Abstract
Cancer treatments such as chemotherapy have been an important part of extending survival in women diagnosed with breast cancer. However, chemotherapy can cause potentially toxic side effects in the brain that impair memory, verbal fluency, and processing speed in up to 30% of women treated. Women report that post-chemotherapy cognitive deficits negatively impact quality of life and may last up to ten years after treatment. Mechanisms underlying these cognitive impairments are not fully understood, but emerging evidence suggests that chemotherapy induces structural changes in the brain, produces neuroinflammation, and reduces adult hippocampal neurogenesis. Dietary approaches that modify inflammation and neurogenesis are promising strategies for reducing chemotherapy-induced cognitive deficits in breast cancer survivors. In this review, we describe the cognitive and neuronal side effects associated with commonly used chemotherapy treatments for breast cancer, and we focus on the often opposing actions of omega-3 fatty acids and added sugars on cognitive function, neuroinflammation, and adult hippocampal neurogenesis. Omega-3 fatty acids administered concurrently with doxorubicin chemotherapy have been shown to prevent depressive-like behaviors and reduce neuroinflammation, oxidative stress, and neural apoptosis in rodent models. In contrast, diets high in added sugars may interact with n-3 FAs to diminish their anti-inflammatory activity or act independently to increase neuroinflammation, reduce adult hippocampal neurogenesis, and promote cognitive deficits. We propose that a diet rich in long-chain, marine-derived omega-3 fatty acids and low in added sugars may be an ideal pattern for preventing or alleviating neuroinflammation and oxidative stress, thereby protecting neurons from the toxic effects of chemotherapy. Research testing this hypothesis could lead to the identification of modifiable dietary choices to reduce the long-term impact of chemotherapy on the cognitive functions that are important to quality of life in breast cancer survivors.
Keywords: Chemotherapy, Omega-3 fatty acids, Added sugars, Cognition, Inflammation, Neurogenesis, Chemobrain
Introduction
Advances in the detection and treatment of breast cancer have increased the 5-year survival rate to nearly 90%. However, chemotherapy is often associated with lingering side effects that can persist for over a decade following treatment, negatively affecting quality of life and reducing survival. One of the most common side effects is chemotherapy-related cognitive impairment, also referred to as “chemobrain” or “chemofog” [1]. Despite an array of studies examining its cause and effects, the mechanism through which chemotherapy affects the brain function remains unclear. Recent studies suggest a mechanism that involves interaction of the immune and neuroendocrine systems, which in turn shape the development of behavioral symptoms in cancer patients [2].
It was first demonstrated approximately 30 years ago that cancer patients being treated with chemotherapy were experiencing cognitive dysfunction [3]. Further studies have confirmed impairments in attention, processing speed, executive function, and working memory as the most common cognitive domains affected by chemotherapy [4, 5]. A difficulty arises when trying to compare studies with different experimental designs, chemotherapeutic agents, cognitive instruments, and patient populations, among other factors. However, the emerging picture is that chemotherapy has the capacity to alter brain function in ways that may result in cognitive deficits for at least a subset of individuals. Identifying patients at increased risk for cognitive deficits and identifying ways to reduce the risk of cognitive dysfunction are important for improving quality of life after cancer. This review will describe the cognitive and neuronal side effects of commonly used chemotherapeutic agents, and explore dietary enrichment with omega-3 fatty acids, as well as lowering dietary sucrose, as potential strategies for reducing these negative outcomes of chemotherapy treatment.
Effects of chemotherapy in the brain
Chemotherapy contributes to cognitive impairment
Over the past 10 years, evidence supporting the behavioral and physiological deficits associated with chemotherapy has grown. In addition, cognitive deficits in breast cancer patients have been reported following diagnosis but prior to chemotherapy initiation [4]. Even with these deficits at baseline, chemotherapy was associated with a further decline in cognitive function across time. This suggests that while it is possible that factors related to the tumor, stress of diagnosis, or surgical intervention may affect cognition, chemotherapy treatment also has a negative impact on cognitive functioning. These chemotherapy-induced cognitive deficits can persist for up to 15 years after completion of treatment [5]; the data from this study suggest that chemotherapy can exacerbate the cognitive deficits linked to aging, which are further associated with changes in frontal brain regions and their subcortical connections.
Chemotherapy induces structural changes and inflammation in the brain
The use of neuroimaging techniques, such as magnetic resonance imaging (MRI) and functional MRI (fMRI), allow the identification of structural and functional differences within the brain. Several imaging studies demonstrate that chemotherapy can induce changes in the brain, including volume reductions and changes in activity patterns. As examples, the use of MRI in breast cancer survivors reveals a reduction in white and gray matter in brain regions related to cognitive function one year after chemotherapy treatment [6]; fMRI demonstrates decreased activation in the pre-frontal cortex and hippocampus of patients receiving chemotherapy when performing cognitive tasks [7]. In a separate study, increased memory impairment and reduced hippocampal volume in cancer patients receiving chemotherapy were associated with increased interleukin-6 (IL-6) and tumor necrosis alpha (TNF-α) cytokine concentration [8].
