Abstract
Background
Diabetes is the leading cause of end-stage renal disease (ESRD) and a significant contributor to mortality in the general population. We examined the associations of hemoglobin A1c (HbA1c) levels with ESRD and death in a population with diabetes and chronic kidney disease (CKD).
Study Design
Cohort study.
Setting & Participants
6,165 patients with diabetes (treated with oral hypoglycemic agents and/or insulin) and CKD stages 1 to 5 at a large health care system.
Predictor
HbA1c level (examined as a categorical and continuous measure).
Outcomes
All-cause and cause-specific mortality ascertained from the Ohio Department of Health mortality files and ESRD ascertained from the US Renal Data System.
Results
During a median 2.3 years of follow-up, 957 patients died (887 pre-ESRD deaths) and 205 patients reached ESRD. In a Cox proportional hazards model, after multivariable adjustment including for kidney function, HbA1c level < 6% was associated with higher risk for death when compared with HbA1c levels of 6% to 6.9% (HR, 1.23; 95% CI, 1.01–1.50). Similarly, HbA1c level ≥ 9% was associated with higher risk for all-cause death (HR, 1.34; 95% CI, 1.06–1.69). In competing-risk models, baseline HbA1c level was not associated with ESRD. For cause-specific mortality, diabetes accounted for >12% of deaths overall and >19% of deaths among those with HbA1c levels > 9%.
Limitations
Small proportion of participants with advanced kidney disease; single-center population.
Conclusions
In this cohort of patients with CKD with diabetes, HbA1c levels < 6% and ≥9% were associated with higher risk for death. HbA1c levels were not associated with ESRD in this specific CKD population. Diabetes-related deaths increased with higher HbA1c levels.
INDEX WORDS: Glycated hemoglobin, HbA1c, end stage renal disease (ESRD), diabetes mellitus, diabetes control, incident ESRD, chronic kidney disease (CKD), death and kidney disease, diabetic nephropathy, mortality
Diabetes is considered as a coronary artery disease equivalent, and the presence of diabetes and chronic kidney disease (CKD) poses the highest risk for death compared to diabetes or CKD alone.1,2 The prevalence of diabetic kidney disease is also increasing, and diabetic nephropathy is the leading cause of end-stage renal disease (ESRD).1,3,4 What constitutes an ideal glycated hemoglobin (hemoglobin A1c [HbA1c]) level has been a matter of debate, and some clinical trials in the general population have reported that intensive glycemic control in diabetic patients is associated with adverse outcomes.5–8 Based on available evidence, the American Diabetes Association has recommended targeting an HbA1c level < 7% for most nonpregnant adults and <8% for those at risk for hypoglycemia, extensive comorbid conditions, and long-standing diabetes.9
Few studies have evaluated associations between HbA1c levels and clinical outcomes in those with CKD. Shurraw et al10 reported that HbA1c levels > 9% were associated with worse clinical outcomes, such as faster kidney disease progression, more cardiovascular events, and increased mortality, among patients with non–dialysis-dependent CKD. In addition, lower HbA1c levels (<6.5%) were associated with higher hazards of death. Findings from a cohort of Taiwanese adults with type 2 diabetes showed that HbA1c levels > 7.0% were associated with increased risk for ESRD compared with HbA1c levels of 6% to 7%, but HbA1c levels < 6.0% were also associated with increased risk for ESRD.11 However, a secondary analysis of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial reported that tighter glycemic control in patients with CKD was associated with a significant increase in cardiovascular and all-cause mortality.12 Although secondary analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation) trial reported a reduced risk for ESRD with intense glucose control, no significant effects of intensive glycemic control on ESRD were noted in other studies.13,14 Patients with CKD are generally at higher risk for hypoglycemia, making a case for avoiding intense glycemic control in this population.15 Hence, given these inconsistent findings in the literature, we examined associations between HbA1c levels and ESRD and death in a cohort of patients with diabetes and non–dialysis-dependent CKD receiving care in a large US health care system.
METHODS
Overview
We conducted an analysis using a pre-existing electronic medical record (EMR)-based CKD registry. The development and validation of this registry at Cleveland Clinic has been described in detail previously.16 This study and the CKD registry were approved by the Cleveland Clinic Institutional Review Board (IRB #09-015). Informed consent was not obtained because these data were developed using electronic medical records and Cleveland Clinic has an opt-in policy for collecting data for research purposes using electronic medical records.
Study Population
Patients who were residents of Ohio and had (1) at least 1 outpatient encounter with a Cleveland Clinic health care provider and either 2 estimated glomerular filtration rate (eGFR) values < 60 mL/min/1.73 m2 more than 90 days apart or International Classification of Diseases, Ninth Revision codes for various kidney diseases, (2) diabetes and were using oral hypoglycemic agents and/or insulin, and (3) HbA1c measured in the year prior to the second eGFR < 60 mL/min/1.73 m2 or a CKD diagnosis were included (Fig S1, available as online supplementary material). Patients younger than 18 years and those who already had ESRD diagnosed (ie, dialysis dependent or having received a kidney transplant) were excluded. Patients who met the inclusion/exclusion criteria from January 1, 2005, to September 15, 2009, were included in this analysis.
