Abstract
Nearly half of the global population are carriers of Helicobacter pylori (H. pylori), a Gram-negative bacterium that persists in the healthy human stomach. H. pylori can be a pathogen and causes development of peptic ulcer disease in a certain state of the macroorganism. It is well established that H. pylori infection is the main cause of chronic gastritis and peptic ulcer disease (PUD). Decontamination of the gastric mucosa with various antibiotics leads to H. pylori elimination and longer remission in this disease. However, the reasons for repeated detection of H. pylori in recurrent PUD after its successful eradication remain unclear. The reason for the redetection of H. pylori in recurrent PUD can be either reinfection or ineffective anti-Helicobacter therapy. The administration of antibacterial drugs can lead not only to the emergence of resistant strains of microorganisms, but also contribute to the conversion of H. pylori into the resting (dormant) state. The dormant forms of H. pylori have been shown to play a potential role in the development of relapses of PUD. The paper discusses morphological H. pylori forms, such as S-shaped, C-shaped, U-shaped, and coccoid ones. The authors proposes the classification of H. pylori according to its morphological forms and viability.
Keywords: Helicobacter pylori, Forms of H. pylori, Dormant forms of H. pylori, Viable forms of H. pylori, Non-viable forms of H. pylori, Physiological states of H. pylori, Culturable forms of H. pylori, Unculturable forms of H. pylor, Resuscitation of dormant H. pylori, Ulcerogenesis
Core tip: The administration of antisecretory and antibacterial drugs can lead to the conversion of Helicobacter pylori (H. pylori) into the resting (dormant) state. C-shaped and U-shaped forms of H. pylori, most likely, are dormant forms of the bacteria. C-shaped and U-shaped forms of H. pylori are capable of reverse transition into the vegetative replicative state and of causing development of recurrence of peptic ulcer disease (PUD). The induction of process reversion occurs under the influence of specific molecules. The identification and study of these compounds will allow development of new drugs aimed at preventing recurrent PUD associated with dormant forms of H. pylori.
INTRODUCTION
Peptic ulcer disease (PUD) is a problem that is traditionally the center of attention of gastroenterologists[1]. Many aspects of this pathology have been well studied. The disease develops as a result of the influence of a set of various exogenous and endogenous etiological factors. There are many theories of PUD development, including vascular, inflammatory-gastritis, allergic, hormonal, motor-primacy, corticovisceral, neurogenic, psychosomatic, acido-peptic and infectious ones. Each of them deserves attention, as it reflects one of the facets of this complex problem. The diversity of the causes that lead to the pathological process allows PUD to be considered as a polyetiologic and polypathogenic disease.
PUD with a frequently recurrent or long-term healing ulcer of the stomach or duodenum generally occurs in the presence of chronic active gastritis or chronic active duodenitis, both of which are associated with Helicobacter pylori (H. pylori) infection. Decontamination of the gastric mucosa (GM) with various antibiotics results in H. pylori eradication and longer remission in PUD[2-6]. However, the reasons for repeated detection of H. pylori in relapsed PUD after its supposedly successful eradication remain unclear. This may be due to either reinfection or ineffective anti-Helicobacter therapy. In most cases, the administration of antibacterial drugs leads to complete H. pylori eradication, but can give rise to resistant bacterial strains and facilitate the conversion of H. pylori into the resting (dormant) forms[7]. It is therefore relevant to study dormant H. pylori forms, as well as their values in ulcerogenesis[8].
H. PYLORI IS ONE OF THE ETIOLOGICAL FACTORS OF PUD
Landmarks in the history of H. pylori studies
The accumulated scientific data can confirm that H. pylori infection is important in the mechanism of PUD development[9]. H. pylori was first reported in 1875 when Bottcher and Letulle observed it on the margins of peptic ulcers[10]. The bacterium did not grow in the artificial nutrient media that were known at that time, and this accidental discovery was long forgotten. In the 1980s, Australian pathologist Robin Warren together with Barry Marshall isolated H. pylori from human gastric mucosal biopsy specimens and cultured it in artificial nutrient media. Warren and Marshall suggested that most gastric ulcers and gastritis in humans might be associated with H. pylori infection[11,12]. Marshall demonstrated the role of H. pylori infection in the development of gastrointestinal diseases in 1983. He drank a culture of the bacterium to prove the etiological role of H. pylori in the development of antral gastritis. Thereafter, he developed H. pylori-associated antral gastritis. After that, many researchers concentrated on the study of H. pylori[13].
There has been gradually increasing evidence that duodenal ulcers and duodenitis are also associated with H. pylori infection[14-16]. In 2005, Warren and Marshall received the Nobel Prize in Physiology or Medicine for the discovery of H. pylori pathogenicity[12] and rekindled interest in the study of this microorganism. Since then, the association of H. pylori with digestive system diseases has been the subject of much research attention[10,16].
Risk of digestive system diseases caused by H. pylori
H. pylori is one of the most common infections worldwide. H. pylori infection is highly prevalent throughout the world, especially in developing countries[10]. Nearly half of the global population are carriers of H. pylori, a Gram-negative bacterium that persists in the human stomach and duodenum[12,17-21]. H. pylori gastric colonization is acquired early in life (almost always before the age of 10 years), and, in the absence of antibiotic therapy, generally persists for life[12,22,23]. The prevalence of H. pylori ranges from 35% to 90% in different populations[21,24-28]. It presents in 70%-90% of the population in developing countries and 35%-40% in developed ones[21,29].
Moscow falls into a group of cities with extremely high H. pylori infection prevalence, with the predominance of virulent bacterial strains[30]. It is reported that 88% of the Moscow working population is infected with H. pylori. Its prevalence is 78% in people younger than 30 years and about 97% in individuals older than 60 years[30]. The prevalence of H. pylori infection is high in developing countries, especially among children. In India, the prevalence of this infection is 22%, 56% and 87% in the 0-4, 5-9 and 10-19 year age groups, respectively[21,31]. H. pylori is usually the numerically dominant gastric microorganism[13]. However, PUD occurs only in a small percentage of H. pylori carriers[32].
H. pylori does not typically cause any adverse effects[13]. Many people infected with H. pylori are shown to remain asymptomatic carriers[33]. It has turned out that H. pylori may behave as a commensal or symbiont, depending upon the circumstances[34-37]. The idea that H. pylori might actually confer benefits to humans has engendered considerable controversy among investigators. The data of the potential importance of health benefits that might be afforded by H. pylori are considered and debated in the review by Cover and Blaser[13]. It has been presumed that the conserved microbiota have specific adaptations that permit persistence at particular locales[13].
In the 1980-1990s, researchers studied the role of H. pylori as an important factor in the etiopathogenesis of PUD. Human gastric colonization by the bacterium H. pylori is a predisposing factor for gastrointestinal diseases, such as gastritis and PUD[13,38]. Strong links exist between PUD and H. pylori infection[39]. So, H. pylori detection rates in PUD vary from 60% to 100%. There is also a strong relationship between H. pylori and duodenal ulcer[21]. H. pylori has been shown to be one of the important local factors involved in the development of ulcerative defect[40,41]. The manifestations associated with chronic H. pylori infection vary considerably among distinct geographic regions and these differences have been attributed at least in part to polymorphisms of H. pylori genes, particularly those encoding virulence factors[15]. H. pylori is an important gastrointestinal pathogen associated with gastritis, PUD, and an increased risk of gastric carcinoma[22]. The presence of H. pylori in the gastroduodenal mucosa and its involvement in the development of chronic gastritis, PUD, carcinoma and other diseases are well documented[9,15,23,42].
Blaser considers that H. pylori shows its pathogenicity, by regulating the expression of different genes to the extent that is dictated by the response of a macroorganism[13,43]. The microorganism and macroorganism create a finely tuned balance system, the resulting impairment of which develops a specific disease with certain clinical signs and prognosis[44]. In the vast majority of cases, long-lasting H. pylori infection induces chronic gastritis, while only some patients develop PUD and gastric cancer. For this reason, the bacterium is considered to be a risk factor for the development and recurrence of PUD[45,46]. Therefore, H. pylori is assigned to the group of pathogenic bacteria. Antibiotic treatment of PUD results in bacterial disappearance and ulcer healing[12]. Marshall and Warren reported that eradication of the bacteria significantly reduced the duodenal ulcer relapse rate[12].
