Abstract
Mindfulness-Based interventions have increased in popularity in psychiatry, but the impact of these treatments on disorder-relevant biomarkers would greatly enhance efficacy and mechanistic evidence. If Generalized Anxiety Disorder (GAD) is successfully treated, relevant biomarkers should change, supporting the impact of treatment and suggesting improved resilience to stress. Seventy adults with GAD were randomized to receive either Mindfulness-Based Stress Reduction (MBSR) or an attention control class; before and after, they underwent the Trier Social Stress Test (TSST). Area-Under-the-Curve (AUC) concentrations were calculated for adrenocorticotropic hormone (ACTH) and pro-inflammatory cytokines. MBSR participants had a significantly greater reduction in ACTH AUC compared to control participants. Similarly, the MBSR group had a greater reduction in inflammatory cytokines’ AUC concentrations. We found larger reductions in stress markers for patients with GAD in the MBSR class compared to control; this provides the first combined hormonal and immunological evidence that MBSR may enhance resilience to stress.
Keywords: Meditation, Anxiety, Resilience, Stress Reactivity, Acute Stress, Mindfulness-Based Intervention, Psychological Stress
1. Introduction
Chronic or repeated psychological stress has been associated with abnormalities in stress hormones and inflammatory markers. These hormonal and immune abnormalities are in turn associated with negative health consequences such as cardiovascular disease risk and metabolic syndrome (Chrousos, 2000; Ridker et al., 2000). For example, chronic over-secretion of cortisol is associated with metabolic and hemodynamic disturbances such as high systolic blood pressure, fasting glucose, and insulin (Kaur, 2014). In addition, higher levels of circulating pro-inflammatory cytokines such as interleukin-6 (IL-6) are associated with other metabolic syndrome elements including higher body-mass index and the development of type 2 diabetes, and with an increased risk for coronary artery disease (Pradhan et al., 2002).
The causal link between hormonal and inflammatory markers and stress suggested by this body of cross-sectional data is supported by studies showing changes in these biomarkers in response to an experimental stress. Paralleling the epidemiological observations of chronically stressed populations, laboratory stress challenge tests have thus been found to provoke similar elevations in stress hormones (cortisol and adrenocorticotropic hormone (ACTH)) and markers of inflammation (tumor necrosis factor-alpha (TNF-alpha), and IL-6) in the bloodstream (Kirschbaum et al., 1993; Pace et al., 2006; von Kanel et al., 2005).
Mindfulness-Based interventions have greatly increased in popularity and have been used to treat anxiety in recent years. However, randomized and adequately controlled trials are needed to validate waitlist-controlled findings and provide additional confirmation of biological effects (Chiesa and Serretti, 2010; Goyal et al., 2014). Given that mindfulness meditation focuses on one’s present experience is often ignored or avoided in Generalized Anxiety Disorder (GAD), we conducted a randomized, controlled study comparing Mindfulness-Based Stress Reduction (MBSR), a standardized and manualized mindfulness meditation training course, with an attention control, Stress Management Education (SME) in individuals with GAD. We measured the effect of MBSR vs. SME on clinical anxiety measures, and found a greater drop in anxiety ratings in most of our measures (see (Hoge et al., 2013) for detailed results). In a group of these patients, we examined resilience to subsequent stress by measuring hormones and inflammatory markers during the laboratory-based Trier Social Stress Test (TSST). Resilience is “the ability of individuals to adapt successfully in the face of acute stress, trauma, or chronic adversity, maintaining or rapidly regaining psychological well-being and physiological homeostasis” (Charney, 2004), and the TSST, which provides a way to measure coping and recovery from a standardized stressor, has been used to assess resilience in the laboratory (Rose et al., 2013). Prior research has demonstrated that patients with GAD, like other chronically stressed populations, have an exaggerated stress hormone response to the TSST or other laboratory stress provocation, compared to healthy controls (Gerra et al., 2000).
