Abstract
Stretching single chromosomal DNA fibers in nanofluidic devices has become a valuable tool for studying the genome and more recently the epigenome. Although nanofluidic technology has been extensively used in single molecular DNA analysis, compared to bare DNA, much less work has been done to elongate chromatin, and only a few studies utilize more biologically relevant samples such as native eukaryotic chromatin. Here, we provide a method for stretching and imaging individual chromatin fibers within a micro- and nanofluidic device. This device was used to electrophoretically stretch and image single native chromatin fibers extracted from human cancer cells (HeLa cells) by attaching the chromatin to microspheres held at the entrance of a nanoslit. To further demonstrate the potential of this device in epigenetics, histone modification H3k79me2 was optically detected by fluorescence microscopy.
INTRODUCTION
Chromatin in eukaryotic cells is comprised of DNA wrapped around histone protein complexes which is packed tightly into a small nuclear compartment. Modifications and packaging of chromatin, as well as its underlying DNA sequence, play a crucial role in gene expression and have profound consequences for human evolution1 and disease.2,3 Therefore, the ability to observe linearized chromatin fibers and DNA molecules has the potential to help identify and map genetic and epigenetic modifications throughout the genome.4–6 Here, a nanofluidic device was employed to unravel the coiled chromatin without popping off the histone proteins so that various features of native chromatin fibers could be observed.
In previous studies, several techniques have been used to stretch nucleic acids for analysis,7,8 which has been largely exploited in the case of prokaryotic DNA, synthetic/reconstituted chromatin, or protein-conjugated DNA. These techniques include optical9–13 and magnetic tweezers,14,15 atomic force microscopy (AFM),16 adsorption onto a modified surface under flow,17–19 and shear flow.20–24 Furthermore, it has been shown that by suppressing the thermal fluctuations of DNA molecules, high resolution optical images can be obtained. This suppression can be achieved with the help of highly confined nanochannels,25–35 by stretching samples in nanoslits with external forces such as an applied electric field36,37 or flow38 or by immobilizing them onto a positively charged or hydrophobic surface.39–41 While many of these nanofluidic techniques should be capable of accommodating more complex structures such as eukaryotic chromatin, these studies and demonstrations are rare. This may be in part due to the inherent challenges of employing nanochannels to achieve high degrees of linearization as eukaryotic chromatin contains highly packaged DNA with various levels of organization and structures. For example, using narrow nanochannels can preclude certain condensed chromatin fibers, such as heterochromatin (diameters of ∼30 nm), from entering the nanochannels. In contrast, using wider nanochannels can hinder effective linearization, especially for highly packed molecules. Despite these challenges, the linearization and study of reconstituted chromatin in nanochannels have been previously reported, indicating that the equilibrium length of the elongated chromatin is roughly one-third that of the equal genomic length of bare DNA.42 However, in order to achieve these equilibrium measurements, no external forces could be employed to further control or manipulate the elongated molecules.
To address some of the limitations inherent in many of these stretching techniques, we establish a method that allows for controllable stretching of long single native chromatin fibers (>0.25 Mbp) in nanofluidic slits for optical image analysis. This approach is simple to execute and can be easily integrated or coupled with other fluidic devices for expanded functionality. With this method, we visualize the density profiles of the DNA, histones, and possible histone modifications in extended single chromatin fibers.
METHODS
Device fabrication
We fabricate nanoslit devices on fused silica substrates by using a differential etching process defining the slit height h = 130 nm (z-axis). The fabricated nanoslits have a length of ls = 90 μm (x-axis) and a width of ws = 10 μm (y-axis). The two ends of each nanoslit are both connected to microchannels that are 1 μm in height and 184 μm in width in order to load samples and anchor microspheres [Figs. 1(a) and S1 (supplementary material)]. The detailed fabrication process is described in our previous work.36
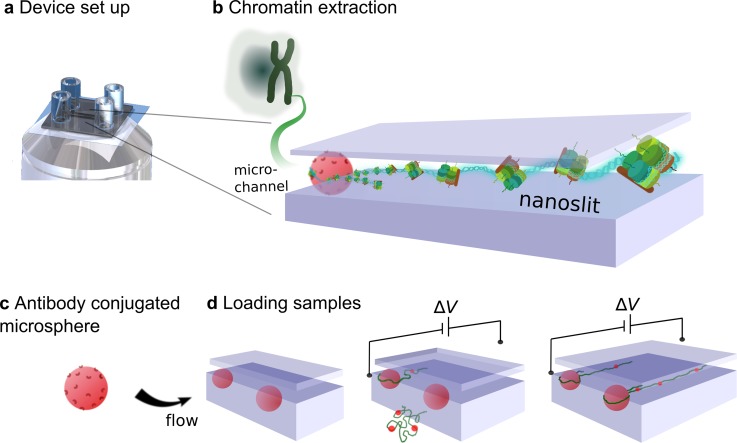
FIG. 1.
