Abstract
The IL-23/IL-17 pathway is important in multiple autoimmune diseases, but its effect on lupus pathology remains unclear with opposing trials in murine models of the disease. Herein we show a disease activity related upregulation of serum IL-23 and IL-23 receptor in patients with SLE as compared to healthy controls. IL-23 when added in SLE T cell in vitro cultures, induced IL-17 and limited IL-2 production while Tfh and DN T cells significantly expanded. To further dissect the role of IL-23 in the expression of autoimmunity and related pathology, we generated IL-23 receptor deficient MRL.lpr mice. These IL-23R−/−MRL.lpr mice displayed attenuated lupus nephritis with a striking decrease in the accumulation of double negative T (DN T) cells in the kidneys and secondary lymphoid organs. Moreover, T cells from IL-23R−/−MRL.lpr mice produced increased amounts of IL-2 and reduced amounts of IL-17 compared to T cells from wild type animals. In vitro IL-23 treatment promoted IL-17 production and downregulated IL-2 production. The IL-23R−/−MRL.lpr had fewer T follicular helper cells (Tfh), B cells and plasma cells, leading to decreased production of anti-dsDNA antibodies. Our results show that IL-23 accounts for main aspects of human and murine lupus including the expansion of DN T cells, decreased IL-2 and increased IL-17 production. We propose that blockade of IL23 should have a therapeutic value in patients with SLE.
Keywords: Murine models of lupus, SLE, interleukins
Introduction
Systemic Lupus Erythematosus (SLE) is an autoimmune disease characterized by an imbalance between pro-inflammatory, such as IFNγ, and regulatory cytokines, such as IL-2 (reviewed in (1)). This imbalance underlies the basic pathophysiologic steps that eventually lead to organ damage: production of autoantibodies, immune complex formation and deposition, inflammatory cell migration and activation in various tissues such as the skin, joints and kidneys. Although a multitude of biologic medications have been used to disrupt cytokine signaling in SLE, only belimumab, an anti-B Lymphocyte stimulator (BLyS), has been shown to be modestly effective, in the treatment of this disease (2).
Based on some early findings that the pro-inflammatory cytokine IL-17 is expressed by T lymphocytes in kidney biopsies of patients with lupus nephritis (3), we proposed that Th17 cells may be important in the pathogenesis of the disease. As generation of these cells depends on Interleukin 23 (IL-23), a member of the IL-12 family (4, 5), we examined the effect of this cytokine in the model of autoimmunity B6.lpr. These mice bear a mutation (lpr) on the Fas gene that results in deficient lymphocyte apoptosis. This leads to massive hyperplasia of their secondary lymphoid organs, expression of variety of autoantibodies, but only mild kidney and skin inflammation. We found that B6.lpr mice that lack the receptor for IL-23 do not develop pathogenic autoantibodies or organ inflammation (6). Moreover, there was a significant reduction of the CD3+CD4−CD8− T (double negative, DN T) cell population that is abnormally expanded in these mice. We then used a neutralizing anti-IL-23 Ab to treat MRL.lpr mice that, as opposed to B6.lpr mice, develop severe nephritis similar to SLE; the treated mice had less severe nephritis than control-treated mice suggesting but not conclusively proving that IL-23 plays a role in the development of autoimmunity and ensuing inflammation (7).
It was further hypothesized based on well-established models of autoimmunity, that the pro-inflammatory effects of IL-23 depend on the production of IL-17A and/or F by differentiated T helper cells (Th17). Targeting though IL-17 directly showed opposing effects in different murine lupus trials (8, 9), suggesting that IL-23 potentially has a broader effect on the development of SLE.
Both MRL.lpr lymphocytes and SLE T cells produce reduced amounts of the regulatory cytokine IL-2 in vitro (10). This is particularly true for the DN T cell population that is expanded in MRL.lpr mice and SLE patients and is reduced in the absence of IL-23R. We therefore hypothesized that IL-23 not only induces IL-17 but also decreases IL-2 production, leading to pro-inflammatory/anti-inflammatory cytokine imbalance.
To answer the question whether IL-23 is central in the pathophysiology of SLE, we evaluated its concentration in the serum of SLE patients, its relation to disease flares and its direct effect on SLE T cells ex vivo. Furthermore, we generated MRL.lpr mice that lack the IL-23 receptor and studied in vivo the development of nephritis. We present for the first-time evidence that IL-23 supports the generation of extra-follicular T helper cells in SLE T cell cultures, promoting the production of anti-dsDNA Ab. Similarly, IL-23 receptor deficiency in MRL.lpr mice prevented the development of nephritis by inhibiting the generation of double negative T cells and extra-follicular T helper cells. Besides the expected effect of IL-23 on IL-17 production, we show that IL-23 critically influences the production of IL-2, limiting the activation of the transcription factor NFκB.
Materials and Methods
Patients
Patients who fulfilled the American College of Rheumatology (ACR) criteria for the diagnosis of SLE (11) were enrolled in the study by donating 50 mL of blood. The BIDMC IRB approved the study protocol and informed consents were obtained from all study subjects. We included 69 patients in the studies (64 female and 5 male, age: 39.8+/−8.9 years, racial distribution: 54% white, 35% african-american, 10% asian, 1% other). SLE disease activity was measured by SLEDAI (12). Medications used included: hydroxychloroquine (86%), prednisone (65%, dose: 5–50 mg/day), mycophenolate mofetil (32%), cyclophosphamide IV (2 patients), belimumab (1 patient), azathioprine (17.4%). We included 27 healthy controls (26 female, 1 male, age: 40+/−8.5 years, racial distribution: 48% white, 41% african-american, 7% asian, 4% other).
T cells were isolated by negative selection using the RosetteSep T Cell Isolation Kit (StemCell Technologies) unless otherwise indicated. PBMCs were isolated using a Ficoll gradient. Serum was isolated from 10 mL serum separator tubes.
Mice
The IL-23R–deficient C57BL/6 mice were generated as previously described (6). All mice were housed at the Beth Israel Deaconess Medical Center pathogen-free animal facility (Boston, MA). Our protocol was approved by the BIDMC IACUC.
