Abstract
During pregnancy and lactation, subcutaneous white adipocytes in the mouse mammary gland transdifferentiate reversibly to milk-secreting epithelial cells. In this study, we demonstrate by transmission electron microscopy that in the post-lactating mammary gland interscapular multilocular adipocytes found close to the mammary alveoli contain milk protein granules. Use of the Cre-loxP recombination system allowed showing that the involuting mammary gland of whey acidic protein-Cre/R26R mice, whose secretory alveolar cells express the lacZ gene during pregnancy, contains some X-Gal-stained and uncoupling protein 1–positive interscapular multilocular adipocytes. These data suggest that during mammary gland involution some milk-secreting epithelial cells in the anterior subcutaneous depot may transdifferentiate to brown adipocytes, highlighting a hitherto unappreciated feature of mouse adipose organ plasticity.
Keywords: pregnancy, mammary gland, adipose organ, electron microscopy, Cre-loxP recombination system
Introduction
In all mammals, adipocytes are located in fat depots that are distributed both in subcutaneous and visceral locations and are characterized by distinctive gross and histological morphological features (Cinti 1999, 2005). In mice subcutaneous fat is organized in two depots, anterior and posterior, close to the limbs. The anterior subcutaneous depot has a complex shape. Its larger portion occupies the interscapular region and is the origin of several smaller depots that reach the anterior part of the body (where they give rise to subclavicular and axillary fat) and the deep dorsal region (where they give rise to subscapular and deep cervical fat). The posterior depot, at the base of the hind legs, has a simpler anatomy, where a single strip of fat that begins in the back at the lumbar level (dorso-lumbar portion) extends to the inguino-crural region (inguinal portion) and to the pubic region (gluteal portion), where it joins the contralateral, symmetric depot.
In adult, non-pregnant female mice, the subcutaneous depots contain the mammary gland “anlagen” (Imagawa et al., 1990; Richert et al., 2000; Silberstein 2001; Hennighausen and Robinson, 2005). In the parenchyma of these depots branched mammary epithelial ducts are connected to three bilateral nipples in the anterior subcutaneous depot and to two bilateral nipples in the posterior subcutaneous depot. During pregnancy, adipose tissue is progressively substituted by epithelial structures that soon will give rise to mammary gland alveoli. In rodents, mammary gland development is so massive that during lactation the subcutaneous depots are almost completely occupied by large, milk-producing alveoli, with a very small number of adipocytes scattered among them. Soon after the end of lactation the alveoli disappear and the subcutaneous depots are again filled with adipocytes.
Previous ultrastructural, lineage-tracing and explant experiments have suggested that the plastic changes occurring in the subcutaneous depots of pregnant mice are to some extent due to the reversible transdifferentiation of white adipocytes to epithelial milk-secreting cells (Morroni et al., 2004; De Matteis et al., 2009; Prokesch et al., 2014). Importantly, epithelial milk-secreting cells are rich in cytoplasmic lipid vacuoles, meeting the definition of adipocytes, i.e. parenchymal adipose organ cells rich in cytoplasmic lipids, regardless of their function. We have proposed calling the epithelial milk-secreting cells derived from adipocyte transdifferentiation “pink adipocytes” (Giordano et al., 2014).
Subcutaneous fat is a mixed tissue composed both of white and thermogenic brown adipocytes (Cinti 1999, 2005; Vitali et al., 2012). The former are more abundant in the posterior depot and the latter in the anterior depot. Notably, external cues, such as cold exposure or beta-adrenergic stimuli, promote the transdifferentiation of some white adipocytes to brown adipocytes, which reconvert to white adipocytes soon after the stimuli are withdrawn (Barbatelli et al., 2010; Rosenwald et al., 2013; Altshuler-Keylin et al., 2016). Histological examination demonstrates that in the anterior depot of lactating mice the mammary alveoli lie close to interscapular brown fat. To assess whether, during mammary gland involution, alveolar epithelial cells transdifferentiate not only to white adipocytes, but also to brown adipocytes, we conducted an ultrastructural and lineage-tracing study.