Definitions and Outcome Measures
Demographics, Comorbid Conditions, and Laboratory Parameters
Demographic details were extracted from the EMR. Diabetes mellitus, hypertension, coronary artery disease, and other comorbid conditions were defined using prespecified criteria and validated. Relevant outpatient laboratory values were obtained from the EMR. Medication details were obtained from the EMR and were validated in the past. The automated chemistry laboratory at Cleveland Clinic runs HbA1c testing on a Roche Integra 800 platform using a method called TinaQuant Gen2, an immune-based turbidimetric assay. It measures both hemoglobin concentration and HbA1c concentration, then calculates the glycated hemoglobin percentage. The laboratory follows the National Glycohemoglobin Standardization Program guidelines for standardizing these measures. Baseline HbA1c measurements in the year prior to the second eGFR < 60 mL/min/1.73 m2 or diagnosis of CKD were used in this study, and when multiple measurements were available for a patient, the result closest to the date of diagnosis of CKD was selected for analytical purposes. For the time-dependent repeated-measures analysis, we included the baseline HbA1c value and the first HbA1c value measured each month during study follow-up. We used carry-forward values to fill in data for months when HbA1c data were not available.
Kidney Function Measures
All creatinine measurements were performed by the modified kinetic Jaffé reaction using an Hitachi D 2400 Modular Chemistry Analyzer thereafter (Roche Diagnostics) at the Cleveland Clinic laboratory. In patients who had at least 2 serum creatinine levels measured 90 days apart during January 2005 to September 15, 2009, at the Cleveland Clinic health system,17 the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation was used to calculate eGFR. Urinary protein studies were not available for the entire study population. Therefore, to be comprehensive and reflect clinical practice, patients who had urine dipstick measurements, urine albumin-creatinine ratio, urine protein-creatinine ratio, and 24-hour urine studies were included to assess whether they had proteinuria. The following cutoffs were considered in determining whether someone had proteinuria: presence of proteinuria ≥ 1+ in dipstick studies, >30 mg/g in those who had urine albumin-creatinine ratio and urine protein-creatinine ratio studies, and proteinuria with protein excretion > 30 mg in 24-hour studies. Urinalysis chemstrip is performed on the iRICELL 3000 using iChem VELOCITY test strips (both Beckman Coulter) or on the AX-4280 using AUTION 9EB test strips (both ARKRAY).
Urine albumin was measured by immunoturbidimetric assay with antigen excess check, and urine creatinine was measured using a multistep enzymatic procedure that produces a quinone imine chromogen on the Roche Modular platform at the Cleveland Clinic laboratory.
Outcome Measures
The primary outcomes of interest were all-cause mortality and ESRD. ESRD was defined as the need for renal replacement therapy: dialysis or transplantation. Mortality details were ascertained from the Ohio Department of Health mortality files, which also provided cause-specific mortality data18; deaths from the Cleveland Clinic EMR were also captured. Incident treated ESRD was ascertained from linkage of our registry with the US Renal Data System (USRDS). Patients were followed up from their date of inclusion in the registry until September 15, 2009.
Statistical Analysis
Baseline characteristics among strata of HbA1c levels (<6, 6%–6.9%, 7%–7.9%, 8%–8.9%, and ≥9%) were compared using χ2 and analysis of variance tests for categorical and continuous variables, respectively. These categories were chosen because they are used in clinical practice and other studies. To evaluate whether unadjusted survival and ESRD among persons with CKD was associated with baseline HbA1c levels, we fitted cumulative incidence functions that adjusted for competing risks using the Fine and Gray method with date of second eGFR < 60 mL/min/1.73 m2 or date of CKD diagnosis as the time of origin. We tabulated causes of death for all deaths (both before and after ESRD).
We evaluated the independent relationship between various baseline HbA1c categories and pre-ESRD mortality using a Cox proportional hazards regression model with HbA1c levels of 6% to 6.9% as the reference group. We also used Fine and Gray’s extension of the Cox regression that models the cumulative incidence to fit competing-risk regression models and evaluate the association between baseline HbA1c levels and ESRD.19 We adjusted for the following covariates in the models: age; race; sex; malignancy; coronary artery disease; congestive heart failure; cerebrovascular disease; peripheral vascular disease; use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, statins, and β-blockers; albumin level; hemoglobin level; body mass index group (underweight, normal, overweight, and obese); smoking; eGFR; and albuminuria. Linearity assumptions for continuous covariates were relaxed as needed by using splines at the 10th, 50th, and 90th percentiles. We tested 2-way interactions between baseline HbA1c level and the following prespecified covariates in the adjusted ESRD and mortality models: age, sex, race, coronary artery disease, and eGFR. We also evaluated the association between baseline continuous HbA1c levels and pre-ESRD mortality and ESRD using splines at the 10th, 50th, and 90th percentiles of HbA1c and plotted continuous HbA1c versus the log hazard of mortality. To incorporate HbA1c results obtained after inception, we fitted a Cox proportional hazards model of mortality with time-dependent repeated measures of HbA1c using the categories defined. Percentages of missing information for individual variables were as follows: body mass index, 3%; serum albumin, 15%; hemoglobin, 21%; and proteinuria, 27%. Because complete case analyses are prone to yielding biased results, we used multiple imputation (SAS proc MI; version 9.4, SAS Institute Inc) with the Markov chain Monte Carlo method and a single chain to impute 5 data sets with complete data. Cox models were performed on each of the 5 imputed data sets, and parameter estimates were combined using SAS MIanalyze. We conducted several sensitivity analyses on the pre-ESRD mortality and ESRD models by excluding: (1) those with malignancy, (2) those with type 1 diabetes, and (3) events in the first 6 months of follow-up.
