Abstract
Apolipoprotein L1 gene (APOL1) renal-risk variants exhibit strong genetic association with a spectrum of non-diabetic kidney diseases in individuals with recent African ancestry. Relationships between APOL1 kidney risk variants and cardiovascular disease (CVD) susceptibility and CVD-related death remain controversial. Some studies detected an increased risk for CVD, whereas others support protection from death and subclinical CVD and cerebrovascular disease. Because treatments for non-diabetic kidney disease may target this gene and its protein products, it remains critical to clarify the potential extra-renal effects of APOL1 kidney risk variants. This review addresses the current literature on APOL1 associations with CVD, cerebrovascular disease, and death. Potential causes of disparate results between studies are discussed.
Keywords: African Americans, apolipoprotein L1 (APOL1), cardiovascular disease (CVD), kidney risk variants, genetic risk, atherosclerosis, nonmodifiable risk factor, racial disparities, chronic kidney disease (CKD), death, mortality, cerebrovascular disease, coronary artery calcification (CAC), review
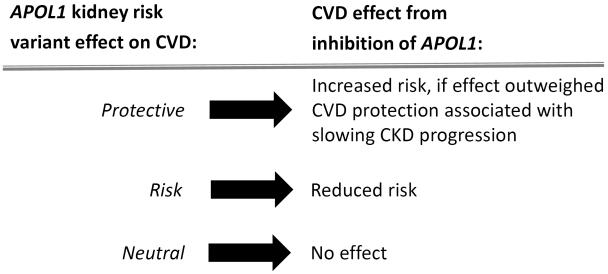
The apolipoprotein L1 gene (APOL1) association with a spectrum of non-diabetic kidney diseases is among the strongest genetic causes of complex disease.1,2,3 Identification of APOL1 has dramatically altered our understanding of susceptibility to glomerulosclerosis in populations with recent African ancestry.4 In contrast, associations of APOL1 kidney risk variants (KRVs) with cardiovascular disease (CVD) and death have been inconsistent; several studies suggest enhanced risk, while a growing body of evidence supports protection.5,6,7,8 APOL1 is expressed in the renal and systemic vasculature, 9,10 and its KRVs are associated with increased plasma small high-density lipoprotein (HDL)–cholesterol particle concentrations.11 These findings support the potential for vascular involvement. Because therapies for APOL1-associated kidney disease will likely target the gene and its protein products, it is critical to fully understand extra-renal effects, including those involving blood vessels (Figure 1). It remains important to halt the development and progression of APOL1-associated chronic kidney disease (CKD) without increasing the potential risk of atherosclerotic complications.
Figure 1.
Postulated effects of APOL1 inhibition on CVD.
Studies Finding an Association of APOL1 and Increased Risk of CVD and Mortality
To assess effects of APOL1 on CVD, Ito et al. examined two study cohorts containing African American participants.12 First, they looked at 1,959 participants in the Jackson Heart Study (JHS), which followed a general African American population for five years. Of participants aged 35-84 years, 284, 892, and 783, respectively, had two, one, and zero APOL1 KRVs. Baseline clinical CVD risk factors were similar between the three genotypic groups. The JHS participants with two APOL1 KRVs had a significant increase in the composite outcome of myocardial infarction (MI), stroke, and therapeutic surgical or endovascular interventions, relative to those with zero KRVs (odds ratio [OR], 2.17; p=9.4×10−4). The increased risk for CVD remained significant in a Cox proportional hazard model that adjusted for age, sex, body mass index (BMI), diabetes, hypertension, smoking, lipids, and CKD (p=0.029). The expected APOL1 associations with CKD, dialysis, and earlier age at onset of kidney disease were present in this population-based report.
In addition, an undisclosed number of JHS participants underwent computed tomography (CT) to measure coronary artery calcified atherosclerotic plaque (coronary artery calcification [CAC]). Surprisingly, despite its association with the composite of MI and stroke, those with two APOL1 KRVs (versus zero KRVs) had lower CAC; however, CT imaging methods, CAC scores, and analysis results were not provided. Effects on CAC reportedly remained significant when participants with CKD were excluded. While lower CAC is protective from CVD events and mortality in all populations,13,14 it has not been associated with an increased risk of CVD as in the JHS. Finally, analyses assessing JHS participants with one APOL1 KRV in additive (zero versus one versus two KRVs) or recessive (zero/one versus two KRVs) models were not provided.
Ito et al. also evaluated 749 African American women aged 50-79 years from the Women’s Health Initiative (WHI), a multi-center randomized control trial of postmenopausal hormone replacement therapy.12 Women with advanced CKD were excluded. The WHI participants with two (n=103) and zero (n=302) APOL1 KRVs were compared for rates of incident CVD and baseline estimated glomerular filtration rate (eGFR) after a mean follow-up of 2.5 years. Those with two APOL1 KRVs had a significantly lower eGFR at baseline; they also had a higher risk of incident CVD (OR, 1.98) during follow-up.