Characteristics of H. pylori
The genus Helicobacter (helix and bacteria) is heterogeneous[47]. The Helicobacter genus now includes at least 26 formally named species, and more that are still being studied[48,49]. Some of them were previously known by other names. Humans have been found to have only 11 Helicobacter species: H. pylori, H. heilmannii, and H. felis in the GM, H. cinaedi (H. westmeadii, H. canadensis), H. fennelliae, H. canis, H. pullorum, and H. rappini in the small intestinal mucosa. Some Helicobacter species have been isolated from the human hepatobiliary system: H. pylori from the liver, H. bilis, H. pullorum, and H. rappini from the bile ducts. H. pylori is the best known bacterium. H. pylori includes several strains[46]. H. pylori strains isolated from unrelated humans exhibit a high level of genetic diversity (reviewed in Blaser MJ and Berg DE[50]). The genetic structure of the pathogenic genes of H. pylori varies largely, which contributes to the differences in virulence among various strains and in clinical symptoms[15]. H. pylori strains differ in resistance to drugs, adhesive specificity and production of cytotoxins.
H. pylori (Figure 1) is a small, Gram-negative, asporogenous, S-shaped or slightly spirally curved, microaerophilic bacterium[51-53].
Figure 1.
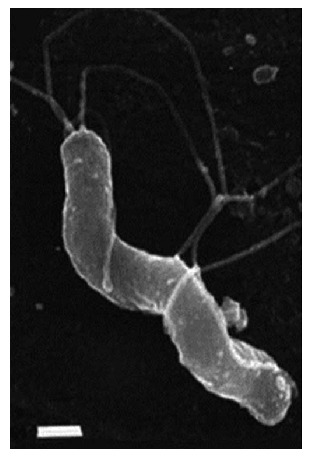
Morphology of Helicobacter pylori. S-shaped H. pylori with five to seven sheathed polar flagella. Field emission SEM, bar = 0.5 μm. (Field emission SEMs courtesy of L. Thompson and negative stains courtesy of S. Danon, School of Microbiology and Immunology, University of New South Wales). From: Helicobacter pylori: Physiology and Genetics. Mobley HLT, Mendz GL, Hazell SL, editors. Washington (DC): ASM Press; 2001. Chapter 6, Morphology and Ultrastructure[54].
Thirty-seven degrees Celsius and pH 4.0-6.0 are the most favorable conditions for the life, growth, and reproduction of H. pylori; although, the species also survives at pH 2.5. H. pylori in vivo and under optimum in vitro conditions exists as an S-shaped bacterium with 1 to 3 turns, 0.5 μm × 5 μm in length, and a tuft of 5 to 7 polar sheathed flagella[54-56]. The bacterial cell is covered with a smooth sheath. The flagellum of H. pylori is 30 nm in diameter, consisting of an internal filament approximately 12 nm in diameter surrounded by a sheath, the outer membrane of which is continuous with the outer membrane of the cell[54-56].
Unipolar flagella are essential for the unique motility of H. pylori[57]. Qin et al[57] employed cryo-electron tomography to visualize intact H. pylori cells, with a particular focus on the flagella. Remarkably, the unipolar flagella of H. pylori are driven by one of the largest flagellar motors found in bacteria. The flagellar motor provides higher torque needed by the bacterium to navigate the viscous environment of the human stomach. Thin sections of H. pylori reveal the typical cell wall detail of a Gram-negative bacterium that consists of outer and inner, or plasma, membranes separated by the periplasm of approximately 30 nm thickness[54]. H. pylori has inherent corkscrew motility. The presence of flagella, a smooth cell sheath, a spiral shape, and corkscrew motility allows this microorganism to move in the mucus thickness along the pH gradient and serves as its virulence factor. In addition, the flagella contribute to the aggregation of H. pylori to colonize the latter on the epithelial surface of GM[58].
The stomach is the major habitat of H. pylori[13], but it may also survive in other environments[13,18,59]. The habitat of H. pylori may be the proximal duodenum or distal esophagus. This is usually accompanied by gastric metaplasia at these sites[13]. A gene that is pathognomonic for duodenal ulcer and called dupA (duodenal ulcer promoting gene), which encompasses the two H. pylori genes of jhp0917 and jhp0918, has been discovered[60]. This gene increases the survival of the microorganism at low pH values. The presence of the dupА gene is associated with a high risk for duodenal ulcer and with a low risk for gastric atrophy and cancer[61].
COCCOID AND DORMANT FORMS OF H. PYLORI
Morphological forms of H. pylori
All living organisms are equipped with mechanisms that allow extended survival in adverse environments. For a number of them, this response involves, besides metabolic adaptations, changes in cell morphology[62]. H. pylori is mainly present as a spiral-shaped form in human gastric biopsy specimens. On aging, the bacterial cells lose their typical spiral-shaped form and convert to coccoid ones (Figure 2)[54]. When influenced by adverse factors (temperature or pH changes, prolonged fasting when cultivated, or use of antibacterial drugs), non-spore-forming microorganisms can be transformed into a latent coccoid form[63].
Figure 2.
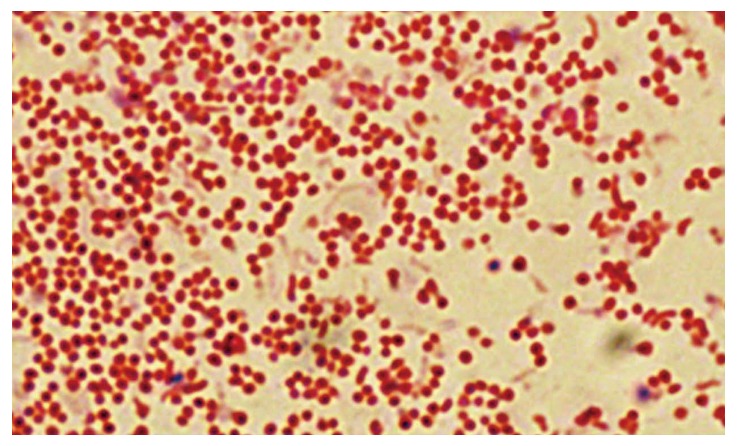
Microscopic images of coccoid forms of Helicobacter pylori after 6-d exposure to antibiotics. From: Faghri et al[65].
The ability of H. pylori to transform from the spiral-shaped form to the coccoid form is one of its most important, but not exclusive properties. Through the course of evolution, H. pylori has evolved special adaptive mechanisms and acquired vital physiological properties allowing it to survive extreme situations in the human organism, when cultivated, and to survive in the external environment[64].
The bacterium transforms from spiral to coccoid under mild circumstances, whereas under extreme ones it is unable to undergo shape modification. This strongly supports the view that transformation into the coccoid form is an active, biologically led process, switched on by the bacterium as a protection mechanism[62,65]. This study demonstrates that the coccoid shape is in fact a manifestation of cell adaptation to less than optimum environments.
Saito et al[66] identified three types of coccoid forms of H. pylori. The authors claim to represent different H. pylori transformation processes and consist of bacteria that are dead, living and cultivated, and viable but non-culturable[62,66]. Controversy exists as to whether these cells are viable, dormant or just dead[54].
The initial stage of H. pylori transformation in the coccoid form is accompanied by the condensation of the protoplasmic matrix and an increase in the periplasm on one side of the microorganism (usually at the pole opposite to the flagellar basal complex). An increase in the volume of the periplasmic space leads to stretching of the cell wall, displacement of the protoplasmic matrix to the periphery, and accumulation of dense material, that results in the formation of C-shaped and/or U-shaped cells (Figure 3)[54].
Figure 3.
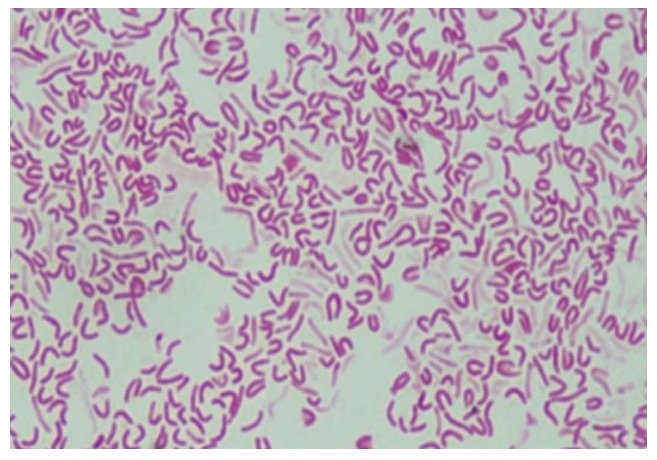
Microscopic images of morphological forms of Helicobacter pylori after exposure to antibiotics: S-shaped, U-shaped, C-shaped and coccoid-shaped. From: Faghri et al[65].