We generated IL-23R–deficient MRL.lpr mice using a backcross-intercross scheme. After 12 generations of breeding, the mice were PCR screened for the Fas/lpr and mutated IL-23R gene. Primer for Fas/lpr genetic screen: 5′ GTAAATAATTGTGCTTCGTCAG-3′, 5′-TAGAAAGGTGCACGGGTGTG- 3′, and 5′-CAAATCTAGGCATTAACAGTG-3′; IL-23R genetic screen: 5′-ACCCCTAGGAATGCTCGTCAAG-3′ and 5′-TGGTTGCCTGCACCAATTTAAAAG-3′; and for homozygous versus heterozygous mutated: 5′-GATCATCTTATGGCTGGTCCTC-3′ and 5′GAGTGAGACAGTGTAGCCACAGAT-3′.
Flow cytometry
2×106 cells were suspended in 50 μl FACS buffer containing fluorescent antibodies. All cells were stained with Zombie aqua (Biolegend). The following antibodies were used for staining: CD45, CD3, CD4, CD8, TCRβ, CXCR5, PD-1, CXCR4, PSGL-1, CD138, CD19, CD25, IL-23R, Thy1.2, CD44, CD62L (BD Pharmingen); For Ki67 (Biolegend) and Foxp3 (eBioscience) measurements, after surface staining, cells were fixed, rendered permeable and intracellularly stained following the manufacturer instructions. Cells were analyzed by flow cytometry (FACS Caliber; BD Biosystems, San Jose, USA).
Cell culture, cytokine and protein measurement
Murine lymphocytes were cultured in RPMI1640 with 10% (v/v) FCS (supplemented with 50 μM 2-ME, 1 mM sodium pyruvate, non-essential amino acids, L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin) at 37°C in a humidified atmosphere of 10% CO2 in culture incubator.
SLE patients or murine lymphocytes were incubated (2 ×106/ml) in plates coated with 5μg/ml anti-CD3/28. IL-23 (25 ng/ml, R&D Systems) was added as described. Before collection, PMA, calcium ionophore (A23187) and 1 μl/ml brefeldin A (Golgi-Plug; BD Pharmingen) were added. The cells were subsequently collected and IL-17, IL-2 and/or IFN-γ production was measured by intracellular staining. For western blots, cells were lysed using the Nuclear Extraction Kit (Abcam) and immunoblotted using anti-NFκBp65 and β-actin antibodies (Santa Cruz)
ELISA was used for the measurement of IL-2, IFN-γ and IL-17 in supernatants (Biolegend). Anti-dsDNA antibody titers in serum and supernatants were also measured using ELISA (Alpha Diagnostic).
Histopathology and tissue cell isolation
The kidneys were fixed in 10% formalin overnight at 4°C, embedded in paraffin, and cut at 6 μM before being stained with H&E and periodic acid-Schiff reagent. We evaluated in a blinded fashion glomerular pathology (in 100 glomeruli/kidney) using a semi-quantitative scale as before (13, 14).
Cells were extracted from murine spleens and lymph nodes by filtering the tissue through a 100-μm BD Biosciences Falcon cell strainer. The extracts were centrifuged at 1200 rpm for 5 min. ACK lysing buffer (Quality Biological) solution was added in the cell pellet to lyse the red cells. The treated cell pellet was subsequently washed once with DMEM cell culture medium and re-suspended in medium for further treatment or staining.
Murine kidney tissue was incubated with collagenase type A (10 μg/ml; Sigma-Aldrich) in cold Dulbecco’s PBS buffer with EDTA (2 μM) for 15 min at 37°C. The digested kidney tissue suspension was strained through a 100-μm BD Biosciences Falcon cell strainer (Fisher Scientific). The cells were centrifuged at 1200 rpm for 5 min and the cell pellet washed and re-suspended for treatment or staining.
BrdU assay
Mice were injected with 5-bromo-deoxyuridine (BrdU; 100 mg/kg i.p. BD Biosciences San Jose, CA) and then sacrificed 3 hours later. BrdU staining was performed using a BrdU labeling kit (BD).
Statistical analysis
Statistical analyses were performed in GraphPad Prism version 6.0 software. Statistical significance was determined by t-tests (two-tailed). Statistical significance was defined as p<0.05. 3–5 mice were used for each experiment as indicated. Subsequently the results were replicated in 2 additional independent experiments using 3–5 mice/experiment.
