Abstract
Pituitary adenoma invasion into the orbit is a rare phenomenon with only 22 cases, including the present case, in the literature. Our case is a 31-year-old man who presented with biopsy-proven atypical pituitary adenoma invading the right orbit after a prior resection. We compare his clinical course with previous cases.and discuss clinical features, radiological features, management considerations, histologic features, and prognosis. Cases are organized by specific pituitary tumor type to aid in determining appropriate management. Early surgical intervention is key, especially in the setting of pathologic features indicating aggressive tumor behavior or worsening visual function, but is generally not indicated in prolactin-secreting adenomas that may respond to medical therapy. The role of radiation therapy is not fully established; however, it should be strongly considered in conjunction with or after surgery, especially in cases where complete resection is not achieved or histological and molecular analysis indicate a high likelihood of recurrence. More uniform and comprehensive data about management and outcomes are needed to determine the optimal treatment approach for this rare entity.
Keywords: orbital invasion, atypical pituitary adenoma, pituitary tumors, ocular oncology
1. Introduction
Pituitary adenomas are benign epithelial tumors derived from the adenohypophysial cells of the pituitary gland and account for approximately 15% of intracranial neoplasms with a prevalence of about 80–90 per 100,000.16 Microadenomas, those measuring <10mm in diameter, account for ~60% of tumors, and macroadenomas (>10mm) account for ~40%.8 These tumors can be further classified as functional (hormone secreting) or non-functional. The clinical presentation will vary depending on the presence of mass effect secondary to enlarging tumor size and/or hormonal irregularities often accompanied by protean signs and symptoms.7 Pituitary adenomas are rarely invasive, but those that possess certain atypical histological, immunohistochemical, or molecular features may be clinically indistinguishable from pituitary carcinoma.10 These atypical or aggressive neoplasms have the potential to expand beyond the sella turcica and produce predictable symptoms consistent with the direction of growth.13 Orbital invasion is rare, with only 22 cases including the present case, in the literature. Tumors with orbital involvement present challenging treatment and management considerations as they frequently recur and require a long-term, coordinated multidisciplinary approach in order to salvagea vision while maintaining quality of life. Treatment and management will differ depending on the degree of expansion, potential effectiveness of preoperative medical therapy based on tumor subtype, ability of the patient to tolerate surgical intervention, use of for post-operative radiation therapy in cases of inadequate tumor resection, and the need for continued hormone suppression or replacement. We present a case of a growth hormone secreting pituitary adenoma extending into the right orbit that presented with constant severe headache and partial third nerve palsy. We also review the literature and organize cases by adenoma subtype.
2. Case report
Initial presentation of pituitary tumor
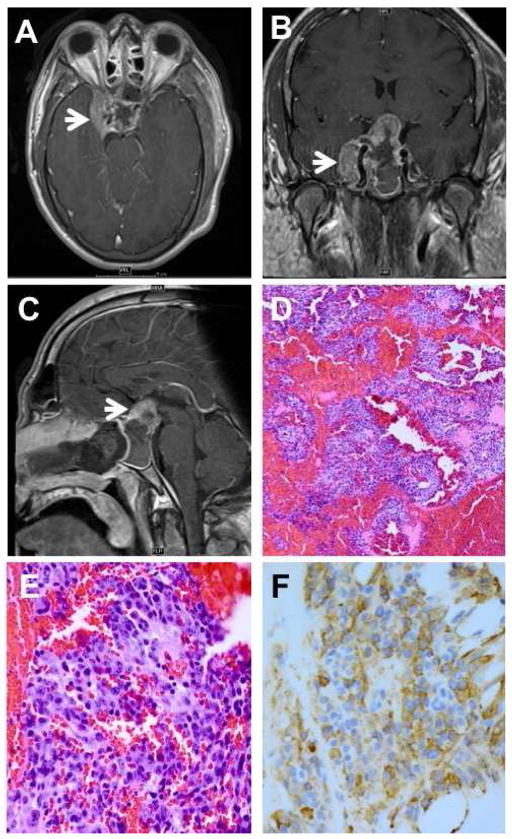