These C-shaped and/or U-shaped forms then convert to the coccoid cells, with an increase in the protoplasmic cylinder and maintenance of the double membrane system (Figures 3 and 4)[54]. In their work, Mouery et al[67] have shown transmission electron micrographs of typical stages of the helical-to-coccoid transformation. C-shaped and/or U-shaped forms of H. pylori are an intermediate state of the bacteria[67-69].
Figure 4.
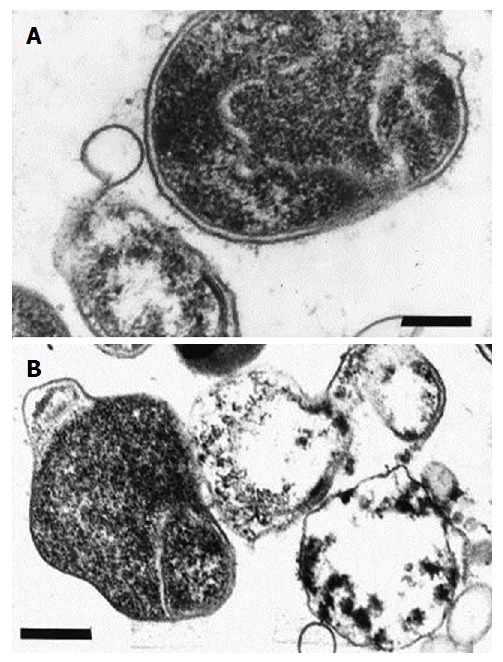
Electronograms of coccoid forms of Helicobacter pylori. A: Initial ingrowth in the periplasmic space resulting in the formation of U-shaped cells; B: Conversion to the coccoid form. Ultrathin section, bar = 0.5 μm. From: Helicobacter pylori: Physiology and Genetics. Mobley HLT, Mendz GL, Hazell SL, editors. Washington (DC): ASM Press; 2001. Chapter 6, Morphology and Ultrastructure[54].
The C-shaped and/or U-shaped forms of H. pylori are an intermediate bacterial type transforming into an inactive phase (dormancy). Dormancy is understood to be a reversible state of bacterial cells with a low metabolic activity, in which they can be for a long time without replication[63,70,71]. In microbiology, this condition has, until recently, been associated with the presence of forms, such as spores and cysts. In the late 20th century, the literature began to discuss the possibility of formation of dormant (resting) forms by non-spore-forming bacteria[63,72] that encompass most Gram-negative pathogenic bacteria, including H. pylori. Dormancy is characterized by the increased resistance of bacterial cells to extreme stresses (deficiency of nutrients, effects of antisecretory drugs, antibiotics, etc.) and favors their survival[73]. The ability of H. pylori to go into a dormant state may be an important factor in the epidemiology and spread of helicobacteriosis. The C-shaped and U-shaped forms of H. pylori can be considered truly a dormant form capable of reinfection[53]. The role of these forms in the pathogenesis and transmission of infection needs to be clarified.
The C-shaped and/or U-shaped forms of H. pylori keep the polar membrane associated with the flagellar basal complex[56,65]. Only a small number of intermediate forms of H. pylori possesses a complete set of flagella and retains metabolic activity to ensure mobility comparable to that of spiral-shaped forms[68].
The fully-formed coccoid forms maintain the basic pattern of a bacterial cell structure (Figure 4). They have a cell wall, cytoplasmic membrane and cytoplasm[56,62,67,69,74]. The coccoid cells differ in details of the cell wall structure, which leads to impairment of recognition of the bacteria by the host immune system (bacterial mimicry)[68].
The accumulated scientific data suggest that there are three morphological forms of H. pylori: (1) S-shaped forms; (2) U-shaped and C-shaped (intermediate or transitional) forms; and (3) Coccoid forms.
The intermediate and coccoid forms can coexist in the mucosa or in the culture in various ratios[75]. Their ratio depends on the time of H. pylori being present under adverse conditions and on the level of exposure to adverse factors. Spiral-shaped forms were predominant in a 3-d culture of H. pylori; about half of the bacteria are coccoid forms at 6 d[76-78]. Mouery et al[67] show the graphical distribution of the ratio of morphological forms of H. pylori after 12, 24 and 48 h of cultivation. The distributions of morphologies of more than 100 cells of each genotype for each time point are shown[67]. The number of coccoid forms increases with the longer time of cultivation. This happens due to the transition of C-shaped and U-shaped forms of H. pylori to coccoid ones.
The C-shaped, U-shaped and coccoid forms of H. pylori lose enzyme activity and show a lower metabolism[79]. The urease activities of resting (dormant) and coccoid cells were found to be lower than those of the spiral-shaped form of H. pylori[80,81]. A significant transformation of H. pylori into coccoid forms may result in loss of urease activity. However, urease-encoding genes continue to be identified in H. pylori by polymerase chain reaction (PCR)[69,81,82]. The minimization of enzyme activity and energy metabolism in the transformable H. pylori forms is adaptive and aimed at preserving the viability of microorganisms[65,83]. Bacterial viability has been confirmed by the fact that the transformed forms of H. pylori can be detected by acridine orange staining[69,84]. The C-shaped, U-shaped and coccoid forms of H. pylori tolerate a wider pH range to a greater extent than the spiral-shaped forms, are resistant to unfavorable factors and antibiotics, and cannot lose virulence. All this creates favorable conditions for the preservation of bacteria in the human body or in the external environment.
The triggers of H. pylori transformation from spiral-shaped to coccoid forms in the environment may be physical factors: higher insolation, low humidity, and lack of food substrates[77,85]. During bacteriological cultivation, transformation into the coccoid forms occurs due to the depletion of adequate components of the nutrient medium[85,86]. H. pylori transforms in the human body due to changes in the habitat conditions upon exposure to antisecretory and antibacterial drugs. Khomeriki et al[69] have studied the time course of changes in the transformation of H. pylori in the GM. They have indicated that the spiral-shaped forms transform into the coccoid ones a few hours after adhesion to the cell surface of GM cells[69].
The conversion of the spiral forms of H. pylori to its C-shaped, U-shaped and coccoid forms in the GM and in the nutrient medium is due to the accumulation of toxic metabolic products of H. pylori vital functions. Reactive oxygen species generated by phagocytes or by H. pylori itself in the presence of specific pyridine nucleotides may trigger the formation of transitional and coccoid forms in the GM of untreated patients[69]. When an unfavorable situation occurs, there is accumulation of factors that induce the conversion of cells in the bacterial populations to a dormant state.
Loginov et al[8] carried out a comparative analysis in which H. pylori in GM biopsy specimens from patients with duodenal ulcer was detected by a quantitative urease test and PCR before and a month after anti-Helicobacter therapy. Prior to anti-Helicobacter therapy, the detection rate of H. pylori in the GM biopsy specimens from patients with PUD was 93.4% and 98.7%, as shown by the quantitative urease test and PCR, respectively (Table 1). The difference of 5.3% in the detection rate of H. pylori may be due to the different sensitivities of these methods.
Table 1.
Detection of Helicobacter pylori in gastric mucosa biopsy specimens from in-patients with duodenal ulcer by quantitative urease test and polymerase chain reaction before and one month after anti-Helicobacter therapy
| Detection of Helicobacter pylori | Quantitative urease test | PCR | Difference between methods |
| Before treatment, % | 93.4 | 98.7 | 5.3 |
| After treatment, % | 11.1 | 24.1 | 13.0 |
PCR: Polymerase chain reaction.
One month after eradication therapy, these patients had H. pylori detected by the quantitative urease test in 11.1% of cases and by PCR in 24.1%. The difference between the methods was 13%, i.e., almost twice that as before the treatment. These findings suggest that H. pylori were not completely eliminated in some patients at 1 mo after the anti-Helicobacter therapy, and the H. pylori diagnosed by PCR were at least partially in a dormant (resting) state and partly in coccoid forms. The low urease activity (or lack thereof) of coccoid H. pylori forms precludes identifying them by the quantitative urease test. The data presented allow us to indirectly suggest that after anti-Helicobacter therapy, the dormant forms of H. pylori are present in patients as a result of their incomplete elimination[7,87].
Viability of dormant and coccoid forms of H. pylori
The pleiomorphic nature of H. pylori has been the subject of intensive debate for many years, with part of the scientific community arguing that the coccoid shape represents a degraded, nonviable form of the cell[62,85,86,88-91]. Evidence supporting the concept that the coccoid forms are degenerate and not capable of growth comes from a number of studies showing that as the cells age, the levels of DNA and RNA and mRNA expression decrease with degradation of the nucleic acids, nonrandom fragmentation of the ribosomal RNA, and no evidence of a membrane potential necessary for processes such as oxidative phosphorylation[54]. There is evidence that the coccoid forms lose their reproductive ability, are unculturable in artificial nutrient media, have no characteristic features under a light microscope, and do not produce urease or produce it in low amounts[65,79,81,86,92,93]. The infectivity of coccoid H. pylori forms is still controversial[94].
