Abstract
Background
Despite the reduced incidence of coronary heart disease (CHD) with intensive risk factor management, people with diabetes and prediabetes remain at increased CHD risk. Diabetes prevention interventions may be needed to reduce CHD risk. This approach was examined in the Diabetes Prevention Program (DPP) and its Outcome Study (DPPOS), a long-term intervention study in 3234 subjects with prediabetes (mean [αSD] age 64±10 yrs) which showed reduced diabetes risk with lifestyle and metformin compared to placebo over 3.2 years.
Methods
The DPPOS offered periodic group lifestyle sessions to all participants and continued metformin in the originally randomized metformin group. Subclinical atherosclerosis was assessed in 2029 participants using coronary artery calcium (CAC) measurements after 14 years of average followup. The CAC scores were analyzed continuously as CAC severity, and categorically as CAC presence (CAC score>0), and reported separately in men and women.
Results
There were no CAC differences between lifestyle and placebo intervention groups, in either sex. CAC severity and presence were significantly lower among men in the metformin versus the placebo group (age-adjusted mean CAC severity: 39.5 vs 66.9 AU, p=0.04; CAC presence: 75% vs 84%, p=0.02), but no metformin effect was seen in women. In multivariate analysis, the metformin effect in men was not influenced by demographic, anthropometric or metabolic factors, by the development of diabetes, or by use/non-use of statin therapy.
Conclusion
Metformin may protect against coronary atherosclerosis in prediabetes and early diabetes among men.
Keywords: coronary artery disease, treatment, clinical trial, diabetes mellitus
Subject Code: Clinical Studies, Coronary Circulation, Diabetes, Type 2, Cardiovascular Disease, Computerized Tomography, Treatment, Coronary Artery Disease
Introduction
The incidence of coronary heart disease (CHD) is increased approximately two fold in type 2 diabetes and CHD remains the single most important cause of morbidity and mortality in diabetes (1). Interventions targeting factors that accelerate coronary atherosclerosis such as dyslipidemia, hypertension, hyperglycemia and a procoagulant state have contributed to the significantly reduced incidence of CHD in the general population in recent years (2). However, despite intensive management of CHD risk factors and parallel reductions in overall rates of CHD in diabetes, there remains considerable diabetes-related excess CHD risk, suggesting that there are limitations to the benefits of these CHD-targeted clinical interventions in established diabetes (3). Greater success in reducing CHD may thus be achieved by initiating preventive approaches as early as possible in the course of diabetes, including diabetes prevention itself. For example, lifestyle or pharmacologic interventions for diabetes prevention, implemented early in the progression from prediabetes to diabetes when clinical CHD events are less common, may be the key to fundamentally altering the increased risk of atherosclerosis in this disease.
The Diabetes Prevention Program (DPP) and its Outcome Study (DPPOS) comprise one of the few clinical trials testing the effects of therapeutic interventions in subjects with prediabetes on long-term health outcomes. DPP demonstrated that intensive lifestyle change or metformin treatment reduced the incidence of diabetes and improved the cardiovascular disease (CVD) risk profile in a cohort of subjects at high risk for diabetes (4). Since the onset of diabetes was systematically ascertained through semi-annual assessments, it was possible to demonstrate that development of diabetes was accompanied by deterioration of cardiovascular risk factors in DPP and DPPOS (5, 6). However, to date there are too few CVD events to assess the effect of our interventions on these outcomes. Coronary artery calcium (CAC) measurements reflect total coronary atherosclerotic burden and provide an effective, non-invasive tool to predict CHD events in cohorts with and without type 2 diabetes, and without known CHD (7,8). We performed measurements of CAC to assess DPP/DPPOS treatment effects on this early marker of subclinical coronary atherosclerosis.
Methods
Written informed consent was obtained from all participants and the studies were approved by each clinical center’s institutional review board (see Fig 1 for CONSORT flow diagram).
Fig 1. Consort diagram.
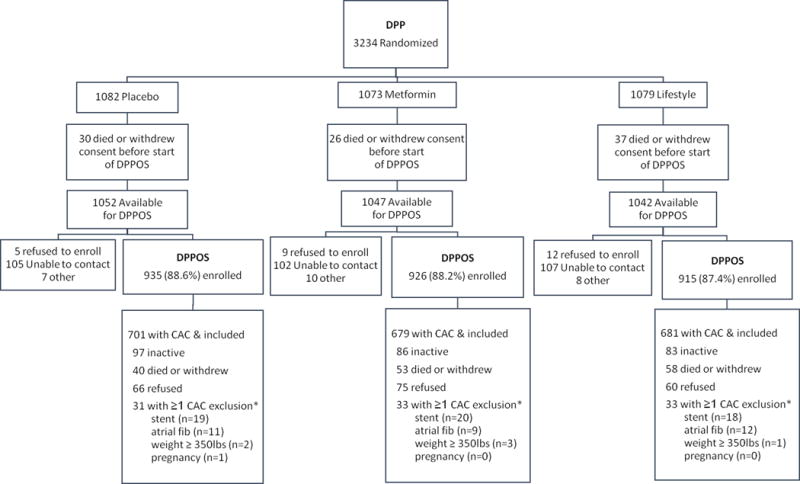
* CAC exclusion criteria are not mutually exclusive.
DPP Design
The Diabetes Prevention Program was a randomized clinical trial comparing metformin treatment or an individual behavioral lifestyle intervention program with placebo, to prevent or delay incident diabetes (4). Inclusion criteria were: age ≥25 years, body mass index (BMI) ≥24 kg/m2 (≥22 kg/m2 in Asian Americans), fasting plasma glucose levels between 95 and 125 mg/dl and impaired glucose tolerance (IGT, 2-hour post-load glucose of 140–199 mg/dl). Those taking medications known to alter glucose tolerance, had experienced a CVD event in the prior 6 months or had illnesses that reduced their ability to participate were excluded. Participants were randomly assigned to one of three interventions: metformin 850 mg twice daily, placebo twice daily, or an intensive program of lifestyle modification. Treatment assignments were stratified according to clinical center and double blinded for the metformin and placebo groups. The goals of lifestyle change were to achieve and maintain a weight reduction of at least 7% of initial body weight through consumption of a low-calorie, low-fat diet and to engage in moderate physical activity for at least 150 min/week. Diabetes was diagnosed on the basis of an annual oral glucose tolerance test or a semiannual fasting plasma glucose test according to American Diabetes Association (ADA) criteria (9). The diagnosis required confirmation by a second test, usually within six weeks. If diabetes was diagnosed and confirmed, diagnoses were reported to the participants and their health care providers were informed. Study metformin or placebo was provided until hyperglycemia worsened to a fasting plasma glucose level ≥140 mg/dl during DPP or an HbA1c >7.0% during DPPOS. When this occurred, study drug was discontinued and diabetes management was transferred to the participant’s own health care provider. Although participants and their providers were informed of study related blood pressure and lipid profile results, all medical management decisions were undertaken by the participants’ health care providers.