Mukamal et al. examined 798 older African Americans in the Cardiovascular Health Study (CHS), a prospective cohort including 5,888 African American and European American participants aged 65 years or older from four US centers; greater than ten-year follow-up was available.15 African American participants with two APOL1 KRVs (n=91) had significantly higher baseline albuminuria than the 707 participants with zero or one KRVs (p<0.001), without statistically significant differences in baseline eGFR or CVD. Changes in eGFR over time did not differ significantly between APOL1 genotype groups; this may have been a result of the advanced age of this cohort. African Americans developing APOL1-related CKD typically do so at earlier ages; the CHS may contain a sample at lower risk of accelerated declines in eGFR, perhaps due to absence of requisite second hits necessary to initiate progressive kidney disease.16 Compared to the group with zero or one APOL1 KRV, the CHS group with two KRVs had significantly higher all-cause mortality (hazard ratio [HR], 1.3; p=0.05), non-cardiovascular mortality (HR, 1.4; p=0.05), and MI (HR, 1.8; p=0.02). In contrast, there were no statistically significant differences in cardiovascular mortality (HR, 1.3; p=0.31), stroke (HR, 1.2; p=0.80), or congestive heart failure (CHF; HR, 1.0; p=0.98). End points were generally similar between African Americans with less than two APOL1 KRVs and European American CHS participants. Table 1 summarizes the major findings from the JHS, WHI, and CHS. None of these studies adjusted for the overall proportion of African ancestry in their cohorts.
Table 1.
Studies finding an association of APOL1 risk for CVD and mortality
| Study: Population |
Comparisons | End Point - Outcome | Comments |
|---|---|---|---|
| JHS^ (12): 1959 African Americans from general population |
African Americans: APOL1 2 KRVs (n=284) vs APOL1 0 KRVs (n=783) |
CV: CVD* - significant positive association | Significant association after CKD adjustment; Dominant and recessive models not reported |
| CV: CAC score - Significant negative association |
Significant association in participants lacking CKD; Dominant and recessive models not reported |
||
| Renal: CKD† - Significant positive association | Earlier age at CKD onset | ||
| WHI^ (12): 749 postmenopausal African American women |
African Americans: APOL1 2 KRVs (n=103) vs APOL1 <2 KRVs (n=646) |
CV: Major adverse CV event - Significant positive association |
None |
| Renal: eGFR - Significant negative association | Significant negative association with hematocrit |
||
| CHS^ (15): 798 older African Americans; 4964 older European Americans |
African Americans: APOL1 2 KRVs (n=91) vs APOL1 <2 KRVs (n=707) |
Mortality: All-cause - Significant positive association |
None |
| Mortality: CVD - Non-significant | Low event numbers | ||
| Mortality: Non-CVD - Significant positive association |
Low event numbers | ||
| CV: MI - Significant positive association | Low event numbers | ||
| CV: Stroke - Non-significant | Low event numbers | ||
| CV: CHF - Non-significant | Low event numbers | ||
| Renal: eGFR - Non-significant | None | ||
| Renal: Albuminuria - Significant positive association |
None | ||
| African Americans with <2 APOL1 KRVs (n=707) vs European Americans (n=4964) |
No significant differences in mortality, CVD, or renal end points |
None |
CHS, Cardiovascular Health Study; JHS, Jackson Health Study; KRV = kidney risk variant, CVD = cardiovascular disease, CKD = chronic kidney disease, CAC = coronary artery calcification, eGFR= estimated glomerular filtration; WHI, Women’s Health Initiative; CHF, congestive heart failure; MI, myocardial infarction; CV, cardiovascular
myocardial infarction, stroke or therapeutic surgical or endovascular procedure;
eGFR <60 ml/min/1.73m2 or urine albumin-creatinine ratio >30 mg/g’
CHS eGFR used cystatin C-based eGFR; eGFR methodology in JHS and WHI was not specified.
Studies Not Finding an Association of APOL1 and Increased Risk of CVD and Mortality
Prior to direct analysis of potential APOL1 effects on CVD, the African American Study of Kidney Disease and Hypertension (AASK) detected a far higher frequency of kidney end points, relative to deaths, after ten-year follow-up of treated hypertension in non-diabetic African Americans with CKD attributed to high blood pressure.17,18 The composite AASK primary end point included death, doubling of serum creatinine concentration, or initiation of dialysis. The AASK investigators reported higher frequencies of doubling of serum creatinine and dialysis, relative to death. Subsequently, APOL1 KRVs were strongly associated with AASK kidney outcomes, rising serum creatinine concentrations, and albuminuria, whereas the blood pressure treatment arm (standard versus intensive control) and class of anti-hypertensive medications were not.19,20 Kidney function declined relatively steadily among AASK participants with two APOL1 KRVs—supporting the presence of an intrinsic kidney disease process, such as primary forms of glomerulosclerosis.21 A recent AASK analysis failed to detect a significant effect of APOL1 kidney risk variants on survival.22 Results suggested that participants in the intensive blood pressure control arm with two APOL1 kidney risk variants might have improved long-term survival; this effect was not seen in those in the less intensive blood pressure control arm.22
Results from four studies detecting protective or neutral effects of APOL1 KRVs on CVD and mortality are summarized in Table 2. The African American–Diabetes Heart Study (AA-DHS) evaluated 717 participants: 91 with two, 350 with one, and 276 with zero APOL1 KRVs.23 The AA-DHS included only type 2 diabetes–affected individuals, and APOL1 KRVs do not associate with classic diabetic kidney disease. Hence, unlike the JHS, WHI, and CHS, where APOL1 KRVs were associated with kidney disease and/or albuminuria at baseline, confounding of APOL1-kidney disease risk on CVD and mortality outcomes were absent.12,15 APOL1 KRVs showed a significant negative association with CT-derived carotid artery calcified atherosclerotic plaque (p=0.02) and a trend toward negative association with CAC (p=0.08) in dominant models adjusting for age, sex, overall African ancestry proportion, hemoglobin A1c, BMI, smoking, hypertension, renin-angiotensin system blockade, and statins. Mortality data in the AA-DHS came from the Social Security Death Index. The survival analysis demonstrated that participants with two APOL1 KRVs lived significantly longer than those with one KRV, and those with one KRV lived longer than those with zero KRVs (additive genetic model; p=0.005).23 Although AA-DHS and JHS both observed lower levels of calcified atherosclerotic plaque with increasing numbers of APOL1 KRVs, results in AA-DHS demonstrated the expected protective effects of less subclinical CVD in the form of calcified plaque, whereas JHS saw paradoxical increases in risk for the composite CVD outcome.12,23 The CVD events in the JHS were adjudicated and are therefore accurate. Similarly, the outcome of death in the AA-DHS was based on the Social Security Death Index and should be reliable.