The existing clinical data support a potential inflammatory mechanism, however, establishment of the neural mechanisms underlying chemotherapy-induced changes in the brain structure and function will need to rely heavily on animal models. Indeed, chemotherapeutic agents used in the clinic are known to alter neurogenesis, induce cell death, and trigger oxidative stress and inflammation, consequently damaging the central nervous system and possibly inducing cognitive and behavioral impairment through both direct and indirect pathways. For example, many commonly used chemotherapeutic agents, such as doxorubicin, do not cross the blood–brain barrier in large quantities; however, they increase TNF-α, which penetrates the central nervous system, increasing reactive oxygen and nitrogen species. These consequently induce microglia to increase local TNF-α levels in the brain, promoting neuronal damage [9]. Additionally, doxorubicin administration impairs inhibitory avoidance, a measure of hippocampal memory, in rats [10], and a separate work demonstrates that it induces TNF-α neurotoxicity [11]. Collectively, these data suggest that chemotherapy-induced inflammation might be playing a crucial role in the development of chemotherapy-related brain changes and resulting cognitive deficits.
Chemotherapy influences adult hippocampal neurogenesis
Besides the indirect inflammatory effects of chemotherapeutic agents, such treatment can directly impact the nervous system. Chemotherapy regimens target cell division in an absolute manner; thus, adult neural stem cell proliferation and adult neurogenesis can be affected together with the malignant cells [12]. In the adult brain, neurogenesis occurs within the hippocampus; neurogenesis is involved in memory formation and spatial processing, and is crucial for optimal cognitive function [13]. Several preclinical studies have correlated chemotherapy-induced cognitive impairment with reduced adult hippocampal neurogenesis (AHN). For example, mice treated with cyclophosphamide display reduced hippocampal neurogenesis and exhibit impaired learning in the passive avoidance and novel object recognition tasks [14]. Cyclophosphamide also inhibits DNA synthesis and increases oxidative stress, inducing behavioral deficits that are inversely correlated with neurogenesis [15]. An inflammatory environment can also impair AHN [16]. Therefore, chemotherapy can act directly on AHN by decreasing hippocampal cell proliferation and neurogenesis, or indirectly by promoting an inflammatory environment that is not optimal for neurogenesis. To date there is evidence in support of both a non-inflammatory- and an inflammatory-based mechanism by which chemotherapy alters the brain structure and function [17], nonetheless, further research is needed to establish a causal relationship and develop effective preventative or treatment strategies.
Although many types of chemotherapy do not freely cross the blood–brain barrier, it is now well established that chemotherapeutic drugs can trigger physiological changes such as increases in oxidative stress and/or the production of pro-inflammatory cytokines in the periphery, which can consequently act on the brain through a variety of mechanisms [2]. There also is growing evidence demonstrating that suppression of AHN can result in detrimental mental health outcomes such as the development of depression, anxiety, and cognitive deficits [12]. Recent studies in rodents suggest that chemotherapy-induced suppression of AHN may underlie the behavioral side effects of breast cancer treatment as well [14].
Potential benefits of increasing dietary omega-3 fatty acids and decreasing added sugars
Dietary approaches that modify inflammation and AHN are promising strategies for reducing chemotherapy-induced cognitive deficits in breast cancer survivors. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), the omega-3 fatty acids (n-3 FAs) found in fish oil and marine sources, are the primary fatty acids associated with anti-inflammatory properties and neuroprotection in animals and humans [18]. DHA is the major fatty acid in the brain and is typically found in levels 250–300 times higher than EPA [18]. DHA has a variety of effects on neuronal membranes including altering permeability and membrane fluidity; these effects were recently summarized in an excellent literature review [18]. EPA and DHA are substrates for lipid mediators important in the inflammatory response, many of which have a wide range of anti-inflammatory and pro-resolving actions [19]. In a recently published report, administration of EPA and DHA concurrently with doxorubicin chemotherapy prevented depressive-like behaviors and reduced neuroinflammation, oxidative stress, and neural apoptosis in male rats [20]. DHA has been shown to be safe and well tolerated at a dose of 1.8 g/day in women with advanced metastatic breast cancer during 20 weeks of doxorubicin-based chemotherapy, and DHA was associated with improved overall survival in a subset of women who highly incorporated DHA into plasma phospholipids [21]. Both EPA and DHA may play important roles in reducing neuroinflammation; DHA appears to have a more potent inhibitory effect on individual inflammatory cytokines, while EPA has broader effects on multiple cytokines in human neurons [18].