DPPOS Design
DPP showed that lifestyle intervention reduced incidence of diabetes by 58% and metformin by 31% compared with placebo during an average follow-up of 3.2 years (4). At DPP-end the placebo and metformin groups were unmasked to their treatment assignment, and all participants were offered the lifestyle intervention in a group format during a one-year bridge period. All surviving consented members (n= 3149) of the three original DPP treatment arms, regardless of diabetes status, were invited to participate in the DPPOS, and 2776 participants (88%) joined (10). Maintenance group lifestyle sessions, offered quarterly to all DPPOS participants, reinforced the basic lifestyle content and the weight loss and physical activity goals. In addition to the maintenance sessions, the original lifestyle group was offered supplementary group programs, reinforcing specific behavioral self-management activities, twice per year. During DPPOS, metformin, now unmasked, continued to be provided to participants randomized to metformin who remained eligible.
Clinical and metabolic variables
Standardized interviewer-administered questionnaires were used to obtain demographic and clinical data. ‘Ever smoking’ was defined as prior use of 100 cigarettes or more. Blood pressure (BP), height, and weight were measured using standardized techniques. HbA1c, lipid profile, serum creatinine and urine albumin/creatinine ratio, and high sensitivity C reactive protein (CRP) and plasma tissue plasminogen activator (tPA) measurements were performed at the Central Biochemistry Laboratory (Northwest Lipid Research Laboratories, University of Washington, Seattle) as previously reported (4, 6). Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (11). All assessments were performed at baseline and annually thereafter except for CRP and tPA which were measured at baseline, DPP year 1 and DPPOS year 1 and 5.
Coronary artery calcium measurements
CAC was measured during Year 10 of DPPOS in 2029 participants at all 25 sites according to previously published methods (12). This represents 74% of the DPPOS cohort (Figure 1). Participants who weighed more than 350 lbs at the time of scanning (6) were ineligible to participate in the CAC substudy because of the inability to acquire the relevant images on conventional equipment, 201 refused consent, 57 had a stent, 32 had atrial fibrillation and 1 was pregnant. At the DPP baseline examination, participants who later underwent CAC studies had slightly higher minority race/ethnicity representation, were slightly younger, had modestly lower BMI and lower systolic BP and slightly fewer were smokers compared to those who did not undergo the procedure. Baseline metabolic measures were not different between tested and untested groups, except for slightly higher HDL-C levels in tested participants. The proportion with CAC measurements did not differ among treatment groups. Chest computed tomography was performed by certified technologists at each site using prospectively electrocardiogram-triggered scan acquisition at 50% of the R-R interval with a multi-detector system, acquiring a block of 4 2.5-mm slices for each cardiac cycle in a sequential or axial scan mode. Subjects were scanned twice and measurement of CAC was calibrated against a phantom of known physical calcium concentration. A radiologist or cardiologist read all computed tomography scans at the central reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA in Torrence, California) in a manner blinded to patient characteristics and treatment assignment. Discrepancies were reviewed and agreement obtained through consensus. For each scan, a total phantom-adjusted averaged Agatston score (13) was calculated, defined as the sum of calcium measures from the left main, left anterior descending, circumflex, and right coronary arteries.
Statistical methods
The outcomes reported in these analyses are based on data obtained as of January 2, 2014 for the 2061 DPPOS participants who had CAC measurements. All treatment group comparisons were conducted based on the original randomized interventions.
CAC was expressed both as a continuous variable (CAC severity) and as a categorical variable, mainly using a cutpoint of 0 (CAC presence), but also using cutpoints of10 and 100 Agatston units (AU). CAC severity was considered the primary measure for this analysis due to greater power in analyzing continuous outcomes. CAC severity was analyzed by Tobit regression (14) of the CAC score using the lifereg procedure in SAS to account for the skewness resulting for the relatively large number of individuals with a CAC score of 0. CAC scores were transformed to log(CAC +1) The Tobit regression coefficient represents the log ratio of the geometric mean CAC score per unit increase in the covariate, assuming some true measurable calcification for all subjects, including those with undetectable levels. The adjusted CAC mean scores were expressed as the geometric mean by back-transforming the adjusted censored mean calculated from the Tobit model. The presence of CAC (CAC>0) was analyzed using logistic regression.
Prespecified analysis of treatment effect modification by demographic subgroups (baseline age, sex, and race) and diabetes status subgroups was conducted using an interaction term in the models for CAC severity. If significant heterogeneity or interaction was detected among subgroups, then under the closure principle (15), the difference between groups can be tested within each subgroup or category at the 0.05 level without the need to adjust for multiple tests. There was an interaction between sex and the metformin vs. placebo effect on CAC presence (p=0.01) and CAC severity (p=0.08). Thus, all analyses were stratified by sex due to the interaction and due to the expected differences between men and women. Other secondary analyses were not adjusted for multiple comparisons and are nominally significant at the 0.05 level.
Results
Cohort Characteristics
Characteristics at baseline and follow-up of the cohort included in this analysis are shown in Table 1. Mean age of participants at the time of scanning was 67±10 years in men and 63±9 years in women, with mean study duration of 13.7±0.08 years since randomization. During DPPOS, 57% of non-diabetic metformin participants took 80% or more of the prescribed metformin dose and 70% took metformin in any amount, compared with 1% of non-diabetic participants in the lifestyle and 3% in the placebo groups taking metformin prescribed outside the study. Mean years of metformin use in the metformin group was 9.6±4.6 years whereas in the placebo and lifestyle groups it was 1.7±2.9 and 1.3±2.5 years respectively. Overall, diabetes developed in 59% of the placebo group as compared to 54% and 51% in the metformin and lifestyle groups respectively at the time of scanning, with the mean duration of diabetes being 5.4±5.3, 4.5±5.1 and 3.8±4.6 years and mean HbA1c 6.0±0.7%, 5.9±0.6% and 5.9±0.6% in the placebo, metformin and lifestyle groups respectively. The mean follow-up BMI was significantly lower in the metformin and lifestyle compared to placebo groups in both sexes, while systolic BP (SBP) was lower in the lifestyle group in men only. Statin use increased significantly over time in DPPOS to >50% of participants, greater in men than in women; this was not different across treatment groups. The proportion of current smokers was low during the study although a much higher proportion had a past history of cigarette smoking. tPA levels were lower in the metformin and lifestyle compared to the placebo groups in both sexes, while CRP was lower and high density lipoprotein cholesterol (HDL-C) was higher in the active treatment groups among women only.
Table 1.