All data analyses were conducted using Linux SAS version 9.4 and R statistical software, version 3.0.1 (The R Foundation for Statistical Computing) with the rms package. The cmprsk package was used for competing-risk analysis in R.
RESULTS
Baseline Patient Characteristics
We included 6,165 patients with non–dialysis-dependent CKD in this analysis (Fig S1). Mean age of the study population was 70.1 ± 11.8 (standard deviation) years, with 46.7% men and 20.7% blacks. Mean body mass index of the study cohort was 32.3 ± 7.2 kg/m2. Prevalences of hypertension, malignancy, and coronary artery disease were 96.3%, 17.0%, and 30.2%, respectively. Mean eGFR was 50.5 ± 16.6 mL/min/1.73 m2, with 58.8% in stage 3a, 24.5% in stage 3b, and 7.4% in stage 4 CKD. Table 1 outlines further details of the study population overall and by HbA1c categories.
Table 1.
Patient Characteristics Across Categories of Baseline HbA1c Levels
| HbA1c
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Factor | N | All Patients | <6% (n = 803) |
6%–6.9% (n = 2,340) |
7%–7.9% (n = 1,596) |
8%–8.9% (n = 723) |
≥9% (n = 703) |
P |
| Age, y | 6,165 | 70.1 ± 11.8 | 70.2 ± 12.0 | 72.5 ± 10.1 | 70.6 ± 11.4 | 68.6 ± 12.2 | 62.7 ± 13.8 | <0.001a |
| Male sex | 6,165 | 46.7 | 46.0 | 46.3 | 48.0 | 46.6 | 46.4 | 0.8b |
| African American | 6,165 | 20.7 | 17.2 | 17.9 | 21.1 | 22.7 | 31.6 | <0.001b |
| Smoking | 5,735 | 8.6 | 7.7 | 7.3 | 8.3 | 9.8 | 13.4 | <0.001b |
| BMI, kg/m2 | 5,979 | 32.3 ± 7.2 | 32.1 ± 7.9 | 31.8 ± 6.7 | 32.4 ± 7.2 | 32.8 ± 7.3 | 33.4 ± 8.0 | <0.001a |
| BMI category | 6,165 | <0.001b | ||||||
| < 18.5 kg/m2 | 0.37 | 0.12 | 0.43 | 0.25 | 0.55 | 0.57 | ||
| 18.5–24.9 kg/m2 | 12.6 | 14.7 | 13.7 | 11.0 | 10.8 | 12.5 | ||
| 25–29.9 kg/m2 | 28.9 | 30.3 | 30.1 | 30.2 | 26.6 | 22.5 | ||
| 30–34.9 kg/m2 | 25.4 | 22.5 | 25.6 | 26.1 | 25.3 | 26.3 | ||
| 35–39.9 kg/m2 | 16.2 | 14.4 | 15.7 | 15.7 | 19.2 | 17.9 | ||
| ≥40 kg/m2 | 13.5 | 14.3 | 11.8 | 13.4 | 14.5 | 17.9 | ||
| Missing | 3.0 | 3.6 | 2.8 | 3.4 | 3.0 | 2.3 | ||
| eGFR, mL/min/1.73 m2 | 6,165 | 50.5 ± 16.6 | 47.7 ± 14.3 | 50.0 ± 13.8 | 50.7 ± 16.6 | 51.2 ± 18.4 | 54.0 ± 23.6 | <0.001a |
| eGFR category | 6,165 | <0.001b | ||||||
| ≥90 mL/min/1.73 m2 | 4.0 | 1.6 | 2.3 | 4.2 | 5.0 | 10.7 | ||
| 60–89 mL/min/1.73 m2 | 4.6 | 2.7 | 4.0 | 4.6 | 6.5 | 7.1 | ||
| 45–59 mL/min/1.73 m2 | 58.8 | 58.7 | 62.6 | 58.8 | 55.9 | 48.9 | ||
| 30–44 mL/min/1.73 m2 | 24.5 | 28.1 | 24.0 | 24.7 | 23.0 | 23.2 | ||
| 15–29 mL/min/1.73 m2 | 7.4 | 7.3 | 7.0 | 7.0 | 9.0 | 8.3 | ||
| <15 mL/min/1.73 m2 | 0.71 | 1.5 | 0.17 | 0.63 | 0.69 | 1.8 | ||
| Hypertension | 6,165 | 96.3 | 96.0 | 96.8 | 96.6 | 95.7 | 94.7 | 0.09b |
| CAD | 6,165 | 30.2 | 28.0 | 29.2 | 33.3 | 32.4 | 26.6 | 0.003b |
| CHF | 6,165 | 10.9 | 12.1 | 9.7 | 10.5 | 12.0 | 13.8 | 0.02b |