Although participants’ ratings of subjective stress were reduced more after MBSR compared to SME in our clinical study, we wanted to examine biomarkers in a separate planned analysis using blood markers previously linked to acute and chronic stress, such as the stress hormones cortisol and ACTH, and markers of inflammation, TNF-alpha and IL-6. We were interested in whether MBSR could improve coping and mitigate the physiological effects of acute stress. In addition, decreases in stress hormones and chronic inflammatory markers after mindfulness meditation, compared to a control intervention, would provide some support to the hypothesis that mindfulness meditation training may contribute to improvements in overall medical (cardiovascular and metabolic) health through reductions in stress related biological responses. We hypothesized that mindfulness meditation would mitigate the previously reported elevated response to acute stress observed in GAD, evidenced by a greater reduction in stress hormones and inflammatory markers with treatment.
2. Methods
2.1. Participants and Procedures
The procedures of the clinical randomized controlled trial have been described in detail previously (Hoge et al., 2013). Briefly, individuals age 18 and older with GAD, as determined by the Structured Clinical Interview for the DSM-IV (SCID) (First et al., 2002), were randomized to either a modified group MBSR or group SME (see below for course descriptions). Exclusion criteria included lifetime history of psychotic disorder, intellectual disability, organic medical disorders (such as endocrine diseases such as Addison’s and Cushing’s or chronic inflammatory diseases), bipolar disorder, post-traumatic stress disorder or obsessive compulsive disorder; current alcohol or substance abuse or dependence; significant suicidal ideation or behaviors; and concurrent psychotherapy for GAD. Additional inclusion criteria for this biomarker ancillary study were completion of the pre- and post-treatment TSST experiments, and the availability of blood specimens with adequate volume from all three TSST time periods: pre-stress, immediate post-stress, and later post-stress. In addition, we excluded patients taking antidepressants and benzodiazepines, as prior data suggest they may artificially alter the hormone response during the TSST (Bremner et al., 2003; Carpenter et al., 2007).
Prior to the start of the intervention class, and after baseline questionnaire measurements, participants came to the lab for a testing day, in which they completed the TSST and all blood testing (see details in section 2.2 below). After the end of the 8-week intervention, they returned to the lab for the second TSST. All study procedures were given ethical approved by the Massachusetts General Hospital/Partners Health Care institutional review board and all participants gave informed written consent before beginning the study.
Seventy-nine participants completed treatment in the parent RCT study (Hoge et al., 2013). Eligible for the biomarker analysis were the 72 participants (MBSR, n=43; SME, n=29) who agreed to blood collection. Some data were missing due to occasional processing or assay problems: intravenous catheter failure (n=1, MBSR), insufficient plasma quantity for multiple assays (n=5 in MBSR, n=4 in SME). Thus, because a minimum of three time points were required to calculate an Area Under the Curve (AUC), some participants’ biomarkers AUC’s could not be calculated. One participant was excluded from the biomarker analysis due to a post-randomization acute medical issue, and another due to lab error. Thus, the final sample size that contained both time points varied slightly for each stress marker: n=67 for ACTH, n=68 for cortisol, n=65 for TNF-alpha, and n=62 for IL-6.
2.2. The TSST
The TSST and blood collection procedures were conducted between 1:00pm and 4:30pm to control for hormonal diurnal variation. The TSST consists of an 8-minute public speaking task and a subsequent 5-minute mental arithmetic task (serial subtraction) performed in front of a panel of “evaluators” dressed in white lab coats and holding clipboards and a large, conspicuous video camera. The TSST procedure followed a detailed script to ensure its systematic and controlled delivery.
Because the TSST was administered before and at the end of the trial, several measures were taken to lower the potential for stress habituation and to improve methodological rigor for the second TSST: 1) the evaluators were switched so that they would be strangers the second time, 2) the TSST was moved to a different room, 3) a different arithmetic task was employed to avoid practice effects and 4) participants were told that their performance on the first speech was in the low range, and that this was their chance to improve their score [26].
