Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract and have gained considerable research and treatment interest, especially in the last two decades. GISTs are driven by mutations commonly found in the KIT gene and less commonly in the platelet-derived growth factor receptor alpha gene, BRAF gene and succinate dehydrogenase gene. GISTs behave in a spectrum of malignant potential, and both the tumor size and mitotic index are the most commonly used prognostic criteria. Whilst surgical resection can offer the best cure, targeted therapy in the form of tyrosine kinase inhibitors (TKIs) has revolutionized the management options. As the first-line TKI, imatinib offers treatment for advanced and metastatic GISTs, adjuvant therapy in high-risk GISTs and as a neoadjuvant agent to downsize large tumors prior to resection. The emergence of drug resistance has altered some treatment options, including prolonging the first-line TKI from 1 to 3 years, increasing the dose of TKI or switching to second-line TKI. Other newer TKIs, such as sunitinib and regorafenib, may offer some treatment options for imatinib-resistant GISTs. New molecular targeted therapies are being evaluated, such as inhibitors of BRAF, heat shock protein 90, glutamine and mitogen-activated protein kinase signaling, as well as inhibitors of apoptosis proteins antagonist and even immunotherapy. This editorial review summarizes the recent research trials and potential treatment targets that may influence our future patient-specific management of GISTs. The current guidelines in GIST management from Europe, North America and Asia are highlighted.
Keywords: Gastrointestinal stromal tumors, KIT gene, Platelet-derived growth factor receptor alpha gene, BRAF gene, Succinate dehydrogenase gene, CD117, Tyrosine kinase inhibitor, Molecular targeted therapy
Core tip: Research in the histogenesis of gastrointestinal stromal tumors (GISTs) identified gene mutations in KIT, platelet-derived growth factor receptor alpha and BRAF. The discovery of tyrosine kinase inhibitors (TKIs) has allowed targeted therapy in metastatic and high-risk resected GISTs. However, the emergence of TKI-resistant GISTs has raised some important treatment issues. Newer TKIs and alternative targeted therapy within the domain of BRAF and the mitogen-activated protein kinase signaling pathway, heat shock protein 90 and succinate dehydrogenase inhibition are being investigated and appear promising. Many clinical trials have been undertaken and are still ongoing to define the best molecular targeted therapy for GISTs. The European, American and Asian guidelines on GISTs provide useful resources for specialists dealing with these interesting tumors.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) account for less than 1% of all gastrointestinal tumors, and the prevalence of histological type comes after adenocarcinoma and lymphoma. GISTs are, however, the most common mesenchymal tumors of the gastrointestinal tract[1]. Historically, GISTs were classified as leiomyomas or leiomyosarcomas due to smooth muscle features observed under light microscopy.
GISTs were first termed in 1983 by Mazur and Clark[2], who discovered that the majority of gastric wall tumors were not derived from smooth muscle and nerve sheath origin using immunohistochemistry. GISTs are believed to arise from the interstitial cells of Cajal or their precursors and are heterogeneous histologically, showing spindle cells (70%), epitheloid cells (20%) and mixed cells (10%)[3]. The histogenesis of GISTs has since gained considerable research and treatment interest.
A systematic review of population-based cohort studies on GISTs by Søreide et al[4] showed that incidence ranges from low 0.43 per 100000 per year in Shanxi Province, China to high 1.6-2.2 per 100000 per year in South Korea. The cohort of 13550 patients from 19 countries gave the reported age ranging from 10-100 years, with median age in the 60 s; both male and female populations had about equal distribution. The anatomical locations of GISTs are frequently the stomach (55.6%) and small bowel (31.8%), and are less commonly found in the colon and rectum (6%), other various locations (5.5%) and esophagus (0.7%)[4].
Primary GISTs are commonly symptomatic (in about 80% cases), presenting with gastrointestinal bleeding or obstructive symptoms and abdominal pain. Incidental asymptomatic GISTs are discovered in less than 20% of cases during other gastrointestinal endoscopy or imaging investigations.
The diagnostic tests for GISTs may include gastrointestinal endoscopy (Figure 1), computed tomography (CT) scan (Figure 2), magnetic resonance imaging (MRI) scan and 18fluoro-deoxyglucose-positron emission tomography (18FDG-PET) scan (Figure 3). Endoscopic ultrasound scan (Figure 4) with fine needle aspiration biopsy (Figure 5) may be useful in confirming GISTs histologically.
Figure 1.
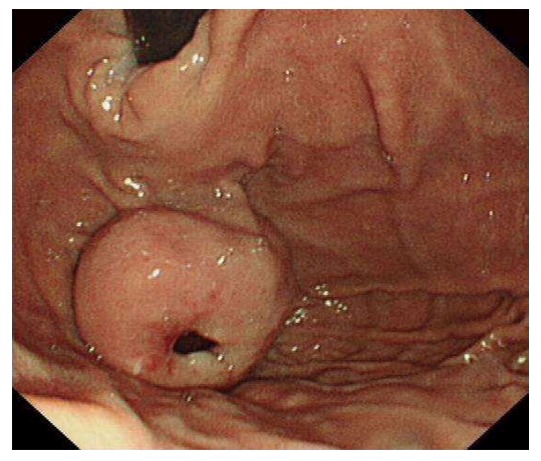
Gastroscopy view of a gastric gastrointestinal stromal tumors. A 4 cm × 4 cm in diameter gastric fundal submucosal tumor with a central ulceration associated with a recent bleed is shown.
Figure 2.
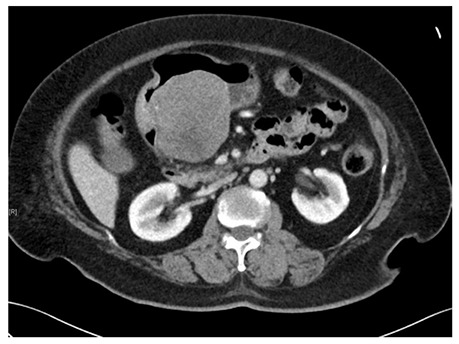
Computed tomography scan image of a gastric gastrointestinal stromal tumors. A submucosal tumor measuring 7.7 cm × 7.6 cm × 7.2 cm in dimension was located on the posterior wall of gastric antrum. An ill-defined hypodensity within the mass could represent an area of necrosis.
Figure 3.