A 31-year-old man presented to his optometrist in February, 2011, with fluctuating headaches and visual changes for 2 months. Spectacles failed to improve his vision, and he was referred to a neurologist who found a pituitary adenoma Five month later he was sent to our institution where he was found to have vision of hand motions OD and 20/100, OS and decreased color vision . He had right upper eyelid ptosis, a right relative afferent pupillary defect, temporal optic nerve pallor OU, and facial features suggestive of acromegaly. A magnetic resonance image (MRI) of the head revealed a large sellar and suprasellar mass measuring 4.1 cm with extension and encasement of the right internal carotid artery and the cavernous sinus and compression of the right optic nerve (Figure 1A-C). His growth hormone level was found to be 55.3 ng/mL (normal = 1 ng/mL). He underwent a transsphenoidal resection of the mass without complication. He was treated postoperatively with octreotide for acromegaly and with hormone replacement therapy for panhypopituitarism. Histological evaluation of the resected specimen revealed a monomorphic population of cells with amphophilic nuclei and sparsely granulated cytoplasm with extensive hemorrhage and no mitotic figures (Figure 1D-E). Immunohistochemical stains showed diffuse reactivity for growth hormone (Figure 1F) and were negative for prolactin, ACTH, LH, FSH, TSH, and cytokeratins AE1/AE3. Additionally, the Ki-67 proliferation index was elevated at 5–10%. Follow-up examination in October, 2011, showed improved VA of 20/40 OD and 20/25 OS.
Figure 1.
A: MRI showing invasion of the right cavernous sinus. B: MRI showing encasement of the carotid artery (arrow) and compression of optic chiasm. C: MRI showing 4.1 cm sellar/suprasellar mass with sellar expansion. D: biopsy revealing pituitary adenoma with extensive hemorrhage. E: monomorphic population of amphophilic and sparsely granulated cells. F: adenoma cells show diffuse reactivity for human growth hormone. (D: hematoxylin and eosin, 25X; E: hematoxylin and eosin, 100X; F: anti-human growth hormone, 100X).
In January, 2012, he presented for rapidly decreasing vision (VA) in both eyes. An MRI at that time demonstrated recurrent tumor involving the sellar and suprasellar areas with extension into the right cavernous sinus along the middle cranial fossa, the right foramen ovale, and the right pterygopalatine fossa. Additionally, there was compression of the anterior visual pathways and chiasm with tumor invading the superior orbital fissure. Subsequently, the patient underwent tumor debulking and stereotactic radiotherapy. That specimen was unavialable for histopathologic review. An MRI demonstrated reduced size of mass after radiation, and his acuity was 20/20 OD.
Recurrent pituitary tumor invasion of the orbit
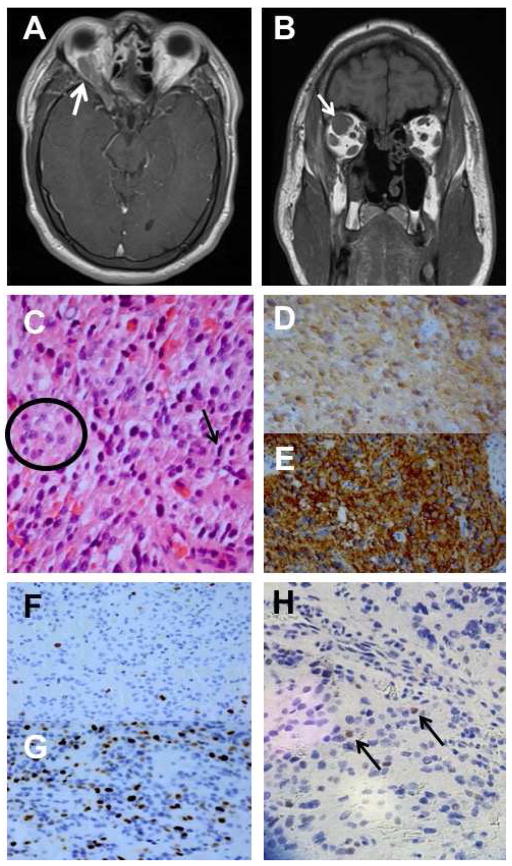
The patient remained stable until May, 2014, when he presented with right-sided headaches and right upper eyelid swelling; however, VA was 20/40 OD and 20/30 OS. MRI demonstrated for the first time extension of the residual pituitary mass into the orbit (Figure 2A-B). He underwent a right orbitotomy, and histological evaluation of the neoplasm showed nuclei with prominent nucleoli and occasional mitotic figures (Figure 2C). Immunohistochemical stains showed positivity for human growth hormone and vimentin and neuroendocrine markers neuron-specific enolase (Figure 2D) and synaptophysin (Figure 2E). Additionally, markers for sarcomas (desmin, myogenin, smooth muscle actin), epithelial tumors (chromogranin, CK8), mesenchymal tumors (CD34), and prolactin were negative. Molecular testing for Ki-67 positivity (Figure 2F) showed an increase to 25% of cells (compared with 5–10% initially in 2011, Figure 2G) and p53 positivity in 5% of cells (Figure 2H). AThe final pathological diagnosis was growth hormone-secreting atypical pituitary adenoma.