In contrast to generally beneficial effects of n-3 FAs on cognitive function, diets high in added sugars may interact with n-3 FAs to diminish their anti-inflammatory actions [22], cause changes in brain n-3 FA metabolism [23], or act independently to promote cognitive deficits [24] and inflammation [25]. Naturally occurring dietary sugars include glucose (starches), lactose (milk), and fructose (fruit). Dietary added sugars are those that have been added to foods during processing to enhance flavor, and commonly include sucrose (table sugar) and fructose. Research surrounding high added sugar intake often employs animal models with high sucrose and/or high fructose diets as a proxy for high dietary added sugar intake [22–25]. Memory deficits associated with sucrose intake are independent of weight gain in rodent models of diet-induced obesity; obese animals on high sucrose diets develop memory deficits whereas obese animals on high fat diets do not [26]. Furthermore, in mice, n-3 FAs decrease weight gain associated with a high fat diet, but not a high sucrose diet [22], suggesting that n-3 FAs interact differently with dietary fats versus sugars. Indeed, a high sucrose diet not only promotes obesity, but also may interfere with the anti-inflammatory effects of high n-3 FAs in adipose tissue [22]. This is relevant to patients being treated for breast cancer because our data suggest that this population consumes up to 32% of kilocalories from added sugars (T. Orchard, unpublished data), an amount that equals or exceeds levels found to be harmful in animal models examining neuroinflammation, neurogenesis, and cognition [27–30].
N-3 FAs and added sugars influence cognition
The effects of n-3 FAs on cognitive symptoms associated with anti-cancer drugs are yet to be investigated in clinical trials, but observational data suggest that n-3 FA intake is associated with less fatigue in breast cancer patients undergoing chemotherapy [31], and fatigue and cognitive dysfunction often co-occur in women treated for breast cancer [32]. Additionally, results of randomized controlled trials in adults who were cognitively healthy or had mild age-related decline suggest that n-3 FAs may reduce depression and improve cognitive function, specifically performance on verbal fluency, recognition, and memory tests [33, 34]. However, results are not consistent across all human studies [35].
Recently, the effects of n-3 FAs on depressive-like behavior in male rats treated with doxorubicin (DOX) have been examined [20]. Rats in the DOX + n-3 FA group were given EPA and DHA (1.5 g/kg) by gavage beginning one week prior to chemotherapy administration and continuing for two weeks during seven injections of doxorubicin (2.5 mg/kg dose). Compared to the DOX alone group, rats receiving DOX + n-3 FA exhibited less depressive-like behavior. Oxidative stress markers were reduced in the brain of mice receiving DOX + n-3 FA compared to DOX alone. Additionally, provision of EPA and DHA partially prevented DOX-induced apoptosis of neurons in the pre-frontal cortex and the hippocampus. To our knowledge, this is the only study to date that has tested n-3 FAs as a treatment for chemotherapy-induced cognitive deficits.
Although there is a paucity of research on n-3 FA treatment for other cognitive deficits associated with chemotherapy, multiple studies have examined the effects of n-3 FAs on learning and memory in animal models of aging with generally favorable results [18]. Because the effects of chemotherapy on brain structure and function resemble the effects seen with aging (e.g., memory impairment, increased inflammation, and oxidative stress) [8, 9], results of these studies may inform future research addressing cognitive deficits resulting from chemotherapy. For example, in aged rats, dietary EPA increased cortical tissue DHA, down-regulated age-related microglial activation, and improved spatial memory [36]. Aged mice fed with n-3 FAs for two months showed improved spatial recognition and novel object recognition memory [37]. Similarly, aged mice fed with n-3 FAs by gavage for 8 weeks performed significantly better than mice fed with olive oil or no supplement on several memory tasks associated with hippocampal function: recognition memory measured by novel object recognition test, spatial memory measured by the Morris water maze and spatial Y-maze, and aversive responses measured by the contextual fear conditioning task [38].
The effects of sucrose or fructose alone on cognitive function have been investigated in a limited number of studies using rodent models. As an example, rats fed a diet with excess sucrose exhibited impaired spatial learning and memory, as well as hippocampal insulin resistance, compared to rats fed with standard chow [26]. Inflammatory cytokines were not specifically evaluated in these experiments, however, fasting blood glucose levels were elevated among sucrose-fed rats compared to rats fed with standard chow, and glucose metabolism abnormalities are associated with increased inflammation. The researchers proposed that development of insulin resistance in the hippocampus led to deficits in hippocampal-dependent cognitive functions. Similarly, another study reported impairment of hippocampal-dependent place recognition memory in rats fed with 10% sucrose solutions in addition to a standard diet or high fat cafeteria style diet for short periods of time (e.g., 5, 11, or 20 days); hippocampal inflammation also increased in animals in the sucrose group [27]. Long-term exposure (eight months) to 10% fructose solution in addition to a standard diet led to weight gain, peripheral insulin resistance, lipid abnormalities, and memory impairments in male rats, compared to a control group with no fructose [28].
