Abstract
Type 1 diabetes (T1D) manifests when the insulin-producing pancreatic beta-cells are destroyed as a consequence of an inflammatory process initiated by lymphocytes of the immune system. The non-obese diabetic (NOD) mouse develops T1D spontaneously and serves as an animal model for human T1D. The heteroreceptor (HR), comprising IL-4Rα and IL-13Rα1, serves both IL-4 and IL-13 cytokines which are believed to function as anti-inflammatory cytokines in T1D. However, whether the HR provides a responsive element to environmental (i.e. physiologic) IL-4/IL-13 in the regulation of peripheral tolerance and the development of T1D has yet to be defined. In this study, NOD mice deficient for the HR have been generated by means of IL-13Rα1 gene disruption and used to determine whether such deficiency affects the development of T1D. Surprisingly, the findings indicate that NOD mice lacking the HR (13R−/−) display resistance to T1D as the rise in blood glucose level (BGL) and islet inflammation were significantly delayed in these HR-deficient relative to HR-sufficient (13R+/+ ) mice. In fact, the frequency and spleen- to-pancreas dynamics of both Th1 and Th17 cells were affected in 13R−/− mice. This is likely due to an increase in the frequency of mTGFβ+FoxP3int Tregs and persistence of CD206+ macrophages in the pancreas as both types of cells confer resistance to T1D upon transfer to 13R+/+ mice. These findings reveal new insights as to the role environmental IL-4/IL-13 and the HR play in peripheral tolerance and the development of T1D.
Introduction
T1D is a chronic disease in which the insulin-producing β-cells are destroyed by an inflammatory process driven by self-reactive T lymphocytes (1, 2). The disease manifests when regulatory T cells (Tregs)1 and anti-inflammatory cytokines no longer control the function of self-reactive effector Th1 and Th17 cells. It has long been known that type II cytokines, such as IL-4 and IL-13, function as anti-inflammatory in mouse and human T1D (3–6). For instance, neonatal Th1 cells unusually upregulate the IL-4Rα/IL-13Rα1 heteroreceptor (HR) (7) and both IL-4 and IL-13 signal death of these inflammatory cells (8). However, adult Th1 cells do not express the IL-4Rα/IL-13Rα1 heteroreceptor (HR) and the conventional IL-4 receptor (IL-4R), comprising IL-4Rα and the common gamma chain (IL-4R/γ), does not signal in these cells (9). It is thus possible that the cytokines utilize the IL-4Rα/IL-13Rα1 heteroreceptor (HR) on other cell types (10) to drive anti-inflammatory function against Th1 cells. Moreover, IL-13 signals only through the HR as the conventional IL-13Rα1/IL-13Rα2 serves as a decoy receptor (11, 12). In addition, as the HR is expressed on antigen presenting cells (APCs) such as dendritic cells (DCs) (13), macrophages (14), and basophils (8) it is possible that the cytokines drive their anti-inflammatory effect against effector T cells by controlling the function of these APCs. To determine whether the anti-inflammatory function of endogenous or environmental IL-4 and IL-13 cytokines contribute to the maintenance of peripheral tolerance and restrain the development of autoimmune or type 1 diabetes, we sought to generate NOD mice in which the HR is non-functional and test for effects on the disease. Mice in which the IL-13Rα1 gene is disrupted, express the conventional IL-4R but the HR does not form (15). These mice, in which both IL-4 and IL-13 cannot signal through the HR, offer a model suitable for assessing the role these cytokines play in the regulation of T1D. To this end, we generated NOD mice deficient for IL-13Rα1 (13R−/−) and determined how the lack of signaling through the HR by environmental IL-4 and IL-13 affect T1D. Unexpectedly, the findings indicate that 13R−/− mice display resistance to T1D relative to 13R+/+ animals. In fact, there was a significant delay in the onset of disease in the 13R−/− mice which parallels with diminished pancreatic infiltration and persistence of healthy islets. Also, at week 6 and 7 of age the mice showed increased frequency of a subset of T regulatory cells (Tregs) which express intermediate (int) levels of FoxP3 and display membrane bound TGFβ (mTGFβ+). As has been shown before (16), these FoxP3intmTGFβ+ are highly suppressive and when transferred into 13R+/+ NOD mice the onset of disease is delayed as in 13R−/− mice. Later on, there was appearance in the pancreas of 13R−/− mice of a CD206-positive population of macrophages which was also suppressive upon transfer into 13R+/+ mice and delayed the onset of disease. These findings highlight the role environmental cytokines and their receptors play in the development of T1D.
Materials and methods
Mice
Wild type 13R+/+ NOD (H-2g7) and NOD.scid mice were purchased from The Jackson Laboratory (Bar Harbor, ME). 13R−/− NOD mice were generated by breeding 13R−/− Balb/c mice (15) onto the NOD background via speed congenic technology based on 58 microsatellite markers on sequences between the Balb/c donor strain and NOD recipient strain. A total of 8 backcrosses with wild type 13R+/+ NOD mice (from Jackson Labs) were performed to ensure homozygosity of NOD alleles. As the targeted ES cells for generation of 13R−/− Balb/c mice were of Balb/c background, the microsatellite markers were between Balb/c and NOD only. 13R+/+FoxP3-GFP NOD mice were previously described (16). 13R−/− NOD mice were bred with 13R+/+FoxP3-GFP NOD animals to generate 13R−/−FoxP3-GFP NOD mice also by speed congenic technology. 13R+/+FoxP3-DTR NOD mice were previously described (17). The NOD.13R−/− mice were bred with 13R+/+FoxP3-DTR NOD mice to generate 13R−/−FoxP3-DTR NOD mice. All animals were used according to the guidelines of the University of Missouri of Columbia Animal Care and Use Committee.
Induction of T1D by adoptive transfer of splenic cells
Splenic cells were isolated from diabetic 13R+/+ or 13R−/− NOD mice and 10 × 106 cells per mouse were injected intravenously (i.v) via the tail vein into NOD.scid mice. The hosts were then monitored for T1D. A mouse is considered diabetic when blood glucose level (BGL) is ≥ 300 mg/dl for 3 consecutive days.
Western blot
Whole cell lysates of intestinal epithelial cells isolated from adult 13R+/+ and 13R−/− mice were run on a gradient (4–12%) polyacrylamide NuPAGE gel. The gel was transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA) and blotted with anti-IL-13Rα1 mAb (IG3) or anti-β-actin (Abcam, Cambridge, MA, USA). Goat anti-mouse IgG-HRP (Santa Cruz Bio) was used as secondary Abs.
Histology
Pancreata were frozen in optimal cutting temperature compound at −80ºC. The cryosections were performed at 8 μm thickness and were cut 200 μm apart to avoid repeated counting of the same islet. At least three non-serial sections per pancreas were stained with hematoxylin-eosin and analyzed by light microscopy. The following criteria was used to score insulitis; mild insulitis, partial infiltration within islet; peri-insulitis, infiltration restricted to islet periphery; severe insulitis, complete infiltration within the islet; healthy, absence of islet infiltration.
