Abstract
Background
Investigation into strenuous activity and kidney function has gained interest given increasing marathon participation.
Study Design
Prospective observational study
Setting & Participants
Runners participating in the 2015 Hartford Marathon
Predictor
Completing a marathon
Outcomes
Acute kidney injury (AKI) as defined by AKI Network (AKIN) criteria. Stage 1 AKI was defined as 1.5- to 2-fold or 0.3 mg/dL increase in serum creatinine within 48 hours of day 0 and stage 2 was defined as a > 2- to 3-fold increase in creatinine. Microscopy score was defined by the number of granular casts and renal tubular epithelial cells.
Measurements
Samples were collected 24 hours pre-marathon (Day 0), immediately post- marathon (Day 1) and 24 hours post-marathon (Day 2). Measurements of serum creatinine, creatine kinase, and urine albumin were completed as well as urine microscopy analysis. Six injury urine biomarkers (IL-6, IL-8, IL-18, kidney injury molecule 1, neutrophil gelatinase-associated lipocalin, and tumor necrosis factor α) and two repair urine biomarkers (YKL-40 and monocyte chemoattractant protein 1) were measured.
Results
22 marathon runners were included. Mean age was 44 years and 41% were male. 82% of runners developed a rise in creatinine equivalent to AKIN-defined AKI stages 1 and 2. 73% had microscopy diagnoses of tubular injury. Serum creatinine, urine albumin, and injury and repair biomarkers peaked on Day 1 and were significantly elevated compared to Day 0 and Day 2. Serum creatine kinase levels continued to significantly rise from Day 0 to Day 2.
Limitations
Small sample size and limited clinical data available at all time points.
Conclusions
Marathon runners developed AKI and urine sediments diagnostic of tubular injury. Rise in injury and repair biomarkers suggests structural damage to renal tubules occurring after marathon. The results of our study should be validated in larger cohorts with longer follow-up of kidney function.
Keywords: Acute kidney injury (AKI), injury biomarkers, repair biomarkers, marathon running, urine microscopy, acute tubular injury, strenuous exercise, serum creatinine, urine albumin, creatine kinase, tubular injury, renal damage
There is limited knowledge regarding the possible deleterious effects of vigorous activity and heat stress on kidney function. Marathon running serves as a human model of strenuous physical exertion due to the intense 26.2-mile run and heat stress involved.1,2
The relationship between marathon running and kidney injury has not been thoroughly evaluated in the literature, but given the rise in marathon participation—with a record high of 550,600 participants in 2014 in the United States—this relationship may become consequential.3 Despite this increasing participation in marathons, the association between marathon running and kidney function has largely been overlooked since runners are generally regarded as healthy athletes with trained physiology to tolerate high states of energy expenditure.4 For example, it has been shown that marathon runners can maximize their oxygen uptake nearly 50% more than healthy non-runners who are half their age.4 Synergistic to this increase in oxygen uptake, cardiac output typically increases three- to five-fold above resting levels to meet the physical demands of marathon running.5 However, while blood flow to the skeletal muscles and the skin significantly increases, renal blood flow may decrease to 25% of resting levels during strenuous activity.5 It is hypothesized that this reduction of blood supply to the kidneys may lead to ischemic tubular damage, as normally kidneys receive 20% of cardiac output.
Another possible mechanism of tubular damage in the runners could be that the rise in core body temperature, which could induce heat stress leading to kidney injury. It has been shown that runners’ rectal temperatures may rise to about 102°F (39°C) in cool running temperatures of about 50°F (10°C) but may exceed 104°F (40°C) in hotter running temperatures of about 95°F (35°C).6 Such a rise in core body temperatures for at least two hours in a standard marathon raises concern for cellular kidney damage. Army recruits, mine workers, and men who exercise vigorously under warm climates have all been noted to develop acute kidney injury (AKI).7,8,9 In general AKI induced by heat stress resolves in complete recovery, but in one study about 10% of those with heat stress–induced AKI went on to develop chronic interstitial nephritis.10
Lastly, while volume depletion might be another reasonable explanation, research indicates that kidney injury can occur even with adequate hydration during running.11 One study suggested that marathon running induces actual structural damage in the kidneys with a rise in serum creatinine and injury biomarkers leading to AKI.12 However, as most studies are using serum creatinine, which is a marker of filtration, the type of structural injury to the kidneys remains unclear and the hypothesis of ischemic damage is yet to be supported by evidence. Since urine microscopy is a hallmark of acute tubular injury (ATI), its use in combination with other conventional and research biomarkers could help elucidate the etiology of kidney injury associated with marathon running.13 Thus we present a prospective observational study evaluating kidney function of runners participating in the Hartford Marathon using both conventional and novel renal biomarkers of injury and repair, to illuminate the relationship between vigorous activity and kidney function.
Methods
Study Design and Participants
Marathon runners participating in the 2015 Hartford Marathon (Connecticut, United States) were enrolled in the study. Recruitment in this prospective observational cohort study was achieved via a survey posted on the Hartford Marathon Registration website and through local running clubs. Runners who were aged 22–63 years and consented for research were included. Other inclusion criteria included a normal body mass index (BMI) of 18.5–24.9 kg/m2, at least three years of running experience, minimum of 15 miles of training per week on average for the last 3 years, completed at least four races that were greater than 20 kilometers in distance, and completed a previous marathon within the last five years within 50%–70% of their World Association of Veteran Athletes performance limit.14 Runners were excluded from the study if they sustained any major running injuries over the last four months, participated in another marathon within 4 weeks prior to race, used NSAIDs within 48 hours prior to or 24 hours post marathon, used statins or anabolic steroids, donated blood within 8 weeks prior to race or had a history of hypothyroidism, kidney disorders, coronary artery disease or convulsive seizures.