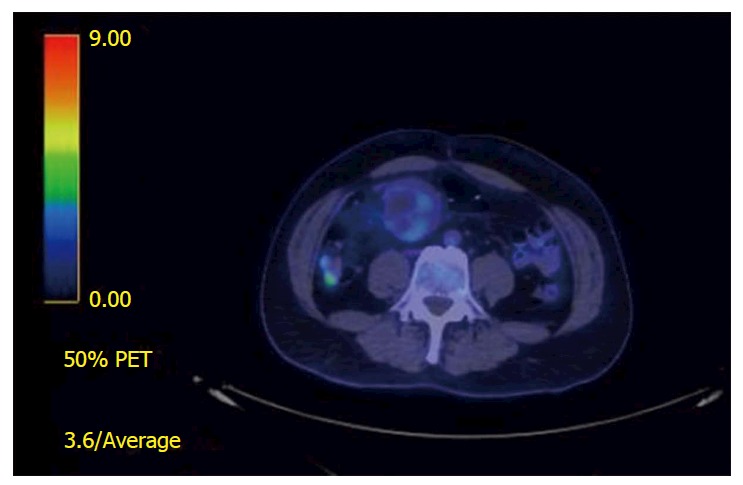
18Fluoro-deoxyglucose-positron emission tomography scan views of a small bowel gastrointestinal stromal tumors. There is a mildly fluoro-deoxyglucose avid mass adjacent to the jejunal anastomosis with SUVmax 3.4 suggestive of a local recurrence.
Figure 4.
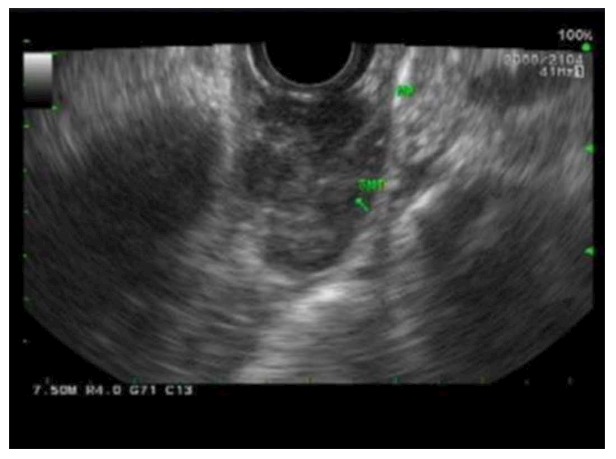
Endoscopic ultrasonography images of esophageal gastrointestinal stromal tumors. A distal esophageal submucosal lesion measuring 2.6 cm × 1.3 cm in diameter was noted to be well circumscribed, heterogeneous with hypoechoic echotexture without disruption of wall architecture and with no perilesional lymph node.
Figure 5.
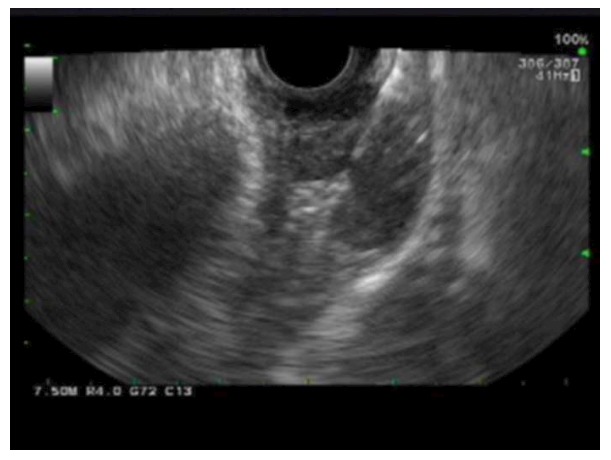
Endoscopic ultrasound scan and fine needle aspiration image of an esophageal gastrointestinal stromal tumors. The procedure was performed using a 22F procore needle with on-site cytotech.
Open or laparoscopic complete surgical R0 resection of GISTs (Figure 6) represent the only potentially curative treatment, but certain high risk features of the resected GISTs give rise to recurrence of the disease. DeMatteo et al[5] reviewed 200 patients with GISTs treated and followed-up at a single institution and found that 46% had primary disease, 47% had metastasis and 7% had isolated local recurrence. Eighty patients with primary disease who underwent complete resection had 5-year survival rate of 54%. Survival was predicted by tumor size, but not by microscopic resection margin. However, tumor recurrence was noted to occur at the original primary tumor site, peritoneum and liver. These data predated the use of tyrosine kinase inhibitors (TKIs). In later years, the treatment options for residual or progressive liver metastases of GISTs included hepatic artery embolization, radiofrequency ablation or liver resection[6-8].
Figure 6.
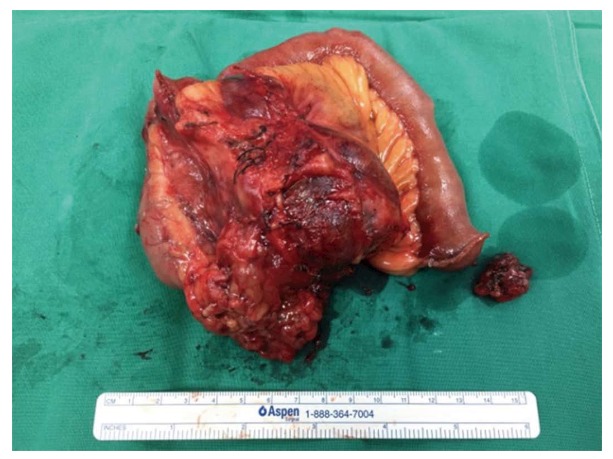
Macroscopic image of a recurrent small bowel gastrointestinal stromal tumors. A picture of a large enbloc resection specimen of a small bowel mesentery and jejunum was taken along a separate smaller metastatic mesenteric nodule.
Historical assessment of the malignant potential in GISTs were based on the criteria of tumor size, mitotic count, proliferating cell nuclear antigen and proliferation index, which allowed classification into low- and high-risk subgroups[9]. Subsequently, different risk stratification systems for GISTs were proposed, such as the National Institutes of Health (NIH) consensus criteria (Fletcher’s criteria based on size and mitotic count) and the Armed Forces Institute of Pathology criteria (Mittinen’s criteria based on size, mitotic count and tumor site) and the 8th edition of the International Union Against Cancer utilizing TNM classification in addition to a grade category based on mitotic count[10-12].