Schematic representation of chromatin stretched by a nanofluidic device. (a) The schematic of device setup. (b) GFP-chromatin fibers extracted from HeLa cells. (c) and (d) DAPI-dye bound to DNA in chromatin fibers is anchored at anti-histone H3 microspheres. (d) The experimental procedures: First, antibody microspheres were loaded and introduced into the entrance of nanoslits by capillary flow. Second, chromatin was inserted and attached by an antibody microsphere by the electrophoretic force on the DNA. Third, tethered chromatin was stretched in a controlled manner by applying an electric field.
Chromatin extraction
HeLa cells expressing the green fluorescent protein (GFP) on histone H2B were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) until they reached roughly 90% confluency [Fig. 1(b)]. Prior to chromatin extraction, cells were washed with Phosphate Buffer Saline (PBS), trypsinized, and then resuspended in a 1% Triton-X in PBS solution. Cell lysis was performed with a Dounce homogenizer, and cellular debris was removed via centrifugation prior to resuspending the nuclei pellets in 0.5 M NaCl in PBS solution. The studied chromatin fibers have a wide distribution in the length ranging from a few micrometers to over eighty micrometers (Fig. S2, supplementary material). Its approximate length was analyzed by imaging 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI) stained DNA in a field of En = 7.5 (V/cm). Figures 2 and S3 (supplementary material) show different conformations of elongated chromatin fibers and the distribution.
FIG. 2.
Conformations of stretched chromatin fibers. Stretching chromatin fibers (a)–(c) in one nanoslit, stained with DAPI. The plot of the fluorescent intensity shows (a) folded/multi-tangles, (b) folded and knots, and (c) uniform linearization single chromatin fibers. The dashed line indicates the averaged background intensity. Pink lines are schematics of the corresponding chromatin polymers.
Histone modification labeling
The histone H3K79me2 antibody was purchased from Active Motif (Cat # 39923) and labeled with Alexa Fluor 647 using the Alexa Fluor 647 Monoclonal Antibody Labeling Kit (Invitrogen, Cat # A20186). The dye to protein ratio was calculated to be 1:1. Antibodies were used in experiments at a concentration of 10 μg/ml. To confirm the selectivity of H3k79me2 for this antibody, we reproduced Active Motif's published control assay. This was achieved by labeling the chromatin with Alex Fluor647-labeled Mouse IgG1 in place of the H3k79me2 antibody. As expected, we observed no binding between H3k79me2 modified chromatin and the IgG1 antibody, demonstrating the specificity of H3k79me2 to the antibody (Fig. S4), consistent with the chromatin immunoprecipitation (ChIP) analysis performed by Active Motif.43
Antibody-conjugated microsphere preparations
The biotin-conjugated anti-Histone H3 antibody (Bioss antibodies) and streptavidin-coated microspheres (0.4 μm in diameter, red fluorescent; Bangs Lab., Inc.) were conjugated for anti-Histone H3 microspheres [Fig. 1(c)]. They were prepared using Phosphate Buffered Saline (PBS, 10 mM Phosphate; 150 mM NaCl, pH7.0) containing 150 mM NaCl and 1% Tween 20 and incubated for 1 h at room temperature.
Microscopy and image analysis
We observed single chromatin fibers in a fluorescence microscopy system consisting of an inverted microscope (Olympus), a 100X oil-immersion lens (Leica, N.A. 1.35), and an EMCCD camera (IXon-897, Andor Technologies) with an equivalent pixel resolution of 160 nm. Images were captured with an exposure time of 0.1 s. We analyze chromatin dynamics from the CCD images using ImageJ (NIH) software and MATLAB (The Mathworks, Natick, MA).
