Abstract
Aims
To determine the association between dietary intake and risk of non-severe hypoglycemia in adolescents with type 1 diabetes.
Methods
Type 1 adolescents were enrolled from Flexible Lifestyle Empowering Change (FL3X) randomized trial and wore a blinded continuous glucose monitoring (CGM) system at baseline for one week in free-living conditions. Dietary intake was calculated as the average from two 24-hour dietary recalls. Non-severe hypoglycemia was defined as having blood glucose <70 mg/dL for ≥10 minutes but not requiring external assistance, categorized as daytime and nocturnal (11PM-7AM). Data were analyzed using logistic regression models.
Results
Among 98 participants with 14,277 hours of CGM data, 70 had daytime hypoglycemia, 66 had nocturnal hypoglycemia, 55 had both, and 17 had neither. Soluble fiber and protein intake were positively associated with both daytime and nocturnal hypoglycemia. Glycemic index, monounsaturated fat, and polyunsaturated fat were negatively associated with daytime hypoglycemia only. Adjusting for total daily insulin dose per kilogram eliminated all associations.
Conclusions
Dietary intake was differentially associated with daytime and nocturnal hypoglycemia. Over 80% of type 1 adolescents had hypoglycemia in a week, which may be attributed to the mismatch between the optimal insulin dose needed for each meal and actually delivered insulin dose without considering quality of carbohydrate and nutrients beyond carbohydrate.
Keywords: Type 1 diabetes, dietary intake, nutrition, hypoglycemia, continuous glucose monitoring
1. Introduction
Hypoglycemia occurs frequently in people with type 1 diabetes with an incidence of over 1–2 episodes per week per patient.1 Youth with type 1 diabetes are particularly vulnerable to hypoglycemia due to unpredictable food consumption, erratic physical activity, and problems with accurate insulin dosing and detecting hypoglycemia.2, 3 Further, their brains are still developing, which put them at risk of cognitive dysfunction and neurological sequelae of hyperglycemia4 and hypoglycemia.5, 6
Hypoglycemia is preventable and nutrition therapy plays a pivotal role in this.7 Current nutrition guidelines are very specific about how to treat hypoglycemia when it occurs.2 However, information regarding whether or how usual dietary intake influences risk of hypoglycemia is limited, particularly for children with diabetes. Medical nutrition therapy designed for adults may not be applicable to children, and it may even conflict with the evidence rising from pediatric populations.7, 8 Further, the literature has primarily focused on postprandial glycemic excursions following experimental meals in clinical trial settings, which is directly related to acute dietary effect on blood glucose after consuming test meals.8–10 Yet, no study has examined the effect of usual dietary intake on risk of hypoglycemia measured by continuous glucose monitor (CGM) in free-living youth with type 1 diabetes, which associates typical dietary patterns with day-to-day glycemic control.
Non-severe hypoglycemia accounts for 88–98% of all hypoglycemic events in patients with diabetes,11 which is defined as a low blood glucose event <70 mg/dL but does not require external assistance.5 Complete quantification of non-severe hypoglycemia in an outpatient setting typically requires CGM. CGM-defined hypoglycemia is recommended by a consensus statement from the American Diabetes Association as an outcome measure, in addition to HbA1c, for evaluating glucose control in people with type 1 diabetes.12 In this study, we aimed to determine the association between usual dietary intake and risk of developing non-severe hypoglycemia in a sample of adolescents with type 1 diabetes who wore CGM in a one-week period at baseline from the Flexible Lifestyle Empowering Change (FL3X) randomized clinical trial (ClinicalTrials.gov identifier: NCT01286350).
2. Participants and methods
2.1. Participants
The FL3X is an 18-month randomized trial with the primary goal of improving glycemic control and quality of life in adolescents with type 1 diabetes through an evidence-based flexible lifestyle intervention. The intervention focuses on increasing adherence to type 1 diabetes self-management including medical therapy, diet, and physical activity. Eligible participants were aged 13–16 years at study entry and had HbA1c 8–13% and duration of diabetes >1 year. Participants were enrolled from two sites: Barbara Davis Center for Childhood Diabetes in Colorado and Cincinnati Children’s Hospital Medical Center in Ohio, coordinated by the University of North Carolina (UNC) at Chapel Hill. Written informed consent was obtained from parents or legal guardians. The current study used baseline data from a subset of 258 adolescents with type 1 diabetes from the FL3X trial who also participated in the ancillary study: Measures of Hypoglycemia and Glycemic Variability Using Continuous Glucose Monitoring. The ancillary study was funded separately from the FL3X trial. Of all 258 participants, 95 had completed baseline data collection when the funding was received, and thus were excluded for the ancillary study. Additional 33 participants were excluded due to participation refusal (n=4), not-completed data collection (n=13), no dietary recall conducted (n=12), and other reasons (n=4). Accordingly, 130 participants who had at least one completed dietary recall comprised our final study sample. The study protocol was approved by the Institutional Review Boards at each participating site. The study was conducted in accordance with the Declaration of Helsinki. For this investigation, data were collected during one week period of time at baseline.
2.2. Measuring blood glucose using CGM
At the baseline visit, the iPro2 CGM system (Medtronic Inc.) with the Enlite sensor was inserted into the abdominal subcutaneous adipose tissue. Participants were carefully instructed on the use and maintenance of the CGM system and were advised to calibrate the sensor before eating and before bed with iPro2 compatible glucometer (OneTouch Ultra2). The Enlite sensor measured interstitial glucose level every five minutes within a range 40–400 mg/dL. On the last day of the CGM wearing week, participants were reminded to send the device back, using the pre-paid box/envelope given at the end of the study visit in the first day. The CGM data were downloaded with CareLink iPro System and uploaded to the coordinating center for data processing. CGM readings were blinded to participants. No alarms for hypoglycemia or hyperglycemia or any communication from the device were available to participants.
2.3. 24-hour dietary and physical activity recalls
Telephone-administered 24-hour dietary recalls were administered to participants (ideally one weekday and one weekend day) to ascertain dietary intake. Interviews were conducted by trained and certified interviewers from the UNC NIH/NIDDK Nutrition Obesity Research Center (NORC) staff (P30DK056350; MPI Mayer-Davis), using the Nutrient Data System for Research software (NDSR Version 2014, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) and the multiple pass interviewing method.13, 14
The validated Previous Day Physical Activity Recall (PDPAR)15, 16 divided the day into half-hour time blocks and queried the dominant activity and the approximate intensity of that activity for each period. The intensity level was categorized as light (slow breathing, little or no movement), moderate (normal breathing and some movement), hard (increased breathing and moderate movement), and vary hard (hard breathing and quick movement). The PDPAR was under the direction of the UNC NORC and administered concurrently with the 24-hour dietary recalls.