The combined effect of high added sugars and low n-3 FAs on cognitive outcomes has not been adequately explored, but one research group has investigated this interaction using a rat model of metabolic syndrome [24]. Male rats were trained on a spatial memory task in the Barnes maze, then assigned to one of four diet groups for six weeks (n-3 FA enriched diet with water or 15% fructose solution, or n-3 FA deficient diet with water or 15% fructose solution) before retesting memory and collecting samples for analysis. Results showed that insulin resistance and hypertriglyceridemia, characteristics of metabolic syndrome, were induced by high fructose consumption. Rats consuming the fructose solution exhibited impairment in spatial memory, which was exacerbated by n-3 deficiency and partially alleviated by n-3 FA enrichment. Of note, a truly low added sugar diet was not evaluated in this study because the background diets all contained moderate levels of added sugars from dextrose and sucrose. Nonetheless, the results suggest that a diet high in n-3 FAs is effective at alleviating some of the deleterious effects of high fructose consumption on memory.
Collectively, these studies suggest that n-3 FAs and added sugars have different and often opposing effects on cognition. High amounts of sucrose and fructose seem to impair memory, while n-3 FAs appear to be protective.
N-3 FAs and added sugars influence neuroinflammation
There is a large body of evidence supporting the anti-inflammatory role of n-3 FAs in humans and animal models [39]. Supplementation with n-3 FAs is known to decrease inflammatory cytokine production in cancer patients [40]. Clinical studies of breast cancer survivors also suggest that dietary intake of n-3 FAs is associated with lower serum inflammatory markers [31]. In contrast to pharmacologic TNF inhibitors, n-3 FAs are generally recognized as safe in doses up to 3 g/day, and are well tolerated by women who are high risk for breast cancer in doses up to 7.5 g/day [41]. Recent studies in a rat model of chemotherapy, suggest that n-3 FAs can ameliorate DOX-induced neuroinflammation; provision of EPA and DHA for three weeks inhibited DOX-induced increases in mRNA expression of inflammatory cytokines, including IL-1β, IL-6, and TNF-α, in brain tissue [20]. In addition, EPA plus DHA supplementation prevented the DOX-induced rise in the protein levels of nuclear factor kappa beta (NF-KB) in the cortex and hippocampus. Similarly, in the mouse models of ischemic brain injury, high EPA and DHA diets reduced protein expression of hippocampal IL-1β [42]. Likewise, in a mouse model of systemic inflammation, transgenic mice treated with a control diet exhibited hippocampal abnormalities and pro-inflammatory responses that were prevented with n-3 FA supplementation [43]. In mouse models, levels of inflammatory cytokines in the hippocampus increase with aging, but this rise is partially alleviated by feeding an EPA plus DHA-enriched diet for two months [37]. Recent data from our research group extend the evidence for benefits of n-3 FAs in reducing neuroinflammation to a postmenopausal mouse model; C57/Bl6 mice receiving high EPA plus DHA diets for only 12 days expressed significantly lower mRNA levels of IL-6, than mice on a control diet [44].
Evidence from RCTs in healthy or overweight adults suggests that added sugars promote systemic inflammation. For example, in overweight adults, short-term consumption of a high sucrose diet modestly increased C-reactive protein (CRP) [25]. Similarly, CRP was increased in healthy young men with consumption of a sugar-sweetened beverage containing 40–80 g/day of either glucose, sucrose, or fructose for three weeks [45].
Rodent studies support the potential of high sucrose diets to induce not only peripheral inflammation, but also neuroinflammation. For example, researchers recently reported increased hippocampal inflammation (e.g., TNF-α and IL-1β) and oxidative stress, in addition to impaired recognition memory, in rats exposed to a 10% sucrose solution for approximately three weeks compared to controls [27]. Similarly, longer-term ingestion of 10% sucrose solution in addition to chow led to increased hippocampal mRNA levels of the inflammatory marker, IL-6, but decreased levels of TNF-α and IL-1β in male rats [29]. A marker of insulin resistance (IRS-1 Ser phosphorylation) was also increased and correlated with IL-6 levels, providing supporting evidence for IL-6-mediated insulin resistance [28]. These studies suggest that inflammation, potentially inducing insulin resistance, may be an underlying mechanism through which a high sugar diet impacts neuroinflammation and in turn, cognition.