Figure 2.
A: MRI showing orbital extension of residual pituitary mass. B: MRI showing enlargement of superior rectus muscle suggesting orbital invasion by sellar mass. C: cells with pleomorphic nuclei with prominent nucleoli (circle) and occasional mitotic figures (arrow). D: positivity for neuron specific enolase. E: positivity for synaptophysin. F: Ki-67 proliferation index of 5–10% from 2011 biopsy. G: Ki-67 proliferation index of 25% from 2014 biopsy. H: p53 positivity in 5% of cells. (C: hematoxylin and eosin, 250X; D: anti-NSE, 25X; E: anti-synaptophysin, 25X; F and G: Ki-67 index, 25X; H: p53 reactivity, 25X).
After this first demonstration of orbital invasion, the patient was treated with additional radiation, somatuline depot and temozolomide. Despite treatment, he continued to have cycles of recurrences of tumor growth within the orbital cavity. Immunohistochemical staining for MGMT was later on performed on the orbital tumor biopsy specimen and was shown to have a high immunoreactivity (80% positive staining). His clinical course included multiple interventions to improve many episodes of vision loss, proptosis, ophthalmoplegia, cranial nerve palsies, and various other pathologic manifestations consistent with an aggressive, difficult to control intracranial neoplasm.
3. Epidemiology and Clinical Features
Among the 22 cases of pituitary adenomas with orbital involvement (Table 1), 11 occurred in women and 11 in men with an age range of 8 to 85 and mean of 33 years. With respect to adenoma subtype, 6/22 were prolactin secreting,12,18,22 4/22 ACTH secreting, 5,14,15 2/22, this case included, GH secreting,22 1/22 TSH secreting,24 4/22 non-funcitonal,2,4,11,20 and 5/22 unspecified.6,11,17,19,23 Of note, one of the cases was mixed prolactin and GH secreting.22 None of the cases reported secreted LH. The most common presenting symptom was headache while progressive vision loss, symptomatic proptosis, diplopia, facial swelling, weight loss, and vomiting were also reported. Presenting signs included third nerve palsy, ptosis, proptosis, afferent pupillary defect, optic disc pallor, anisocoria, optic disc edema, bitemporal hemianopia, and sixth nerve palsy. Prior to presenting with orbital invasion, four cases (including the present case) reported previous surgical interventions that included resection of pituitary adenoma, although many other cases did not indicate whether there was prior surgery. 2,5,14 It is unclear whether surgical manipulation of surrounding anatomical structures increased the potential for orbital expansion.
Table 1.