According to the NIH criteria for primary GISTs, the distribution of risk is categorized as very low risk (15%), low risk (30%), intermediate risk (22%) and high risk (33%)[10]. Table 1 shows the commonly used criteria for assessing malignant risk of GISTs. Other factors associated with a higher malignant risk of GISTs are the presence of necrosis, high cellularity, invasion to serosa or adjacent structure and rich vascularity. In addition, factors associated with a higher risk of recurrence of GISTs are now recognized to be incomplete R1 or R2 resection margin, tumor rupture and spillage during surgery.
Table 1.
National Institutes of Health vs Armed Forces Institute of Pathology criteria for assessing malignant risk of gastrointestinal stromal tumors
| Degree of risk | NIH criteria | AFIP criteria |
| Unknown | - | < 2 cm and ≤ 5 mitotic index |
| Very low | < 2 cm and < 5 mitotic index | ≤ 5 cm and ≤ 5 mitotic index |
| Low | 2-5 cm and < 5 mitotic index | Gastric: > 5 cm and ≤ 5 mitotic index |
| Others: 2-5 cm and ≤ 5 mitotic index | ||
| Intermediate or moderate | 5-10 cm and < 5 mitotic index | Gastric: > 10 cm and ≤ 5 mitotic index or > 2-5 cm and > 5 mitotic index |
| > 5 cm and 6-10 mitotic index | Others: 5-10 cm and ≤ 5 mitotic index | |
| High | > 5 cm and > 5 mitotic index | Gastric: > 5 cm and > 5 mitotic index |
| > 10 cm and any mitotic index | Others: > 10 cm and > 5 mitotic index | |
| Any size and > 10 mitotic index |
Mitotic index = number of mitoses per 50 high-power field. AFIP: Armed Forces Institute of Pathology; NIH: National Institutes of Health.
GENETIC MUTATIONS IN GISTs
The landmark article by Hirota et al[13] discovered that GISTs express the proto-oncogene KIT and that this KIT gene mutation provides growth stimulation of GISTs. c-KIT, also known as CD117, is a protein and a type of a receptor tyrosine kinase found on the surface of a variety of cell types; it is also a type of tumor marker. The binding of stem cell factor to the extracellular domain of c-KIT induces receptor dimerization and activation of downstream signaling pathways responsible for pro-growth signals within the cells.
Another landmark article by Heinrich et al[14] later discovered GISTs lacking KIT expression have mutations related to platelet-derived growth factor receptor alpha (PDGFRA). Overall, KIT or PDGFRA mutations are found in 85% and 5% of GISTs respectively.
Agaram et al[15] later discovered BRAF mutation in imatinib-naïve and imatinib-resistant GISTs. This BRAF mutation in GISTs is quite rare, accounting < 1% of cases[16]. It is noted that these KIT, PDGFRA and BRAF gene mutations are mutually exclusive.
“Wild-type” GISTs were previously referred to GISTs lacking any mutation in KIT and PDGFRA. This “wild-type” terminology should be avoided now that new mutations have been discovered in BRAF genes and in genes encoding the protein succinate dehydrogenase (SDH). About 12%-15% of adult GISTs and 90% of pediatric GISTs lacking KIT, PDGFRA or BRAF mutations are classified into SDH-deficient and non-SDH-deficient groups. The SDH-deficient group includes Carney triad (GISTs, pulmonary chondroma and extra-adrenal paraganglioma) and Carney-Stratakis syndrome (GISTs and paraganglioma)[17].
The vast majority of KIT mutations are localized in exon 11 (juxtamembrane domain; about 70%), exon 9 (extracellular dimerization motif; 10%-15%), exon 13 (tyrosine kinase 1 domain; 1%-3%), and exon 17 (tyrosine kinase 2 domain and activation loop; 1%-3%)[18]. Secondary KIT mutations in exons 13, 14, 17 and 18 are commonly identified in post-imatinib biopsy specimens, after the patients have developed the acquired resistance. The mutations of PDGFRA are noted to be localized in exon 12, 14 and 18, and more specifically as 18 D842V. The mutation of BRAF is identified and localized to exon 15 V600E[15]. Mutations of the SDH gene are found to be localized to subunit B, C and D[17]. Table 2 summarizes the frequency of different genetic mutations in GISTs.
Table 2.
Frequency of genetic mutations in gastrointestinal stromal tumors
| KIT mutation (about 85%) | PDGFRA mutation (about 5%) | BRAF mutation (< 1%) | SDH mutation (12%-15% adult, 90% pediatric GIST) |
| Exon 11 (about 70%) | Exon 18 (about 5%) | Exon 15 V600E | Subunit B, C and D |
| Exon 9 (10%-15%) | Exon 12 (1%) | ||
| Exon 13 (1%-3%) | Exon 14 (< 0.5%) | ||
| Exon 17 (1%) | Exon 18 D842V (about 0%) |
PDGFRA: Platelet-derived growth factor receptor alpha; SDH: Succinate dehydrogenase.
TKIs AND BIOLOGICAL THERAPY IN GISTs
Whilst complete surgical resection of GISTs can offer the best cure, targeted therapy in the form of TKIs has altered our management options. A landmark case report by Joensuu et al[18] described the effect of a TKI called STI571 in a patient with a metastatic GIST, for which the evaluation of MRI and 18FDG-PET scans showed a very dramatic reduction of the GIST.
STI571 was the first TKI, also called imatinib, approved by the United States Food and Drug Administration (FDA) in 2002 for the treatment of unresected or metastatic GISTs. In 2008, imatinib was approved for adjuvant use in high-risk resected GISTs patients to prevent recurrence[19]. In 2012, the FDA granted the extension of standard 1 year imatinib therapy to 3 years, due to increase in overall patient survival[20,21]. An important study demonstrated that imatinib when used as a neoadjuvant therapy decreased the tumor volume and was associated with improved complete surgical resection in the locally advanced primary GISTs[22].
In a trial examining the relationship between kinase genotype and treatment outcome for 428 patients treated with either 400 mg or 800 mg daily doses of imatinib confirmed the favorable impact of KIT exon 11 genotype, when compared with KIT exon 9 and wild-type genotype for patients with advanced GISTs[23].