Flow cytometry
Antibodies
The antibodies used in this study were: anti-CD3e (145-2C11)- allophycocyanin, anti-CD4 (RM4-5)-PerCp5.5, anti-CD19 (1D3)-PE-Cy7, anti-CD11b (M1/70)-FITC, anti-CD11c (HL3)-PE, anti-CD11c (HL3)-allophycocyanin, anti-CD45 (30-F11)-allophycocyanin-Cy7, antit-IL-17A-PE, anti-IFNγ-allophycocyanin, anti-IL-4-PE, anti-IL-10-allophycocyanin (BD Biosciences); anti-CD206 (C068C2)-allophycocyanin, anti-CD206 (C068C2)-PE (BioLegend); F4/80 (BM8)-PerCp 5.5 (eBiosciences); and anti-TGF-β1-biotin (BAF240). Chicken IgY (R&D systems) was used as isotype control for anti-TGF-β1 antibody.
Cell surface staining
Splenocytes, pancreatic cells, pancreatic lymph node cells or purified CD4+ T cells were incubated with mouse IgG (Sigma-Adrich) for 15 mins to block FcRγs. The cells were then stained with test Abs in 0.5% BSA in PBS for 30 mins on ice. For biotin coupled Abs, the cells were stained with PE-conjugated streptavidin for 30 mins on ice.
Intracellular cytokine staining
Splenic and pancreatic CD4+ T cells were purified by negative selection using a Miltenyi-microbead kit.
The splenic CD4+ T cells from normal and diabetic mice were then stimulated with anti-CD3 (2C11) (10 μg/ml) and anti-CD28 (PV1) (1 μg/ml) for 72 h with addition of Brefeldin A (10 μg/ml) during the last 4 hours of incubation. The cells were first stained for CD4 and CD3 markers, then permeabilized with Fix/Perm buffer (eBioscience) and incubated with fluorchrome-labeled anti-IFNγ, anti-IL-4, and anti-IL-17 antibodies. Detection of IFNγ, IL-4 and IL-17 used a Beckman Coulter Cyan (Brea, CA) and the data was analyzed using FlowJo 10.0.8 software (Tree Star).
The pancreatic CD4+ T cells from normal mice were also stimulated with anti-CD3 and anti-CD28 antibodies as above but for the pancreatic CD4+ T cells from diabetic mice the stimulation used PMA (50 ng/ml) and ionomycin (500 ng/ml) instead of anti-CD3 and anti-CD28 antibodies. Also, the incubation lasted only 4 hours with addition of Brefeldin A (10 μg/ml) at the start of the culture.
Isolation of Tregs and Macrophages from the pancreas
Tregs
Pancreata were harvested from 6 week-old 13R−/−FoxP3-GFP NOD female mice and treated with collagenase as described (16). FoxP3hi or FoxP3int Tregs were gated on CD4 and CD25 and sorted on the basis of GFP expression.
Macrophages
Pancreata were harvested from 10 week-old 13R−/−NOD female mice and treated with collagenase as described (16). The cells were stained with anti-CD11b, F4/80 and anti-CD206 antibody and sorted as CD11b+F4/80+CD206+ and CD11b+F4/80+CD206− cells.
Cell sorting was performed on Beckman Coulter MoFlo XDP sorter.
Suppression of T1D by Tregs and macrophages
Twelve week-old 13R+/+ NOD female mice were given i.v either 4 × 105 Tregs or 5 × 105 macrophages per mouse and the hosts were monitored for blood glucose levels twice a week for 15 to 25 weeks.
Depletion of Tregs by Diphtheria toxin
Diphtheria toxin (DT; Sigma-Aldrich) was administered i.p. to six week old 13R−/−FoxP3-DTR NOD mice (300 ng/mouse/injection) for 10 consecutive days and then monitored daily for BGL for 2 weeks.
Measurement of cytokines by ELISA
IL-10 and IL-6 were detected using anti-cytokine antibodies by ELISA according to BD Pharmingen’s standard protocol. The OD450 was read on SpectraMax 190 counter (Molecular Devices, Sunnyvale, CA, USA) and data analysis used SOFTmax PRO 3.1.1 software. Graded amount of rIL-10 and rIL-6 (PeproTech) were included for construction of a standard curve. Cytokine concentration was extrapolated from the linear portion of the standard curve.
Quantitative PCR analysis
Pancreatic CD11b+CD11c− cells were isolated using CD11c and CD11b magnetic microbeads (Miltenyi) respectively. Total RNA was extracted using TRI RNA isolation reagent (Sigma-Aldrich). Quantitative PCR for the targets of interest was performed using Power SYBR Green kit and the StepOnePlus instrument (all from Applied Biosystems). The primers used were:
Arg1
Forward: 5′-GATTATCGGAGCGCCTTTCT-3′
Reverse: 5′-CCACACTGACTCTTCCATTCTT-3′
FIZZ1
Forward: 5′-TCCCAGTGAATACTGATGAGA-3′
Reverse: 5′-CCACTCTGGATCTCCCAAGA-3′
CD206
Forward: 5′-CTGCAGATGGGTGGGTTATC-3′
Reverse: 5′-GGCATTGATGCTGCTGTTATG-3′
GAPDH
Forward: 5′-AACTTTGGCATTGTGGAAGG-3′
Reverse: 5′-GGATGCAGGGATGATGTTCT-3′
TNFα
Forward: 5′-TCTCATGCACCACCATCAAGGACT-3′
Reverse: 5′-TGACCACTCTCCCTTTGCAGAACT-3′,
IL-1β
Forward: 5′-AGCTGAAAGCTCTCCACCTCAA-3′
Reverse: 5′-TGTCGTTGCTTGGTTCTCCTTG-3′
IL-12Rβ2
Forward: 5′-TGTGGGGTGGAGATCTCAGT-3′
Reverse: 5′-TCTCCTTCCTGGACACATGA-3′.
Statistical analysis
P values were calculated using the two-tailed Student’s t test or Mann-Whitney U Test. Comparison of more than two groups was performed using Kruskal-Wallis test. Prism Software v4.0c (GraphPad) was used in all statistical analyses.
Online supplemental material
Supplemental Fig. 1 shows that the frequency of FoxP3hi Tregs in SP, PLN and PN is similar in both 13R−/− and 13R+/+ mice. Supplemental Fig. 2 shows that targeted depletion of FoxP3 Tregs in 13R−/− mice nullifies resistance to T1D. Supplemental Fig. 3 shows that 13R deficiency does not affect the frequency of innate and adaptive immune cells in both the SP and PN at 5 weeks of age. Supplemental Fig. 4 shows that co-transfer of FoxP3int and CD206+ Mϕ into 13R+/+ mice is more effective in delaying T1D than either transfer alone.