Table 2.
Studies not finding an association of APOL1 risk and CVD and mortality
| Study: Population |
Comparisons | End Point - Outcome | Comments |
|---|---|---|---|
| AA-DHS^ (23): 717 African Americans with T2DM |
African Americans with APOL1 2 KRVs (n=91) vs APOL1 1 KRVs (n=350) vs APOL1 0 KRVs (n=276) |
Mortality: All-cause - Significant negative association | p=0.005 (fully-adjusted additive model) |
| CV: CAC score - Non-significant trend toward negative association |
p=0.08 (fully-adjusted dominant model) | ||
| CV: Carotid artery calcium - significant negative association | p=0.02 (fully-adjusted dominant genetic model) | ||
| Renal: eGFR - Non-significant | None | ||
| Renal: Albuminuria - Non-significant | None | ||
| Wake Forest - Emory Dialysis Cohort (28): 725 prevalent African American HD patients |
African Americans with non-DM ESKD APOL1 2 KRVs (n=129) vs APOL1 <2 KRVs (n=146) |
HD survival: Non-DM ESKD - Significant positive association |
p=0.0235 (fully-adjusted additive model); p=0.0385 (fully-adjusted recessive model) |
| African Americans with DM-attributed ESKD APOL1 2 KRVs (n=82) vs APOL1 <2 KRVs (n=368) |
HD survival: DM ESKD - Non-significant | None | |
| SPRINT*^ (24): 2460 African Americans with HTN |
African Americans with APOL1 2 KRVs (n=361) vs APOL1 <2 KRVs (n=2210) |
CV: CVD* - Non-significant | Prevalent CVD (incident data unavailable) |
| Renal: eGFR - Significant negative association | Prevalent data | ||
| Renal: Albuminuria - Significant positive association | Prevalent data | ||
| AA-DHS MIND and SPRINT MIND meta- analysis*^ (29): 680 African Americans with cerebral MRI |
African Americans with APOL1 2 KRVs (n=87) vs APOL1 <2 KRVs (n=593) |
MRI: GMV - Significant positive association | All intracranial measurements associations were also significant in AA-DHS alone but none were in SPRINT before meta-analysis |
| MRI: WMLV - Significant negative association | |||
| Renal: eGFR - Significant negative association | None | ||
| Renal: Albuminuria - Significant positive association | None |
African American-Diabetes Heart Study (AA-DHS); AA-DHS MIND, AA-DHS Memory in Diabetes; KRV = kidney risk variant, CVD = cardiovascular disease, CAC = coronary artery calcification, eGFR = estimated glomerular filtration rate, ESKD = end-stage kidney disease, MRI = magnetic resonance imaging, GMV = gray matter volume, WMLV = white matter lesion volume, SPRINT = Systolic Blood Pressure Intervention Trial; SPRINT MIND, SPRINT Memory and Cognition in Decreased Hypertension; T2DM, type 2 diabetes mellitus; HD, hemodialysis; HTN, hypertension; DM, diabetes mellitus
CVD defined as myocardial infarction, positive cardiac stress test, coronary/carotid/peripheral artery revascularization, ≥50% stenosis of major artery, abdominal aortic aneurysm ≥5cm, CAC score ≥400 Agatston units;
AA-DHS and SPRINT used creatinine-based eGFR
In contrast to longitudinal analyses in the JHS, WHI, CHS, and AA-DHS, cross-sectional associations between APOL1 and CVD were assessed in 2,571 hypertensive African American Systolic Blood Pressure Intervention Trial (SPRINT) participants lacking diabetes.24 A multicenter randomized trial, SPRINT was designed to determine optimal systolic blood pressure targets in high-risk hypertensive patients; the sample was enriched for hypertensive individuals with CKD, those with prior CVD (except stroke), and ethnic minorities.25,26 The main SPRINT CVD outcome included MI, positive cardiac stress testing, coronary/carotid/peripheral vascular revascularization, ≥50% stenosis of a major artery, abdominal aortic aneurysm ≥5 cm, CAC score ≥400 Agatston units, ankle-brachial index ≤0.9, and left ventricular hypertrophy. In addition to this definition, a simpler CVD outcome limited to prior MI and coronary or carotid artery revascularization was employed. Analyses controlled for age, sex, BMI, number of blood pressure medicines, statins, smoking, overall African ancestry proportion, eGFR, and urine albumin-creatinine ratio (UACR). Among African American SPRINT participants, 14% had two APOL1 KRVs (n=361) and 86% (n=2210) had less than two KRVs. Although presence of two APOL1 KRVs was positively associated with greater UACR and reduced eGFR, it was not associated with prevalent CVD based on either the main trial definition or the simplified criteria.