Sample Collection and Measurement
Urine and blood samples were collected at three different time points: 24 hours pre-marathon (Day 0), immediately (within 30 minutes) post-marathon (Day 1) and 24 hours post-marathon (Day 2). Six injury biomarkers (interleukin 6 [IL-6], IL-8, IL-18, kidney injury molecule 1 [KIM-1], neutrophil gelatinase-associated lipocalin [NGAL] and tumor necrosis factor α [TNF-α]), and two repair biomarkers (human cartilage glycoprotein 39 [YKL-40] and monocyte chemoattractant protein 1 [MCP-1]) were measured. Serum creatinine and creatine kinase, urine albumin and urine microscopy were also evaluated at each time point. Only two participants refused to provide urine samples on Day 1.
Urine and blood samples were transported on ice to the Yale University biorepository within 2 hours after collection at Quinnipiac University (Day 0, and Day 2) and the Hartford Marathon (Day 1). Upon arrival to the biorepository, samples were centrifuged at 5000g for 10 minutes at 4°C, separated into 1 ml aliquots and immediately stored at -80°C until biomarker measurement. All laboratory personnel were blinded to patient information.
Conventional Biomarker Measurement
Blood pressure, heart rate, pulse oximetry and respiratory rate were measured on Day 0 and Day 2, but only heart rate and pulse oximetry were measured on Day 1. EDTA plasma samples were used as inputs for the measurement of serum creatine kinase and serum creatinine. Serum creatinine was measured via spectrophotometry using the Jaffe reaction by Quest Diagnostics Laboratory, and serum creatine kinase was also measured via spectrophotometry by the Yale New Haven Hospital Laboratory. Urine albumin, urine sodium and urine creatinine were measured enzymatically via Randox technology by Yale New Haven Laboratory. Urine test strips/ dipsticks were used for urinalysis via an automated analyzer by Siemens Clinitek diagnostics.
Novel Urinary Biomarker Measurement
Urinary biomarker measurements were analyzed as concentrations in ng/ml for NGAL (intra-assay coefficient of variation [CV]: 5.2%) and in pg/mL for the following injury and repair biomarkers: IL-6 (CV: 3%), IL-8 (CV: 2.6%), IL-18 (CV: 5.5%), KIM-1 (CV: 8%), TNF-α (CV: 6.1%), YKL-40 (CV: 6.2%) and MCP-1 (CV:5.8%). All were measured using the Meso Scale Discovery platform (Meso Scale diagnostics, Gaithersburg, MD), which uses electrochemiluminescence detection combined with patterned arrays.
Urine Microscopy
Urine microscopy was performed within 2 hours after sample collection. After centrifugation and aliquoting, about 0.5 ml of urine were left in the test tube. Test tubes were gently agitated manually and a pipette was used to transfer one drop on a glass slide followed by the application of a cover slip with minimal trapping of air bubbles. Samples were examined under low power (×10) followed by high power (×40) on bright field microscopy. Examining at least ten fields per each power field, urine sediments were analyzed for the presence and number of renal tubule epithelial cells (RTEC), and granular casts. Granular casts and RTEC per high-power field were quantified, if present, as 1–5, 6–10 and >10 and as 1–5, 6–20 and >20, respectively. Urine sediment pictures were taken using Apple i-phone 6s camera. An experienced second-year nephrology trainee (S.G.M.) prepared and examined the microscopy slides, and captured images of identified pathology. A nephrology attending physician, expert in urine microscopy (M.A.P.), and the nephrology trainee jointly discussed and determined the final urine microscopy findings (using procedures outlined in http://patr.yale.edu/resources/#page3).
Outcome Definitions
Acute kidney injury was defined using the AKI Network (AKIN) criteria.15 Stage-1 AKI was defined as a 1.5- to 2-fold increase or 0.3 mg/dL increase in serum creatinine from Day 0 to peak creatinine value on either Day 1 or Day 2 and stage-2 AKI was defined as a >2- to 3-fold increase in serum creatinine.
Urine microscopy score was based on the number of RTEC and granular casts seen under high power. These scores were used to differentiate ATI from AKI due to decreased kidney perfusion.16 A score of ≥ 2 on Day 1 or Day 2 was defined as having positive urine microscopy findings. The scoring system is shown in Table S1 (provided as online supplementary material).
Statistical Analysis
All analyses were two tailed and p-values less than 0.05 were considered significant. Descriptive statistics for continuous variables were reported as mean ± standard deviation or median (interquartile range [IQR]) and for categorical variables as frequency (%). Marathon runners’ characteristics were analyzed using Mann–Whitney Wilcoxon tests for continuous variables and chi-squared test for categorical variables. Pearson correlations were used to evaluate bivariate relationships between clinical and novel biomarkers. Analyses were performed using SAS 9.4 software for Windows (SAS Institute, Cary, NC).
This study was approved by the Yale Human Investigation Committee (HIC protocol number: 1509016483) and all participants gave informed consent.