Results
Ablation of 13R confers resistance to T1D in NOD mice
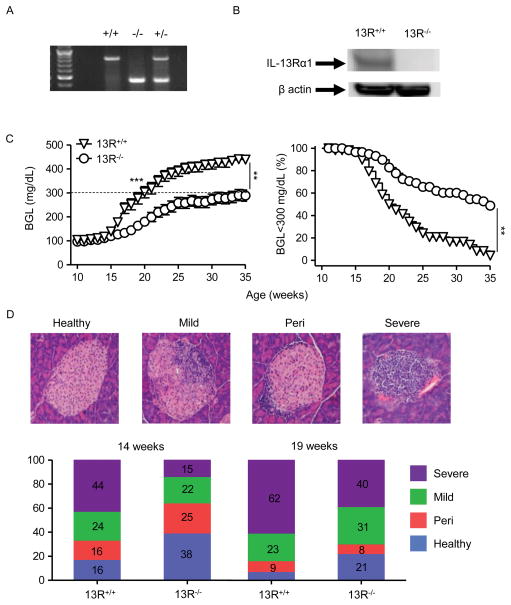
NOD mice were bred with 13R−/− Balb/c mice (15) to generate a NOD strain in which IL-13Rα1 gene is disrupted and the HR cannot form. This was carried out by speed congenic breeding which yielded female NOD mice homozygous for IL-13Rα1 deficiency (Fig. 1A). Also, when intestinal cells, which usually display high levels of the HR (14), were tested for IL-13Rα1 protein expression by Western blot using anti-IL-13Rα1 mAb (14) the 13R−/− NOD mice did not show any significant staining when compared to 13R+/+ NOD mice (Fig. 1B). The 13R−/− mice were then monitored for blood glucose level (BGL) from week 10 to 35 of age to determine whether the HR plays a role in the development of T1D. The results show that 13R−/− mice display significant resistance to T1D compared to 13R+/+ animals (Fig. 1C). Indeed, up to week 14 of age the 13R−/− mice had a mean BGL of 111.5 ± 5.5 mg/dl which is similar to the 117.3 ± 7.5 mg/dl observed in 13R+/+ mice (Fig. 1C, left panel). However, by week 19 the average BGL in 13R+/+ was 277.7 ± 23.8 which is significantly higher than the 163.2 ± 15.0 BGL observed in 13R−/− mice (Fig. 1C, left panel). In fact, by week 19, out of 62 13R+/+ mice 29 were diabetic (BGL ≥ 300mg/dl) while only 8 out of 62 13R−/− mice had a BGL ≥ 300mg/dl at the same time point. At the conclusion of the monitoring period (week 35) while 49% (30 out of 62) of the 13R−/− mice remain with a BGL below 300mg/dl, only 5% (3 out of 62) of 13R+/+ mice showed a BGL ≤ 300 mg/dl (Fig. 1C, right panel). In other words, at week 35 of age 95% of 13R+/+ mice were diabetic compared to 51% of 13R−/− mice indicating that in the absence of the HR, the mice display resistance to T1D.
Figure 1. 13R−/− NOD mice display resistance to T1D.
(A) PCR analysis showing bands for IL-13Rα1 in wild-type 13R+/+ (828 bp), homozygous 13R−/− (466bp) and heterozygous 13R+/− (828 and 466 bp) NOD mice. (B) Shows expression of IL-13Rα1 in intestinal cells from 13R+/+ and 13R−/− mice as determined by Western blot using an anti–IL-13Rα1 Ab. (C) The left panel shows BGL in female 13R+/+ and 13R−/− NOD mice from week 10 to 35 of age. Each group included 62 mice. The right panel shows the percentage of mice with BGL lower than 300 mg/dl among the 62 animals enrolled in the 10–35 week monitoring period. **p<0.005 and ***p<0.0001 as determined by Mann-Whitney U test. (D) The top panel shows H & E staining (100x) of pancreatic sections from either normal, hyperglycemic or diabetic mice covering the scoring scale used for classification of islet infiltration. From left to right the islet are scored as healthy, mild, peri or severely infiltrated. The bottom panel shows islet infiltration severity scores for 13R+/+ and 13R−/− mice at 14 weeks (onset of differential BGL from C) and 19 weeks (onset of diabetes in 13R+/+ mice from C) of age (n = 7 per group).
These results are supported by histologic analysis of pancreatic islet infiltration as the 13R−/− mice had more healthy and less severely infiltrated islets compared to the 13R+/+ animals both at week 14 and 19 of age (Fig. 1D). Indeed, the 13R−/− mice have less insulitis by week 14 (period when 13R+/+ mice group begins to get hyperglycemic) and 19 (period when 13R+/+ mice group begins to be diabetic) (Figure 1D). While genetic contamination from IL-13Rα1−/− Balb/c breeder mice used to generate IL-13Rα1−/− NOD mice is always a possibility, the use of a large number of mice from different crossings for disease monitoring indicate that 13R receptor likely plays a role in the resistance to T1D. In all, 13R−/− mice are more resistant to T1D than their 13R+/+ counterparts.
13R alters the expression of CD4+ T cell signature cytokines in both the spleen and pancreas at the diabetic stage
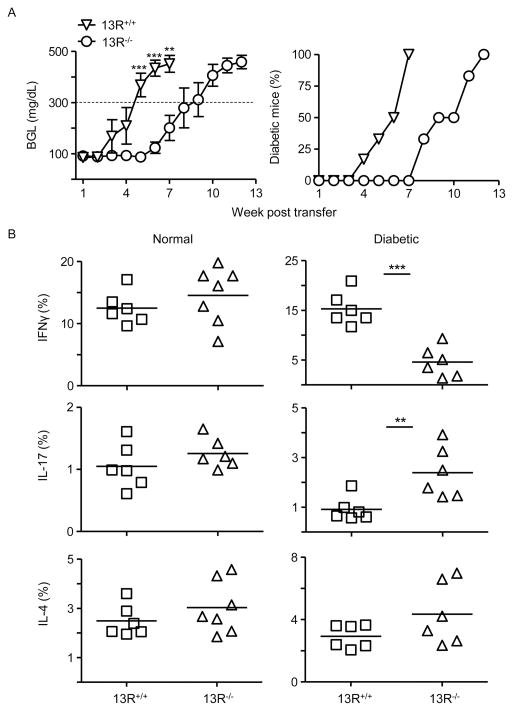
The delay of the onset of T1D in 13R−/− mice could be related to alteration of the function of Th1 and/or Th17 inflammatory cells. To test this premise we began by analyzing bulk splenocytes for transfer of diabetes into NOD.scid mice. The results show that cells from diabetic 13R−/− mice have reduced diabetogenic function relative to splenocytes from 13R+/+ mice (Fig. 2A). Indeed, the BGL in the host mice rose much faster in the animals recipient of 13R+/+ splenocytes compared to those given cells from 13R−/− mice (Fig. 2A). In fact, by week 7 post transfer while all NOD.scid hosts (100% or 6 of 6) recipient of splenocytes from 13R+/+ donors became diabetic (BGL ≥ 300mg/dl) none (0 of 6) of those given splenocytes from 13R−/− had T1D at this time point and the disease manifested after a 6-week delay (Fig. 2A). We then sought to determine whether alteration of T cell function by the HR affects Th1 and/or Th17 cells. To this end, the frequency of cytokine producing splenic and pancreatic Th1 and Th17 cells before (normal) and after (diabetic) development of disease was determined in 13R−/− mice and compared to 13R+/+ animals. The findings show that 13R−/− mice displayed no significant difference in the frequency of IFNγ-, IL-17- or IL-4-producing T cells whether in the spleen (Fig. 2B) or in the pancreas (Fig. 3A and 3B) when compared to 13R+/+ mice.