Results
IL-23 promoted IL-17 production and generation of CD3+CD4−CD8− double negative T cells in SLE patients
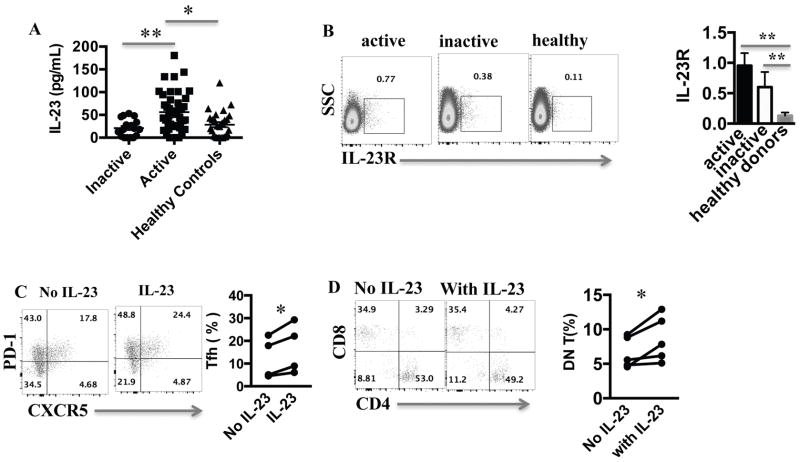
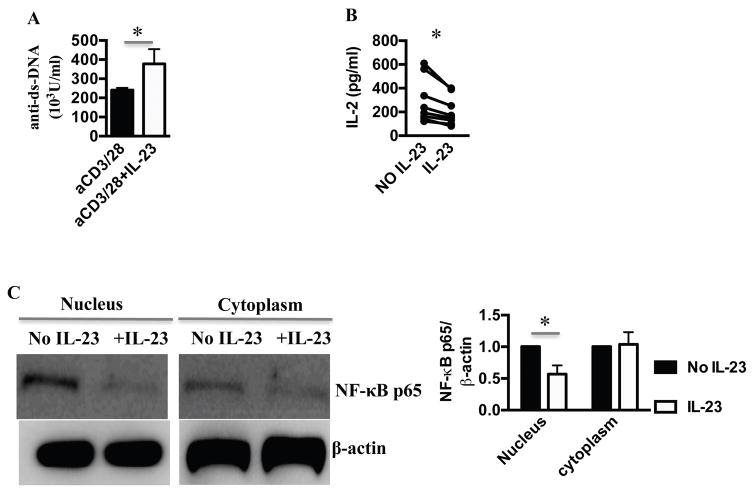
To address the role of IL-23 in SLE patients, we first measured IL-23 levels in the sera of patients and sex, age, and race matched healthy donors. Patients with SLEDAI (12) scores of more than 4 were defined as active. IL-23 was significantly elevated in patients with active disease compared to patients with inactive disease and healthy controls (Fig. 1A). IL-23 levels did not correlate with the type of medications the patients were taking or their dose. Specifically, IL-23 levels did not correlate with prednisone dose (Pearson r=−0.17, p=0.19). We noted a non-statistically significant positive correlation between IL-23 and anti-dsDNA antibody titers (Pearson r=0.25, p=0.054). We found no other statistically significant correlation between serum IL-23 levels and specific disease manifestations, or laboratory values. We then measured IL-23 receptor expression on T cells. Contrary to the serum IL-23 concentration, IL-23R expression on T cells of active and inactive SLE was similar and higher than healthy controls (Fig. 1B). We then asked how IL-23 affects SLE T cells in vitro. Treatment with IL-23 resulted in the doubling of the percentage of T cells that expressed Tfh markers (Fig. 1C) and an increase in DN T cells numbers (Fig. 1D). DN T cells did not express Tfh markers (Supplementary Figure.1A). To address whether the IL-23 induced increase in Tfh results in B cell hyperactivity, we incubated T and B cells from patients with active autoantibody-positive SLE, in the presence or absence of IL-23. After stimulation of T cells with anti-CD3/CD28, we measured the production of anti-dsDNA antibodies by B cells. There was a 2-fold increase of anti-dsDNA antibody production in the presence of IL-23 (Fig. 2A), suggesting a key role of IL-23 in anti-dsDNA antibody production in these limited number of patients. To dissect the effect of IL-23 on T cell function, we measured the cytokine profile of activated SLE T cells cultured in the presence or absence of IL-23. As expected IL-23 increased the production of IL-17, but limited the production of IFN-γ (Supplementary Fig. 1B) and IL-2 by SLE T cells (Fig. 2B), a critical cytokine that boosts Treg and inhibits Tfh. IL-23 did not induce IL-17A+IFN-γ+double positive T cells (Supplementary Fig. 1C) as observed in other disease states. Because IL-2 production by SLE T cells is due to deficient transcription of the IL-2 gene (15), we asked whether IL-23 influences the recruitment of transcription factors in the nucleus of activated SLE T cells. We found that the IL-2 enhancer NFκBp65 (16) recruitment in SLE T cell nuclei is suppressed in the presence of IL-23 (Fig 2C). NFκBp65 is known to be decreased in SLE T cells and its forced replenishment restores IL-2 production (14). Our data therefore suggest that chronic exposure of SLE T cells to IL-23 may account for the decreased production of IL-2 by limiting translocation of necessary transcription factors.
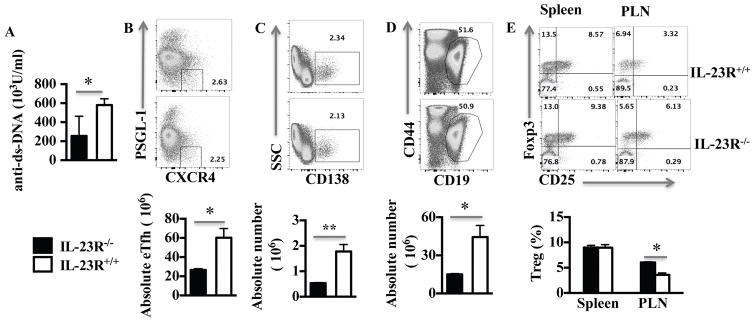
Figure 1. IL-23 is elevated in SLE and promotes the generation of Tfh and DN T cells.
(A) IL-23 level was measured from sera of healthy donors, active and inactive SLE patients. (B) CD3+ T cells from healthy donors, active and inactive SLE patients were stained with an anti-IL-23R antibody. A representative dot plot and cumulative results are shown here. (C) SLE T cells were stimulated with anti-CD3/CD28 antibodies with or without IL-23 for 5 days and then stained for the Tfh markers (CD3+CD4+PD-1+CXCR5+) (representative dot plot and cumulative results from 5 patients). (D) SLE T cells were stimulated with anti-CD3/aCD28 antibodies with or without IL-23 for 5 days and then stained with CD3, CD4 and CD8 (gated for CD3+, representative experiment and cumulative data from 5 patients). * p < 0.05, ** p < 0.01. Error bars represent SD.
Figure 2. IL-23 promoted the production of autoantibodies, while limiting IL-2.
(A) 3×104 CD4 T cells and 3×104 B cells from SLE patients were stimulated with anti-CD3/28 in the absence or presence of 25 ng/ml IL-23 for 6 days, and anti-dsDNA antibodies were measured by ELISA (n=6) (B) SLE T cells were stimulated with anti-CD3/CD28 antibodies in the presence of or absence of IL-23 for 16 hours, and IL-2 production in the supernatant was determined by ELISA (n=8). (C) 4×106 T cells from SLE patients were stimulated with anti-CD3/28 in the absence or presence of 50ng/ml IL-23 for 6 hours; nuclear and cytoplasmic proteins were extracted and relative to β-actin expression of NFκBp65 was measured by western blot (representative blot and cumulative, data n=5 patients). * p < 0.05, ** p < 0.01. Error bars represent SD.
