10.1016/j.vaccine.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiratsuchi T, Schneck J, Kawamura A, et al. Human CD1 dimeric proteins as indispensable tools for research on CD1-binding lipids and CD1-restricted T cells. J Immunol Methods. 2009;345:49–59. doi: 10.1016/j.jim.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lepus CM, Gibson TF, Gerber SA, et al. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac−/−, Balb/c-Rag1−/−gammac−/−, and C.B-17-scid/bg immunodeficient mice. Hum Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Goff RD, Zhou D, et al. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–9. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 28.Blum-Tirouvanziam U, Servis C, Habluetzel A, et al. Localization of HLA-A2. 1-restricted T cell epitopes in the circumsporozoite protein of Plasmodium falciparum. J Immunol. 1995;154:3922–31. [PubMed] [Google Scholar]
- 29.Zhang M, Kaneko I, Tsao T, et al. A highly infectious Plasmodium yoelii parasite, bearing Plasmodium falciparum circumsporozoite protein. Malar J. 2016;15:201. doi: 10.1186/s12936-016-1248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richie TL, Billingsley PF, Sim BK, et al. Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines. Vaccine. 2015;33:7452–61. doi: 10.1016/j.vaccine.2015.09.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollingdale MR, Sedegah M. Development of whole sporozoite malaria vaccines. Expert Rev Vaccines. 2016;18:1–10. doi: 10.1080/14760584.2016.1203784. [DOI] [PubMed] [Google Scholar]