There are opposing data regarding the viability of the C-shaped, U-shaped and coccoid form of H. pylori. A study by Khomeriki and Morozov[69] indicated that the structural transformation of spiral-shaped forms of H. pylori into the coccoid forms is not always a sign of functional disintegration of the microorganism. There is evidence confirming the viability of the transformed forms of H. pylori[81]. They maintain cell structure, exhibit respiratory activity, support protein metabolism and expression, and, in some cases, are capable of reverse transition into the vegetative spiral-shaped bacteria (on their passage through animals)[92]. Cell respiration is detected in up to 40% after 45 d in vitro cultivation of H. pylori[54,95-97]. The findings of Poursina et al[94] suggest that the induced coccoid form of H. pylori is not a passive entity but can actively infect a human by expression of the virulence genes after a long stay in the stomach and may contribute to the development of chronic and severe disease. Flow cytometry analyses show that the majority of the induced coccoids (90%-99.9%) are viable[94].
There is evidence of the viability of the transformed forms of H. pylori obtained using biochemical methods. The cultures consisting of intermediate and coccoid forms have been found to retain oxidative metabolism at the same level as spiral-shaped forms for several months[98]. They maintain a high level of alkaline and acid phosphatases and a stable ATP level that increases if a number of fresh nutrient medium is added to the old culture[80-82,99]. Incorporation of a bromodeoxyuridine label into the transformed forms is suggestive of their continuing synthesis of new DNA[65,69]. So far, it is unconfirmed whether these data indicate the viability of H. pylori or the persistence of cells as ‘‘bags of enzymes’’[98].
The contradictory data on the viability of the transformable forms of H. pylori are likely due to the fact that H. pylori coexists in coccoid and transitional (intermediate C-shaped and U-shaped) forms under unfavorable conditions in the human body or culture media. It is impossible to isolate data on the viability of intermediate and coccoid forms co-existing in the same culture. Apparently, one part of transformed cells in the population of H. pylori truly is degenerative, dead cells[100] (most likely it is the coccoid forms), and the other is dormant cells, reversible forms (most likely it is the C-shaped and U-shaped forms). There is evidence confirming the concept of viability of H. pylori dormant forms that indicate saving of cellular integrity and DNA synthesis in 3-mo-old cultures[54].
Available literature data may suggest the existence of the following conditions for various forms of H. pylori (Figure 5): (1) Viable and culturable (spiral-shaped forms of H. pylori) states; (2) Dormant (resting) and culturable (most likely it is the C-shaped and U-shaped forms of H. pylori) states; and (3) Non-viable and unculturable (most likely it is the coccoid forms of H. pylori) states.
Figure 5.
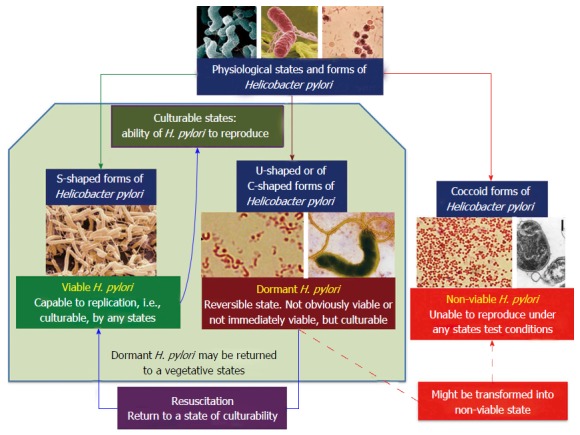
Major physiological states and forms of Helicobacter pylori[101]. H. pylori: Helicobacter pylori.
However, there are not enough solid data to associate the particular morphological type (C-shaped, U-shaped and coccoid forms) of H. pylori with the functional characteristics of viability and culturability[65]. As a rule, the literature presents data on the viability of either spiral or coccoid forms. When describing the latter, the presence of dormant (transitional, intermediate, resting) forms is not generally taken into account. The C-shaped and U-shaped forms of H. pylori are most likely in a dormant (resting) state and can be a viable and culturable (Figure 5)[101].
And if so, once under favorable conditions, the dormant (resting) forms of H. pylori can revert to a vegetative spiral-shaped form. By using electron microscopy, Konstantinova et al[102] have shown that there are various defects in the cell wall of the transformed forms of H. pylori. The authors point out that before the reversion of the dormant forms of H. pylori to vegetative forms, there is a need for certain conditions for the repair of cellular damages.
“Reanimated” H. pylori can play an important role in the development of recurrent PUD after anti-Helicobacter treatment[81,96]. The “revived” forms of H. pylori are able to colonize the GM to subsequently develop peptic ulcer relapse[78,95]. Continuation of investigations in this area may reveal new important aspects of the pathogenesis of H. pylori infection and to find new ways to treat diseases associated with this microorganism[69,89,96].
Figure 5 suggests a classification of major physiological states and forms of H. pylori that is a hypothetical scheme and requires further evidence. Existing conflicting data on viability and culturability of various forms of H. pylori fit well with this scheme and pass into the category of comparable data.
DORMANT FORMS OF H. PYLORI IN THE DEVELOPMENT OF RECURRENT PUD
The main challenge is to prove the reversion of transformable forms of H. pylori into a normal replicative state. There is still no clear separation between the true “revivals” of transformed forms of H. pylori that are usually present in the population and secondary infection with the microorganism.
Genetic typing of the same strain of the microorganism has become possible with advances in molecular diagnosis. By using the PCR-based restriction fragment length polymorphism (PCR-RFLP) analysis, H. pylori strains in patients with duodenal ulcer were genotyped before and 1 mo after anti-Helicobacter therapy with incomplete elimination[7,8,87,103,104]. The flaA gene (1.5 kb in size) encoding the flagellar protein is one of the polymorphic ones in H. pylori. This gene is used in genetic typing of H. pylori strains. Identical H. pylori strains were detected in the same patient before and after the anti-Helicobacter therapy. At that, heterogeneous H. pylori strains were found in different patients. The given data suggested that there was neither superinfection nor reinfection with a new strain at 1 mo after anti-Helicobacter therapy.
Warren and Marshall reported that eradication of the bacteria markedly reduced the relapse rate of duodenal ulcer[12]. H. pylori eradication decreases the recurrence rate of PUD from 50% to 0%-10% of cases per year[105]. Current treatment modalities allow eradication of the H. pylori bacterium in up to 90% of cases (less if there is clarithromycin resistance)[106]. During the first years after effective anti-Helicobacter therapy, the rate of H. pylori reinfection in adults is 0%-35%[107]. The rate of H. pylori reinfection varies according to geographical area[106]. Reinfection in developed countries is less common, in 0%-7% of cases[108]. In regions with higher socioeconomic status and lower prevalence of H. pylori, it is only 1.68% of cases[106]. The H. pylori reinfection rate in Lithuania is relatively high (the annual rate being 3.36%), probably because of the high prevalence of H. pylori[105]. This could indirectly reflect differences in the socioeconomic status between Western and Eastern European countries[106]. In contrast, in developing countries, the reinfection rate could be much higher and has been reported to reach 9.63%[106,109,110].
In some cases, recrudescence or reinfection of H. pylori may occur[106]. According to Loginov et al[7], 18.4% of H. pylori-positive patients were identified among those with H. pylori-associated duodenal ulcer a year after successful treatment. The recurrence rate of PUD in these patients was 14.3%, which comprised 2.6% of the total number of patients included in the study patients. Reinfection of H. pylori is observed rarely and occurs during later periods. Reinfection is considered when H. pylori is found after confirmed H. pylori eradication. H. pylori strains genetically different from the original ones are generally identified in reinfection.
PCR-RFLP was used to detect H. pylori strains in patients with duodenal ulcer before and 1 year after anti-Helicobacter therapy[7,103,104]. H. pylori strains were genotyped by the flaA gene. Genetic typing of H. pylori strains revealed both cases of the same strain of the bacterium in a patient before and 1 year after anti-Helicobacter therapy, as well as cases of its different strains. Gastroduodenoscopy (EGD) at 1 year after treatment revealed that all H. pylori-positive patients had a pattern of exacerbation of chronic antral gastritis. At that, 1 mo after anti-Helicobacter therapy, these patients were found to have no signs of any gastric and duodenal changes during EGD.