2.3. Treatments
MBSR is an 8-week group-based intervention with a single weekend “retreat” day and daily home practice guided by audio recordings. In-class practices (breath-awareness, a body-scan, and gentle Hatha yoga) are used to cultivate awareness of internal present-moment experiences with an accepting, non-judgmental stance. The SME class was designed as an attention control intervention for MBSR to control for the non-specific effects of treatment, such as group support, attention from the instructor, and participants’ expectations. The course is taught in a didactic format, and provides lectures on overall health and wellness such as diet, exercise, sleep, and time management. Importantly, SME does not contain any meditation or other mind-body intervention (Hoge et al., 2013).
2.4. Blood collection
An intravenous catheter was placed at time 0, and then the participant rested while pre-stress blood samples were collected (time +5, +10, +15, and +20 minutes). At +22 minutes, the speech task instructions were read to the participant. At the end of the 8-minute speech preparation time, blood was collected (time +28), and the participant was led to the testing room with the audience of evaluators. After the speech and arithmetic tasks, the subject was led back to the phlebotomy room where post-stress blood samples were collected (+40, +45, +50, +55, and +80 minutes).
To assess effects of acute stress on the Hypothalamic-Pituitary-Adrenal (HPA) axis, we measured blood levels of cortisol and ACTH. To measure effects of acute stress inflammation, we assessed IL-6 and TNF-alpha.
2.5. Statistical Analyses
To be consistent with earlier published trials measuring hormone and cytokine response to the TSST, we compared the pattern of blood markers during stress pre- and post-treatment using an area under the curve (AUC) calculation (Pruessner et al., 1997; Wirtz et al., 2007). We calculated AUC with respect to increase for blood markers of stress for patients with cortisol, ACTH, TNF-alpha, and IL-6, with time point +5 as the baseline blood level, using all time points that were available (Pruessner et al., 2003). Then, we conducted a series of analyses of variance for repeated measures (repeated-measure ANOVA) with time (pre-treatment and post-treatment) as the repeated measure, treatment arm as between-subjects factor, and the AUCs as the dependent variables to examine the effect of group on the change in AUCs between baseline and endpoint. Statistical significance was set to alpha=0.05 (two-sided) for all analyses, and all analyses were conducted using Stata 12.1 (Stata Corporation, College Station, TX).
3. Results
Demographic characteristics by treatment group are presented in Table 1; there were no significant differences in gender, age, or race distribution. After randomization, 11 participants from the SME group dropped out, and 3 from the MBSR group dropped. Mean values and changes in AUC concentrations for hormone and cytokine levels between before and after the 8-week treatment, are shown in Table 2.
Table 1.
Demographic and Clinical Characteristics
| Characteristic | MBSR | SME | p-value† |
|---|---|---|---|
|
| |||
| (n=42) | (n=28) | ||
| Gender: n (%) | 0.63 | ||
| Male | 24 (57) | 14 (50) | |
| Female | 18 (43) | 14 (50) | |
| Race: n (%) | 1.0 | ||
| White | 34 (81) | 24 (86) | |
| Black | 3 (7) | 2 (7) | |
| Asian | 4 (10) | 2 (7) | |
| Other | 1 (2) | 0 | |
| Age (years): Mean (SD) | 40 (14) | 38 (11) | 0.48 |
| Comorbid Depression, Current: n (%) | 5 (11) | 5 (17) | 0.73 |
categorical variables compared with Fisher’s Exact test, continuous variables with t-test
Table 2.