Less data are available from clinical studies to shed light on the combined effects of added sugars and n-3 FAs on neuroinflammatory outcomes. However, one randomized controlled trial (RCT) was recently completed in 20 adults with spinal cord injury suggesting that a diet low in refined sugars and starches, supplemented with 1500 mg EPA and 750 mg DHA daily, in addition to other dietary supplements thought to be anti-inflammatory, could reduce serum inflammatory markers (e.g., interleukin one beta [IL-1β] and interferon gamma) and improve depression scores, as measured by the CES-D (Center for Epidemiologic Studies Depression) scale [46].
N-3 FAs and added sugars influence adult hippocampal neurogenesis
While it is understood that certain dietary components (e.g., n-3 FAs, added sugar) can influence cognition and mood, to our knowledge, the relationship between diet and hippocampal neurogenesis is yet to be explored in human trials. However, there is a growing body of evidence from animal studies supporting the hypothesis that diet can significantly influence brain function through AHN.
Numerous rodent experiments have demonstrated that diets enriched with n-3 FAs can contribute to increases in AHN. For example, eight weeks of n-3 supplementation increased AHN measured by BrdU+ staining compared to control groups [38]. Other research supports a positive correlation between the number of BrdU-labeled hippocampal newborn neurons in aged rats and the DHA content of red blood cell phospholipids [47]; furthermore, in this study hippocampal neurogenesis increased 34% in aged rats after consumption of a DHA diet versus control diet for 16 months, suggesting that DHA may support hippocampal newborn neuron production and/or survival. The mechanisms underlying effects of n-3 FAs on AHN are unclear, but may involve regulation of nuclear transcription factors. Researchers reported that activation of nuclear receptors for which n-3 FAs are ligands (e.g., peroxisome proliferator-activated receptor alpha [PPARα]) protected mice from a reduction in AHN induced by whole-brain irradiation [48]. Additionally, evidence from cell culture studies suggests that EPA and DHA have distinct effects on neural stem cells; EPA seems to induce neural stem cell proliferation via direct effects on endocannabinoid pathways [49].
In contrast to the potential benefits of n-3 FAs, data from rodent studies suggest that high sugar consumption is linked to reductions in AHN. Researchers compared male rats fed with diets supplemented with various sugar solutions or plain water for four weeks; rats fed with sucrose and fructose had significantly fewer newborn hippocampal neurons than rats fed with glucose or plain water, indicative of reduced AHN among the sucrose and fructose-fed groups [50]. Similarly, male mice fed with diets supplemented with 15% fructose water displayed reduced hippocampal neurogenesis along with reduced hippocampal-dependent cognitive function (i.e., spatial learning, episodic memory) compared to mice fed with the same diet with plain water [30]. Importantly, however, was the finding that if mice were switched from the high fructose diet to plain water for an additional twelve weeks, they experienced partial regain of cognitive functioning. Collectively, these findings suggest that high added sugar intake can lead to reductions in AHN, but that the reductions may not be permanent if sugar intake is subsequently reduced. The combined effects of added sugars and n-3 FAs on AHN in human or animal models are yet to be explored.
Conclusion
As cancer treatments continue to improve and extend the lives of patients, it is becoming increasingly important to focus on improving the quality of life for cancer survivors through symptom management. Cognitive impairments associated with chemotherapy are well documented, and can significantly compromise quality of life. Evidence suggests that chemotherapy influences cognition through reductions in hippocampal neurogenesis and neuroinflammation, which affect memory and cognitive function. This review highlights dietary modifications that may have the potential to protect against or ameliorate some of the negative cognitive side effects so commonly associated with chemotherapy treatment. In numerous rodent models, measures of cognitive function have been increased through dietary enrichment with n-3 FAs, and decreased through provision of diets high in added sugar. These effects are frequently accompanied by supporting changes in neuroinflammation and neurogenesis (Fig. 1). These encouraging findings point to diets high in n-3 FAs and low in added sugar content as potentially protective against the cognitive side effects associated with chemotherapy, possibly working through mechanisms associated with inflammation, oxidative stress, and neural protection. It remains to be seen whether the cognitive benefits of a diet rich in n-3 FAs paired with low added sugars extend to animal models that include chemotherapy. If this is indeed the case, the next step forward involves translation of this research from the bench to the bedside to ascertain the efficacy of these dietary modifications among adults receiving chemotherapy.
Fig. 1.