Materials and methods
Animals and tissues
Adult wild type C57BL/6 mice were purchased from Charles River Laboratories (Calco, Italy). ROSA26R (R26R) mice were purchased from and genotyped as described by The Jackson Laboratory (Bar Harbor, ME, USA). Whey acidic protein (WAP)-Cre mice (Wagner et al., 1997) were kindly provided by C. Dickson (London Research Institute, London, UK). All animals were housed in plastic cages in constant environmental conditions (12 h light/dark cycle at 22 °C) with ad libitum access to food and water. Handling was limited to cage cleaning. All efforts were made to minimize animal suffering and to reduce the number of animals used. Experiments were approved by the Animal Ethics Committee of University of Ancona (Politecnica delle Marche) and were carried out in accordance with EC Council Directive 86/609/EEC of 24 November 1986.
Female double transgenic mice were obtained from crosses between R26R hemizygous mice and WAP-Cre hemizygous mice. They were genotyped for the presence of Cre by PCR analysis using primers Cre1 (5′-ATGTCCAATTTACTGACC-3′) and Cre2 (5′-CGCCGCATAACCAGTGAAAC-3′), which yielded a 356 bp product. WAP-Cre/R26R mice were killed 10 days into lactation (n=3) and on day 1, 3, 5 or 10 of mammary gland involution (n=3 for each time point). Cre-Mediated Recombination Analysis was performed as described previously (Morroni et al., 2004).
For morphological analyses, mice were anesthetized with 100 mg/kg ketamine (Ketavet, Farmaceutici Gellini, Aprilia, Italy) in combination with 10 mg/kg xylazine (Rompum, Bayer AG, Leverkusen, Germany) and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. Specimens for light microscopy and immunohistochemistry were paraffin-embedded.
Transmission electron microscopy
Small subcutaneous fat and mammary gland specimens from perfused animals were further fixed in 2% glutaraldehyde/2% formaldehyde in 0.1 M PB for 4 h at room temperature, postfixed in 1% osmium tetroxide, dehydrated in acetone, and embedded in epoxy resin. Semithin sections were stained with toluidine blue. Thin sections were obtained with an MTX ultramicrotome (RMC, Tucson, AZ, USA), stained with lead citrate and examined with a transmission electron microscope (CM10, Philips, Eindhoven, The Netherlands).
β-galactosidase histochemistry
For β-galactosidase (Gal) detection, double transgenic animals were perfused intracardially with 2% paraformaldehyde/0.25% glutaraldehyde in phosphate buffered saline (PBS), pH 7.3. Mammary glands were dissected out and fixed in the same fixative (for up to 90 min). After an overnight wash in PBS, tissues were preincubated in 2 mM MgCl2/0.01% sodium deoxycholate/0.02% Nonidet P-40 in PBS, pH 8.5, for 2 h at room temperature, then incubated in X-Gal solution: 5 mM potassium ferrocyanide containing 1 mg/ml X-Gal (Sigma-Aldrich, St Louis, MO, USA) in the form of 40 mg/ml solution in dimethylformamide, at 30°C for 24 h (WAP- Cre/R26R) or 5 h (aP2-Cre/R26R). PBS at pH 8.5 was used to avoid detection of endogenous β-Gal, which is active at pH values <5. After X-Gal incubation, tissues were postfixed in 4% paraformaldehyde in 0.1 M PB, dehydrated, and embedded in paraffin.