2.4. Other data
Standardized questionnaires were used to collect self-reported data including race, highest level of parental education, duration of diabetes, insulin delivery method, and insulin dose. Weight, height, and HbA1c level were measured or assayed according to standardized protocols. Body mass index (BMI) was computed and converted to a BMI z score using the Center for Disease Control/National Center for Health Statistics 2000 reference curves.17
2.5. Statistical analysis
No severe hypoglycemic events were reported during the study week. Non-severe hypoglycemic events were defined as having CGM reading <70 mg/dL for 10 minutes or more.18, 19 They were further categorized into daytime and nocturnal non-severe hypoglycemia. This distinction is important because current insulin analogues and subcutaneous delivery methods do not adequately mimic normal physiologic patterns of insulin secretion20 and sleep attenuates counter-regulatory responses to hypoglycemia.21 Further, dietary intake and exercise22 as two major determinants of blood glucose occur mainly in the daytime. Accordingly, dietary intake is likely to influence hypoglycemia risk differently during the day and night. Hypoglycemia that occurred between 11:00 PM and 7:00 AM was defined as nocturnal hypoglycemia.18, 23
Usual daily dietary intake in the study week was averaged from two 24-hour dietary recalls. Macronutrients of interest were total carbohydrate, total protein, animal protein, plant protein, total fat, saturated fat (SFA), monounsaturated fat (MUFA), polyunsaturated fat (PUFA), ratio of MUFA to SFA (MUFA/SFA), and ratio of PUFA to SFA (PUFA/SFA). Total fiber, soluble fiber (e.g., fiber in oat bran, barley, seeds, nuts, and lentils), insoluble fiber, glycemic index (GI), and glycemic load (GL) were also studied.
Patients with no dietary recall, one recall only, and two recalls were compared. Further, for those with two dietary recalls, patient characteristics and average daily dietary intake were compared among four groups of participants: no hypoglycemia, daytime hypoglycemia only, nocturnal hypoglycemia only, and both daytime and nocturnal hypoglycemia. Differences were evaluated by the Wilcoxon-Mann-Whitney test for two-group comparison and Kruskal-Wallis test for comparison of three or four groups.
Among those with two dietary recalls, logistic regression models were used to identify dietary predictors of daytime hypoglycemia (those with ≥1 episode of daytime hypoglycemia versus those without, regardless of nocturnal hypoglycemia), and nocturnal hypoglycemia (those with ≥1 episode of nocturnal hypoglycemia versus those without, regardless of daytime hypoglycemia). Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. All adjusted models included total calories, CGM wear time, and average number of meals per day. Other covariates were also adjusted if associated P value was ≤0.2, including age, gender, race (white, non-white), highest parental education (four-year college or more, some college or less), duration of diabetes, BMI z score, hours with vigorous or moderate physical activity per day, hours with electronic media time or TV time per day, and HbA1c level. Finally, insulin delivery method (pump versus multiple daily injection) and insulin dose per kilogram were added to the fully adjusted model.
3. Results
3.1. Patient characteristics
Among 258 adolescent participants from the FL3X trial at baseline, 128 had no dietary recall; 32 had only one recall while 98 had two recalls (Supplementary Table 1). The demographics, clinical characteristics, and dietary intake of these three groups were not different.
Among 98 participants who had two 24-hour dietary recalls, 17 of them had no non-severe hypoglycemia during the study week and 55 developed both daytime and nocturnal non-severe hypoglycemia (Table 1). Participants with non-severe hypoglycemia were not different from those without in terms of age, gender, race, diabetes duration, BMI z score, insulin delivery method, insulin dose per kilogram, parental education, and physical activity. However, lower HbA1c level was seen in participants with non-severe hypoglycemia. Regarding dietary intake, descriptively, total fiber including both soluble fiber and insoluble fiber intake were higher in participants with non-severe hypoglycemia than those without. Conversely, the GI of the diet was higher in participants without non-severe hypoglycemia. Participants with both daytime and nocturnal non-severe hypoglycemia had lower MUFA intake compared to participants with only daytime or nocturnal non-severe hypoglycemia or without non-severe hypoglycemia.
Table 1.
Patient characteristics and average daily dietary intake according to category of non-severe hypoglycemia during one week at baseline visit
| No hypoglycemia (N=17) |
Daytime hypoglycemia only (N=15) |
Nocturnal hypoglycemia* only (N=11) |
Daytime and nocturnal hypoglycemia* (N=55) |
P** | |
|---|---|---|---|---|---|
| Characteristics, % or mean ± SD | |||||
| Age, years | 14.12 ± 1.22 | 14.33 ± 1.11 | 14.27 ± 1.01 | 14.33 ± 1.16 | 0.90 |