Identification of the pattern of chronic antral gastritis and different strains of the bacterium a year after anti-Helicobacter therapy performed in patients with duodenal ulcer could reveal a case of reinfection with a new H. pylori strain in the patient successfully treated against H. pylori. Identification of the pattern of chronic antral gastritis and the same strain of H. pylori a year after anti-Helicobacter therapy in patients with duodenal PUD most likely suggests that the bacterium is transformed from a dormant (resting) state into the vegetative form. Hence, for successful therapy, it is essential not only to eradicate the spiral forms of H. pylori, but to eliminate its viable dormant forms.
Factors contributing to the transformation of dormant (resting) forms of bacteria into the vegetative ones
Mukamolova et al[111] have identified specific bacterial cytokines from Mycobacterium tuberculosis, Mycobacterium avium, Micrococcus luteus, etc., and showed their important role in the activation and reproduction of the dormant forms of the bacterium[111-114]. Cultivation of M. luteus in the presence of a small number of colony-forming cells in the starving population has been ascertained to greatly facilitate the resuscitation of dormant cells[115]. The authors have suggested that the viable cells are able to secrete certain substances promoting the transition of dormant forms into an active reproduction state[111,112,116]. A 16-17 kDa protein, named resuscitation-promoting factor (Rpf), has been isolated[112-115]. The protein promoted the “revival” of the starving cells and reduced the lag phase of an active culture in both the depleted and enriched nutrient media. Using M. luteus as an example, Rpf has been shown to stimulate the “reanimation” of dormant cells. Rpf is a pheromone and belongs to the bacterial cytokines[111].
Structural changes in the reversion of coccoid forms of H. pylori in the vegetative state have not been studied and their triggers are unknown. There is no evidence that there are cytokine factors for the activation of H. pylori reversion and growth. The slightly acidic environment (pH of 5 to 3.5) is known to be a factor that activates the process of protein synthesis in H. pylori. Interestingly, in acting on coccoid and spiral-shaped forms, the same acidic pH values induce the synthesis of various proteins in them[78,117]. Some of the H. pylori proteins, heat shock protein (Hsp) in particular, have a trophic effect on the bacteria themselves and can cause rearrangement of the cell cytoskeleton, which may be a trigger for the reverse transformation of dormant forms into vegetative ones. Hsp synthesis is enhanced under the influence of a number of environmental factors.
These subcellular proteins belong to the chaperones essential for viability of the entire cellular profile of proteins involved in the processes of assembling for a variety of high-molecular-weight proteins[118]. H. pylori possesses two of the most studied chaperones: HspA (smaller) and HspB (larger), which are associated with urease assembling. HspA differs in its properties from analogous proteins of other bacteria. Being strong antigens, H. pylori chaperones take a certain part in the activation of lymphocytes, the regulation of cytokine and chemokine expression, the induction of apoptosis, etc. However, heat shock proteins play a much more important role in the induction of an autoimmune response due to the fact that they are highly antigenically similar to the orthologic structures of the GM[119]. It is possible that cytokine factors of the macroorganism can play an important role in the activation of bacterium dormant forms.
CONCLUSION
It is necessary to continue studies aimed at identifying specific cytokines or other metabolites of H. pylori, which are able to activate the transition of dormant forms of the microorganism into the vegetative spiral state. This will be able to design new anti-Helicobacter drugs to prevent the activation of dormant H. pylori forms, as well as recurrent duodenal ulcer.
ACKNOWLEDGMENTS
The authors thank Professor Pisarev VM for helpful discussions.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Reshetnyak VI and Reshetnyak TM declare no conflicts of interest exist in relation to this publication.
Peer-review started: April 8, 2017
First decision: April 21, 2017
Article in press: June 19, 2017
P- Reviewer: Abadi ATB, Kim GH, Yamada S S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
References
- 1.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–5204. doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai K, Suda H, Ido Y, Takeichi T, Okuda A, Hasuda K, Hattori M. Comparative study: Vonoprazan and proton pump inhibitors in Helicobacter pylori eradication therapy. World J Gastroenterol. 2017;23:668–675. doi: 10.3748/wjg.v23.i4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin LC, Hsu TH, Huang KW, Tam KW. Nonbismuth concomitant quadruple therapy for Helicobacter pylori eradication in Chinese regions: A meta-analysis of randomized controlled trials. World J Gastroenterol. 2016;22:5445–5453. doi: 10.3748/wjg.v22.i23.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghotaslou R, Leylabadlo HE, Asl YM. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J Methodol. 2015;5:164–174. doi: 10.5662/wjm.v5.i3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Past, present and future. World J Gastrointest Pathophysiol. 2014;5:392–399. doi: 10.4291/wjgp.v5.i4.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reshetniak VI, Dudik TV, Solov’eva NA, Zhukhovitskiĭ VG, Il’chenko AA, Kaprel’iants AS. [Eradication of Helicobacter pylori in patients with duodenal ulcer following the short course of treatment with azitromycin and amoxycillin] Antibiot Khimioter. 2002;47:16–19. [PubMed] [Google Scholar]
- 7.Loginov AS, Reshetniak VI, Mukamolova GV, Fedukova NG, Il’chenko AA, Dudik TV, Kaprel’iants AS. [The possibility of Helicobacter pylori being present in the resting state in the gastric mucosa of peptic acid patients after treatment] Ter Arkh. 1999;71:13–16. [PubMed] [Google Scholar]
- 8.Loginov AS, Reshetnyak VI, Dudik TV, Vostroknutova GN, Il’chenko AA, Kaprel’yants AS. Diagnostic methods for detecting forms and strains of Helicobacter pylori and evaluation of its eradication. Bull Exp Biol Med. 2001;132:802–806. doi: 10.1023/a:1013006717904. [DOI] [PubMed] [Google Scholar]
- 9.Testerman TL, Morris J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol. 2014;20:12781–12808. doi: 10.3748/wjg.v20.i36.12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandes YC, Bonatto Gda R, Bonatto MW. Recurrence rate of Helicobacter pylori in patients with peptic ulcer five years or more after successful eradication. Arq Gastroenterol. 2016;53:152–155. doi: 10.1590/S0004-28032016000300006. [DOI] [PubMed] [Google Scholar]
- 11.Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 12.Steensma DP, Kyle RA, Shampo MA. J. Robin Warren: Helicobacter pylori and peptic ulcer. Mayo Clin Proc. 2016;91:e129–e130. doi: 10.1016/j.mayocp.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 13.Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863–1873. doi: 10.1053/j.gastro.2009.01.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddique I, Al-Qabandi A, Al-Ali J, Alazmi W, Memon A, Mustafa AS, Junaid TA. Association between Helicobacter pylori genotypes and severity of chronic gastritis, peptic ulcer disease and gastric mucosal interleukin-8 levels: Evidence from a study in the Middle East. Gut Pathog. 2014;6:41. doi: 10.1186/s13099-014-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen YL, Mo XQ, Huang GR, Huang YQ, Xiao J, Zhao LJ, Wei HY, Liang Q. Gene polymorphisms of pathogenic Helicobacter pylori in patients with different types of gastrointestinal diseases. World J Gastroenterol. 2016;22:9718–9726. doi: 10.3748/wjg.v22.i44.9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidd M, Modlin IM. A century of Helicobacter pylori: paradigms lost-paradigms regained. Digestion. 1998;59:1–15. doi: 10.1159/000007461. [DOI] [PubMed] [Google Scholar]