RESULTS
In previous work, we presented a model that quantitatively described the dynamics of stretching tethered single DNA molecules in nanoslits.36 In this work, we show that this method and device can also be used to study more complex and biologically relevant samples. To demonstrate this, we electrophoretically stretched single native chromatin fibers using 130 nm high nanoslits. This slit height was found to be small enough to produce a sufficient confinement and linearization while minimizing undesired surface interactions between surface-passivated poly(n-vinylpyrrolidone) (PVP) molecules44 and different states of chromatin fibers. The native chromatin was extracted from HeLa cells expressing green fluorescent protein (GFP) on histone H2B [Fig. 1(a) and Movie S1 (supplementary material)]. The chromatin's DNA was stained with DAPI (deep blue) [Fig. 1(b)]. The native chromatin fibers were attached to 0.4 μm diameter antibody-coated microspheres, which were fixed at the entrance of a nanoslit. Note that we held the bare antibody-coated microspheres at the slit entrance via capillary flow before they were bonded to the molecules [Fig. 1(c)]. We then loaded the chromatin solution in the channel with a small-applied electric field, and the molecules spontaneously attached to the anchored spheres. As we applied an electrophoretic force, the chromatin fibers were stretched into the nanoslits, in response to the external field [Fig. 1(d)].
To observe the distribution and conformation of elongated chromatin obtained by this technique, we measured the intensity profiles of fluorescently labeled DNA along the chromatin fibers. As Fig. 2 shows, the conformation of chromatin fibers, such as full single linearized, folded, knots or multiple aggregated/tangled chromatin, can be characterized. Linearized fibers display relatively uniform intensity profiles, whereas folded and/or knotted fibers appear non-uniform. To minimize the likelihood of multiple tangled chromatin fibers, we loaded highly diluted chromatin samples (∼10 pM) into the device.
To keep chromatin fibers intact during elongation, we applied a relatively low electric field En = 7.5 (V/cm) to stretch the molecules as shown in Movie S1. We can easily estimate how much force is applied on an extended chromatin fiber. Assuming that the charge of a histone octamer is 142 e−, 146 bp of DNA are wrapped around each histone octamer,45 the charge density of DNA is 1.47 e−/nm,46 and an average separation distance is 200 bp between histone complexes along the chromatin fiber. The stretched biopolymer chain is characterized by a gradient force,47 where the tension is minimal at the free end and increases toward the tethered end. We calculate the maximum tension on a 30 μm length chromatin fiber to be around 4.5 pN, which contains the electrophoretic forces applied on both the negatively charged DNA chain and the positively charged histone proteins. Indeed, Fig. 3(a) shows a typical length measurement when the chromatin fiber stretches and relaxes by repeatedly application of the electric field. We observed that the extension length is repeatable and constant when the field is applied and the process is reversible. This verifies that this force range can stretch chromatin into linear 30-nm diameter compact fibers and the more extended “beads on a string” conformation, but it does not yield progressively longer fibers due to nucleosomal disruption (which is expected at higher fields).8,10,11 Since we can easily control the electric field and applied forces, this technique is able to linearize chromatin molecules while ensuring that the histones remain intact, preserving any modifications they may have for downstream optical detection.
FIG. 3.
Force-extension relations of HeLa chromatin fibers in the low, intermediate, and large regimes. (a) The extension length as a function of time (blue circle) at three repeating low external pulse fields, En = 7.5 V/cm. When the field is on, the chromatin is elongated. After turning off the fields, the chromatin relaxes. During the events, the extended length is nearly the same (red dashed line). (b) In the intermediate force regime, Fmax = 15 pN, three force-extension curves show different levels of coinciding and hysteresis loops between stretch (blue filled triangle) and release curves (red filled circle). (c) In the large force regime, Fmax = 30 pN, the stretch and release curves do not coincide, and the process displays hysteresis.