| Male | 47.06 | 40.00 | 63.64 | 52.73 | 0.67 |
| White | 100.0 | 93.33 | 90.91 | 87.27 | 0.53 |
| Diabetes duration, years | 6.59 ± 3.71 | 6.29 ± 3.75 | 8.01 ± 5.13 | 5.83 ± 3.52 | 0.55 |
| HbA1c, % | 10.21 ± 1.08 | 9.61 ± 1.07 | 9.84 ± 1.56 | 9.01 ± 0.94 | 0.001 |
| HbA1c, mmol/mol | 88.05 ± 11.81 | 81.57 ± 11.70 | 84.01 ± 17.01 | 74.97 ± 10.28 | 0.001 |
| BMI z score | 1.00 ± 1.03 | 0.47 ± 1.10 | 0.40 ± 0.92 | 0.62 ± 0.87 | 0.30 |
| On insulin pump | 64.71 | 80.00 | 63.64 | 74.07 | 0.69 |
| Insulin dose per kg, unit | 1.03 ± 0.37 | 1.06 ± 0.37 | 0.98 ± 0.26 | 0.98 ± 0.28 | 0.97 |
| Parental education with 4-year college or more | 64.71 | 60.00 | 63.64 | 67.27 | 0.96 |
| Exercise level | |||||
| Vigorous, hours/day | 0.91 ± 1.65 | 0.72 ± 0.82 | 1.34 ± 1.06 | 0.83 ± 0.95 | 0.24 |
| Moderate, hours/day | 2.25 ± 2.00 | 2.20 ± 1.70 | 3.14 ± 1.70 | 2.60 ± 1.69 | 0.24 |
| Electronic media time, hours/day | 2.91 ± 2.14 | 2.43 ± 1.30 | 2.80 ± 2.01 | 2.89 ± 2.37 | 0.99 |
| Television, hours/day | 1.31 ± 1.01 | 2.12 ± 1.13 | 1.66 ± 1.11 | 1.90 ± 1.72 | 0.36 |
| Average number of daily meals | 4.71 ± 1.23 | 4.73 ± 1.56 | 5.55 ± 1.08 | 4.81 ± 1.12 | 0.12 |
| Nutrients, mean ± SD, % of total energy | |||||
| % calorie from fat | 36.87 ± 6.82 | 34.99 ± 6.27 | 37.46 ± 6.56 | 34.01 ± 5.50 | 0.07 |
| % calorie from carbohydrate | 47.79 ± 8.80 | 49.20 ± 8.76 | 48.07 ± 8.64 | 49.05 ± 5.69 | 0.51 |
| % calorie from protein | 15.30 ± 4.41 | 15.85 ± 3.82 | 14.43 ± 3.34 | 16.93 ± 3.74 | 0.16 |
| % calorie from SFA | 12.33 ± 3.61 | 12.30 ± 3.19 | 12.49 ± 3.02 | 12.27 ± 3.02 | 0.95 |
| % calorie from MUFA | 12.67 ± 2.25 | 11.71 ± 2.36 | 13.14 ± 2.57 | 11.45 ± 2.55 | 0.04 |
| % calorie from PUFA | 8.91 ± 2.90 | 7.95 ± 2.51 | 8.93 ± 2.84 | 7.25 ± 2.38 | 0.07 |
| Nutrients, mean ± SD, grams per 1000 kcal | |||||
| Total carbohydrate | 120.66 ± 22.72 | 123.51 ± 22.01 | 123.00 ± 21.07 | 124.86 ± 14.81 | 0.49 |
| Total fiber | 6.47 ± 1.78 | 7.98 ± 2.65 | 8.62 ± 2.62 | 9.12 ± 3.90 | 0.01 |
| Soluble fiber | 2.13 ± 0.50 | 2.77 ± 1.12 | 2.70 ± 0.73 | 2.99 ± 1.16 | 0.006 |
| Insoluble fiber | 4.27 ± 1.41 | 5.18 ± 1.76 | 5.89 ± 2.02 | 6.06 ± 2.97 | 0.04 |
| Total protein | 37.50 ± 10.10 | 39.26 ± 8.76 | 36.96 ± 7.77 | 41.59 ± 9.09 | 0.17 |
| Animal protein | 24.81 ± 11.51 | 26.53 ± 9.06 | 23.23 ± 8.30 | 26.71 ± 8.95 | 0.65 |
| Plant protein | 12.68 ± 3.06 | 12.72 ± 2.48 | 12.73 ± 3.81 | 14.89 ± 4.52 | 0.09 |
| Total fat | 41.87 ± 7.79 | 40.09 ± 6.53 | 42.39 ± 6.72 | 38.70 ± 6.27 | 0.09 |
| SFA | 14.00 ± 4.35 | 14.18 ± 3.68 | 14.04 ± 3.41 | 14.01 ± 3.43 | 0.93 |
| MUFA | 14.37 ± 2.61 | 13.36 ± 2.56 | 14.96 ± 2.64 | 12.97 ± 2.85 | 0.03 |
| PUFA | 10.16 ± 3.40 | 9.05 ± 2.84 | 10.14 ± 2.92 | 8.28 ± 2.75 | 0.06 |
| Glycemic load (glucose reference) | 72.24 ± 15.44 | 68.03 ± 13.37 | 69.48 ± 11.77 | 69.33 ± 10.01 | 0.84 |
| Glycemic index (glucose reference) | 63.48 ± 3.69 | 59.32 ± 3.61 | 61.33 ± 4.57 | 60.10 ± 4.27 | 0.01 |
| MUFA/SFA ratio | 1.07 ± 0.22 | 1.00 ± 0.31 | 1.11 ± 0.27 | 0.96 ± 0.24 | 0.08 |
| PUFA/SFA ratio | 0.86 ± 0.43 | 0.75 ± 0.32 | 0.83 ± 0.31 | 0.69 ± 0.41 | 0.20 |
Abbreviation: MUFA, monounsaturated fat; PUFA, polyunsaturated fat; SD, standard deviation; SFA, saturated fat.
10 min or more with low blood glucose <70 mg/dL between 11PM and 7AM defined nocturnal hypoglycemia.
P value from Kruskal-Wallis test. P values <0.05 were highlighted in bold.
3.2. Usual dietary intake and risk of daytime non-severe hypoglycemia
Fully adjusted models show that total carbohydrate and the GL were not associated with risk of daytime non-severe hypoglycemia (Fig. 1a, Table 2). Every five units higher in the GI of the diet was associated with 68% lower risk of daytime non-severe hypoglycemia (OR, 0.32; 95% CI 0.14–0.73). Intake of soluble fiber, not total fiber, was positively related to the risk of daytime non-severe hypoglycemia (Fig. 1b); the OR (95% CI) for every five grams more intake of soluble fiber with risk of daytime non-severe hypoglycemia was 7.86 (0.98–62.19). Higher total protein intake by 10 grams per day was associated with higher risk of daytime non-severe hypoglycemia (OR, 1.47; 95% CI 1.00–2.17; Fig. 1c). No meaningful difference between animal and plant protein was found. However, type of fat was important. Intake of total fat or SFA was not related to risk of daytime non-severe hypoglycemia while consumption of unsaturated fat was protective (Fig. 1d). Consuming five grams more MUFA (OR, 0.55; 95% CI 0.30–1.00) and PUFA (OR, 0.47; 95% CI 0.24–0.90) per day were associated with lower risk of daytime non-severe hypoglycemia. An inverse association of MUFA/SFA ratio and PUFA/SFA ratio with risk of daytime non-severe hypoglycemia was also found. Adjusting for insulin delivery method did not change results. However, after accounting for insulin dose per kilogram, all associations disappeared except for PUFA/SFA ratio. Of note, we did not have information on insulin dose at each meal, so the insulin dose used here was the self-reported total daily dose at baseline.
Fig. 1. Daily macronutrients intake and daytime non-severe hypoglycemia.