Figure 2. Splenic CD4+ T cells from diabetic 13R−/− mice are less pathogenic and produce diminished IFNγ but heightened IL-17 cytokine relative to cells from 13R+/+ animals.
(A) Shows the mean BGL ± SEM (left panel) and the percentage of diabetic NOD.scid host mice (right panel ) after transfer of splenic cells from diabetic 13R+/+ or 13R−/− NOD mice. Each group included 6 mice. **p<0.005, ***p<0.0001 as determined two-tailed Student’s t-test. (B) Shows intracellular cytokine production by CD3+CD4+ splenic T cells from normal (BGL < 140 mg/dl) and diabetic (BGL ≥ 300 mg/dl) 13R+/+ and 13R−/− mice after in vitro stimulation with anti-CD3 and anti-CD28 antibodies. *p<0.05, ***p<0.0005 as determined by two-tailed Student’s t-test.
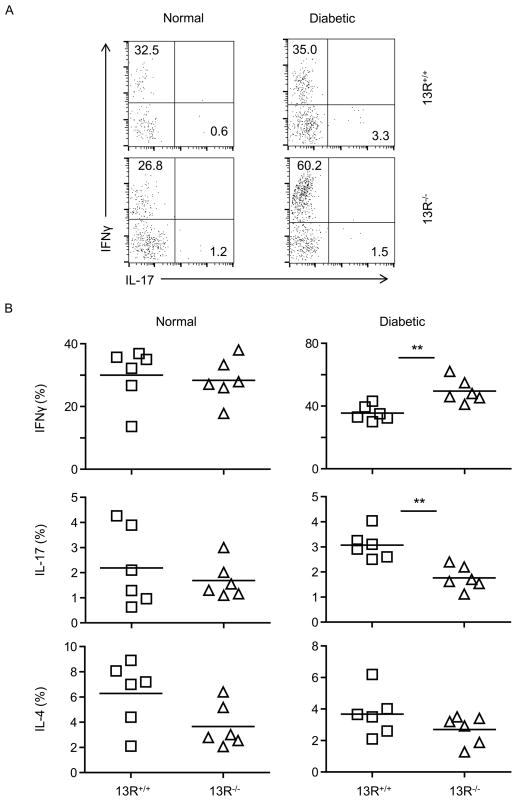
Figure 3. Pancreatic CD4+ T cells from diabetic 13R−/− mice produce more IFNγ but diminished IL-17 cytokine relative to cells from 13R+/+ animals.
Pancreatic cells from normal (BGL < 140 mg/dl) and diabetic (BGL ≥ 300 mg/dl) 13R+/+ or 13R−/− mice were stimulated with PMA and ionomycin, and stained for surface CD3 and CD4 as well as intracellular IFNγ, IL-14, and IL-17 cytokines. (A) Shows a representative FACS plot experiment depicting intracellular production of IFNγ and IL-17 by CD3+CD4+ cells. (B) Shows the percentage of CD3+CD4+ T cells producing IFNγ, IL-17 or IL-4 from normal and diabetic NOD mice. Each point represents an individual mouse. The horizontal line indicates the mean percentage from 6 individually tested mice. **p<0.005 as determined by two-tailed Student’s t-test.
However, at the diabetic stage, 13R−/− mice had decreased frequency of IFNγ-producing Th1 cells in the spleen (Fig. 2B) but increased frequency of these cells in the pancreas (Fig. 3A and 3B) when compared to 13R+/+ mice. As for IL-17-producing Th17 cells, their frequency was increased in the spleen (Fig. 2B) but decreased in the pancreas (Fig. 3A and 3B) in 13R−/− relative to 13R+/+ mice. The frequency of IL-4- producing T cells was similar in both strains at normal and diabetic stages (Fig. 2 and 3). In all, 13R affects the dynamics of Th1 and Th17 relocation from the spleen to the pancreas during the diabetic stage.
Accumulation of protective FoxP3int Treg in the pancreas of 13R−/− mice
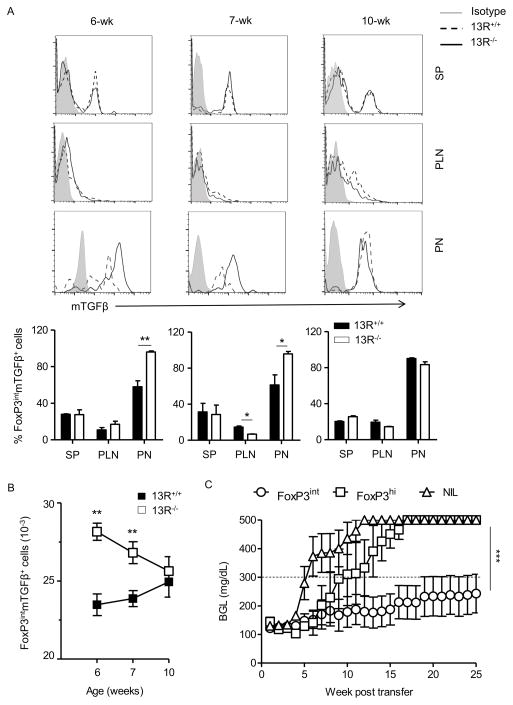
The differential frequency of Th1 and Th17 cells in the pancreas during the diabetic stage could be due to regulatory mechanisms by Tregs. The rationale for this statement stems from earlier studies showing that prior to week 6 of age the NOD mouse expands FoxP3int Tregs, a subset of CD4 T cells expressing intermediate (int) levels of FoxP3, high levels of RORγt and utilize membrane bound TGFβ (mTGFβ) to carry out suppressive functions (16). To test these premises, the spleen (SP), pancreatic lymph nodes (PLN) and pancreas (PN) were harvested from 13R−/− mice on week 6, 7, and 10 of age and their CD4 T cells were tested for expression of mTGFβ and intermediate (FoxP3int Tregs) as well as high levels (FoxP3hi) of FoxP3 without mTGFβ. The results show that while FoxP3hi (mTGFβ−) Tregs were similar in 13R−/− relative to 13R+/+ mice (Fig. S1), the FoxP3int (mTGFβ+) cells were significantly higher in the PN of 13R−/− relative to 13R+/+ mice at week 6 and 7 of age (Fig. 4A). Indeed, the percentages of FoxP3hi Tregs among total CD4 T cells in the SP, PLN and PN were similar with no significant difference between 13R+/+ and 13R−/− mice (Fig. S1). However, the percentages of FoxP3int Tregs were significantly higher in the PN but not the SP or PLN of 13R−/− compared to 13R+/+ mice both at week 6 and 7 of age (Fig. 4A). These differences are also significant when the absolute numbers of FoxP3int cells in the PN are compared between the 2 strains (Fig. 4B). Interestingly, however, there was a decline in the FoxP3int Treg number over time in the 13R−/− mice and by week 10 both strains had similar numbers of these cells (Fig. 4B). To determine whether these FoxP3int Tregs contribute to resistance against T1D, 6-week old 13R−/−FoxP3-DTR NOD mice were given diphtheria toxin (DT) and monitored for rise in BGL. Interestingly, the mice began to show a rise in BGL within 4 days after DT treatment and their BGL kept spiking for the 14 day monitoring period (Fig. S2A). However, age-matched animals that did not receive any DT treatment preserved normoglycemia during the same monitoring period. These findings suggest that Tregs contribute to resistance against disease in 13R−/− NOD mice. Furthermore, transfer of FoxP3int into 13R+/+ mice induces delay of disease onset (Fig. 4C). Indeed, when FoxP3int and FoxP3hi Tregs were sorted from the pancreas of 6 week-old 13R−/−FoxP3-GFP NOD mice, transferred into 12-week old 13R+/+ mice and the hosts were monitored for BGL, the mice recipient of FoxP3int Tregs displayed significant delay in disease onset relative to hosts recipient of FoxP3hi counterparts or animals that did not receive any cell transfer (NIL) (Figure 4C). Interestingly, the transferred FoxP3int Tregs settled in the pancreas but not the spleen (Fig. S2B), a pattern similar to FoxP3int Tregs in 13R−/− mice further asserting their contribution to the delay of disease onset.