The American College of Surgeons Oncology Group led a trial studying the long-term outcome of patients categorized as high risk of recurrence who underwent complete gross GISTs resection followed by adjuvant imatinib at 400 mg/d for 1 year. After a median follow-up of 7.7 years, the 1-, 3- and 5-year overall survival rates were 99%, 97% and 83% respectively, which compared favorably with a historical 5-year overall survival rate of 35%. The 1-, 3- and 5-year recurrence-free survival rates were 96%, 60% and 40% respectively. On univariate analysis, age and mitotic rate were associated with overall survival. On multivariate analysis, the recurrence-free survival rate was lower with increasing tumor size, small bowel site, KIT exon 9 mutation, high mitotic rate, and older age[24].
TKIs other than imatinib are considered as second-generation TKIs, and include sunitinib, regorafenib, sorafenib, nilotinib, dasatinib and pazopanib. Table 3 summarizes the implication of different mutations in GISTs and their response to TKI therapy.
Table 3.
Implication of gastrointestinal stromal tumors mutations and response to targeted therapy
| Imatinib[23] | Sunitinib[25] | Regorafenib[28] | |
| KIT mutation | |||
| Exon 11 | OR 63% | CB 34% | Increased sensitivity |
| Exon 9 | OR 37%. Intermediate sensitivity. Higher dose 800 mg more effective in metastatic disease than 400 mg daily | CB 34% | Unknown |
| Exon 13 | OR 40%. Sensitivity as primary mutation. Resistance as secondary mutation | CB 100% | Unknown |
| Exon 14 | Resistance as secondary mutation | Unknown | Unknown |
| Exon 17 | OR 25%. Primary mutation sensitive in vitro. Resistance as secondary mutation | CB 0% | Unknown |
| PDGFRA mutation | |||
| Exon 18 | OR 50% | CB 0% | Unknown |
| Exon 12 | Increased sensitivity | CB 0% | Unknown |
| Exon 14 | Increased sensitivity in vitro | Unknown | Unknown |
| Exon 18 D842V | Decreased sensitivity | Decreased sensitivity | Unknown |
| BRAF mutation | Resistance | Resistance | Unknown |
| SDH mutation | Decreased sensitivity | Unknown | Increased sensitivity |
| No KIT, PDGFRA or BRAF mutation | OR 28% | CB 56% | Some activity |
Objective response (OR) is defined as a complete or partial response by Response Evaluation Criteria for Solid Tumors (RECIST) criteria, excludes non-evaluable patients. Clinical benefit (CB) is defined as response or stable disease for 6 mo or more according to RECIST. PDGFRA: Platelet-derived growth factor receptor alpha; SDH: Succinate dehydrogenase.
Sunitinib was approved by the FDA for the treatment of imatinib-resistant GISTs in 2006 and is considered as second-line TKI[25]. Heinrich et al[26] discovered clinical activity of sunitinib after imatinib failure is significantly influenced by both primary and secondary mutations in the predominant pathogenic kinases that implicate the optimum treatment of patients with GISTs.
Regorafenib was approved by the FDA in 2013 to treat advanced GISTs that cannot be surgically removed and are resistant to other TKIs, and it is considered as third-line TKI[27]. The long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic or unresectable GISTs after failure of imatinib and sunitinib showed benefit in patients with primary KIT exon 11 mutations and SDH-deficient GISTs[28].
The use of other TKIs, apart from imatinib, sunitinib and regorafenib, is still being evaluated and remains debated. In a Korean clinical trial in 2012, sorafenib was shown to maintain disease control in one-third of the patients with metastatic GISTs who had otherwise failed with two or more TKIs[29].
In a phase I study of single-agent nilotinib or in combination with imatinib in patients with imatinib-resistant GISTs showed some partial clinical response but required phase II doses for further evaluation[30].
In a phase II study of imatinib-resistant GISTs treated with dasatinib, there was a significant activity by objective response rate but it did not meet the predefined 6 mo progression-free survival rate of 30%[31]. There is an American phase II clinical trial of dasatinib in advanced sarcoma including GISTs patients and a European phase II trial of dasatinib as first-line therapy in GISTs patients[32,33]. Both trials have stopped recruiting participants and the conclusion of the study is expected in the future.
In a phase II French trial, Mir et al[34] showed that pazopanib plus best supportive care improves progression-free survival compared with best supportive care alone in patients with advanced GISTs resistant to imatinib and sunitinib. This trial provides reference outcome data for future studies of targeted inhibitors in the third-line setting for this group of patients.
Other TKIs identified in clinical trials include masitinib (AB1010), crenolanib (CP-868,596), AZD2171, vatalanib (PTK787), OSI-930, TKI258 and DCC-2618 (Table 4). A biologics inhibitor of KIT and PDGFRA called olaratumab (IMC-3G3) was trialed (NCT01316263) but the development was put on hold and the stage 2 of this study was not completed.
Table 4.
Potential treatment targets for gastrointestinal stromal tumors
| Category | Name | ClinicalTrials.gov Identifier |
| TKI of KIT and PDGFRA | Masitinib (AB1010) | NCT00998751 (U)[57] |
| Crenolanib (CP-868,596) | NCT02847429 (R), NCT01243346 (C)[58] | |
| AZD2171 | NCT00385203 (C)[59] | |
| Vatalanib (PTK787) | NCT00117299 (C), NCT00655655 (A) | |
| OSI-930 | NCT00513851 (C) | |
| TKI258 | NCT01478373 (C), NCT01440959 (C) | |
| DCC-2618 | NCT02571036 (R) | |
| Biologic inhibitors of KIT and PDGFRA | Olaratumab (IMC-3G3) | NCT01316263 (C) |
| HSP90 inhibitors | Retaspimycin (IPI-5040) | NCT00276302 (C), NCT00688766 (T) |
| BIIB021 (CNF2024) | NCT00618319 (C) | |
| Ganetespib (STA-9090) | NCT01039519 (C) | |
| AUY922 | NCT01389583 (R), NCT01404650 (C) | |
| AT13387 | NCT01294202 (C) | |
| Inhibitors of pathways downstream of KIT and PDGFRA | RAS/RAF/MEK/ERK/MAPK inhibitors: MEK162 | NCT01991379 |
| AKT inhibitors: perifosine | NCT00455559 (C)[60] | |
| mTOR inhibitors: everolimus (RAD001) | NCT01275222 (C), NCT00510354 (C), NCT02071862 (R) | |
| mTOR inhibitors: temsirolimus (Torisel) | NCT00700258 (R) | |
| Cell cycle inhibitors | Alvocidib (Flavopiridol) | NCT00098579 (C) |
| Insulin-like growth factor pathway inhibitors | OSI-906 | NCT01560260 (C)[61] |
R: Recruiting; T: Terminated; C: Completed; A: Active, not recruiting; U: Unknown. PDGFRA: Platelet-derived growth factor receptor alpha.