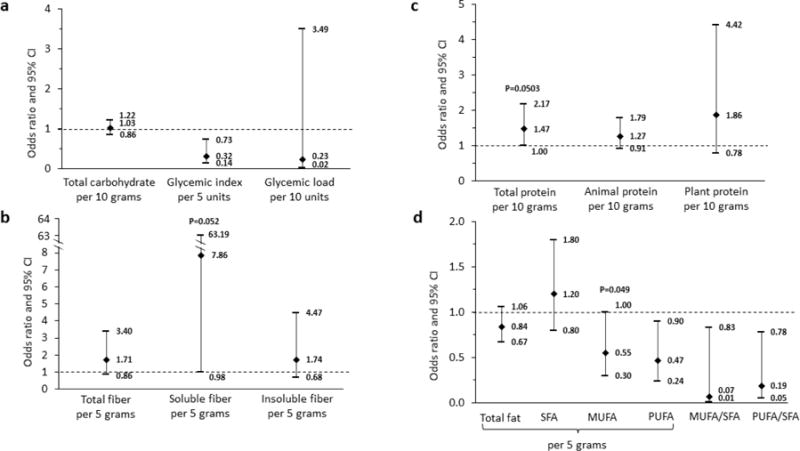
The models were adjusted for continuous glucose monitor wear time, total energy intake per day, average number of meals per day, diabetes duration, HbA1c, daily electronic media time in hours, and TV time in hours. SFA, saturated fat; MUFA, monounsaturated fat; PUFA, polyunsaturated fat.
Table 2.
Dietary intake and risk of daytime non-severe hypoglycemia*
| Nutrients | Model 1 Unadjusted |
Model 2 Partially adjusted |
Model 3 Fully adjusted |
Model 4 Adding insulin delivery method |
Model 5 Further adding insulin dose/kg |
|---|---|---|---|---|---|
| Carbohydrate | |||||
| Total carbohydrate, per 10 grams | 0.98 (0.93–1.03) | 1.11 (0.95–1.29) | 1.03 (0.86–1.22) | 1.03 (0.86–1.22) | 0.93 (0.75–1.17) |
| Total fiber, per 5 grams | 1.07 (0.74–1.53) | 1.48 (0.87–2.53) | 1.71 (0.86–3.40) | 1.73 (0.87–3.47) | 1.26 (0.54–2.90) |
| Soluble fiber. per 5 grams | 1.68 (0.53–5.33) | 8.44 (1.34–53.28) | 7.86 (0.98–63.19) | 8.07 (0.98–66.51) | 5.02 (0.29–87.96) |
| Insoluble fiber, per 5 grams | 1.03 (0.62–1.69) | 1.37 (0.69–2.71) | 1.74 (0.68–4.47) | 1.76 (0.68–4.57) | 1.09 (0.36–3.29) |
| Glycemic index, per 5 units | 0.44 (0.24–0.79) | 0.40 (0.21–0.76) | 0.32 (0.14–0.73) | 0.32 (0.14–0.74) | 0.35 (0.12–1.04) |
| Glycemic load, per 10 units | 0.52 (0.22–1.23) | 0.69 (0.08–6.19) | 0.23 (0.02–3.49) | 0.24 (0.02–3.53) | 0.04 (<0.001–2.50) |
| Protein | |||||
| Total protein, per 10 grams | 0.99 (0.85–1.16) | 1.23 (0.91–1.65) | 1.47 (1.00–2.17) | 1.47 (1.00–2.17) | 1.34 (0.85–2.12) |
| Animal protein, per 10 grams | 0.99 (0.82–1.20) | 1.11 (0.84–1.47) | 1.27 (0.91–1.79) | 1.28 (0.91–1.80) | 1.26 (0.83–1.92) |
| Plant protein, per 10 grams | 0.96 (0.61–1.49) | 1.65 (0.78–3.49) | 1.86 (0.78–4.42) | 1.87 (0.79–4.47) | 1.35 (0.40–4.51) |
| Fat | |||||
| Total fat, per 5 grams | 0.94 (0.88–1.00) | 0.81 (0.66–0.99) | 0.84 (0.67–1.06) | 0.84 (0.67–1.06) | 1.00 (0.76–1.30) |
| SFA, per 5 grams | 0.93 (0.79–1.09) | 1.05 (0.76–1.46) | 1.20 (0.80–1.80) | 1.20 (0.80–1.81) | 1.79 (0.92, 3.48) |
| MUFA, per 5 grams | 0.80 (0.67–0.97) | 0.54 (0.33–0.88) | 0.55 (0.30–1.00) | 0.55 (0.30–1.00) | 0.93 (0.47–1.83) |
| PUFA, per 5 grams | 0.68 (0.51–0.92) | 0.55 (0.34–0.90) | 0.47 (0.24–0.90) | 0.47 (0.24–0.90) | 0.55 (0.27–1.11) |
| MUFA/SFA ratio, per 1 unit | 0.16 (0.03–0.94) | 0.17 (0.03–1.04) | 0.07 (0.01–0.83) | 0.07 (0.006, 0.83) | 0.16 (0.008–3.29) |
| PUFA/SFA ratio, per 1 unit | 0.41 (0.13–1.26) | 0.39 (0.13–1.20) | 0.19 (0.05–0.78) | 0.19 (0.05–0.78) | 0.15 (0.03, 0.86) |
Abbreviation. MUFA, monounsaturated fat; PUFA, polyunsaturated fat; SFA, saturated fat.
Values were odds ratios (95% CIs). Significant results (P<0.05) were highlighted in bold, but p value for total protein in the fully adjusted model (Model 3) was 0.0503.
Model 1. Unadjusted.
Model 2. Adjusted for continuous glucose monitor wear time, total energy intake per day, and average number of meals per day.
Model 3. Model 2 + diabetes duration, HbA1c, daily electronic media time in hours, and TV time in hours.
Model 4. Model 3 + insulin delivery method (pump versus multiple daily injection).
Model 5. Model 4 + insulin dose per kilogram.
3.3. Usual dietary intake and risk of nocturnal non-severe hypoglycemia
Total carbohydrate, the GI, and the GL of the diet were not associated with risk of nocturnal non-severe hypoglycemia (Table 3, Fig. 2a), according to results from the fully adjusted model. Similar to daytime non-severe hypoglycemia, soluble fiber intake per 5 grams was positively associated with risk of nocturnal non-severe hypoglycemia (OR 8.57, 95% CI 1.33–55.07; Fig. 2b). Higher total protein intake by 10 grams was associated with higher risk of nocturnal non-severe hypoglycemia (OR 1.36, 95% CI 0.99–1.86; Fig. 2c). Dietary fat intake was not related to risk of nocturnal non-severe hypoglycemia, including both saturated and unsaturated fat (Fig. 2d). Adjusting for insulin delivery method did not change results. After accounting for insulin dose per kilogram, the positive association of soluble fiber and total protein with risk of nocturnal non-severe hypoglycemia was no longer statistically significant. Unexpectedly, PUFA/SFA ratio was negatively associated with risk of nocturnal non-severe hypoglycemia.