Biophysicists, of course, may want to study higher force extensions which may alter chromatin conformations. For this purpose, we looked at the force-induced response of chromatin fibers in an intermediate force regime (∼15 pN) and a higher force regime (∼30 pN) by applying a maximum external field of En = 25 and 50 (V/cm), respectively. In the intermediate force regime [Fig. 3(b)], the stretch and release halves of a force-extension cycle for different fibers can exhibit both reversible and irreversible (hysteresis) curves. This diversity of behavior from various chromatin fibers is in part due to transitions from somewhat tangled states to untangled states and transitions between heterochromatin and euchromatin or even possibly from histone disruption. Given the unknown and heterogeneous nature of native eukaryotic chromatin, various combinations and extents of these phenomena are possible, resulting in richer and more complex dynamics in the intermediate force regime. However, when the maximum external field is increased to 50 (V/cm), a very pronounced hysteresis loop appears, showing a clear difference between the stretch and release curves as shown in Fig. 3(c). This trend is consistent with the work by previous researchers,11,48,49 which describes the hysteresis as irreversible unfolding of the chromatin fiber due to lost linker histones (histones H1) and/or histone octamers. Although native chromatin fibers may seem challenging due to inhomogeneous persistence lengths and size distributions of the resulting fragments compared with DNA (Fig. S5, supplementary material), our device provides the possibility to more broadly study and characterize the mechanical properties of native chromatin fibers.
To illustrate the broader utility in stretching native chromatin fibers in our device, we visualized epigenetic modifications via the gene silencing mark, dimethylated histone H3 lysine 79 (H3k79me2), as well as the DNA and histone distributions along tethered chromatin fibers (Fig. 4). The samples were labeled with red Alexa647-tagged antibodies targeting histone modification H3K79me2 [Fig. 4(a)]. This direct visualization allowed us to study the relationship between these distributions and the chromatin's structure, which is indicated by the intensity of epigenetic marks, Histone H2B, or DNA. We find that the H3K79me2 marks have high co-localization with the denser regions of nucleosomes or DNA [Fig. 4(b) and Table S1 in supplementary material], suggesting that the histone modification pattern is strongly correlated with DNA density and the chromatin's state.50,51 This demonstrates the ability to use our method to study, identify, and possibly map epigenetic modifications in important samples, a capability which would be valuable when coupling to other integrated fluidic technologies.
FIG. 4.
Chromatin elongation for epigenetic mark detection. (a) The schematic and selected cropped fluorescence images of elongated chromatin fibers, where the scale bar is 5 μm. GFP-H2B (green) chromatin fibers stained with DAPI on DNA (blue) having H3k79me2 histone modification sites labeled with the Alexa647-antibody (red). Overlapping red, blue, and green signals are shown in white. (b) The histone modification, higher DNA stained, and/or higher GFP-histone constituent regions are distinguished by the degree of fluorescent staining on the graph. Pie-chart graphs show co-localization of H3k79me2 epigenetic marks (red) with higher DNA stained regions (blue) and with higher GFP-histone fluorescent intensity regions (green). The overlap coefficient of co-localization between two marks can be calculated by , where S1 and S2 are the signal positions of epigenetic marks and high DNA stained or high GFP-H2B regions along the chain.
It may be of interest to compare our system to several noteworthy alternative techniques for stretching DNA and chromatin fibers (Table I). DNA curtains is a very high throughput visualization tool for analyzing the statistical distributions of single molecules.52 As the sample is anchored, the sample dynamics can be monitored and additional reagents can be introduced without sample loss. However, there is much less control over the individual molecules as there is no isolation or confinement of strands, which limits the degree of linearization that can be achieved. Furthermore, this requires the end-modification of molecules of interest, which may perturb the sample or feature of interest. Another interesting technique is the nanochannel array, which has evolved into a powerful tool for genetics such as with genetic rearrangements or mapping.25 However, despite the high degree of linearization and precision in mapping sequence motifs, this technique has not been demonstrated with more complex fibers such as chromatin.
TABLE I.