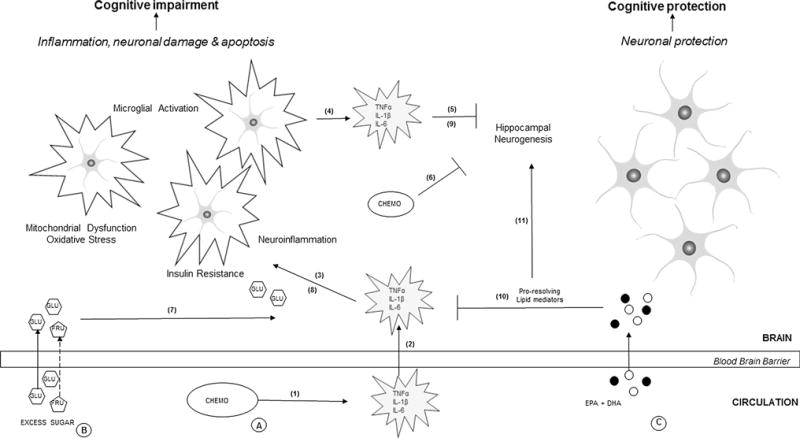
Proposed effects of chemotherapy and dietary added sugars versus omega-3 fatty acids in the brain. A Chemotherapy (Chemo), such as Doxorubicin, (1) induces inflammation in the peripheral circulation. (2) Inflammatory cytokines such as TNFα, IL-1β, and IL-6 cross the blood–brain barrier and stimulate (3) neuroinflammation, mitochondrial dysfunction, production of reactive oxygen species, and nitrogen species resulting in the activation of microglia. (4) Microglia in turn produce local inflammatory cytokines promoting neurotoxicity. (5) An inflammatory environment impairs adult hippocampal neurogenesis (AHN). (6) Additionally, the small amounts of chemotherapeutic drugs that cross the blood–brain barrier can directly decrease hippocampal cell proliferation and neurogenesis. Collectively, these effects can lead to cognitive impairment and other behavioral deficits such as fatigue and depression. B High amounts of dietary added sugars [glucose (GLU) and fructose (FRU)], (7) may contribute to inflammation, (8) promote insulin resistance mediated by cytokines, and (9) reduce neurogenesis, potentially aggravating effects of chemotherapy. C The long-chain n-3 fatty acids, EPA & DHA, may protect neurons from chemotherapy-induced damage via effects of pro-resolving lipid mediators on the regulation of transcription factors involved in (10) neuroinflammation [e.g., nuclear factor kappa beta (NF-KB)] and (11) neurogenesis [e.g., peroxisome proliferator activator receptor alpha (PPARα)] in the cortex and hippocampus, resulting in the protection of neuronal function and cognition. The overall balance of pro- and anti-inflammatory signals influences neuronal and cognitive outcomes
Acknowledgments
Maryam Lustberg, MD, MPH for her expertise in clinical implications of chemotherapy and her contributions to preliminary data. TO and ACD received funding on this topic from NCI (R01CA18997); the funding source provides salary support for TO, ACD, and MMGD but was not directly involved in any aspect of the preparation of this review paper.
Abbreviations
- MRI
Magnetic resonance imaging
- fMRI
Functional magnetic resonance imaging
- IL-6
Interleukin-6
- TNF-α
Tumor necrosis factor alpha
- AHN
Adult hippocampal neurogenesis
- EPA
Eicosapentaenoic acid
- DHA
Docosahexaenoic acid
- n-3 FA
Omega-3 fatty acid
- DOX
Doxorubicin
- RCT
Randomized controlled trial
- IL-1β
Interleukin one beta
- CES-D
Center for Epidemiologic Studies Depression
- NF-KB
Nuclear factor kappa beta
- CRP
C-reactive protein
- PPAR-α
Peroxisome proliferator-activated receptor alpha
Footnotes
Author contributions
TSO & ACD were involved in conceptualization of the manuscript; TSO, ACD, MMGD, and KRW were involved in writing the manuscript; all authors were involved in revision of the manuscript. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest: The authors declare that they have no conflict of interests.