Figure 4. The frequency of protective FoxP3int Tregs is higher in 13R−/− than 13R+/+ mice.
Cells from the spleen (SP), pancreas (PN) and pancreatic lymph nodes (PLN) of 13R+/+FoxP3-GFP or 13R−/−FoxP3-GFP female NOD mice were stained with antibodies to CD4, CD25, and membrane bound TGFβ (mTGFβ) and the frequency of CD4+CD25+GFPint(FoxP3int)mTGFβ+ cells was analyzed by flow cytometry at the age of 6, 7 and 10 weeks. In (A) the histograms show representative FACS plots for expression of mTGFβ by cells gated on CD4 and CD25 and FoxP3int at the indicated weeks for the SP, PLN and PN. Each bar graph show the percentage ± SD of FoxP3int and mTGFβ expression by cells gated on CD4 and CD25 compiled from 3 independent experiments. (B) Shows the absolute number of FoxP3intmTGFβ+ cells ± SEM compiled from 3 independent experiments. *p<0.05, **p<0.005 as determined by two-tailed Student’s t-test. (C) Shows the mean BGL ± SEM in 13R+/+ NOD mice (8 per group) given sorted FoxP3int or FoxP3hi Tregs (4 × 105 cells/mouse) at the age of 12 weeks. A group of mice that did not receive Tregs (NIL) was included for control purposes. ***p<0.0001 as determined by Kruskal-Wallis test.
13R−/− but not 13R+/+ mice develop CD11bhiF4/80hi macrophages protective against T1D
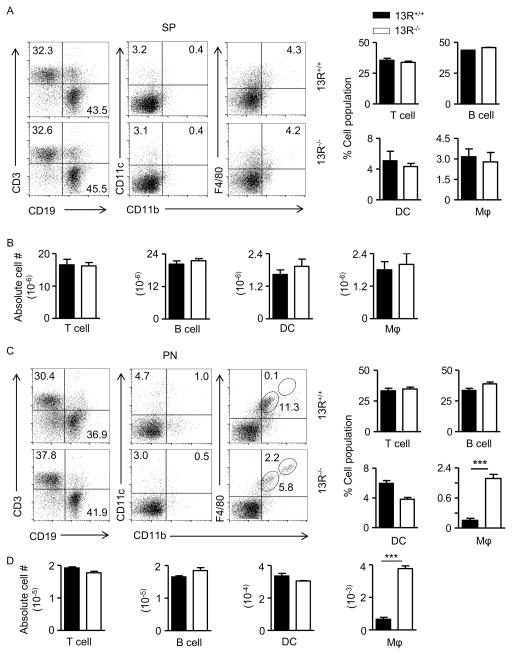
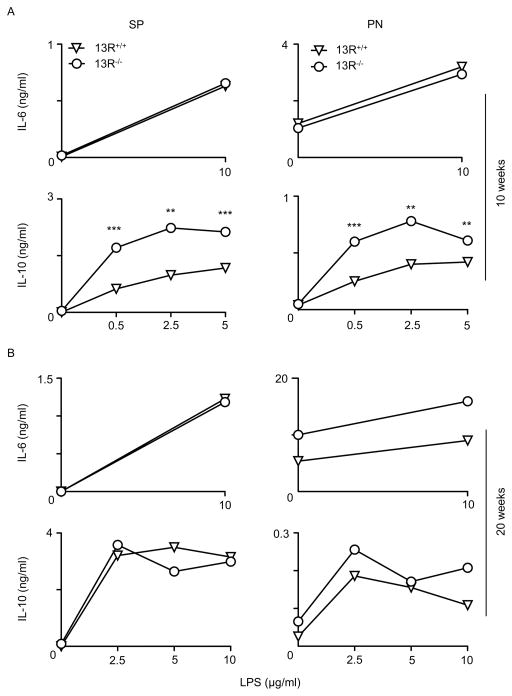
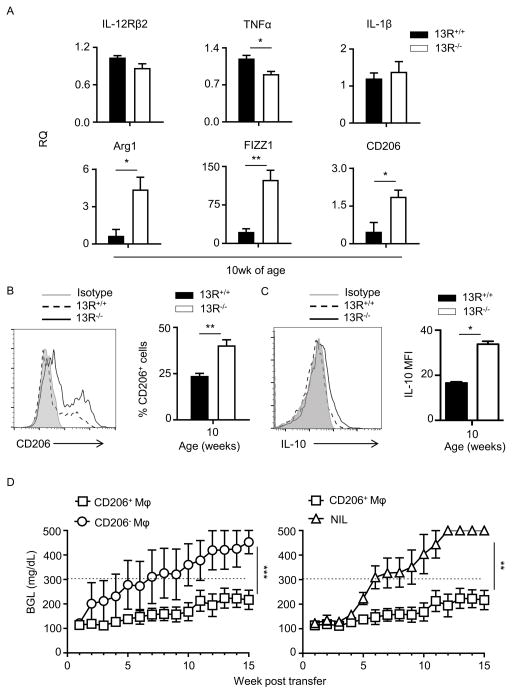
Besides Tregs, antigen presenting cells (APCs) also play a major role in the activation or modulation of effector cells. To determine whether 13R alters the activation of effector T cells through regulation of APC function, we began by evaluating the frequency of DCs, macrophages and B cells prior to (week 5 of age), as well as during (week 10 of age), the insulitis stage. The results indicate that during insulitis there was a significant increase in the frequency of the CD11bhiF4/80hi subset of macrophages in the pancreas of 13R−/− relative to 13R+/+ mice (Fig. 5). Indeed, while at week 5 of age the frequencies and absolute numbers of B cells (CD19), DCs (CD11c) and macrophages (CD11b/F4/80) in the spleen as well as the pancreas were similar in both strains (Fig. S3), by week 10 there was an increase in the frequency and absolute number of the CD11bhiF4/80hi subset of macrophages in the pancreas (Fig. 5C and D) but not in the spleen (Fig. 5A and B). Results compiled from several experiments show that the increase in CD11bhiF4/80hi macrophages in 13R−/− mice is statistically significant indicating that the HR plays a role in the accumulation of these cells in the pancreas. In all, 13R−/− mice display an increased frequency of fully mature CD11bhiF4/80hi macrophages in the pancreas during a period of time where insulitis is progressive. To determine whether these macrophages play a role in the resistance against T1D, we began by analyzing the type of cytokines the cells produce. The results indicate that splenic and pancreatic CD11b+CD11c− monocyte/macrophage populations from 10 week-old 13R−/− mice produce similar amounts of IL-6 when compared to their counterparts from 10 week-old 13R+/+ mice (Fig. 6A). However, both splenic and pancreatic CD11b+CD11c− monocyte/macrophage populations produce significantly higher amounts of IL-10 relative to their counterparts from 10 week-old 13R+/+ mice (Fig. 6A). By week 20 of age, production of IL-6 and IL-10 by splenic and pancreatic CD11b+CD11c− monocyte/macrophage populations was similar with non-significant differences in either strain (Fig. 6B). This suggest that these macrophages are most likely modulatory M2-type cells which would contribute to resistance against T1D (18–22). To test this postulate, we began by assessing the CD11b+CD11c− pancreatic