Characteristics of the cohort at DPP baseline* and during follow-up#
| DPP Baseline* | Characteristics During Follow-up† | |||
|---|---|---|---|---|
| MEN | All | Placebo | Metformin | Lifestyle |
| N | 643 | 215 | 215 | 213 |
| Age at baseline and scan (y) | 53.4 ± 10.0 | 66.4 (65.1, 67.7) | 66.7 (65.5, 68.0) | 68.2 (66.7, 69.6) |
| BMI (kg/m2) | 31.6 ± 5.3 | 31.9 (31.1, 32.7) | 30.8 (30.1, 31.4)‡ | 30.2 (29.5, 31.0)‡ |
| HbA1c (%) | 5.92 ± 0.49 | 6.10 (6.01, 6.19) | 5.88 (5.79, 5.96)‡ | 5.87(5.78, 5.95)‡ |
| Systolic BP (mm Hg) | 125 ± 14 | 123 (122, 124) | 123 (122, 124) | 120 (119, 121)‡║ |
| Non-HDL-C (mg/dl) | 163 ± 34 | 139 (136, 143) | 135 (132, 138) | 138 (134, 141) |
| HDL-C (mg/dl) | 40 ± 9 | 43 (41, 44) | 44 (43, 46) | 44 (43, 45) |
| Median CRP (g/l) | 1.8 [0.9, 3.5] | 1.9 [1.2, 3.7] | 1.7 [2.1, 2.9] | 1.6 [2.2, 2.9]‡ |
| Median Urine Alb/Cr | 4.7 [3.3, 8.5] | 6.4 [4.4, 11.0] | 5.9 [4.0, 11.6] | 6.4 [4.8, 12.6] |
| eGFR | 94 ± 15 | 88 (86, 90) | 87 (85, 89) | 88 (86, 90) |
| tPA | 12.5 ± 4.7 | 11.8 (11.3, 12.4) | 10.4 (9.8, 11.0)‡ | 9.3 (8.8, 9.7)‡║ |
| Statin use | 5.9% | 63% | 58% | 55% |
| Median statin use duration (y) | n/a | 3 [0, 8] | 3 [0, 8] | 2 [0, 6] |
| Antihypertensive use | 19% | 73% | 70% | 62% |
| % Ever smoked | 50% | 54% | 51% | 51% |
| % Current smoker | 6.2% | 5.8% | 1.9% | 1.9% |
| Total metformin use (y) | n/a | 1.9 (1.5, 2.3) | 9.5 (8.8, 10.1)‡ | 1.0 (0.7, 1.3)║ |
| Incident diabetes | n/a | 61% | 53% | 50%‡ |
| Diabetes duration (y) § | n/a | 5.8 | 4.3 | 3.7‡║ |
| Median diabetes duration (y) | n/a | 4.6 [0, 11] | 1.5 [0, 10] | 0.1 [0, 8] |
| WOMEN | ||||
| N | 1418 | 486 | 464 | 468 |
| Age at baseline and scan (y) | 49.1 ± 9.3 | 62.3 (61.5, 63.1) | 63.4 (62.6, 64) | 62.7 (61.8, 63.6) |
| BMI (kg/m2) | 34.5 ± 6.5 | 34.7 (34.1, 35.3) | 33.7 (33.1, 34.3)‡ | 33.3 (32.7, 33.9)‡ |
| HbA1c (%) | 5.92 ± 0.49 | 6.02 (5.96, 6.08) | 5.95 (5.9, 6.0) | 5.95 (5.9, 6.0) |
| Systolic BP (mm Hg) | 122 ± 15 | 121 (120, 122) | 121 (120, 122) | 120 (119, 121) |
| Non-HDL-C (mg/dl) | 156 ± 36 | 142 (139, 144) | 141 (138, 143) | 140 (137, 142) |
| HDL-C (mg/dl) | 49 ± 12 | 51 (50, 52) | 53 (52, 54)‡ | 53 (52, 54)‡ |
| Median CRP (g/l) | 5.0 [2.5, 9.1] | 5.1 [2.4, 8.4] | 3.8 [2.0, 7.5]‡ | 4.2 [1.9, 6.9]‡ |
| Median Urine Alb/Cr | 5.8 [4.0, 9.8] | 7.3 [5.4, 11.5] | 7.5 [5.4, 11.2] | 7.6 [5.3, 12.8] |
| eGFR | 100 ± 16 | 93 (92, 94) | 92 (91, 94) | 93 (91, 94) |
| tPA | 10.7 ± 3.9 | 10.2 (9.8, 10.5) | 8.5 (8.1, 8.8)‡ | 8.6 (8.3, 8.9)‡ |
| Statin use | 3.8% | 52% | 54% | 49% |
| Median statin use duration (y) | n/a | 1 [0, 6] | 1 [0, 5] | 0 [0, 5] |
| Antihypertensive use | 14% | 67% | 68% | 64% |
| %Ever smoked | 34% | 37% | 31% | 35% |
| %Current smoker | 5.8% | 5.1% | 3.1% | 4.0% |
| Total metformin use (y) | n/a | 1.7 (1.4, 1.9) | 9.6 (9.2, 10.0)‡ | 1.4(1.2, 1.7)║ |
| Incident diabetes | n/a | 58% | 54% | 51%‡ |
| Diabetes duration (y) § | n/a | 5.1 (4.6, 5.6) | 4.6 (4.2, 5.1) | 3.8 (3.4, 4.2)‡║ |
| Median diabetes duration (y) | n/a | 3.7 [0, 10.5] | 1.7 [0, 10.1] | 0.5 [0, 8]‡║ |
Characteristics at baseline are expressed as mean ± SD or % as appropriate. No difference detected among treatment groups at baseline except for HDL-C in women (PLA = 47.5, MET=49.1, ILS=49.1) and smoking in women (PLA=8.0%, MET=4.7%, ILS=4.5%)
Characteristics during follow-up based on all annual visits prior to CAC measurement are expressed as mean (95% CI), or median [IQR] over follow-up for continuous variables (as appropriate), and any report of medication over follow-up except for tPA and CRP which reflect data collected from DPP Year 1 and DPPOS Years 1 and 5. Ever smoked includes self-reported prior smoking of 100 cigarettes at baseline and during year of CAC measurement. Current smoker reflects baseline and at CAC measurement.
p<0.05 vs. placebo;
p<0.05 vs. metformin. Significant effects emboldened.
Diabetes duration calculated as years since diabetes diagnosis for participants with diabetes. Otherwise duration is set at 0.
CAC Severity and Presence
The principal aim of this study was to evaluate treatment effects on the CAC score using our primary measure, CAC severity, derived from the logarithmically transformed CAC score ([log(CAC+1)]), as well as our secondary measure, CAC presence (CAC>0). As shown in Table 2, CAC severity was significantly greater in men than in women (p<0.001). In men, CAC severity was significantly lower in the metformin than in the lifestyle group in unadjusted analyses and 41% lower in the metformin than placebo group after age-adjustment. These effects persisted whether metformin-group participants were defined as ever having used metformin or as currently using metformin (data not shown). No effect of metformin to lower CAC severity was seen in women. Similarly, among men, but not in women, the presence of CAC>0 was significantly lower in the metformin group than in the other two groups (10.7% lower versus placebo and 11.7% lower versus lifestyle), but this was not the case for the CAC>10 or CAC>100 categories (Fig 2). There were no differences in CAC prevalence by categories between the three treatment groups. Differences in CAC severity, but not CAC presence, were observed between race/ethnic groups, and by age categories, diabetes status and statin use subgroups in both men and women (Table 2).