Moreover, an analysis of APOL1 kidney risk variant effects on Atherosclerosis Risk in Communities (ARIC) study outcomes showed significant relationships with the rate of decline in eGFR, development of ESKD, and diabetes mellitus (models adjusted for socioeconomic status and co-morbid conditions); however, relationships with CVD and survival were not observed in fully-adjusted models.27
Patient survival based on APOL1 was also assessed in a cohort of 725 African Americans with end-stage kidney disease (ESKD) on hemodialysis (HD) from the Wake Forest and Emory University out-patient dialysis programs.28 Many dialysis-related deaths are CVD-related; hence, mortality on dialysis was chosen to be a potential surrogate for CVD. Analyses adjusted for age at dialysis initiation, sex, number of comorbid conditions from the Centers for Medicare & Medicaid Services (CMS) Medical Evidence Report, and presence of an arteriovenous fistula or graft (versus a permanent catheter) at dialysis initiation, to represent access to pre-dialysis nephrology care. Among the 275 individuals with non-diabetic ESKD, those with two APOL1 KRVs (compared to those with fewer than two) had younger age at dialysis initiation, fewer comorbid conditions, and longer median survivals on dialysis. Results were consistent after full adjustment and when patients were stratified into groups with age <50 or ≥50 years at dialysis initiation to account for the earlier age at ESKD in those with APOL1 high risk genotypes. In contrast, significant APOL1 effects on survival were not observed in the 450 patients with diabetes-attributed ESKD.
Effects of APOL1 KRVs on the brain and cognitive performance were assessed in 517 AA-DHS Memory in Diabetes (MIND) and 2,568 African American SPRINT Memory and Cognition in Decreased Hypertension (also MIND) participants; 483 of the AA-DHS MIND and 197 of the SPRINT MIND participants underwent cerebral magnetic resonance imaging (MRI).29 In AA-DHS MIND, the presence of two APOL1 KRVs was positively associated with cerebral gray matter volume, and negatively associated with white matter lesion volume (higher values for the latter reflect more severe cerebral small vessel disease) and cerebrospinal fluid volume (a measure of cerebral atrophy). APOL1 was not significantly associated with white matter volume or performance on cognitive testing. The SPRINT sample was less well powered than AA-DHS MIND due to the smaller number with a cerebral MRI; however, directions of associations were consistent with those in AA-DHS MIND. A meta-analysis revealed that APOL1 KRVs were positively associated with gray matter volume and negatively associated with white matter lesion volume. Although APOL1 KRVs were associated with kidney disease and albuminuria in AA-DHS MIND and SPRINT participants, protective associations with cerebral volumes were detected. The reason that APOL1 KRVs were associated with kidney disease in AA-DHS MIND, but not in the parent AA-DHS, likely reflects recruitment of an additional 220 MIND study participants for a cerebral MRI. The importance of these results is that cerebral white matter lesion volume reflects leukoaraiosis on MRI.30 This volume increases as a result of cerebral hypoperfusion with subsequent ischemia related to white matter small vessel disease. Reductions in cerebral small vessel disease are protective to the brain; thus, they likely relate to the APOL1 association with larger gray matter volume reflecting preservation of neuronal cell bodies and other brain cells.
Accounting for Disparate Results Among Studies
It is widely accepted that albuminuria and reduced eGFR independently increase the risk of subclinical CVD and clinical CVD events.31,32,33 Reciprocal relationships appear to exist, whereby CVD also associates with the development of CKD.34 Therefore, studies in which APOL1 KRVs are associated with the presence of CKD and/or albuminuria are at risk for confounding with CVD. In contrast, studies where APOL1 KRVs are not associated (or weakly associated) with CKD have lesser likelihoods of confounding. Whether statistical adjustment is capable of fully accounting for the effects of CKD on the related CVD variable is unknown.
Three studies reported that increasing numbers of APOL1 KRVs were associated with a higher risk for CVD events or death.12,15 In contrast, three other studies reported reduced risk of death, subclinical CVD, and/or cerebrovascular disease in those with increasing numbers of APOL1 KRVs.23,28,29 Results in the AASK suggest the potential for minimal effects of APOL1 on CVD,18 while cross-sectional results in SPRINT were neutral (i.e., no significant relationships were seen).24 Inaccuracy in estimating GFR in African Americans could result in misclassification of some participants with CKD as having normal kidney function, thereby affecting results.35 Inclusion of prevalent patients with CKD or on dialysis, rather than incident patients, can lead to survival bias and might contribute to lower rates of subclinical CVD and cerebrovascular disease in African Americans.36 Chen et al. and Kovesdy et al. reported higher mortality rates in African Americans with CKD, compared to European Americans.37,38 However, lower mortality rates and reduced rates of coronary heart disease were reported in African Americans (versus European Americans) without CKD in the US Veterans Health Administration.38 The “perfect” clinical study does not exist and every report has limitations. This includes all studies discussed in this review. However, a closer inspection of study populations, designs, and end points may provide clues to the different outcomes between these well-described cohorts.