Blood Markers of Acute Stress
| During the TSST | MBSR | SME | p-value* | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| pre | post | change | pre | post | change | ||
| HPA axis markers, mean (SD) | |||||||
| Cortisol AUC | 913 (252) | 761 (290) | −152 (256)†† | 1040 (383) | 951 (430) | −89 (328) | 0.38 |
| ACTH AUC | 1979 (916) | 1688 (646) | −290 (541)†† | 2148 (1145) | 2348 (1778) | 200 (792) | 0.007 |
| Cytokines, mean (SD) | |||||||
| TNF-alpha AUC | 480 (247) | 417 (204) | −64 (284) | 317 (186) | 390 (235) | 73 (188) | 0.033 |
| IL-6 AUC | 157 (145) | 120 (114) | −37 (156) | 63 (47) | 96 (70) | 33 (62)† | 0.036 |
p-value of time × treatment type in ANOVA
within-group comparison of pre-post scores with p<0.05,
within-group comparison of pre-post scores with p<0.01
3.1. Endocrine Markers
The repeated-measure ANOVA with cortisol AUC as the dependent variable (F(1,131)=4.50, p<0.0001) found a main effect of time, (F(1,63)= 11.04, p<0.001) but no significant treatment arm X time interaction, (F(1,63)=0.77, p=0.38). However, there was a significant treatment arm X time interaction, F(1,55)=7.75, p=0.007 with the ACTH AUC’s over the course of the treatment, with participants in the MBSR group exhibiting a reduction in their ACTH AUC, while those in the SME group increased (see Table 2).
3. 2. Immunological Markers
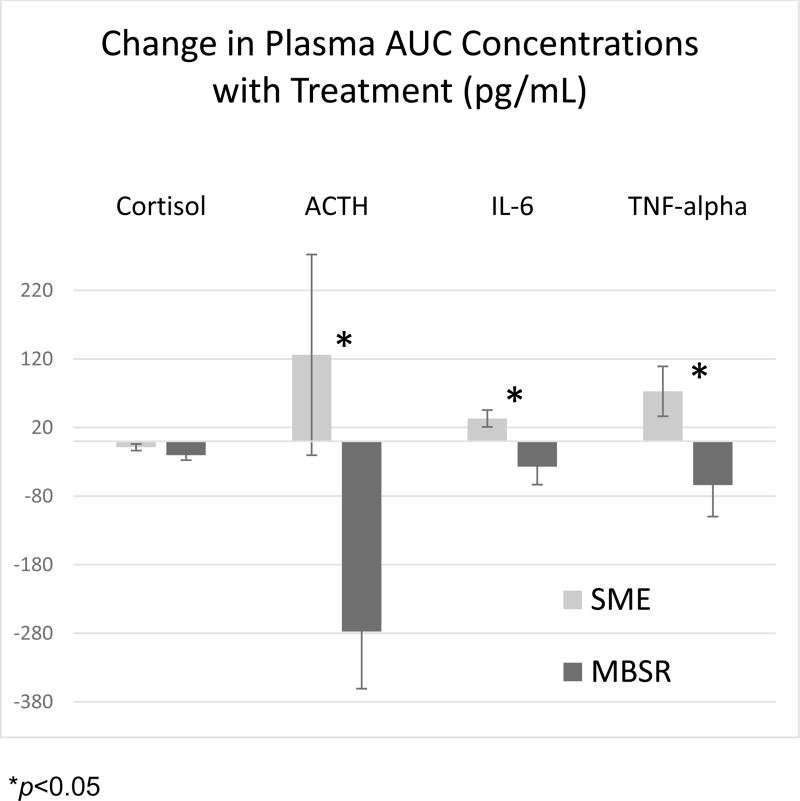
Comparison of the cytokine (IL-6 and TNF-alpha) AUC concentrations measured during the pre-treatment and post-treatment TSST’s showed a decrease after treatment in the MBSR group, but an increase in the SME group (see Table 2). Calculations using a repeated-measure ANOVA with IL-6 AUC as the dependent variable (F(1,134) =1.82, p<0.01) found no main effect of time, F(1,59)= 0.01, p=0.91 but a significant treatment arm X time interaction, F(1,59)=4.69, p=0.034. Similarly, a repeated-measure ANOVA with TNF-alpha AUC (F(1,136)=2.03, p<0.01) found no main effect of time, F(1,63)= 0.02, p=0.89 but a significant treatment arm X time interaction, F(1,63)=4.57, p=0.036. This difference further suggests that MBSR participants had a greater increase in stress resilience, as measured by response to the TSST stressor the second time (Table 2 and Figure 1).