Table 3.
Dietary intake and risk of nocturnal non-severe hypoglycemia*
| Nutrients | Model 1 Unadjusted |
Model 2 Partially adjusted |
Model 3 Fully adjusted |
Model 4 Adding insulin delivery method |
Model 5 Further adding insulin dose/kg |
|---|---|---|---|---|---|
| Carbohydrate | |||||
| Total carbohydrate, per 10 grams | 0.96 (0.92–1.01) | 0.98 (0.86–1.13) | 0.95 (0.82–1.11) | 0.94 (0.80–1.10) | 0.92 (0.76–1.13) |
| Total fiber, per 5 grams | 1.11 (0.78–1.58) | 1.68 (0.98–2.85) | 1.73 (0.97–3.08) | 1.58 (0.88–2.86) | 1.38 (0.67–2.82) |
| Soluble fiber. per 5 grams | 1.47 (0.49–4.38) | 8.43 (1.44–49.46) | 8.57 (1.33–55.07) | 7.08 (1.08–46.57) | 5.06 (0.51–50.40) |
| Insoluble fiber, per 5 grams | 1.14 (0.70–1.85) | 1.69 (0.85–3.34) | 1.76 (0.82–3.75) | 1.56 (0.72–3.38) | 1.27 (0.51–3.18) |
| Glycemic index, per 5 units | 0.71 (0.42–1.18) | 0.79 (0.46–1.36) | 0.83 (0.47–1.46) | 0.86 (0.48–1.53) | 0.57 (0.26–1.24) |
| Glycemic load, per 10 units | 0.45 (0.19–1.03) | 0.37 (0.04–3.07) | 0.27 (0.03–2.69) | 0.27 (0.03–2.93) | 0.10 (0.004–2.66) |
| Protein | |||||
| Total protein, per 10 grams | 0.96 (0.82–1.11) | 1.24 (0.93–1.66) | 1.36 (0.99–1.86) | 1.38 (0.99–1.91) | 1.35 (0.92–1.98) |
| Animal protein, per 10 grams | 0.95 (0.79–1.13) | 1.12 (0.86–1.47) | 1.21 (0.91–1.60) | 1.23 (0.92–1.64) | 1.21 (0.86–1.70) |
| Plant protein, per 10 grams | 0.93 (0.61–1.43) | 1.65 (0.81–3.39) | 1.66 (0.77–3.54) | 1.56 (0.71–3.45) | 1.66 (0.59–4.63) |
| Fat | |||||
| Total fat, per 5 grams | 0.94 (0.88–1.00) | 0.96 (0.80–1.14) | 0.97 (0.79–1.18) | 0.98 (0.79–1.20) | 1.00 (0.78–1.28) |
| SFA, per 5 grams | 0.91 (0.78–1.07) | 1.12 (0.81–1.53) | 1.18 (0.84–1.67) | 1.17 (0.82–1.67) | 1.60 (0.93–2.74) |
| MUFA, per 5 grams | 0.85 (0.71–1.02) | 0.95 (0.62–1.45) | 0.93 (0.57–1.52) | 1.00 (0.60–1.68) | 1.17 (0.62–2.18) |
| PUFA, per 5 grams | 0.72 (0.55–0.95) | 0.71 (0.46–1.10) | 0.70 (0.44–1.13) | 0.71 (0.44–1.16) | 0.51 (0.26–1.00) |
| MUFA/SFA ratio, per 1 unit | 0.45 (0.09–2.33) | 0.41 (0.07–2.33) | 0.30 (0.05–1.96) | 0.38 (0.06–2.57) | 0.18 (0.02–1.89) |
| PUFA/SFA ratio, per 1 unit | 0.56 (0.19–1.64) | 0.51 (0.17–1.54) | 0.42 (0.13–1.39) | 0.41 (0.12–1.38) | 0.20 (0.04–0.90) |
Abbreviation. MUFA, monounsaturated fat; PUFA, polyunsaturated fat; SFA, saturated fat.
Values were odds ratios (95% CIs). Significant results (P<0.05) were highlighted in bold.
Model 1. Unadjusted.
Model 2. Adjusted for continuous glucose monitor wear time, total energy intake per day, and average number of meals per day.
Model 3. Model 2 + diabetes duration, HbA1c, daily electronic media time in hours, and TV time in hours.
Model 4. Model 3 + insulin delivery method (pump versus multiple daily injection).
Model 5. Model 4 + insulin dose per kilogram.
Fig. 2. Daily macronutrients intake and nocturnal non-severe hypoglycemia.

The models were adjusted for continuous glucose monitor wear time, total energy intake per day, average number of meals per day, diabetes duration, HbA1c, daily electronic media time in hours, and TV time in hours. SFA, saturated fat; MUFA, monounsaturated fat; PUFA, polyunsaturated fat.
4. Discussion
In adolescents with type 1 diabetes, non-severe hypoglycemia was common. Over 80% of the study participants developed non-severe hypoglycemia within a week. The mismatch between the insulin dose delivered and the insulin dose actually needed for each freely-consumed meal may be responsible. We found that insulin dosing may need to consider soluble fiber, total protein, MUFA, PUFA, and the GI, in addition to carbohydrate counting recommended by current guidelines. Insulin delivery method did not influence dietary associations with hypoglycemia. Our study suggests that how to inject correct insulin dose at correct time to match every freely consumed meal to reduce clinically unfavorable events such as hypoglycemia remains challenging in type 1 adolescents.
Our analyses revealed that quality of carbohydrate (i.e., soluble fiber and glycemic index) needs to be considered for insulin dosing. Previous literature demonstrated that the reduction of postprandial glucose responses after carbohydrate-rich meals was mainly driven by soluble fiber, not insoluble fiber, via hindering macronutrient absorption and slowing gastric emptying.24 However, none of the published studies in type 1 diabetes populations in the CGM context differentiated two types of fiber. Nonetheless, Maahs et al.25 reported that every one gram increase in total dietary fiber intake was associated with 2.4 to 6.5 mg/dL lower postprandial blood glucose up to 4 hours, in free-living adolescents with type 1 diabetes. Lafrance et al.26 found that high-fiber diet decreased mean blood glucose, but did not increase the incidence of hypoglycemia. However, Lafrance et al. conducted their study in well-controlled patients with type 1 diabetes on intensive insulin therapy in a clinical trial setting. Further, the fiber content in the test breakfast of Lafrance et al.’s study was approximately 50 grams/1000 kcal, which was substantially higher than that in our study.