monocyte/macrophages for up-regulation of markers known to be associated with M1 and M2 macrophage phenotypes. The results show that while pancreatic CD11b+CD11c− monocyte/macrophages from both 13R−/− and 13R+/+ mice displayed a similar pattern of expression for M1 associated IL-12Rβ2, TNFα and IL-1β markers, the macrophages from 13R−/− mice had significantly higher mRNA expression than cells from 13R+/+ mice for Arg1, FIZZ1 and CD206 M2-associated markers (22–24). To ensure that this phenotype is evident and parallels with the appearance of pancreatic CD11bhiF4/80hi at week 10 of age (Fig. 5), we assessed CD206 expression at the protein level in both 13R+/+ and 13R−/− mice at week 10 of age. The results show CD206 (C-Type Mannose Receptor 1) protein expression is significantly increased in 13R−/− relative to 13R+/+ mice (Fig. 7B). This suggests that the absence of 13R allows for M2-type macrophages to accumulate in the pancreas as inflammation progresses. These M2-type macrophages (CD11b+F4/80+CD206+) likely play a role in the resistance to disease because they produce anti-inflammatory IL-10 cytokine (Fig. 7C). Indeed, when CD206+ macrophages from 13R−/− mice were adoptively transferred into 12-week old 13R+/+ mice, the hosts were resistant to T1D (Fig. 7D). By week 7 after cell transfer the average BGL was 311 ± 109 mg/dl for mice recipients of CD206−Mϕ but only 157 ± 26 for animals given CD206+ Mϕ (Fig. 7D, left panel). At this point, 4 out 8 mice (50%) were diabetic (BGL≥ 300 mg/dl) in the CD206− Mϕ group while only 2 out of 9 in the CD206+ Mϕ group had diabetes. At the end of the monitoring period, 7 out of 8 mice were diabetic in the CD206− recipient group while 4 out 9 had diabetes at the 15 week time point. A similar disease delay was observed when the CD206+ recipient group was compared to NOD mice that did not receive macrophage transfer (Fig. 7D, right panel). Interestingly, the CD206+ Mϕ synergize with Foxp3int Tregs as co-transfer of CD206+ Mϕ and FoxP3int Tregs into 13R+/+ mice was more effective in delaying T1D than either transfer alone (Fig. S4). These results indicate that the 13R hinders the development of protective CD11b+F4/80+CD206+ Mϕ that seem to take over for FoxP3int Tregs in disease suppression.
Figure 5. 13R−/− but not 13R+/+ mice develop CD11bhiF4/80hi macrophages in the pancreas.
Splenic (A and B) and pancreatic (C and D) cells from 10-week old 13R−/− and 13R+/+ female NOD mice were stained with antibodies to cell specific surface markers and the percentages (A and C) and absolute numbers (B and D) of T cells (CD3), B cells (CD19), DCs (CD11c), and macrophages (CD11b and F4/80) were determined by gating on CD45 (hematopoietic marker) and the corresponding cell specific marker. In (A) and (C) the FACS plots show a representative experiment while the bar graphs show the mean percentage ± SEM of the indicated cell type compiled from 3 independent experiments. In (B and D) the bars show the mean absolute number ± SEM of the indicated cell type compiled from the 3 independent experiments of A and C, respectively. ***p<0.0005 as determined two-tailed Student’s t-test.
Figure 6. 13R increases IL-10 secretion by Macrophages.
CD11b+ cells were isolated from the SP and PN of 13R+/+ and 13R−/− NOD mice (6 per group) at week 10 (A) and or 20 (B) of age. CD11c− CD11b+ macrophages were isolated from both organs, stimulated with LPS for 48 hours, and the supernatant was then tested for IL-10 by ELISA. Each point represents the mean ± SEM from 3 independent experiments. **p<0.005, ***p<0.0005 as determined by two-tailed Student’s t-test.
Figure 7. 13R−/− mice develop protective pancreatic macrophages which delay T1D upon transfer to 13R+/+ mice.