Peroxidase immunohistochemistry
Immunohistochemistry was performed on 3 μm-thick paraffin-embedded sections of interscapular brown fat and mammary gland. After dewaxing, sections were thoroughly rinsed in PBS, reacted with 0.3% H2O2 (in PBS; 30 min) to block endogenous peroxidase, rinsed again with PBS and incubated in a 3% blocking solution (in PBS; 60 min). Then, they were incubated with the primary antibody against the uncoupling protein 1 (UCP1; in PBS; overnight at 4 °C). UCP1 was localized using a rabbit polyclonal antibody against a synthetic peptide conjugated to KLH, corresponding to the amino acids 145-159 of human UCP1, with N-terminal cysteine added (Abcam, Cambridge, UK, Cat# ab10983 RRID:AB_2241462). After a thorough rinse in PBS, sections were incubated in a 1:200 v/v biotinylated secondary antibody solution (in PBS; 30 min). The biotinylated HRP- conjugated secondary antibody (Vector Laboratories, Burlingame, CA) was goat anti-rabbit IgG. Histochemical reactions were performed using Vectastain ABC Kit (Vector Laboratories) and Sigma Fast 3,3′-diaminobenzidine (Sigma-Aldrich) as the substrate. Sections were finally counterstained with hematoxylin, dehydrated and mounted in Entellan. Staining was never observed when the primary antibody was omitted.
Results
Light microscopy
By gross anatomical examination, interscapular brown adipose tissue (BAT) from non-pregnant mice was clearly visible in the anterior adipose depot due to its brownish color (Fig. 1A). In lactating mice it was barely recognizable due to its white fat-like macroscopic appearance (Fig. 1B). Light microscopy disclosed that it contained multilocular adipocytes with large lipid droplets (a sign of weak thermogenic activity) exhibiting scarce or no expression of UCP1, the thermogenic protein (Ricquier, 2005) (Fig. 1C and D). These findings agree with the reduced BAT activity that is seen during pregnancy and lactation (Abelenda and Puerta, 1987; Trayhurn and Wusteman, 1987; Imai-Matsumara et al., 1990). The BAT lobules were surrounded and infiltrated by well- differentiated mammary alveoli without clear boundaries between the adipose and the epithelial component (Fig. 1D). A progressive reduction in the number of the alveoli and a progressive expansion of BAT areas were detected in the first few days of mammary gland involution (data not shown). Quantitative data from posterior subcutaneous fat of B6 and Sv129 mice indicate that in warm-acclimated animals 5-10% of the parenchyma consists of BAT-like tissue (Vitali et al., 2012). Interestingly, serial sections of the posterior glands showed that there was no BAT-like tissue in the glands during lactation (data not shown).
Figure 1.
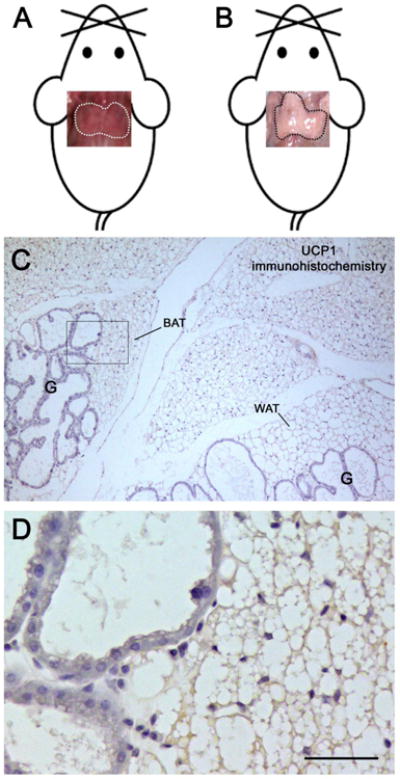
The mouse anterior subcutaneous depot during lactation. Upper panels, pictures of the gross anatomy of the interscapular depot fitted on mouse templates. In non-pregnant female mice (A), the interscapular fat (dotted area) is easily recognized by its brownish color; after 10 days of lactation (B) it turns white and the depot acquires a white fat-like macroscopic appearance. In sections processed for immunohistochemistry (C), interscapular multilocular adipocytes containing large lipid droplets exhibit very weak UCP1 expression. Mammary gland alveoli (G) are found close to both brown (BAT) and white (WAT) adipose tissue lobules. At higher magnification (D), no clear boundaries are visible between the secretory epithelium and the adipose parenchyma; note the weak and homogenous UCP1 staining in multilocular adipocytes. D is the enlargement of the area framed in C.