Another important trait of carbohydrate quality is the GI. Existing evidence indicates that consistent consumption of a low-GI diet may reduce insulin requirement and improve average blood glucose.27, 28 If usual carbohydrate-to-insulin ratio is used, the risk of hypoglycemia may be increased with consuming low-GI diet in type 1 diabetes. This is consistent with our finding that the GI was inversely associated with risk of daytime non-severe hypoglycemia. However, discerning the independent effect of the GI from fiber is difficult,7 because foods rich in fiber generally have a low GI, although not all foods with a low GI necessarily have a high fiber content.29 Nonetheless, the negative GI-hypoglycemia relationship remained in our data even after adjusting for fiber or other major macronutrients (data not shown). Previous studies consistently reported that low-GI foods or diet lowered mean blood glucose concentrations and reduced peak glucose excursion compared to high-GI foods or diet in people with type 1 diabetes.26, 27, 30, 31 However, the relationship between the GI and hypoglycemia risk was much less consistent. Nansel et al.27 found increased incidence of hypoglycemia with low-GI diet in children with type 1 diabetes while other studies reported no difference between low- and high-GI diet regarding hypoglycemia risk.26, 31 Notably, these studies used various definitions of low- and high-GI diet, monitored different length of blood glucose, and assigned test meals at different timing. All these may have led to inconsistent findings regarding the relationship between the GI and hypoglycemia risk.
We found that higher protein intake was associated with higher risk of non-severe hypoglycemia in adolescents with type 1 diabetes. In healthy individuals32 or people with type 2 diabetes,33 ingested protein appears to increase both insulin and glucagon secretion, usually resulting in no change or lower glucose concentration. However, the findings in people with type 1 diabetes are different, because type 1 patients rely exclusively on exogenous insulin. A review of experimental studies in type 1 diabetes led by Bell et al.28 stated that protein tends to increase glucose concentrations in the late postprandial period. This conflicts with the positive protein-hypoglycemia association that we identified in an outpatient setting. The studies included in the Bell et al.’s review are clinical trials with predesigned meals and insulin dosing strategy primarily based on carbohydrate counting. In an outpatient environment, individual experience commonly leads to adjustments for meals high in fat or protein, although current guidelines account only for carbohydrate.34 Thus, some study participants may bolus more insulin than needed for high-protein meals, with or without correct carbohydrate counting,35 resulting in greater hypoglycemia risk. However, this is speculative. Without mealtime insulin dose matched to each meal, we are unable to determine when and how the mismatch between dietary intake and insulin dose occur. Evidence has also shown that the glycemic effect of protein depends on carbohydrate content of the meal in type 1 diabetes,28 but we did not find an interaction between carbohydrate and protein in relation to hypoglycemia risk (data not shown). Of note, although a bedtime snack containing protein and carbohydrate is commonly consumed by patients to prevent nocturnal hypoglycemia, currently, there are no clear clinical practice guidelines on the optimal composition of a bedtime snack.7, 36, 37
Our data also revealed that fat quality mattered in terms of managing hypoglycemia. To our knowledge, we did not find any diet and CGM study that distinguished fat types. Rather, dietary fat as a whole was considered. Studies from Wolpert et al.,38 Smart et al.8 and other investigators39, 40 demonstrated that meals containing carbohydrate and that are also high in dietary fat can cause sustained late high postprandial blood glucose up to or over 5 hours. The relevant mechanisms are delayed gastric emptying, impaired insulin sensitivity, and enhanced hepatic glucose production.41 If individuals with type 1 diabetes do not adjust insulin dose for dietary fat, they may be more likely to have hyperglycemia instead of hypoglycemia. However, these findings from previous studies could not explain the difference between saturated and unsaturated fat. In a randomized trial conducted among obese adults with type 2 diabetes, a low carbohydrate diet that was high in unsaturated fat and low in saturated fat reduced glucose variability measured by 48-hour CGM. However, adjusting for glucose variability did not change our results (data not shown). Also, the differential effect on insulin resistance between saturated and unsaturated fat does not apply here; unsaturated fat causes less profound insulin resistance than saturated fat.42
Another notable finding from our study is that dietary intake was a stronger predictor of non-severe hypoglycemia in the daytime than nighttime. Similarly, an early large study on type 1 children by Beregszàszi et al.43 did not find a difference in food consumption in the daytime between participants with nocturnal hypoglycemia and those without. The GI-hypoglycemia relationship of our study was also consistent with the Nansel et al.’s study.27 They reported no difference in the mean blood glucose and hypoglycemia risk in the night between consuming a low-GI and a high-GI diet in type 1 children, although the mean blood glucose was lower and hypoglycemia risk was higher in the daytime.27 Probably, the main reason is that the failure of insulin replacement to mimic normal insulin secretion of pancreas causes a mismatch between nighttime insulin requirements and blood glucose, leading to nocturnal hypoglycemia.20, 44
The insulin dose data we used were self-reported usual total insulin dose at baseline visit. We did not have information on insulin dose and injection time at each meal. The high hypoglycemia risk in our participants may be related to both incorrect meal-based insulin dose and injection time. Optimal amount and delivery patterns of insulin for meals high in fat or protein or varying in the GI are not yet fully understood. Also, the insulin injection time depends on blood glucose concentration, meal composition, exercise, and type of insulin.45 Therefore, appropriate insulin dosing is complex and requires substantial behavioral support.