Figure 1.
Change in plasma area-under-the-curve (AUC) concentration with treatment (pg/mL)
4. Discussion
We found that mindfulness meditation training was associated with an attenuated stress response to laboratory stress in GAD, with evidence from both HPA axis hormones and inflammatory markers, raising the possibility that mindfulness meditation may imbue some resilience to stressful psychological challenges. The TSST is a well-validated, widely used laboratory-based model of psychological stress that can be used in a controlled fashion to understand the effects of potential real-world stressors (Kirschbaum et al., 1993). Therefore, the present findings help elucidate potential benefits of meditation training on psychological resilience in an at risk population with a preexisting anxiety disorder, GAD.
Our findings are consistent with other work demonstrating that meditation training may enhance biological resilience to laboratory stress. For example, Pace et al. demonstrated that healthy participants who did a high amount of compassion meditation practice had a faster drop in cortisol after the TSST than healthy participants who did a low amount of compassion meditation practice (Pace et al., 2010).
Interestingly, participants who did not practice meditation (those in the SME group) experienced increased stress in anticipation of the second TSST, rather than a habituation-related decreased level of stress, as has been suggested by studies of repeat TSSTs with healthy adults (Schommer et al., 2003). This observation is consistent with research with participants who were depressed, traumatized, or had ‘high exhaustion’; these groups had either a higher cortisol reactivity to the TSST compared to controls, or an increased sensitivity to the TSST when it was given a second time (Britton et al., 2012; Kudielka et al., 2006; Peckins et al., 2012). For example, when experiencing the TSST a second time, patients with a history of depression who did not receive treatment evidenced an increase in anticipatory (pre-stressor) anxiety (Britton et al., 2012), representing a model for increased risk for cumulative stress related effects in individuals with affective disorders.
The value of an intervention that can improve resilience to psychological stress in this vulnerable population cannot be overestimated. Multiple studies show that psychological stress can contribute to the onset, exacerbation, or relapse of anxiety disorders (Lteif and Mavissakalian, 1995; Watanabe et al., 2005). Although there are some limitations to using a lab stress to model life stressors, even improved laboratory stress coping has been directly linked to better mental health outcomes: Aschbacher et al. demonstrated that worse psychological coping during the TSST was associated with greater depression symptoms in the subsequent year (Aschbacher et al., 2012). Looking at the question clinically, studies involving patients with remitted depression found that mindfulness meditation training was associated with decreased risk for relapse to depression, suggesting that mindfulness meditation may improve coping and resilience (Segal et al., 2010; Teasdale et al., 2000).
Self-report of emotional states (such as anxiety, in this case) can be highly biased and unreliable, especially in GAD; some patients with GAD may have limited access to or difficulty describing internal processes (Salters-Pedneault et al., 2006). A recent review of TSST data found that only about 25% of studies found a correlation between stress hormone levels and self-reported emotional stress (Campbell and Ehlert, 2012). Biological measures of stress and anxiety used in this study are valuable since they overcome some of the limitations due to the inherent variability of self-report data associated with individual factors.