| CBVD | 6,165 | 11.2 | 10.7 | 11.6 | 12.2 | 10.7 | 8.8 | 0.2b |
| PVD | 6,165 | 4.9 | 3.5 | 4.3 | 5.3 | 6.8 | 5.7 | 0.02b |
| Hyperlipidemia | 6,165 | 91.7 | 87.0 | 91.9 | 92.7 | 93.6 | 92.2 | <0.001b |
| Malignancy | 6,165 | 17.0 | 21.5 | 19.4 | 15.9 | 11.6 | 12.1 | <0.001b |
| Statin use | 6,165 | 79.1 | 70.1 | 79.7 | 81.5 | 83.1 | 78.1 | <0.001b |
| ACEi/ARB use | 6,165 | 89.0 | 86.3 | 88.2 | 89.5 | 90.9 | 91.7 | 0.003b |
| β-Blocker use | 6,165 | 62.6 | 64.9 | 61.4 | 63.2 | 64.7 | 60.2 | 0.2b |
| Oral hypoglycemic use | 6,165 | 87.8 | 89.4 | 92.0 | 87.3 | 83.5 | 77.4 | <0.001b |
| Insulin use | 6,165 | 41.0 | 27.3 | 25.9 | 44.7 | 63.2 | 75.5 | <0.001b |
| Albumin, g/dl | 5,214 | 4.1 ± 0.42 | 4.1 ± 0.47 | 4.1 ± 0.40 | 4.1 ± 0.40 | 4.1 ± 0.43 | 4.0 ± 0.43 | <0.001a |
| Hemoglobin, mg/dl | 4,856 | 12.6 ± 1.7 | 12.3 ± 1.9 | 12.6 ± 1.6 | 12.6 ± 1.7 | 12.7 ± 1.7 | 12.7 ± 1.7 | <0.001a |
| Proteinuria | 4,498 | 39.4 | 31.2 | 32.8 | 39.1 | 48.2 | 60.9 | <0.001b |
Note: Values for categorical variables are given as column percentage; for continuous variables, as mean ± standard deviation.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CAD, coronary artery disease; CBVD, cerebrovascular disease; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; PVD, peripheral vascular disease.
P by analysis of variance.
P by Pearson χ2 test.
HbA1c, ESRD, and Mortality
Categorical Analysis
During a median follow-up of 2.3 years, 957 patients died (887 pre-ESRD deaths) and 205 patients reached ESRD. Unadjusted competing-risk analyses (Fig 1) showed differences in the incidence of ESRD across different HbA1c levels (P < 0.001) and also differences in all-cause mortality (P < 0.05; Fig 1). In multivariable Cox proportional hazards regression, HbA1c level was independently associated with pre-ESRD mortality. HbA1c level < 6% was associated with higher risk for death when compared with HbA1c levels of 6% to 6.9%, as was an HbA1c level ≥ 9% (Table 2). All 2-way interaction terms between HbA1c level and age, sex, race, coronary artery disease, and eGFR were nonsignificant. In the adjusted competing-risk model of ESRD, baseline HbA1c levels < 6% and ≥9% were not significantly different from HbA1c levels of 6% to 6.9% (Table 3). The interaction between HbA1c level and eGFR was statistically significant (P < 0.01), suggesting that HbA1c level < 6% was associated with lower risk among those with lower eGFRs, and HbA1c level ≥ 9% was associated with higher risk only among those with higher eGFRs (Table S1). Interactions between HbA1c level and age, sex, race, and coronary artery disease were not statistically significant. In the multivariable mortality model with time-dependent repeated measures of HbA1c, results were similar to those in the primary analysis (Table S2).
Figure 1.