Results
Study Participants
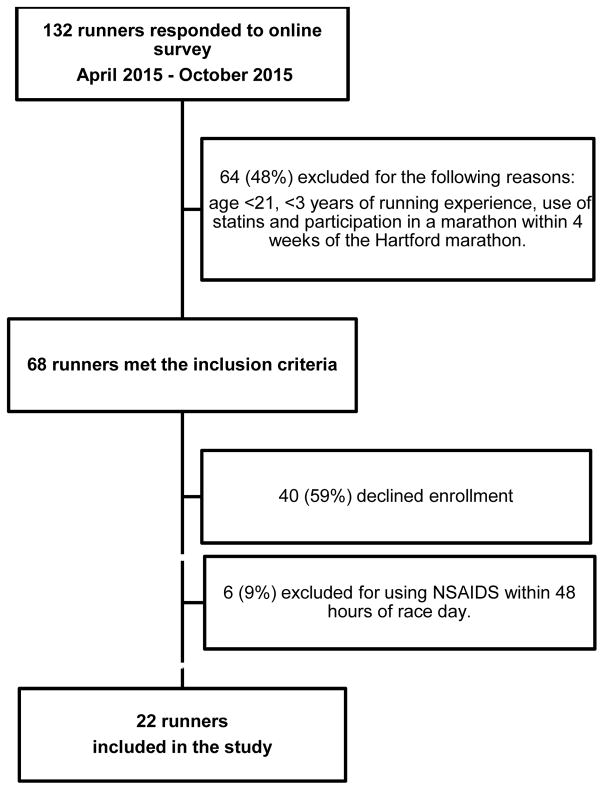
Out of the 132 individuals who responded to the online survey, 68 individuals met the inclusion criteria and 22 runners agreed to participate and consented to the study. The study cohort is shown in Figure 1. A total of 9 (41%) men and 13 (59%) women were included; mean age was 44.2 ±12.9 (standard deviation) years and mean BMI was 22.4 ±2.4 kg/m2 (Table 1). Runners had a median running experience of 12 (IQR, 5.0–15.0) years and participated in a median of 5 (IQR, 2–16) prior marathons. Runners trained an average of 31.8 ±10.4 miles/week, and overall the cohort was fairly healthy without comorbidities except for two runners with hypertension and one with type I diabetes. Six runners (27%) consumed NSAIDS within two weeks of the race but not within 48 hours of race day and 11 (50%) were taking herbal supplements. On the day of the marathon the weather was sunny with an average temperature of 62°F. All runners completed the 26.2 miles with an average marathon finishing time of 4.02 ±0.64 hours.
Figure 1.
Enrollment chart of runners in the study cohort
Table 1.
Characteristics of marathon runners and post-marathon outcomes
| Characteristics and Outcomes | Value |
|---|---|
| Baseline characteristics | |
| Age (years) | 44.2 ±12.9 |
| Male sex | 9 (41%) |
| Height (cm) | 169.7±7.8 |
| Weight (kg) | 65.0 ±11.6 |
| BMI (kg/m2) | 22.4 ±2.4 |
| Hypertension | 2 (9%) |
| DM type I | 1(5%) |
| Self-reported NSAID use | 6 (27%) |
| Herbal Supplements use | 11 (50%) |
| Running Experience (years) | 12.0 [5.0–15.0] |
| Average Miles per week | 31.8 ±10.4 |
| Best Marathon Finishing Time (hours) | 4.2 ±0.6 |
| Number of Prior Marathons | 5 [2–16] |
| Postmarathon Outcomes | |
| Hartford Marathon finishing time (hours) | 4.02 ±0.64 |
| ≥AKI stage 1* | 18 (82%) |
| Positive urine microscopy findings (score ≥2) | 16 (73%) |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation if normally distributed or median [interquartile range] if nonnormally distributed.
AKI: acute kidney injury; BMI: body mass index; DM: diabetes mellitus; NSAID: non-steroidal anti-inflammatory drug.
Only one runner developed stage two AKI.
Conventional Biomarkers
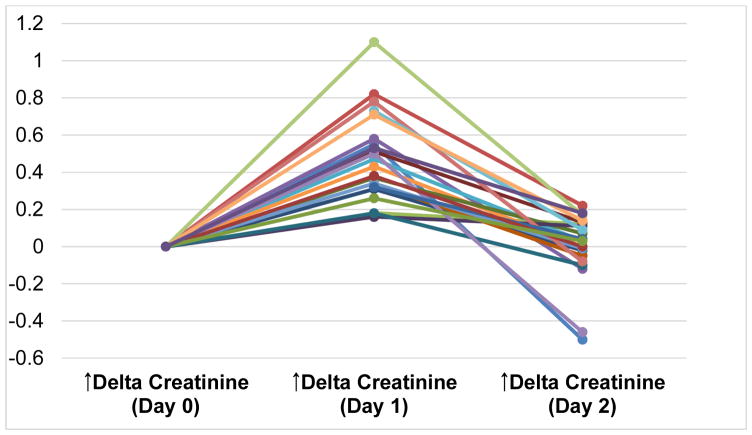
Vital signs of runners across the three study time points are shown in Table 2. Serum creatinine concentration peaked on Day 1 in all runners, as shown in Figure 2. Median creatinine values on Days 0, 1 and 2 were 0.81 (IQR, 0.76–0.95) mg/dL, 1.28 (IQR, 1.09–1.54) mg/dL, and 0.90 (IQR, 0.80–0.90) mg/dL, respectively (Table 3). Eighty two percent of runners developed at least stage one AKI by AKIN criteria and one runner developed stage two AKI. Along with serum creatinine, urine albumin also peaked on Day 1 as shown in Table 3. However, serum creatine kinase continued to rise 24 hours post-marathon, with Day 2 values significantly higher compared to Day 0 and Day 1 (Table 3). Fractional excretion of sodium was less than 1% across all three time points but did significantly decrease from Day 0 to Days 1 and 2 (Table 3). There were no significant correlations between creatine kinase and both conventional and novel biomarkers across the time points except for YKL-40, which positively correlated with creatine kinase on Day 1 (r=0.51; p<0.05), and IL-8, which negatively correlated with creatine kinase on day 1 (r=−0.51; p<0.05; Table S2).