Decreased lupus disease severity and accumulation of DN T cells in kidney in IL-23R−/− MRL.lpr mice
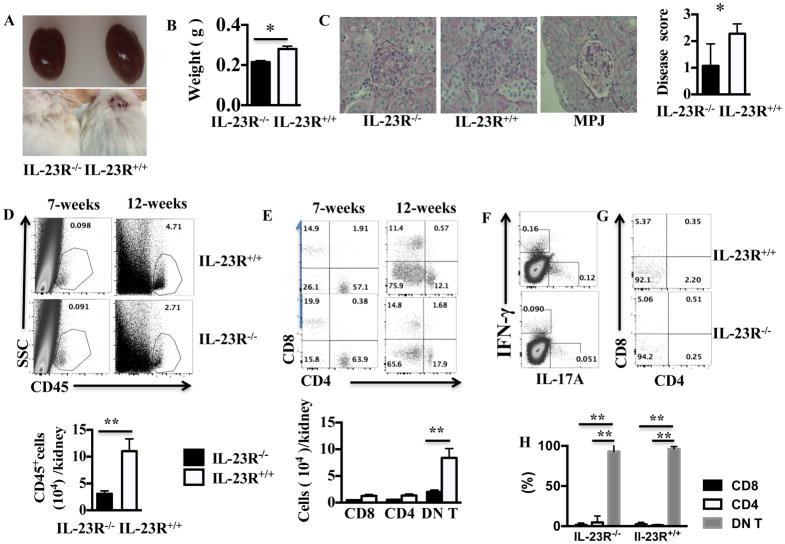
To address mechanistically the effect of IL-23 on lupus pathogenesis we generated IL-23R−/− MRL.lpr mice after back-crossing the B6IL-23R−/− with MRL.lpr for 12 generations. As opposed to B6.lpr mice that develop autoantibodies but not severe autoimmune disease, these mice develop severe nephritis that is similar to SLE and therefore can be far more useful in addressing the effect of IL-23 on both the development of autoimmunity and the ensuing inflammatory organ damage. By 3 months of age, IL-23R+/+ MRL.lpr mice developed enlarged kidneys and severe skin lesions, while IL-23R−/− MRL.lpr mice did not (Fig. 3A and B). On microscopic analysis, we found that the IL-23R−/− MRL.lpr mice had significantly attenuated glomerulonephritis as compared to IL-23R+/+ MRL.lpr mice (Fig. 3C representative H&E staining and cumulative scores). We then performed flow cytometric analysis of kidney infiltrating lymphocytes and found that IL-23R deficiency resulted in decreased kidney infiltration of CD45+ cells (Fig. 3D). Among the infiltrating lymphocytes, the majority were DN T cells (Fig. 3E). To further evaluate the phenotype of infiltrating T cells we stained the kidney derived lymphocytes for expression of IL-17 and IFN-γ (Fig. 3F left panel). We found that the IL-23R−/− MRL.lpr had a two-fold decrease in both IL-17 and IFN-γ expressing cells vs IL-23R+/+ MRL.lpr. Of note, the majority of IL-17+ cells were DN T cells (Fig. 3F, G, H). Taken together these data suggest that IL-23R deficiency fundamentally changes the inflammatory infiltrate in the kidneys of MRL.lpr mice and prevents tissue damage.
Figure 3. IL-23R deficiency prevented the development of nephritis in MRL.lpr mice.
(A) Kidneys (top panel) and skin (lower panel) from a representative pair of 12-week old female IL-23R+/+ and IL-23R −/− MRL.lpr mice is shown here. (B) Harvested kidneys from IL-23R+/+ and IL-23R−/− weight is shown (n=3). (C) Representative H&E staining of kidney sections and cumulative results of kidney scores in IL-23R+/+, and IL-23R−/− MRL.lpr are shown here (n=4). H&E staining of kidney section from healthy MRL.MPJ mice is shown for comparison. (D) Kidneys from 7-week-old and 12-week old IL-23R+/+ and IL-23R+/+ MRL.lpr mice were harvested and cells were isolated. Frequency of CD45+ cells among total live kidney cells (upper panel) and absolute number of CD45+ cells (lower panel) are shown here. (E) Kidneys from 7-week and 12-week old IL-23R+/+ MRL.lpr and IL-23R−/− MRL.lpr mice were harvested, cells were isolated and analyzed with flow cytometry for CD4 and CD8 expression after gating for CD45+CD3+T cells. The relative percentage to total T cells (upper panel) and the absolute number of the major T cell subsets (lower panel) are shown here (n=3). (F–G) Cells were isolated from the kidneys of 3 mice and were stimulated with PMA and Ionomycin in the presence of Golgi plug for 4 hours; then the cells were fixed and subjected to surface staining for TCRβ, CD3, CD4, CD8 and intra-cellular staining for IL-17 and IFN-γ. The expression of IFN-γ and IL-17 in TCRβ+CD3+ cells (F) and the expression of CD4 and CD8 on IL-17+ cells (G), (H) (cumulative results (lower panel), n=3) is shown here. *= p<0.05, ** =p<0.01. Error bar represents SD. Data are representative of three independent experiments.
IL-23R deficiency resulted in decreased accumulation of DN T cells in secondary lymphoid organs
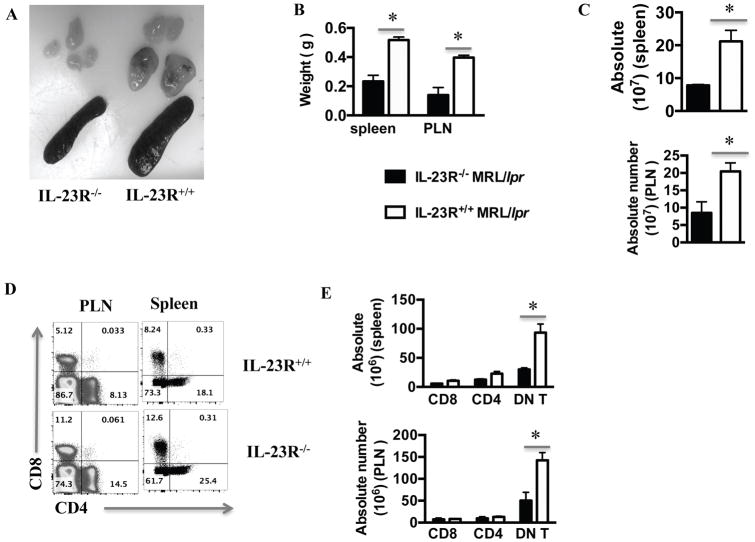
IL-23R+/+ MRL.lpr mice had significantly larger spleens and peripheral lymph nodes than IL-23R−/− MRL.lpr mice (Fig. 4A–B), with both spleen and lymph nodes being profoundly more hyper-cellular (Fig. 4C). As we examined the cellular composition, we found that IL-23R deficiency affected primarily the DN T cells by reducing both their relative frequency and their absolute numbers (Fig. 4D, E). The relative proportion of CD4+ and CD8+ T cells increased in the absence of IL-23R, but their total numbers were not statistically significantly different in the presence or absence of the IL-23R (Fig. 4D and E). Taken together, these data suggest that genetic deletion of the IL-23 receptor in MRL.lpr mice reduced the numbers of DN T cells in both the kidneys and peripheral lymphoid organs.