Ayptical Pituitary Adenoma with Orbital Invasion
| Author/ Year |
Age (years)/ Sex |
History | Presentation | Previous Pituitary Surgery |
Treatment | Visual Outcome |
Clinical Outcome |
Other | |
|---|---|---|---|---|---|---|---|---|---|
| Prolactin | |||||||||
|
| |||||||||
| Ross/1989 | 8/M | NS | Acute headache, blurred vision, vomitting, L ptosis and papilledema | No | Meds, surgery, and Rx | NS | Stable 2 years post Rx | ||
| Karcioglu/2003 | 26/M | Progressive VL OU x 1m | 20/200 OD, 20/40 OS, APD OD, papilledema OU | No | Meds only | 20/40 OD 20/25 OS |
Stable 4 years post dx | Tx with BC | |
| Karcioglu/2003 | 24/F | Prolactinoma dx 21 years prior, RP proptosis OD | CF OD, 20/20 OS, APD & pale ON OD | Yes | Meds, partial resection x 2 | NS | Lost to followup 23 yrs after dx | Tx with BC | |
| Karcioglu/2003 | 35/M | RP proptosis OD | APD OD, proptosis OD | No | Enucleation, partial resection exenteration | NS | Deceased 1m post surgery | ||
| Karcioglu/2003 | 33/F | Prolactinoma dx 2 years prior | 20/20 OD, 20/100 OS, APD OS | No | Meds, partial resection, exenteration | NS | Deceased 4 years after dx | Tx with BC | |
| Vaudaux/2003 | 30/M | 4 years of bulging eye | Proptosis, headache, acromegaly, nl VA | No | Meds only | 20/16 OS | Stable with meds 1 year post dx | Tx with BC, also GH secreting | |
|
| |||||||||
| ACTH | |||||||||
|
| |||||||||
| Dhaliwal/2014 | 28/F | Partial ACTH PA resection 7m prior | 20/20 OU, diplopia, pain OS | Yes | Partial resection, stereo Rx, | 20/20 OU | Stable diplopia 9m post surgery | Tx with k etoconazole after1st resection | |
| Dhaliwal/2014 | 85/F | Glaucoma, bilateral trabeculectomy | Nonspecific visual changes | No | Partial resection | 20/40 OU | Stable VA 3m post surgery | ||
| Choong/2014 | 68/F | Worsening HA and diplopia | CN III and VI palsies, VA NS | No | Partial resection | NS | Persistent CN VI palsy | ||
| Kim/2016 | 28/M | ACTH PA resection 9 years prior, Rx 4years prior to orbital involvment | HA, 20/20 OU, nl CV, diplopia, mild temporal ON pallor OU | Yes | Resection, Rx x 3 | 20/20 OU | Stable VA, visual symptoms throughout Tx | ||
|
| |||||||||
| GH | |||||||||
|
| |||||||||
| Naguib/2016 | 31/M | Fluctuating HA and visual changes x 2m | HM OD, 20/100 OS, decreased CV OU, RUL ptosis, APD OD, temporal ON pallor OU, acromegaly | Yes | Multiple resections, stereo Rx, meds | CF OD/NS OS | CF OD, general disability | Tx with octreotide | |
|
| |||||||||
| TSH | |||||||||
|
| |||||||||
| Yovos/1981 | 17/F | NS | ON atrophy, bitemporal VF defects | NS | Surgery, Rx | NS | NS | ||
|
| |||||||||
| Nonfx | |||||||||
|
| |||||||||
| Jackson/1962 | NS/M | NS | Nonspecific visual changes, ON atrophy | NS | NS | NS | NS | ||
| Daita/1987 | 50/M | NS | 20/200 unspecified, ON atrophy, bitiemporal VF defects | NS | Surgery, Rx | NS | NS | ||
| Spiegel/1994 | 25/F | NS | CF unspecified, ON atrophy, unilateral constriction | NS | Surgery, Rx | HM | NS | ||
| Bernardini/2001 | 47/F | NS | No VA loss | NS | Surgery | NS | NS | ||
|
| |||||||||
| NS | |||||||||
|
| |||||||||
| Jackson/1962 | NS/M | NS | NS | NS | NS | NS | NS | ||
| De Divitiis/1973 | 29/F | NS | Unspecified VA loss, bitemporal hemianopia | NS | NS | NS | NS | ||
| Wray/1977 | 71/F | NS | Diplopia, unspecifid CN palsy, unspecified VA loss | NS | NS | NS | NS | ||
| Sammartino/1979 | 12/M | NS | Diplopia, unspecified CN palsy, unspecified VA loss | NS | Surgery | NS | NS | ||
| Ortiz/1992 | 16/M | 4w of HA, proptosis and WL | VA 20/20 OU, 6mm proptosis OS | No | Sugery, Rx | NLP OS | 20m post surgery was stable | ||
M, male; F, female; NS, not specified; OD, R, Right; OS, L, Left; Rx, radiation therapy; VL, vision loss; m, month; w, week; APD, afferent pupillary defect; dx, diagnosis; tx, treatment; BC, bromocriptine; RP, rapidly progressive; ON, optic nerve; HA, headache; nl, normal; VA, visual acuity; GH, growth hormone; ACTH, adrenocorticotropic hormone; TSH, thyroid stimulating hormone; nonfx, nonfunctional; CN, cranial nerve; CF, count fingers; HM, hand motion; VF, visual field; WL, weight loss; NLP, no light perception; RUL, right upper lid