Comparison of methods of long (3 kbp–1 Mbp) native eukaryotic chromatin stretching.
| Method | Degree of linearization | Ratio of linearized single chromatin | Surface/Bead immobilization | Precision | Advantage | References |
|---|---|---|---|---|---|---|
| Molecular combing | 80%–90%a | No data | Required | 76 bp (with super-resolution localization)a | High throughput | 40 and 56 |
| Nano-squeezing | Uncontrollable: 40%–80%a | ∼10%a | No | No data | Simple, inexpensive fabrication | 27 and 53 |
| Nanochannel array | ∼15%b (200 × 200 nm2 channels) | ∼99%a | No | 1.3 kbpa | High throughput | 25, 28, and 42 |
| Nanoslit bead-anchoring | Controllable: 40%–95% | ∼36% | Required | 300 bpa | High accuracy, controllable extension | This work36,37 |
Statistical results from the DNA sample.
Statistical results from the reconstituting chromatin sample.
Two recent reports have performed analysis on native eukaryotic chromatin. Cerf et al. used Polydimethylsiloxane (PDMS) stamps to assemble and stretch large-scale arrays of chromatin.40 In contrast to the DNA curtains, this process results in uniformly spaced arrays of single elongated molecules. However, the assembly and transfer method cannot be integrated into fluidic devices for added functionality, and once stretched and stamped, molecules that are not straight or fully elongated cannot be adjusted as there are no external forces present. Matsuoka et al.4,27,53 also used a PDMS device to linearize and squeeze chromatin fibers in nanoscale channels by applying tensile strain. Although this technique results in highly linearized and confined molecules for high-resolution microscopy, this too has some drawbacks. In particular, the sub-micron channels are formed randomly by cracking the PDMS substrate where the size, location, and number of channels cannot be controlled. In addition, chromatin molecules are captured randomly in and throughout the nanochannels and subject to non-uniform hydrodynamic flow during changes in applied strain, which can significantly impact the yield of elongated chromatin. Finally, since elongated molecules are mechanically confined, they cannot be studied dynamically or have additional reagents or proteins introduced without displacing the sample.
In contrast to the above technologies, our nanoslit device allows us to control and modulate the degree of linearization (up to 95%) by anchoring the molecule to an antibody coated microsphere, which requires no modification of the native eukaryotic chromatin. Furthermore, the nanoslit aids in this extension by confining the molecule and providing a restricted region of observation. This allows to us to do both multicolor imaging of near static molecules and modulation of the applied forces to study the biophysical properties of single chromatin.
Although our work shows how single native eukaryotic chromatin fibers can be controllably stretched in nanoslits, we envisage that super-resolution optical microscopy should make it a useful design for observing detailed protein-DNA interactions during force-extension dynamics and increasing the accuracy of histone modification localization. Moreover, because it allows effective linearization of chromatin and the ability to preserve histone modification information, it should be possible to study and identify the epigenetic marks through genomic mapping in large-scale structure variations.54 More broadly, due to the simplicity of the device and its use, integrating this with other on-chip functions should be easily achievable, making it possible to extend this technique to microfluidics which deals with the material extracted from a single cell6,21,55 for use as a diagnostic in cancer detection/identification. Thus, the stretching of native chromatin offers a simple experimental platform to probe both the mechanical behavior of chromatin and the epigenetic information it contains.
SUPPLEMENTARY MATERIAL
See supplementary material for more statistical results of the studied native chromatin samples and Movie S1 demonstrating the single GFP-fluorescent chromatin stretching at small fields (7.5 V/cm).
ACKNOWLEDGMENTS
This work was accomplished under guidance of Professor Harold G. Craighead. The authors thank Dr. Jaime J. Benitez, Dr. Christopher Wallin, Dr. Neil Y. C. Lin, Dr. K. K. Sriram, Dr. Yii-Lih Lin, Professor John T. Lis, and Professor David Newquist for helpful discussions and insightful suggestions. The HeLa cell Histone-GFP expressing cell line was provided by Professor Paul Soloway and extracted by Harvey C. Tian. This research was supported by the National Institutes of Health Grant (No. R01 DA030329-03). This work was performed in part at the Cornell Nanofabrication Facility (CNF) of NNIN supported by NSF (No. ECCS-0335765).