- 17.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 18.van Amsterdam K, van Vliet AH, Kusters JG, van der Ende A. Of microbe and man: determinants of Helicobacter pylori-related diseases. FEMS Microbiol Rev. 2006;30:131–156. doi: 10.1111/j.1574-6976.2005.00006.x. [DOI] [PubMed] [Google Scholar]
- 19.Cellini L, Grande R, Di Campli E, Di Bartolomeo S, Di Giulio M, Traini T, Trubiani O. Characterization of an Helicobacter pylori environmental strain. J Appl Microbiol. 2008;105:761–769. doi: 10.1111/j.1365-2672.2008.03808.x. [DOI] [PubMed] [Google Scholar]
- 20.Rudnicka K, Graczykowski M, Tenderenda M, Chmiela M. [Helicobacter pylori morphological forms and their potential role in the transmission of infection] Postepy Hig Med Dosw (Online) 2014;68:219–229. doi: 10.5604/17322693.1092705. [DOI] [PubMed] [Google Scholar]
- 21.Mohammed SA. Prevalence of Helicobacter pylori among patients with different gastrointestinal disorders in Saudi Arabia. Med J Indones. 2017;25:214. [Google Scholar]
- 22.Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 23.Perry S, de la Luz Sanchez M, Yang S, Haggerty TD, Hurst P, Perez-Perez G, Parsonnet J. Gastroenteritis and transmission of Helicobacter pylori infection in households. Emerg Infect Dis. 2006;12:1701–1708. doi: 10.3201/eid1211.060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Júnior R, Fernandes AB, Santos FSD, Silva JHG, Loiola RPD, Silva JG, Pereira JO. Soroprevalência da infecção por Helicobacter pylori em uma amostra rural do Estado do Amazonas, Brasil Rev Pan-Amazônica de Saúde 2012; 3: 33-36. [Google Scholar]
- 25.Fonseca FM, Etchebehere RM, Oliveira AG. Helicobacter pylori infection in patients undergoing upper endoscopy at University Hospital in Uberaba, Minas Gerais, Brazil. JMPHC. 2013;4:33–35. [Google Scholar]
- 26.Oliveira JG, Ferreira CH, Camerin AC, Rota CA, Meurer L, Silveira TR. Prevalence of infection with cagA-positive Helicobacter pylori strains among children and adolescents in southern Brazil. Arq Gastroenterol. 2014;51:180–185. doi: 10.1590/s0004-28032014000300003. [DOI] [PubMed] [Google Scholar]
- 27.Bor S, Kitapcioglu G, Kasap E. Prevalence of gastroesophageal reflux disease in a country with a high occurrence of Helicobacter pylori. World J Gastroenterol. 2017;23:525–532. doi: 10.3748/wjg.v23.i3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wijetunge S, Kotakadeniya R, Noordeen F, Buharideen SM, Samarasinghe B, Dharmapala A, Galketiya KB. Prevalence of Helicobacter pylori in benign gastric ulcers in a cohort of Sri Lankan patients. Ceylon Med J. 2015;60:152–154. doi: 10.4038/cmj.v60i4.8224. [DOI] [PubMed] [Google Scholar]
- 29.Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19 Suppl 1:1–5. doi: 10.1111/hel.12165. [DOI] [PubMed] [Google Scholar]
- 30.German SV, Zykova IE, Modestova AV, Yermakov NV. [Prevalence of infection Helicobacter pylori in Moscow population] Russian J Gastroenterol, Hepatol, Coloproctol. 2010;20:25–30. [Google Scholar]
- 31.Das JC, Paul N. Epidemiology and pathophysiology of Helicobacter pylori infection in children. Indian J Pediatr. 2007;74:287–290. doi: 10.1007/s12098-007-0046-6. [DOI] [PubMed] [Google Scholar]
- 32.Algood HM, Cover TL. Helicobacter pylori persistence: an overview of interactions between H. pylori and host immune defenses. Clin Microbiol Rev. 2006;19:597–613. doi: 10.1128/CMR.00006-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rauws EA, Tytgat GN. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990;335:1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- 34.Blaser MJ, Parsonnet J. Parasitism by the “slow” bacterium Helicobacter pylori leads to altered gastric homeostasis and neoplasia. J Clin Invest. 1994;94:4–8. doi: 10.1172/JCI117336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaser MJ. Ecology of Helicobacter pylori in the human stomach. J Clin Invest. 1997;100:759–762. doi: 10.1172/JCI119588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Axon A. Helicobacter pylori is not a commensal. Curr Opin Gastroenterol. 1999;15:1–4. [Google Scholar]
- 37.Blaser MJ. Hypothesis: the changing relationships of Helicobacter pylori and humans: implications for health and disease. J Infect Dis. 1999;179:1523–1530. doi: 10.1086/314785. [DOI] [PubMed] [Google Scholar]
- 38.Blaser MJ. Helicobacter pylori and gastric diseases. BMJ. 1998;316:1507–1510. doi: 10.1136/bmj.316.7143.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ierardi E, Goni E, Losurdo G, Di Mario F. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2014;19 Suppl 1:27–31. doi: 10.1111/hel.12157. [DOI] [PubMed] [Google Scholar]
- 40.Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol. 2014;20:12767–12780. doi: 10.3748/wjg.v20.i36.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.da Costa DM, Pereira Edos S, Rabenhorst SH. What exists beyond cagA and vacA? Helicobacter pylori genes in gastric diseases. World J Gastroenterol. 2015;21:10563–10572. doi: 10.3748/wjg.v21.i37.10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim A, Servetas SL, Kang J, Kim J, Jang S, Cha HJ, Lee WJ, Kim J, Romero-Gallo J, Peek RM Jr, Merrell DS, Cha JH. Helicobacter pylori bab Paralog Distribution and Association with cagA, vacA, and homA/B genotypes in American and South Korean clinical isolates. PLoS One. 2015;10:e0137078. doi: 10.1371/journal.pone.0137078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- 44.Shkitin VA, Shpirna AI, Starovoitov GN. [Role of Helicobacter pylori in human pathology] Klinicheskaya Mikrobiologiya i Antimikrobnaya Khimioterapiya. 2002;4:128–145. [Google Scholar]
- 45.Yamaoka Y. Pathogenesis of Helicobacter pylori-Related Gastroduodenal Diseases from Molecular Epidemiological Studies. Gastroenterol Res Pract. 2012;2012:371503. doi: 10.1155/2012/371503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J, He C, Chen M, Wang Z, Xing C, Yuan Y. Association of presence/absence and on/off patterns of Helicobacter pylori oipA gene with peptic ulcer disease and gastric cancer risks: a meta-analysis. BMC Infect Dis. 2013;13:555. doi: 10.1186/1471-2334-13-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen RJ. Helicobacter-species classification and identification. Br Med Bull. 1998;54:17–30. doi: 10.1093/oxfordjournals.bmb.a011667. [DOI] [PubMed] [Google Scholar]
- 48.Whary MT, Fox JG. Natural and experimental Helicobacter infections. Comp Med. 2004;54:128–158. [PubMed] [Google Scholar]
- 49.Mateos-Muñoz B, Pérez-de-la-Serna J, Ruiz-de-León A, Serrano-Falcón B, Casabona-Francés S, Velasco-Cerrudo A, Rey-Díaz-Rubio E. Enterohepatic Helicobacter other than Helicobacter pylori. Rev Esp Enferm Dig. 2013;105:477–484. doi: 10.4321/s1130-01082013000800006. [DOI] [PubMed] [Google Scholar]
- 50.Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767–773. doi: 10.1172/JCI12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bardakhch’ian EA, Lomov SIu, Kharlanova NG, Kamneva NV. [Role of Helicobacter pylori in different extragastroduodenal diseases] Eksp Klin Gastroenterol. 2005;(3):20–27. [PubMed] [Google Scholar]
- 52.Chumpitaz Conde J, Gutiérrez Manay J, Córdova Acosta R, Sánchez Medina M, Vásquez Valverde N, Rivadeira Malaga C, Beteta Del Carpio O, Solano Mendoza L, Marocho Chahuayo L, Pareja Cuadros E, et al. [Isolation of helicobacter pylori in dental plaque in patients with gastritis at “Angamos” clinic] Rev Gastroenterol Peru. 2006;26:373–376. [PubMed] [Google Scholar]
- 53.Dubois A. Intracellular Helicobacter pylori and gastric carcinogenesis: an “old” frontier worth revisiting. Gastroenterology. 2007;132:1177–1180. doi: 10.1053/j.gastro.2007.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Rourke J, Bode G. Morphology and ultrastructure (Chapter 6). In: Mobley HLT, Mendz GL, Hazell SL, editors. Helicobacter pylori: physiology and genetics. Washington (DC): ASM Press. 2001 Available from: https://www.ncbi.nlm.nih.gov/books/NBK2452/ [PubMed] [Google Scholar]