Our study is the largest investigation so far that examined the association of usual dietary intake with risk of non-severe hypoglycemia in adolescents with type 1 diabetes. We used blinded CGM to document hypoglycemic events, which could more objectively and completely capture hypoglycemia compared to traditional methods (e.g., daily blood glucose check and hypoglycemic symptoms). Nonetheless, misclassification of hypoglycemia may exist due to the inaccuracy of CGM data. Other limitations should also be noted. First, we did not have mealtime insulin dose data and did not ask how participants calculated insulin dose. Therefore, we were unable to determine how and when the mismatch happened between dietary intake and dosed insulin. Second, participants are not representative of all youth with type 1 diabetes and had an HbA1c range of 8–13% and duration of diabetes >1 year. Third, two days of 24-hour recalls may not capture usual dietary intake, and reporting bias may occur. Fourth, the small sample size precludes assessment of potential interactions among nutrients and effect modifications by baseline glycemic status, diabetes duration, and pubertal status. Fifth, we did not consider the severity of hypoglycemia which is related to duration of low blood glucose <70 mg/dL and the lowest glucose concentration within a hypoglycemic episode. Sixth, residual confounding may be likely. For example, moderate or vigorous exercise, a known factor related to hypoglycemia in children with type 1 diabetes,36 was not included in the final models according to our pre-specified covariates inclusion criterion requiring P ≤0.2, although further accounting for it did not make a difference (data not shown). More precise and objective measurement of physical activity would be important in future studies. Finally, the definition of nocturnal hypoglycemia is arbitrary, although 11PM-7AM threshold is commonly used in the literature.18, 23
In conclusion, we found that different nutrients had different association with the occurrence of non-severe hypoglycemia in free-living adolescents with type 1 diabetes. Also, dietary intake was differentially associated with risk of non-severe hypoglycemia between daytime and nighttime. To reduce hypoglycemia risk, current insulin dosing recommendations mainly based on carbohydrate counting may need to be modified. Calculating insulin dose may consider several other nutrition factors including soluble fiber, total protein, MUFA, PUFA, and the GI. Further research is required to better understand the relationship among dietary intake, insulin dosing, and risk of hypoglycemia, especially in type 1 adolescents. Such studies need to collect insulin dose and time of injection for each meal during the study period.
Supplementary Material
Acknowledgments
Funding: This study was supported by NIH/NIDDK (1UC4DK101132) and Helmsley Charitable Trust.
VWZ is funded by the Sanofi Global Nutrition Scholars program at the University of North Carolina at Chapel Hill. CMS is an employee of the American Heart Association. JJ is an employee of Sanofi US. DMM is on the advisory board for Insulet, consults for Abbott Diabetes Care, and his non-profit employer has received research funding from Medtronic, Dexcom, and Roche.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registration: ClinicalTrials.gov identifier: NCT01286350.
Declaration of interest: All other authors declare no conflict of interest.
References
- 1.Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10(12):711–722. doi: 10.1038/nrendo.2014.170. [DOI] [PubMed] [Google Scholar]
- 2.Ly TT, Maahs DM, Rewers A, Dunger D, Oduwole A, Jones TW. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2014;15(Suppl 20):180–192. doi: 10.1111/pedi.12174. [DOI] [PubMed] [Google Scholar]
- 3.Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook Authors Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–2054. doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mauras N, Mazaika P, Buckingham B, et al. Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes. 2015;64(5):1770–1779. doi: 10.2337/db14-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannonen R, Tupola S, Ahonen T, Riikonen R. Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia. Dev Med Child Neurol. 2003;45(4):262–268. doi: 10.1017/s0012162203000501. [DOI] [PubMed] [Google Scholar]
- 7.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36(11):3821–3842. doi: 10.2337/dc13-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smart CE, Evans M, O’Connell SM, et al. Both dietary protein and fat increase postprandial glucose excursions in children with type 1 diabetes, and the effect is additive. Diabetes Care. 2013;36(12):3897–3902. doi: 10.2337/dc13-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pankowska E, Blazik M, Groele L. Does the fat-protein meal increase postprandial glucose level in type 1 diabetes patients on insulin pump: the conclusion of a randomized study. Diabetes Technol Ther. 2012;14(1):16–22. doi: 10.1089/dia.2011.0083. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Lopez JM, Gonzalez-Rodriguez M, Pazos-Couselo M, Gude F, Prieto-Tenreiro A, Casanueva F. Should the amounts of fat and protein be taken into consideration to calculate the lunch prandial insulin bolus? Results from a randomized crossover trial. Diabetes Technol Ther. 2013;15(2):166–171. doi: 10.1089/dia.2012.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostenson CG, Geelhoed-Duijvestijn P, Lahtela J, Weitgasser R, Markert Jensen M, Pedersen-Bjergaard U. Self-reported non-severe hypoglycaemic events in Europe. Diabet Med. 2014;31(1):92–101. doi: 10.1111/dme.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maahs DM, Buckingham BA, Castle JR, et al. Outcome Measures for Artificial Pancreas Clinical Trials: A Consensus Report. Diabetes Care. 2016;39(7):1175–1179. doi: 10.2337/dc15-2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beaton GH, Milner J, Corey P, et al. Sources of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Am J Clin Nutr. 1979;32(12):2546–2559. doi: 10.1093/ajcn/32.12.2546. [DOI] [PubMed] [Google Scholar]
- 14.Posner BM, Smigelski C, Duggal A, Morgan JL, Cobb J, Cupples LA. Validation of two-dimensional models for estimation of portion size in nutrition research. J Am Diet Assoc. 1992;92(6):738–741. [PubMed] [Google Scholar]
- 15.Clark BK, Pavey TG, Lim RF, Gomersall SR, Brown WJ. Past-day recall of sedentary time: Validity of a self-reported measure of sedentary time in a university population. J Sci Med Sport. 2016;19(3):237–241. doi: 10.1016/j.jsams.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Kozey Keadle S, Lyden K, Hickey A, et al. Validation of a previous day recall for measuring the location and purpose of active and sedentary behaviors compared to direct observation. Int J Behav Nutr Phys Act. 2014;11 doi: 10.1186/1479-5868-11-12. 12-5868-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Danne T, Philotheou A, Goldman D, et al. A randomized trial comparing the rate of hypoglycemia–assessed using continuous glucose monitoring–in 125 preschool children with type 1 diabetes treated with insulin glargine or NPH insulin (the PRESCHOOL study) Pediatr Diabetes. 2013;14(8):593–601. doi: 10.1111/pedi.12051. [DOI] [PubMed] [Google Scholar]