Table 2.
CAC severity and prevalence according to age, race/ethnicity, diabetes/non-diabetes status and interventions stratified by sex
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | Placebo | Metformin | Lifestyle | N | Placebo | Metformin | Lifestyle | |
|
| ||||||||
| N | 215 | 215 | 213 | 486 | 464 | 468 | ||
| CAC Severity (AU) Geometric Mean (95% CI)* | ||||||||
|
| ||||||||
| Overall - unadjusted | 627 | 63.7 (41.3, 98.3) | 40.2 (26.1, 61.9)‡ | 70.1 (45.4, 108.2) | 1402 | 5.3 (3.6, 7.8) | 6.1 (4.2, 9.0) | 6.0 (4.1, 8.8) |
|
| ||||||||
| Overall – age adjusted | 627 | 66.9 (45.3, 98.8) | 39.5 (26.7, 58.4)† | 58.3 (39.4, 86.4) | 1402 | 4.7 (3.3, 6.8) | 4.8 (3.4, 7.0) | 5.2 (6.3, 7.5) |
|
| ||||||||
| Raceab | ||||||||
| Caucasian | 354 | 112.0 (69.6, 180.3) | 81.2 (52.3, 126.1) | 82.9 (52.2, 131.5) | 713 | 5.8 (3.5, 9.5) | 6.7 (4.0, 11.0) | 6.5 (3.9, 10.9) |
| African Am | 102 | 38.2 (13.6, 108.5) | 17.8 (5.8, 54.4) | 33.8 (10.7, 107.5) | 320 | 3.8 (1.8, 8.1) | 3.3 (1.5, 6.9) | 4.8 (2.3, 10.0) |
| Hispanic | 101 | 31.0 (10.7, 90.0) | 10.5 (3.3, 33.6) | 13.6 (4.1, 45.7) | 226 | 5.2 (2.2, 11.8) | 4.3 (1.9, 10.0) | 5.7 (2.5, 12.8) |
| Am Indian | 12 | NE | NE | NE | 101 | 1.4 (0.1, 10.2) | 2.1 (0.2, 15.6) | 1.1 (0.1, 8.9) |
| Asian | 58 | NE | NE | NE | 42 | NE | NE | NE |
|
| ||||||||
| Age (y) at randomizationab | ||||||||
| 25–44 | 124 | 17.6 (5.6, 56.5) | 3.0 (0.8, 10.2)† | 8.2 (2.6, 26.1) | 468 | 1.3 (0.5, 2.9) | 1.4 (0.6, 3.2) | 2.5 (1.2, 5.4)† |
| 45–59 | 344 | 68.0 (41.0, 112.6) | 51.6 (31.2, 85.3) | 67.3 (39.0, 115.9) | 748 | 7.0 (4.3, 11.3) | 7.0 (4.3, 11.4) | 5.7 (3.4, 9.5) |
| 60+ | 159 | 200 (101.9, 391.6) | 178.9 (92.6, 344.8) | 273.8 (152.0, 493.2) | 186 | 40.0 (18.5, 86.2) | 36.5 (17.8,74.8) | 34.6 (17.4, 68.7) |
|
| ||||||||
| Diabetes Statusabd | ||||||||
| Diabetes | 344 | 65.9 (40.1, 108.2) | 46.2 (27.1, 78.9) | 72.5 (41.9, 125.2) | 761 | 5.8 (3.6, 9.2) | 4.3 (2.6, 7.1) | 7.0 (4.3, 11.5) |
| No Diabetes | 283 | 65.6 (35.2, 122.3) | 34.5 (19.4, 61.1) | 46.0 (26.3, 80.6) | 641 | 3.4 (2.0, 6.0) | 5.4 (3.2, 9.2) | 3.8 (2.2, 6.4) |
|
| ||||||||
| Statin Useab | ||||||||
| Statin | 119.8 (76.0, 188.8) | 73.8 (45.9, 118.3) | 98.5 (60.5, 160.4) | 8.3 (5.2, 13.2) | 8.7 (5.4, 13.8) | 8.7 (5.4, 13.8) | ||
| No Statin | 25.8 (13.2, 50.5) | 17.2 (9.1, 32.7) | 31.5 (17.0, 58.6) | 2.5 (1.4, 4.5) | 2.5 (1.4, 4.4) | 3.1 (1.7, 5.3) | ||
|
| ||||||||
| CAC Presence (%) | ||||||||
|
| ||||||||
| Overall | 84% | 75%†‡ | 85% | 50% | 53% | 52% | ||
|
| ||||||||
| Race | ||||||||
| Caucasian | 89% | 83% | 89% | 53% | 56% | 53% | ||
| African Am | 76% | 62% | 83% | 50% | 49% | 51% | ||
| Hispanic | 79% | 58% | 63% | 51% | 53% | 60% | ||
| Am Indian | NE | NE | NE | 27% | 39% | 28% | ||
| Asian | NE | NE | NE | NE | NE | NE | ||
|
| ||||||||
| Age (y) | ||||||||
| 25–44 | 71% | 40%† | 60% | 30% | 33% | 40% | ||
| 45–59 | 85% | 80% | 87% | 55% | 57% | 52% | ||
| 60+ | 91% | 88% | 97% | 81% | 81% | 81% | ||
|
| ||||||||
| Diabetes Status | ||||||||
| Diabetes | 84% | 77% | 86% | 52% | 52% | 54% | ||
| No Diabetes | 84% | 72%‡ | 84% | 46% | 54% | 49% | ||
|
| ||||||||
| Statin Use | ||||||||
| Statin | 88% | 80%‡ | 89% | 58% | 60% | 59% | ||
| No Statin | 76% | 68% | 79% | 40% | 43% | 45% | ||
CAC severity is represented as geometric mean CAC score (95% CI) for each treatment group overall and by subgroups and adjusted for age in the race, diabetes and statin subgroups.
Significant differences between groups are emboldened and noted as
p<0.05 vs placebo;
p<0.05 vs lifestyle; NE = cells with <20 not estimated
p<0.05 for difference in CAC severity among men subgroups.
p<0.05 for difference in CAC severity among women subgroups.
p<0.10 for interaction of subgroup X treatment group among men in CAC severity.
p<0.10 for interaction of subgroup X treatment group among women in CAC severity.
Fig 2. Distribution of CAC Scores by Treatment Group and Sex.