4. Radiological Features
Computerized tomography (CT) can be used as an initial imaging modality, especially in cases when immediate surgical intervention may be indiated. Otherwise, MRI is the imaging modality of choice. CT may reveal a sellar/suprasellar mass, whereas MRI may reveal a heterogeneous or homogeneous mass in the sella. Invasion into the orbit will be demonstrated by tissue extension into the superior orbital fissure or optic foramen. Further orbital invasion can occur along the path of the rectus muscles or adjacent to the optic nerve along the optic canal.
5. Medical Intervention
Prior to medical intervention, Serum levels for TSH, T3/T4, prolactin, FSH, LH, GH, insulin like growth factor, ACTH and random cortisol should be obtained. As demonstrated in this series, clinical signs of aberrant pituitary hormone function (i.e. acromegaly, amenorrhea, galactorrhea) are not always apparent ,particularly early in the disease course.
Overall, medical interventions prior to surgery are intended to reduce mass effect, restore optimal pituitary function, and suppress hormone hypersecrtion.21 Patients presenting with sudden enlargement (pituitary apoplexy) or other signs of significant tumor burden must first be stabilized. This can be achieved by treatment with high-dose glucocorticoids to minimize any inflammation and potentially improve ophthalmoplegia and visual symptoms.15 Appropriate medical intervention prior to histologic evaluation may be warranted in the setting of clinically-evident hormone hypersecretion. In most other cases, however, surgical intervention is considered the treatment of choice except in cases of suspected prolactin-secreting adenomas that are responsive to dopamine agonists and may decrease in size with treatment, reducing the need for surgery.3 Other adenoma subtypes should be surgically excised and histologically classified to determine appropriate pharmacotherapy and assess the need for radiation therapy.
6. Surgical Indications and Techniques
Transphenoidal resection is currently the widely accepted surgical approach for pituitary adenomas.3 Orbital extension however introduces other considerations needed to preserve salvageable orbital structures and preserve vision as complete tumor resection is difficult to achieve.5 For example, in the current series 7/22 cases reported incomplete resection after initial surgery. Although surgical intervention is the indicated first-line therapy for GH, ACTH, and non-functional pituitary adenomas, in cases of orbital invasion deterioration of vision or worsening ocular signs and symptoms also should prompt surgical evaluation. After initial mass resection from the orbit, any remaining tumor may be treated with radiation therapy if the residual tumor is small and optic nerve damage can be avoided. Otherwise, patients with continued ocular symptoms will require repeat orbitotomies. Recurrences of worsening ocular symptoms, particularly with incomplete resection, were common in this series, and subsequent tumor debulking may necessitate other surgical approaches including frontotemporal craniotomy.2,12,18
7. Pathology
Pituitary tumor pathology exists on a spectrum from typical adenoma, to atypical adenoma, and finally pituitary adenocarcinoma, with each entity exhibiting features indicative of a certain degree of pathogenicity. This is based on the 2004 WHO classification that often does not correlate with clinical behavior as neither all typical adenomas have a benign clinical course, nor do all atypical adenomas have a tendency to recur or invade surrounding structures. We refer to the lesions as “atypical” while acknowledging this designation as a likely oversimplification. Additionally, we cannot classify many of the earlier cases as technically “atypical” due to a lack of reported data, but it is reasonable to presume the lesions fall into an atypical or “aggressive” pituitary designation given the invasion of surrounding structures.