The JHS, WHI, and CHS detected significant positive associations between APOL1 KRVs and CVD events and/or death. The potential for confounding by APOL1 association with CKD and/or albuminuria was present in all studies, and none adjusted for global African ancestry proportion. APOL1 KRVs were significantly associated with baseline parameters of kidney disease in both JHS and WHI.12 Of note, APOL1 did not associate with the progression of CKD over time in JHS—potentially reducing confounding effects. The main JHS analysis focused on participants with the most extreme genotypes, comparing two versus zero APOL1 KRVs; neither additive nor dominant models were presented for association with CVD or death. Those models might have provided interesting results, given subsequent reports.23,28 Although the WHI attempted to recruit healthy post-menopausal women without CKD, significant APOL1 associations with eGFR and hematocrit were evident, the latter potentially reflecting subclinical CKD.12 In the CHS, APOL1 KRVs were significantly associated with baseline albuminuria (but not eGFR).15 Relatively few CVD events were observed during the thirteen-year CHS follow-up period: of 91 participants with high risk APOL1 genotypes, there were 19 MIs, 12 strokes, and 23 CVD-related deaths. Relative to the other reports, the CHS evaluated an older study population. It is unclear what effects advanced age may have had on conclusions; APOL1-associated kidney disease typically develops in the fourth to fifth decades.1 Finally, considering the clinical CVD associations with APOL1 reported in JHS, the negative relationship between APOL1 KRVs with CAC (subclinical atherosclerosis) appear paradoxical.12 Lower degrees of subclinical CVD are widely accepted as protective from CVD.13,14 Thus, the APOL1 association with lower CAC despite greater numbers of CVD events in JHS participants with high-risk genotypes is difficult to reconcile. Details of the APOL1 association analysis with CAC were not provided.
In contrast, the AA-DHS was not confounded by APOL1 associations with kidney disease or albuminuria.23 Not only were reduced levels of subclinical CVD seen based on CT vascular imaging in those with higher numbers of APOL1 KRVs (paralleling JHS results), this finding translated to the expected improved survival in those with lower levels of subclinical CVD. Potential limitations of the AA-DHS are that results may not be generalizable to populations lacking diabetes, and adjudicated CVD events were not captured. In this regard, SPRINT may be informative. The SPRINT study excluded individuals with diabetes and prior strokes.24 However, just as in the JHS, WHI, and CHS, APOL1 KRVs were associated with baseline kidney disease in SPRINT. This association was weak and likely related to SPRINT inclusion criteria that permitted recruitment of patients with low level proteinuria (<1 g/d) and reduced eGFR. Compared with the 13% of African Americans in the general population who possess two APOL1 KRVs (i.e., have high risk for CKD genotypes), a similar percentage of African American SPRINT participants had two KRVs (14%). Thus, the relatively weak APOL1-association with kidney disease in SPRINT participants, whose KRV frequencies mirrored the general African American population, could reduce the confounding between CKD and CVD. Despite recruitment of participants enriched for prior CVD or at high risk for future CVD in SPRINT, APOL1 was not significantly associated with prevalent CVD. SPRINT limitations include the lack of longitudinal follow-up; however, those analyses are underway given early cessation of SPRINT due to significant reductions in CVD with more intensive lowering of systolic blood pressure.26
Potential support for vascular protection related to APOL1 KRVs was seen with improved survival of African Americans with non-diabetic etiologies of ESKD.28 The effects of APOL1 KRVs on the brain also appeared to parallel those in the systemic vasculature. Similar to the lower levels of calcified atherosclerotic plaque in African Americans with increasing numbers of APOL1 KRVs, lesser degrees of cerebral white matter small vessel disease (and larger volumes of gray matter) were present in African Americans with greater numbers of APOL1 KRVs.29 This observation was robust to adjustment for APOL1 KRV association with CKD, which is important because these protective relationships were observed despite association between APOL1 with non-diabetic ESKD28 and with parameters of kidney disease in AA-DHS MIND.29
We note that many of the studies in Table 2 (relative to those in Table 1) included participants with type 2 diabetes. Several cellular pathways appear to differ between those with diabetic and non-diabetic forms of kidney disease, including autophagy. The APOL1 protein contains a single BH3 domain; members of the so-called BH3-only family are involved in the autophagy pathway,39 which is down-regulated in diabetic kidney disease. Consistent with this, several modifiers of kidney disease progression in AASK had effects in obese individuals with APOL1 kidney risk alleles.40
Study End Points
These studies employed potentially related, but markedly different, end points. As such, end point selection may have contributed to the differing conclusions. The JHS evaluated the composite end point of MI, stroke, or therapeutic surgical or endovascular procedure, whereas the WHI evaluated all major adverse cardiovascular events.12 The CHS studied MI, stoke, CHF, total mortality, CVD mortality, and non-CVD mortality.15 The possible APOL1 KRV effect of increasing risk for MI in these three studies would be expected to yield higher rates of CVD mortality. However, CHS saw significantly higher all-cause mortality and non-CVD mortality, whereas effects on CVD mortality were non-significant.15 In contrast, the AA-DHS and a study of hemodialysis patients with non-diabetic ESKD assessed overall participant survival (all cause-mortality); independent CVD events were not measured.23,28 These two studies detected lower rates of death with increasing numbers of APOL1 KRVs. It was presumed that the majority of deaths in African Americans with type 2 diabetes and with ESKD would be CVD-related, but this was not formally tested. Subclinical CVD differs from adjudicated CVD events.