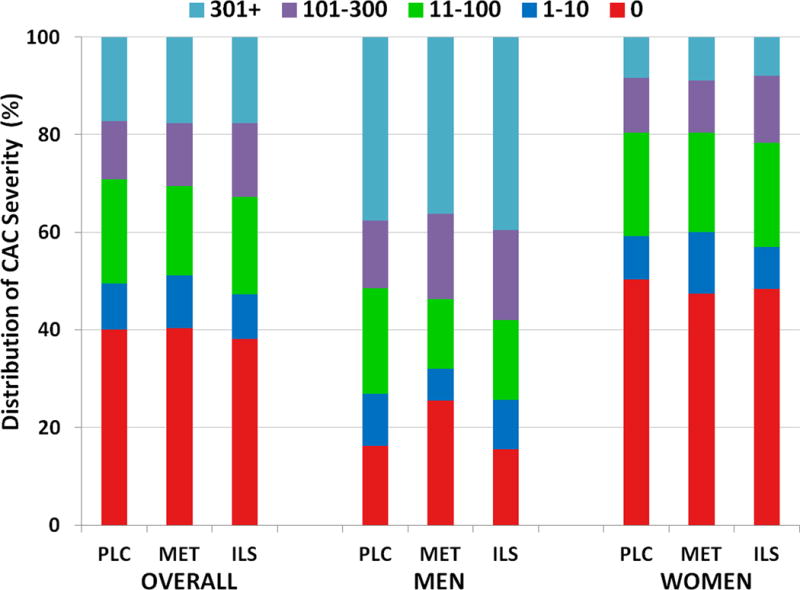
Each bar depicts the percent prevalence of CAC severity by color-coded severity category. There were no differences between CAC severity categories among treatment groups overall (p=0.69) or among men (p=0.08) or women (p=0.50)
By age groups, both CAC severity and CAC>0 were significantly lower in the 25–44 year age group among men in the metformin versus placebo groups, with a tendency in this direction in the older age groups. CAC severity was significantly higher in the lifestyle than the placebo group among women in the 25–44 year age group. CAC severity and presence among men in the metformin group tended to be lower in both those with and without diabetes and in all race/ethnic subgroups although this did not reach significance (except for CAC>0 in those without diabetes). Among women, there was an interaction between diabetes status and treatment group for CAC severity (p=0.04). CAC severity was higher among statin users for both men and women.
Baseline age, SBP, eGFR (inversely) and non-HDL-C correlated with CAC severity in men and women and in all three treatment groups, as did mean SBP and eGFR during follow-up, but there was no relationship with HbA1c levels and only weak associations with BMI, HDL-C, tPA and CRP (Fig 3A and B).
Fig 3. Spearman correlations of CAC severity and covariates among all participants and by treatment group and sex.
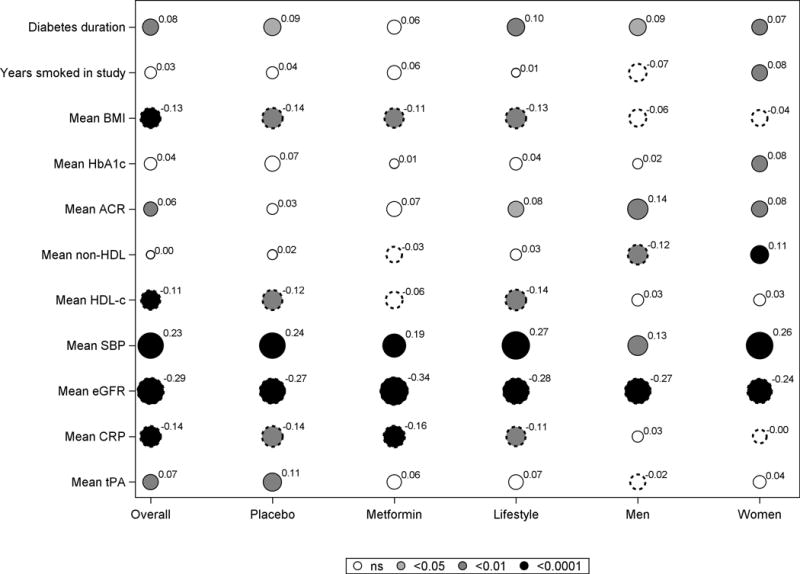
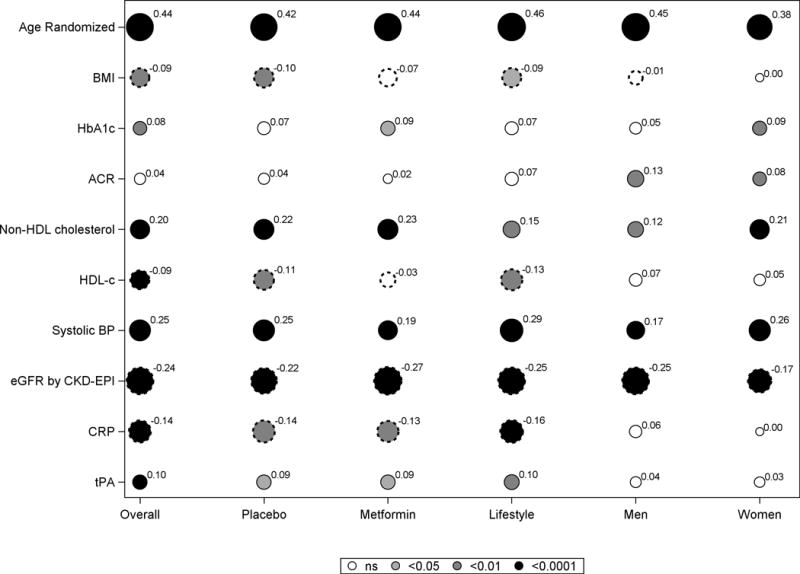
A. Baseline covariates
B. Mean covariates values during follow-up
Each circle represents the correlation coefficient of the bivariate analysis between CAC severity and the covariate, with the diameter of the circle proportionate to the correlation shown as a superscript to the circle. The color shading in the circle depicts the p value (black for p <0.0001; Dark grey for p <0.01; light grey for p <0.05 and white for p>0.05). Smooth circles indicates a positive correlation while scalloped circles depict a negative correlation.
Models adjusting for demographic and CVD risk factors, diabetes status and duration and the effects of interventions on CAC severity stratified by sex
To determine whether the lower CAC severity in men with metformin could be accounted for by demographic or cardiovascular risk factors, or by diabetes development, their effects were examined in a series of multivariate models stratified by sex, comparing CAC severity in the metformin group versus placebo or lifestyle groups (Fig 4). After adjustment for age, the lower CAC severity in men in the metformin versus placebo groups (Figure 4 upper left panel) was not attenuated by controlling for differences in race/ethnicity, baseline and follow-up risk factors, or diabetes status. Compared to men in the lifestyle group (Figure 4 lower left panel), CAC severity was also lower in the metformin group in the unadjusted model, but with little change in the point estimate in all adjusted models.