Figure 4. IL-23R−/− MRL.lpr mice had decreased T cell accumulation in secondary lymphoid organs.
(A) A representative pair of harvested spleens and peripheral lymph nodes (PLNs) from 12-week old IL-23R+/+ and IL-23R−/− MRL.lpr mice are shown here. (B) Spleen and PLN weights from the two groups (n=3) are plotted here. (C) Total number of splenocytes and PLN cells from the two groups (n=3) are shown here. (D–E) Cells from spleens and lymph nodes were isolated from IL-23R+/+ and IL-23R−/− MRL.lpr mice, stained for Thy1.2, CD3, CD4 and CD8, and analyzed with flow-cytometry. Representative plot of CD4 and CD8 staining of Thy1.2+CD3+ splenocytes and PLN cells from both groups (D) and cumulative results (E, n=3). *= p< 0.05. Error bar represents SD. Data are representative of three independent experiments.
IL-23R deficiency resulted in impaired T cell-dependent autoimmune humoral responses
Murine and human lupus is characterized by a profound increase in the production of auto-antibodies, that are thought to be pathogenic. B cell activation and eventual production of class switched autoantibodies is assisted by their interaction with T cells (17). It has been shown that not only CD4+ but also DN T cells (18, 19) can be effective in providing help to B cells to produce anti-dsDNA antibodies. We found that serum anti-dsDNA autoantibody levels were reduced by more than 60% in the IL-23R deficient mice as compared to wild type mice (Fig. 5A). This finding was in accordance with the observation that extra follicular helper cells (eTfh), plasma cells and B cells were all reduced in absolute numbers (Fig. 5B, C, D). Treg cells were proportionally higher in the lymph nodes but not the spleens of IL-23R−/− MRL.lpr vs wild type mice (Fig. 5F). These results clearly demonstrate that IL-23R signaling is important for T cell-dependent humoral responses in lupus prone mice.
Figure 5. IL-23R−/− MRL.lpr mice displayed decreased spontaneous humoral responses.
(A). Anti-dsDNA antibodies were measured in the serum of 12-week-old IL-23R−/− and IL-23R+/+MRL.lpr mice (representative experiment and cumulative data from 5 mice/group). (B) Splenocytes were isolated from 12-week old IL-23R−/− and IL-23R+/+ MRL.lpr mice and stained for PSGL-1, CXCR4, Thy 1.2, CD8, CD4, CD44, CD62L. The eTfh cells (Thy1.2+CD8−CD62L−CD44+CD4+PSGL-1−CXCR4+) cells were measured (representative experiment, gated for Thy1.2+CD4+CD8-CD44+CD62L− (upper panel), and cumulative results (lower panel), n=3). (C) The same samples as in B were stained for CD138, CD44 and CD3 (representative dot plot of CD138+ cells after gating from CD3−CD44+ CD19low (upper panel), and cumulative results (lower panel), n=3). (D) The same samples as in B were stained for CD19, CD44 and CD3 (representative dot plot of CD44−CD19+ cells after gating from CD3−(upper panel), and cumulative results (lower panel), n=3). (E) Cells from the spleens and PLN of IL-23R+/+ and IL-23R−/− MRL.lpr mice were stained for TCRβ, CD4, CD8 and CD25 followed by intracellular staining for Foxp3 (representative experiment of CD25+Foxp3+ cells after gating for CD3+CD4+CD8− (upper panel), and cumulative results (lower panel) (n=3) *= p< 0.05, ** =p < 0.01. Error bar represents SD. Data are representative of three independent experiments.
IL-23R signaling influences IL-17 production and DN T cell generation
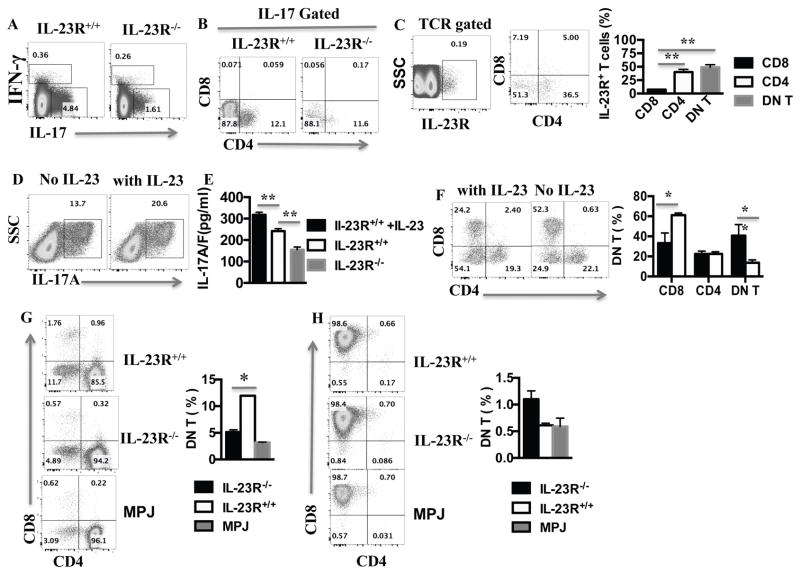
As expected, we found that Th17 cells were decreased in IL-23R deficient compared to sufficient mice (Fig. 6A). In both IL-23R deficient and sufficient animals, DN T cells were the main IL-17 producing cells when tested directly ex vivo (Fig. 6B). To explain the IL-23R influenced preferential production of IL-17 by DN T cells, we stained MRL.lpr splenocytes for IL-23 receptor and found that the majority of T cells expressing IL-23R were DN T cells with a minority being CD4+, while very few CD8+ were IL-23R+ (Fig. 6C). We then tested whether exogenous IL-23 (25 ng/mL) can boost the production of IL-17 (Fig. 6D) when added to MRL.lpr lymphocytes in culture. Indeed, there was an increase in both IL-17+ T cells and IL-17 secretion after addition of IL-23 (Fig. 6D and E).