(A) CD11c−CD11b+ macrophages were sorted from the pancreas of 10 week-old female 13R+/+ and 13R−/− NOD mice after depletion of CD11c cells. RNA was then extracted by Trizol and used to measure mRNA expression for markers associated with M1 and M2 macrophages. Each bar represents the mean RQ ± SEM compiled from 3 independent experiments. *p<0.05, **p<0.005 as determined by two-tailed Student’s t-test. (B) Pancreatic cells from 10-week-old female 13R+/+ and 13R−/− NOD mice were stained with antibodies to CD11b, F4/80 and CD206 and the cells were analyzed for CD206 expression. The plots show a representative experiment for expression of CD206 by cells gated on CD11b+ and F4/80+. The bar graphs show the percent mean ± SD of CD11b+F4/80+CD206+ cells compiled from three independent experiments. **p<0.005 as determined by two-tailed Student’s t-test. (C) CD11b+cells were isolated from the pancreas of 10-week-old female 13R1+/+ and 13R1−/−NOD mice. The cells were then stimulated with LPS (0.5μg/ml) for 24 hours and stained with antibodies to CD11b, F4/80, CD206 and intracellular IL-10. The plot shows a representative experiment for IL-10 expression by cells gated on CD11b, F4/80, and CD206. The bar graph shows the percent mean ± SD of CD11b+F4/80+CD206+IL-10+ cells compiled from three independent experiments. *p<0.05 as determined by two-tailed Student’s t-test. (D) Pancreatic cells were harvested from 10 week-old 13R−/− female NOD mice and used to sort two populations of CD11b+F4/80+ macrophages (Mϕ) on the basis of CD206 expression. The CD206+ and CD206− Mϕ were separately transferred (5 × 105 cells/mouse) into 12-week old 13R+/+ mice to test for resistance to T1D. The hosts given CD206+ (n = 9) and CD206− (n =8) cells were then monitored for BGL twice a week for 15 weeks. A group of mice (n = 7) which did not receive any cell transfer (NIL) was included for control purposes. A mouse is considered diabetic when the BGL is ≥300 mg/dl for three consecutive tests. **p<0.005 and ***p<0.0001 as determined by Mann-Whitney U test.
Discussion
IL-4 and IL-13 are viewed as anti-inflammatory cytokines (25, 26) and function to modulate autoimmunity (27–30). To gain insights on the mechanism by which these cytokines counter T1D, a NOD mouse deficient for IL-4Rα/IL-13Rα1 HR, which serves both cytokines (31, 32), was generated and monitored for development of disease. Surprisingly, these animals displayed resistance to disease and the onset of T1D was significantly delayed relative to mice in which the HR is intact. Furthermore, the splenocytes from diabetic 13R−/− mice were not as efficient as spleen cells from 13R+/+ mice in transferring disease into NOD.scid mice, suggesting a state of tolerance. Examination of the frequency of Th1 and Th17 cells in the spleen of 13R−/− diabetic mice indicated a decrease in Th1 but an increase in Th17 cells relative to 13R+/+ mice. In contrast, the frequency of Th1 cells in the pancreas was significantly increased while the percentage of Th17 cells was much lower in 13R−/− relative to 13R+/+ mice. Given that 13R controls IL-12 production by DCs (33) and IL-12R expression by naïve T cells (13), it is possible that its deficiency interferes with the generation of Th1 cells leading to delayed accumulation of these cells in the pancreas. Furthermore, diminished Th1 cells in the spleen would favor the differentiation of Th17 cells as IFNγ is known to influence differentiation of Th17 cells (34, 35). Also, HR-deficiency perhaps affects the dynamics of spleen-to-pancreas trafficking of effector T cells. Alternatively, as effector T cells can display different patterns of sensitivity to suppression by Tregs (36–40), it is possible that the Th17 effectors are more sensitive than Th1 cells to suppressive factors prevalent in the pancreas. The latter suggestion finds support by reports showing that mTGFβ+FoxP3int Tregs (16) are indeed more frequent in the 13R−/− pancreatic milieu during the insulitis stage. In fact, these FoxP3int Tregs were able to drive resistance against T1D when they were transferred to 13R+/+ NOD mice. As subsets of Tregs, including FoxP3int Tregs, utilize mTGFβ to carry out suppressive function (16, 41), it may be that Th17 and Th1 effectors display differential expression of the TGFβ receptor leading to discrepancy on their sensitivity to suppression by FoxP3Int Tregs. Furthermore, a subset of macrophages expressing CD206, an M2 marker for these cells, were found in the pancreas of the 13R−/− T1D-resistant, but not the 13R+/+ T1D susceptible mice. These CD206+ M2-like macrophages are suppressive and confer resistance to T1D when transferred to 13R+/+ mice and this is likely due to the production of anti-inflammatory IL-10 cytokine and arginase 1 enzyme. Again, these macrophages and their cytokine(s) may interfere with spleen-to-pancreas trafficking of Th1 and Th17 effector cells and/or inflict differential suppressive function on the 2 subsets of effector cells. The presence of CD206+ M2-like macrophages in the pancreas of 13R−/− mice is intriguing as expression of IL-13Rα1 is usually associated with the M2 macrophage phenotype (14). On a speculative basis, it is possible that CD206+ macrophages traffic to the pancreas more efficiently in the absence of IL-13Rα1. Alternatively, signaling through the conventional IL-4R under inflammatory circumstance may trigger apoptosis of the CD206 macrophage subset.
In summary, interference with HR function in NOD mice confers resistance against T1D mainly by supporting the development of suppressive FoxP3int Tregs and sustaining the persistence of CD206+ macrophages in the pancreas. This suggests that in wild type mice the HR serves to counter these events, perhaps by interference with signaling pathways involved in Treg and macrophage development and survival.
Supplementary Material
Acknowledgments
Funding. This work was supported by grants R01DK093515 and RO1NS057194 from the National Institutes of Health (to HZ), by funds from the Leda J. Sears Trust and by the J. Lavenia Edwards endowment. M.M.M. was supported by T32 Training Grant GM008396 from the National Institute of General Medical Sciences.
Footnotes
Abbreviations. Arg1, arginase 1; BGL, blood glucose level; DC, dendritic cell; DT, diphtheria toxin; FIZZ1, found in inflammatory zone 1; hi, high; int, intermediate; MFI, mean fluorescence intensity; Mϕ, macrophage; mTGFβ, membrane TGFβ; PLN, lymph node; PN, pancreas; SP, spleen; T1D, type 1 diabetes; Tregs, T regulatory cells; HR, IL-4Rα/IL-13Rα1 heteroreceptor; 13R, IL-13Rα1.