Fig 4. Adjusted treatment effects of metformin vs. placebo (MET vs PLAC) and metformin vs. lifestyle (MET vs. ILS) on CAC severity stratified by sex.
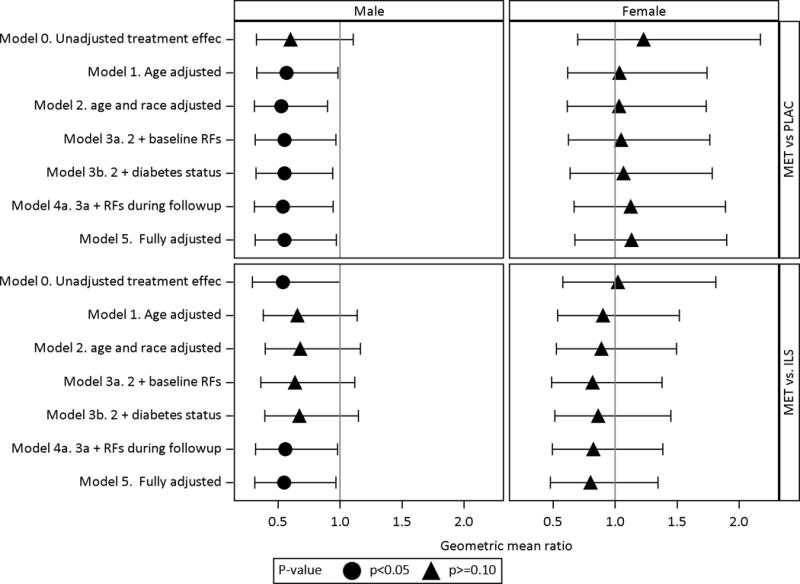
The treatment effects are stratified by sex along the columns, displayed along the rows and expressed as geometric mean ratio of metformin compared to placebo (MET vs. PLAC) and metformin compared to lifestyle (MET vs. ILS). The geometric mean ratio is calculated by taking the anti-log of the treatment effect coefficient from obtained the Tobit regression model with ln(CAC+1) as the outcome. Adjustments to the treatment effects are sequentially modeled as defined below. Baseline risk factors include family history of premature CVD, BMI, HbA1c, ln(ACR), non-HDL-C, HDLc, SBP, eGFR by CKD-epi, ln(CRP), ever smoke status. Risk factors during follow-up include mean levels for BMI, HbA1c, ln (ACR), non-HDL-C, BMI, ln(CRP), SBP, ACR, eGFR and years of statin.
MODEL 0 Unadjusted treatment effect
MODEL 1 Adjusted for age only
MODEL 2 Adjusted for demographics: Model 1 + demographics (age, race/ethnicity)
MODEL 3a Adjusted for baseline RFs: Model 2 + baseline risk factors (Family history of premature CVD, BMI, HbA1c, ACR, non-HDL-C, HDL-C, SBP, eGFR by CKD-epi, CRP, ever smoke status)
MODEL 3b Adjusted for demographics and diabetes status: Model 2 + diabetes status at the time of the scan
MODEL 4a Adjusted for demographics, risk factors at baseline and during follow-up : Model 3a + risk factors during follow-up
MODEL 4b Adjusted for demographics, risk factors at baseline and diabetes status: Model 3a + diabetes status
MODEL 5 Fully adjusted: Model 4A + diabetes status and duration
Discussion
We evaluated the effect of lifestyle intervention and metformin on CAC severity and presence in the long-term DPP/DPPOS. The major findings in this analysis are that in men, but not in women, CAC severity and presence were lower in the metformin group compared to the placebo group, with a similar tendency in the metformin versus the lifestyle group. A tendency toward lower CAC in the men of the metformin group was also evident in race/ethnicity and age subgroups and was most prominent in younger men; these factors did not attenuate the effect of metformin in multivariate analysis. There were no CAC differences between the lifestyle and placebo groups.
This study evaluated CAC cross-sectionally an average of 13–14 years after baseline randomization, and suggests that compared to the placebo group, metformin treatment may have reduced early stages of plaque development in men. Notably there was also a higher prevalence of CAC in this cohort than has been reported in other population studies such as the Multi-Ethnic Study of Subclinical Atherosclerosis. Possible explanations include the lower proportion of DPP subjects from minority race/ethnic groups (who have a lower prevalence of CAC compared to Whites), and the higher prevalence of DPP participants with metabolic syndrome (which has been shown to be associated with higher CAC) than those without this syndrome (16, 17).
Metformin has been demonstrated to reduce CHD events compared to diet (18) and sulfonylurea treatments (19) and versus placebo in insulin treated subjects with type 2 diabetes (20).This is the first demonstration that metformin may have a beneficial effect on coronary atherosclerosis in a prediabetic population. Recently, a one-year treatment program using metformin in subjects with human immunodeficiency virus infection and metabolic syndrome demonstrated reduce CAC progression with metformin compared to placebo (21). By contrast, metformin treatment had no effect on carotid intimal media thickness (CIMT) in the latter study or in a larger study of non-diabetic patients with CHD (22), raising the possibility that coronary calcification may be a more sensitive marker of a metformin effect on atherosclerosis than is CIMT.
The mechanism(s) for protective effects of metformin on atherosclerotic vascular disease are not well understood. In addition to its antihyperglycemic action, metformin has been shown to improve endothelial function (23), and favorably alter the lipid profile (24) as well as lower markers of inflammation and procoagulation (6). Although we observed associations between CAC and baseline non-HDL-C, SBP, eGFR, CRP, tPA and HDL-C, adjustment for cardiovascular risk factors did not account for the effect of metformin on CAC in men. Our current findings could reflect other effects of metformin on the pathogenesis of atherosclerosis, perhaps directly on the vessel wall. Recently, evidence for direct anti-atherogenic actions of metformin involving AMPK-mediated inhibition of monocyte-to-macrophage differentiation (25), inhibition of vascular senescence, and down-regulation of angiotensin II type 1 receptors have been demonstrated in experimental models (26). In addition metformin has been shown to inhibit vascular calcification in rat smooth muscle cells (27).
The sex difference in the effect of metformin on CAC that we observed has not been reported in studies of metformin’s preclinical or clinical effects on CVD. CAC severity was considerably lower in women than in men in our study, making it more difficult to identify an effect of metformin in women. On the other hand, over half of the women had measurable CAC yet there was no suggestion of an effect of metformin on CAC presence in women. At entry to the study 36% of women were premenopausal and since atherogenesis proceeds more slowly in premenopausal women, especially in those who remained without diabetes, this might have contributed to the lack of effect of metformin in women. However we were not able to detect an effect of metformin based on a stratification of the female population into those aged ≥45 compared to <45 years at randomization (data not shown).Intriguingly, a similar sex dimorphism has previously been seen in DPP in terms of the prevention of metabolic syndrome (28) wherein metformin had a profound effect in men (p=0.002) yet no effect in women (p> 0.20). This raises the possibility of hormonal interactions; for example it has been shown that metformin reduces testosterone levels in men (29) but not women (30).