Genetic Risk for Atherosclerosis in African Americans
In contrast to CKD, recent African ancestry is protective from the development of calcified atherosclerotic plaque (subclinical atherosclerosis, including CAC) in admixed populations with and without diabetes.41,42 European ancestry increases the risk for CAC. Therefore, we typically adjust for global African ancestry proportion in genetic studies evaluating subclinical CVD in African Americans. Despite more severe conventional CVD risk factors (e.g., hypertension, albuminuria, higher low-density lipoprotein cholesterol, and poorer glycemic control among patients with diabetes), African Americans have substantially lower levels of CAC and calcified atherosclerotic plaque in the aorta and carotid arteries relative to European Americans.43,44,45 This radiologic observation has great clinical relevance. It translates to an approximate 50% reduction in MIs among African Americans with equal access to healthcare as European Americans in the Kaiser Permanente Health Maintenance Organization and Veterans Health Administration.46,47 African Americans with ESKD are also known to live significantly longer on CMS-supported renal replacement therapy and have fewer MIs relative to European Americans with ESKD.48
The majority of reports from our group observed APOL1-associated protection from the development of subclinical CVD or cerebrovascular disease. It is possible that part of the increased risk of CVD and MI reported by JHS, WHI, and CHS was affected by the APOL1 association with baseline CKD or albuminuria.12,15 Potentially protective effects of APOL1 KRVs on death, CVD, and subclinical cerebrovascular disease are supported by AA-DHS, AA-DHS MIND, and studies in prevalent patients receiving hemodialysis.18,23,28,29 Therefore, we feel that there is a need for additional studies to elucidate relationships between APOL1 KRVs and CVD. Ideally, studies will incorporate designs that are only minimally confounded by the APOL1 association with progressive kidney disease, perhaps large population-based samples without over-representation of patients with advanced CKD (controlling for participant age and relevant CVD risk factors) and studies in cohorts with type 2 diabetes where APOL1 is not associated with kidney disease (additionally controlling for diabetes duration and glycemic control). These studies will need to be well-powered with sufficient follow-up time to detect extra-renal effects of APOL1, which are likely weaker than those for kidney disease. Future studies should also consider overall African ancestry proportion, since it strongly and reproducibly associates with the risk of subclinical CVD. It should be kept in mind that even if studies support that APOL1 kidney risk variants confer some protection from CVD, the effect will have to be weighed against the powerful CVD risk that is conferred by CKD itself. If APOL1 inhibitors are identified, their clinical role needs to balance mitigation of CKD progression (with its attendant risk for CVD and mortality outcomes) versus potential direct adverse CVD effects of such inhibition. Nephrologists are respected for treating many of our patient’s medical problems, not simply kidney disease. In a similar fashion, while we focus on eradicating APOL1-associated kidney disease, we must remain vigilant for the vascular effects of APOL1.
Acknowledgements
Support: National Institutes of Health grants R01 DK071891, R01 DK070941, R01 DK084149, and R01 NS075107 to Dr Freedman.
Wake Forest University Health Sciences and Dr Freedman have filed for a patent related to APOL1 genetic testing. Dr Freedman receives research support from Novartis Pharmaceuticals and is a consultant for Ionis Pharmaceuticals.
Footnotes
Financial Disclosure: The other authors declare that they have no relevant financial interests.
Peer Review: Evaluated by 2 external peer reviewers, a Co-Editor, and Editor-in-Chief Levey.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, Bekele E, Bradman N, Wasser WG, Behar DM, Skorecki K. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128(3):345–350. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR. The Apolipoprotein L1 (APOL1) Gene and Nondiabetic Nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freedman BI, Cohen AH. Hypertension-attributed nephropathy: what's in a name? Nat Rev Nephrol. 2016;12(1):27–36. doi: 10.1038/nrneph.2015.172. [DOI] [PubMed] [Google Scholar]
- 5.Lipkowitz MS. Apolipoprotein L1: from obscurity to consistency to controversy. Kidney Int. 2015;87(1):14–17. doi: 10.1038/ki.2014.319. [DOI] [PubMed] [Google Scholar]
- 6.Farrall M. Cardiovascular twist to the rapidly evolving apolipoprotein L1 story. Circ Res. 2014;114(5):746–747. doi: 10.1161/CIRCRESAHA.114.303354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruzel-Davila E, Wasser WG, Aviram S, Skorecki K. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transplant. 2016;31(3):349–58. doi: 10.1093/ndt/gfu391. [DOI] [PubMed] [Google Scholar]