Table 2.
Vital signs of marathon runners by time points
| Vital Signs | Day 0: Premarathon | Day 1: Immediately postmarathon | Day 2: Postmarathon | Overall Pa |
|---|---|---|---|---|
| Systolic BP (mm Hg) | 118 ±6 | N/A | 113 ±6 | 0.01 |
| Diastolic BP (mm Hg) | 75 ±8 | N/A | 72 ±7 | 0.05 |
| Heart rate | 62 ±9 | 126 ±5 | 63 ±12 | <0.001 |
| Respiratory rate | 16 ±3 | NA | 15 ±2 | 0.03 |
| Pulse Oximetry, % | 98 ±1 | 98 ±1 | 99 ±1 | 0.09 |
Note: Unless otherwise indicated, values are given as mean ± standard deviation.
Overall p-values were obtained via Kruskal-Wallis Test.
BP, blood pressure; N/A, not applicable (indicates that vital sign was not measured).
Figure 2.
Absolute change in serum creatinine per runner compared to baseline value on Day 0
Table 3.
Levels of conventional biomarkers by time point
| Conventional Biomarker | Day 0: Premarathon (n=22) | Day 1: Immediately Postmarathon (n=22b) | Day 2: Postmarathon (n=22) | Overall Pa |
|---|---|---|---|---|
| Serum Creatinine (mg/dL) | 0.81 (0.76, 0.95) | 1.28 (1.09, 1.54)* | 0.90 (0.80, 0.90) | <0.001 |
| Serum creatine kinase (U/l) | 86 (74, 159) | 268 (242, 344) | 722 (434, 844)* | <0.001 |
| FENa (%) | 0.82 (0.61, 1.11)* | 0.23 (0.11, 0.29) | 0.22 (0.18, 0.41) | <0.001 |
| Urine albumin (mg/dL) | 0.50 (0.50, 0.50) | 3.50 (1.69, 6.53)* | 0.54 (0.50, 0.68) | <0.001 |
| Urine albumin-creatinine ratio (mg/g) | 0.01 (0.01, 0.01) | 0.02 (0.02, 0.05)* | 0.01 (0.00, 0.01) | <0.001 |
| Urine Microscopy score ≥1 | 2 (9%)* | 13 (65%) | 13 (59%) | <0.001 |
| Urinalysis | ||||
| Specific Gravity | 1.02 (1.01, 1.02) | 1.02 (1.01, 1.03) | 1.02 (1.01, 1.02) | 0.2 |
| pH | 7.0 (6.0, 7.0)* | 5.5 (5.5, 7.0) | 6.0 (5.5, 6.5) | 0.002 |
| Blood | 0.3 | |||
| 0 | 19 (86%) | 13 (65%) | 18 (82%) | |
| 1 | 2 (9%) | 4 (20%) | 3 (14%) | |
| 2 | 0 | 1 (5%) | 0 | |
| 3 | 0 | 2 (10%) | 0 | |
| 4 | 1 (5%) | 0 | 1 (5%) | |
| Nitrite positive | 0 | 1 | 0 | 0.3 |
| Protein | 0.03 | |||
| 0 | 21 (95%) | 12 (60%) | 18 (82%) | |
| 1 | 1 (5%) | 2 (10%) | 4 (18%) | |
| 2 | 0 | 4 (20%) | 0 | |
| 3 | 0 | 1 (5%) | 0 | |
| 4 | 0 | 1 (5%) | 0 | |
| Ketones | <0.001 | |||
| 0 | 22 (100%) | 9(45%) | 17 (77%) | |
| 1 | 0 | 8 (40%) | 5 (23%) | |
| 2 | 0 | 3 (15%) | 0 | |
| Leukocyte Esterase | 0.7 | |||
| 0 | 16 (73%) | 15 (75%) | 18 (82%) | |
| 1 | 4 (18%) | 4 (20%) | 1 (5%) | |
| 2 | 1 (5%) | 0 | 1 (5%) | |
| 3 | 1 (5%) | 1 (5%) | 2 (9%) |
FENa, fractional excretion of sodium;
Note: Values for categorical variables are given as number (percentage); for continuous variables, as median [interquartile range].
Overall p-values were obtained via Kruskal-Wallis Test for continuous variables, and X2 test statistic was used for categorical variables.
Only 20 urine samples were available on Day 1 since 2 runners refused to provide urine.
Value is significantly different compared to the other two time points.