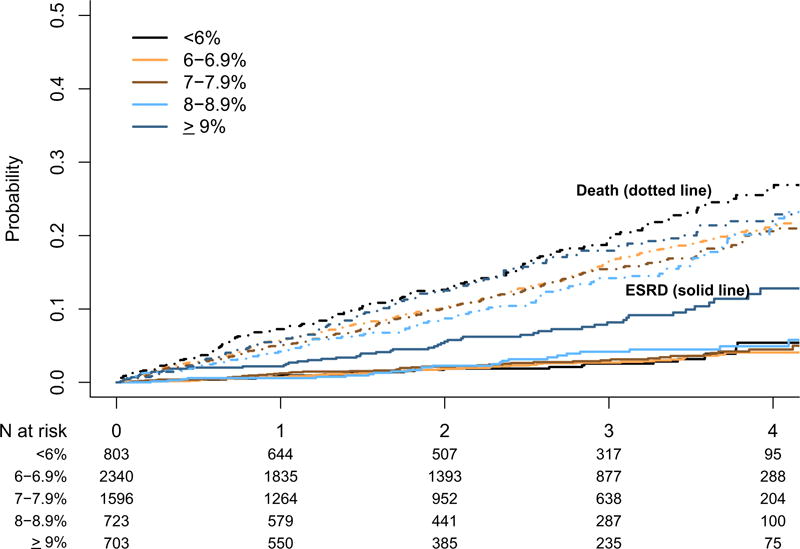
Cumulative incidence curves for end-stage renal disease (ESRD) and death among patients with chronic kidney disease across hemoglobin A1c categories using competing risks.
Table 2.
Associations Between HbA1c and Pre-ESRD Mortality in CKD
| HbA1c < 6% | HbA1c 7%–7.9% | HbA1c 8%–8.9% | HbA1c > 9% | |
|---|---|---|---|---|
| Unadjusted | 1.27 (1.04–1.54) | 0.96 (0.81–1.14) | 0.92 (0.73–1.15) | 1.15 (0.92–1.43) |
| Adjusted for | ||||
| 1) Age, race, sex | 1.35 (1.11–1.64) | 1.00 (0.85–1.19) | 1.01 (0.80–1.27) | 1.60 (1.27–2.00) |
| 2) 1 + comorbid conditions, BMI group, albumin, hemoglobin, smoking | 1.28 (1.05–1.56) | 0.95 (0.80–1.13) | 0.99 (0.78–1.25) | 1.41 (1.12–1.78) |
| 3) 2 + ACEi/ARB, statin, β-blocker, eGFR, proteinuria | 1.23 (1.01–1.50) | 0.94 (0.79–1.12) | 0.96 (0.76–1.22) | 1.34 (1.06–1.69) |
Note: Values are given as hazard ratio (95% confidence interval). Estimates combined using MIanalyze on the 5 imputed data sets. Reference category is 6% to 6.9%.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c.
Table 3.
Associations of HbA1c With ESRD: Competing-Risk Model With Death as Competing Risk
| HbA1c < 6% | HbA1c 7%–7.9% | HbA1c 8%–8.9% | HbA1c ≥ 9% | |
|---|---|---|---|---|
| Unadjusted | 1.10 (0.68–1.78) | 1.18 (0.80–1.73) | 1.31 (0.82–2.11) | 3.15 (2.17–4.57) |
| Adjusted for | ||||
| 1) Age, race, sex | 0.98 (0.61–1.59) | 1.05 (0.73–1.55) | 1.07 (0.66–1.73) | 1.95 (1.32–2.87) |
| 2) 1 + comorbid conditions, BMI group, albumin, hemoglobin, smoking | 0.87 (0.53–1.41) | 0.99 (0.67–1.47) | 1.02 (0.62–1.68) | 1.76 (1.15–2.67) |
| 3) 2 + ACEi/ARB, statin, β-blocker, eGFR, proteinuria | 0.58 (0.32–1.02) | 0.92 (0.62–1.37) | 0.65 (0.38–1.12) | 1.35 (0.88–2.09) |
Note: Values given as subdistribution hazard ratio (95% confidence interval). Estimates combined using MIanalyze on the 5 imputed data sets. Reference category is 6% to 6.9%.
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HbA1c, hemoglobin A1c.
Continuous Analysis (using baseline HbA1c data)
When considered as a continuous variable, HbA1c level was significantly associated with pre-ESRD mortality (Fig 2). The relationship was nonlinear, with very low and high HbA1c levels having the higher risk for mortality and risk being lowest at HbA1c levels of about 7% to 8%. In the analysis of continuous HbA1c versus ESRD, neither the main effect nor any of the splines were significantly associated with the outcome.
Figure 2.