- 8.Reiner AP, Susztak K. APOL1 Variants: From Parasites to Kidney Function to Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2016;36(2):219–220. doi: 10.1161/ATVBAHA.115.306794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhavan SM, O'Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22(11):2119–2128. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, Saleem MA, Satchell SC, Banas B, Mathieson PW, Kretzler M, Hemal AK, Rudel LL, Petrovic S, Weckerle A, Pollak MR, Ross MD, Parks JS, Freedman BI. Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol. 2015;26(2):339–348. doi: 10.1681/ASN.2013091017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez OM, Judd SE, Irvin MR, Zhi D, Limdi N, Palmer ND, Rich SS, Sale MM, Freedman BI. APOL1 nephropathy risk variants are associated with altered high-density lipoprotein profiles in African Americans. Nephrol Dial Transplant. 2016;31(4):602–8. doi: 10.1093/ndt/gfv229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito K, Bick AG, Flannick J, Friedman DJ, Genovese G, Parfenov MG, Depalma SR, Gupta N, Gabriel SB, Taylor HA, Jr., Fox ER, Newton-Cheh C, Kathiresan S, Hirschhorn JN, Altshuler DM, Pollak MR, Wilson JG, Seidman JG, Seidman C. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res. 2014;114(5):845–850. doi: 10.1161/CIRCRESAHA.114.302347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 14.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. J Am Coll Cardiol. 2004;43(9):1663–1669. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 15.Mukamal KJ, Tremaglio J, Friedman DJ, Ix JH, Kuller LH, Tracy RP, Pollak MR. APOL1 Genotype, Kidney and Cardiovascular Disease, and Death in Older Adults. Arterioscler Thromb Vasc Biol. 2016;36(2):398–403. doi: 10.1161/ATVBAHA.115.305970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman BI, Skorecki K. Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol. 2014;9(11):2006–2013. doi: 10.2215/CJN.01330214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appel LJ, Wright JT, Jr., Greene T, Kusek JW, Lewis JB, Wang X, Lipkowitz MS, Norris KC, Bakris GL, Rahman M, Contreras G, Rostand SG, Kopple JD, Gabbai FB, Schulman GI, Gassman JJ, Charleston J, Agodoa LY, for the African American Study of Kidney Disease and Hypertension Collaborative Research Group Long-term Effects of Renin-Angiotensin System-Blocking Therapy and a Low Blood Pressure Goal on Progression of Hypertensive Chronic Kidney Disease in African Americans. Archives of Internal Medicine. 2008;168(8):832–839. doi: 10.1001/archinte.168.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alves TP, Wang X, Wright JT, Jr., Appel LJ, Greene T, Norris K, Lewis J. Rate of ESRD exceeds mortality among African Americans with hypertensive nephrosclerosis. J Am Soc Nephrol. 2010;21(8):1361–1369. doi: 10.1681/ASN.2009060654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, Astor BC, Bottinger EP, Iyengar SK, Klotman PE, Freedman RG, Zhang W, Parekh RS, Choi MJ, Nelson GW, Winkler CA, Kopp JB. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, Feldman HI, Parekh RS, Kusek JW, Greene TH, Fink JC, Anderson AH, Choi MJ, Wright JT, Jr., Lash JP, Freedman BI, Ojo A, Winkler CA, Raj DS, Kopp JB, He J, Jensvold NG, Tao K, Lipkowitz MS, Appel LJ. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tin A, Grams ME, Estrella M, Lipkowitz M, Greene TH, Kao WH, Li L, Appel LJ. Patterns of Kidney Function Decline Associated with APOL1 Genotypes: Results from AASK. Clin J Am Soc Nephrol. 2016;11(8):1353–9. doi: 10.2215/CJN.12221115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ku E, Lipkowitz MS, Appel LJ, et al. Strict blood pressure control and associates with decreased mortality risk by APOL1 genotype. Kidney International. 2016 doi: 10.1016/j.kint.2016.09.033. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman BI, Langefeld CD, Lu L, Palmer ND, Carrie SS, Bagwell BM, Hicks PJ, Xu J, Wagenknecht LE, Raffield LM, Register TC, Jeffrey CJ, Bowden DW, Divers J. APOL1 associations with nephropathy, atherosclerosis, and all-cause mortality in African Americans with type 2 diabetes. Kidney Int. 2015;87(1):176–81. doi: 10.1038/ki.2014.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langefeld CD, Divers J, Pajewski NM, Hawfield AT, Reboussin DM, Bild DE, Kaysen GA, Kimmel PL, Raj DS, Ricardo AC, Wright JT, Jr., Sedor JR, Rocco MV, Freedman BI. Apolipoprotein L1 gene variants associate with prevalent kidney but not prevalent cardiovascular disease in the Systolic Blood Pressure Intervention Trial. Kidney Int. 2015;87(1):169–75. doi: 10.1038/ki.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC, Jr., Johnson KC, Killeen AA, Lewis CE, Oparil S, Reboussin DM, Rocco MV, Snyder JK, Williamson JD, Wright JT, Jr., Whelton PK. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT) Clin Trials. 2014;11(5):532–546. doi: 10.1177/1740774514537404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright JT, Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grams ME, Rebholz CM, Chen Y, Rawlings AM, Estrella MM, Selvin E, Appel LJ, Tin A, Coresh J. Race, APOL1 Risk, and eGFR Decline in the General Population. J Am Soc Nephrol. 2016;27(9):2842–2850. doi: 10.1681/ASN.2015070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Langefeld CD, Comeau ME, Bonomo JA, Rocco MV, Burkart JM, Divers J, Palmer ND, Hicks PJ, Bowden DW, Lea JP, Krisher JO, Clay MJ, Freedman BI. APOL1 renal-risk genotypes associate with longer hemodialysis survival in prevalent nondiabetic African American patients with end-stage renal disease. Kidney Int. 2016;90(2):389–95. doi: 10.1016/j.kint.