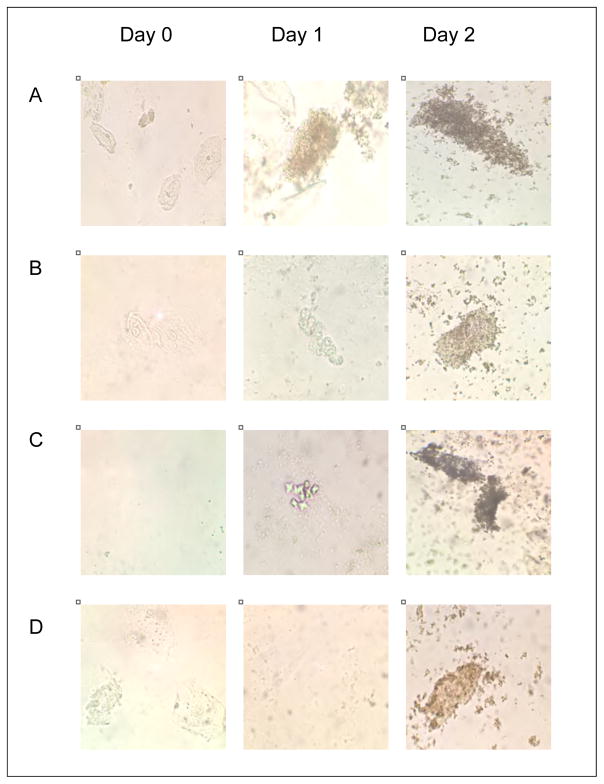
Urine microscopy revealed minimal findings on Day 0, but Days 1 and 2 revealed significant increase in score among runners, with 9%, 65%, and 59% having positive scores on each day, respectively (p<0.001). A total of 16 (73%) runners were scored as having positive microscopy findings on Day 1 or Day 2. No crystals, including urate crystals, were seen on urine sediment. Representative urine microscopy images taken from runners on Days 1 and 2 are shown in Figure 3.
Figure 3.
Representative urine microscopy images of samples from four runners by time point
Injury Biomarkers
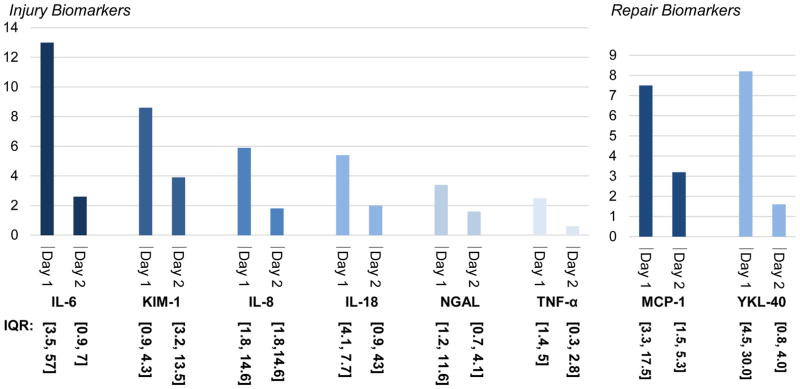
Urine biomarkers of injury were significantly elevated on Day 1 compared to Days 0 and 2 (Table 4). The highest-fold increases on Day 1 as compared to baseline (Day 0) values were seen in IL-6, KIM-1 and IL-8 as seen in Figure 4A.
Table 4.
Levels of injury and repair biomarkers by time point
| Urinary Biomarker | Day 0: Premarathon | Da 1: Immediately Postmarathon | Day 2: Postmarathon | Overall Pa |
|---|---|---|---|---|
| Injury Biomarkers | ||||
| KIM-1 (pg/ml) | 132.59* (67.61, 219.98) | 723.32 (459.36, 1970.64) | 702.42 (123.27, 1098.67) | <0.001 |
| TNF-α (pg/ml) | 0.02 (0.01, 0.04) | 0.09* (0.04, 0.18) | 0.02 (0.00, 0.04) | 0.001 |
| IL-18 (pg/ml) | 6.43 (4.24, 12.26) | 45.89* (23.42, 63.45) | 16.95 (4.84, 29.98) | <0.001 |
| IL-6 (pg/ml) | 0.05 (0.03, 0.15) | 0.96* (0.38, 2.24) | 0.19 (0.08, 0.30) | <0.001 |
| IL-8 (pg/ml) | 4.80 (0.92, 20.65) | 43.87* (9.93, 123.00) | 9.31 (2.88, 23.11) | 0.01 |
| NGAL (ng/ml) | 8.00 (4.15, 30.48) | 37.64* (19.03, 84.61) | 18.49 (9.25, 33.69) | 0.001 |
| Repair Biomarkers | ||||
| YKL-40 (pg/ml) | 96.25 (43.96, 124.31) | 865.13* (466.84, 1764.28) | 202.97 (55.91, 398.81) | <0.001 |
| MCP-1 (pg/ml) | 39.56 (26.12, 79.29) | 264.47* (131.12, 702.01) | 186.28(55.91, 366.74) | <0.001 |
Note: Unless otherwise indicated, values are given as median [interquartile range].
IL, interleukin; KIM-1, kidney injury molecule 1; MCP-1, monocyte chemoattractant protein-1; NGAL, neutrophil gelatinase-associated lipocalin; TNF-α, tumor necrosis factor α; YKL-40, human cartilage glycoprotein 39.
Overall p-values were obtained via Kruskal-Wallis Test.
Value is significantly different compared to the other two time points
Figure 4.
Fold increase in biomarkers compared to baseline on Day 0
Repair Biomarkers
Both YKL-40 and MCP-1 peaked on Day 1 as seen in Table 3. MCP-1 had a 7.5-fold increase and YKL-40 had an 8.2-fold increase on Day 1 after marathon running as compared to baseline levels (Figure 4B).