Pituitary adenomas will stain positively for neuroendocrine markers such as neuron- specific enolase and synaptophysin. Subtypes of pituitary adenomas can be determined with IHC positivity for human growth hormone, prolactin, ACTH, FSH, LH or TSH. Expression of certain mutated genes and molecular markers indicative of increased cell cycle activity can be used to differentiate pituitary tumor pathologies. Irregular p53 tumor suppressor gene expression is not seen in typical adenomas, and therefore its presence indicates transformation into the atypical category. Other aberrant gene expression in atypical pituitary tumors include pituitary tumor transforming gene (PTTG), O-6-Methylguanine-DNA Methyltransferase (MGMT), and matrix metallopeptidase 9 (MMP-9).10 The Ki-67 proliferation index, a marker for increased cell cycle activity, will show 1% activity in typical pituitary tumors, 3–4.5% in atypical and 12% in adenocarcinoma.10 In the present case the Ki-67 index was 25% in the 2014 biopsy, an increase from 5–10% demonstrated in 2011. This increase in Ki-67 expression over time in conjunction with the clinically evident aggressive tumor behavior further supports the use of this marker in predicting tumor behavior and in considering radiation therapy post surgically.
8. Treatment Outcome and Prognosis
Because data are limited with respect to pituitary adenomas with orbital invasion, it is useful to discuss patterns of care and survival outcomes from the Surveillance, Epidemiology, and End Results (SEER) database for patients with atypical pituitary adenomas and pituitary adenocarcinomas. A recent review of the SEER database found at total of 117 cases diagnosed between 1973 and 2008. 9 Specifically, data from 83 atypical pituitary adenomas and 7 pituitary adenocarcinomas were used to compare survival and treatment outcomes. This review found worse survival in patients with pituitary carcinoma than in patients with atypical, invasive adenoma. Additionally, survival advantages were found in patients diagnosed before age 65 and in women. Although no statistical difference in survival was found between those treated with radiation therapy and those who were not, the authors of the series did find a trend towards improved survival. They concluded, therefore, that radiation therapy should be considered especially in patients with a high Ki-67 percentage and in those with incomplete resections because of the high likelihood of recurrence.
In our review of atypical adenomas with orbital invasion, 9/22 patients received surgery and radiation, 7/22 surgery only, 2/22 medication only (prolactinomas), and in 4/22 treatment was not specified. Overall, uniform data regarding final visual and prognostic outcomes are lacking, but some useful conclusions can be gathered from the available details. 7/22 cases reported VA of 20/40 or better at final follow up. These positive visual outcomes predictably correlated with an overall better clinical outcome and tended to be in the most recent case reports (i.e. since 2000) that emphasized the need for early surgical intervention. With regards to surgical intervention, 3/22 reported the need for repeat surgery owing to recurrence or worsening visual function, and 4/22 reported orbital invasion after a previous pituitary adenoma resection; however, many of the case reports did not specify if prior surgeries were performed.
Another positive prognostic factor appeared to be responsiveness to dopamine agonists in the case of prolactin-secreting adenomas. Four cases reported using such medical intervention, with 3/4 reporting a generally favorable visual and clinical outcome. This positive outcome is expected as dopamine agonists shrink the tumor size, relieving symptoms and in 2/4 cases avoiding surgery.
The role of radiation therapy cannot be adequately assessed in this series as prognostic data are lacking in those cases that employed radiation, and in the cases with the best clinical outcomes radiation therapy was less likely to be employed. This was probably due to individual management decisions rather than a specific protocol. One case17 did, however, have a favorable outcome with post-surgical radiation of residual tumor. Additionally, a recent case successfully maintained vision at 20/20 in the affected eye with one initial surgical resection followed by 3 rounds of radiotherapy performed years apart in response to interval tumor growth.14
Recent studies have demonstrated that MGMT expression is predictive of responsiveness to the alkylating agent temozolomide in the treatment of pituitary invasive adenomas and carcinomas refractory to conventional therapy. There is an inverse relationship between MGMT immunoreactivity and temozolomide response. Low MGMT expression (below 50% reactivity by immunohistochemical staining) correlates with good response to temozolomide, while high MGMT expression is associated with resistance.25 This was evidenced by our patient who had a tumor with high MGMT expression that progressed despite temozolomide treatment.