References
- 1.Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol Nurs Forum. 2012;39(1):E31–E40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- 2.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silberfarb PM, Philibert D, Levine PM. Psychosocial aspects of neoplastic disease: II. Affective and cognitive effects of chemotherapy in cancer patients. Am J Psychiatry. 1980;137(5):597–601. doi: 10.1176/ajp.137.5.597. [DOI] [PubMed] [Google Scholar]
- 4.Hurria A, Rosen C, Hudis C, Zuckerman E, Panageas KS, Lachs MS, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54(6):925–931. doi: 10.1111/j.1532-5415.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamada TH, Denburg NL, Beglinger LJ, Schultz SK. Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22(1):48–54. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, et al. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109(1):146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Apple AC, Schroeder MP, Ryals AJ, Voss JL, Gitelman D, et al. Reduced prefrontal activation during working and long-term memory tasks and impaired patient-reported cognition among cancer survivors postchemotherapy compared with healthy controls. Cancer. 2016;122(2):258–268. doi: 10.1002/cncr.29737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(Suppl):S109–S116. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osburg B, Peiser C, Domling D, Schomburg L, Ko YT, Voigt K, et al. Effect of endotoxin on expression of TNF receptors and transport of TNF-alpha at the blood-brain barrier of the rat. Am J Physiol Endocrinol Metab. 2002;283(5):E899–E908. doi: 10.1152/ajpendo.00436.2001. [DOI] [PubMed] [Google Scholar]
- 10.Liedke PE, Reolon GK, Kilpp B, Brunetto AL, Roesler R, Schwartsmann G. Systemic administration of doxorubicin impairs aversively motivated memory in rats. Pharmacol Biochem Behav. 2009;94(2):239–243. doi: 10.1016/j.pbb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Tangpong J, Cole MP, Sultana R, Joshi G, Estus S, Vore M, et al. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis. 2006;23(1):127–139. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Pereira Dias G, Hollywood R, Bevilaqua MC, da Luz AC, Hindges R, Nardi AE, et al. Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms. Neuro Oncol. 2014;16(4):476–492. doi: 10.1093/neuonc/not321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Kim JS, Song MS, Kim SH, Kang SS, Bae CS, et al. Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol Learn Mem. 2010;93(4):487–494. doi: 10.1016/j.nlm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Manda K, Bhatia AL. Prophylactic action of melatonin against cyclophosphamide-induced oxidative stress in mice. Cell Biol Toxicol. 2003;19(6):367–372. doi: 10.1023/b:cbto.0000013342.17370.16. [DOI] [PubMed] [Google Scholar]
- 16.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, et al. Mechanisms of chemotherapy-induced behavioral toxicities. Front Neurosci. 2015;9:131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA. DPA and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu YQ, Dang RL, Tang MM, Cai HL, Li HD, Liao DH, et al. Long Chain Omega-3 Polyunsaturated Fatty Acid Supplementation Alleviates Doxorubicin-Induced Depressive-Like Behaviors and Neurotoxicity in Rats: Involvement of Oxidative Stress and Neuroinflammation. Nutrients. 2016;8(4):E243. doi: 10.3390/nu8040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bougnoux P, Hajjaji N, Ferrasson MN, Giraudeau B, Couet C, Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br J Cancer. 2009;101(12):1978–1985. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma T, Liaset B, Hao Q, Petersen RK, Fjaere E, Ngo HT, et al. Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice. PLoS ONE. 2011;6(6):e21647. doi: 10.1371/journal.pone.0021647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taha AY, Gao F, Ramadan E, Cheon Y, Rapoport SI, Kim HW. Upregulated expression of brain enzymatic markers of arachidonic and docosahexaenoic acid metabolism in a rat model of the metabolic syndrome. BMC Neurosci. 2012;13:131. doi: 10.1186/1471-2202-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agrawal R, Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012;590(10):2485–2499. doi: 10.1113/jphysiol.2012.230078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sørensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr. 2005;82(2):421–427. doi: 10.1093/ajcn.82.2.421. [DOI] [PubMed] [Google Scholar]
- 26.Jurdak N, Kanarek RB. Sucrose-induced obesity impairs novel object recognition learning in young rats. Physiol Behav. 2009;96(1):1–5. doi: 10.1016/j.physbeh.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 27.Beilharz JE, Maniam J, Morris MJ. Short exposure to a diet rich in both fat and sugar or sugar alone impairs place, but not object recognition memory in rats. Brain Behav Immun. 2014;37:134–141. doi: 10.1016/j.bbi.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Wu HW, Ren LF, Zhou X, Han DW. A high-fructose diet induces hippocampal insulin resistance and exacerbates memory deficits in male Sprague-Dawley rats. Nutr Neurosci. 2015;18(7):323–328. doi: 10.1179/1476830514Y.0000000133. [DOI] [PubMed] [Google Scholar]