References
- 1. Orlando L. and Willerslev E., Science 345(6196), 511–512 (2014). 10.1126/science.1256515 [DOI] [PubMed] [Google Scholar]
- 2. Barski A., Cuddapah S., Cui K. R., Roh T. Y., Schones D. E., Wang Z. B., Wei G., Chepelev I., and Zhao K. J., Cell 129(4), 823–837 (2007). 10.1016/j.cell.2007.05.009 [DOI] [PubMed] [Google Scholar]
- 3. Bernstein B. E., Meissner A., and Lander E. S., Cell 128(4), 669–681 (2007). 10.1016/j.cell.2007.01.033 [DOI] [PubMed] [Google Scholar]
- 4. Matsuoka T., Kim B. C., Moraes C., Han M., and Takayama S., Biomicrofluidics 7(4), 041301 (2013). 10.1063/1.4816835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy-Sakin M., Grunwald A., Kim S., Gassman N. R., Gottfried A., Antelman J., Kim Y., Ho S. O., Samuel R., Michalet X., Lin R. R., Dertinger T., Kim A. S., Chung S., Colyer R. A., Weinhold E., Weiss S., and Ebenstein Y., ACS Nano 8(1), 14–26 (2014). 10.1021/nn4050694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aguilar C. A. and Craighead H. G., Nat. Nanotechnol. 8(10), 709–718 (2013). 10.1038/nnano.2013.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dorfman K. D., King S. B., Olson D. W., Thomas J. D. P., and Tree D. R., Chem. Rev. 113(4), 2584–2667 (2013). 10.1021/cr3002142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lavelle C., Victor J. M., and Zlatanova J., Int. J. Mol. Sci. 11(4), 1557–1579 (2010). 10.3390/ijms11041557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bintu L., Ishibashi T., Dangkulwanich M., Wu Y. Y., Lubkowska L., Kashlev M., and Bustamante C., Cell 151(4), 738–749 (2012). 10.1016/j.cell.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brower-Toland B. D., Smith C. L., Yeh R. C., Lis J. T., Peterson C. L., and Wang M. D., Proc. Natl. Acad. Sci. U.S.A. 99(4), 1960–1965 (2002). 10.1073/pnas.022638399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cui Y. and Bustamante C., Proc. Natl. Acad. Sci. U.S.A. 97(1), 127–132 (2000). 10.1073/pnas.97.1.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soltani M., Lin J., Forties R. A., Inman J. T., Saraf S. N., Fulbright R. M., Lipson M., and Wang M. D., Nat. Nanotechnol. 9(6), 448–452 (2014). 10.1038/nnano.2014.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heller I., Sitters G., Broekmans O. D., Farge G., Menges C., Wende W., Hell S. W., Peterman E. J. G., and Wuite G. J. L., Nat. Methods 10(9), 910–U132 (2013). 10.1038/nmeth.2599 [DOI] [PubMed] [Google Scholar]
- 14. Kruithof M., Chien F. T., Routh A., Logie C., Rhodes D., and van Noort J., Nat. Struct. Mol. Biol. 16(5), 534–540 (2009). 10.1038/nsmb.1590 [DOI] [PubMed] [Google Scholar]
- 15. Bancaud A., Silva N. C. E., Barbi M., Wagner G., Allemand J. F., Mozziconacci J., Lavelle C., Croquette V., Victor J. M., Prunell A., and Viovy J. L., Nat. Struct. Mol. Biol. 13(5), 444–450 (2006). 10.1038/nsmb1087 [DOI] [PubMed] [Google Scholar]
- 16. Zhu R., Howorka S., Proll J., Kienberger F., Preiner J., Hesse J., Ebner A., Pastushenko V. P., Gruber H. J., and Hinterdorfer P., Nat. Nano 5(11), 788–791 (2010). 10.1038/nnano.2010.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bensimon A., Simon A., Chiffaudel A., Croquette V., Heslot F., and Bensimon D., Science 265(5181), 2096–2098 (1994). 10.1126/science.7522347 [DOI] [PubMed] [Google Scholar]