It was notable that there was no reduction in the prevalence of clinically significant CAC e.g. CAC>100 among men in the metformin as compared to the placebo group, i.e. the effect of metformin on CAC was most evident in those with lower CAC scores. Although lower CAC scores are more susceptible to obesity-associated scanning artifacts, CAC scores at the lower end of the range are associated with a significant increase in the CHD event rate despite this (31). These findings could imply that the effect of metformin involves smaller and more recently calcifying plaques, rather than well-established lesions. This is supported by the observation that the most prominent difference in CAC severity or presence was found in younger men, who would be expected to have atherosclerotic lesions earlier in their development than among older men. Whether this means that metformin would have less clinical efficacy in older men must await testing of the effect of metformin on clinical outcomes.
It was also of interest to find that lower CAC in the metformin group was evident regardless of whether diabetes had developed or not. Although metformin has a durable effect to delay diabetes development, and its use in prediabetes has been endorsed by the ADA (32), it is unknown whether use of metformin prior to development of diabetes has benefit for vascular complications. The current findings provide initial evidence that metformin may have a favorable effect on atherosclerosis both in the prediabetes phase as well as in the early period after development of diabetes in men, suggesting that the effect of metformin on CAC in men does not depend on diabetes prevention. In this regard it should be noted that the eligibility for DPP required both impaired glucose tolerance and a fasting glucose >95 mg/dl which would have excluded prediabetic subjects with milder degrees of dysglycemia. Therefore these findings cannot be generalized to all subjects with prediabetes. Overall 54% of the DPP/DPPOS cohort had developed diabetes at the time of scanning, but despite the presence of only modest hyperglycemia and a relatively short diabetes duration we found that CAC severity was increased in those who developed diabetes compared to those who did not. Since incident diabetes was identified in the DPP/DPPOS study by semi-annual testing which allows for a narrow definition of the timing of its biochemical onset, this suggests that progression of dysglycemia to diabetes influences atherosclerosis. Taken together these findings support the notion that use of metformin within a few years before or after diabetes development has a beneficial effect on early stages of atherosclerosis in men.
The absence of an effect of lifestyle intervention on CAC in the face of the metformin finding is somewhat surprising since the lifestyle group experienced more significant reductions in the development of dyslipidemia, hypertension and metabolic syndrome as well as greater lowering of CRP levels than did the metformin group (6, 28 33). Further, lifestyle change had a greater long-term effect on diabetes prevention than metformin treatment during the first 10 years of follow-up (33). Our findings extend those reported in a shorter 1 year Mediterranean diet intervention trial with a 3 year follow-up in a small study of subjects with CHD, which found no effect on CAC progression (34). Our findings are not concordant with the results of the Da Qing diabetes prevention trial, which found a significant reduction in mortality largely due to reduced CVD in the lifestyle intervention compared to the control group after 23 years of follow-up. However this observation was seen in women but not men, and there were asymmetric losses-to-follow-up and differences in age and smoking behavior between the two intervention groups (35).It remains possible that there may be long-term effects on CVD outcomes with lifestyle intervention that are not reflected by its effect on CAC measured 10–13 years post randomization. One important difference between metformin and lifestyle in the DPP/DPPOS is intensity of exposure over the period of observation Metformin treatment was consistently used throughout the entire follow-up period, whereas the intensive period of lifestyle intervention averaged only 3.2 years, following which net exposure was reduced, leading to reduced efficacy as measured by a diminution of weight loss.
Medication use might also have confounded these observations. During the post-DPP time period, lipid lowering and antihypertensive therapy were increasingly prescribed in all treatment groups, and at the time of the CAC scan lipid lowering medications were used by 58% and antihypertensive medications by 67% of the entire cohort. These factors may have limited or obscured a beneficial effect of the lifestyle intervention on CAC. Indeed, as others have shown, we found that CAC severity (total plaque burden) was greater in statin users than non-users in all treatment groups, and it has been suggested that statin use may be associated with plaque stabilization (36). Nevertheless, CAC severity was lower in men from the metformin group despite the possible confounding effects of lipid-lowering and antihypertensive therapies, and was in fact less prevalent in men taking statin medications in the metformin group. These observations suggest that metformin may slow or delay atherosclerosis independently of the effect of modern cardio-prevention strategies in these subjects.
There are several limitations to this study. First, although metformin treatment was almost exclusive to the metformin intervention group, small numbers of participants from the other two intervention groups were taking out-of-study metformin. Second, the nature of the lifestyle intervention in the three treatment groups changed in the transition from DPP to DPPOS. Third, the study cohort was selected based on glucose and body weight entry criteria and those weighing more than 350 lbs were excluded, so that the results cannot be generalized to the entire prediabetic population. Finally because CAC was measured only once at year 14, it was not possible to directly measure the effect of our interventions on CAC over time. Interpretation of the effect of the interventions were therefore based on the assumption that there were no differences in baseline CAC given the randomization of subjects into intervention groups at baseline. We found no differences in cardiometabolic risk factors at baseline between treatment groups except for slightly lower HDL-C and higher smoking rates in women in the placebo group only.
In summary, these findings add support to the evidence base that metformin may protect against atherosclerotic vascular disease early in diabetes development and potentially extends the range of this action to include high-risk male prediabetic subjects. Whether these findings translate into beneficial effects on CVD events will require ongoing follow-up.
Supplementary Material
Clinical Perspective.
What is new?
Despite intensive risk factor management, cardiovascular disease (CVD) remains a major cause of morbidity and mortality in diabetes raising the question of whether diabetes prevention interventions may be important in reducing CVD risk in diabetes
We found that men, but not women with prediabetes treated with metformin for an average duration of 14 years in the Diabetes Prevention Program Outcome Study had lower coronary calcium scores than their placebo group counterparts.
No difference in coronary calcium scores was observed in the group receiving a lifestyle intervention as compared to the placebo group.
What are the clinical implications?
Several studies in subjects with diabetes have suggested that metformin treatment is associated with a reduction in CVD events.
These findings provide the first evidence that metformin may protect against coronary atherosclerosis in men with prediabetes, although demonstration that metformin reduces CVD events in these subjects is needed before firm therapeutic implications of these findings can be made
The reason for an absence of an effect in women is unclear; women have less coronary atherosclerosis and coronary calcium than men and therefore a metformin effect may be more difficult to identify in women
Acknowledgments
The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS.
Funding: During the DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the Office of Research on Women’s Health, the National Center for Minority Health and Human Disease, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, Lipha (Merck-Sante) provided medication and LifeScan Inc. donated materials during the DPP and DPPOS. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. A complete list of Centers, investigators, and staff can be found in the Appendix.