CURRENT RESEARCH IN GISTs
The emergence of TKI-resistant GISTs has led to further research in understanding of this treatment failure and the alternative signaling mechanism conferring GIST survival. The research to find new drugs, particularly targeted therapy, is being evaluated.
Agaram et al[15] found that BRAF mutations appear to be associated with a higher malignant risk and resistance to TKI compared to KIT and PDGFRA mutations. Kinase inhibitors targeting BRAF may be considered as an effective therapeutic option in this GISTs subset. Falchook et al[35] published the first report on BRAF inhibitor, dabrafenib (GSK2118436), which showed prolonged anti-tumor activity in V600E BRAF-mutated GIST patients. There is presently no trial in GISTs looking at BRAF inhibitors.
In a phase II trial study of heat shock protein (HSP)90 inhibitor, BIIB021, given to patients with GISTs refractory to imatinib and sunitinib, promising response was shown[36]. This result encourages future development of HSP90 inhibitors in TKI-resistant GISTs. A next phase study evaluating BIIB021 in GISTs is therefore warranted.
Testing for germline mutations in SDH is presently recommended for patients with GISTs lacking mutations in KIT, PDGFRA and BRAF[37]. There is an ongoing phase II trial of vandetanib in children and adults with “wild-type” GISTs but it is currently not recruiting participants and the estimated study conclusion will be available in 2023[38]. Another study currently recruiting participants is the glutamine inhibitor CB-839 trial in solid tumors including SDH-deficient GISTs[39].
Ran et al[40] recently reported the combined inhibition of mitogen activated kinase (MAPK) and KIT signaling synergistically destabilizes the transcription factor called ETV1 and suppresses GIST growth. The combination of MAPK inhibitors and TKIs to target ETV1 may provide an effective therapeutic strategy in GISTs clinical management. There is currently a trial recruiting participants to study MEK162 in combination with imatinib in patients with untreated advanced GISTs[41].
In another emerging target category, Falkenhorst et al[42] discovered inhibitor of apoptosis proteins (IAPs) such as XIAP and survivin are commonly dysregulated in GISTs. Future study to assess the combination of imatinib with an IAP antagonist such as YM155 to enhance the pro-apoptotic activity in GISTs is therefore needed.
There was a clinical trial study looking at the role of immunotherapy by combining pegylated-interferon α-2b with imatinib for treatment of stage III/IV GISTs that yielded highly promising clinical outcomes. The trial was terminated early in 2012 in preparation for a larger future trial[43]. Table 4 summarizes the potential treatment targets in GISTs under clinical trials. The trials’ information was obtained from https://clinicaltrials.gov/ct2/home online.
CURRENT TREATMENT GUIDELINES FOR GISTs
Most countries have their own clinical practice guidelines for GISTs, such as the American National Comprehensive Cancer Network (NCCN) (2010 update), the European Society of Medical Oncology (ESMO) (2012), the French National Federation of Cancer Centers consensus guidelines (in French) (2005), the Japan Society of Clinical Oncology (2008), the Korean GISTs guidelines (2012 Update), the Canadian Advisory Committee on GISTs statement (2006) and the Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland (AUGIS) (2009)[44-50]. Most recently, the Asian Consensus Guidelines (2016) for the Diagnosis and Management of GISTs was published to promote optimal care for Asian populations[51].
The NCCN task force report update on GISTs management is quite comprehensive and detailed, and covers over 41 pages. It described the epidemiology from the Surveillance, Epidemiology and End Results data from the National Cancer Institute, the clinical presentation, the pathology and differential diagnosis using immunohistochemistry and gene expression profiling, the recommendations for diagnosing GISTs, the significance of KIT and PDGFRA mutation status, the recommendations for mutational analysis, the management of adult vs pediatric patients with GISTs, the principles of surgery for GISTs, the need for multidisciplinary management for primary, recurrent or metastatic GISTs and the imaging of GISTs[44].
The ESMO clinical practice guidelines on GISTs describes the incidence of GISTs in Europe, the strategy to diagnose GISTs, the stage classification and risk assessment (does not recommend TNM classification), the staging procedure using CT, MRI and FDG-PET scan, the treatment planning involving multidisciplinary team for localized and metastatic disease, the response evaluation and optimal follow-up for different risk categories[45].
In the Asian consensus guidelines for diagnosis and management of GISTs, some points were highlighted. Firstly, it recommends the minimal 3-year treatment with imatinib before and after surgery for high-risk GISTs. Secondly, it recommends early evaluation of tumor response after 1 mo of neoadjuvant imatinib treatment, when genotyping is not feasible for primary gastric GISTs. Thirdly, it suggested a prospective study on the feasibility and efficacy of high-dose imatinib therapy in Asian patients. Lastly, it recommends imatinib rechallenge instead of discontinuing TKI treatment if third-line regorafenib is not available or failed[51].
In summary of these published treatment guidelines, the general consensus is complete surgical resection of GISTs as the first step when possible. Surgery is potentially curative for primary GISTs that have not metastasized and the probability of recurrence will depend on the malignant potential risk stratification criteria.
GIST cases that are initially inoperable may be given neoadjuvant therapy with the first-line TKI imatinib to improve resectability. Following complete removal of primary GISTs, patients with a higher risk of tumor recurrence may consider adjuvant therapy with first-line TKI. Patients with metastatic GIST disease, even if removed, will benefit from TKI to maintain disease control.
For patients with imatinib-resistant GISTs, sunitinib is a second-line drug treatment whilst regorafenib is the third-line drug for imatinib- or sunitinib-resistant GISTs. Some drugs approved for other conditions may be prescribed off-label for GISTs at a physician’s discretion but with a caveat, and clinicians are advised to follow the local guidelines. New molecular targeted drugs are being tested in many clinical trials and some are still under development. An algorithm for the management of GISTs based on the summary of current guidelines is included (Figure 7).