Bar: 3 cm in A, 250 μm in B, 40 μm in C.
Transmission electron microscopy
To assess the fate of alveolar epithelial cells during mammary gland involution and their relationships with the interscapular BAT parenchyma, the peripheral interscapular BAT, which contains both brown adipocytes and alveoli (Fig. 2A and B), was examined by transmission electron microscopy. Several multilocular adipocytes containing numerous typical “brown” mitochondria as well as milk protein granules were detected on days 1, 3, 5 and 10 of gland involution (Fig. 2C and D). These ultrastructural features could represent an intermediate phenotype of epithelial to brown cell transdifferentiation.
Figure 2.
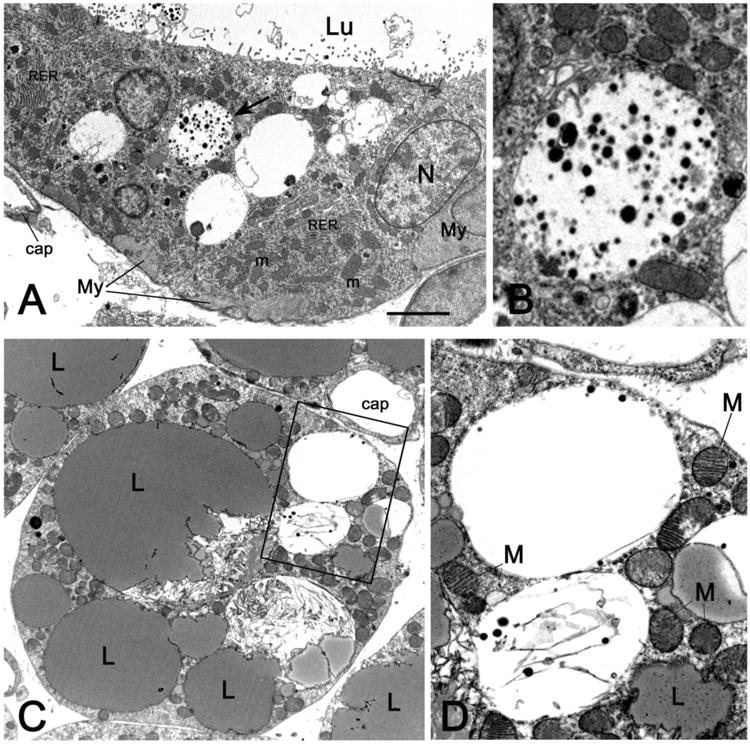
Transmission electron microscopy images from specimens of peripheral anterior mammary gland in contact with interscapular BAT on day 1 of mammary gland involution. In A, the epithelial cells bordering the alveolar lumen (Lu) contain numerous mitochondria (m), stacked rough endoplasmic reticulum (RER), and large vacuoles containing milk protein secretory granules; the vacuole indicated by the arrow is enlarged in B. C: a multilocular adipocyte containing several “brown-like” mitochondria (M) packed with laminar cristae and two milk protein secretory vacuoles (enlarged in D). Cap: capillary, My: myoepithelial cells, N: nucleus, L: lipid droplet. Bar: 1 μm in A and C, 0,3 μm in B and D.
Lineage-tracing analysis
To obtain further evidence of the transdifferentiation of epithelial glandular cells into brown adipocytes, the Cre-loxP system was used to drive β-gal expression in the secretory epithelium under the control of cell-type specific promoters (Soriano, 1999; Birling et al., 2009). β-gal expression was induced in WAP-Cre/R26R double transgenic mice by the promoter of WAP, a protein specific of milk-producing cells (15). The alveoli of the lactating gland stained for X-Gal, the β-gal substrate, whereas the brown adipocytes were negative (data not shown). However, in the post-lactation period, several X-Gal-positive multilocular cells were detected between BAT and the involuting glands in the mixed areas (Fig. 3A). To confirm that these multilocular adipocytes were indeed brown adipocytes, their UCP1 expression was evaluated by immunohistochemistry in the sections previously reacted for X-Gal. The results showed that the X-Gal-positive multilocular cells were also positive for UCP1 (Fig 3B), thus confirming their brown phenotype. Brown adipocytes of the central part of interscapular fat and located at a distance from the alveoli, were UCP1-positive, but always X-Gal-negative (data not shown).