The observation of higher stress markers during the TSST in our and other’s data may help explain the chronically high levels of circulating stress hormones and cytokines observed in individuals with anxiety disorders, who experience repeated stress due to their symptoms over months and years (Arranz et al., 2007; Mantella et al., 2008). This link between negative medical health outcomes and chronic elevations in inflammatory markers and stress hormones may help explain the higher rates of cardiovascular disease and metabolic syndrome in patients with anxiety disorders (Carroll et al., 2009; Roest et al., 2010). For example, the impairing, distressing, and often chronic generalized anxiety disorder (GAD) is associated with a greater risk of cardiovascular disease mortality (Tully et al., 2013). Thus, if this relationship is at all causal, successful treatment of anxiety disorders such as GAD may result not only in improved psychological health but also improved medical health, increasing the public health relevance of anxiety disorder treatment. Furthermore, some data suggest that normalization of stress and inflammation pathways may accompany successful treatment of chronic anxiety or conditions with chronic stress (Doering et al., 2007); however determination of mechanistic pathways may be difficult due to confounding effects of pharmacological treatments and medical illness on these systems.
There are some limitations of this study. First, conclusions are limited due to relatively small sample size, and exclusion criteria for study entry may limit the generalizability of our findings. Also, it is not clear why there were no significant differences in cortisol AUC changes between the groups, when the closely related ACTH changes were significantly different. One possibility is that due to the slower half-life of cortisol (compared to ACTH) and therefore longer persistence in the blood, cortisol may not be as reliable when measurements are taken close together (King et al., 2009). For example, in a study by Jezova et al., ACTH was found to rise with stress but not cortisol measurements taken at the exact same time (Jezova et al., 2013). Further, our hormonal mean plasma levels showed more variability than other published reports; however, the large majority of experiments using the TSST utilized healthy populations such as college students, and not a psychiatrically disordered population such as our GAD sample. Lastly, we did not include measures of potential confounds including religious belief, stressful life events, or socioeconomic status, however, since participants were randomized between MBSR vs. SME, we do not expect the two groups to significantly differ on these variables.
In conclusion, these findings suggest that mindfulness meditation training, a relatively inexpensive and low-stigma treatment approach, may be a helpful strategy to decrease biological stress reactivity and improve resilience to stressors in patients with GAD. Future work should focus on the impact of mindfulness meditation on “real life” stress and anxiety disorder severity and relapse.
Highlights.
Individuals with Generalized Anxiety Disorder who completed mindfulness meditation training had a greater drop in stress-related adrenocorticotropic hormone.
Individuals with Generalized Anxiety Disorder who completed mindfulness meditation training also had a greater drop in proimflammatory cytokines.
Findings suggest that mindfulness meditation training may have helped participants cope better with subsequent stress.
Acknowledgments
Funding/support: This study was primarily supported by grant K23AT4432 from the National Center on Complementary and Alternative Medicine, National Institutes of Health (Hoge, P.I.). The Highland Street Foundation provided additional support including to Ms. Palitz, Mr. Schwarz, Ms. Owens, and Drs. Bui, Pollack and Simon.
The authors would like to thank Zayda Vallejo for her work in teaching the study courses, and the MGH hospital volunteers who assisted in this study, and to acknowledge nursing support from the Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Footnotes
Trial Registration: www.clinicaltrails.gov Identifier: NCT01033851.
Authorship Contributions: EAH, MHP, NMS, MEO and JMJ were responsible for the study conception and design; EAH, EB, SAP, and NRS analyzed and interpreted the data.
All authors participated in the drafting the article or with revising it critically for important intellectual content and all authors gave final approval of the version to be published.