Discussion
In this prospective study with sample collection along with urine microscopy, we discovered that both serum creatinine and urine albumin significantly increased after marathon participation, with most runners (82%) developing at least stage-1 AKI. Given that serum creatinine can be affected by multiple non-renal factors, such as muscle breakdown and volume shifts, we also assessed injury biomarkers in runners.17,18,19 There are several injury biomarkers that are being developed in the research setting. The biomarker NGAL has been identified to be an early injury biomarker of AKI by genomic, transcriptomic, and proteomic techniques.20,21 It is a neutrophil derived marker, which is also produced by renal tubular cells in the setting of various types of injury.22 Cut-off values ranging from 50 to 100 ng/ml have been used to accurately diagnose AKI in multiple different clinical settings such as post cardiac surgery, and kidney transplantation.23,24,25 Another injury biomarker, KIM-1, is extensively expressed in renal proximal tubular cells post injury and at much lower levels in lymphocytes.26,27 It has been shown to have considerable predictive value in early detection of AKI in multiple clinical settings.28 Similarly, specific interleukins, which are mainly produced by macrophages but also expressed in renal tubular cells, have been identified to detect AKI with substantial accuracy.29,30 In intensive care patients, IL-18 was found to be a marker of AKI and mortality.31 Alongside IL-18 , both IL-6 and IL-8 have also been identified as markers for predicting AKI post cardiac surgery.32,33 Lastly, TNF-α, which is mainly produced by macrophages, has also been associated with AKI in patients with severe sepsis.34
Similar to creatinine, these injury biomarkers were significantly increased among the marathon runners in the present study. This suggests that there is parenchymal damage (primarily tubular) in the kidney secondary to strenuous exercise. However, given non-renal sites of production for several of these biomarkers, it is also plausible that this rise in biomarkers is not specific to kidney injury as increased systemic production could lead to increased levels in the urine.22,27,29,30
Interestingly, despite the rise in creatine kinase, as would be expected from muscle breakdown during running, there was no significant positive correlation between creatine kinase levels and injury or conventional biomarkers. Given that kidney injury does not correlate with muscle breakdown, we hypothesize that heat stress and rise in core body temperature along with systemic inflammation are likely associated with AKI in marathon runners. It has been shown that runners have significant rise in rectal temperatures post marathon running.35 However, since we did not measure core body temperatures before and after running, our hypothesis regarding heat stress needs to be evaluated in future studies. Despite prior work showing that most marathon runners maintain adequate hydration, fractional excretion of sodium values in our cohort suggest dehydration leading to decreased kidney perfusion could have also contributed to the rise in creatinine.11 However, it has also been shown that low fractional excretion of sodium states are present in other conditions such as ATI, given the presence of vasoconstriction in early ATI.36
In this study, urine microscopy reveals that the main etiology of AKI in marathon runners is ATI. Given that ATI occurs due to ischemia, which usually occurs in the clinical context of background comorbidities or multiple organ failure, runner ATI is distinct in that it is in healthy individuals.37 Despite a reduction in cystatin C-estimated glomerular filtration rate (eGFR) directly after marathon running, one study showed return to baseline in renal parameters two weeks after running, suggesting that despite the development of acute injury post running, there is no persistent reduction in filtration function.38 However there are no studies to evaluate renal structure and damage in runners beyond two weeks, and hence it remains unclear whether runners develop chronic sequela from repetitive renal insults while running. However, despite rigorous training with about 32 miles per week and a median of 12 years of running experience of those in our cohort, there was no evidence of CKD in our participants. In addition, there is no published evidence of an increase in CKD prevalence among marathon runners. The possibility of ascertainment bias also exists, since CKD is an asymptomatic disease with most runners being healthy individuals who may not undergo routine annual health examination.
In parallel to the strenuous physical state and heat stress that is experienced by athletes such as marathon runners, agricultural workers in Central America may have similar working environments but with notable differences that may explain the variance in the prevalence of CKD in both populations.39 On average sugarcane workers spend about 4 hours per day in 95–108°F temperatures.40,41 The intensity of the work is magnified because workers are paid not by the hour but rather by the number of sugarcanes they cut at the end of the day.42 Similarly in this study, runners completed the Hartford marathon in an average of 4 hours, with likely comparable levels of intensity of physical labor but under much cooler temperatures of 62°F. Hence the increase in the prevalence of CKD in Central America might be secondary to field workers’ exposure to higher external ambient temperatures, as well as more frequent heat stress injury. Agricultural workers have been shown to have acute decreases in kidney function and progression to CKD associated with dehydration, systemic inflammation, and oxidative stress.43 It is also possible that compared to agricultural workers, marathon runners have controlled ischemic preconditioning throughout their training, which may improve the kidney’s ability to better tolerate repeated injury.44 Agricultural workers, however, have ischemic injury in a more uncontrolled setting with limited access to hydration and health resources, which may hinder their ability to adapt to recurrent injury.
Given our small sample size and lack of multivariable analyses, we can only speculate that marathon runners adapt well to injury as AKI development was only transient. This is despite 23% of runners in our cohort having NGAL levels >90 ng/ml, which approach levels traditionally seen in critically ill patients such as those with hepatorenal syndrome or those immediate following cardiac surgery (Table S3).45,46,47 This highlights that elevation in biomarkers is only part of the story of renal effects of running, as NGAL represents the level of injury and inflammation but does not highlight the cascade of repair processes triggered in response to injury. The latter was captured in runners by the significant rise in both repair biomarkers, YKL-40 and MCP-1, immediately post-marathon. A glycoprotein, YKL-40 has been shown to play an important role in cytoprotection and repair especially in recovery from AKI.48,49,50 Also, MCP-1 has been shown to be essential for macrophage recruitment and healing especially after ischemia reperfusion injury.51,52 It is possible that in contrast to American runners, repetitive injury in Mesoamericans is met with inadequate reparative responses leading to sequela of fibrosis and progression from acute to chronic injury.