- 55.Goodwin CS, McCulloch RK, Armstrong JA, Wee SH. Unusual cellular fatty acids and distinctive ultrastructure in a new spiral bacterium (Campylobacter pyloridis) from the human gastric mucosa. J Med Microbiol. 1985;19:257–267. doi: 10.1099/00222615-19-2-257. [DOI] [PubMed] [Google Scholar]
- 56.Jones DM, Curry A, Fox AJ. An ultrastructural study of the gastric campylobacter-like organism ‘Campylobacter pyloridis’. J Gen Microbiol. 1985;131:2335–2341. doi: 10.1099/00221287-131-9-2335. [DOI] [PubMed] [Google Scholar]
- 57.Qin Z, Lin WT, Zhu S, Franco AT, Liu J. Imaging the motility and chemotaxis machineries in Helicobacter pylori by cryo-electron tomography. J Bacteriol. 2016 doi: 10.1128/JB.00695-16. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sidebotham RL, Baron JH. Hypothesis: Helicobacter pylori, urease, mucus, and gastric ulcer. Lancet. 1990;335:193–195. doi: 10.1016/0140-6736(90)90279-e. [DOI] [PubMed] [Google Scholar]
- 59.Sasaki K, Tajiri Y, Sata M, Fujii Y, Matsubara F, Zhao M, Shimizu S, Toyonaga A, Tanikawa K. Helicobacter pylori in the natural environment. Scand J Infect Dis. 1999;31:275–279. doi: 10.1080/00365549950163572. [DOI] [PubMed] [Google Scholar]
- 60.Talebi Bezmin Abadi A, Perez-Perez G. Role of dupA in virulence of Helicobacter pylori. World J Gastroenterol. 2016;22:10118–10123. doi: 10.3748/wjg.v22.i46.10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makarenko EV, Voropaeva AV, Matveyenko ME. [The effect of Helicobacter pylori genotypes on morphological parameters of the gastric mucosa in patients with duodenal ulcer and chronic gastritis] Vestnik VGMU. 2009;8:88–96. [Google Scholar]
- 62.Azevedo NF, Almeida C, Cerqueira L, Dias S, Keevil CW, Vieira MJ. Coccoid form of Helicobacter pylori as a morphological manifestation of cell adaptation to the environment. Appl Environ Microbiol. 2007;73:3423–3427. doi: 10.1128/AEM.00047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaprelyants AS, Gottschal JC, Kell DB. Dormancy in non-sporulating bacteria. FEMS Microbiol Rev. 1993;10:271–285. doi: 10.1111/j.1574-6968.1993.tb05871.x. [DOI] [PubMed] [Google Scholar]
- 64.Bode G, Mauch F, Malfertheiner P. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol Infect. 1993;111:483–490. doi: 10.1017/s0950268800057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faghri J, Poursina F, Moghim S, Zarkesh Esfahani H, Nasr Esfahani B, Fazeli H, Mirzaei N, Jamshidian A, Ghasemian Safaei H. Morphological and Bactericidal Effects of Different Antibiotics on Helicobacter pylori. Jundishapur J Microbiol. 2014;7:e8704. doi: 10.5812/jjm.8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saito N, Konishi K, Sato F, Kato M, Takeda H, Sugiyama T, Asaka M. Plural transformation-processes from spiral to coccoid Helicobacter pylori and its viability. J Infect. 2003;46:49–55. doi: 10.1053/jinf.2002.1047. [DOI] [PubMed] [Google Scholar]
- 67.Mouery K, Rader BA, Gaynor EC, Guillemin K. The stringent response is required for Helicobacter pylori survival of stationary phase, exposure to acid, and aerobic shock. J Bacteriol. 2006;188:5494–5500. doi: 10.1128/JB.00366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams CL. Helicobacter pylori: bacteriology and laboratory diagnosis. J Infect. 1997;34:1–5. doi: 10.1016/s0163-4453(97)80002-3. [DOI] [PubMed] [Google Scholar]
- 69.Khomeriki SG, Morozov IA. The role of coccoid forms of Helicobacter pylori in pathogenetic mechanisms and persistence of Helicobacter infection. Russian J Gastroenterol, Hepatol, Coloproctol. 2001;11 Suppl 13:99–102. [Google Scholar]
- 70.Anuchin AM, Mulyukin AL, Suzina NE, Duda VI, El-Registan GI, Kaprelyants AS. Dormant forms of Mycobacterium smegmatis with distinct morphology. Microbiology. 2009;155:1071–1079. doi: 10.1099/mic.0.023028-0. [DOI] [PubMed] [Google Scholar]
- 71.Kudykina YK, Shleeva MO, Artsabanov VY, Suzina NE, Kaprel’iants AS. [Generation of dormant forms by Mycobacterium smegmatis in the poststationary phase during gradual acidification of the medium] Mikrobiologiia. 2011;80:625–636. [PubMed] [Google Scholar]
- 72.Kell DB, Kaprelyants AS, Weichart DH, Harwood CR, Barer MR. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Van Leeuwenhoek. 1998;73:169–187. doi: 10.1023/a:1000664013047. [DOI] [PubMed] [Google Scholar]
- 73.Tutelyan AV, Gaponov AM, Pisarev VM, El-Registan GI. [Microbial dormancy and prevention of healthcare-associated infections] Ter Arkh. 2015;87:103–108. doi: 10.17116/terarkh20158711103-109. [DOI] [PubMed] [Google Scholar]
- 74.Sarem M, Corti R. [Role of Helicobacter pylori coccoid forms in infection and recrudescence] Gastroenterol Hepatol. 2016;39:28–35. doi: 10.1016/j.gastrohep.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 75.West AP, Millar MR, Tompkins DS. Survival of Helicobacter pylori in water and saline. J Clin Pathol. 1990;43:609. doi: 10.1136/jcp.43.7.609-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Catrenich CE, Makin KM. Characterization of the morphologic conversion of Helicobacter pylori from bacillary to coccoid forms. Scand J Gastroenterol Suppl. 1991;181:58–64. [PubMed] [Google Scholar]
- 77.Moshkowitz M, Gorea A, Arber N, Konikoff F, Berger S, Gilat T. Morphological transformation of Helicobacter pylori during prolonged incubation: association with decreased acid resistance. J Clin Pathol. 1994;47:172–174. doi: 10.1136/jcp.47.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benaissa M, Babin P, Quellard N, Pezennec L, Cenatiempo Y, Fauchère JL. Changes in Helicobacter pylori ultrastructure and antigens during conversion from the bacillary to the coccoid form. Infect Immun. 1996;64:2331–2335. doi: 10.1128/iai.64.6.2331-2335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faizullina RA, Abdullina EV. Factors of pathogenicity and virulence of Helicobacter pylori in the development of Helicobacter-associated gastroduodenal pathology. Prakticheskaya Meditsina. 2011;1:74–78. [Google Scholar]
- 80.Nilius M, Ströhle A, Bode G, Malfertheiner P. Coccoid like forms (CLF) of Helicobacter pylori. Enzyme activity and antigenicity. Zentralbl Bakteriol. 1993;280:259–272. doi: 10.1016/s0934-8840(11)80964-3. [DOI] [PubMed] [Google Scholar]
- 81.Can F, Karahan C, Dolapci I, Demirbilek M, Tekeli A, Arslan H. Urease activity and urea gene sequencing of coccoid forms of H. pylori induced by different factors. Curr Microbiol. 2008;56:150–155. doi: 10.1007/s00284-007-9047-y. [DOI] [PubMed] [Google Scholar]
- 82.Hua J, Ho B. Is the coccoid form of Helicobacter pylori viable? Microbios. 1996;87:103–112. [PubMed] [Google Scholar]
- 83.Jiesong H, Megraud F. Evidence of the viability of non cultutable coccoidal forms of Helicobacter felis. Gut. 1995;37 Suppl 1:376. [Google Scholar]
- 84.Catrenich CE, Chestnut MH. Character and origin of vacuoles induced in mammalian cells by the cytotoxin of Helicobacter pylori. J Med Microbiol. 1992;37:389–395. doi: 10.1099/00222615-37-6-389. [DOI] [PubMed] [Google Scholar]
- 85.Sörberg M, Nilsson M, Hanberger H, Nilsson LE. Morphologic conversion of Helicobacter pylori from bacillary to coccoid form. Eur J Clin Microbiol Infect Dis. 1996;15:216–219. doi: 10.1007/BF01591357. [DOI] [PubMed] [Google Scholar]
- 86.Kusters JG, Gerrits MM, Van Strijp JA, Vandenbroucke-Grauls CM. Coccoid forms of Helicobacter pylori are the morphologic manifestation of cell death. Infect Immun. 1997;65:3672–3679. doi: 10.1128/iai.65.9.3672-3679.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loginov AS, Il'chenko AA, Mukamolova GV, Fedukova NG, Dudik TV, Kaprel'iants AS, Reshetniak VI. Comparative efficacy of different methods of detecting H. pylori in patients with peptic ulcer. Ross Gastroenterol Zh. 1998;3:3–11. [Google Scholar]