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedman BI, Gadegbeku CA, Bryan RN, Palmer ND, Hicks PJ, Ma L, Rocco MV, Smith SC, Xu J, Whitlow CT, Wagner BC, Langefeld CD, Hawfield AT, Bates JT, Lerner AJ, Raj DS, Sadaghiani MS, Toto RD, Wright JT, Jr., Bowden DW, Williamson JD, Sink KM, Maldjian JA, Pajewski NM, Divers J. APOL1 renal-risk variants associate with reduced cerebral white matter lesion volume and increased gray matter volume. Kidney Int. 2016;90(2):440–9. doi: 10.1016/j.kint.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao D, Cooper L, Cai J, Toole JF, Bryan NR, Hutchinson RG, Tyroler HA. Presence and severity of cerebral white matter lesions and hypertension, its treatment, and its control. The ARIC Study. Atherosclerosis Risk in Communities Study. Stroke. 1996;27(12):2262–2270. doi: 10.1161/01.str.27.12.2262. [DOI] [PubMed] [Google Scholar]
- 31.Wannamethee SG, Shaper AG, Perry IJ. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke. 1997;28(3):557–563. doi: 10.1161/01.str.28.3.557. [DOI] [PubMed] [Google Scholar]
- 32.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134(8):629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 33.McCullough PA, Jurkovitz CT, Pergola PE, McGill JB, Brown WW, Collins AJ, Chen SC, Li S, Singh A, Norris KC, Klag MJ, Bakris GL, for the KEEP Investigators Independent Components of Chronic Kidney Disease as a Cardiovascular Risk State: Results From the Kidney Early Evaluation Program (KEEP) Archives of Internal Medicine. 2007;167(11):1122–1129. doi: 10.1001/archinte.167.11.1122. [DOI] [PubMed] [Google Scholar]
- 34.Elsayed EF, Tighiouart H, Griffith J, Kurth T, Levey AS, Salem D, Sarnak MJ, Weiner DE. Cardiovascular Disease and Subsequent Kidney Disease. Archives of Internal Medicine. 2007;167(11):1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 35.Delanaye P, Mariat C, Maillard N, Krzesinski JM, Cavalier E. Are the creatinine-based equations accurate to estimate glomerular filtration rate in African American populations? Clin J Am Soc Nephrol. 2011;6(4):906–912. doi: 10.2215/CJN.10931210. [DOI] [PubMed] [Google Scholar]
- 36.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19(7):1403–1410. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen TK, Estrella MM. APOL1 risk variants and death among African American hemodialysis patients: survival of the fittest? Kidney Int. 2016;90(2):249–252. doi: 10.1016/j.kint.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 38.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, Molnar MZ, Kalantar-Zadeh K. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132(16):1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhaorigetu S, Wan G, Kaini R, Jiang Z, Hu CA. ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy. 2008;4(8):1079–1082. doi: 10.4161/auto.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen TK, Choi MJ, Kao WH, Astor BC, Scialla JJ, Appel LJ, Li L, Lipkowitz MS, Wolf M, Parekh RS, Winkler CA, Estrella MM, Crews DC. Examination of Potential Modifiers of the Association of APOL1 Alleles with CKD Progression. Clin J Am Soc Nephrol. 2015;10(12):2128–35. doi: 10.2215/CJN.05220515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wassel CL, Pankow JS, Peralta CA, Choudhry S, Seldin MF, Arnett DK. Genetic ancestry is associated with subclinical cardiovascular disease in African-Americans and Hispanics from the multi-ethnic study of atherosclerosis. Circ Cardiovasc Genet. 2009;2(6):629–636. doi: 10.1161/CIRCGENETICS.109.876243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Divers J, Palmer ND, Lu L, Register TC, Carr JJ, Hicks PJ, Hightower RC, Smith SC, Xu J, Cox AJ, Hruska KA, Bowden DW, Lewis CE, Heiss G, Province MA, Borecki IB, Kerr KF, Chen YD, Palmas W, Rotter JI, Wassel CL, Bertoni AG, Herrington DM, Wagenknecht LE, Langefeld CD, Freedman BI. Admixture mapping of coronary artery calcified plaque in african americans with type 2 diabetes mellitus. Circ Cardiovasc Genet. 2013;6(1):97–105. doi: 10.1161/CIRCGENETICS.112.964114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111(10):1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 44.Lee TC, O'Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41(1):39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 45.Freedman BI, Hsu FC, Langefeld CD, Rich SS, Herrington DM, Carr JJ, Xu J, Bowden DW, Wagenknecht LE. The impact of ethnicity and sex on subclinical cardiovascular disease: the Diabetes Heart Study. Diabetologia. 2005;48(12):2511–2518. doi: 10.1007/s00125-005-0017-2. [DOI] [PubMed] [Google Scholar]
- 46.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA. 2002;287(19):2519–2527. doi: 10.1001/jama.287.19.2519. [DOI] [PubMed] [Google Scholar]
- 47.Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003;26(8):2392–2399. doi: 10.2337/diacare.26.8.2392. [DOI] [PubMed] [Google Scholar]
- 48.Young BA, Rudser K, Kestenbaum B, Seliger SL, Andress D, Boyko EJ. Racial and ethnic differences in incident myocardial infarction in end-stage renal disease patients: The USRDS. Kidney Int. 2006;69(9):1691–1698. doi: 10.1038/sj.ki.5000346. [DOI] [PubMed] [Google Scholar]