- 18. Greene E. C., Wind S., Fazio T., Gorman J., and Visnapuu M. L., Method Enzymol. 472, 293–315 (2010). 10.1016/S0076-6879(10)72006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cerf A., Cipriany B. R., Benitez J. J., and Craighead H. G., Anal. Chem. 83(21), 8073–8077 (2011). 10.1021/ac202506j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perkins T. T., Smith D. E., Larson R. G., and Chu S., Science 268(5207), 83–87 (1995). 10.1126/science.7701345 [DOI] [PubMed] [Google Scholar]
- 21. Oana H., Nishikawa K., Matsuhara H., Yamamoto A., Yamamoto T. G., Haraguchi T., Hiraoka Y., and Washizu M., Lab Chip 14(4), 696–704 (2014). 10.1039/C3LC51111A [DOI] [PubMed] [Google Scholar]
- 22. Ladoux B., Quivy J. P., Doyle P., du Roure O., Almouzni G., and Viovy J. L., Proc. Natl. Acad. Sci. U.S.A. 97(26), 14251–14256 (2000). 10.1073/pnas.250471597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Finkelstein I. J., Visnapuu M. L., and Greene E. C., Nature 468(7326), 983–987 (2010). 10.1038/nature09561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tegenfeldt J. O., Bakajin O., Chou C. F., Chan S. S., Austin R., Fann W., Liou L., Chan E., Duke T., and Cox E. C., Phys. Rev. Lett. 86(7), 1378–1381 (2001). 10.1103/PhysRevLett.86.1378 [DOI] [PubMed] [Google Scholar]
- 25. Lam E. T., Hastie A., Lin C., Ehrlich D., Das S. K., Austin M. D., Deshpande P., Cao H., Nagarajan N., Xiao M., and Kwok P. Y., Nat. Biotechnol. 30(8), 771–776 (2012). 10.1038/nbt.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sheats J., Reifenberger J. G., Cao H., and Dorfman K. D., Biomicrofluidics 9(6), 064119 (2015). 10.1063/1.4938732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuoka T., Kim B. C., Huang J. X., Douville N. J., Thouless M. D., and Takayama S., Nano Lett. 12(12), 6480–6484 (2012). 10.1021/nl304063f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lim S. F., Karpusenko A., Sakon J. J., Hook J. A., Lamar T. A., and Riehn R., Biomicrofluidics 5(3), 034106 (2011). 10.1063/1.3613671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reisner W., Morton K. J., Riehn R., Wang Y. M., Yu Z. N., Rosen M., Sturm J. C., Chou S. Y., Frey E., and Austin R. H., Phys. Rev. Lett. 94(19), 196101 (2005). 10.1103/PhysRevLett.94.196101 [DOI] [PubMed] [Google Scholar]
- 30. Freitag C., Noble C., Fritzsche J., Persson F., Reiter-Schad M., Nilsson A. N., Graneli A., Ambjornsson T., Mir K. U., and Tegenfeldt J. O., Biomicrofluidics 9(4), 044114 (2015). 10.1063/1.4923262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Michaeli Y. and Ebenstein Y., Nat. Biotechnol. 30(8), 762–763 (2012). 10.1038/nbt.2324 [DOI] [PubMed] [Google Scholar]
- 32. Tegenfeldt J. O., Prinz C., Cao H., Chou S., Reisner W. W., Riehn R., Wang Y. M., Cox E. C., Sturm J. C., Silberzan P., and Austin R. H., Proc. Natl. Acad. Sci. U.S.A. 101(30), 10979–10983 (2004). 10.1073/pnas.0403849101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yeh J.-W., Taloni A., Chen Y.-L., and Chou C.-F., Nano Lett. 12(3), 1597–1602 (2012). 10.1021/nl2045292 [DOI] [PubMed] [Google Scholar]
- 34. Jo K., Dhingra D. M., Odijk T., de Pablo J. J., Graham M. D., Runnheim R., Forrest D., and Schwartz D. C., Proc. Natl. Acad. Sci. U.S.A. 104(8), 2673–2678 (2007). 10.1073/pnas.0611151104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim Y., Kim K. S., Kounovsky K. L., Chang R., Jung G. Y., dePablo J. J., Jo K., and Schwartz D. C., Lab Chip 11, 1721–1729 (2011). 10.1039/c0lc00680g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeh J.-W. and Szeto K., ACS Macro Lett. 5, 1114–1118 (2016). 10.1021/acsmacrolett.6b00639 [DOI] [PubMed] [Google Scholar]