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller R, Krahl T, Sarvetnick N. Pancreatic expression of interleukin-4 abrogates insulitis and autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 1996;184:1093–1099. doi: 10.1084/jem.184.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, Kronenberg M, Koezuka Y, Delovitch TL, Gombert JM, Leite-De-Moraes M, Gouarin C, Zhu R, Hameg A, Nakayama T, Taniguchi M, Lepault F, Lehuen A, Bach JF, Herbelin A. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–1062. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- 5.Kretowski A, Mysliwiec J, Kinalska I. In vitro interleukin-13 production by peripheral blood in patients with newly diagnosed insulin-dependent diabetes mellitus and their first degree relatives. Scand J Immunol. 2000;51:321–325. doi: 10.1046/j.1365-3083.2000.00693.x. [DOI] [PubMed] [Google Scholar]
- 6.Newcomb DC, Boswell MG, Zhou W, Huckabee MM, Goleniewska K, Sevin CM, Hershey GK, Kolls JK, Peebles RS., Jr Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J Allergy Clin Immunol. 2011;127:1006–1013. e1001–1004. doi: 10.1016/j.jaci.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, Lee HH, Bell JJ, Gregg RK, Ellis JS, Gessner A, Zaghouani H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity. 2004;20:429–440. doi: 10.1016/s1074-7613(04)00072-x. [DOI] [PubMed] [Google Scholar]
- 8.Dhakal M, Miller MM, Zaghouani AA, Sherman MP, Zaghouani H. Neonatal Basophils Stifle the Function of Early-Life Dendritic Cells To Curtail Th1 Immunity in Newborn Mice. J Immunol. 2015;195:507–518. doi: 10.4049/jimmunol.1500027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Paul WE. Impaired interleukin 4 signaling in T helper type 1 cells. J Exp Med. 1998;187:1305–1313. doi: 10.1084/jem.187.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 11.Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300:1527–1528. doi: 10.1126/science.1085458. [DOI] [PubMed] [Google Scholar]
- 12.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Jr, Donnelly RP, Wynn TA. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeman CM, Dhakal M, Zaghouani AA, Cascio JA, Wan X, Khairallah MT, Chen W, Zaghouani H. Developmental expression of IL-12Rbeta2 on murine naive neonatal T cells counters the upregulation of IL-13Ralpha1 on primary Th1 cells and balances immunity in the newborn. J Immunol. 2013;190:6155–6163. doi: 10.4049/jimmunol.1202207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhakal M, Hardaway JC, Guloglu FB, Miller MM, Hoeman CM, Zaghouani AA, Wan X, Rowland LM, Cascio JA, Sherman MP, Zaghouani H. IL-13Ralpha1 is a surface marker for M2 macrophages influencing their differentiation and function. Eur J Immunol. 2014;44:842–855. doi: 10.1002/eji.201343755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haymaker CL, Guloglu FB, Cascio JA, Hardaway JC, Dhakal M, Wan X, Hoeman CM, Zaghouani S, Rowland LM, Tartar DM, VanMorlan AM, Zaghouani H. Bone marrow-derived IL-13Ralpha1-positive thymic progenitors are restricted to the myeloid lineage. J Immunol. 2012;188:3208–3216. doi: 10.4049/jimmunol.1103316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartar DM, VanMorlan AM, Wan X, Guloglu FB, Jain R, Haymaker CL, Ellis JS, Hoeman CM, Cascio JA, Dhakal M, Oukka M, Zaghouani H. FoxP3+RORgammat+ T helper intermediates display suppressive function against autoimmune diabetes. J Immunol. 2010;184:3377–3385. doi: 10.4049/jimmunol.0903324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan X, Guloglu FB, VanMorlan AM, Rowland LM, Jain R, Haymaker CL, Cascio JA, Dhakal M, Hoeman CM, Tartar DM, Zaghouani H. Mechanisms underlying antigen-specific tolerance of stable and convertible Th17 cells during suppression of autoimmune diabetes. Diabetes. 2012;61:2054–2065. doi: 10.2337/db11-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsa R, Andresen P, Gillett A, Mia S, Zhang XM, Mayans S, Holmberg D, Harris RA. Adoptive Transfer of Immunomodulatory M2 Macrophages Prevents Type 1 Diabetes in NOD Mice. Diabetes. 2012;61:2881–2892. doi: 10.2337/db11-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of Clinical Investigation. 122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J. Pancreatic beta-Cells Limit Autoimmune Diabetes via an Immunoregulatory Antimicrobial Peptide Expressed under the Influence of the Gut Microbiota. Immunity. 2015;43:304–317. doi: 10.1016/j.immuni.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Kambara K, Ohashi W, Tomita K, Takashina M, Fujisaka S, Hayashi R, Mori H, Tobe K, Hattori Y. In vivo depletion of CD206+ M2 macrophages exaggerates lung injury in endotoxemic mice. Am J Pathol. 2015;185:162–171. doi: 10.1016/j.ajpath.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 24.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proceedings of the National Academy of Sciences. 2013;110:17253–17258. doi: 10.1073/pnas.1308887110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 26.O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 27.Ochoa-Reparaz J, Rynda A, Ascon MA, Yang X, Kochetkova I, Riccardi C, Callis G, Trunkle T, Pascual DW. IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J Immunol. 2008;181:954–968. doi: 10.4049/jimmunol.181.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cihakova D, Barin JG, Afanasyeva M, Kimura M, Fairweather D, Berg M, Talor MV, Baldeviano GC, Frisancho S, Gabrielson K, Bedja D, Rose NR. Interleukin-13 protects against experimental autoimmune myocarditis by regulating macrophage differentiation. Am J Pathol. 2008;172:1195–1208. doi: 10.2353/ajpath.2008.070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min B, Legge KL, Pack C, Zaghouani H. Neonatal exposure to a self-peptide-immunoglobulin chimera circumvents the use of adjuvant and confers resistance to autoimmune disease by a novel mechanism involving interleukin 4 lymph node deviation and interferon gamma-mediated splenic anergy. J Exp Med. 1998;188:2007–2017. doi: 10.1084/jem.188.11.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Homann D, Holz A, Bot A, Coon B, Wolfe T, Petersen J, Dyrberg TP, Grusby MJ, von Herrath MG. Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity. 1999;11:463–472. doi: 10.1016/s1074-7613(00)80121-1. [DOI] [PubMed] [Google Scholar]
- 31.Hilton DJ, Zhang JG, Metcalf D, Alexander WS, Nicola NA, Willson TA. Cloning and characterization of a binding subunit of the interleukin 13 receptor that is also a component of the interleukin 4 receptor. Proc Natl Acad Sci U S A. 1996;93:497–501. doi: 10.1073/pnas.93.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 33.Lee HH, Hoeman CM, Hardaway JC, Guloglu FB, Ellis JS, Jain R, Divekar R, Tartar DM, Haymaker CL, Zaghouani H. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205:2269–2280. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 35.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens GL, McHugh RS, Whitters MJ, Young DA, Luxenberg D, Carreno BM, Collins M, Shevach EM. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 37.Wohlfert EA, Callahan MK, Clark RB. Resistance to CD4+CD25+ regulatory T cells and TGF-beta in Cbl-b−/− mice. J Immunol. 2004;173:1059–1065. doi: 10.4049/jimmunol.173.2.1059. [DOI] [PubMed] [Google Scholar]
- 38.Bopp T, Palmetshofer A, Serfling E, Heib V, Schmitt S, Richter C, Klein M, Schild H, Schmitt E, Stassen M. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+ CD25+ regulatory T cells. J Exp Med. 2005;201:181–187. doi: 10.1084/jem.20041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sojka DK, Hughson A, Sukiennicki TL, Fowell DJ. Early kinetic window of target T cell susceptibility to CD25+ regulatory T cell activity. J Immunol. 2005;175:7274–7280. doi: 10.4049/jimmunol.175.11.7274. [DOI] [PubMed] [Google Scholar]
- 40.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008;181:7350–7355. doi: 10.4049/jimmunol.181.10.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, Yu P, Zaghouani H. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–7316. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.