- 29.Djordjevic A, Bursac B, Velickovic N, Vasiljevic A, Matic G. The impact of different fructose loads on insulin sensitivity, inflammation, and PSA-NCAM-mediated plasticity in the hippocampus of fructose-fed male rats. Nutr Neurosci. 2015;18(2):66–75. doi: 10.1179/1476830513Y.0000000098. [DOI] [PubMed] [Google Scholar]
- 30.Cisternas P, Salazar P, Serrano FG, Montecinos-Oliva C, Arredondo SB, Varela-Nallar L, et al. Fructose consumption reduces hippocampal synaptic plasticity underlying cognitive performance. Biochim Biophys Acta. 2015;1852(11):2379–2390. doi: 10.1016/j.bbadis.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alfano CM, Imayama I, Neuhouser ML, Kiecolt-Glaser JK, Smith AW, Meeske K, et al. Fatigue, inflammation, and omega-3 and omega-6 fatty acid intake among breast cancer survivors. J Clin Oncol. 2012;30(12):1280–1287. doi: 10.1200/JCO.2011.36.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Askren MK, Jung M, Berman MG, Zhang M, Therrien B, Peltier S, et al. Neuromarkers of fatigue and cognitive complaints following chemotherapy for breast cancer: a prospective fMRI investigation. Breast Cancer Res Treat. 2014;147(2):445–455. doi: 10.1007/s10549-014-3092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurko-Mauro K. Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr Alzheimer Res. 2010;7(3):190–196. doi: 10.2174/156720510791050911. [DOI] [PubMed] [Google Scholar]
- 34.Sinn N, Milte CM, Street SJ, Buckley JD, Coates AM, Petkov J, et al. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. 2012;107(11):1682–1693. doi: 10.1017/S0007114511004788. [DOI] [PubMed] [Google Scholar]
- 35.Rogers PJ, Appleton KM, Kessler D, Peters TJ, Gunnell D, Hayward RC, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 36.Kelly L, Grehan B, Chiesa AD, O’Mara SM, Downer E, Sahyoun G, et al. The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol Aging. 2011;32(12):2318.e1–e15. doi: 10.1016/j.neurobiolaging.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Labrousse VF, Nadjar A, Joffre C, Costes L, Aubert A, Gregoire S, et al. Short-term long chain omega3 diet protects from neuroinflammatory processes and memory impairment in aged mice. PLoS ONE. 2012;7(5):e36861. doi: 10.1371/journal.pone.0036861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cutuli D, De Bartolo P, Caporali P, Laricchiuta D, Foti F, Ronci M, et al. n-3 polyunsaturated fatty acids supplementation enhances hippocampal functionality in aged mice. Front Aging Neurosci. 2014;6:220. doi: 10.3389/fnagi.2014.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Experimental evidence of omega-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res Int. 2013;2013:743171. doi: 10.1155/2013/743171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finocchiaro C, Segre O, Fadda M, Monge T, Scigliano M, Schena M, et al. Effect of n-3 fatty acids on patients with advanced lung cancer: a double-blind, placebo-controlled study. Br J Nutr. 2012;108(2):327–333. doi: 10.1017/S0007114511005551. [DOI] [PubMed] [Google Scholar]
- 41.Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y, et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr. 2010;91(5):1185–1194. doi: 10.3945/ajcn.2009.29036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lalancette-Hebert M, Julien C, Cordeau P, Bohacek I, Weng YC, Calon F, et al. Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke. 2011;42(10):2903–2909. doi: 10.1161/STROKEAHA.111.620856. [DOI] [PubMed] [Google Scholar]
- 43.Crupi R, Cambiaghi M, Deckelbaum R, Hansen I, Mindes J, Spina E, et al. n-3 fatty acids prevent impairment of neurogenesis and synaptic plasticity in B-cell activating factor (BAFF) transgenic mice. Prev Med. 2012;54(Suppl):S103–S108. doi: 10.1016/j.ypmed.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 44.Gaudier-Diaz MM, Lustberg MB, Cole RM, Belury MA, DeVries AC, Orchard TS. Effects of Sucrose and Omega-3 Fatty Acids on Chemotherapy-Induced Neuroinflammation in a Mouse Model of Breast Cancer. J Acad Nutr Diet. 2015;115(9):A25. [Google Scholar]
- 45.Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr. 2011;94(2):479–485. doi: 10.3945/ajcn.111.013540. [DOI] [PubMed] [Google Scholar]
- 46.Allison DJ, Ditor DS. Targeting inflammation to influence mood following spinal cord injury: a randomized clinical trial. J Neuroinflammation. 2015;12:204. doi: 10.1186/s12974-015-0425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tokuda H, Kontani M, Kawashima H, Kiso Y, Shibata H, Osumi N. Differential effect of arachidonic acid and docosahexaenoic acid on age-related decreases in hippocampal neurogenesis. Neurosci Res. 2014;88:58–66. doi: 10.1016/j.neures.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Ramanan S, Zhao W, Riddle DR, Robbins ME. Role of PPARs in Radiation-Induced Brain Injury. PPAR Res. 2010;2010:234975. doi: 10.1155/2010/234975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dyall SC, Mandhair HK, Fincham RE, Kerr DM, Roche M, Molina-Holgado F. Distinctive effects of eicosapentaenoic and docosahexaenoic acids in regulating neural stem cell fate are mediated via endocannabinoid signalling pathways. Neuropharmacology. 2016;107:387–395. doi: 10.1016/j.neuropharm.2016.03.055. [DOI] [PubMed] [Google Scholar]
- 50.van der Borght K, Kohnke R, Goransson N, Deierborg T, Brundin P, Erlanson-Albertsson C, et al. Reduced neurogenesis in the rat hippocampus following high fructose consumption. Regul Pept. 2011;167(1):26–30. doi: 10.1016/j.regpep.2010.11.002. [DOI] [PubMed] [Google Scholar]