Figure 7.
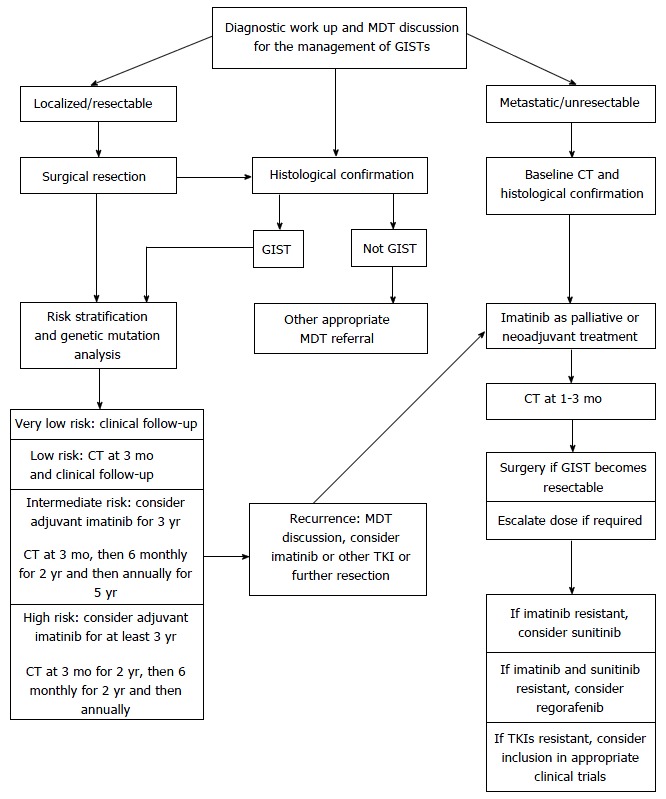
Algorithm for the management of gastrointestinal stromal tumors. GIST: Gastrointestinal stromal tumor; CT: Computed tomography; TKI: Tyrosine kinase inhibitor.
PROGNOSIS
Data from pooled analysis of 2560 patients diagnosed with operable GISTs who were not given adjuvant therapy gave the estimated 15-year recurrence-free survival after surgery at 59.9%[52]. Whilst in a trial previously alluded to with resected GISTs deemed high risk were subsequently treated with imatinib showed the 5-year overall survival rate of 83%[24]. In a different follow-up study to assess the long-term survival of 695 patients with metastatic GISTs who were treated with imatinib, the estimated 10-year overall survival is 23%[53].
There was an interesting Dutch study which highlighted that severe fatigue occurred in 30% of patients with GISTs and in 33% of patients with GISTs who took TKIs. The disabling fatigue was associated with psychological distress and physical function[54]. In another survey study, the long term functional outcomes of laparoscopic resection of gastric GISTs were investigated by utilizing the Gastrointestinal Quality of Life Index (GIQLI). Most patients reported no change in symptoms and the GIQLI scores were within the normal range, with minimal effect on long-term quality of life[55].
FUTURE GIST TREATMENT TRENDS
About 5 years ago, Dematteo[56] proposed a concept of personalized therapy for GISTs. With accumulating research data in biology, such as genetic mutations and adjuvant or neoadjuvant therapy with systemic drugs, it is considered true that personalized assessment and therapy may appear to be the future trend for GIST management.
Complete surgical resection of GISTs is the gold standard of primary treatment when possible, with or without the adjunct of molecular targeted drug therapy. Through the understanding of the mutations of GISTs and addressing treatment resistance with TKIs, new treatment ideas such as combination trials of TKI plus other drugs, TKI plus surgery in specified sequences, newer line TKIs, inhibitors of BRAF, HSP inhibitors, inhibitors of downstream pathways such as MAPK, IAP inhibitors and immunotherapy may play important roles as molecular targeted therapy in the future.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Lim KT and Tan KY declare no conflicts of interest related to this publication.
Peer-review started: March 2, 2017
First decision: April 21, 2017
Article in press: June 19, 2017
P- Reviewer: Dogusoy GB, Meshikhes AWN, Misiakos EP S- Editor: Qi Y L- Editor: Filipodia E- Editor: Li D
References
- 1.Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1–12. doi: 10.1007/s004280000338. [DOI] [PubMed] [Google Scholar]
- 2.Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7:507–519. doi: 10.1097/00000478-198309000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 4.Søreide K, Sandvik OM, Søreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016;40:39–46. doi: 10.1016/j.canep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 5.DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51–58. doi: 10.1097/00000658-200001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi K, Gupta S, Trent JC, Vauthey JN, Krishnamurthy S, Ensor J, Ahrar K, Wallace MJ, Madoff DC, Murthy R, et al. Hepatic artery chemoembolization for 110 gastrointestinal stromal tumors: response, survival, and prognostic factors. Cancer. 2006;107:2833–2841. doi: 10.1002/cncr.22336. [DOI] [PubMed] [Google Scholar]
- 7.Pawlik TM, Vauthey JN, Abdalla EK, Pollock RE, Ellis LM, Curley SA. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg. 2006;141:537–543; discussion 543-544. doi: 10.1001/archsurg.141.6.537. [DOI] [PubMed] [Google Scholar]
- 8.DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540–547; discussion 540-547. doi: 10.1097/00000658-200110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol. 1995;103:41–47. doi: 10.1093/ajcp/103.1.41. [DOI] [PubMed] [Google Scholar]
- 10.Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O’Leary TJ, Remotti H, Rubin BP, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 11.Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 12.Brierley JD, Gospodarowicz, Wittekind C. TNM classification of malignant tumours. International union against cancer (UICC) 8th ed. New York: Wiley-Blackwell; 2016. pp. 127–130. [Google Scholar]
- 13.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 15.Agaram NP, Wong GC, Guo T, Maki RG, Singer S, DeMatteo RP, Besmer P, Antonescu CR. Novel V600E BRAF Mutations in Imatinib-Naive and Imatinib-Resistant Gastrointestinal Stromal Tumors. Genes Chromosomes Cancer. 2008;47:853–859. doi: 10.1002/gcc.20589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agaimy A, Terracciano LM, Dirnhofer S, Tornillo L, Foerster A, Hartmann A, Bihl MP. V600E BRAF mutations are alternative early molecular events in a subset of KIT/PDGFRA wild-type gastrointestinal stromal tumours. J Clin Pathol. 2009;62:613–616. doi: 10.1136/jcp.2009.064550. [DOI] [PubMed] [Google Scholar]
- 17.Gaal J, Stratakis CA, Carney JA, Ball ER, Korpershoek E, Lodish MB, Levy I, Xekouki P, van Nederveen FH, den Bakker MA, et al. SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol. 2011;24:147–151. doi: 10.1038/modpathol.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052–1056. doi: 10.1056/NEJM200104053441404. [DOI] [PubMed] [Google Scholar]
- 19.Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, McGreevey LS, Chen CJ, Van den Abbeele AD, Druker BJ, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21:4342–4349. doi: 10.1200/JCO.2003.04.190. [DOI] [PubMed] [Google Scholar]
- 20.FDA approves Gleevec for expanded use in patients with rare gastrointestinal cancer. FDA News Release online 2012-01-31. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm289760.htm.