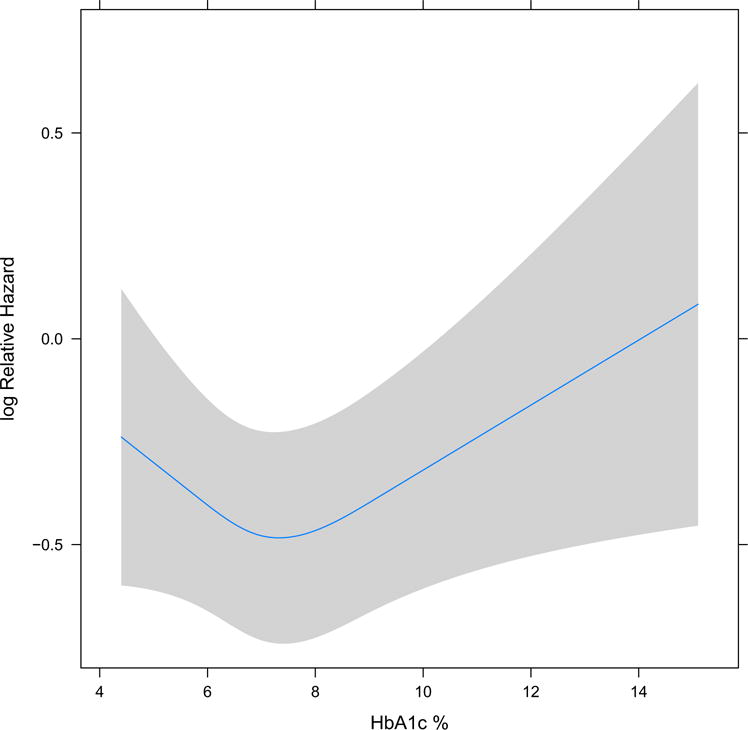
Associations of hemoglobin A1c (HbA1c) with all-cause mortality (baseline HbA1c considered as continuous measure with splines at the 10th, 50th, and 90th percentile: 5.8, 6.9, and 9.1 respectively; main effect P = 0.07, nonlinear P = 0.04).
Causes of Death
Cause-of-death details were available from the Ohio Department of Health mortality data for 942 patients. Table 4 shows causes of death overall and by HbA1c categories.
Table 4.
Cause of Death Overall and by HbA1c Level for Patients in Whom Cause-of-Death Details Available
| HbA1c
|
||||||
|---|---|---|---|---|---|---|
| Factor | Overall (N = 942) |
<6% (n = 153) |
6%–6.9% (n = 346) |
7%–7.9% (n = 225) |
8%–8.9% (n = 99) |
≥9% (n = 119) |
| Cardiovascular diseases | 38.6 | 38.6 | 38.7 | 34.7 | 47.5 | 38.7 |
| Ischemic heart disease | 22.5 | 18.3 | 23.4 | 18.2 | 31.3 | 26.1 |
| Heart failure | 1.9 | 2.6 | 2.0 | 1.8 | 0.0 | 2.5 |
| Cerebrovascular disease | 3.3 | 3.9 | 2.6 | 4.9 | 2.0 | 2.5 |
| All other cardiovascular disease | 10.9 | 13.7 | 10.7 | 9.8 | 14.1 | 7.6 |
| Malignant neoplasms | 19.5 | 25.5 | 19.1 | 16.0 | 23.2 | 16.8 |
| All other diseases | 40.4 | 34.0 | 39.9 | 48.9 | 29.3 | 43.7 |
| Diabetes mellitus | 12.2 | 5.9 | 9.8 | 17.3 | 10.1 | 19.3 |
| Chronic lower respiratory diseases | 3.7 | 3.3 | 3.8 | 4.9 | 4.0 | 1.7 |
| Nephritis, nephrotic syndrome, and nephrosis | 2.2 | 2.0 | 2.3 | 3.1 | 0.0 | 2.5 |
| Septicemia | 2.2 | 1.3 | 2.9 | 2.2 | 2.0 | 1.7 |
| Influenza and pneumonia | 2.2 | 2.6 | 1.4 | 2.7 | 3.0 | 2.5 |
| Chronic liver disease and cirrhosis | 1.3 | 3.3 | 0.87 | 0.44 | 0.0 | 1.3 |
| Alzheimer disease | 1.3 | 0.65 | 1.7 | 1.8 | 1.0 | 0.0 |
| Pneumonitis due to solids and liquids | 0.96 | 0.0 | 1.2 | 1.8 | 0.0 | 0.84 |
| Parkinson disease | 0.42 | 0.0 | 0.58 | 0.89 | 0.0 | 0.0 |
| All other diseases | 13.9 | 15.0 | 15.3 | 13.8 | 9.1 | 12.6 |
| Non–disease-related deaths | 1.4 | 2.0 | 2.3 | 0.44 | 0.0 | 0.84 |
Note: Values are given as column percentages.
Abbreviation: HbA1c, hemoglobin A1c.
Sensitivity Analyses
Sensitivity analysis excluding those with malignancy (n = 1,048) yielded similar results to the primary analysis and is shown in Table S3. Sensitivity analysis excluding those with type 1 diabetes (n = 575) yielded qualitatively similar findings as in the primary analyses, with HbA1c levels < 6% and ≥9% having mortality hazard ratios (HRs) of 1.28 (95% confidence interval [CI], 1.002–1.64) and 1.43 (95% CI, 1.08–1.88), respectively, when compared with HbA1c levels of 6% to 6.9%. In the ESRD model excluding type 1 diabetes, other HbA1c levels were not significantly different from HbA1c levels of 6% to 6.9%. Sensitivity analysis excluding events during the first 6 months of follow-up yielded similar results to those in the primary analysis (Table S3).