- 19.Tsujino D, Nishimura R, Morimoto A, Tajima N, Utsunomiya K. A crossover comparison of glycemic variations in Japanese patients with type 1 diabetes receiving insulin glargine versus insulin detemir twice daily using continuous glucose monitoring (CGM): J COLLECTION (Jikei COmparison of Lantus and LEvemir with Cgm for Thinking Insulin OptimizatioN) Diabetes Technol Ther. 2012;14(7):596–601. doi: 10.1089/dia.2011.0235. [DOI] [PubMed] [Google Scholar]
- 20.Brunton SA. Nocturnal hypoglycemia: answering the challenge with long-acting insulin analogs. MedGenMed. 2007;9(2):38. [PMC free article] [PubMed] [Google Scholar]
- 21.Merl V, Kern W, Peters A, et al. Differences between nighttime and daytime hypoglycemia counterregulation in healthy humans. Metabolism. 2004;53(7):894–898. doi: 10.1016/j.metabol.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Physical activity/exercise and diabetes. Diabetes Care. 2004;27(Suppl 1):S58–62. doi: 10.2337/diacare.27.2007.s58. [DOI] [PubMed] [Google Scholar]
- 23.Nimri R, Atlas E, Ajzensztejn M, Miller S, Oron T, Phillip M. Feasibility study of automated overnight closed-loop glucose control under MD-logic artificial pancreas in patients with type 1 diabetes: the DREAM Project. Diabetes Technol Ther. 2012;14(8):728–735. doi: 10.1089/dia.2012.0004. [DOI] [PubMed] [Google Scholar]
- 24.Weickert MO, Pfeiffer AF. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008;138(3):439–442. doi: 10.1093/jn/138.3.439. [DOI] [PubMed] [Google Scholar]
- 25.Maahs DM, Mayer-Davis E, Bishop FK, Wang L, Mangan M, McMurray RG. Outpatient assessment of determinants of glucose excursions in adolescents with type 1 diabetes: proof of concept. Diabetes Technol Ther. 2012;14(8):658–664. doi: 10.1089/dia.2012.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafrance L, Rabasa-Lhoret R, Poisson D, Ducros F, Chiasson JL. Effects of different glycaemic index foods and dietary fibre intake on glycaemic control in type 1 diabetic patients on intensive insulin therapy. Diabet Med. 1998;15(11):972–978. doi: 10.1002/(SICI)1096-9136(1998110)15:11<972::AID-DIA704>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Nansel TR, Gellar L, McGill A. Effect of varying glycemic index meals on blood glucose control assessed with continuous glucose monitoring in youth with type 1 diabetes on basal-bolus insulin regimens. Diabetes Care. 2008;31(4):695–697. doi: 10.2337/dc07-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell KJ, Smart CE, Steil GM, Brand-Miller JC, King B, Wolpert HA. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38(6):1008–1015. doi: 10.2337/dc15-0100. [DOI] [PubMed] [Google Scholar]
- 29.Riccardi G, Rivellese AA, Giacco R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr. 2008;87(1):269S–274S. doi: 10.1093/ajcn/87.1.269S. [DOI] [PubMed] [Google Scholar]
- 30.Ryan RL, King BR, Anderson DG, Attia JR, Collins CE, Smart CE. Influence of and optimal insulin therapy for a low-glycemic index meal in children with type 1 diabetes receiving intensive insulin therapy. Diabetes Care. 2008;31(8):1485–1490. doi: 10.2337/dc08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammed NH, Wolever TM. Effect of carbohydrate source on post-prandial blood glucose in subjects with type 1 diabetes treated with insulin lispro. Diabetes Res Clin Pract. 2004;65(1):29–35. doi: 10.1016/j.diabres.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed M, Nuttall FQ, Gannon MC, Lamusga RF. Plasma glucagon and alpha-amino acid nitrogen response to various diets in normal humans. Am J Clin Nutr. 1980;33(9):1917–1924. doi: 10.1093/ajcn/33.9.1917. [DOI] [PubMed] [Google Scholar]
- 33.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78(4):734–741. doi: 10.1093/ajcn/78.4.734. [DOI] [PubMed] [Google Scholar]
- 34.American Diabetes Association. Standards of Medical Care in Diabetes-2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34(1):3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spiegel G, Bortsov A, Bishop FK, et al. Randomized nutrition education intervention to improve carbohydrate counting in adolescents with type 1 diabetes study: is more intensive education needed? J Acad Nutr Diet. 2012;112(11):1736–1746. doi: 10.1016/j.jand.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smart CE, Annan F, Bruno LP, Higgins LA, Acerini CL. Nutritional management in children and adolescents with diabetes. Pediatr Diabetes. 2014;15(Suppl 20):135–153. doi: 10.1111/pedi.12175. [DOI] [PubMed] [Google Scholar]
- 37.Desjardins K, Brazeau AS, Strychar I, Rabasa-Lhoret R. Are bedtime nutritional strategies effective in preventing nocturnal hypoglycaemia in patients with type 1 diabetes? Diabetes Obes Metab. 2014;16(7):577–587. doi: 10.1111/dom.12232. [DOI] [PubMed] [Google Scholar]
- 38.Wolpert HA, Atakov-Castillo A, Smith SA, Steil GM. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care. 2013;36(4):810–816. doi: 10.2337/dc12-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodefalk M, Aman J, Bang P. Effects of fat supplementation on glycaemic response and gastric emptying in adolescents with Type 1 diabetes. Diabet Med. 2008;25(9):1030–1035. doi: 10.1111/j.1464-5491.2008.02530.x. [DOI] [PubMed] [Google Scholar]
- 40.Neu A, Behret F, Braun R, et al. Higher glucose concentrations following protein- and fat-rich meals – the Tuebingen Grill Study: a pilot study in adolescents with type 1 diabetes. Pediatr Diabetes. 2015;16(8):587–591. doi: 10.1111/pedi.12224. [DOI] [PubMed] [Google Scholar]
- 41.Wolever TM, Mullan YM. Sugars and fat have different effects on postprandial glucose responses in normal and type 1 diabetic subjects. Nutr Metab Cardiovasc Dis. 2011;21(9):719–725. doi: 10.1016/j.numecd.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Xiao C, Giacca A, Carpentier A, Lewis GF. Differential effects of monounsaturated, polyunsaturated and saturated fat ingestion on glucose-stimulated insulin secretion, sensitivity and clearance in overweight and obese, non-diabetic humans. Diabetologia. 2006;49(6):1371–1379. doi: 10.1007/s00125-006-0211-x. [DOI] [PubMed] [Google Scholar]
- 43.Beregszaszi M, Tubiana-Rufi N, Benali K, Noel M, Bloch J, Czernichow P. Nocturnal hypoglycemia in children and adolescents with insulin-dependent diabetes mellitus: prevalence and risk factors. J Pediatr. 1997;131(1 Pt 1):27–33. doi: 10.1016/s0022-3476(97)70121-5. [DOI] [PubMed] [Google Scholar]
- 44.Yale JF. Nocturnal hypoglycemia in patients with insulin-treated diabetes. Diabetes Res Clin Pract. 2004;65(Suppl 1):S41–6. doi: 10.1016/j.diabres.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 45.American Diabetes Association. Insulin administration. Diabetes Care. 2004;27(Suppl 1):S106–9. doi: 10.2337/diacare.27.2007.s106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