Figure 6. IL-23R deficiency limited the production of IL-17 and the generation of DN T cells.
Spleens were harvested from IL-23R−/− MRL.lpr and IL-23R+/+MRL.lpr mice as indicated, and single cell suspension was prepared. (A) IFN-γ and IL-17 produced by T cells were analyzed by intracellular staining and cytometry after gating on live TCRαβ+ T cells (see Experimental Procedures) (B) IL-17-producing T cells were analyzed for CD4 and CD8 expression by cytometry after gating on TCRβ+IL-17+; (C) TCRβ+ splenocytes from IL-23R+/+ MRL.lpr were stained with anti-IL-23R antibody and evaluated using flow cytometry (cumulative results (right panel), n=3 ). (D) Splenocytes from IL-23R+/+ MRL.lpr were stimulated with anti-CD3/anti-CD28 antibodies for 3 days with or without IL-23; then the cells were harvested to analyzed for IL-17 expression by cytometry via gating on TCRβ+ T; (E) The supernatants from the 3 day-cell cultures described in D including a IL-23R−/− control were used for IL-17A/F level measurement with ELISA (n=3 mice in each plot). (F) 4×106 splenocytes from 8-week-old MRL.lpr mice were stimulated with anti-CD3/28 in the presence or absence of 25ng/ml IL-23 for 2 days; the cells were then harvested and analyzed for CD4 and CD8 expression by cytometry after gating on CD3+ T cells (representative plot of 3 mice-left panel and cumulative results-right panel). (G–H) CD4+ (G) and CD8+ (H) T cells were sort-purified from the spleens of 12-week-old IL-23R+/+ MRL.lpr, IL-23R−/−MRL.lpr and MRL.MPJ mice. They then were stimulated with anti-CD3/CD28 for 3 days before being (re)stained for CD3, CD4 and CD8 expression (representative experiment left panel, cumulative data from 3 mice/group, right panel). *= p< 0.05. Error bar represents SD. Data are representative of three independent experiments.
We observed that addition of IL-23 in the culture of total splenocytes with anti-CD3/CD28 antibodies resulted in increased numbers of DN T cells (Fig. 6F). We then cultured purified splenic CD4+ and CD8+ T cells in vitro from IL-23R−/− and IL-23R+/+ MRL.lpr mice. We found that >10% CD4+ T cells from wild type MRL.lpr mice become DN T cells, significantly higher than both IL-23R−/− MRL.lpr CD4+ and control MRL.MPJ CD4+ cells (Fig. 6G). Of note, very few CD8+ T cells became DN T cells either in the presence of absence of IL-23R (Fig. 6H).
Given the effect of IL-23R deficiency on DN T cell numbers we postulated that not only there is a decrease in generation of DN T cells but also that DN T cells may proliferate less and/or die at a higher rate in the absence of IL-23R. To this end, we isolated lymphocytes from IL-23R−/− and IL-23R+/+ MRL.lpr mice, and analyzed the proportion of Ki67+ cells using flow-cytometry. As shown in supplementary figure 2A and 2B, in both spleen and peripheral lymph nodes, DN T cells from IL-23R+/+ MRL.lpr mice exhibited increased proliferation as compared with those from IL-23R−/− MRL.lpr. CD8+ T cells exhibited the opposite, while there was no difference in CD4+ T cell proliferation between the two strains. To assess DN T cell proliferation dynamically, we injected Brdu intraperitoneally into the two strains of mice and found that the proliferation of T cells from spleens was not significantly different between the two groups of mice (Supplementary Fig. 2C). However, CD4+ T cells from peripheral lymph nodes of IL-23R+/+ MRL.lpr mice exhibited decreased proliferation compared to those from IL-23R−/− controls. In contrast, DN T cells from IL-23R+/+ MRL.lpr mice displayed more proliferation than those from IL-23R−/− mice (Supplementary Fig. 2D). These results demonstrated that IL-23R deficiency resulted in decreased DN T cell proliferation. The effect on CD4+ and CD8+ T cells was opposite although not consistent between the spleens and lymph nodes.
Next, we asked whether deficiency of IL-23R influences T cell death in MRL.lpr mice. To this end we isolated lymphocytes from spleen and lymph nodes of IL-23R−/− MRL.lpr and IL-23R+/+ controls. IL-23R deficiency resulted in increased death of DN T cells in the spleen as compared with Il-23R+/+ animals (Supplementary Fig. 3A), while in peripheral lymph nodes there was no significant differences among the groups (Supplementary Fig. 3B). There was no apparent effect of IL-23R on single positive T cell death. Taken together, these data suggest that IL-23R signaling enhances DN T cell accumulation in MRL.lpr mice by increasing their generation from CD4 cells, promoting their ability to proliferate and decreasing their demise.