Three cases14,18,22 reported no visual dysfunction at any point in the clinical course, and in one22 of those cases radiographic evidence revealed invasion via the superior orbital fissure rather than via the optic foramen. Despite limited radiographic data from all case reports, those authors hypothesized that lack of visual dysfunction, and therefore a better visual outcome, may be correlated to invasion via the superior orbital fissure rather than through the optic foramen.
9. Conclusion
Atypical or aggressive pituitary adenomas with orbital invasion are uncommon, but may result in serious ophthalmic deficits and system complications. Early diagnosis and management are key in reducing morbidity. This diagnosis should especially be considered in a patient with previous pituitary pathology presenting with acute or progressive ocular dysfunction. Treatment and management differ depends on tumor subtype and histopathology indicative of aggressive behavior and increased chance of recurrence. Optimal management of atypical pituitary adenomas is not clearly defined, but surgical and medical intervention with or without adjuvant radiotherapy is current practice. We found that a high Ki-67 proliferation index was suggestive of aggressive tumor behavior, although other cases are needed to support this observation. Additional series of patients with uniform and comprehensive data with respect to pathologic features, treatment course, and outcomes may provide guidelines for optimal management.
10. Method of Literature Search
Search of Pubmed was performed using the following key words: pituitary, adenoma, orbital, invasion, and atypical. Articles cited in the reference lists of the previous case reports were also used. Articles in languages other than English were not included. Date of search spanned from Aug 1961 to August 2016.
Acknowledgments
Supported in part by P30EY06360 and an unrestricted departmental grant from Research to Prevent Blindness, Inc, New York, NY
Footnotes
11. Disclosure
The authors report no proprietary or commercial interest in any product or concept discussed in this article. This case was presented in part at the Eastern Ophthalmic Pathology Society (EOPS) meeting in Baltimore, Maryland November 6–8, 2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bengtsson D, Schrøder HD, Andersen M, et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689–1698. doi: 10.1210/jc.2014-4350. [DOI] [PubMed] [Google Scholar]
- 2.Bernardini FP, Kersten RC, Moin M, Kulwin DR. Unsuspected recurrent pituitary adenoma presenting as an orbital mass. Ophthal Plast Reconstr Surg. 2001;17(2):140–143. doi: 10.1097/00002341-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Chanson P, Salenave S. Diagnosis and treatment of pituitary adenomas. Minerva Endocrinol. 2004;29(4):241–275. [PubMed] [Google Scholar]
- 4.Daita G, Yonemasu Y, Hashizume A. Unilateral exophthalmos caused by an invasive pituitary adenoma. Neurosurgery. 1987;21(5):716–718. doi: 10.1227/00006123-198711000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Dhaliwal JS, Seibold LK, Kleinschmidt-Demasters BK, et al. Orbital invasion by ACTH-secreting pituitary adenomas. Ophthal Plast Reconstr Surg. 30(2):e28–30. doi: 10.1097/IOP.0b013e31829164cb. [DOI] [PubMed] [Google Scholar]
- 6.de Divitiis E, Cerillo A. Pituitary adenoma with intra-orbital extension. Apropos of a case. Neurochirurgie. 1973;19(6):561–566. [PubMed] [Google Scholar]
- 7.Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613–619. doi: 10.1002/cncr.20412. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez A, Karavitaki N, Wass JAH. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK) Clin Endocrinol (Oxf) 2010;72(3):377–382. doi: 10.1111/j.1365-2265.2009.03667.x. [DOI] [PubMed] [Google Scholar]