Declaration of Interest
Dr. Hoge reports grants from National Institutes of Health (K23AT4432) during the conduct of the study. Dr. Bui received funding from the National Institute of Health, the American Foundation for Suicide Prevention, the Highland Street Foundation, and from the U.S. Department of Defense during the conduct of the study. Ms. Palitz and Mr. Schwarz received funding from the National Institute of Health and the Highland Street Foundation during the conduct of the study. Ms. Owens received funding from Highland Street Foundation during the conduct of the study. Dr. Johnston has nothing to disclose. Dr. Simon received funding from the National Institute of Health, the American Foundation for Suicide Prevention, the Highland Street Foundation, and from the U.S. Department of Defense during the conduct of the study. Dr. Pollack reports grants from NIH during the conduct of the study; he was also an advisory board member or consultant for Eli Lilly, Medavante, Otsuka, and Transcept; Dr. Pollack also had equity in Medavante, Mensante Corporation, Mindsite, and Targa; he has received royalties from or holds patents on the SIGH-A and SAFER interviews.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arranz L, Guayerbas N, De la Fuente M. Impairment of several immune functions in anxious women. J Psychosom Res. 2007;62(1):1–8. doi: 10.1016/j.jpsychores.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, Epel E, Wolkowitz OM, Prather AA, Puterman E, Dhabhar FS. Maintenance of a positive outlook during acute stress protects against pro-inflammatory reactivity and future depressive symptoms. Brain Behav Immun. 2012;26(2):346–352. doi: 10.1016/j.bbi.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Adil J, Khan S, Nazeer A, Afzal N, McGlashan T, Elzinga B, Anderson GM, Heninger G, Southwick SM, Charney DS. Cortisol response to a cognitive stress challenge in posttraumatic stress disorder (PTSD) related to childhood abuse. Psychoneuroendocrinology. 2003;28(6):733–750. doi: 10.1016/s0306-4530(02)00067-7. [DOI] [PubMed] [Google Scholar]
- Britton WB, Shahar B, Szepsenwol O, Jacobs WJ. Mindfulness-based cognitive therapy improves emotional reactivity to social stress: results from a randomized controlled trial. Behav Ther. 2012;43(2):365–380. doi: 10.1016/j.beth.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37(8):1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Anderson GM, Wilkinson CW, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D, Phillips AC, Thomas GN, Gale CR, Deary I, Batty GD. Generalized anxiety disorder is associated with metabolic syndrome in the Vietnam experience study. Biol Psychiatry. 2009;66(1):91–93. doi: 10.1016/j.biopsych.2009.02.020. [DOI] [PubMed] [Google Scholar]
- Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161(2):195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- Chiesa A, Serretti A. A systematic review of neurobiological and clinical features of mindfulness meditations. Psychol Med. 2010;40(8):1239–1252. doi: 10.1017/S0033291709991747. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S50–55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Doering LV, Cross R, Vredevoe D, Martinez-Maza O, Cowan MJ. Infection, depression, and immunity in women after coronary artery bypass: a pilot study of cognitive behavioral therapy. Altern Ther Health Med. 2007;13(3):18–21. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Nov, (SCID-I/P) [Google Scholar]
- Gerra G, Zaimovic A, Zambelli U, Timpano M, Reali N, Bernasconi S, Brambilla F. Neuroendocrine responses to psychological stress in adolescents with anxiety disorder. Neuropsychobiology. 2000;42(2):82–92. doi: 10.1159/000026677. [DOI] [PubMed] [Google Scholar]
- Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, Berger Z, Sleicher D, Maron DD, Shihab HM, Ranasinghe PD, Linn S, Saha S, Bass EB, Haythornthwaite JA. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174(3):357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge EA, Bui E, Marques L, Metcalf CA, Morris LK, Robinaugh DJ, Worthington JJ, Pollack MH, Simon NM. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786–792. doi: 10.4088/JCP.12m08083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezova D, Hlavacova N, Makatsori A, Duncko R, Loder I, Hinghofer-Szalkay H. Increased anxiety induced by listening to unpleasant music during stress exposure is associated with reduced blood pressure and ACTH responses in healthy men. Neuroendocrinology. 2013;98(2):144–150. doi: 10.1159/000354202. [DOI] [PubMed] [Google Scholar]
- Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- King AP, Abelson JL, Britton JC, Phan KL, Taylor SF, Liberzon I. Medial prefrontal cortex and right insula activity predict plasma ACTH response to trauma recall. Neuroimage. 2009;47(3):872–880. doi: 10.1016/j.neuroimage.2009.05.088. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, von Kanel R, Preckel D, Zgraggen L, Mischler K, Fischer JE. Exhaustion is associated with reduced habituation of free cortisol responses to repeated acute psychosocial stress. Biol Psychol. 2006;72(2):147–153. doi: 10.1016/j.biopsycho.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Lteif GN, Mavissakalian MR. Life events and panic disorder/agoraphobia. Compr Psychiatry. 1995;36(2):118–122. doi: 10.1016/s0010-440x(95)90106-x. [DOI] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Reynolds CF, Lenze EJ. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33(6):773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pace TW, Negi LT, Sivilli TI, Issa MJ, Cole SP, Adame DD, Raison CL. Innate immune, neuroendocrine and behavioral responses to psychosocial stress do not predict subsequent compassion meditation practice time. Psychoneuroendocrinology. 2010;35(2):310–315. doi: 10.1016/j.psyneuen.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckins MK, Dockray S, Eckenrode JL, Heaton J, Susman EJ. The longitudinal impact of exposure to violence on cortisol reactivity in adolescents. J Adolesc Health. 2012;51(4):366–372. doi: 10.1016/j.jadohealth.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AD, Manson JE, Rossouw JE, Siscovick DS, Mouton CP, Rifai N, Wallace RB, Jackson RD, Pettinger MB, Ridker PM. Inflammatory biomarkers, hormone replacement therapy, and incident coronary heart disease: prospective analysis from the Women’s Health Initiative observational study. JAMA. 2002;288(8):980–987. doi: 10.1001/jama.288.8.980. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Gaab J, Hellhammer DH, Lintz D, Schommer N, Kirschbaum C. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology. 1997;22(8):615–625. doi: 10.1016/s0306-4530(97)00072-3. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56(1):38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Rose RD, Buckey JC, Jr, Zbozinek TD, Motivala SJ, Glenn DE, Cartreine JA, Craske MG. A randomized controlled trial of a self-guided, multimedia, stress management and resilience training program. Behav Res Ther. 2013;51(2):106–112. doi: 10.1016/j.brat.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Salters-Pedneault K, Roemer L, Tull MT, Rucker L, Mennin DS. Evidence of broad deficits in emotion regulation associated with chronic worry and generalized anxiety disorder. Cognitive Therapy and Research. 2006;30(4):469–480. [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Bieling P, Young T, MacQueen G, Cooke R, Martin L, Bloch R, Levitan RD. Antidepressant monotherapy vs sequential pharmacotherapy and mindfulness-based cognitive therapy, or placebo, for relapse prophylaxis in recurrent depression. Arch Gen Psychiatry. 2010;67(12):1256–1264. doi: 10.1001/archgenpsychiatry.2010.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68(4):615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- Tully PJ, Cosh SM, Baune BT. A review of the affects of worry and generalized anxiety disorder upon cardiovascular health and coronary heart disease. Psychol Health Med. 2013;18(6):627–644. doi: 10.1080/13548506.2012.749355. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Kudielka BM, Hanebuth D, Preckel D, Fischer JE. Different contribution of interleukin-6 and cortisol activity to total plasma fibrin concentration and to acute mental stress-induced fibrin formation. Clin Sci (Lond) 2005;109(1):61–67. doi: 10.1042/CS20040359. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Nakao K, Tokuyama M, Takeda M. Prediction of first episode of panic attack among white-collar workers. Psychiatry Clin Neurosci. 2005;59(2):119–126. doi: 10.1111/j.1440-1819.2005.01345.x. [DOI] [PubMed] [Google Scholar]
- Wirtz PH, von Kanel R, Emini L, Suter T, Fontana A, Ehlert U. Variations in anticipatory cognitive stress appraisal and differential proinflammatory cytokine expression in response to acute stress. Brain Behav Immun. 2007;21(6):851–859. doi: 10.1016/j.bbi.2007.02.003. [DOI] [PubMed] [Google Scholar]