Footnotes
Clinical Trial Registration: Diabetes Prevention Program Outcomes Study (DPPOS) NCT00038727; https://clinicaltrials.gov/ct2/show/NCT00038727
Disclosures: Kieren Mather received a research grant from Novo IIT and has also medication or supply donations with aggregate value >$10K for other research studies from Novo, Sanofi, Merck, and Abbott. The other authors declare no conflict of interest.
References
- 1.Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 3.ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC, Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Diabetes Prevention Program Research Group. Goldberg RB, Temprosa M, Haffner S, Orchard TJ, Ratner RE, Fowler SE, Mather K, Marcovina S, Saudek C, Matulik MJ, Price D. The Effect of Progression from Impaired Glucose Tolerance to Diabetes on Cardiovascular Risk Factors and its Amelioration by Lifestyle and Metformin Intervention: The Diabetes Prevention Program Randomized Trial. Diabetes Care. 2009;32:726–732. doi: 10.2337/dc08-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg RB, Temprosa MG, Mather KJ, Orchard TJ, Kitabchi AE, Watson KE, for the Diabetes Prevention Program Research Group* Lifestyle and Metformin Interventions Have a Durable Effect to Lower CRP and tPA Levels in the Diabetes Prevention Program Except in Those Who Develop Diabetes. Diabetes Care. 2014;37:2253–2260. doi: 10.2337/dc13-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 8.Khaleeli E, Peters SR, Bobrowsky K, Oudiz RJ, Ko JY, Budoff MJ. Diabetes and the associated incidence of subclinical atherosclerosis and coronary artery disease: implications for management. Am Heart J. 2001;141:637–644. doi: 10.1067/mhj.2001.113224. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of Medical Care in Diabetes—2015: 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2015;38(Supplement 1):S8–S16. doi: 10.2337/dc15-S005. [DOI] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, Sidney S, Bild DE, Williams OD, Detrano RC. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of multi-ethnic study of atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 13.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 14.Ma S, Liu A, Carr J, Post W, Kronmal R. Statistical modeling of Agatston score in multi-ethnic study of atherosclerosis (MESA) PLoS One. 2010;5:e12036. doi: 10.1371/journal.pone.0012036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcus R, Peritz E, Gabriel KR. On closed testing procedures with special reference to ordered analysis of variance. Biometrika. 1976;63:655–660. [Google Scholar]
- 16.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 17.Bertoni AG, Wong ND, Shea S, Ma S, Liu K, Preethi S, Jacobs DR, Jr, Wu C, Saad MF, Szklo M. Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2007;11:2951–2956. doi: 10.2337/dc07-1042. [DOI] [PubMed] [Google Scholar]
- 18.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 19.Hong J, Zhang Y, Lai S, Lv A, Su Q, Dong Y, Zhou Z, Tang W, Zhao J, Cui L, Zou D, Wang D, Li H, Liu C, Wu G, Shen J, Zhu D, Wang W, Shen W, Ning G, SPREAD-DIMCAD Investigators Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36:1304–1311. doi: 10.2337/dc12-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kooy A, de Jager J, Lehert P, Bets D, Wulffelé MG, Donker AJ, Stehouwer CD. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–625. doi: 10.1001/archinternmed.2009.20. [DOI] [PubMed] [Google Scholar]
- 21.Fitch K, Abbara S, Lee H, Stavrou E, Sacks R, Michel T, Hemphill L, Torriani M, Grinspoon S. Effects of lifestyle modification and metformin on atherosclerotic indices among HIV-infected patients with the metabolic syndrome. AIDS. 2012;26:587–597. doi: 10.1097/QAD.0b013e32834f33cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preiss D, Lloyd SM, Ford I, McMurray JJ, Holman RR, Welsh P, Fisher M, Packard CJ, Sattar N. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:116–124. doi: 10.1016/S2213-8587(13)70152-9. [DOI] [PubMed] [Google Scholar]
- 23.Mather KJ, Verma S, Anderson TJ. Improved endothelial function with metformin in type 2 diabetes mellitus. J Am Coll Cardiol. 2001;37:1344–1350. doi: 10.1016/s0735-1097(01)01129-9. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg R, Temprosa M, Otvos J, Brunzell J, Marcovina S, Mather K, Arakaki R, Watson K, Horton E, Barrett-Connor E. Lifestyle and Metformin Treatment Favorably Influence Lipoprotein Subfraction Distribution in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2013;98:3989–3998. doi: 10.1210/jc.2013-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasamsetti SB, Karnewar S, Kanugula AK, Raj AT, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK mediated inhibition of STAT3 activation: Potential role in atherosclerosis. Diabetes. 2015;64:2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
- 26.Forouzandeh F, Salazar G, Patrushev N, Xiong S, Hilenski L, Fei B, Alexander RW. Metformin beyond diabetes: pleiotropic benefits of metformin in attenuation of atherosclerosis. J Am Heart Assoc. 2014;3:e001202. doi: 10.1161/JAHA.114.001202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao X, Li H, Tao H, Wu N, Yu L, Zhang D, Lu X, Zhu J, Lu Z, Zhu Q. Metformin inhibits vascular calcification in female rat aortic smooth muscle cells via the AMPK-eNOS-NO pathway. Endocrinology. 2013;154:3680–3689. doi: 10.1210/en.2013-1002. [DOI] [PubMed] [Google Scholar]
- 28.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler SE, Diabetes Prevention Program Research Group The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–619. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozata M, Oktenli C, Bingol N, Ozdemir IC. The effects of metformin and diet on plasma testosterone and leptin levels in obese men. Obes Res. 2001;9:662–667. doi: 10.1038/oby.2001.90. [DOI] [PubMed] [Google Scholar]
- 30.Vrbikova J, Hill M, Starka L, Cibula D, Bendlova B, Vondra K, Sulcová J, Snajderová M. The effects of long-term metformin treatment on adrenal and ovarian steroidogenesis in women with polycystic ovary syndrome. Eur J Endocrinol. 2001;144:619–628. doi: 10.1530/eje.0.1440619. [DOI] [PubMed] [Google Scholar]
- 31.Budoff MJ, McClelland RL, Nasir K, Greenland P, Kronmal RA, Kondos GT, Shea S, Lima JA, Blumenthal RS. Cardiovascular events with absent or minimal coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2009;158:554–561. doi: 10.1016/j.ahj.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Diabetes Association. Standards of Medical Care in Diabetes-Prevention or Delay of Type 2 Diabetes. Diabetes Care. 2015;38(Supplement 1):S31–S32. [Google Scholar]
- 33.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Haffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;14:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehmann N, Paul A, Moebus S, Budde T, Dobos GJ, Michalsen A. Effects of lifestyle modification on coronary artery calcium progression and prognostic factors in coronary patients - 3-year results of the randomized SAFE-LIFE trial. Atherosclerosis. 2011;219:630–636. doi: 10.1016/j.atherosclerosis.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 35.Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, Yang W, Zhang B, Shuai Y, Hong J, Engelgau MM, Li H, Roglic G, Hu Y, Bennett PH. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2:474–480. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 36.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