- 9.Hansen TM, Batra S, Lim M, et al. Invasive adenoma and pituitary carcinoma: a SEER database analysis. Neurosurg Rev. 2014;37(2):279-85-6. doi: 10.1007/s10143-014-0525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ironside JW. Best Practice No 172: pituitary gland pathology. J Clin Pathol. 2003;56(8):561–568. doi: 10.1136/jcp.56.8.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson H. Orbital tumours. J Neurosurg. 1962;19:551–567. doi: 10.3171/jns.1962.19.7.0551. [DOI] [PubMed] [Google Scholar]
- 12.Karcioglu ZA, Aden LB, Cruz AAV, Zaslow L, Saloom RJ. Orbital invasion with prolactinoma: a clinical review of four patients. Ophthal Plast Reconstr Surg. 2002;18(1):64–71. doi: 10.1097/00002341-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Lee KC, Kim SH. Cranial nerve palsies accompanying pituitary tumour. J Clin Neurosci. 2007;14(12):1158–1162. doi: 10.1016/j.jocn.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Kim Y-H, Kim JH, Yang HK, Hwang J-M. Preserved visual function with an orbital invasion of pituitary adenoma. Br J Neurosurg. 2016:1–3. doi: 10.3109/02688697.2016.1139051. Epub ahead. [DOI] [PubMed] [Google Scholar]
- 15.Lee SL, Choong K. Pituitary macroadenoma with invasion into cavernous sinus, cranial nerve palsies. [Accessed January 30, 2016];Endocrine Today. http://www.healio.com/endocrinology/neuroendocrinology/news/print/endocrine-today/%257B6996d2f6-ffec-4d90-8150-38973813f8d3%257D/pituitary-macroadenoma-with-invasion-into-cavernous-sinus-cranial-nerve-palsies. Published 2010.
- 16.Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol. 2011;7(5):257–266. doi: 10.1038/nrendo.2011.40. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz JM, Stein SC, Nelson P, Manning AB. Pituitary adenoma presenting as unilateral proptosis. Arch Ophthalmol (Chicago, Ill 1960) 1992;110(2):282–283. doi: 10.1001/archopht.1992.01080140138041. [DOI] [PubMed] [Google Scholar]
- 18.Ross RJ, McEniery JM, Grossman A, Doniach I, Besser GM, Savage MO. Massive prolactinoma with galactorrhoea in a prepubertal boy. Postgrad Med J. 1989;65(764):403–406. doi: 10.1136/pgmj.65.764.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sammartino A, Bonavolontà G, Pettinato G, Loffredo A. Exophthalmos caused by an invasive pituitary adenoma in a child. Ophthalmol J Int d’ophtalmologie Int J Ophthalmol Zeitschrift für Augenheilkd. 1979;179(2):83–89. doi: 10.1159/000308871. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel PH, Karcioglu ZA. Orbital invasion by pituitary adenoma. Am J Ophthalmol. 1994;117(2):270–271. doi: 10.1016/s0002-9394(14)73095-8. [DOI] [PubMed] [Google Scholar]
- 21.Vance ML. Pituitary adenoma: a clinician’s perspective. Endocr Pract. 2008;14(6):757–763. doi: 10.4158/EP.14.6.757. [DOI] [PubMed] [Google Scholar]
- 22.Vaudaux J, Portmann L, Maeder P, Borruat F-X. Orbital invasion by a pituitary macroadenoma without visual loss: case report and review of the literature. Eye (Lond) 2003;17(9):1032–1034. doi: 10.1038/sj.eye.6700481. [DOI] [PubMed] [Google Scholar]
- 23.Wray SH. Neuro-ophthalmologic manifestations of pituitary and parasellar lesions. Clin Neurosurg. 1977;24:86–117. doi: 10.1093/neurosurgery/24.cn_suppl_1.86. [DOI] [PubMed] [Google Scholar]
- 24.Yovos JG, Falko JM, O’Dorisio TM, Malarkey WB, Cataland S, Capen CC. Thyrotoxicosis and a thyrotropin-secreting pituitary tumor causing unilateral exophthalmos. J Clin Endocrinol Metab. 1981;53(2):338–343. doi: 10.1210/jcem-53-2-338. [DOI] [PubMed] [Google Scholar]
- 25.Bengtsson D, Schroder HD, Andersen M, et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689–98. doi: 10.1210/jc.2014-4350. [DOI] [PubMed] [Google Scholar]