Figure 3.
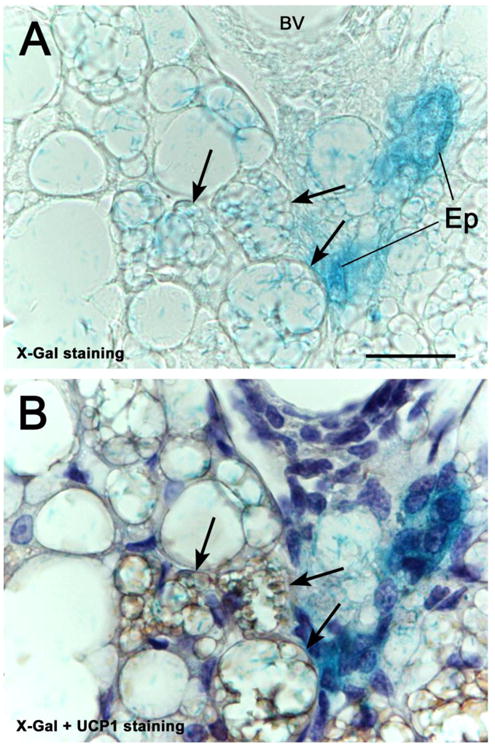
X-Gal staining of the peripheral anterior mammary gland in contact with interscapular BAT of a WAP-Cre/R26R mouse 10 days post-lactation. In A, not only mammary epithelial structures (Ep), but also some multilocular adipocytes (arrows) show the blue X-Gal precipitate. UCP1 immunohistochemistry and hematoxylin counterstaining, performed in the same section (B), show that the X-Gal-positive multilocular adipocytes also exhibit UCP1 immunoreactivity (arrows). bV: blood vessel.
Bar: 25 μm.
Discussion
Our data suggest that, after lactation, some milk-producing alveolar epithelial cells in the anterior subcutaneous depot mammary gland convert to UCP1-positive multilocular adipocytes. Importantly, in WAP-Cre/R26R double transgenic mice only the interscapular brown adipocytes found in close proximity to the involuting glands contained milk granules and stained for X-Gal, suggesting that a subpopulation of brown adipocytes is involved in the process. These data also raise the intriguing question whether during mammary gland development some interscapular brown adipocytes transdifferentiate to alveolar epithelial cells, as already documented in white adipocytes (Morroni et al., 2004; De Matteis et al., 2009; Prokesch et al., 2014). If this were the case, the present findings would demonstrate a further, distinctive type of cell plasticity of the mouse adipose organ, reversible brown to “pink” transdifferentiation (Giordano et al., 2014). The evidence that clusters of brown adipocytes are also found in the posterior subcutaneous depot in non-pregnant animals, and that no residual BAT-like tissue is detected at these sites during lactation, suggests that reversible brown to pink transdifferentiation may also take place in this depot. Brown to pink transdifferentiation could provide a novel perspective on the BAT inactivation and reduced non-shivering thermogenesis that are seen in rodents during pregnancy and lactation (Abelenda and Puerta, 1987; Trayhurn and Wusteman, 1987; Imai-Matsumara et al., 1990). Human isolated mature adipocytes has been recently shown to undergo a spontaneous process of transdifferentiation to fibroblast-like cells that can subsequently be committed to different phenotypes (Maurizi et al., 2016). The distinctive in vitro and in vivo plastical abilities of mammalian adipocytes can be exploited for therapeutic purposes. White to brown adipocyte conversion, especially in visceral depots, holds considerable potential for the treatment of obesity and metabolic syndrome (Giordano et al., 2016). In parallel, white and/or brown adipocyte to epithelial cell transdifferentiation in the mammary gland may be of interest in mammary gland pathology, especially neoplastic diseases. Interestingly, loss of PPARγ—a transcription factor involved both in adipogenesis (Hallenborg et al., 2015) and alveologenesis (Jain et al., 1998)-in mammary secretory epithelial cells creates a pro-breast tumorigenic environment during mammary gland involution (Apostoli et al., 2014); the process may to some extent be related to impaired epithelial cell to white adipocyte transdifferentiation. MiR-27a, an inhibitor of brown adipogenesis (Sun and Trajkovski, 2014), has been reported to promote breast cancer growth and metastasis in humans (Tang et al., 2012) and could be a factor involved in the still unknown relationship between brown fat and breast cancer progression that has been observed in some experimental models (Jones et al., 2011).