- 88.Andersen LP, Rasmussen L. Helicobacter pylori-coccoid forms and biofilm formation. FEMS Immunol Med Microbiol. 2009;56:112–115. doi: 10.1111/j.1574-695X.2009.00556.x. [DOI] [PubMed] [Google Scholar]
- 89.Bumann D, Habibi H, Kan B, Schmid M, Goosmann C, Brinkmann V, Meyer TF, Jungblut PR. Lack of stage-specific proteins in coccoid Helicobacter pylori cells. Infect Immun. 2004;72:6738–6742. doi: 10.1128/IAI.72.11.6738-6742.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Enroth H, Wreiber K, Rigo R, Risberg D, Uribe A, Engstrand L. In vitro aging of Helicobacter pylori: changes in morphology, intracellular composition and surface properties. Helicobacter. 1999;4:7–16. doi: 10.1046/j.1523-5378.1999.09034.x. [DOI] [PubMed] [Google Scholar]
- 91.Harvey P, Leach S. Analysis of coccal cell formation by Campylobacter jejuni using continuous culture techniques, and the importance of oxidative stress. J Appl Microbiol. 1998;85:398–404. doi: 10.1046/j.1365-2672.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 92.Cellini L, Allocati N, Angelucci D, Iezzi T, Di Campli E, Marzio L, Dainelli B. Coccoid Helicobacter pylori not culturable in vitro reverts in mice. Microbiol Immunol. 1994;38:843–850. doi: 10.1111/j.1348-0421.1994.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 93.Eaton KA, Catrenich CE, Makin KM, Krakowka S. Virulence of coccoid and bacillary forms of Helicobacter pylori in gnotobiotic piglets. J Infect Dis. 1995;171:459–462. doi: 10.1093/infdis/171.2.459. [DOI] [PubMed] [Google Scholar]
- 94.Poursina F, Faghri J, Moghim S, Zarkesh-Esfahani H, Nasr-Esfahani B, Fazeli H, Hasanzadeh A, Safaei HG. Assessment of cagE and babA mRNA expression during morphological conversion of Helicobacter pylori from spiral to coccoid. Curr Microbiol. 2013;66:406–413. doi: 10.1007/s00284-012-0280-7. [DOI] [PubMed] [Google Scholar]
- 95.Barer MR, Gribbon LT, Harwood CR, Nwoguh CE. The viable but non-culturable hypothesis and medical microbiology. Rev Med Microbiol. 1993;4:183–191. [Google Scholar]
- 96.Cellini L, Robuffo I, Di Campli E, Di Bartolomeo S, Taraborelli T, Dainelli B. Recovery of Helicobacter pylori ATCC43504 from a viable but not culturable state: regrowth or resuscitation? APMIS. 1998;106:571–579. [PubMed] [Google Scholar]
- 97.Donelli G, Matarrese P, Fiorentini C, Dainelli B, Taraborelli T, Di Campli E, Di Bartolomeo S, Cellini L. The effect of oxygen on the growth and cell morphology of Helicobacter pylori. FEMS Microbiol Lett. 1998;168:9–15. doi: 10.1111/j.1574-6968.1998.tb13248.x. [DOI] [PubMed] [Google Scholar]
- 98.Gribbon LT, Barer MR. Oxidative metabolism in nonculturable Helicobacter pylori and Vibrio vulnificus cells studied by substrate-enhanced tetrazolium reduction and digital image processing. Appl Environ Microbiol. 1995;61:3379–3384. doi: 10.1128/aem.61.9.3379-3384.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Andersen LP, Dorland A, Karacan H, Colding H, Nilsson HO, Wadström T, Blom J. Possible clinical importance of the transformation of Helicobacter pylori into coccoid forms. Scand J Gastroenterol. 2000;35:897–903. doi: 10.1080/003655200750022922. [DOI] [PubMed] [Google Scholar]
- 100.Mai U, Geis G, Leying H, Ruhl G, Opferkuch W. Dimorphism of Campylobacter pylori. Gastroduodenal pathology and Campylobacter pylori. In: Megraud F, Lamuliatte H, editors. Amsterdam: Elsevier Science; 1989. pp. 29–33. [Google Scholar]
- 101.Kell DB, Kenny LC. A Dormant Microbial Component in the Development of Preeclampsia. Front Med (Lausanne) 2016;3:60. doi: 10.3389/fmed.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Konstantinova ND, Zhukhovitskii VG, Didenko LV, Andreevskaya SG. Ultrastructural organization of Helicobacter pylori under natural conditions and during ex vivo culturing. Bull Exp Biol Med. 2001;131:299–301. doi: 10.1023/a:1017680205786. [DOI] [PubMed] [Google Scholar]
- 103.Loginov AS, Kaprel’iants AS, Reshetniak VI, Vostroknutova GN, Dudik TV, Ilchenko AA. On the possibility of genetic typing of H.pylori in gastric mucosa bioptates. Ross Gastroenterol Zh. 1999;4:5–9. [Google Scholar]
- 104.Dudik TV, Solov’eva NA, Zhukhovitskiĭ VG, Kirillov MIu, Kaprel’iants AS, Reshetniak VI. [Methods of Helicobacter pylori detection] Ross Gastroenterol Zh. 2001;(2):77–89. [PubMed] [Google Scholar]
- 105.O’Connor HJ, Kanduru C, Bhutta AS, Meehan JM, Feeley KM, Cunnane K. Effect of Helicobacter pylori eradication on peptic ulcer healing. Postgrad Med J. 1995;71:90–93. doi: 10.1136/pgmj.71.832.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jonaitis L, Kiudelis G, Slepavicius P, Kupcinskas L. High rate of Helicobacter pylori reinfection in Lithuanian peptic ulcer patients. World J Gastrointest Pathophysiol. 2016;7:181–185. doi: 10.4291/wjgp.v7.i1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miehlke S, Lehn N, Meining A, Bästlein E, Mannes GA, Stolte M, Bayerdörffer E. Helicobacter pylori reinfection is rare in peptic ulcer patients cured by antimicrobial therapy. Eur J Gastroenterol Hepatol. 1996;8:1161–1163. doi: 10.1097/00042737-199612000-00005. [DOI] [PubMed] [Google Scholar]
- 108.Ivashkin VT, Megro F, Lapina TL. Moscow: Publishing house “Triada-X”; 1999. Helicobacter pylori: revolution in gastroenterology; p. 255. [Google Scholar]
- 109.Yan TL, Hu QD, Zhang Q, Li YM, Liang TB. National rates of Helicobacter pylori recurrence are significantly and inversely correlated with human development index. Aliment Pharmacol Ther. 2013;37:963–968. doi: 10.1111/apt.12293. [DOI] [PubMed] [Google Scholar]
- 110.Harris AW, Misiewicz JJ, editors . London: Blackwell Healthcare Communication; 1996. Helicobacter pylori; p. 66. [Google Scholar]
- 111.Mukamolova GV, Kaprelyants AS, Young DI, Young M, Kell DB. A bacterial cytokine. Proc Natl Acad Sci USA. 1998;95:8916–8921. doi: 10.1073/pnas.95.15.8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shleeva MO, Mukamolova GV, Telkov MV, Berezinskaia TL, Syroeshkin AV, Biketov SF, Kaprel’iants AS. [Formation of nonculturable Mycobacterium tuberculosis and their regeneration] Mikrobiologiia. 2003;72:76–83. [PubMed] [Google Scholar]
- 113.Mukamolova GV, Murzin AG, Salina EG, Demina GR, Kell DB, Kaprelyants AS, Young M. Muralytic activity of Micrococcus luteus Rpf and its relationship to physiological activity in promoting bacterial growth and resuscitation. Mol Microbiol. 2006;59:84–98. doi: 10.1111/j.1365-2958.2005.04930.x. [DOI] [PubMed] [Google Scholar]
- 114.Shleeva M, Kondratieva T, Rubakova E, Vostroknutova G, Kaprelyants A, Apt A. Reactivation of dormant “non-culturable” Mycobacterium tuberculosis developed in vitro after injection in mice: both the dormancy depth and host genetics influence the outcome. Microb Pathog. 2015;78:63–66. doi: 10.1016/j.micpath.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 115.Votyakova TV, Kaprelyants AS, Kell DB. Influence of Viable Cells on the Resuscitation of Dormant Cells in Micrococcus luteus Cultures Held in an Extended Stationary Phase: the Population Effect. Appl Environment Microbiol. 1994;60:3284–3291. doi: 10.1128/aem.60.9.3284-3291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mukamolova GV, Turapov OA, Kazarian K, Telkov M, Kaprelyants AS, Kell DB, Young M. The Rpf gene of Micrococcus luteus encodes an essential secreted growth factor. Mol Microbiol. 2002;46:611–621. doi: 10.1046/j.1365-2958.2002.03183.x. [DOI] [PubMed] [Google Scholar]
- 117.Mizoguchi H, Fujioka T, Kishi K, Nishizono A, Kodama R, Nasu M. Diversity in protein synthesis and viability of Helicobacter pylori coccoid forms in response to various stimuli. Infect Immun. 1998;66:5555–5560. doi: 10.1128/iai.66.11.5555-5560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 119.Kansau I, Labigne A. Heat shock proteins of Helicobacter pylori. Aliment Pharmacol Ther. 1996;10 Suppl 1:51–56. doi: 10.1046/j.1365-2036.1996.22164005.x. [DOI] [PubMed] [Google Scholar]