Our study is unique in that, to our knowledge, it is the first to evaluate urine microscopy in parallel with conventional and novel biomarkers of injury and repair in marathon runners. We have shown that AKI in runners is secondary to structural injury, mainly ATI, as evidenced by serum creatinine levels, urine microscopy analysis, and levels of novel biomarkers of injury and repair. We acknowledge the limitations of our study, namely that our sample size is small, and hence is subject to confounding. In addition, our study had a short follow up time of 48 hours and no long-term outcomes are captured. Last, the presence of organic compounds such as ketones could have led to false-positive serum creatinine measurements, since ketones produce similar color changes as creatinine when using the Jaffe methodology to determine creatinine values.53
In this study, we have shown that marathon runners develop a rise in creatinine that is equivalent to AKI stages 1 and 2 based on AKIN criteria with urine sediments that are diagnostic of ATI. This is accompanied by an increase in injury and repair biomarkers, further indicating structural damage in the kidney. The results of our study are mainly hypothesis generating and should be further validated in larger cohorts.
Supplementary Material
Table S1: Microscopy scoring system.
Table S2: Pearson and Spearman correlation between biomarker levels and creatine kinase levels for each time point.
Table S3: Distribution of NGAL levels and training of marathon runners.
Acknowledgments
The authors thank the runners who participated in this study and the Hartford Marathon Foundation for their collaboration. The authors also thank Karen Myrick, David Jou, Amanda La Falce, Kelly Malloy, Gabriela Narowska, Selin Isguven, and Joe El-Khoury for their assistance with data collection and sample processing.
Support: This study was supported by the Quinnipiac University Faculty Scholarship grant. Dr Parikh was supported by the K24 grant (K24DK090203) from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr Mansour was supported by the T32 training grant (T32DK007276) from the National Institutes of Health.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: SGM, RWP, TGM, CRP; data acquisition: SGM, MAP, CRP; data analysis and interpretation: SGM, CRP; statistical analysis: SGM, CRP, GV; supervision and mentorship: CRP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. CRP takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by two external peer reviewers, a Statistical Editor, a Co- Editor and Editor-in-Chief Levey.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams WC, Fox RH, Fry AJ. Thermoregulation during marathon running in cool, moderate and hot environments. Journal of Applied Physiology. 1975;38(6):1030–1037. doi: 10.1152/jappl.1975.38.6.1030. [DOI] [PubMed] [Google Scholar]
- 2.Boppart MD, Asp S, Wojtaszewski JFP. Marathon running transiently increases c-Jun NH2-terminal kindase and p38y activities in human skeletal muscle. The Journal of Physiology. 2000;526(3):663–669. doi: 10.1111/j.1469-7793.2000.00663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annual Reports. Running USA. [Accessed November, 2016]; www.runningusa.org/marathon-report-2016.
- 4.Costill DL. Physiology of marathon running. JAMA. 1972;221(9):1024–1029. [PubMed] [Google Scholar]; Billat VL, Petot H, Landrain M. Cardiac output and performance during a marathon race in middle-aged recreational runners. Scientific World J. 2012:1–9. doi: 10.1100/2012/810859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poortmans JR. Exercise and renal function. Sports Med. 1984;1(2):125–153. doi: 10.2165/00007256-198401020-00003. [DOI] [PubMed] [Google Scholar]
- 6.Adams WC, Fox RH, Fry AJ, MacDonald IC. Thermoregulation during marathon running in cool, moderate, and hot environments. J Applied Physiol. 1975;38(6):1030–1037. doi: 10.1152/jappl.1975.38.6.1030. [DOI] [PubMed] [Google Scholar]
- 7.Kew MC, Abrahams C, Levin NW. The effects of heat stroke on the function and structure of the kidney. Quart J Med. 1967;36:277–300. [PubMed] [Google Scholar]
- 8.Shilbolet S, Coll R, Gilat T. Heat stroke: Its clinical picture and mechanisms in thirty-six cases. Quart J Med. 1967;36:524–548. [PubMed] [Google Scholar]
- 9.Vertel RM, Knochel TP. Acute renal failure due to heat injury. Amer J Med. 1967;67:350–376. doi: 10.1016/0002-9343(67)90196-9. [DOI] [PubMed] [Google Scholar]
- 10.Kew MC, Abrahams C, Seftel C. Chronic interstitial nephritis as a consequence of heat stroke. Quart J Med. 1970;39:189–199. [PubMed] [Google Scholar]
- 11.Clarkson PM. Exertional rhabdomyolysis and acute renal failure in marathon runners. Sports Med. 2007;37(4–5):361–363. doi: 10.2165/00007256-200737040-00022. [DOI] [PubMed] [Google Scholar]
- 12.McCullough PA, Chinnaiyan KM, Gallagher MJ, et al. Changes in renal markers and acute kidney injury after marathon running. Nephrology. 2011;16:194–199. doi: 10.1111/j.1440-1797.2010.01354.x. [DOI] [PubMed] [Google Scholar]
- 13.Perazella MA, Coca SG, Kanbay M. Diagnostic value of urine microscopy for differential diagnosis of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2008;3(6):1615–1619. doi: 10.2215/CJN.02860608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young BW, Medic N, Weir PL. Explaining performance in elite middle-aged runners: contributions from age and from ongoing and past training factors. Journal of Sport and Exercise Psychology. 2008;30:737–754. doi: 10.1123/jsep.30.6.737. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perazella MA, Coca SG, Hall IE. Urine microscopy is associated with severity and worsening of acute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. 2010;5(3):402–408. doi: 10.2215/CJN.06960909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi K, Yuen PST, Eisner C, et al. Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol. 2009;20:217–1221. doi: 10.1681/ASN.2008060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parikh CR, Garg AX. Acute kidney injury: better biomarkers and beyond. Kidney Int. 2008;73:801–803. doi: 10.1038/ki.2008.17. [DOI] [PubMed] [Google Scholar]
- 19.Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med. 2008;36:S159–165. doi: 10.1097/CCM.0b013e318168c652. [DOI] [PubMed] [Google Scholar]