- 21.Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, Ramadori G, Hohenberger P, Duyster J, Al-Batran SE, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012;307:1265–1272. doi: 10.1001/jama.2012.347. [DOI] [PubMed] [Google Scholar]
- 22.Andtbacka RH, Ng CS, Scaife CL, Cormier JN, Hunt KK, Pisters PW, Pollock RE, Benjamin RS, Burgess MA, Chen LL, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol. 2007;14:14–24. doi: 10.1245/s10434-006-9034-8. [DOI] [PubMed] [Google Scholar]
- 23.Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, Ryan CW, von Mehren M, Blanke CD, Rankin C, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMatteo RP, Ballman KV, Antonescu CR, Corless C, Kolesnikova V, von Mehren M, McCarter MD, Norton J, Maki RG, Pisters PWT, Demetri GD, Brennan MF, Owzar K, the American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team for the Alliance for Clinical Trials in Oncology. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor (GIST): ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg. 2013;258:422–429. doi: 10.1097/SLA.0b013e3182a15eb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FDA Approves New Treatment for Gastrointestinal and Kidney Cancer. FDA News Release online 2006-01-26. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108583.htm.
- 26.Heinrich MC, Maki RG, Corless CL, Antonescu CR, Harlow A, Griffith D, Town A, McKinley A, Ou WB, Fletcher JA, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FDA approves Stivarga for advanced gastrointestinal stromal tumors. FDA News Release online 2013-02-15. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm340958.htm.
- 28.Ben-Ami E, Barysauskas CM, von Mehren M, Heinrich MC, Corless CL, Butrynski JE, Morgan JA, Wagner AJ, Choy E, Yap JT, et al. Long-term follow-up results of the multicenter phase II trial of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of standard tyrosine kinase inhibitor therapy. Ann Oncol. 2016;27:1794–1799. doi: 10.1093/annonc/mdw228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park SH, Ryu MH, Ryoo BY, Im SA, Kwon HC, Lee SS, Park SR, Kang BY, Kang YK. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs. 2012;30:2377–2383. doi: 10.1007/s10637-012-9795-9. [DOI] [PubMed] [Google Scholar]
- 30.Demetri GD, Casali PG, Blay JV, von Mehren M, Morgan JA, Bertulli R, Ray-Coquard I, Cassier P, Davey M, Borghaei H, et al. A Phase I Study of Single-Agent Nilotinib (AMN107) or in Combination with Imatinib in Patients with Imatinib-Resistant Gastrointestinal Stromal Tumors. Clin Cancer Res. 2009;15:5910–5916. doi: 10.1158/1078-0432.CCR-09-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trent JC, Wathen K, von Mehren M, Samuels BL, Staddon AP, Choy E, Butrynski JE, Chugh R, Chow WA, Rushing DA, et al. A phase II study of dasatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST) J Clin Oncol. 2011;29 Suppl:Abstr 10006. [Google Scholar]
- 32.Trial of Dasatinib in Advanced Sarcomas. First received 2007-04-20. Last updated 2016-10-12. Available from: https://clinicaltrials.gov/ct2/show/NCT00464620.
- 33.Dasatinib as First-Line Therapy in Treating Patients With Gastrointestinal Stromal Tumors. First received 2007-12-05. Last updated 2017-02-17. Available from: https://www.clinicaltrials.gov/ct2/show/NCT00568750?term=NCT00568750&rank=1.
- 34.Mir O, Cropet C, Toulmonde M, Cesne AL, Molimard M, Bompas E, Cassier P, Ray-Coquard I, Rios M, Adenis A, et al. Pazopanib plus best supportive care versus best supportive care alone in advanced gastrointestinal stromal tumours resistant to imatinib and sunitinib (PAZOGIST): a randomised, multicentre, open-label phase 2 trial. Lancet Oncol. 2016;17:632–641. doi: 10.1016/S1470-2045(16)00075-9. [DOI] [PubMed] [Google Scholar]
- 35.Falchook GS, Trent JC, Heinrich MC, Beadling C, Patterson J, Bastida CC, Blackman SC, Kurzrock R. BRAF mutant gastrointestinal stromal tumor: first report of regression with BRAF inhibitor dabrafenib (GSK2118436) and whole exomic sequencing for analysis of acquired resistance. Oncotarget. 2013;4:310–315. doi: 10.18632/oncotarget.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson MA, Okuno SH, Keohan ML, Maki RG, D’Adamo DR, Akhurst TJ, Antonescu CR, Schwartz GK. Phase II study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal tumors. Ann Oncol. 2013;24:252–257. doi: 10.1093/annonc/mds275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janeway KA, Kim SY, Lodish M, Nosé V, Rustin P, Gaal J, Dahia PL, Liegl B, Ball ER, Raygada M, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci USA. 2011;108:314–318. doi: 10.1073/pnas.1009199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phase II Trial of Vandetanib in Children and Adults with Wild-Type Gastrointestinal Stromal Tumors. First received 2013-12-14. Last updated 2017-02-7. Available from: https://clinicaltrials.gov/ct2/show/NCT02015065?term=SDH and GIST&rank=1.
- 39.Study of the Glutaminase Inhibitor CB-839 in Solid Tumors. First received 2014-02-14. Last updated 2016-08-18. Available from: https://clinicaltrials.gov/ct2/show/NCT02071862?term=SDH and GIST&rank=2.