- 37. Sriram K. K., Yeh J. W., Lin Y. L., Chang Y. R., and Chou C. F., Nucl. Acids Res. 42(10), e85 (2014). 10.1093/nar/gku254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marie R., Pedersen J. N., Bauer D. L. V., Rasmussen K. H., Yusuf M., Volpi E., Flyvbjerg H., Kristensen A., and Mir K. U., Proc. Natl. Acad. Sci. U.S.A. 110(13), 4893–4898 (2013). 10.1073/pnas.1214570110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sriram K. K., Chang C. L., Kumar U. R., and Chou C. F., Biomicrofluidics 8(5), 052102 (2014). 10.1063/1.4892515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cerf A., Tian H. C., and Craighead H. G., ACS Nano 6(9), 7928–7934 (2012). 10.1021/nn3023624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ebenstein Y., Gassman N., Kim S., Antelman J., Kim Y., Ho S., Samuel R., Michalet X., and Weiss S., Nano Lett. 9(4), 1598–1603 (2009). 10.1021/nl803820b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Streng D. E., Lim S. F., Pan J. H., Karpusenka A., and Riehn R., Lab Chip 9(19), 2772–2774 (2009). 10.1039/b909217j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.See http://www.activemotif.com/catalog/details/39143/histone-h3-dimethyl-lys79-antibody-pab for detail information.
- 44. Yeh J.-W., Taloni A., Sriram K. K., Chen Y.-L., and Chou C.-F., e-print arXiv:1502.05115.
- 45. Luger K., Mader A. W., Richmond R. K., Sargent D. F., and Richmond T. J., Nature 389, 251–260 (1997). 10.1038/38444 [DOI] [PubMed] [Google Scholar]
- 46. Keyser U. F., Koeleman B. N., Van Dorp S., Krapf D., Smeets R. M. M., Lemay S. G., Dekker N. H., and Dekker C., Nat. Phys. 2(7), 473–477 (2006). 10.1038/nphys344 [DOI] [Google Scholar]
- 47. Marko J. F. and Siggia E. D., Macromolecules 28(26), 8759–8770 (1995). 10.1021/ma00130a008 [DOI] [Google Scholar]
- 48. Marko J. F. and Siggia E. D., Biophys. J. 73(4), 2173–2178 (1997). 10.1016/S0006-3495(97)78248-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Collepardo-Guevara R. and Schlick T., Nucl. Acids Res. 40(18), 8803–8817 (2012). 10.1093/nar/gks600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fierz B. and Muir T. W., Nat. Chem. Biol. 8(5), 417–427 (2012). 10.1038/nchembio.938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilson C. B., Rowell E., and Sekimata M., Nat. Rev. Immunol. 9(2), 91–105 (2009). 10.1038/nri2487 [DOI] [PubMed] [Google Scholar]
- 52. Fazio T., Visnapuu M. L., Wind S., and Greene E. C., Langmuir 24(18), 10524–10531 (2008). 10.1021/la801762h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim B. C., Moraes C., Huang J. X., Matsuoka T., Thouless M. D., and Takayama S., Small 10(19), 4020–4029 (2014). 10.1002/smll.201400147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reisner W., Larsen N. B., Silahtaroglu A., Kristensen A., Tommerup N., Tegenfeldt J. O., and Flyvbjerg H., Proc. Natl. Acad. Sci. U.S.A. 107(30), 13294–13299 (2010). 10.1073/pnas.1007081107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Benitez J. J., Topolancik J., Tian H. C., Wallin C. B., Latulippe D. R., Szeto K., Murphy P. J., Cipriany B. R., Levy S. L., Soloway P. D., and Craighead H. G., Lab Chip 12(22), 4848–4854 (2012). 10.1039/c2lc40955k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Neely R. K., Dedecker P., Hotta J. I., Urbanaviciute G., Klimasauskas S., and Hofkens J., Chem. Sci. 1(4), 453–460 (2010). 10.1039/c0sc00277a [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See supplementary material for more statistical results of the studied native chromatin samples and Movie S1 demonstrating the single GFP-fluorescent chromatin stretching at small fields (7.5 V/cm).