- 20.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 21.Supavekin S, Zhang W, Kucherlapati R, et al. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63(5):1714–1724. doi: 10.1046/j.1523-1755.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 22.Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008 Sep;52(3):595–605. doi: 10.1053/j.ajkd.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–8. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 24.Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–91. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Mishra J, Ma Q, Kelly C, et al. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr Nephrol. 2006;21:856–63. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- 26.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–44. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 27.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24(11):3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 28.Shao X, Tian L, Xu W, et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One. 2014;9(1):e84131. doi: 10.1371/journal.pone.0084131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Emmanuilidis K, Weighardt H, Matevossian E, et al. Differential regulation of systemic IL-18 and IL-12 release during postoperative sepsis: High serum IL-18 as an early predictive indicator of lethal outcome. Shock. 2002;18:301–305. doi: 10.1097/00024382-200210000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease & quest. Kidney international. 2004;66(2):480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 31.Parikh CR, Abraham E, Ancukiewicz M, et al. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WR, Garg AX, Coca SG, et al. Plasma IL-6 and IL-10 concentrations predict AKI and long-term mortality in adults after cardiac surgery. J Am Soc Nephrol. 2015;26(12):3123–32. doi: 10.1681/ASN.2014080764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liangos O, Kolyada A, Tighiouart H, et al. Interleukin-8 and acute kidney injury following cardiopulmonary bypass: a prospective cohort study. Nephron Clin Pract. 2009;113(3):c148–154. doi: 10.1159/000232595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashad DI, Elsayed ET, Helmy TA, et al. Study of the role of tumor necrosis factor-α3(-3083G/A) and interleukin-10 (-1082 G/A) polymorphisms as potential risk factors to acute kidney injury in patients with severe sepsis using high-resolution melting curve analysis. Ren Fail. 2016;27:1–6. doi: 10.1080/0886022X.2016.1244081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pugh LGCE, Corbett JL, Johnson RH. Rectal temperatures, weight losses, and sweat rates in marathon running. Journal of Applied Physiology. 1967;23(3):347–352. doi: 10.1152/jappl.1967.23.3.347. [DOI] [PubMed] [Google Scholar]
- 36.Brosius FC, Lau K. Low fractional excretion of sodium in acute renal failure: role of timing of the test and ischemia. Am J Nephrol. 1986;6(6):450. doi: 10.1159/000167251. [DOI] [PubMed] [Google Scholar]
- 37.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121(11):4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hewing B, Schattke S, Spethmann S, et al. Cardiac and renal function in a large cohort of amateur marathon runners. Cardiovasc Ultrasound. 2015;13:13. doi: 10.1186/s12947-015-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiner DE, McClean MD, Kaufman JS. The Central American epidemic of CKD. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.05050512. [DOI] [PubMed] [Google Scholar]
- 40.Wijkstrom J, Leiva R, Elinder CG, et al. Clinical and pathological characterization of Mesoamerican nephropathy: a new kidney disease in Central America. Am J kidney Dis. 2013;62(5):908–918. doi: 10.1053/j.ajkd.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Tabanino R, Jarquin E, Wesseling C, et al. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador- A cross-shift study of workers at risk of Mesoamerican nephropathy. Environmental Research. 2015;142:746–755. doi: 10.1016/j.envres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Laws RL, Brooks DR, Amador JJ, et al. Changes in kidney function among Nicaraguan sugarcane workers. International Journal of Occupational and Environmental Health. 2015;21(3):241–250. doi: 10.1179/2049396714Y.0000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paula-Santos U, Zanetta DMT, Terra-Filho M, et al. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015;87:792–799. doi: 10.1038/ki.2014.306. [DOI] [PubMed] [Google Scholar]
- 44.Zarbock A, Schmidt C, Van Aken H, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313(21):2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 45.Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury n critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–32. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belcher JM, Sanyal AJ, Peixoto AJ, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60(2):622–632. doi: 10.1002/hep.26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasse M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J kidney Dis. 2009;54(6):1012–1024. doi: 10.1053/j.ajkd.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt IM, Hall IE, Kale S, et al. Chitinase-like protein Brp-39/YKL-40 modulates the renal response to ischemic injury and predicts delayed allograft function. J Am Soc Nephrol. 2013;24(2):309–19. doi: 10.1681/ASN.2012060579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Peng H, Sun HX, et al. Chitinase 3-like 1 suppresses injury and promotes fibroproliferative responses in mammalian lung fibrosis. Science Translational Medicine. 2014;6(240) doi: 10.1126/scitranslmed.3007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yadav A, Saini V, Arora S. MCP-1: chemoattractant with a role beyond immunity: a review. Clin Chim Acta. 2010;411:1570–1579. doi: 10.1016/j.cca.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Stroo I, Claessen N, Teske GJD. Deficiency for the chemokine monocyte chemoattractant protein-1 aggravates tubular damage after renal ischemia/reperfusion injury. PLoS One. 2015;10:e0123203. doi: 10.1371/journal.pone.0123203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Husdan H, Rapoport A. Estimation of creatinine by Jaffe reaction. A comparison of three methods. Clin Chem. 1968;14(3):222–237. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Microscopy scoring system.
Table S2: Pearson and Spearman correlation between biomarker levels and creatine kinase levels for each time point.
Table S3: Distribution of NGAL levels and training of marathon runners.