IL-23R deficiency resulted in increased IL-2 production
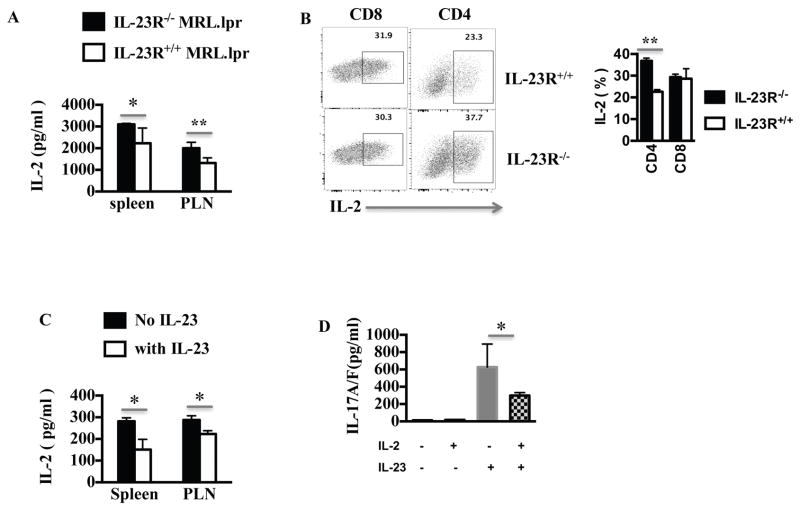
As discussed above, lupus T cells are characterized by poor production of IL-2 upon stimulation, while exogenous IL-2 ameliorates the disease (13). We therefore asked whether IL-23R deficiency will affect IL-2 production. In Fig. 7A, we show that IL-23R−/− lymphocytes produced higher levels of IL-2 when activated in vitro than wild type MRL.lpr mice. Using intracellular staining we found that the IL-23 deficiency promoted IL-2 production by CD4 T cells with minimal effect on CD8 (Fig. 7B) and no effect on the very low IL-2 producing DN T cells (Supplementary Fig. 4). Then, we cultured IL-23 sufficient MRL.lpr lymphocytes in the presence of IL-23 and measured IL-2 production (Fig. 7C). IL-23 resulted in a two and three-fold decrease in the production of IL-2 by spleen and lymph node cells respectively. To further analyze the effect of the altered cytokine milieu in MRL.lpr mice, we cultured wild type MRL.lpr lymphocytes with or without IL-23 and/or IL-2. We observed that the positive effect of IL-23 on IL-17 production can be limited in the presence of IL-2 (Fig. 7D). These data taken together, show that IL-23 not only affects IL-17 production but fundamentally alters the cytokine milieu leading to a low IL-2/high IL-17 pro-inflammatory state.
Figure 7. IL-23 limited the production of IL-2 in MRL.lpr mice.
(A) 4×106 cell-suspension were prepared from spleens or PLNs of IL-23R+/+ or IL-23R−/− MRL.lpr mice and were stimulated with anti-CD3/CD28 antibodies for 16 hours. IL-2 levels in the supernatant were measured by ELISA (n=3). (B) 4×106 cell-suspension were prepared from spleens of IL-23R+/+ or IL-23R−/− MRL.lpr mice were stimulated with anti-CD3/CD28 antibodies for 16 hours. PMA, Ionomycin and Brefeldin A were added 4 hours prior to harvesting and then IL-2 expression was analyzed by flow-cytometry after gating on live TCRαβ+ CD4+ or CD8+ T cells. (C) 4×106 cells from spleens or lymph nodes (PLN) of IL-23R+/+ MRL.lpr mice were stimulated with anti-CD3/CD28 with or without IL-23 (25ng/ml) for 3 days and the level of IL-2 in the supernatant was measured by ELISA (n=3). (D) 4×106 cells from spleens of IL-23R+/+MRL.lpr mice were stimulated with anti-CD3/CD28 antibodies with or without IL-23 (25ng/ml) and/or IL-2 for 3 days. Subsequently IL-17A/F level in the supernatant was measured by ELISA (cumulative results, n=3 mice). *= p < 0.05, **= p < 0.01. Error bar represents SD. Data are representative of three independent experiments.
Discussion
In this study, we showed for the first time that IL-23 and its receptor are upregulated in SLE and may account at least in part for the expansion of extra-follicular Tfh cells, the decreased IL-2 and aberrant anti-dsDNA antibody production. In murine lupus, deficiency of IL-23R attenuated the severity of nephritis by inducing the counter-inflammatory cytokine IL-2, limiting the expansion of DN T cells and inhibiting critical steps in the production of pathogenic autoantibodies.
DN T cells are present in the thymus, and are generated at an early stage in the development of mature T cells. DN T cells are also generated in the periphery as the result of downregulation/internalization of CD4 or CD8. It has been shown that activated CD8 cells from healthy individuals convert to DN T cells (20). In MRL.lpr mice DN T cells are expanded due to deficient apoptosis and have pro-inflammatory features producing IL-17 among other cytokines (3). DN T cells from SLE patients share certain features with the MRL.lpr DN T cells: they produce IL-17, infiltrate the kidneys (3) and provide help to B cells (19). Our data show that CD4+ T cells from lupus-prone mice and patients with SLE can become DN T cells upon exposure to IL-23. Moreover, we found that IL-23R deficiency results in decreased proliferation and increased death of DN T cells suggesting an effect of IL-23 on DN T cells at various stages of their development. The underlying mechanism may reflect a direct effect of STAT3-mediated pro-survival signal (21) to DN T cells or an indirect one by influencing IL-2 production which can alter both cell activation but also post activation cell death (22).
IL-23R deficiency resulted in decreased production of anti-dsDNA antibodies in MRL.lpr mice, while in vitro exposure of T and B cells from SLE patients to IL-23 resulted in higher T-dependent anti-dsDNA production. The production of these potentially pathogenic autoantibodies depends on the activity of T follicular helper cells (Tfh) (23). Circulating Tfh-like cells have been well described in SLE and are associated with disease activity (24). Our data show that IL-23 significantly increases Tfh and Tfh like cell numbers in both mice and in in vitro SLE lymphocyte cultures, potentially due to the observed decrease in IL-2 production. Future studies are needed to further dissect the molecular mechanisms whereby IL-23R leads to expansion of Tfh in lupus-prone mice and SLE patients.