Acknowledgments
This work was supported by grants from Marche Polytechnic University (Contributi Ricerca Scientifica) and the Italian Ministry of University (PRIN 2010-11 to S.C.).
Footnotes
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jcp.25858]
The authors do not have conflict of interest to declare.
Literature cited
- Abelenda M, Puerta ML. Inhibition of diet-induced thermogenesis during pregnancy in the rat. Pflugers Arch. 1987;409:314–317. doi: 10.1007/BF00583482. [DOI] [PubMed] [Google Scholar]
- Altshuler-Keylin S, Shinoda K, Hasegawa Y, Ikeda K, Hong H, Kang Q, Yang Y, Perera RM, Debnath J, Kajimura S. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab. 2016;24:402–419. doi: 10.1016/j.cmet.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostoli AJ, Skelhorne-Gross GE, Rubino RE, Peterson NT, Di Lena MA, Schneider MM, Senqupta SK, Nicol CJ. Loss of PPARγ expression in mammary secretory epithelial cells creates a pro-breast tumorigenic environment. Int J Cancer. 2014;134:1055–1066. doi: 10.1002/ijc.28432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- Birling MC, Gofflot F, Warot X. Site-specific recombinases for manipulation of the mouse genome. Methods Mol Biol. 2009;561:245–263. doi: 10.1007/978-1-60327-019-9_16. [DOI] [PubMed] [Google Scholar]
- Cinti S. The Adipose Organ. Milan: Kurtis; 1999. [Google Scholar]
- Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- De Matteis R, Zingaretti MC, Murano I, Vitali A, Frontini A, Giannulis I, Barbatelli G, Marcucci F, Bordicchia M, Sarzani R, Raviola E, Cinti S. In vivo physiological transdifferentiation of adult adipose cells. Stem Cells. 2009;27:2761–2768. doi: 10.1002/stem.197. [DOI] [PubMed] [Google Scholar]
- Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S. White, brown and pink adipocytes: The extraordinary plasticity of the adipose organ. Eur J Endocrinol. 2014;170:159–171. doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- Giordano A, Frontini A, Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat Rev Drug Discov. 2016;15:405–424. doi: 10.1038/nrd.2016.31. [DOI] [PubMed] [Google Scholar]
- Hallenborg P, Petersen RK, Kouskoumvekaki I, Newman JW, Madsen L, Kristiansen K. The elusive endogenous adipogenic PPARγ agonists: Lining up the suspects. Prog Lipid Res. 2015;61:149–162. doi: 10.1016/j.plipres.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Information networks in the mammary gland. Nat Rev Mol Cell Biol. 2005;9:715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- Imagawa W, Bandyopadhyay GK, Nandi S. Regulation of mammary epithelial cell growth in mice and rats. Endocr Rev. 1990;11:494–523. doi: 10.1210/edrv-11-4-494. [DOI] [PubMed] [Google Scholar]
- Imai-Matsumara K, Matsumura K, Morimoto A, Nakayama T. Suppression of cold-induced thermogenesis in full-term pregnant rats. J Physiol. 1990;425:271–281. doi: 10.1113/jphysiol.1990.sp018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Pulikuri S, Zhu Y, Qi C, Kanwar YS, Yeldandi AV, Rao MS, Reddy JK. Differential expression of the peroxisome proliferator-activated receptor gamma (PPARgamma) and its coactivators steroid receptor coactivator-1 and PPAR-binding protein PBP in the brown fat, urinary bladder, colon, and breast of the mouse. Am J Pathol. 