DISCUSSION
In this observational analysis of a large cohort of patients with CKD receiving treatment for diabetes with either oral hypoglycemic medications and/or insulin, we noted a U-shaped association in that HbA1c levels < 6% and ≥9% were independently associated with increased mortality compared with patients with HbA1c levels of 6% to 6.9%. By contrast, HbA1c levels were not independently associated with the incidence of ESRD in this CKD population. However, associations between HbA1c level and ESRD were modified by baseline eGFR, and higher HbA1c levels appeared to be associated with higher risk for ESRD in those with relatively preserved kidney function. For cause-specific mortality, diabetes accounted for at least 12% of deaths overall, and among those with HbA1c levels ≥ 9%, nearly 20% of deaths were attributed to diabetes.
Previous studies have examined associations between HbA1c levels and death in CKD populations. In a large database analysis from the Canadian province of Alberta, HbA1c levels < 6.5% and >9% were associated with higher risks for death.10 Another report from Taiwan indicated that HbA1c level > 9% was associated with ESRD, cardiovascular events, and death in those with CKD stages 3 to 4, but not those with CKD stage 5.11 A secondary analysis of the ACCORD trial that compared the effects of HbA1c targets < 6.0% versus 7% to 7.9% reported higher risks for cardiovascular and all-cause mortality in their diabetic population with lower HbA1c levels.13 Our findings add to the existing literature and highlight the potential risk of targeting lower HbA1c levels (<6%) in patients with CKD while at the same time highlighting the potential harmful effects of higher HbA1c levels. Cumulatively, the available data are in agreement with the KDIGO (Kidney Disease: Improving Global Outcomes) guideline statements on glycemic targets of HbA1c of 7.0% (graded 1A) and not treating to an HbA1c target < 7.0% in patients who are at risk for hypoglycemia.20 It is also worth noting that ~25% of our study population had HbA1c levels > 8%, suggesting an opportunity for better glycemic control in this population.
The observed harmful associations with higher HbA1c levels could be attributed to the macrovascular complications associated with diabetes. As for the inferior outcomes among patients with very low HbA1c concentrations, previous analysis reported that patients with CKD were at higher risk for sustaining hypoglycemia and the risk for death was higher within 1 day after hospitalization for hypoglycemic episodes.15 Although hypoglycemia could explain the higher risk that we observed in those with HbA1c levels < 6%, we cannot corroborate this hypothesis in our data because hypoglycemia-related deaths are not separately reported in the Ohio Department of Health mortality files. In the general population, diabetes remains the 7th leading cause of death in the United States.21 In this study population of patients with CKD and diabetes, diabetes accounted for at least 12% of deaths. Of note, the proportion of diabetes-related deaths increased as HbA1c levels worsened, thus highlighting the detrimental impact of uncontrolled diabetes mellitus in those with CKD.
Higher HbA1c levels have been associated with higher risk for kidney disease progression in some previous analyses, but not in others. In a meta-analysis, Coca et al14 reported that for those with type 2 diabetes, lower HbA1c levels were associated with reduced risk for micro- and macroalbuminuria, but its effects on ESRD were uncertain. More recently, a secondary analysis of the ADVANCE trial reported that intensive glucose control led to a long-term reduction in ESRD while not increasing the risk for cardiovascular events or death.13 We did not note a general association between HbA1c level and ESRD in those with pre-existing CKD, but this relationship appeared to be modified by eGFR; that is, patients with higher HbA1c levels had higher risks for ESRD if they had relatively preserved kidney function. It is important to note that 89% of our study population were using renin–angiotensin–aldosterone system (RAAS) blockers; hence, it is unclear whether the relationship between intense HbA1c control and kidney disease progression varied based on the use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Prior reports either refrained from reporting the use of RAAS inhibitors or did not adjust for their use in multivariable models; clearly, RAAS inhibitors have been shown to reduce the risk for ESRD in those with diabetic CKD.22
Certain limitations of our study deserve mention. Being an observational study, patients achieved their HbA1c levels as a result of their care and treatment and other health-related behavior rather than by randomization into specific glycemic control target groups. Hence, a causal relationship cannot be established. Our study population is derived from the EHRs of a large integrated health system and may not be generalizable to other settings. Providers may have differed in their treatment recommendations and patients may have differed in their treatment adherence. Whether these findings can be extrapolated to community-based CKD populations is unknown. The pragmatic clinical data from within a single health system carry the limitation that we cannot account for care obtained outside of Cleveland Clinic health system. We also lacked information for duration of diabetes and other diabetes-related complications such as diabetic neuropathy, diabetic retinopathy, and macrovascular diabetic complications. In addition, we lacked information relating to newer noninsulin injectable antidiabetic drugs. Furthermore, we included a limited number of patients with CKD stage 5, for whom the reliability of HbA1c measurements is unclear. Additionally, we did not have detailed and longitudinal medication data to examine any differences across medication subgroups or from medication changes over time.
However, the strengths of this study include a large diverse clinical population of patients with CKD stages 3 and 4 and availability of information for all-cause mortality, cause-specific death details (which to our knowledge have not been reported before), and ESRD incidence from merging our data with outside state and federal data sources, which provided validated end points for our analysis.