Besides the aforementioned effects of IL-23, we showed that IL-23 affects the production of key cytokines. IL-23 promoted the production of IL-17 as expected, but also limited the production of IL-2 by impairing the il-2 gene enhancer NFκBp65. NFκBp65 is known to be deficiently recruited in SLE T cell nuclei and contribute to the limited production of IL-2 by activated SLE T cells (16). Low dose IL-2 has been claimed to suppress autoimmunity and pathology in mice and in uncontrolled trials in humans (25, 26). It has been suggested that the effect of low dose IL-2 in abrogating lupus disease activity depends on empowering regulatory T cells. Our data suggest that IL-23 deficiency in lupus prone mice induces IL-2 production and Treg generation. The reciprocal relation between IL-23/IL-17 on one hand and IL-2/Treg on the other may be key in the ongoing inflammatory response in SLE. This observation furthermore explains why IL-23 targeting is efficacious in mitigating lupus nephritis in MRL.lpr mice as opposed to IL-17A targeting which does not increase IL-2/Treg or improve lupus nephritis.
IL-23, a cytokine that is elevated in the serum of patients with active SLE, emerges therefore as a key molecule that dictates the balance between pro- and anti-inflammatory cytokines in this disease. We propose that targeting IL-23 may restore this balance in SLE, limiting both autoantibody production and cell infiltration in organ targets. Furthermore, as biologic therapies move to dual cytokine targeting, our studies point to the possibility of combining low dose IL-2 along with blockade of IL-23 as a higher clinical value therapeutic intervention.
Supplementary Material
Acknowledgments
This work was supported by NIAMS R01AR060849 (VCK) and R01AI085567 (GCT).
The authors thank Michele Finell for help with patient recruitment and sample collection.
Footnotes
None of the authors have any relevant financial interests related to these studies.
References
- 1.Tsokos GC. Systemic lupus erythematosus. The New England journal of medicine. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Furie R, Petri M, Zamani O, Cervera R, Wallace DJ, Tegzova D, Sanchez-Guerrero J, Schwarting A, Merrill JT, Chatham WW, Stohl W, Ginzler EM, Hough DR, Zhong ZJ, Freimuth W, van Vollenhoven RF, Group BS. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis and rheumatism. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, Kyttaris VC, Juang YT, Tsokos GC. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. Journal of immunology. 2008;181:8761–8766. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. gammadelta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–363. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 6.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. Journal of immunology. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyttaris VC, Kampagianni O, Tsokos GC. Treatment with anti-interleukin 23 antibody ameliorates disease in lupus-prone mice. Biomed Res Int. 2013;2013:861028. doi: 10.1155/2013/861028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt T, Paust HJ, Krebs CF, Turner JE, Kaffke A, Bennstein SB, Koyro T, Peters A, Velden J, Hunemorder S, Haag F, Steinmetz OM, Mittrucker HW, Stahl RA, Panzer U. Function of the Th17/interleukin-17A immune response in murine lupus nephritis. Arthritis & rheumatology. 2015;67:475–487. doi: 10.1002/art.38955. [DOI] [PubMed] [Google Scholar]
- 9.Amarilyo G, Lourenco EV, Shi FD, La Cava A. IL-17 promotes murine lupus. Journal of immunology. 2014;193:540–543. doi: 10.4049/jimmunol.1400931. [DOI] [PubMed] [Google Scholar]
- 10.Linker-Israeli M, Bakke AC, Kitridou RC, Gendler S, Gillis S, Horwitz DA. Defective production of interleukin 1 and interleukin 2 in patients with systemic lupus erythematosus (SLE) Journal of immunology. 1983;130:2651–2655. [PubMed] [Google Scholar]
- 11.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis and rheumatism. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 12.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis and rheumatism. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 13.Mizui M, Koga T, Lieberman LA, Beltran J, Yoshida N, Johnson MC, Tisch R, Tsokos GC. IL-2 protects lupus-prone mice from multiple end-organ damage by limiting CD4−CD8− IL-17-producing T cells. Journal of immunology. 2014;193:2168–2177. doi: 10.4049/jimmunol.1400977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards LJ, Mizui M, Kyttaris V. Signal transducer and activator of transcription (STAT) 3 inhibition delays the onset of lupus nephritis in MRL/lpr mice. Clinical immunology. 2015;158:221–230. doi: 10.1016/j.clim.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. Journal of immunology. 2001;166:4216–4222. doi: 10.4049/jimmunol.166.6.4216. [DOI] [PubMed] [Google Scholar]
- 16.Herndon TM, Juang YT, Solomou EE, Rothwell SW, Gourley MF, Tsokos GC. Direct transfer of p65 into T lymphocytes from systemic lupus erythematosus patients leads to increased levels of interleukin-2 promoter activity. Clinical immunology. 2002;103:145–153. doi: 10.1006/clim.2002.5192. [DOI] [PubMed] [Google Scholar]
- 17.Datta SK. Major peptide autoepitopes for nucleosome-centered T and B cell interaction in human and murine lupus. Ann N Y Acad Sci. 2003;987:79–90. doi: 10.1111/j.1749-6632.2003.tb06035.x. [DOI] [PubMed] [Google Scholar]
- 18.Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4−8− T helper cell lines that express the gamma delta T-cell antigen receptor. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:7020–7024. doi: 10.1073/pnas.87.18.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. Journal of immunology. 1989;143:103–112. [PubMed] [Google Scholar]
- 20.Crispin JC, Tsokos GC. Human TCR-alpha beta+ CD4− CD8− T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. Journal of immunology. 2009;183:4675–4681. doi: 10.4049/jimmunol.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong H, Zhang ZG, Tian XQ, Sun DF, Liang QC, Zhang YJ, Lu R, Chen YX, Fang JY. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10:287–297. doi: 10.1593/neo.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9:757–766. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 24.Choi JY, Ho JH, Pasoto SG, Bunin V, Kim ST, Carrasco S, Borba EF, Goncalves CR, Costa PR, Kallas EG, Bonfa E, Craft J. Circulating follicular helper-like T cells in systemic lupus erythematosus: association with disease activity. Arthritis & rheumatology. 2015;67:988–999. doi: 10.1002/art.39020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizui M, Tsokos GC. Low-Dose IL-2 in the Treatment of Lupus. Curr Rheumatol Rep. 2016;18:68. doi: 10.1007/s11926-016-0617-5. [DOI] [PubMed] [Google Scholar]
- 26.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X, Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Zhang X, Su Y, Guo J, Shen N, Morand EF, Yu D, Li Z. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.