- 40.Ran L, Sirota I, Cao Z, Murphy D, Chen Y, Shukla S, Xie Y, Kaufmann MC, Gao D, Zhu S, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumour growth. Canc Discov. 2015;5:304–315. doi: 10.1158/2159-8290.CD-14-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MEK162 in Combination With Imatinib Mesylate in Patients With Untreated Advanced Gastrointestinal Stromal Tumor (GIST). First received 2013-11-18. Last updated 2016-08-31. Available from: https://clinicaltrials.gov/ct2/show/NCT01991379?term=MEK and GIST&rank=1.
- 42.Falkenhorst J, Grunewald S, Mühlenberg T, Marino-Enriquez A, Reis A, Corless C, Heinrich M, Treckmann J, Podleska LE, Schuler M, et al. Inhibitor of Apoptosis Proteins (IAPs) are commonly dysregulated in GIST and can be pharmacologically targeted to enhance the pro-apoptotic activity of imatinib. Oncotarget. 2016;7:41390–41403. doi: 10.18632/oncotarget.9159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen LL, Chen X, Choi H, Sang H, Chen LC, Zhang H, Gouw L, Andtbacka RH, Chan BK, Rodesch CK, et al. Exploiting antitumor immunity to overcome relapse and improve remission duration. Cancer Immunol Immunother. 2012;61:1113–1124. doi: 10.1007/s00262-011-1185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, Pisters PW, Raut CP, Riedel RF, Schuetze S, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8 Suppl 2:S1–S41; quiz S42-S44. doi: 10.6004/jnccn.2010.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii21–iii26. doi: 10.1093/annonc/mdu255. [DOI] [PubMed] [Google Scholar]
- 46.Blay JY, Landi B, Bonvalot S, Monges G, Ray-Coquard I, Duffaud F, Bui NB, Bugat R, Chayvialle JA, Rougier P, et al. [Recommendations for the management of GIST patients] Bull Cancer. 2005;92:907–918. [PubMed] [Google Scholar]
- 47.Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, Otani Y, Shimada Y, Takahashi F, Kubota T. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008;13:416–430. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 48.Kang YK, Kang HJ, Kim KM, Sohn T, Choi D, Ryu MH, Kim WH, Yang HK. Clinical practice guideline for accurate diagnosis and effective treatment of gastrointestinal stromal tumor in Korea. Cancer Res Treat. 2012;44:85–96. doi: 10.4143/crt.2012.44.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blackstein ME, Blay JY, Corless C, Driman DK, Riddell R, Soulières D, Swallow CJ, Verma S. Gastrointestinal stromal tumours: consensus statement on diagnosis and treatment. Can J Gastroenterol. 2006;20:157–163. doi: 10.1155/2006/434761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reid R, Bulusu R, Carroll N, Eatock M, Geh I, Judson I, O’Dwyer P, Warren B, Seddon B, Hill G. 2009. Guidelines for the management of gastrointestinal stromal tumours (GIST) pp. 1–55. Available from: http://www.augis.org/wp-content/uploads/2014/05/GIST_Management_Guidelines_180809.pdf. [Google Scholar]
- 51.Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN, et al. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat. 2016;48:1155–1166. doi: 10.4143/crt.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–274. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]
- 53.Heinrich M, Rankin C, Blanke CD, Demetri GD, Borden EC, Ryan CW, von Mehren M, Blackstein ME, Priebat DA, Tap WD, et al. Correlation of Long-term Results of Imatinib in Advanced Gastrointestinal Stromal Tumors With Next-Generation Sequencing Results: Analysis of Phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944–952. doi: 10.1001/jamaoncol.2016.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poort H, van der Graaf WT, Tielen R, Vlenterie M, Custers JA, Prins JB, Verhagen CA, Gielissen MF, Knoop H. Prevalence, Impact, and Correlates of Severe Fatigue in Patients With Gastrointestinal Stromal Tumors. J Pain Symptom Manage. 2016;52:265–271. doi: 10.1016/j.jpainsymman.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Dressler JA, Palazzo F, Berger AC, Stake S, Chaudhary A, Chojnacki KA, Rosato EL, Pucci MJ. Long-term functional outcomes of laparoscopic resection for gastric gastrointestinal stromal tumors. Surg Endosc. 2016;30:1592–1598. doi: 10.1007/s00464-015-4384-6. [DOI] [PubMed] [Google Scholar]
- 56.Dematteo RP. Personalized therapy: prognostic factors in gastrointestinal stromal tumor (GIST) J Gastrointest Surg. 2012;16:1645–1647. doi: 10.1007/s11605-012-1944-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Cesne A, Blay JY, Bui BN, Bouché O, Adenis A, Domont J, Cioffi A, Ray-Coquard I, Lassau N, Bonvalot S, et al. Phase II study of oral masitinib mesilate in imatinib-naïve patients with locally advanced or metastatic gastro-intestinal stromal tumour (GIST) Eur J Cancer. 2010;46:1344–1351. doi: 10.1016/j.ejca.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 58.Heinrich MC, Griffith D, McKinley A, Patterson J, Presnell A, Ramachandran A, Debiec-Rychter M. Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin Cancer Res. 2012;18:4375–4384. doi: 10.1158/1078-0432.CCR-12-0625. [DOI] [PubMed] [Google Scholar]
- 59.Judson I, Scurr M, Gardner K, Barquin E, Marotti M, Collins B, Young H, Jürgensmeier JM, Leahy M. Phase II study of cediranib in patients with advanced gastrointestinal stromal tumors or soft-tissue sarcoma. Clin Cancer Res. 2014;20:3603–3612. doi: 10.1158/1078-0432.CCR-13-1881. [DOI] [PubMed] [Google Scholar]
- 60.Conley AP, Araujo D, Ludwig J, Ravi V, Samuels BL, Choi H, Thall PF, Patel S, Benjamin R, Trent J. A randomized phase II study of perifosine (P) plus imatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST) J Clin Oncol. 2009;27 Suppl 15:10563. [Google Scholar]
- 61.Songdej N, von Mehren M. GIST treatment options after tyrosine kinase inhibitors. Curr Treat Options Oncol. 2014;15:493–506. doi: 10.1007/s11864-014-0295-3. [DOI] [PubMed] [Google Scholar]