In summary, we report increased risk for all-cause mortality among patients with diabetes and CKD who had HbA1c levels < 6% and among those who had HbA1c levels ≥ 9%. By contrast, HbA1c level was not associated with ESRD in this study population. The proportion of deaths due to diabetes increased as HbA1c levels increased. Clinical trials comparing different diabetes control targets and specific medication strategies in patients with established CKD are warranted.
Supplementary Material
Table S1: Competing risks model of ESRD with 2-way interaction of HbA1c level and eGFR.
Table S2: Associations between time-dependent repeated measures of HbA1c and pre-ESRD mortality.
Table S3: Associations between HbA1c and pre-ESRD mortality and ESRD: sensitivity analyses.
Figure S1: Flow chart showing how patients were selected to be included.
Acknowledgments
Results of this study were presented as an abstract at the 2016 Kidney Week of the American Society of Nephrology in Chicago, IL, November 18, 2016.
Support
Dr Navaneethan is supported by a grant from the National Institutes of Health (NIH; National Institute of Diabetes and Digestive and Kidney Diseases-R01DK101500). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIH did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Footnotes
Financial Disclosure
Dr Navaneethan has served as consultant for Bayer and Boeringher-Ingelheim. The other authors declare that they have no relevant financial interests.
Contributions
Research idea and study design: SDN, JDS, SEJ, SA, JVN; data acquisition: SA; data analysis/interpretation: SDN, JDS, SEJ, SA, WCW, JVN; statistical analysis: SA, JDS. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. SDN takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review
Evaluated by 2 external peer reviewers, a statistician, and an Acting Editor-in-Chief.
SUPPLEMENTARY MATERIAL
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2016.11.018) is available at www.ajkd.org
Because an author of this article was an editor for AJKD at the time of manuscript submission, the peer-review and decision-making processes were handled entirely by a member of the Editorial Board (Luxia Zhang, MD) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Information for Authors & Journal Policies.
References
- 1.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Muntner P, Lloyd A, et al. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet. 2012;380(9844):807–814. doi: 10.1016/S0140-6736(12)60572-8. [DOI] [PubMed] [Google Scholar]
- 3.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.USRDS. Incidence, prevalence, patient characteristics, and treatment modalities. 2016;2016(08/04) [Google Scholar]
- 5.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 6.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 8.Cefalu WT. Glycemic targets and cardiovascular disease. N Engl J Med. 2008;358(24):2633–2635. doi: 10.1056/NEJMe0803831. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes-2016. Diabetes Care. 2016;39(suppl 1):S39–S45. doi: 10.2337/dc16-er09. [DOI] [PubMed] [Google Scholar]
- 10.Shurraw S, Hemmelgarn B, Lin M, et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171(21):1920–1927. doi: 10.1001/archinternmed.2011.537. [DOI] [PubMed] [Google Scholar]
- 11.Liao LN, Li CI, Liu CS, et al. Extreme levels of HbA1c increase incident ESRD risk in Chinese patients with type 2 diabetes: competing risk analysis in national cohort of Taiwan Diabetes Study. PLoS One. 2015;10(6):e0130828. doi: 10.1371/journal.pone.0130828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papademetriou V, Lovato L, Doumas M, et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87(3):649–659. doi: 10.1038/ki.2014.296. [DOI] [PubMed] [Google Scholar]
- 13.Wong MG, Perkovic V, Chalmers J, et al. Long-term benefits of intensive glucose control for preventing end-stage kidney disease: ADVANCE-ON. Diabetes Care. 2016;39(5):694–700. doi: 10.2337/dc15-2322. [DOI] [PubMed] [Google Scholar]
- 14.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172(10):761–769. doi: 10.1001/archinternmed.2011.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1121–1127. doi: 10.2215/CJN.00800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navaneethan SD, Jolly SE, Schold JD, et al. Development and validation of an electronic health record-based chronic kidney disease registry. Clin J Am Soc Nephrol. 2011;6(1):40–49. doi: 10.2215/CJN.04230510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navaneethan SD, Schold JD, Arrigain S, Kirwan JP, Nally JV., Jr Body mass index and causes of death in chronic kidney disease. Kidney Int. 2016;89(3):675–682. doi: 10.1016/j.kint.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A proportional hazards models for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 20.KDIGO. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. 2013;2016(08/04) http://www.kdigo.org/clinical_practice_guidelines/pdf/CKD/KDIGO_2012_CKD_GL.pdf. Accessed January 30, 2017. [Google Scholar]
- 21.Centers for Disease Control and Prevention. Leading causes of death-2014. 2016;2016(08/04) [Google Scholar]
- 22.Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015;385(9982):2047–2056. doi: 10.1016/S0140-6736(14)62459-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Competing risks model of ESRD with 2-way interaction of HbA1c level and eGFR.
Table S2: Associations between time-dependent repeated measures of HbA1c and pre-ESRD mortality.
Table S3: Associations between HbA1c and pre-ESRD mortality and ESRD: sensitivity analyses.
Figure S1: Flow chart showing how patients were selected to be included.