1998;153:349–354. doi: 10.1016/s0002-9440(10)65577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LP, Buelto D, Tago E, Owusu-Boaitey KE. Abnormal mammary adipose tissue environment of Brca1 mutant mice show a persistent deposition of highly vascularized multilocular adipocytes. J Cancer Sci Ther. 2011 doi: 10.4172/1948-5956.s2-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morroni M, Giordano A, Zingaretti MC, Boiani R, De Matteis R, Kahn BB, Nisoli E, Tonello C, Pisoschi C, Luchetti MM, Marelli M, Cinti S. Reversible transdifferentiation of secretory epithelial cells into adipocytes in the mammary gland. Proc Natl Acad Sci USA. 2004;101:16801–16806. doi: 10.1073/pnas.0407647101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi G, Poloni A, Mattiucci D, Santi S, Maurizi A, Izzi V, Giuliani A, Mancini S, Zingaretti C, Perugini J, Severi I, Falconi M, Vivarelli M, Rippo MR, Corvera S, Giordano A, Leoni P, Cinti S. Human white adipocytes convert into “rainbow” adipocytes in vitro. J Cell Physiol. 2016 doi: 10.1002/jcp.25743. in press. [DOI] [PubMed] [Google Scholar]
- Prokesch A, Smorlesi A, Perugini J, Manieri M, Ciarmela P, Mondini E, Trajanoski Z, Kristiansen K, Giordano A, Bogner-Strauss JG, Cinti S. Molecular aspects of adipo-epithelial transdifferentiation in mouse mammary gland. Stem Cells. 2014;32:2756–2766. doi: 10.1002/stem.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert MM, Schwertfeger KL, Ryder JW, Anderson SM. An atlas of mouse mammary gland development. J Mammary Gland Biol Neoplasia. 2000;5:227–241. doi: 10.1023/a:1026499523505. [DOI] [PubMed] [Google Scholar]
- Ricquier D. Respiration uncoupling and metabolism in the control of energy expenditure. Proc Nutr Soc. 2005;64:47–52. doi: 10.1079/pns2004408. [DOI] [PubMed] [Google Scholar]
- Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- Silberstein GB. Postnatal mammary gland morphogenesis. Microsc Res Tech. 2001;52:155–162. doi: 10.1002/1097-0029(20010115)52:2<155::AID-JEMT1001>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sun L, Trajkovski M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism. 2014;63:272–282. doi: 10.1016/j.metabol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Tang W, Zhu J, Su S, Wu W, Liu Q, Yu F. MiR-27 as a prognostic marker for breast cancer progression and patient survival. PLoS ONE. 2012;7:e51702. doi: 10.1371/journal.pone.0051702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayhurn P, Wusteman MC. Sympathetic activity in brown adipose tissue in lactating mice. Am J Physiol. 1987;253:E515–E520. doi: 10.1152/ajpendo.1987.253.5.E515. [DOI] [PubMed] [Google Scholar]
- Vitali A, Murano I, Zingaretti MC, Frontini A, Ricquier D, Cinti S. The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J Lipid Res. 2012;53:619–629. doi: 10.1194/jlr.M018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner KU, Wall RJ, St-Onge L, Gruss P, Wynshaw-Boris A, Garrett L, Li M, Furth PA, Hennighausen L. Cre-mediated gene deletion in the mammary gland. Nucleic Acids Res. 1997;25:4323–4330. doi: 10.1093/nar/25.21.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]


